Abstract
Standard local control theory, which describes Ca2+ release during excitation–contraction coupling (ECC), assumes that all ryanodine receptor 2 (RyR2) complexes are equivalent. Findings from our laboratory have called this assumption into question. Specifically, we have shown that the RyR2 complexes in ventricular myocytes are different, depending on their location within the cell. This has led us to hypothesize that similar differences occur within the rat atrial cell. To test this hypothesis, we have triple-labelled enzymatically isolated fixed myocytes to examine the distribution and colocalization of RyR2, calsequestrin (Casq), voltage-gated Ca2+ channels (Cav1.2), the sodium–calcium exchanger (Ncx) and caveolin-3 (Cav3). A number of different surface RyR2 populations were identified, and one of these groups, in which RyR2, Cav1.2 and Ncx colocalized, might provide the structural basis for ‘eager’ sites of Ca2+ release in atria. A small percentage of the dyads containing RyR2 and Cav1.2 were colocalized with Cav3, and therefore could be influenced by the signalling molecules it anchors. The majority of the RyR2 clusters were tightly linked to Cav1.2, and, whereas some were coupled to both Ca 1.2 and Ncx, none were with Ncx alone. This suggests that Cav1.2-mediated Ca2+ -induced Ca2+ release is the primary method of ECC. The two molecules studied that were found in the interior of atrial cells, RyR2 and Casq, showed significantly less colocalization and a reduced nearest-neighbour distance in the interior, compared with the surface of the cell. These differences might result in a higher excitability for RyR2 in the interior of the cells, facilitating the spread of excitation from the periphery to the centre. We also present morphometric data for all of the molecules studied, as well as for those colocalizations found to be significant.
Key words: Atrial myocyte, Excitation–contraction coupling, Ryanodine receptor, Cav1.2, Sodium–Calcium exchanger, Caveolin-3, Calsequestrin
Introduction
Excitation–contraction coupling (ECC) in both atrial and ventricular myocytes depends on Ca2+-induced Ca2+ release (CICR). During CICR, a small influx of Ca2+ through voltage-gated calcium channels (Cav1.2) initiates a much larger release of Ca2+ from type 2 ryanodine receptors (RyR2) located in the adjacent junctional sarcoplasmic reticulum (SR) (Fabiato, 1983). In ventricular cells, a well-developed transverse and axial tubular system (TATS) spreads depolarization into the myocyte, resulting in a nearly synchronous SR Ca2+ release throughout the entire cell (Wier et al., 1995). Most atrial cells, however, lack such tubules and show a unique spatiotemporal pattern of Ca2+ release during ECC, characterized by a subsarcolemmal ring of elevated Ca2+ concentration, which variably propagates into the central bulk of the myocyte in direct proportion to cellular Ca2+ load and Ca2+ influx (Berlin, 1995; Huser et al., 1996; Mackenzie et al., 2001). Mackenzie and colleagues (Mackenzie et al., 2001) also showed that there are so-called ‘eager sites’ (those sites that respond very quickly to rises in Ca2+ concentration) along the periphery of atrial myocytes; these are separated by one or more ‘failure sites’, which exhibit weak regenerative Ca2+ responses with approximately one-quarter the magnitude of the ‘eager’ sites. Cav1.2 channels are primarily distributed along the periphery of atrial myocytes and are highly colocalized with RyR2 (Carl et al., 1995). However, although this colocalization explains the responsiveness of peripheral sites to electrical depolarization, it does not explain why the so-called eager and failure sites, both classified as junctional RyR2, respond so differently to electrical stimulation.
One of the many proteins associated with RyR2 in a macromolecular complex is calsequestrin (Casq) (Bers, 2004). Multiple studies have shown that Casq acts as a luminal Ca2+ sensor (Beard et al., 2005) and a regulatory protein that alters the open probability of RyR2 (Gyorke et al., 2004; Terentyev et al., 2007). This protein is distributed primarily along Z-lines and is highly colocalized with RyR2 throughout the ventricular myocyte (Scriven et al., 2000); however, its localization in atrial myocytes, particularly with respect to RyR2, is unknown.
Caveolin-3 (Cav3), the dominant isoform of caveolin in cardiomyocytes, is responsible for the formation of lipid rafts and caveolae in the cell membrane, but it is also found outside of these two structures (Patel et al., 2008). Rafts and/or caveolae have been postulated to play an important role in ECC; in the adult rat ventricular myocyte, removing cholesterol and disrupting the caveolae and/or rafts using methyl-β-cyclodextrin (MBC) reduced both the amplitude of the [Ca2+]i transient and the contraction (Calaghan and White, 2006). The use of MBC in rat arterial smooth muscle and neonatal cardiomyocytes, which lack a defined TATS, much like atrial myocytes, resulted in a reduction in the frequency, width and amplitude of Ca2+ sparks, without modulation of the current through the Cav1.2 channels (Lohn et al., 2000). Their data suggest that caveolin might have a role in regulating local SR Ca2+ release, and might indicate the presence of Cav1.2 channels near Cav3.
The type 1 sodium–calcium exchanger (Ncx) is known to be the sole member of the Ncx family of proteins present in the cardiomyocyte (Quednau et al., 1997) and has long been implicated in the regulation of ECC. First viewed as a possible alternative mechanism by which to activate CICR, it is now largely agreed that Ncx acts as a modulator of the process in cardiac myocytes (Bers, 2008). Structural data (Scriven et al., 2005) demonstrate that, whereas both couplons (Cav1.2 and RyR2) and Ncx molecules are closely associated with Cav3 on the surface of rat ventricular myocytes, they are only sparsely colocalized with one another within the cell interior. However, other studies have shown that Ncx is separate from Cav3, both in developing (Lin et al., 2009) and adult (Cavalli et al., 2007) ventricular cardiomyocytes. Owing to a lack of a well-developed TATS, Ncx is largely distributed along the cell periphery in atria (Bootman et al., 2006) but might colocalize with proteins such as RyR2 and Cav1.2 channels there. If so, it could act as an important modulator of ECC.
Results
Controls
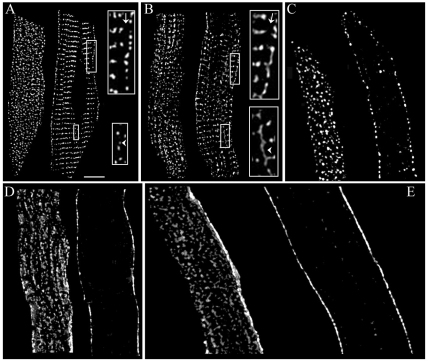
Before beginning the triple-labelling experiments, a series of controls was performed in order to ensure the validity of our protocols. First, rat atrial myocytes were labelled with antibodies against RyR2, Cav1.2, Casq, Ncx or Cav3 individually. This was done in order to examine not only the distribution of each protein, but also to ensure that the labelling characteristics did not change when the cells were incubated with multiple antibodies simultaneously. Deconvolved images from the surface and interior (left- and right-hand panels respectively, for each pair of images) of the labelled cells are shown in Fig. 1. At the surface, antibodies against both RyR2 and Casq (Fig. 1A,B, left-hand panels) displayed highly punctate labelling, spread fairly evenly across the membrane. In the interior of the myocyte (Fig. 1A,B, right-hand panels), the labelling of these two proteins had a similar ladder-like pattern. Discrete clusters of both RyR2 and Casq lined up largely along the Z-lines but also appeared in some longitudinal elements between Z-lines (arrows). We also confirmed the presence of a small subsarcolemmal gap in the RyR2 labelling (Carl et al., 1995; Mackenzie et al., 2001), and show that this phenomenon also exists for the Casq distribution in atrial cells (arrowheads).
Fig. 1.
Single-labelling of atrial myocytes. An atrial myocyte segment labelled with antibodies against RyR2 (A), Casq (B), Cav1.2 (C), Ncx (D) or Cav3 (E). For each antibody, the image shown is either a 1.5-μm-thick section taken from the cell surface (left panels), or a single 250-nm plane taken from approximately the middle of the cell (right panels). For the interior segments of RyR2 (A) and Casq (B), there is a discrete subsarcolemmal ‘gap’ with no labelling of either protein (upper inset, arrows), whereas the lower inset shows that some longitudinal elements of labelling are present (arrowheads). Scale bar: 5 μm.
Fig. 1C–E shows the distribution of Cav1.2, Ncx and Cav3 proteins, respectively. At the surface (Fig. 1C, left-hand panel), clusters of Cav1.2 were spread evenly across the membrane. Ncx labelling on the surface (Fig. 1D, left-hand panel), by contrast, was dense but somewhat uneven, and appeared as even larger aggregates than those of Cav1.2. Note that the very bright staining at one side of the membrane is an artefact created by imaging the sarcolemma in parallel with the optical axis of the microscope (edge effect). An antibody directed against the Cav3 protein also collected in large clusters on the sarcolemmal membrane (Fig. 1E, left-hand panel) that appeared to be oriented in longitudinal striations. Cav1.2, Ncx and Cav3 proteins were only expressed to a very small degree in the interior of atrial myocytes (Fig. 1C–E, right-hand panels) and the antibodies against these three proteins label only faintly in this region. This labelling probably represents either synthesis and/or degradation of the proteins or the presence of rudimentary t-tubules in some atrial cells.
The ability of the Zenon kits to eliminate cross-reaction between the secondary antibodies against two monoclonal primary antibodies was tested using primary antibodies against proteins that are known to localize to distinct regions of the cell (RyR2 and the nuclear pore complex). We labelled myocytes simultaneously with these two antibodies using the manufacturer's protocol and were able to confirm that there was no cross-talk between the two labels (supplementary material Fig. S1). The results of these control experiments confirmed that we could label with two monoclonal antibodies simultaneously without fear of cross-reaction.
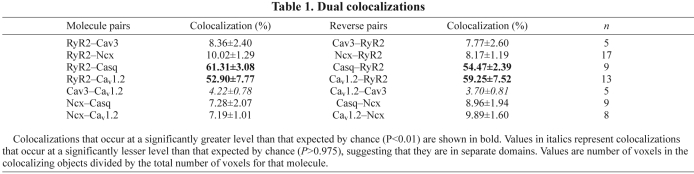
The final step in confirming the validity of our triple-labelling experiments was to compare the colocalization values between pairs of proteins that occurred in more than one experiment, to see whether they were the same as one another. We found no significant differences (see supplementary material Table S1 for a detailed analysis), and, for this reason, pooled the results for the dual colocalizations that were common to more than one experiment (Table 1). With the completion of these control experiments and calculations, we were confident that the labelling characteristics of each protein involved in our triple-labelling experiments are independent of one another and that our results are a true indication of the structural organization of atrial myocytes and the association of these proteins within the cells.
Table 1.
Dual colocalizations
Triple-labelling experiments
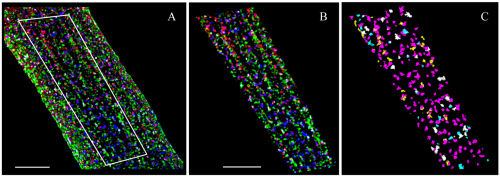
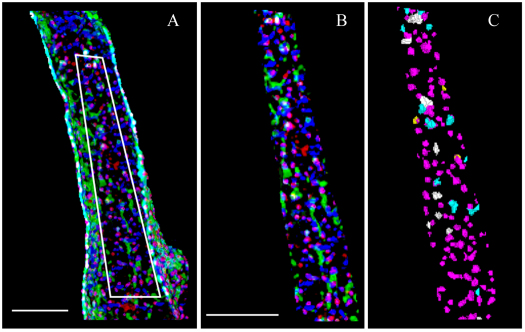
The first of three triple-labelling experiments reported here examines the potential association between RyR2, Ncx and Cav1.2 in rat atria. Fig. 2 shows a section from a representative atrial cell, with RyR2 in blue, Ncx in green and Cav1.2 in red. Each image (A–C) is the same 1-μm-thick layer from the surface of the myocyte. Fig. 2A shows the entire field of view; this exhibited a significant edge effect because of the abundance of Ncx labelling. In this region, the cell curvature caused an increase in apparent colocalization owing to the reduced resolution along the z-axis (Scriven et al., 2008). To prevent the edge effect from causing errors in our calculations, we used the cell segment shown in Fig. 2B (outlined segment in Fig. 2A) to measure colocalization. Fig. 2C shows the colocalization between the three molecules. The primary association appears to be between RyR2 and Cav1.2 (magenta; Table 1). There were also a significant number of RyR2–Cav1.2–Ncx triplets (white; Table 2). Neither the Ncx–RyR2 (cyan) nor the Cav1.2–Ncx (yellow) represent significant colocalizations (Table 1). Ncx seems to be mainly organized into large clusters that are either completely separate from the RyR2–Cav1.2 unit, and do not associate with either protein (e.g. Fig. 2B), or that are integral to the RyR2–Cav1.2 unit.
Fig. 2.
Triple-labelling of RyR2, Ncx and Cav1.2. An atrial myocyte labelled with antibodies against RyR2 (blue), Ncx (green) and Cav1.2 (red). All images are 1-μm-thick slices from the cell surface. (A) View of the entire cell, showing the surface distribution and colocalization of RyR2, Ncx and Cav1.2. (B) Segment of the cell surface from the area shown in A. (C) The same segment, showing only the object colocalization of RyR2–Cav1.2–Ncx (white), RyR2–Cav1.2 (magenta), Ncx–RyR2 (cyan) and Ncx–Cav1.2 (yellow). Scale bars: 5 μm.
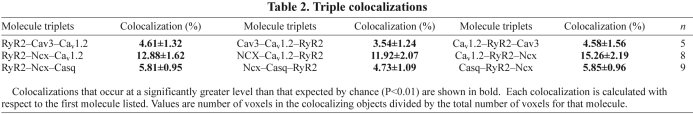
Table 2.
Triple colocalizations
Fig. 3 shows triple labelling of RyR2 (blue), Casq (red) and Ncx (green) in a 1-μm-thick segment from the cell surface of a representative atrial myocyte. The full cell is shown in Fig. 3A, the outlined segment shows the area that excluded the edges and was used for the analysis. Fig. 3B is an expanded version of the boxed region in Fig. 3A, whereas Fig. 3C shows the object colocalization between the three molecules. The most dominant form of association between these proteins was RyR2 with Casq alone (magenta), with about 50% of the voxels colocalized (Table 1). There were a significant number of RyR2–Casq–Ncx triplets (white; Table 2), as well as a few dual colocalizations [Ncx–RyR2 (cyan) and Ncx–Casq (yellow)] neither of which is significant (Table 1). It is worth noting that there was a large amount of Ncx that was not colocalized with either RyR2 or Casq (Fig. 3B).
Fig. 3.
Triple-labelling of RyR2, Ncx and Casq. An atrial myocyte segment labelled with antibodies against RyR2 (blue), Ncx (green) and Casq (red). All images are 1-μm-thick slices from the cell surface. (A) View of the entire cell, showing surface distribution and colocalization of RyR2, Ncx and Casq. (B) Segment of the cell surface from the area shown in A. (C) The same segment, showing only the object colocalization of RyR2–Ncx–Casq (white), RyR2–Casq (magenta), RyR2–Ncx (cyan) and Ncx–Casq (yellow). Scale bars: 5 μm.
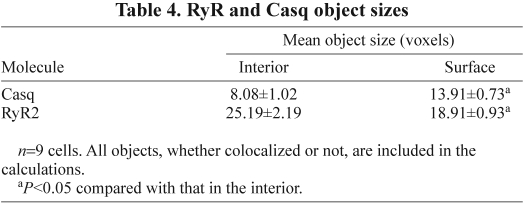
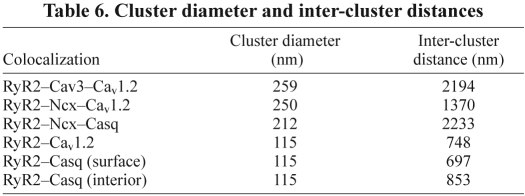
RyR2 and Casq are the only molecules studied that have a substantial presence in the interior of the atrial cell. A comparison of the surface and interior colocalization of RyR2 and Casq (Table 3), showed that there was significantly less colocalization in the interior than at the surface, irrespective of whether one compares object colocalization or the number of voxels in the colocalized objects. In addition, the size of both the RyR2 and Casq objects differs significantly between the interior and surface, with Casq smaller in the interior, whereas the RyR2 was larger (Table 4). Furthermore, the median nearest-neighbour distances for both RyR and Casq were significantly less (P<0.05) in the interior of the cell than they were on the surface (Table 5), although neither the cluster diameter nor the inter-cluster distance differed between the interior and surface (Table 6).
Table 3.
RyR–Casq colocalization
Table 4.
RyR and Casq object sizes
Table 5.
Median nearest-neighbour distances
Table 6.
Cluster diameter and inter-cluster distances
In Fig. 4, we show an atrial myocyte labelled with antibodies against RyR2 (blue), Cav1.2 (red) and Cav3 (green). Fig. 4A shows a 1-μm-thick layer, from the bottom cell surface, with a marked edge effect. In Fig. 4B, we display the segment outlined in Fig. 4A that excludes the edge regions. Object colocalization is shown in Fig. 4C, with the triple colocalization RyR2–Cav1.2–Cav3 (white; Table 2) and the colocalization between RyR2 and Cav1.2 (magenta; Table 1) being highly significant. Colocalization of RyR2 with Cav3 (cyan) was not significant, whereas the observed values for the Cav3–Cav1.2 pair (yellow) were much less than those expected by chance (Table 1), indicating that these two molecules are probably in separate domains. The large colocalization between RyR2 and Cav1.2 was reflected in the large number of tightly coupled magenta clusters with a few triplets (white) interspersed.
Fig. 4.
Triple-labelling of RyR2, Cav3 and Cav1.2. An atrial myocyte labelled with antibodies against RyR2 (blue), Cav3 (green) and Cav1.2 (red). All images are 1-μm-thick slices from the cell surface. (A) View of the entire cell, showing surface distribution and colocalization of RyR2, Cav3 and Cav1.2. (B) Segment of the cell surface from the area shown in A. (C) The same segment, showing only the object colocalization of RyR2–Cav3–Cav1.2 (white), RyR2–Cav1.2 (magenta), Cav3–Cav1.2 (yellow) and RyR2–Cav3 (cyan). Scale bars: 5 μm.
Our analysis technique (Fletcher et al., 2010) allowed us to derive three useful metrics for describing the positioning of the molecules within the atrial cell; these are the median nearest-neighbour distance of the individual molecules (Table 5); the cluster diameter, a measure of how closely associated the colocalized molecules are (Table 6); and the inter-cluster distance, a measure of how separated the colocalizations are. The values in Table 6 include only those colocalizations that were statistically significant (see Tables 1 and 2).
Discussion
A number of our results correspond well with earlier findings in rat ventricular myocytes (Scriven et al., 2000). The association of RyR2 with Ncx in atrial cells was not significantly different from that expected by chance, and atrial RyR2 had large and significant colocalizations with both Cav1.2 and Casq. The nearest-neighbour distances for the surface RyR2 and Cav1.2 (Table 5), as well as the inter-cluster distance for the RyR2–Cav1.2 (Table 6), were remarkably similar to those found in the ventricle (Scriven et al., 2010), with differences of 20 nm or less in their values. In addition, the nearest-neighbour distance for Cav3 indicated that its density was higher than that for either RyR2 or Cav1.2, which agrees with our earlier finding from the ventricle surface (Scriven et al., 2005). Ncx had a nearest-neighbour value (and thus density) similar to that of Cav3, although we do not have values from the ventricle surface that we can compare it with. Results that differ from those in the ventricle involve Cav3: RyR2 and Cav3 alone were not significantly colocalized, unlike ventricular myocytes, where surface Cav3 seems to associate equally with RyR2 and Cav1.2, presumably at the dyads (Scriven et al., 2005). In atrial cells, there was a small but significant population of RyR2, Cav1.2 and Cav3 triplets (Table 2).
The role of Ncx in ECC has been subject of an extended debate. Numerous models with various assumptions have produced conflicting results (Sher et al., 2008), and, although it is well established that Ncx plays a major role in Ca2+ extrusion in the cardiomyocyte, its contribution to the Ca2+ influx, which could trigger CICR, is uncertain. Evidence from rabbit neonatal ventricular cells, which do not have a well-developed TATS, as for atrial myocytes, shows that they have a heightened level of RyR2–Ncx colocalization (Dan et al., 2007) and that they rely on Ncx-mediated CICR (Huang et al., 2008) during their early development. Our results indicate that this mechanism is absent in the mature atria; as there was no significant colocalization between RyR2 and Ncx, couplons consisting solely of these two molecules (and excluding Cav1.2) are unlikely to exist and cannot contribute to CICR. As ~50% of the RyR2 colocalized only with Cav1.2 channels, it is probable that Cav1.2-mediated CICR is the primary method of initiating Ca2+ release in the atrial myocyte.
We found a statistically significant number (~13%) of RyR2–Cav1.2–Ncx triplets on the atrial surface, with a spacing of approximately twice that of the simple RyR2–Cav1.2 couplon (Table 6). This result raises questions as to the role of Ncx in these triplets and why only a limited number of dyads are involved. Bers (Bers, 2008) argues that, if the Ncx faces the restricted space of the dyadic cleft, the high Ca2+ concentrations that exist during the action potential would limit the role of Ncx to Ca2+ extrusion. Simulations by Noble and colleagues (Sher et al., 2008) indicate that, although Ncx primarily acts as a agent of Ca2+ extrusion and does not significantly contribute to Ca2+ influx, it might increase the probability of Cav 1.2 opening by reducing [Ca2+] in the dyadic space, thus decreasing Ca2+-dependent inactivation. Furthermore, Noble's group found that at least 10%, but no more than 18%, of the Ncx should be colocalized with RyR2 for their model to reproduce previously measured currents. Although the model was designed for ventricular tissue, these findings suggest that the RyR2, Cav1.2 and Ncx triplets represent a subgroup of dyads with increased excitability. We hypothesize that these dyads form the structural basis for the ‘eager sites’ of Ca2+ release (Mackenzie et al., 2001) at the surface of atrial myocytes.
Another small (~3%) but significant group of triplets is RyR2–Ncx–Casq, whose sparsity is reflected in their large intercluster distances (Table 6). Although it is possible that these represent RyR2–Ncx dyads modulated by Casq, this seems unlikely given the absence of this type of dyad without Casq. We believe that this is a subgroup of the RyR2–Cav1.2–Ncx conglomeration, in which the Ncx is close enough (probably in the dyad) to colocalize with Casq. As Casq does not always colocalize with RyR2, we can split the RyR2–Cav1.2–Ncx group into two – one in which the RyR2 excitability is modulated by Casq, and one in which it is not. Confirmation of this hypothesis would require quadruple labelling so that we can look at all four proteins simultaneously.
If we assume that Casq acts as both a luminal Ca2+ sensor for, and a Po inhibitor of, RyR2 channels when it is bound (Gyorke et al., 2004; Terentyev et al., 2007), the lower colocalization of RyR2 and Casq in the interior of atrial myocytes means it is probable that they are more excitable than their surface counterparts. This would facilitate the diffusion of Ca2+ waves from the periphery to the centre, which is necessary in cells lacking a t-tubular structure. In addition, the nearest-neighbour distances for both RyR2 and Casq are significantly less in the interior than on the surface of the cell (Table 5), which would increase the likelihood of inward diffusion of the Ca2+ waves. Woo and colleagues (Woo et al., 2003a) noted a larger number of combined sparks (i.e. sparks that consisted of two to five single sparks) in the centre of atrial cells compared with that evident in their periphery, which could support a role for Casq as a modulator of excitability. They also reported that spontaneous Ca2+ sparks are more frequent in the cell periphery than in the interior, but attributed this to an interaction between the α1c C-terminal tail of Cav1.2 and RyR2 (Woo et al., 2003b), which occurs only in the cell periphery, rather than to any effect of Casq. A second significant finding is that the sizes of the Casq clusters (both colocalized and not) are significantly smaller in the interior of the cell compared with at the periphery (Table 4). Although it is difficult to make an exact correspondence between voxel numbers and molecule density, it seems probable that there is much less Casq within the cell than along its edge, which might represent smaller stores of Ca2+. By contrast, RyR2 clusters are larger in the interior than in the exterior. It is not clear whether this is due to the limited space available at the surface or to some functional difference.
An indication of the position of Ncx in relation to the RyR2–Cav1.2 is given by the cluster diameters of RyR2–Ncx–Cav1.2 and RyR2–Ncx–Casq, which were approximately twice those of RyR2–Cav1.2 and RyR2–Casq (Table 6). This suggests that although Ncx is close enough to the RyR2–Cav1.2 couplon to influence [Ca2+] within the dyadic space, it is further away from RyR than is Cav1.2, and it is not in contact with these molecules. This means that it is unlikely that either fluorescence resonance energy transfer (FRET) or immunoprecipitation could be used to confirm this result. Furthermore, a recent paper (Nichols et al., 2010) has shown that, despite their well-known close association in both the ventricular and atrial cardiomyocyte, RyR2 and Cav1.2 do not co-immunoprecipitate, making it unlikely that RyR2 and Ncx would do so.
The two significant dual colocalizations, RyR–Cav1.2 and RyR–Casq (Table 1), as measured in different experiments, have colocalization values that are not significantly different from each other and sum to greater than 100% of the total RyR. In addition, they have identical cluster diameters and similar intercluster distances (Table 6). In the ventricle, electron micrographs show that Casq is associated with the couplon (Franzini-Armstrong et al., 1987), so it is probable that there is significant overlap between the two groups, and that they form the triplet RyR–Cav1.2–Casq. If there is complete overlap between the two groups, and all of the RyR is in the triplet, summing the significant colocalizations indicates that over 20% of the RyR would not be accounted for. There are a number of possibilities: not all of the RyR is in the triplet, and the three associations RyR–Cav1.2, RyR–Casq and RyR–Cav1.2–Casq are all present; some of the RyR is by itself; or RyR is associated with a molecule that we have not tested for. These possibilities are not mutually exclusive. As shown above, ‘naked’ RyR is definitely present in the interior of the atrial cell, so it is not far-fetched to suggest its existence on the atrial surface.
A surprising finding was that there was no significant pairing between Cav3 with either RyR2 or Cav1.2: the only significant association of Cav3 that we found was in the triplet RyR2–Cav1.2–Cav3, which we suspect is due to dyads being close to a group of Cav3 molecules. The proportion of total RyR2 colocalized with Cav3 alone is not statistically significant, which suggests that Cav1.2 channels in the RyR2–Cav1.2–Cav3 group are immediate neighbours of Cav3, rather than occupying lipid rafts or caveolae at these sites. Thus, a small proportion of the dyads are close enough to be affected by signalling molecules associated with Cav3, but the cluster diameters (Table 6) indicate that, although Cav3 is close to the couplon, it is probably not in physical contact with the sarcolemmal Cav1.2. The functional consequences of this association are difficult to estimate; there are multiple signalling molecules (e.g. β-adrenergic and A1 receptors) associated with Cav3. Cholesterol removal, which disrupts caveolae and lipid rafts, has been shown to cause a reduction in the frequency, width and amplitude of Ca2+ sparks (Lohn et al., 2000), although it is not clear whether this effect is due to a disruption of signalling between Cav3-associated molecules and the dyad or to the deleterious effects of cholesterol removal from the membrane. If it is the former, molecules associated with Cav3 might alter the open probability of RyR2, resulting in a change in the spark characteristics.
Because they were derived from different experiments, one could theorise that the two triplets RyR2–Cav1.2–Ncx and RyR2–Cav1.2–Cav3 actually represent the quadruplet RyR2–Cav1.2–Cav3–Ncx. However, as the number of RyR2–Cav1.2–Cav3 triplets is far fewer than those of RyR2–Cav1.2–Ncx (Table 2), and their intercluster distances are very different (Table 6), it is unlikely that the groups match up. Even if significant numbers of the quadruplet did exist, there would still be a large amount of unmatched RyR2–Cav1.2–Ncx.
Finally, isolated Cav1.2 was significantly less colocalized with Cav3 than one would expect by chance, implying that these two molecules are specifically directed to separate cellular domains. This differs from ventricular tissue, where Cav3 and Cav1.2 are colocalized on the cell surface (Scriven et al., 2005; Balijepalli et al., 2006).
Conclusions
We have provided evidence to support our hypothesis that atrial RyR2 complexes are heterogeneous and can be targeted to specific intracellular domains. In particular, we have identified a number of surface populations of RyR2, on the basis of its binding partners, that we believe are structurally and functionally distinct. These are: RyR with Cav1.2 and Casq (couplons modulated by Casq); RyR2 with Cav1.2 and Cav3 (couplons modulated by molecules associated with Cav3). RyR2 with Cav1.2 and Ncx (the eager sites); and RyR2 with Cav1.2, Casq and Ncx (eager sites modulated by Casq). In addition, the following might also be present: RyR2 with Cav1.2, RyR2 with Casq, and RyR2 alone; however, their relative proportions are unclear. We also conclude that Cav1.2-mediated CICR is the dominant form in atrial myocytes and that there is no evidence to support a RyR2–Ncx-mediated CICR. Finally, we have found that the RyR and Casq within the cell interior is different from that on the surface, and these differences might assist in the propagation of the Ca2+ wave into the cell interior.
One of the central tenets of local control theory – that all couplons are functionally equivalent – is challenged by the discovery of the different RyR2 complexes shown here. Although each individual RyR2 complex might be independently controlled, their molecular constituents and binding partners are highly unlikely to be identical.
Materials and Methods
All chemicals were purchased from Sigma–Aldrich unless otherwise stated. Animal handling was performed in accordance with the guidelines of the Canadian Council on Animal Care.
Cell isolation and preparation
Atrial myocytes were isolated from freshly excised hearts using the method of Rodrigues and Severson (Rodrigues and Severson, 1997). Briefly, adult male Wistar rats weighing 200–250 g were given 200 units of heparin (Organon Canada, Toronto, ON) intraperitoneally at 15–20 minutes prior to killing with sodium pentobarbital (20 mg per 100 g of body weight; MTC Pharmaceuticals, Cambridge, ON). The heart was immediately excised and hung for retrograde Langendorff perfusion with warm (37°C) Joklik MEM (M0518) supplemented with 23 mM NaHCO3, 1.2 mM MgSO4, and 1 mM DL-carnitine (final concentrations), and equilibrated with 95% O2 and 5% CO2. Perfusion was adjusted to give a flow of 7 ml/minute and maintained for 5 minutes to drain the heart of blood. It was then switched to a Joklik solution containing 162 units/ml type II collagenase (Worthington Biochemical, Lakewood, NJ). Once the heart softened, the atria were separated from the ventricles, minced and filtered through a Nitex nylon mesh (200 μm). Preparations contained ~30–50% rod-shaped quiescent cells, which were incubated for 20 minutes at 37°C in M-199 (M4530) supplemented with 25 mM HEPES (pH 7.4), 1 mM DL-carnitine, 0.1 mM insulin, 0.56 mM penicillin, 0.14 mM streptomycin sulphate, 2 mM EGTA and 0.01 g/ml fatty-acid-free BSA (final concentrations). Cells were then fixed for 10 minutes in freshly made 2% paraformaldehyde. Fixation was quenched with 100 mM glycine (pH 7.4) for 10 minutes, after which cells were washed three times for 10 minutes each in PBS (137 mM NaCl, 8 mM NaH2PO4, 2.7 mM KCl and 1.5 mM KH2PO4, pH 7.4). Cells were then permeabilized with 0.1% Triton X-100 for 10 minutes followed by three 10-minute washes in PBS.
Immunolabelling
Primary antibodies were against: RyR2 (mouse monoclonal; MA3-916, Affinity BioReagents, Golden, CO); calsequestrin (rabbit polyclonal; PA1-913, Affinity BioReagents); Cav3 (mouse monoclonal; 610420, BD Biosciences); Ncx (mouse monoclonal; R3F1, Swant, Bellinzona, Switzerland); and Cav1.2 [rabbit polyclonal; CNC1, a gift from William Catterall (Hell et al., 1993)]. Secondary antibodies were affinity purified and highly cross-adsorbed to minimize species cross-reactivity, and were either goat anti-rabbit-IgG or goat anti-mouse-IgG conjugated to Alexa Fluor 350, 488 or 594 (Invitrogen).
Incubations involving one monoclonal and one polyclonal primary antibody were performed sequentially overnight at 4°C. All labelling experiments involving two monoclonal primary antibodies were performed using mouse IgG1 Zenon labelling kits (Invitrogen). Experiments involving one polyclonal and two monoclonal antibodies were performed in one of two ways. In the first method, cells were incubated with one monoclonal antibody overnight at 4°C, and then with an anti-mouse-IgG secondary antibody for 1.5 hours at room temperature. The second monoclonal primary was conjugated to its fluorophore using a Zenon labelling kit and then added to cells for 1 hour at room temperature. The third primary antibody, a polyclonal, was added to cells overnight at 4°C and then an anti-rabbit-IgG secondary antibody conjugated to a chosen fluorophore was added for 1.5 hours at room temperature. In the second method, cells were incubated with the polyclonal antibody overnight at 4°C and then with an anti-rabbit-IgG secondary antibody for 1.5 hours at room temperature. The myocytes were then incubated simultaneously with the two monoclonal antibodies that had been conjugated to their appropriate fluorophore using a mouse IgG1 Zenon labelling kit. For each triple labelling experiment, an appropriate control experiment was performed in order to ensure no cross-reaction between the monoclonal antibodies.
Control experiments
We used the Zenon kit (Invitrogen) to label monoclonal antibodies against the RyR and the nuclear pore complex (NPC). These two molecules are known to be in separate domains. Supplementary material Fig. S1 shows the results from this experiment. RyR and its corresponding secondary antibody (Alexa-Fluor-594-conjugated; shown in red) and NPC (Alexa-Fluor-488-conjugated; shown in green) do indeed stay localized to separate regions of the cell – the Z lines (arrow) and nuclear pore region (arrowhead), respectively. There is negligible red labelling in the nuclear region and/or green labelling at the Z-lines, indicating no cross-reaction between the secondary antibodies. Colocalization analysis of these two proteins confirms what we see in Fig. 2 (0.32% RyR colocalizes with NPC; 6.88% NPC colocalizes with RyR; whole-cell values). The higher percentage of NPC with RyR is a result of a much lower density of NPC as compared with RyR, and also of colocalization along the z-axis where RyR lies on top of, and underneath, the nuclear membrane. We also performed this experiment by labelling RyR overnight at 4°C, then adding a goat Alexa-Fluor-594-conjugated anti-mouse IgG1 secondary antibody for 1.5 hours at room temperature. This was followed by incubation with a NPC antibody that had been conjugated to a goat Alexa-Fluor-488-conjugated anti-mouse IgG secondary antibody with the Zenon labelling kit. The results were identical to the image in supplementary material Fig. S1 (data not shown), and therefore confirmed a second protocol that could be used to label cells with two monoclonal antibodies without cross-reaction.
Imaging, deconvolution and analysis
Images were captured using a Zeiss AxioObserver inverted microscope with a 63×/1.4 NA oil immersion objective and were then passed through a narrow bandpass filter (Semrock, Rochester, NY) specific for the chosen fluorophore. Images were captured on a thermoelectrically cooled charged-couple device (CCD) camera with an 80% quantum efficiency (SITe SI502AB chip). The z-position of the sample was controlled by a PZM2000 piezo stage (Applied Scientific Instrumentation, Eugene, OR). The pixel size of these images was 95 nm in the x- and y-planes, and images were acquired at 250-nm intervals in the z-plane.
Images were deconvolved using the algorithm developed by Carrington and colleagues (Carrington et al., 1995). All images were dark-current- and background-subtracted, and flat-field corrected, to allow for non-uniformity in illumination and camera sensitivity across the field of view. After deconvolution, the images were aligned using fiduciary markers that emitted in all wavelengths. Finally, images were peeled, one layer of voxels at a time, and numbered from 0 (surface) to 12 (centre) (Scriven et al., 2005). Because of some uncertainty in the exact position of the surface, the −1 layer (immediately outside the surface) was also included. Colocalization and labelling density (number of lit voxels over the total number of voxels) were measured as a function of the distance into the cell; ‘surface’ and ‘interior’ represented the values obtained from layers −1 to +2 and +4 to +10, respectively. Interior colocalization and density was only calculated for the RyR2–Casq pair, as no other proteins had significant labelling in the interior. All values were obtained from cell segments chosen to eliminate possible edge effects (Scriven et al., 2008). Voxels or blobs encompassing all three antibodies (e.g. A with B and C) were counted as triply colocalized and separate from the dual colocalizations (e.g. A with B, B with C, and C with A) given that the triple grouping was thought to be functionally different from the doublets (Fletcher et al., 2010).
Determining that colocalization has occurred between two or more proteins is not sufficient as one has also to determine whether the measured colocalization is significant or if it could have occurred purely by chance. We have recently published techniques to analyze and determine the statistical significance of images using three fluorophores (Fletcher et al., 2010) using both voxel colocalization and an object-based ‘blob’ colocalization. Briefly, this latter approach splits the images into three-dimensional blobs, each centred on one of the local maxima within the image. Two or more blobs are colocalized when the maxima of the blobs falls within the body of the other. Colocalization of blob objects gives a measure of how groups of voxels (and thus molecules) interact with one another and omits colocalizations that occur owing to peripheral overlap between the edges of the blobs. Significance was calculated by generating a series of images in which the blobs were placed at random throughout the region of interest; the values generated were then compared with that of the observed value. Values with P≤0.025 were regarded as being significantly colocalized, P≥0.975 as being significantly separate and 0.025<P<0.975 as occurring by chance. A number of useful metrics can be derived from this approach: the median nearest-neighbour distance is the median distance between blob centres for a particular molecule, the cluster diameter is the minimum-sized sphere that can be drawn about the maxima of two or more colocalized blobs, and the intercluster distance is the median distance between the centre of these spheres.
Supplementary Material
Acknowledgments
We thank William Catterall for the gift of the anti-Cav1.2 antibody (National Institutes of Health grant R01 HL085372) and Qixia Yu for technical assistance. This work was supported by grants from the Canadian Institutes of Health Research (MOP12875), the Heart and Stroke Foundation of British Columbia and the Yukon, and the Natural Sciences and Engineering Research Council of Canada (to E.D.W.M.).
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/124/7/1167/DC1
References
- Balijepalli R. C., Foell J. D., Hall D. D., Hell J. W., Kamp T. J. (2006). Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signalling complex is required for β2-adrenergic regulation. Proc. Natl. Acad. Sci. USA 103, 7500-7505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard N. A., Casarotto M. G., Wei L., Varsanyi M., Laver D. R., Dulhunty A. F. (2005). Regulation of ryanodine receptors by calsequestrin: effect of high luminal Ca2+ and phosphorylation. Biophys. J. 88, 3444-3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin J. R. (1995). Spatiotemporal changes of Ca2+ during electrically evoked contractions in atrial and ventricular cells. Am. J. Physiol. Heart Circ. Physiol. 269, H1165-H1170 [DOI] [PubMed] [Google Scholar]
- Bers D. M. (2004). Macromolecular complexes regulating cardiac ryanodine receptor function. J. Mol. Cell. Cardiol. 37, 417-429 [DOI] [PubMed] [Google Scholar]
- Bers D. M. (2008). Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 70, 23-49 [DOI] [PubMed] [Google Scholar]
- Bootman M. D., Higazi D. R., Coombes S., Roderick H. L. (2006). Calcium signalling during excitation–contraction coupling in mammalian atrial myocytes. J. Cell Sci. 119, 3915-3925 [DOI] [PubMed] [Google Scholar]
- Calaghan S., White E. (2006). Caveolae modulate excitation-contraction coupling and β2-adrenergic signalling in adult rat ventricular myocytes. Cardiovasc. Res. 69, 816-824 [DOI] [PubMed] [Google Scholar]
- Carl S. L., Felix K., Caswell A. H., Brandt N. R., Ball W. J., Jr, Vaghy P. L., Meissner G., Ferguson D. G. (1995). Immunolocalization of sarcolemmal dihydropyridine receptor and sarcoplasmic reticular triadin and ryanodine receptor in rabbit ventricle and atrium. J. Cell Biol. 129, 672-682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington W. A., Lynch R. M., Moore E. D., Isenberg G., Fogarty K. E., Fay F. S. (1995). Superresolution three-dimensional images of fluorescence in cells with minimal light exposure. Science 268, 1483-1487 [DOI] [PubMed] [Google Scholar]
- Cavalli A., Eghbali M., Minosyan T. Y., Stefani E., Philipson K. D. (2007). Localization of sarcolemmal proteins to lipid rafts in the myocardium. Cell Calcium 42, 313-322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan P., Lin E., Huang J., Biln P., Tibbits G. F. (2007). Three-dimensional distribution of cardiac Na+-Ca2+ exchanger and ryanodine receptor during development. Biophys. J. 93, 2504-2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. (1983). Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. Cell Physiol. 245, C1-C14 [DOI] [PubMed] [Google Scholar]
- Fletcher P. A., Scriven D. R. L., Schulson M. N., Moore E. D. W. (2010). Multi-image colocalization and its statistical significance. Biophys. J. 99, 1996-2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Kenney L. J., Varriano-Marston E. (1987). The structure of calsequestrin in triads of vertebrate skeletal muscle: a deep-etch study. J. Cell Biol. 105, 49-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyorke I., Hester N., Jones L. R., Gyorke S. (2004). The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys. J. 86, 2121-2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell J. W., Yokoyama C. T., Wong S. T., Warner C., Snutch T. P., Catterall W. A. (1993). Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel alpha 1 subunit. J. Biol. Chem. 268, 19451-19457 [PubMed] [Google Scholar]
- Huang J., Hove-Madsen L., Tibbits G. F. (2008). Ontogeny of Ca2+-induced Ca2+ release in rabbit ventricular myocytes. Am. J. Physiol. Cell Physiol. 294, C516-C525 [DOI] [PubMed] [Google Scholar]
- Huser J., Lipsius S. L., Blatter L. A. (1996). Calcium gradients during excitation-contraction coupling in cat atrial myocytes. J. Physiol. 494, 641-651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E., Hung V. H., Kashihara H., Dan P., Tibbits G. F. (2009). Distribution patterns of the Na+-Ca2+ exchanger and caveolin-3 in developing rabbit cardiomyocytes. Cell Calcium 45, 369-383 [DOI] [PubMed] [Google Scholar]
- Lohn M., Furstenau M., Sagach V., Elger M., Schulze W., Luft F. C., Haller H., Gollasch M. (2000). Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ. Res. 87, 1034-1039 [DOI] [PubMed] [Google Scholar]
- Mackenzie L., Bootman M. D., Berridge M. J., Lipp P. (2001). Predetermined recruitment of calcium release sites underlies excitation-contraction coupling in rat atrial myocytes. J. Physiol. 530, 417-429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols C. B., Rossow C. F., Navedo M. F., Westenbroek R. E., Catterall W. A., Santana L. F., McKnight G. S. (2010). Sympathetic stimulation of adult cardiomyocytes requires association of AKAP5 with a subpopulation of L-type calcium channels. Circ. Res. 107, 747-756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H. H., Murray F., Insel P. A. (2008). Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu. Rev. Pharmacol. Toxicol. 48, 359-391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednau B. D., Nicoll D. A., Philipson K. D. (1997). Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. Cell Physiol. 272, C1250-C1261 [DOI] [PubMed] [Google Scholar]
- Rodrigues B., Severson D. L. (1997). Preparation of cardiomyocytes. In Biochemical Techniques in the Heart (ed. McNeil J. H.), pp. 101-115 Boca Raton, FL: CRC Press; [Google Scholar]
- Scriven D. R. L., Dan P., Moore E. D. W. (2000). Distribution of proteins implicated in excitation–contraction coupling in rat ventricular myocytes. Biophys. J. 79, 2682-2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriven D. R. L., Klimek A., Asghari P., Bellve K., Moore E. D. W. (2005). Caveolin-3 is adjacent to a group of extradyadic ryanodine receptors. Biophys. J. 89, 1893-1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriven D. R. L., Lynch R. M., Moore E. D. (2008). Image acquisition for colocalization using optical microscopy. Am. J. Physiol. Cell Physiol. 294, C1119-C1122 [DOI] [PubMed] [Google Scholar]
- Scriven D. R. L., Asghari P., Schulson M. N., Moore E. D. W. (2010). An analysis of Cav1.2 and ryanodine receptor clusters in rat ventricular myocytes. Biophys. J. 99, 3923-3929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sher A. A., Noble P. J., Hinch R., Gavaghan D. J., Noble D. (2008). The role of the Na+/Ca2+ exchangers in Ca2+ dynamics in ventricular myocytes. Prog. Biophys. Mol. Biol. 96, 377-398 [DOI] [PubMed] [Google Scholar]
- Terentyev D., Viatchenko-Karpinski S., Vedamoorthyrao S., Oduru S., Gyorke I., Williams S. C., Gyorke S. (2007). Protein protein interactions between triadin and calsequestrin are involved in modulation of sarcoplasmic reticulum calcium release in cardiac myocytes. J. Physiol. 583, 71-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier W. G., Lopez-Lopez J. R., Shacklock P. S., Balke C. W. (1995). Calcium signalling in cardiac muscle cells. Ciba Found. Symp. 188, 146-160 [DOI] [PubMed] [Google Scholar]
- Woo S. H., Cleemann L., Morad M. (2003a). Spatiotemporal characteristics of junctional and nonjunctional focal Ca2+ release in rat atrial myocytes. Circ. Res. 92, e1-e11 [DOI] [PubMed] [Google Scholar]
- Woo S. H., Soldatov N. M., Morad M. (2003b). Modulation of Ca2+ signalling in rat atrial myocytes: possible role of the alpha1C carboxyl terminal. J. Physiol. 552, 437-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.