Abstract
HLA–DM (DM) plays a critical role in antigen presentation through major histocompatibility complex (MHC) class II molecules. DM functions as a molecular chaperone by keeping class II molecules competent for antigenic peptide loading and serves as an editor by favoring presentation of high-stability peptides. Until now, DM has been thought to exert these activities only in late endosomal/lysosomal compartments of antigen-presenting cells. Here we show that a subset of DM resides at the cell surface of B cells and immature dendritic cells. Surface DM engages in complexes with putatively empty class II molecules and controls presentation of those antigens that rely on loading on the cell surface or in early endosomal recycling compartments. For example, epitopes derived from myelin basic protein that are implicated in the autoimmune disease multiple sclerosis are down-modulated by DM, but are presented in the absence of DM. Thus, this novel concept of functional DM on the surface may be relevant to both protective immune responses and autoimmunity.
Keywords: antigen presentation/autoimmunity/MHC class II/multiple sclerosis/peptide editing
Introduction
Antigen presentation to CD4+ T-helper cells requires the concerted activities of at least three types of molecules (Karlsson et al., 1994): antigenic peptide receptors encoded in the major histocompatibility complex (MHC) class II and two specialized accessory molecules, the invariant chain (Ii) and HLA–DM (DM), denoted H2-M in the murine system. Initially, homotrimers of Ii chaperone newly synthesized MHC class II αβ dimers (Anderson and Miller, 1992; Romagnoli and Germain, 1994) via a central segment, denoted CLIP (class II associated Ii peptides; Riberdy et al., 1992), and flanking regions of CLIP (Vogt et al., 1995; Park et al., 1995; Siebenkotten et al., 1998). By virtue of a sorting signal, Ii targets αβ dimers to endocytic compartments for encounter of internalized exogenous antigen (Bakke and Dobberstein, 1990; Lotteau et al., 1990). Endosomal proteases cleave Ii and generate intermediary αβ–CLIP complexes (Denzin and Cresswell, 1995; Riese et al., 1996). CLIP as a peptide has been shown to occupy the antigen binding groove (Ghosh et al., 1995), thus preventing binding of cognate peptide. In the context of some class II alleles a self-release mechanism initiated by an N–terminal effector sequence of CLIP is functional and allows antigenic peptide loading (Kropshofer et al., 1995a,b).
To remove CLIP in a pan-allelic fashion and to assist in subsequent loading steps, antigen-presenting cells (APCs) recruit a second chaperone, DM (Cho et al., 1991; Kelly et al., 1991). Although the structure of DM is very similar to that of classical class II dimers, its α1 and β1 domains do not form a groove (Fremont et al., 1998; Mosyak et al., 1998) and do not accommodate peptides (Kropshofer et al., 1997a). DM accumulates in endosomal/lysosomal compartments where it engages in complexes with other class II molecules (Karlsson et al., 1994; Sanderson et al., 1994, 1996). In these complexes DM is thought to stabilize a strained, transition-state conformer of the class II peptide binding groove that is open, thus favoring peptide exchange (Kropshofer et al., 1997a,b). This explains why DM is able to catalyze the release of CLIP (Denzin and Cresswell, 1995; Sloan et al., 1995) and binding of cognate peptide in an enzyme-like fashion (Vogt et al., 1996). As a successor to Ii, DM chaperones empty αβ dimers by preventing them from aggregation and inactivation in the absence of appropriate peptide (Denzin et al., 1996; Kropshofer et al., 1997a). Moreover, DM functions as a peptide editor (Kropshofer et al., 1997b): DM releases peptides with an intrinsically low kinetic stability whereas it tolerates high-stability peptides occupying the class II binding groove (Kropshofer et al., 1996; Weber et al., 1996). This quality control mechanism leads to the situation where the majority of naturally processed peptides form long-lived complexes with class II molecules on the cell surface (Lanzavecchia et al., 1992).
In B cells and dendritic cells (DC) it has been established that antigenic peptides can be loaded either on newly synthesized class II molecules in lysosome-like organelles, named MIICs (class II containing compartments; Peters et al., 1991), or on recycling class II molecules in early endosomes and on the cell surface (Reid and Watts, 1990; Roche et al., 1993; Pinet et al., 1995; Cella et al., 1997). DM was generally assumed only to be active in MIICs, where it is enriched (Lindstedt et al., 1995; Marks et al., 1995), but not in compartments of the so-called alternative or recycling pathway as, so far, attempts to detect DM on the cell surface have failed (Denzin et al., 1994; Sandersson et al., 1994). As a consequence antigens loaded on recycling rather than newly synthesized class II molecules would escape the chaperone and editing activity of DM.
The present report provides evidence that B cells and immature DC express DM at the cell surface. Surface DM appears to have a major impact on antigen presentation via the alternative pathway in that it down-regulates presentation of low-stability ligands, such as epitopes implicated in the autoimmune disease multiple sclerosis (MS).
Results
DM counteracts loading and presentation of exogenous myelin basic protein or peptide
To explore whether DM may play a role outside conventional MIICs, we first focused on presentation of myelin basic protein (MBP). MBP is considered a candidate autoantigen in MS and has been shown to be loaded onto surface and recycling DR molecules (Pinet et al., 1995; Vergelli et al., 1995). Processing and determinant capture by recycling class II molecules without passage through MIICs appears to be essential for those antigens, such as MBP, that are sensitive to excessive proteolysis by the diverse set of endosomal/lysosomal proteases (Pinet and Long, 1998).
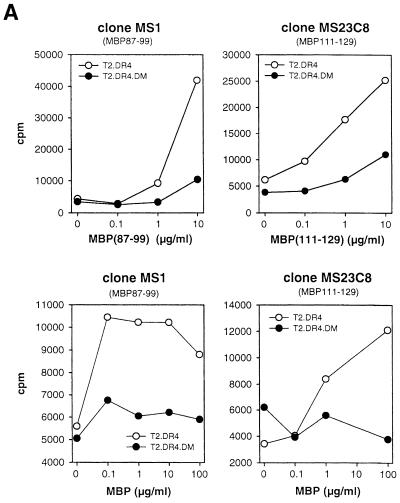
DM-negative T2.DR4 and DM-positive T2.DR4.DM transfectants were pulsed either with MBP protein or the peptides MBP(87–99) and MBP(111–129), which are the immunodominant MBP autoepitopes in the context of DR4 (DRB1*0401) (Muraro et al., 1997). As a read-out system, proliferation of DR4-restricted autoreactive T–cell clones (TCCs) MS23C8 and MS1, specific for MBP(111–129) and MBP(87–99), respectively, was analyzed. MBP peptides and MBP protein were well recognized by both TCCs on T2.DR4 cells, but in the context of DM-positive T2.DR4.DM cells presentation of MBP peptides was strongly reduced and MBP protein was not presented at all (Figure 1A). Thus, DM counteracts presentation of immunodominant MBP epitopes. Differences in expression or loading capacity of DR4 of both cell lines could not account for the down-regulation, as both cell lines expressed similar levels of surface DM (Figure 2A), and DR4 purified from either of the transfectants bound MBP(87–99) (Figure 1B) and other peptides (data not shown) equally well, excluding the possibility that T2.DR4.DM cells differ from T2.DR4 cells by carrying stably bound endogenous peptides that prevent binding of MBP-derived peptides.
Fig. 1. DM counteracts presentation of autoepitopes in the alternative pathway. (A) DM down-regulates immunodominant MBP epitopes. T2.DR4 and T2.DR4.DM lymphoblastoid cell lines were pulsed for 3 h with various amounts of MBP(87–99) or MBP(111–129) peptides (upper panel) and MBP protein (lower panel). Proliferation of the T–cell clones MS1 and MS23C8 recognizing MBP(87–99) and MBP(111–129), respectively (Muraro et al., 1997), was evaluated by measuring [3H]thymidine incorporation. The results of one of three experiments are shown. (B) DR4 from T2.DR4 and T2.DR4.DM have equal binding capacity. DR4 was purified from both T2 transfectants and analyzed for binding of AMCA-labeled MBP(87–99) and control peptide HA(307–319)-Asp309 (5 μM each) for 3 h at pH 6.5 and 37°C by high-performance size exclusion chromatography, as described (Kropshofer et al., 1996). (C) DM counteracts binding of MBP peptides. T2.DR4 and T2.DR4.DM cells were pulsed with N–terminally biotinylated HA peptide (10 μM) at 37°C for 180 min. After 30 min either no peptide or the indicated amounts of the competitor peptides MBP(87–99) or MBP(111–129) were added. HA peptide loading obtained after 180 min was quantified in a sandwich immunoassay based on time-resolved Europium fluorescence. DM increased the IC50 of MBP(87–99) from 5 to 20 μM and the IC50 of MBP(111–129) from 5 to >100 μM, where IC50 is the dose necessary to reduce HA peptide binding by 50%.
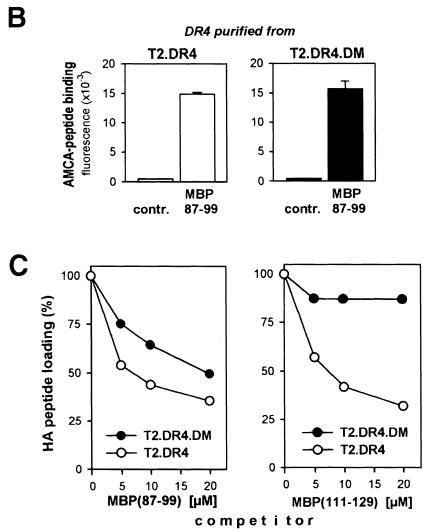
Fig. 2. DM is expressed on the cell surface of B cells in association with DR molecules. (A) Flow cytometric analysis. DM expression on the surface of the transfectant T2.DR4.DM and the B-LCLs WT-100 and Jesthom, as detected by staining with the anti-DM rabbit antiserum 11323 (Busch et al., 1998; Liu et al., 1998), upper panel, black. As a negative control, T2.DR4 cells were used. For comparison, DR expression was determined by staining with the mAb L243, lower panel, black. Staining was performed either with biotinylated goat anti-rabbit IgG and streptavidin–R-phycoerythrin or with goat anti-mouse IgG–R-phycoerythrin. Background control was obtained with an irrelevant rabbit antiserum (upper panel, white) or with an irrelevant IgG2a mAb (lower panel, white). (B) DM is bound to DR on the cell surface. T2.DR4, T2.DR4.DM and Jesthom cells were incubated with the biotinylated mAbs 1B5 and L243 at 4°C, thereby allowing binding to surface DR molecules only. Cells were lysed either in 1% NP-40 or in 1% CHAPS. Antibody–DR complexes were precipitated with NeutrAvidin–agarose beads at 4°C, boiled in Laemmli sample buffer and probed for DRα and co-precipitating DMβ by Western blot analysis with the mAbs 1B5 and DM.K8, respectively. #To exclude post-lysis artefacts by putative capture of intracellular DR–DM complexes, a lysate from the B-LCL Jesthom containing DR–DM complexes was added to the T2.DR4 cell lysate prior to precipitation with NeutrAvidin–agarose beads.
To prove that DM interferes directly with MBP peptide loading we tested competition of MBP peptides MBP(87–99) and MBP(111–129) against biotinylated HA(307–319) (HA) peptide. HA peptide, which is derived from influenza virus hemagglutinin, is another antigen known to be loaded onto recycling DR molecules (Pinet et al., 1994, 1995). To this end, we pre-loaded T2.DR4 and T2.DR4.DM cells with labeled exogenous HA peptide and added increasing amounts of MBP peptide as a competitor. In accordance with the T–cell proliferation data, we found reduced competition against HA peptide of both MBP peptides in the presence of DM (Figure 1C). The reduction was more pronounced with MBP(111–129), which is consistent with MBP(111–129) binding less stably than MBP(87–99) to DR4 (Muraro et al., 1997) and agrees with the fact that DM preferentially removes low-stability ligands (Kropshofer et al., 1996; Weber et al., 1996). We conclude that DM-mediated down-regulation of MBP presentation is a consequence of DM acting as a negative editor of both MBP epitopes.
DM is located on the cell surface
The observation that DM controls exogenous peptide loading or processing of whole protein antigens in the alternative pathway suggests that DM may have access to class II molecules on the cell surface. So far, surface expression of DM has widely been excluded, but here we are able to provide evidence for surface DM by flow cytometry with the antiserum 11323 raised against recombinant DM (Busch et al., 1998; Liu et al., 1998): T2.DR4.DM cells stained positive for surface DM, whereas T2.DR4 cells did not (Figure 2A). Likewise, DM was found on the surface of B lymphoblastoid cell lines (B–LCLs), such as WT-100 and Jesthom (Figure 2A), and on the surface of immature DC (cf. Figure 6A). Quantitation by cell surface biotinylation and quantitative Western blotting revealed that the surface DM fraction of WT–100 and Jesthom comprises ∼10–15% of total DM (Table I). The same technique allowed us to detect roughly two-thirds of the DR molecules and ∼4–6% of the lysosomal resident lamp–1 on the cell surface, which is in agreement with the literature (Pieters et al., 1991; Rohrer et al., 1996).
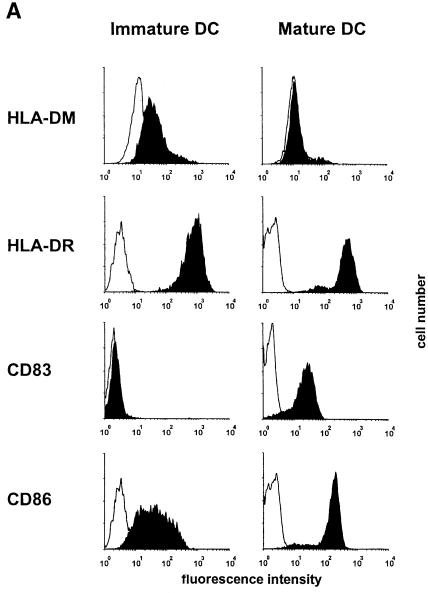
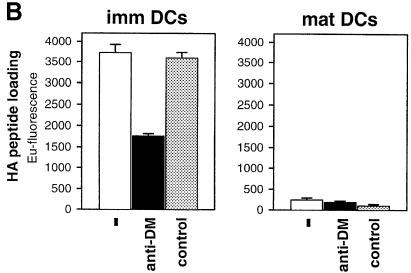
Fig. 6. Dendritic cells (DC) down-regulate surface DM upon maturation. Immature DC were generated by culturing PBMC in GM-CSF (800 U/ml) and IL-4 (1000 U/ml). For induction of maturation a cytokine cocktail consisting of TNF–α (10 ng/ml), IL–1β (10 ng/ml), IL–6 (1000 U/ml) and prostaglandin E–2 (1 μg/ml) was added. (A) Flow cytometric analysis. Immature DC at day 5 of in vitro culturing and mature DC at day 8 were analyzed by cytofluorometry using monoclonal antibodies towards CD83 (Pharmingen) and CD86 (Pharmingen). Isotype controls were run in parallel. Staining for DM and DR was performed as described in the legend to Figure 2. (B) Exogenous peptide loading by immature DC is DM dependent and abolished upon maturation. A total of 106 immature and mature DC (DRB1 *0101) were pulsed with biotinylated HA peptide (10 μM) for 30 min at 37°C with and without anti-DM serum 11323 (5 μl) or a control serum. Binding was quantified via sandwich immunoassay, as described in the legend to Figure 1.
Table I. Quantitation of DM on the cell surface.
| Cell type | Cell surface expression (%) |
||
|---|---|---|---|
| HLA–DM | HLA–DR | lamp-1 | |
| WT-100 | 15 ± 2 | 67 ± 3 | 6 ± 1 |
| Jesthom | 10 ± 1 | 58 ± 1 | 4 ± 1 |
WT-100 and Jesthom cells were surface biotinylated by lysis in 1% NP-40. Biotinylated surface proteins were separated from unbiotinylated proteins by precipitation with NeutrAvidin–agarose. The precipitated material was probed for DR, DM and lamp–1 by quantitative Western blotting with the mAb 1B5, the mAb DM.K8 or the anti-lamp-1 antiserum, respectively, and compared with the respective amounts in the total cell lysate prior to NeutrAvidin depletion. Quantitation was performed on a Lumi-Imager F1 (Roche) by plotting band intensities versus graded amounts of the cell lysate, thereby generating a standard curve for each protein to be analyzed. Lumi-Imager values used for calculation were within the linear range of the standard curve. Values for each protein are given as a percentage of cell surface expression versus total cell expression. The mean ± SD of two experiments is shown.
Surface DM is associated to class II molecules
DM from late endosomal/lysosomal compartments engages in complexes with peptide-receptive class II molecules that are empty (Kropshofer et al., 1997b). We wondered whether this also holds true for surface DM. We incubated Jesthom, T2.DR4.DM and T2.DR4 as a control at 4°C with biotinylated L243, which binds exclusively to surface DR molecules under these conditions. After detergent lysis with either NP–40 or CHAPS, surface DR and putative associated proteins were retrieved with NeutrAvidin beads and analyzed by Western blotting (Figure 2B). In CHAPS lysates of Jesthom and T2.DR4.DM cells DM co-immunoprecipitated with DR from the cell surface. However, when NP–40 was used no association of DM and DR was observed, as described earlier (Sanderson et al., 1996; Kropshofer et al., 1997b). To exclude the possibility that intracellular DR or DM was precipitated, the antibody 1B5 recognizing the lumenal tail of the DRα chain (Adams et al., 1983) was used as a negative control. As expected, 1B5 did not precipitate either DR or DM (Figure 2B). As a further control, we added a Jesthom lysate containing DR–DM complexes (cf. Figure 2B) to the T2.DR4 homogenate, but we could not detect DM co-precipitating with DR captured by biotinylated L243, thus ruling out post-lysis capture of DR–DM complexes. Together these data demonstrate that surface DM engages in complexes with DR molecules. This is reminiscent of endosomal/lysosomal DM that associates with empty class II molecules.
DM from the cell surface facilitates loading of exogenous peptide
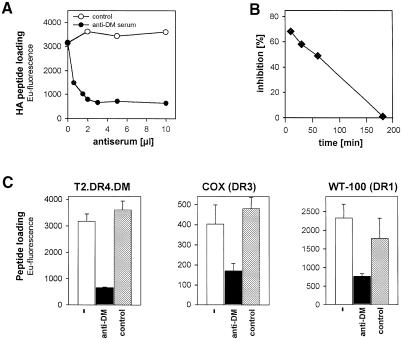
Previous reports have shown in vitro that DM catalyzes peptide exchange most efficiently at acidic pH but is also active at neutral pH (Denzin et al., 1995; Sherman et al., 1995). To demonstrate that DM is functional at neutral pH in vivo, we attempted to block DM activity on T2.DR4.DM cells. Treatment of T2.DR4.DM cells with the anti-DM serum diminished HA peptide loading by up to 80% in a dose-dependent manner (Figure 3A). Specificity was controlled by using an unrelated control serum (Figure 3A) or DM-negative T2.DR4 cells (Figure 4A). To determine whether the target of the blockade was surface DM or internal DM, we pre-incubated T2.DR4.DM cells with the anti-DM serum on ice and removed the serum before pulsing with peptide to avoid internalization of the antibody prior to peptide exposure. Inhibition of HA peptide loading was maximal at the very beginning of the pulse and declined with a half-time of ∼90 min (Figure 3B). This result underlines the fact that surface DM rather than the internal MIIC pool of DM is responsible for mediating capture of exogenous antigen.
Fig. 3. DM from the cell surface facilitates peptide loading. (A) Inhibition of exogenous peptide loading by anti-DM antiserum. T2.DR4.DM cells were co-incubated with 15 μM biotinylated HA peptide and increasing amounts of the anti-DM serum 11323. Cells were incubated for 3 h at 37°C, lysed in 1% NP-40 and DR4–HA complexes quantified as described in the legend to Figure 1. An irrelevant rabbit antiserum was used as a control. The anti-DM serum did not interfere with peptide loading in the context of T2.DR4 cells (cf. Figure 4A; data not shown). (B) Anti-DM serum blocks DM from the cell surface. T2.DR4.DM cells were pre-incubated for 30 min on ice with the anti-DM antiserum 11323 (5 μl) or a control serum, thereby preventing internalization. Unbound serum was removed by washing the cells with RPMI/10% FCS. Cells were pulsed with biotinylated HA peptide (15 μM) at 37°C for the indicated periods of time. DR4–HA complexes were quantified as described above. The percentage of inhibition was determined at each time point by comparing HA peptide loading obtained with anti-DM versus the control serum. (C) Surface DM mediates loading in the context of various DR allelic products. T2.DR4.DM, COX (DRB1 *0301) and WT-100 (DRB1 *0101) cells were incubated for 3 h at 37°C in the absence or presence of the anti-DM antiserum (3 μl) and pulsed with biotinylated HA peptide, ApoB100(2877–2894) peptide or A2(103–117) peptide (15 μM each), respectively. An irrelevant rabbit antiserum was used as a control. Cells were lysed in 1% NP-40 and bound peptide was quantified as described in the legend to Figure 1. The mean of two experiments is shown; error bars indicate SDs.
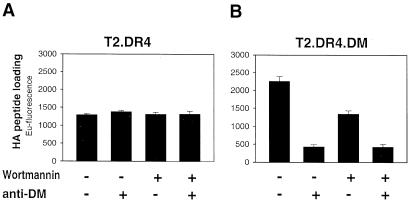
Fig. 4. DM-mediated loading of exogenous peptide is accomplished on the surface and after endocytosis. T2.DR4 (A) or T2.DR4.DM (B) cells were pre-treated for 30 min at 37°C with or without the endocytosis inhibitor wortmannin (0.5 μM) and pulsed for 30 min at 37°C with biotinylated HA peptide (15 μM) in the absence and presence of the anti-DM serum 11323 (5 μl). DR4–HA complexes were quantified as described in the legend to Figure 1. The mean fluorescence of triplicates of two independent experiments with similar results is presented; error bars indicate SDs. The same results were obtained after treatment with glutaraldehyde, another agent interfering with endocytosis (data not shown).
Involvement of surface DM in antigenic peptide loading was not restricted to T2 transfectants or to DR4: treatment with the 11323 DM serum also reduced antigenic peptide binding to DR3 and DR1 by ∼60–70%, as shown with ApoB 100(2877–2894) peptide from apolipoprotein B100 and HA peptide in the context of the B-LCLs COX and WT-100, respectively (Figure 3C). To investigate whether a similar effect was seen with an alternative technique, we analyzed WT-100 cells by flow cytometry using the monoclonal antibody (mAb) UL-5A1, which recognizes DR1 associated to the self-peptide A2(103–117) (Wölpl et al., 1998). Treatment with 11323 diminished surface DR1-A2(103–117) complexes by ∼30–40% (data not shown), emphasizing that surface DM participates in antigenic peptide capture, irrespective of the class II allele or peptide being involved.
Surface DM mediates loading on the cell surface and via endocytosis
From the above findings it is still an open question whether DM-mediated loading in the alternative pathway relies on endocytosis. Wortmannin has been shown to be a specific and very potent inhibitor of phosphatidylinositol-3kinases, interfering with fluid-phase- and receptor-mediated endocytosis (Li et al., 1995; Simonsen et al., 1998). When we treated T2.DR4.DM cells with wortmannin, HA peptide loading declined by ∼40% (Figure 4B). The residual 40% of loading seems to be due to surface DM activity (cf. Figure 3A) because it could be blocked by consecutive treatment with wortmannin and the anti-DM serum (Figure 4B). Fixation of cells with glutaraldehyde, another means to interfere with endocytosis, led to similar results (data not shown). In T2.DR4 cells, however, loading was not impaired by wortmannin, the anti-DM serum (Figure 4A) or glutaraldehyde treatment (data not shown). Thus, in contrast to loading in the absence of DM, surface DM-mediated peptide loading is partially dependent on internalization into endocytic compartments.
Class II molecules loaded with exogenous peptide and DM co-fractionate in light-density organelles
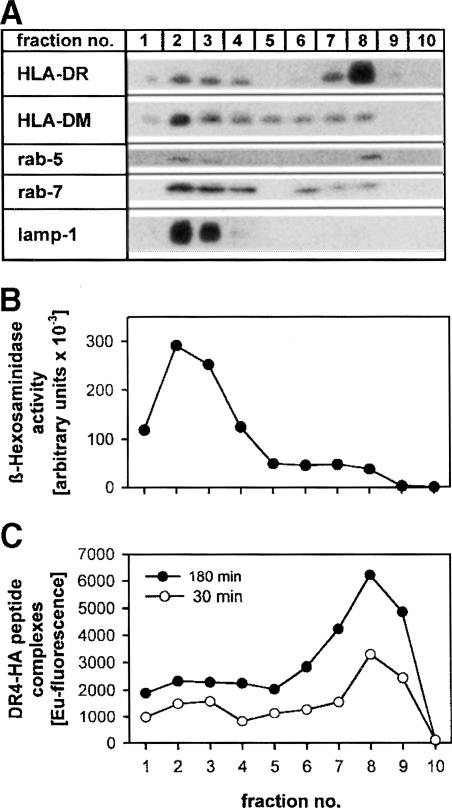
To visualize the cellular compartments carrying class II molecules associated with exogenous peptide, we pulsed T2.DR4.DM cells with HA peptide for either 30 or 180 min and fractionated the cells on a Percoll density gradient. Two major groups of compartments could be separated (Figure 5): fractions 5–9 contain both plasma membrane and early endosomes, which are representative for the alternative pathway, whereas late endosomes and lysosomes were distributed in fractions 1–4. This was substantiated by the activity profile of β–hexosaminidase (Figure 5B), a marker enzyme of lysosomes and late endosomes (Castellino and Germain, 1995), the distribution of the lysosomal marker lamp–1, the late endosomal marker Rab–7 (Novick and Zerial, 1997) and the early endosomal marker Rab–5 (Figure 5A). DM was mainly found in high-density fractions 1–4, but a subset of DM was detected in fractions 5–8 containing membranes from the plasma membrane and early endosomes (Figure 5A), consistent with flow cytometry data (cf. Figure 2A). As expected from the results of Figure 4, the majority of DR4–HA peptide complexes was found in fractions 5–8, irrespective of whether the cells had been pulsed with HA peptide for 30 or 180 min. Hence, this result is compatible with the view that it is mainly the cell surface and early endosomes where DM facilitates capture of exogenous HA peptide by DR4.
Fig. 5. DR4 loaded with exogenous HA peptide co-fractionates with light-density fractions containing plasma membrane and early endosomes. T2.DR4.DM cells were pulsed with 15 μM biotinylated HA peptide for either 30 min or 3 h at 37°C and then subjected to subcellular fractionation on a 27% Percoll density gradient. (A) Subcellular fractions were analyzed for DR, DM, Rab–5 (specific for early endosomes), Rab–7 (specific for late endosomes) and lamp–1 (specific for lysosomes and late endosomes) by Western blotting. The vast majority of HLA–DR molecules co-fractionate with Rab–5, whereas HLA–DM mainly co-fractionates with Rab–7 and lamp–1. (B) Activity profile of the lysosomal and late endosomal marker enzyme β–hexosaminidase of the Percoll fractions. (C) Distribution of DR4–HA peptide complexes after a 30 or 180 min pulse, as detected by the described sandwich immunoassay after Percoll fractionation.
Dendritic cells carry functional DM on the surface, which is down-regulated upon maturation
Finally, we sought to extend our studies to DC, as immature DC efficiently capture antigen and load it onto recycling class II molecules (Cella et al., 1997). Flow cytometric analysis of DC derived from peripheral blood monocytes (PBMC) revealed up-regulation of the co-stimulatory molecule CD86 and the activation marker CD83 upon tumor necrosis factor–α (TNF–α)- and interleukin–1 (IL–1)-induced maturation, while the expression of DR remained almost unchanged (Figure 6A). Most importantly, immature DC displayed sizeable levels of DM on the surface, comparable to B cells (cf. Figure 2A), whereas in mature DC no surface DM was detectable (Figure 6A). Developmental down-regulation of surface DM was reflected by exogenous peptide loading: immature DC bound HA peptide very efficiently, whereas maturation led to complete loss of binding (Figure 6B). Treatment with the anti-DM serum reduced HA peptide binding on immature DC by ∼50–60%, which is reminiscent of the effect on B–cell loading (cf. Figure 6B). We conclude that surface DM is functional in antigenic peptide loading of immature DC.
Discussion
It is common knowledge that DM facilitates antigenic peptide loading in high-density endosomal and lysosomal organelles of APCs (Kropshofer et al., 1997a; Vogt and Kropshofer, 1999). This classical pathway relies on newly synthesized class II molecules and involves antigens that need to be unfolded and probably proteolyzed intracellularly prior to capture by αβ dimers (Wolf and Ploegh, 1995). If this were the only way in which self- or foreign antigens are processed, the immune system would lose the possibility to present and recognize labile epitopes that are easily destroyed by proteases under the harsh conditions of lysosome-like compartments (Griffin et al., 1997; Pinet and Long, 1998). B cells and DC solve this problem by keeping available a cohort of peptide-receptive class II molecules on the cell surface and in early or recycling endosomes (Adorini et al., 1989; Reid and Watts, 1990; Pinet et al., 1995; Cella et al., 1997; Santambrogio et al., 1999a). Well established examples of antigens that depend on this alternative or recycling pathway are RNase A (Nadimi et al., 1991; Escola et al., 1995), HA (Pinet et al., 1994), p56-65 from mycobacteria (Geluk et al., 1997) and MBP (Pinet et al., 1995; Vergelli et al., 1997). Studies involving these antigens were frequently performed with DM-negative transfectants and, therefore, it remained uncertain whether DM plays a role in the alternative pathway.
This study demonstrates for the first time that a sizeable subset of DM molecules is expressed on the cell surface of B cells and immature DC, and has a profound impact on the capacity and selectivity of these APCs to present antigenic peptides in a DR-restricted fashion. We could show that at steady state the surface pool of DM comprises ∼10–15% of the total amount of DM in Epstein–Barr virus (EBV)-transformed B cells (Table I). Estimates based on flow cytometry and quantitative Western blotting suggested that immature DC bear at least similar levels of surface DM (Figures 2A and 6A). From the knowledge that B cells carry ∼106 DR molecules in total and that the cellular DR:DM ratio is ∼20:1 (Schafer et al., 1996; Kropshofer et al., 1997a) it follows that ∼5000 DM molecules are expressed at the B–cell surface. This is quite a high number in light of the fact that only 100–300 class II molecules are sufficient for T–cell activation and that DM acts as a catalyst with one DM molecule facilitating peptide exchange of several class II molecules (Vogt et al., 1996).
In support of our data, DM has been suggested to be expressed on the surface of Langerhans cells (Andersson et al., 1998). However, previous attempts failed to detect DM on the surface of human B cells (Denzin et al., 1994; Sanderson et al., 1994), giving rise to the assumption that antigenic peptide loading in the alternative pathway would proceed without the involvement of DM. Limitations in the sensitivity of the techniques employed or exceptionally low DM levels in the respective APCs may have impaired the search for surface DM in the above studies. Moreover, albeit that DM is mainly active at the acidic pH of endosomes and lysosomes, it is also functional at neutral pH at the cell surface. In line with previous reports (Denzin et al., 1995; Sherman et al., 1995), we observed that at neutral pH (6.5–7.5) DM retained ∼25–35% of its activity at pH 5.0 (data not shown).
Co-precipitation studies revealed that surface DM engages in complexes with DR molecules (Figure 2B). This finding suggests that the cell surface harbors DR–DM complexes that, most likely, are equivalent to those described for endosomal/lysosomal compartments where DM keeps ‘empty’ DR molecules receptive for antigen capture (Kropshofer et al., 1997a). An abundance of empty class II molecules has recently been described for immature DC (Santambrogio et al., 1999a). In turn, it is conceivable that empty class II molecules function as a type of scavenger for DM molecules that arrive at the cell surface. One might imagine that DM reaches the plasma membrane by direct sorting from the trans-Golgi network. Alternatively, DM may be delivered to the cell surface via endosomal transport vesicles or via fusion of whole MIIC with the plasma membrane, as suggested previously (Raposo et al., 1996; Wubbolts et al., 1996; Pond and Watts, 1997).
In further support of antigen-receptive class II–DM complexes in the alternative pathway, our results demonstrate that surface DM is critically involved in loading exogenous peptides: (i) in B cells peptide loading onto various DR allelic products was blocked 60–80% by an anti-DM serum (Figure 3), indicating that the majority of peptide binding was controlled by surface DM; (ii) similar to B cells, exogenous HA peptide loading of immature DC was reduced by 50–60% after blocking surface DM (Figure 6B); and (iii) the kinetics of loading was considerably faster in T2.DR4.DM than in T2.DR4 cells (Figure 4; data not shown). Exogenous peptide antigens may bind directly to surface DR molecules or, after endocytosis, to DR in early endosomes (Barnes and Mitchell, 1995). We addressed the issue of at which of these two locations DM takes part in loading and found that formation of DR4–HA peptide complexes declined by ∼50% after inhibition of endocytosis by treatment with wortmannin (Figure 4B) or glutaraldehyde fixation (data not shown), suggesting that surface DM exerts half of its activity directly on the surface and the other half after internalization into early endosomal compartments. This is clearly different from loading in the absence of DM since loading was not impaired by interference with endocytosis in cells deficient for DM (Figure 4A). In accordance with the above data, the majority of DR4–HA peptide complexes co-fractionated with a subset of DM molecules in light-density organelles including plasma membrane and early endosomes after Percoll density gradient fractionation (Figure 5). The involvement of DM in loading on the cell surface also provides a rationale for the observation in H2-M knock-out mice, which were inferior to wild-type mice in presenting exogenous peptide antigens (Martin et al., 1996; Miyazaki et al., 1996; Tourne et al., 1997).
Furthermore, our data provide direct evidence for quality control of loading by surface DM. We could show that the DR4-restricted immunodominant autoepitope MBP(111–129), which is implicated in MS, is prevented from binding to DR4 in the presence of DM, whereas it binds in the absence of DM (Figure 1C). We could exclude an impact of pre-bound endogenous self-peptides by which T2.DR4 differ from T2.DR4.DM cells: purified DR4 derived from both cell lines bound peptide equally well in vitro (Figure 1B). Interference with MBP(111–129) binding by DM was also observed when endocytosis was blocked by fixation with glutaraldehyde (data not shown), suggesting that peptide editing by DM can occur on the cell surface. DM was also found to counteract the other MBP auto– epitope, MBP(87–99) (Figure 1), and COL(259–273), an immunodominant autoepitope from collagen type II implicated in rheumatoid arthritis (data not shown).
In agreement with this, expression of DM led to a decline in the proliferation of autoreactive T–cell clones specific for the above MBP epitopes (Figure 1A, upper panel). Importantly, this impact of DM editing on T–cell activation was also seen with exogenous MBP, a protein that is known to be processed via the alternative pathway (Pinet et al., 1995): DM abolished T–cell proliferation normally induced by whole MBP protein in the absence of DM (Figure 1A, lower panel). In agreement with the kinetic proofreading model, whereby peptides with high kinetic stability resist removal by DM whereas low-stability peptides undergo release by DM (Kropshofer et al., 1997b), DM counteracted presentation of MBP(111–129), an immuno-dominant peptide that binds poorly to DR4 (Muraro et al., 1997). Strikingly, intermediate to poor binding to the restricting MHC class II molecule is a property shared by other epitopes associated with autoimmune diseases (Liu and Wraith, 1995): e.g. collagen-derived peptide COL(259–273) associated with rheumatoid arthritis (Raddrizzani et al., 1999), and acetylated MBP(1–9), which is the major encephalitogenic epitope in the mouse model of MS (Fairchild et al., 1993; Mason and McConnell, 1994).
How can it be explained that low-stability peptides such as MBP(111–129) do give rise to activated autoreactive T cells in MS when DM counteracts their presentation? It is possible that the expression or activity of DM in the alternative pathway is abnormally reduced in the respective APCs, e.g. astroglial and microglia cells in MS (Martin et al., 1992). This may be accomplished by expression of too low levels of DM, down-regulation of DM at the transcriptional level or up-regulation of DO, which appears to inhibit DM activity at high DO concentrations (Denzin et al., 1997; A.B.Vogt, G.J.Hämmerling and H.Kropshofer, unpublished data). It will be a future challenge to focus on DM expression and activity of APCs in autoimmune diseases.
Our finding that surface DM facilitates binding of exogenous MHC class II ligands may be of particular relevance in the light of the recent reports that DC display ectoproteases on their surface (Amoscato et al., 1998) and that immature DC are able to perform extracellular processing of antigen with subsequent presentation of the resulting peptides (Santambrogio et al., 1999b). It is very likely that such an extracellular processing pathway utilizes DM and is complemented by our finding that DC express surface DM only in their immature state, probably in order to facilitate antigenic peptide capture, as shown with HA peptide (Figure 6B). Upon cytokine-induced maturation, DC were revealed to down-regulate their surface DM and concomitantly lost the ability to load peptide (Figure 6B). This opens up the possibility that surface DM may play a role at sites of inflammation or during necrosis of cells where antigenic polypeptides or peptides may be generated extracellularly. These antigens could be captured directly from the extracellular milieu by empty class II molecules of B cells and immature DC kept antigen receptive by surface DM. Loading of extracellularly processed antigen facilitated by surface DM may represent a mechanism that would allow more efficient and prompt mounting of an immune response, especially in the case of epitopes that are very sensitive to endosomal/lysosomal proteases. In particular, the presentation of autoantigen in inflammatory foci of specific organs, e.g. the brain in demyelinating diseases such as MS, may be an example of such an environment (Martin et al., 1992). Taken together, these observations enlarge the importance of DM as a central regulator in antigen processing in that they ascribe to DM an as yet unexpected role on the cell surface.
Materials and methods
Cell lines
The EBV-transformed homozygous B-cell lines WT-100 (DRB1*0101) and Jesthom (DRB1*0101), COX (DRB1*0301) and the T × B hybrid cell line T2 stably transfected with DRB1*0401 (T2.DR4) or DRB1*0401 and DM (T2.DR4.DM) (Denzin et al., 1994) were maintained in roller bottles at 37°C in RPMI 1640 containing 20 mM HEPES and 10% heat-inactivated fetal calf serum (FCS) (Conco Lab Division).
Generation of immature and mature DC
Dendritic cells were generated by culturing PBMC obtained from leukapheresis of a human donor as described (Thurner et al., 1999). Briefly, PBMC were cultured in complete RPMI medium containing human serum. After 1 h all non-adherent cells were removed and fresh complete medium was added. From day 1 onwards, cells were cultured in complete medium containing granulocyte-macrophage colony-stimulating factor (GM-CSF) (800 U/ml) and IL-4 (1000 U/ml). On day 5, all non-adherent cells were harvested as immature DC. For the generation of mature DC the harvested cells were replated in fresh complete medium containing GM–CSF and IL–4. On day 6, maturation was induced by adding a cytokine cocktail consisting of TNF–α (10 ng/ml), IL–1β (10 ng/ml), IL–6 (1000 U/ml) and prostaglandin E–2 (1 μg/ml) to the culture medium. Finally, on day 8 mature DC were harvested.
Peptides and proteins
Peptides were synthesized and labeled at the N–terminus with 7–amino-4-methyl-coumarin-3-acetic acid (AMCA), or biotin as described (Kropshofer et al., 1995a). The following peptides were used: HA(307–319), PKYVKQNTLKLAT, from influenza virus hemagglutinin; COL(259–273), GIAGFKGEQGPKGEHPG, from human collagen type II; ApoB100(2577–2594), ISNQLTLDSNTKYFHK; MBP(87–99), VHFFKNIVTPRTP, and MBP(111–129), LSRFSWGAEGQRPGFGYGG, from human MBP; and A2(103–117), VGSDWRFLRGYHQYA (Chicz et al., 1992). MBP was purified from human brain as described (Vergelli et al., 1997).
Antibodies
The hybridoma cell lines L243 (anti-DRαβ; Lampson and Levy, 1980), 1B5 (anti-DRα; Adams et al., 1983), DM.K8 (anti-DMβ; Vogt et al., 1996) and the rabbit antiserum 11323 (anti-DMαβ; Busch et al., 1998; Liu et al., 1998) have been described previously.
Flow cytometric analysis
Cells were stained with the anti-DM antiserum 11323 followed by biotinylated goat anti-rabbit IgG and by streptavidin–R-phycoerythrin (Southern Biotechnology Associates). Alternatively, cells were stained with mAbs directed against DR (L243), CD83 (Pharmingen) or CD86 (Pharmingen) followed by goat anti-mouse IgG–R-phycoerythrin or goat anti-mouse IgG–fluorescein isothiocyanate (Southern Biotechnology Associates). Analysis was performed on a FACScan flow cytometer (Becton Dickinson). Background fluorescence was evaluated using an irrelevant antiserum or an irrelevant isotype-matched mAb.
Quantification of DM and DR on the cell surface
A total of 2.5 × 107 cells were washed twice in isotonic buffer (250 mM sucrose, 30 mM sodium phosphate, 5 mM MgCl2, 0.1 mM CaCl2 pH 7.6). Sulfo-NHS-SS-Biotin (Pierce) was added at a concentration of 3 mg/ml in isotonic buffer. Cells were rotated at 4°C for 15 min for biotin surface labeling followed by two washes with 50 mM glycine in isotonic buffer to stop biotinylation. Cells were then lysed in lysis buffer (20 mM Tris, 5 mM MgCl2 pH 7.6) containing 1% NP-40 and a protease inhibitory cocktail (leupeptin, phenylmethylsulfonyl fluoride, chymostatin and pepstatin) at 4°C for 1 h. The lysate was cleared by two centrifugation steps at 13 000 r.p.m. Biotinylated surface proteins were separated from unbiotinylated proteins by precipitation with NeutrAvidin–agarose beads (Pierce). NeutrAvidin beads were washed four times with washing buffer (100 mM sodium phosphate, 50 mM NaCl, 1% NP-40 pH 6.5). Surface proteins were then eluted by boiling the beads twice in 2× Laemmli sample buffer, subjected to Western blotting and quantified by densitometry. As a reference, the cell lysate prior to NeutrAvidin depletion was used.
Western blot analysis
Samples were separated by SDS–PAGE (12%) and transferred onto Immobilon PVDF membranes (Millipore). After the transfer the PVDF membrane was blocked with Blocking reagent (Boehringer) and then incubated with the respective primary antibody. Binding of antibody was detected by incubation with horseradish peroxidase-conjugated secondary antibodies (Dianova) followed by enhanced chemiluminescence with Super-Signal™ or Super-Signal™ Ultra (Pierce).
Immunoprecipitation of cell surface proteins
Cells were washed twice with ice-cold RPMI/10% FCS and 7 μg of the respective biotinylated antibody were added. Cells were then rotated at 4°C for 45 min, washed twice with isotonic buffer and finally lysed in a hypotonic buffer (20 mM HEPES, 5 mM MgOAc pH 6.4) containing 1% detergent (NP-40 or CHAPS) and a protease inhibitory cocktail. The lysate was cleared by two centrifugation steps at 13 000 r.p.m. To retrieve the antibody complexes the supernatant was rotated with 10 μl NeutrAvidin–agarose beads (Pierce) for 2 h at 4°C. The beads were washed four times with washing buffer (100 mM sodium phosphate, 50 mM NaCl, 1% NP-40 or 1% CHAPS pH 6.5). Immunoprecipitates were eluted by boiling the beads twice in 2× Laemmli sample buffer for 5 min and analyzed by Western blotting.
In vivo loading assay
Cells (1.5 × 106) were washed and resuspended in 300 μl of medium (RPMI plus 10% FCS). Biotinylated HA(307–319) was added as a reporter peptide. Cells were rotated at 37°C for 3 h, washed and lysed in 20 mM Tris pH 7.4, 5 mM MgCl2, 1% NP-40 supplemented with protease inhibitors. Quantitation of HLA–DR–HA peptide complexes was performed by sandwich immunoassay. The lysate was diluted 10–fold in phosphate-buffered saline containing 0.05% Tween–20 and 1% bovine serum albumin and incubated in an L243-coated microtiter plate (Nunc) for 3 h. Plates were developed by incubation for 45 min with 0.1 μg/ml streptavidin–Europium (Perkin Elmer) according to the manufacturer's protocol and time-resolved fluorescence was quantified using the VICTOR multilabel counter (Perkin Elmer).
In the experiments where endocytosis had to be blocked, 0.5 μM wortmannin (Sigma) was added to the cells 30 min prior to the addition of biotinylated peptide.
Subcellular fractionation
A total of 2 × 108 T2.DR4.DM cells were washed twice in ice-cold RPMI plus 10% FCS. Biotinylated HA was added to a final concentration of 15 μM. The cells were rotated at 37°C either for 30 min or 3 h, washed twice with isotonic buffer and then resuspended in 10 ml of homogenization buffer (250 mM sucrose, 10 mM triethanolamine, 10 mM acetic acid, 5 mM MgCl2 pH 7.5). After 15 min incubation on ice the cells were passed six times through a ball-bearing homogenizer (EMBL, Heidelberg; gap 18 μm). The homogenate was spun for 5 min at 1000 g. The supernatant was mixed with Percoll (Amersham-Pharmacia) and a sucrose buffer up to a final concentration of 27% Percoll, 250 mM sucrose and 10 mM Tris. After centrifugation in a Beckman Type 45 rotor at 30 000 r.p.m. for 45 min the gradient was divided into 10 fractions. Membranes were pelleted by diluting the individual fractions with sucrose buffer (250 mM sucrose, 10 mM Tris pH 7.5) followed by ultracentrifugation in a Beckman SW28 rotor at 25 000 r.p.m. for 90 min. Pelleted membranes were lysed in 1% NP-40 and subsequently analyzed either by marker analysis or by the in vivo loading assay described.
Marker analysis
The lysosomal marker enzyme β-hexosaminidase (Mane et al., 1989) was assayed by a published procedure (Green et al., 1987). Lamp–1 (lysosomes and late endosomes; Mane et al., 1989), rab7 (late endosomes; Novick and Zerial, 1997), rab5 (plasma membrane and early endosomes; Novick and Zerial, 1997), HLA–DR and HLA–DM were assayed by Western blotting. The rabbit anti-lamp-1 serum was a gift of Dr Carlsson (Uppsala, Sweden). The rabbit anti-rab7 and anti-rab5 antisera were a gift of Dr Faigle (Paris, France). HLA–DR and HLA–DM molecules were detected by the mAbs 1B5 and DM.K8, respectively.
T–cell proliferation assay
MS1 and MS23C8 T–cell clones were generated by stimulation with MBP(87–99) and MBP(111–129) peptides, respectively. Both clones are DRB1*0401 (DR4) restricted. Proliferation assays were carried out by using T2.DR4 or T2.DR4.DM transfectants as APCs. APCs were irradiated (30 000 Rad), plated at 10 000 cells/well, and pulsed with various concentrations of MBP peptides or MBP protein in 100 μl/well of media at 37°C for 3 h. Media consisted of Iscove's modified Dulbecco's medium (IMDM) (Gibco-BRL), 5% heat-inactivated pooled human serum, 1% penicillin/streptomycin and 2% glutamine. Cells were washed twice, and T cells were added at 10 000 cells/well in serum-free media (X-vivo 15; BioWhittaker). Cells were co-cultured for 72 h in a total volume of 200 μl. During the last 16 h of culture 1 μCi of [3H]thymidine (Dupont) per well was added. [3H]thymidine incorporation was measured in a scintillation counter (Betaplate; Pharmacia).
Acknowledgments
Acknowledgements
We are grateful to Dr D.M.Zaller for providing us with the 11323 anti-DM serum, to Dr J.S.Blum for T2.DR4 transfectants and to Dr P.Cresswell for T2.DR4.DM transfectants. We thank Dr Bill Biddison for helpful comments on the manuscript, Dr R.Pipkorn for synthesis, labeling and purification of peptides, N.Anders for performing some of the Western blot analysis, and K.Potzies, K.U.Seng and A.Schäfer for expert technical assistance.
References
- Adams T.E., Bodmer, J.G. and Bodme, W.F. (1983) Production and characterization of monoclonal antibodies recognizing the α-chain subunits of human Ia alloantigens. Immunology, 50, 613–624. [PMC free article] [PubMed] [Google Scholar]
- Adorini L., Appella, E., Doria, G., Cardinaux, F. and Nagy, Z.A. (1989) Competition for antigen presentation in living cells involves exchange of peptides bound by class II MHC molecules. Nature, 342, 800–803. [DOI] [PubMed] [Google Scholar]
- Amoscato A.A., Prenovitz, D.A. and Lotze, M.T. (1998) Rapid extracellular degradation of synthetic class I peptides by human dendritic cells. J. Immunol., 161, 4023–4032. [PubMed] [Google Scholar]
- Anderson M.S. and Miller, J. (1992) Invariant chain can function as a chaperone protein for class II major histocompatibility complex molecules. Proc. Natl Acad. Sci. USA, 89, 2282–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson T., Patwardhan, A., Emilson, A., Carlsson, K. and Scheynius, A. (1998) HLA–DM is expressed on the cell surface and colocalizes with HLA–DR and invariant chain in human Langerhans cells. Arch. Dermatol. Res., 290, 674–680. [DOI] [PubMed] [Google Scholar]
- Bakke O. and Dobberstein, B. (1990) MHC class II-associated invariant chain contains a sorting signal for endosomal compartments. Cell, 63, 707–715. [DOI] [PubMed] [Google Scholar]
- Barnes K.A. and Mitchell, R.N. (1995) Detection of functional class II-associated antigen: role of a low density endosomal compartment in antigen processing. J. Exp. Med., 181, 1715–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch R., Doebele, R.C., von Scheven, E., Fahrni, J. and Mellins, E.D. (1998) Aberrant intermolecular disulfide bonding in a mutant HLA–DM molecule: Implications for assembly, maturation and function. J. Immunol., 160, 734–743. [PubMed] [Google Scholar]
- Castellino F. and Germain, R.N. (1995) Extensive trafficking of MHC class II-invariant chain complexes in the endocytic pathway and appearance of peptide-loaded class II in multiple compartments. Immunity, 2, 73–88. [DOI] [PubMed] [Google Scholar]
- Cella M., Engering, A., Pinet, V., Pieters, J. and Lanzavecchia, A. (1997) Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature, 388, 782–787. [DOI] [PubMed] [Google Scholar]
- Chicz R.M., Urban, R.G., Lane, W.S., Gorga, J.C., Stern, L.J., Vignali, D.A.A. and Strominger, J.L. (1992) Predominant naturally processed peptides bound to HLA–DR1 are derived from MHC-related molecules and are heterogeneous in size. Nature, 358, 764–768. [DOI] [PubMed] [Google Scholar]
- Cho S., Attaya, M. and Monaco, J.J. (1991) New class II-like genes in the murine MHC. Nature, 353, 573–576. [DOI] [PubMed] [Google Scholar]
- Denzin L.K. and Cresswell, P. (1995) HLA–DM induces CLIP dissociation from MHC class II αβ dimers and facilitates peptide loading. Cell, 82, 155–165. [DOI] [PubMed] [Google Scholar]
- Denzin L.K., Robbins, N.F., Carboy-Newcomb, C. and Cresswell, P. (1994) Assembly and intracellular transport of HLA–DM and correction of the class II antigen-processing defect in T2 cells. Immunity, 1, 595–606. [DOI] [PubMed] [Google Scholar]
- Denzin L.K., Hammond, C. and Cresswell, P. (1996) HLA–DM interactions with intermediates in HLA–DR maturation and a role for HLA–DM in stabilizing empty HLA–DR molecules. J. Exp. Med., 184, 2153–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzin L.K., Sant'Angelo, D.B., Hammond, C., Surman, M.J. and Cresswell, P. (1997) Negative regulation by HLA–DO of MHC class II-restricted antigen processing. Science, 278, 106–109. [DOI] [PubMed] [Google Scholar]
- Escola J.M., Grivel, J.C., Chavrier, P. and Gorvel, J.P. (1995) Different endocytic compartments are involved in the tight association of class II molecules with processed hen egg lysozyme and ribonuclease A in B cells. J. Cell Sci., 108, 2337–2345. [DOI] [PubMed] [Google Scholar]
- Fairchild P.J., Wildgoose, R., Atherton, E., Webb, S. and Wraith, D.C. (1993) An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int. Immunol., 5, 1151–1158. [DOI] [PubMed] [Google Scholar]
- Fremont D.H., Crawford, F., Marrack, P., Hendrickson, W.A. and Kappler, J. (1998) Crystal structure of mouse H2-M. Immunity, 9, 385–393. [DOI] [PubMed] [Google Scholar]
- Geluk A., van Meijengaarden, K.E., de Vries, R.R.P., Sette, A. and Ottenhoff, T.H.M. (1997) A DR17-restricted T cell epitope from a secreted Mycobacterium tuberculosis antigen binds to DR17 molecules at neutral pH. Eur. J. Immunol., 27, 842–847. [DOI] [PubMed] [Google Scholar]
- Ghosh P., Amaya, M., Mellins, E. and Wiley, D.C. (1995) The structure of HLA–DR3 complexed with CLIP, an intermediate in peptide loading. Nature, 387, 457–462. [DOI] [PubMed] [Google Scholar]
- Green S.A., Zimmer, K.P., Griffiths, G. and Mellman, I. (1987) Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J. Cell Biol., 105, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J.P., Chu, R. and Harding, C.V. (1997) Early endosomes and a late endocytic compartment generate different peptide–class II MHC complexes via distinct processing mechanisms. J. Immunol., 158, 1523–1532. [PubMed] [Google Scholar]
- Karlsson L., Peleraux, A., Lindstedt, R., Liljedahl, M. and Peterson, P.A. (1994) Reconstitution of an operational MHC class II compartment in nonantigen-presenting cells. Science, 266, 1569–1572. [DOI] [PubMed] [Google Scholar]
- Kelly A.P., Monaco, J.J., Cho, S. and Trowsdale, J. (1991) A new human HLA class II-related locus. Nature, 353, 571–573. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Vogt, A.B. and Hämmerling, G.J. (1995a) Structural features of invariant chain CLIP region controlling rapid release from HLA–DR molecules and inhibition of peptide binding. Proc. Natl Acad. Sci. USA, 92, 8313–8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropshofer H., Vogt, A.B., Stern, L.J. and Hämmerling, G.J. (1995b) Self-release of CLIP in peptide loading of HLA–DR molecules. Science, 270, 1357–1359. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Vogt, A.B., Moldenhauer, G., Hammer, J., Blum, J.S. and Hämmerling, G.J. (1996) Editing of the HLA–DR peptide repertoire by HLA–DM. EMBO J., 15, 6144–6154. [PMC free article] [PubMed] [Google Scholar]
- Kropshofer H., Hämmerling, G.J. and Vogt, A.B. (1997a) How HLA–DM edits the MHC class II peptide repertoire: survival of the fittest?Immunol. Today, 18, 77–82. [DOI] [PubMed] [Google Scholar]
- Kropshofer H., Arndt, S.O., Moldenhauer, G., Hämmerling, G.J. and Vogt, A.B. (1997b) HLA–DM acts as a molecular chaperone and rescues empty HLA–DR molecules at lysosomal pH. Immunity, 6, 293–302. [DOI] [PubMed] [Google Scholar]
- Lampson L.A. and Levy, R. (1980) Two populations of Ia-like molecules on a human B cell line. J. Immunol., 125, 293–299. [PubMed] [Google Scholar]
- Lanzavecchia A., Reid, P.A. and Watts, C. (1992) Irreversible association of peptides with class II MHC molecules in living cells. Nature, 357, 249–252. [DOI] [PubMed] [Google Scholar]
- Li G., D'Souza-Schorey, C., Barbieri, M.A., Roberts, R.L., Klippel, A., Williams, L.T. and Stahl, P.D. (1995) Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl Acad. Sci. USA, 92, 10207–10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstedt R., Liljedahl, M., Péléraux, A., Peterson, P.A. and Karlsson, L. (1995) The MHC class II molecule H2-M is targeted to an endosomal compartment by a tyrosine-based targeting motif. Immunity, 3, 561–572. [DOI] [PubMed] [Google Scholar]
- Liu G.Y. and Wraith, D.C. (1995) Affinity for class II MHC determines the extent to which soluble peptides tolerize autoreactive T cells in naive and primed adult mice—implications for autoimmunity. Int. Immunol., 7, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Liu S.H., Marks, M.S. and Brodsky, F.M. (1998) A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J. Cell Biol., 140, 1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotteau V., Teyton, L., Peleraux, A., Nilsson, T., Karlsson, L., Schmid, S.L., Quaranta, V. and Peterson, P.A. (1990) Intracellular transport of class II MHC molecules directed by the invariant chain. Nature, 348, 600–605. [DOI] [PubMed] [Google Scholar]
- Mane S.M., Marzella, L., Bainton, D.F., Holt, V.K., Cha, Y., Hildreth, J.E. and August, J.T. (1989) Purification and characterization of human lysosomal membrane glycoproteins. Arch. Biochem. Biophys., 268, 360–378. [DOI] [PubMed] [Google Scholar]
- Marks M.S., Roche, P.A., van Donselaar, E., Woodruff, L., Peters, P.J. and Bonifacino, J.S. (1995) A lysosomal targeting signal in the cytoplasmic tail of the β chain directs HLA–DM to MHC class II compartments. J. Cell Biol., 131, 351–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R., McFarland, H.F. and McFarlin, D.E. (1992) Immunological aspects of demyelinating diseases. Annu. Rev. Immunol., 10, 153–187. [DOI] [PubMed] [Google Scholar]
- Martin W.D., Hicks, G.G., Mendiratta, S.K., Leva, H.I., Ruley, H.E. and Van Kaer, L. (1996) H2-M mutant mice are defective in the peptide loading of class II molecules, antigen presentation and T cell repertoire selection. Cell, 84, 543–550. [DOI] [PubMed] [Google Scholar]
- Mason K. and McConnell, H.M. (1994) Short-lived complexes between myelin basic protein peptides and I-Ak. Proc. Natl Acad. Sci. USA, 91, 12463–12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Wolf, P., Tourne, S., Waltzinger, C., Dierich, A., Barois, N., Ploegh, H., Benoist, C. and Mathis, D. (1996) Mice lacking H2-M complexes, enigmatic elements of the MHC class II peptide-loading pathway. Cell, 84, 531–541. [DOI] [PubMed] [Google Scholar]
- Mosyak L., Zaller, D.M. and Wiley, D.C. (1998) The structure of HLA–DM, the peptide exchange catalyst that loads antigen onto class II MHC molecules during antigen presentation. Immunity, 9, 377–383. [DOI] [PubMed] [Google Scholar]
- Muraro P.A., et al. (1997)Immunodominance of a low-affinity major histocompatibility complex-binding myelin basic protein epitope (residues 111–129) in HLA–DR4 (B1*0401) subjects is associated with a restricted T cell receptor repertoire. J. Clin. Invest., 100, 339–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadimi F., Moreno, J., Momburg, F., Heuser, A., Fuchs, S., Adorini, L. and Hämmerling, G.J. (1991) Antigen presentation of hen egg-white lysozyme but not of ribonuclease A is augmented by the major histocompatibility complex class II-associated invariant chain. Eur. J. Immunol., 21, 1255–1263. [DOI] [PubMed] [Google Scholar]
- Novick P. and Zerial, M. (1997) The diversity of Rab proteins in vesicle transport. Curr. Opin. Cell Biol., 9, 496–504. [DOI] [PubMed] [Google Scholar]
- Park S.J., Sadegh-Nasseri, S. and Wiley, D.C. (1995) Invariant chain made in Escherichia coli has an exposed N-terminal segment that blocks antigen binding to HLA–DR1 and a trimeric C-terminal segment that binds empty HLA–DR1. Proc. Natl Acad. Sci. USA, 21, 11289–11293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters P.J., Neefjes, J.J., Oorschot, V., Ploegh, H.L. and Geuze, H.J. (1991) Segregation of MHC class II molecules from MHC class II molecules in the Golgi complex for transport to lysosomal compartment. Nature, 349, 669–676. [DOI] [PubMed] [Google Scholar]
- Pieters J., Horstmann, H., Bakke, O., Griffiths, G. and Lipp, J. (1991) Intracellular transport and localization of MHC class II molecules and associated invariant chain. J. Cell Biol., 115, 1213–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinet V.M. and Long, E.O. (1998) Peptide loading onto recycling HLA–DR molecules occurs in early endosomes. Eur. J. Immunol., 28, 799–804. [DOI] [PubMed] [Google Scholar]
- Pinet V., Malnati, M.S. and Long, E.O. (1994) Two processing pathways for the MHC class II-restricted presentation of exogenous influenza virus antigen. J. Immunol., 152, 4852–4560. [PubMed] [Google Scholar]
- Pinet V., Vergelli, M., Martin, R., Bakke, O. and Long, E.O. (1995) Antigen presentation mediated by recycling of surface HLA–DR molecules. Nature, 375, 603–606. [DOI] [PubMed] [Google Scholar]
- Pond L. and Watts, C. (1997) Characterization of transport of newly assembled T cell stimulatory MHC class II–peptide complexes from MHC class II compartments to the cell surface. J. Immunol., 519, 543–553. [PubMed] [Google Scholar]
- Raddrizzani L., Bono, E., Vogt, A.B., Kropshofer, H., Gallazzi, F., Sturniolo, T., Hämmerling, G.J., Sinigaglia, F. and Hammer, J. (1999) Identification of destabilizing residues in HLA class II-selected bacteriophage display libraries edited by HLA–DM. Eur. J. Immunol., 29, 660–668. [DOI] [PubMed] [Google Scholar]
- Raposo G., Nijman, H.W., Stoorvogel, W., Liejendekker, R., Harding, C.V., Melief, C.J. and Geuze, H.J. (1996) B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med., 183, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P.A. and Watts, C. (1990) Cycling of cell-surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature, 346, 655–657. [DOI] [PubMed] [Google Scholar]
- Riberdy J.M., Newcomb, J.R., Surman, M.J., Barbosa, J.A. and Cresswell, P. (1992) HLA–DR molecules from an antigen-processing mutant cell line are associated with invariant chain peptides. Nature, 360, 474–477. [DOI] [PubMed] [Google Scholar]
- Riese R.J., Wolf, P.R., Bromme, D., Nathin, L.R., Villadangos, J.A., Ploegh, H.L. and Chapman, H.A. (1996) Essential role for cathepsin S in MHC class II-associated invariant chain processing and peptide loading. Immunity, 4, 357–365. [DOI] [PubMed] [Google Scholar]
- Roche P.A., Teletski, C.I., Stang, E., Bakke, O. and Long, E.O. (1993) Cell surface HLA–DR-invariant chain complexes are targeted to endosomes by rapid internalization. Proc. Natl Acad. Sci. USA, 90, 8581–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J., Schweizer, A., Russell, D. and Kornfeld, S. (1996) The targeting of lamp-1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine motif relative to the membrane. J. Cell Biol., 132, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnoli P. and Germain, R.N. (1994) The CLIP region of invariant chain plays a critical role in regulating major histocompatibility complex class II folding, transport and peptide occupancy. J. Exp. Med., 180, 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson F., Kleijmeer, M., Kelly, A., Verwoerd, D., Tulp, A., Neefjes, J., Geuze, H.J. and Trowsdale, J. (1994) Accumulation of HLA–DM, a regulator of antigen presentation, in MHC class II compartments. Science, 266, 1566–1569. [DOI] [PubMed] [Google Scholar]
- Sanderson F., Thomas, C., Neefjes, J. and Trowsdale, J. (1996) Association between HLA–DM and HLA–DR in vivo. Immunity, 4, 87–96. [DOI] [PubMed] [Google Scholar]
- Santambrogio L., Sato, A.K., Fischer, F.R., Dorf, M.E. and Stern, L.J. (1999a) Abundant empty class II molecules on the surface of immature dendritic cells. Proc. Natl Acad. Sci. USA, 96, 15050–15055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santambrogio L., Sato, A.K., Carven, G.J., Belyanskaya, S.L., Strominger, J.L. and Stern, L.J. (1999b) Extracellular antigen processing and presentation by immature dendritic cells. Proc. Natl Acad. Sci. USA, 96, 15055–15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer P.H., Green, J.M., Malapati, S., Gu, L. and Pierce, S.K. (1996) HLA–DM is present in one-fifth the amount of HLA–DR in the class II peptide-loading compartment where it associates with leupeptin-induced peptide (LIP)–HLA–DR complexes. J. Immunol., 157, 5487–5495. [PubMed] [Google Scholar]
- Sherman M.A., Weber, D.A. and Jensen, P.E. (1995) DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity, 3, 197–205. [DOI] [PubMed] [Google Scholar]
- Siebenkotten I.M., Carstens, C. and Koch, N. (1998) Identification of a sequence that mediates promiscuous binding of invariant chain to MHC class II allotypes. J. Immunol., 160, 3355–3362. [PubMed] [Google Scholar]
- Simonsen A., et al. (1998)EEA1 links PI (3)K function to Rab5 regulation of endosomal fusion. Nature, 394, 494–498. [DOI] [PubMed] [Google Scholar]
- Sloan V.S., Cameron, P., Porter, G., Gammon, M., Amaya, M., Mellins, E. and Zaller, D.M. (1995) Mediation by HLA–DM of dissociation of peptides from HLA–DR. Nature, 375, 802–806. [DOI] [PubMed] [Google Scholar]
- Thurner B., et al. (1999)Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J. Immunol. Methods, 223, 1–15. [DOI] [PubMed] [Google Scholar]
- Tourne S., Miyazaki, T., Wolf, P., Ploegh, H., Benoist, C. and Mathis, D. (1997) Functionality of major histocompatibility complex class II molecules in mice doubly deficient for invariant chain and H-2M complexes. Proc. Natl Acad. Sci. USA, 94, 9255–9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergelli M., Pinet, V., Vogt, A.B., Kalbus, M., Malnati, M., Riccio, P., Long, E.O. and Martin, R. (1997) HLA–DR-restricted presentation of purified myelin basic protein is independent of intracellular processing. Eur. J. Immunol., 27, 941–951. [DOI] [PubMed] [Google Scholar]
- Vogt A.B. and Kropshofer, H. (1999) HLA–DM—an endosomal and lysosomal chaperone for the immune system. Trends Biochem. Sci., 24, 150–154. [DOI] [PubMed] [Google Scholar]
- Vogt A.B., Stern, L.J., Amshoff, C., Dobberstein, B., Hämmerling, G.J. and Kropshofer, H. (1995) Interference of distinct invariant chain regions with superantigen contact area and antigenic peptide binding groove of HLA–DR. J. Immunol., 155, 4757–4765. [PubMed] [Google Scholar]
- Vogt A.B., Kropshofer, H., Moldenhauer, G. and Hämmerling, G.J. (1996) Kinetic analysis of peptide loading onto HLA–DR molecules mediated by HLA–DM. Proc. Natl Acad. Sci. USA, 93, 9724–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D.A., Evavold, B.D. and Jensen, P.E. (1996) Enhanced dissociation of HLA–DR-bound peptides in the presence of HLA–DM. Science, 274, 618–620. [DOI] [PubMed] [Google Scholar]
- Wolf P.R. and Ploegh, H.L. (1995) How MHC class II molecules acquire peptide cargo: biosynthesis and trafficking through the endocytic pathway. Annu. Rev. Cell Dev. Biol., 11, 267–306. [DOI] [PubMed] [Google Scholar]
- Wölpl A., Halder, T., Kalbacher, H., Neumeyer, H., Siemoneit, K., Goldmann, S.F. and Eiermann, T.H. (1998) Human monoclonal antibody with T–cell-like specificity recognizes MHC class I self-peptide presented by HLA–DR1 on activated cells. Tissue Antigens, 51, 258–269. [DOI] [PubMed] [Google Scholar]
- Wubbolts R.W., Fernandez-Borja, M., Oomen, L., Verwoerd, D., Janssen, H., Calafat, J., Tulp, A., Dusseljee, S. and Neefjes, J. (1996) Direct vesicular transport of MHC class II molecules from lysosomal structures to the cell surface. J. Cell Biol., 135, 611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]