Abstract
It has recently been reported that N–ethylmaleimide-sensitive fusion ATPase (NSF) can fuse protein-free liposomes containing substantial amounts of 1,2–dioleoylphosphatidylserine (DOPS) and 1,2–dioleoyl-phosphatidyl–ethanolamine (DOPE) (Otter-Nilsson et al., 1999). The authors impart physiological significance to this observation and propose to re-conceptualize the general role of NSF in fusion processes. We can confirm that isolated NSF can fuse liposomes of the specified composition. However, this activity of NSF is resistant to inactivation by N–ethylmaleimide and does not depend on the presence of α–SNAP (soluble NSF-attachment protein). Moreover, under the same conditions, either α–SNAP, other proteins apparently unrelated to vesicular transport (glyceraldehyde-3–phosphate dehydrogenase or lactic dehydrogenase) or even 3 mM magnesium ions can also cause lipid mixing. In contrast, neither NSF nor the other proteins nor magnesium had any significant fusogenic activity with liposomes composed of a biologically occurring mixture of lipids. A straightforward explanation is that the lipid composition chosen as optimal for NSF favors non-specific fusion because it is physically unstable when formed into liposomes. A variety of minor perturbations could then trigger coalescence.
Keywords: fusion/lipid/NSF/SNARE/vesicle
Introduction
The N–ethylmaleimide (NEM)-sensitive fusion ATPase (NSF) utilizes the energy made available by ATP hydrolysis to disrupt otherwise stable complexes of vesicle-associated (v–)SNAREs (SNAP receptors) and target membrane-associated (t–)SNAREs (Söllner et al., 1993a; Hayashi et al., 1995; Banerjee et al., 1996; Hay et al., 1997; Ungermann et al., 1998). Its established function in membrane fusion, based on this activity, is to maintain a supply of uncombined SNARE proteins within the same membrane, to allow SNARE pairing between membranes and to allow v–SNAREs to be recycled to donor membranes unaccompanied by cognate t–SNAREs (Mayer et al., 1996; Hanson et al., 1997; Otto et al., 1997; Littleton et al., 1998). Once this is achieved, NSF is no longer needed for a single round of fusion to occur, as shown for homotypic fusion of yeast vacuoles as well as other experimental systems (Banerjee et al., 1996; Colombo et al., 1996; Mayer et al., 1996; Xu et al., 1999). While ATP hydrolysis by NSF is needed for its role in fusion in many cell-free transport reactions (Block et al., 1988; Beckers et al., 1989; Sztul et al., 1993; Rodriguez et al., 1994), recently Warren and colleagues have found that ATP hydrolysis by NSF is not required for NSF to fulfill its role in post-mitotic Golgi stack re-assembly (Müller et al., 1999). This raises the possibility that, in addition to its role in recycling SNARE complexes, NSF may participate in some additional processes leading to fusion by an unknown mechanism. The activity of NSF in post-mitotic fusion, although ATP hydrolysis independent, was sensitive to NEM.
In this context, the recent report that NSF and α–SNAP (which binds NSF to SNARE complexes) can together rapidly and efficiently fuse certain protein-free liposomes (Otter-Nilsson et al., 1999) takes on special interest and warrants further examination. The recent report claims that fusion of liposomes requires a combination of NSF and α–SNAP and requires hydrolysis of ATP. Sensitivity of purified NSF to NEM was not reported. Fusion activity of NSF was only reported using donor and acceptor liposomes consisting of atypical phospholipid compositions as compared with cellular membranes. Specifically, donor liposomes contained an equimolar mixture of 1,2–dioleoyl-phosphatidylethanolamine (DOPE) and 1,2–dioleoyl-phosphatidylserine (DOPS), and acceptor liposomes consisted of equimolar amounts of DOPE and 1,2–dioleoyl-phosphatidylcholine (DOPC) or DOPS. Fusion activity was also reported for the NSF homolog p97, bound to its co-factor p47, and purified as a complex from rat liver cytosol.
Here we characterize the fusogenic activity of NSF with regard to ATP hydrolysis, NEM sensitivity and lipid background.
Results
NSF-mediated fusion of liposomes is strictly dependent on the lipid composition
To characterize the lipid requirements of NSF-mediated liposome fusion we prepared the following lipid mixtures: (i) DOPE:DOPS (50:50) donor and DOPE:DOPC (50:50) acceptor liposomes (as used by Otter-Nilsson et al., 1999); (ii) 1–palmitoyl-2–oleoyl phosphatidylcholine (POPC): DOPS (85:15) donor and acceptor liposomes (as used for SNARE-mediated fusion of liposomes by Weber et al., 1998); and (iii) liposomes made from a lipid mixture similar to rat liver Golgi membranes (van Meer, 1998). Small unilamellar protein-free liposomes were prepared by extrusion through 50 nm pore filters.
Liposome fusion was monitored by a well established lipid mixing assay (Struck et al., 1981; Otter-Nilsson et al., 1999). Donor liposomes were supplemented with equimolar amounts of two fluorescently headgroup-labeled lipids [0.8% each of N–(7–nitro-2,1,3–benzoxadiazole-4–yl)-1,2–dipalmitoyl phosphatidylethanolamine (NBD-DPPE) and N–(lissamine rhodamine B sulfonyl)-1,2–dipalmitoyl phosphatidylethanolamine (rhodamine-DPPE)]. When these fluorescent lipids are in close proximity, energy transfer results in a decreased emission of NBD fluorescence. As a consequence of fusion of fluorescently labeled donor liposomes with unlabeled acceptor liposomes, the distance between NBD- and rhodamine-labeled lipids increases. Therefore, the efficiency of energy transfer from NBD-DPPE to rhodamine-DPPE is diminished, which in turn results in an increase of NBD fluorescence.
Fusion measurements were initiated by the addition of donor liposomes to pre-warmed buffer followed by the addition of acceptor liposomes and NSF in a final concentration within the linear response (5 μg/ml). A molar ratio of donor to acceptor of 1:5 was maintained throughout all experiments. After 10 min, liposomes were detergent solubilized (0.1% Triton X–100) to obtain maximal dilution of fluorescent lipids. The fusion activity is expressed as a percentage of maximal NBD fluorescence obtained after addition of detergent.
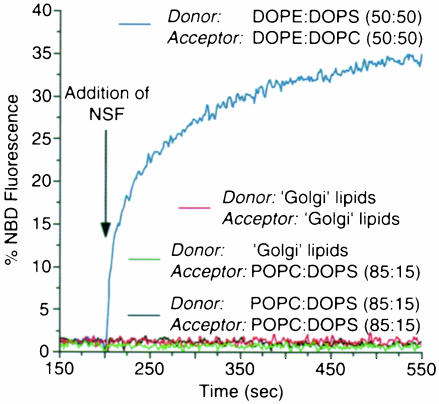
As shown in Figure 1, the addition of NSF to DOPE: DOPS (50:50) donor and DOPE:DOPC (50:50) acceptor liposomes resulted in a rapid and efficient increase of NBD fluorescence (blue curve). NSF-mediated fusion could also be observed when both donor and acceptor liposomes were composed of equimolar amounts of DOPE and DOPS. However, in contrast to the findings of Otter-Nilsson et al. (1999), fusion activity was completely abolished after heat denaturation, and addition of α–SNAP only slightly increased the fusion efficiency (data not shown). This increase was most likely to be due to a fusion activity directly associated with α–SNAP (see below and Figure 3). Liposomes composed of POPC:DOPS (85:15) did not fuse upon addition of NSF (Figure 1, black curve). To mimic the lipid composition of a cellular membrane, we made use of liposomes consisting of a lipid mixture similar to rat liver Golgi membranes (van Meer, 1998). A potential lipid asymmetry between the outer and inner leaflet was not reconstituted. As with POPC:DOPS liposomes, significant fusion activity of NSF was not detectable (Figure 1, red curve). Likewise, NSF-mediated fusion activity could not be observed when a combination of ‘Golgi’ donor liposomes and POPC:DOPS acceptor liposomes was analyzed (Figure 1, green curve). The addition of α–SNAP (in the presence of Mg2+–ATP) did not change these results.
Fig. 1. NSF-mediated liposome–liposome fusion is restricted to specific lipid compositions. Donor and acceptor liposomes with the lipid compositions indicated were mixed at a molar ratio of 1:5 in a cuvette at 37°C (for details see Materials and methods). Donor liposomes were supplemented with rhodamine- and NBD-labeled DPPE (0.8 mol% each). NSF was added to a final concentration of 5 μg/ml at the time indicated. After 10 min the reaction was terminated by adding Triton X–100 at a final concentration of 0.1% (w/v). Data are expressed as a percentage of maximal NBD fluorescence after addition of detergent.
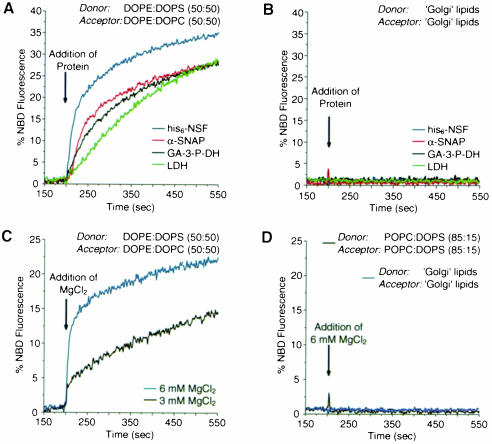
Fig. 3. Factors mediating fusion of DOPE:DOPS donor and DOPE:DOPC acceptor liposomes. Donor and acceptor liposomes were mixed with various reagents and fusion was followed by the increase of NBD fluorescence and analyzed as described in the legend to Figure 1. (A) Donor (DOPE:DOPS; 50:50) and acceptor (DOPE:DOPC; 50:50) liposomes were premixed. NSF (blue curve), α–SNAP (red curve), glyceraldehyde-3–phosphate dehydrogenase (GA-3-P-DH, black curve) or lactic dehydrogenase (LDH, green curve) were added at a final concentration of 5 μg/ml. (B) NSF (blue curve), α–SNAP (red curve), glyceraldehyde-3-phosphate dehydrogenase (GA-3-P-DH, black curve) or lactic dehydrogenase (LDH, green curve) were added to premixed ‘Golgi’-like donor and acceptor liposomes at a final concentration of 5 μg/ml. (C) MgCl2 was added at the concentration indicated to a premix of DOPE:DOPS (50:50) donor and DOPE:DOPC (50:50) acceptor liposomes (black curve, 3 mM; blue curve, 6 mM). (D) MgCl2 (final concentration of 6 mM) was added to either ‘Golgi’ lipid donor and acceptor liposomes (blue curve) or POPC:DOPS (85:15) donor and acceptor liposomes (black curve).
SNARE-mediated membrane fusion does not depend on a specific lipid composition
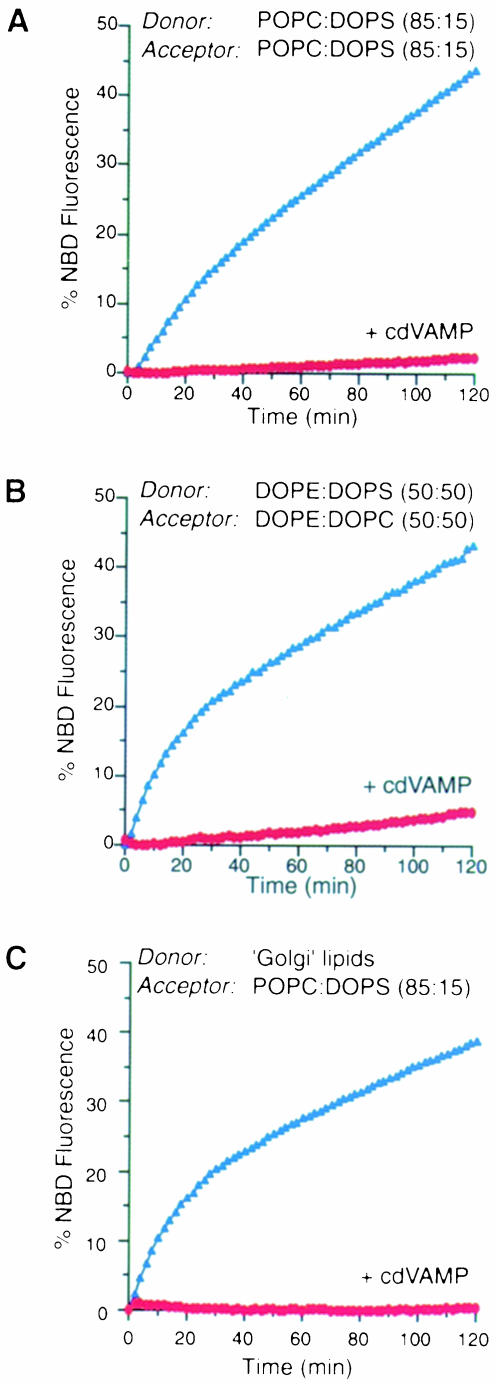
To compare the lipid requirements of NSF-mediated liposome fusion (see above) with those for SNARE-mediated membrane fusion (Weber et al., 1998) we prepared v– and t–SNARE-containing liposomes with the same lipid compositions used to study NSF-dependent fusion. As illustrated in Figure 2, isolated SNAREs fused POPC:DOPS (85:15; donor and acceptor) liposomes, DOPE:DOPS (50:50; donor) and DOPE:DOPC (50:50; acceptor) liposomes, and liposomes consisting of a lipid composition similar to rat liver Golgi membranes (donor) and POPC:DOPS (85:15; acceptor). SNAREs were also capable of fusing liposomes containing Golgi-like lipids on both the donor and the acceptor side albeit with reduced efficiency (∼50%; data not shown). This lower efficiency was likely to be due to aggregation of t–SNARE liposomes (which in turn reduced the concentration of accessible t–SNARE liposomes) and does not necessarily indicate a specific lipid requirement in acceptor liposomes. In all cases, fusion was entirely dependent on free SNAREs since the addition of the cytosolic domain of VAMP (which prevents trans-SNARE complex formation) abolished the signal. These results indicate that, in contrast to NSF, SNAREs can fuse a variety of liposomes without an apparent requirement for a specific lipid composition.
Fig. 2. SNARE-mediated membrane fusion does not depend on a specific lipid composition. SNARE-containing liposomes of the lipid compositions indicated were reconstituted as described previously (Weber et al., 1998), see also Materials and methods. (A) POPC:DOPS (85:15; donor and acceptor); (B) DOPE:DOPS (50:50; donor) and DOPE:DOPC (50:50; acceptor); (C) ‘Golgi’ lipids (donor) and POPC:DOPS (85:15; acceptor). Lipid recovery and efficiency of protein reconstitution were found to be similar under all conditions. v– and t–SNARE-containing liposomes were mixed on ice and incubated at 37°C for the times indicated. Where indicated, the cytosolic domain of VAMP was added at a final concentration of 0.4 mg/ml. Fluorometric recordings were obtained as described previously (Weber et al., 1998).
DOPE-containing liposomes are inherently metastable
To determine whether fusion of liposomes containing high amounts of phosphatidylethanolamine (PE) is uniquely mediated by NSF, we tested other components for their ability to fuse DOPE:DOPS (50:50) donor and DOPE: DOPC (50:50) acceptor liposomes. Although one of the proteins tested (bovine serum albumin; data not shown) did not cause fusion of liposomes, other proteins, such as α–SNAP, lactic dehydrogenase (Morero et al., 1985) and glyceraldehyde-3–phosphate dehydrogenase (Sirover, 1999) were capable of mediating fusion of these liposomes (Figure 3A). Moreover, as shown in Figure 3C, elevated levels of MgCl2 (3 mM and above) also caused liposome fusion. In all cases these fusogenic activities were not reduced by the presence of chelating agents (EGTA or EDTA), excluding the possibility that Ca2+ contaminations contributed to lipid mixing (data not shown). Neither of the above proteins nor MgCl2 significantly fused liposomes made of the rat liver Golgi lipid mixture or POPC:DOPS (85:15) (Figure 3B and D).
NSF-mediated fusion of DOPE-containing liposomes does not require ATP hydrolysis
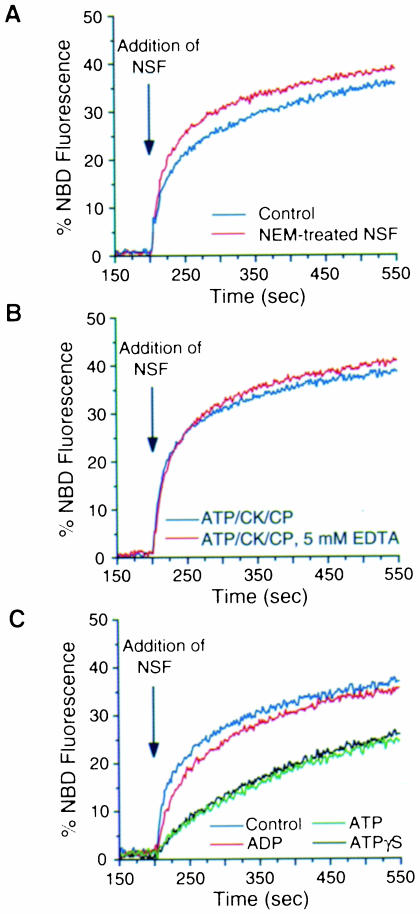
NSF is characterized by a high sensitivity to NEM, a feature that allowed its identification and isolation in an assay reconstituting vesicle-mediated intra-Golgi transport (Block et al., 1988). NSF-mediated hydrolysis of ATP is inhibited by NEM (Nagiec et al., 1995), depends on the presence of free Mg2+ ions (Whiteheart et al., 1994) and is required for intra-Golgi transport (Whiteheart et al., 1994) and for SNARE complex disassembly (Söllner et al., 1993b). We therefore tested whether ATP hydrolysis by NSF is also required for liposome fusion and whether the putative fusion activity is sensitive to NEM. To analyze the sensitivity of NSF-mediated fusion activity to NEM, we pre-incubated NSF for 30 min on ice in the presence of 2 mM NEM, followed by the addition of dithiothreitol (DTT) to a final concentration of 3 mM to quench free NEM. A control sample was treated with NEM that had been pre-incubated with DTT for 10 min. NEM-treated NSF or control NSF was then added to a premix of DOPE:DOPS (50:50) donor and DOPE:DOPC (50:50) acceptor liposomes. As illustrated in Figure 4A, NSF-mediated fusion is not inhibited by pretreatment with NEM. To ensure that normal NSF activity was inactivated by NEM under the conditions employed, aliquots of NEM-treated NSF were tested in the in vitro transport assay reconstituting intra-Golgi transport (Balch et al., 1984). In this assay NEM-treated NSF could not support transport with NEM-treated membranes, whereas the control sample was fully functional (data not shown).
Fig. 4. Fusion of liposomes is not dependent on the ATPase activity of NSF. (A) NSF-mediated fusion of liposomes is not inhibited by NEM. NSF was pre-incubated with 2 mM NEM for 30 min on ice followed by the addition of DTT (final concentration of 3 mM) to quench free NEM. A control sample was incubated for 30 min on ice with NEM that had been pre-incubated with DTT. NEM-treated NSF (red curve) or control NSF (blue curve) was added to a premix of DOPE:DOPS (50:50) donor and DOPE:DOPC (50:50) acceptor liposomes and lipid mixing was monitored. (B) NSF-mediated fusion of liposomes is not inhibited by EDTA. NSF was added to a premix of DOPE:DOPS (50:50) donor and DOPE:DOPC (50:50) acceptor liposomes in fusion buffer containing 1 mM MgCl2, 0.5 mM ATP, 10 IU/ml creatine kinase and 10 mM creatine phosphate as ATP-regenerating system, in the presence (red curve) or absence (blue curve) of 5 mM EDTA. (C) Nucleotide requirements for NSF-mediated fusion. NSF-bound nucleotide was exchanged against either ADP, ATP or ATPγS as described in Materials and methods. Exchange efficiency was monitored employing [α–33P]ATP and found to be >90% for each condition. A control sample of NSF was not subjected to nucleotide exchange. NSF in the presence of the various nucleotides was added to premixed liposomes and fusion monitored as described above.
In another set of experiments, we further investigated a potential requirement for ATP hydrolysis in NSF-mediated liposome fusion. First, we conducted fusion assays in the absence of free magnesium (i.e. in the presence of 5 mM EDTA, 0.5 mM ATP, 10 mM creatine phosphate and 10 U/ml creatine kinase), which is required for NSF-mediated ATP hydrolysis (Whiteheart et al., 1994). As shown in Figure 4B, NSF-mediated liposome fusion remained un– affected under this condition. Secondly, we performed nucleotide exchange experiments where NSF-bound nucleo– tide was exchanged for either ADP, ATP or ATPγS. NSF was labeled with [α–33P]ATP and nucleotide exchange efficiencies were monitored by analyzing displacement of [α–33P]ATP-derived radioactivity. In each case, nucleotide exchange was found to be >90% (for experimental details see Materials and methods). When NSF–ATP and NSF–ATPγS were compared in the liposome fusion assay, no significant difference in fusion activity could be observed (Figure 4C). However, both initial kinetics and overall fusion efficiency were reduced compared with NSF protein not subjected to the nucleotide exchange procedure described. In contrast, when ADP was bound, initial kinetics and overall efficiency were almost identical to untreated NSF protein, consistent with the observation that purified recombinant NSF is largely in its ADP form (Hanson et al., 1997). Analysis of aliquots of the various fusion reactions revealed the presence of comparable NSF quantities. These results unambiguously demonstrate that ATP hydrolysis is not required for NSF-mediated fusion of liposomes. Moreover, it appears that the ADP form of NSF is more potent with respect to fusogenic activity.
Discussion
We can confirm that NSF has fusogenic activity towards liposomes of the lipid composition specified by Otter-Nilsson et al. (1999). However, as opposed to their findings, this activity does not require α–SNAP as an auxiliary factor and is completely abolished by heat inactivation. Moreover, under the same conditions, α–SNAP alone, unrelated proteins (glyceraldehyde-3–phosphate dehydrogenase or lactic dehydrogenase) or even 3 mM magnesium ions can fuse these liposomes. This demonstrates that these liposomes are prone to fusion, as determined by lipid mixing. We cannot rigorously rule out the possibility that a contamination in the NSF preparation contributes to its fusogenic activity or that the observed fusion activity is restricted only to lipid mixing (i.e. does not involve content mixing). In any case, none of the tested proteins, or magnesium, had a significant fusogenic activity with liposomes composed of a rat liver Golgi lipid mixture. In contrast, fusion of liposomes bearing neuronal v– and t–SNAREs was tolerant towards a wide variation of lipid composition, occurring with liposomes composed of a rat liver Golgi lipid mixture, among other compositions. Importantly, in our study NSF fusion activity is not dependent on ATP hydrolysis and, strikingly, NEM treatment does not cause inactivation of NSF fusion activity. In fact, our data indicate that the ADP form of NSF is more fusogenic than the ATP-bound form.
Otter-Nilsson et al. (1999) also report that a complex of the NSF homolog p97 with its cofactor p47 (purified from rat liver cytosol) is fusogenic with the same unusual lipid composition as used for NSF assays. Using a sample kindly provided by this group, we can confirm this observation but as with NSF find that the p97–p47 fusion activity is NEM resistant and Mg2+ independent. In contrast, the fusion activity of p97 with biological membranes is both NEM sensitive and Mg dependent (Rabouille et al., 1995; Kondo et al., 1997), a finding that also holds true for its yeast homolog Cdc48p (Frohlich et al., 1995).
In a recent report by Müller et al. (1999) an ATP hydrolysis-independent but NEM-sensitive activity of NSF was required for post-mitotic Golgi re-assembly. We conclude that this activity (and NSF's previously understood activity in promoting fusion via SNARE activation) is unrelated to the activity of NSF in liposome fusion (Otter-Nilsson et al., 1999). While it cannot be excluded that rare lipid microdomains of a composition matching that chosen by Otter-Nilsson et al. (1999) for their studies form at sites of membrane fusion, our studies indicate that this would be a prerequisite for their observations to be physiologically relevant, and even then it would appear that other proteins or even simple salts could substitute. In this context, it is also of note that bivalent cations such as calcium can adhere and fuse lipid bilayers that contain substantial amounts of phosphatidylserine (PS) and PE (Kachar et al., 1986; Hui et al., 1988). Furthermore, PE is well known to be a non-bilayer-forming lipid, thereby promoting lipid bilayer merger (Cullis and de Kruijff, 1978; Vidal and Hoekstra, 1995). Indeed, the straightforward explanation is that the lipid composition optimal for such non-specific fusion simply forms liposomes that are physically unstable, so that a variety of minor perturbations can cause coalescence.
Furthermore, recent studies demonstrate that NSF can interact in a nucleotide-dependent manner with a variety of different substrates, including t–SNAREs (Hanson et al., 1995), cis–v–t–SNARE complexes (Ungermann et al., 1998), β–arrestin (McDonald et al., 1999) and certain subunits of AMPA-receptors (for reviews see Lin and Sheng, 1998; Osten and Ziff, 1999). Most of these NSF interactions and the associated reactions, which apparently cause conformational changes in the substrate proteins, are temporarily and spatially separated from actual membrane fusion events (Banerjee et al., 1996; Colombo et al., 1996; Mayer et al., 1996; Nichols et al., 1997; Ungermann et al., 1998; Xu et al., 1999). Interestingly, SNAREpins (trans–v/t–SNARE complexes that fuse membrane largely independent of their lipid composition) are not an effective substrate for disruption by NSF, nor is a single round of fusion stimulated by SNAP and NSF (T.Weber, F.Parlati, J.A.McNew, R.J.Johnston, B.Westermann, T.H.Söllner and J.E.Rothman, manuscript in preparation). These observations, together with the data in this paper make it extremely unlikely that NSF and SNAPs are directly involved in bilayer fusion in cells. However, it cannot be excluded that in addition to maintaining a supply of uncombined SNARE proteins, NSF plays a more subtle role in lipid bilayer fusion, e.g. by changing the state of a fusion pore containing SNAREs.
Materials and methods
Materials
All lipids were purchased from Avanti Polar Lipids as chloroform solutions. Glyceraldehyde-3–phosphate dehydrogenase (rabbit muscle), l–lactic dehydrogenase (rabbit muscle), N–ethylmaleimide and OptiPrep were obtained from Sigma. ATP, ATPγS and ADP were purchased from Roche Molecular Biochemicals. [α–33P]ATP (3000 Ci/mmol) was obtained from NEN Life Science Products.
Protein expression and purification
Expression and purification of His6-NSF and His6-α–SNAP were performed as described (Whiteheart et al., 1994). VAMP2-His6 was purified as described by Weber et al. (1998), thrombin-cleavable syntaxin1A–SNAP–25 complex was purified according to Parlati et al. (1999). Protein concentrations were estimated with the Bio-Rad Protein Assay using γ–globulin as a standard.
NEM treatment of NSF
A 50 mM NEM stock solution (dissolved in water) was freshly prepared. NEM was then added to NSF at a final concentration of 2 mM. After a 30 min incubation on ice, DTT was added at a final concentration of 3 mM to quench any remaining NEM.
Lipid stock solutions
Lipid stock solutions were prepared by diluting the corresponding lipids in chloroform. The following lipid stock solutions were used at a final concentration of 15 mM: DOPE:DOPS, molar ratio of 50:50; DOPE:DOPC, molar ratio of 50:50; and POPC:DOPS, molar ratio of 85:15. The Golgi-like lipid mixture consisted of 43 mol% phosphatidylcholine (PC; bovine liver), 19 mol% PE (bovine liver), 10 mol% phosphatidylinositol (PI; bovine liver), 5 mol% PS (bovine brain), 16 mol% cholesterol (wool grease) and 7 mol% sphingomyelin (SM; bovine brain) at a final concentration of 3 mM. Donor lipid mixes were prepared by including 0.8 mol% each of headgroup-labeled NBD-DPPE and rhodamine-DPPE to the DOPE:DOPS and ‘Golgi’ stock solutions, equally reducing the respective mole percentages of each lipid. POPC:DOPS donor lipid mixes were prepared by adding 0.8 mol% of each fluorescent lipid at the expense of POPC. Stock solutions were stored in teflon-sealed glass vials at –20°C.
Preparation of protein-free liposomes
Small unilamellar liposomes were prepared as described by Otter-Nilsson et al. (1999). Briefly, lipid solutions were transferred to 10 ml glass vials and dried under a gentle stream of nitrogen, followed by 3 h under vacuum. Lipid films were resuspended by vortexing in fusion buffer (10 mM HEPES–KOH pH 6.5, 140 mM NaCl, 10 mM KOAc, 1 mM MgCl2) to a final concentration of 15 mM. After 10 rapid freeze–thaw cycles (30 s in liquid nitrogen, 30 s in a 37°C waterbath and 5 s vortexing), liposomes of an average size of 50 nm were formed by extrusion with a handheld extrusion apparatus (Lipofast; Avestin) through 50 nm polycarbonte filters (41 passages). Freshly prepared liposomes were stored at 4°C and were used within a maximum of 24 h.
Reconstitution of proteoliposomes
Liposomes were formed in the presence of either the v–SNARE VAMP or a preformed t–SNARE complex consisting of thrombin-cleavable syntaxin1A and SNAP–25, respectively, as described (Parlati et al., 1999; J.A.McNew, T.Weber, F.Parlati, R.J.Johnston, T.H.Söllner and J.E.Rothman, manuscript in preparation), except that liposome flotation was performed in OptiPrep gradients. After overnight dialysis, liposomes were mixed 1:1 with a 60% OptiPrep solution and overlaid with 20 and 0% OptiPrep in reconstitution buffer [25 mM HEPES–KOH pH 7.4, 100 mM KCl, 10% (w/v) glycerol, 1 mM DTT], respectively. After centrifugation, proteoliposomes were collected at the 0–20% interface.
Liposome fusion assays
Fusion of protein-free liposomes was performed as described by Otter–Nilsson et al. (1999), in a total volume of 2 ml. NBD fluorescence was followed in a Luminescence Spectrometer (Perkin Elmer LS50B) at the following wavelengths: excitation, 465 nm (slit width 10 nm); emission, 530 nm (slit width 10 nm); and monitored in 1 s intervals. Cross-polarized filters and an excitation cut-off filter at 515 nm were used to eliminate potential light scattering. Donor liposomes were added to fusion buffer at 37°C in a thermostatted continuously stirred cuvette. A stirring bar device was used to ensure homogeneity of samples. After 100 s acceptor liposomes were added at a ratio of donor to acceptor of 1:5 (final lipid concentration of 500 μM). After an additional 100 s the protein or reagent to be tested was added. After 10 min, 50 μl of a 4% (w/v) Triton X–100 solution were added to terminate the reaction and allow normalization; NBD fluorescence of mixed liposomes was set to 0%, maximal NBD fluorescence after addition of detergent was set to 100%. Data were not corrected for detergent quenching of NBD fluorescence.
Fusion of SNARE-containing liposomes was performed in 96–well plates as described (Weber et al., 1998) under the conditions indicated in the figure legends. N–dodecyl-maltoside was added to a final concentration of 0.5% (w/v) to determine the 100% signal used to scale the fusion reaction.
ATP exchange assays
His6-tagged NSF (2.6 mg/ml) was diluted 10–fold in fusion buffer, reducing the nucleotide concentration to 50 μM. Loading of [α–33P]ATP onto NSF (final concentration 517 nM based on hexamer) was performed with 1 μCi [α–33P]ATP in the presence of 2 mM EDTA for 15 min at RT in a final volume of 0.1 ml. Exchange of nucleotides was performed by adding ATP, ATPγS or ADP to a final concentration of 2.5 mM. Following the addition of MgCl2 (final concentration 10 mM) the sample was further incubated for 15 min at RT. An aliquot of each sample was subjected to a filter assay as described (Matveeva et al., 1997). Radioactivity recovered in the absence of competing unlabeled nucleotide was set to 100%. Liposome fusion assays employing samples from the various nucleotide exchange reactions were performed by adding 40 μl of each sample to DOPE:DOPS (donor) and DOPE:DOPC (acceptor) liposomes in the presence of 0.5 mM nucleotide (either ATP, ATPγS or ADP).
Acknowledgments
Acknowledgements
We wish to thank Drs Hoekstra, Otter-Nilsson and Nilsson for communicating to us the precise conditions used in their published fusion assays of NSF, including lipid compositions, and for providing a sample of the same p47–p97 preparation from rat liver cytosol whose fusion activity was published in their paper. We would also like to thank Dr Latterich for providing samples and for helpful discussions. This work was supported by an NIH grant (to J.E.R.), postdoctoral fellowships of the European Molecular Biology Organization (to B.B. and T.W.), the Human Frontiers Science Program Organization (to W.N.), the Swiss National Science Foundation (to T.W.), the Medical Research Council of Canada (to F.P.) and the National Institutes of Health (to J.A.M.)
References
- Balch W.E., Dunphy, W.G., Braell, W.A. and Rothman, J.E. (1984) Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell, 39, 405–416. [DOI] [PubMed] [Google Scholar]
- Banerjee A., Barry,V.A., DasGupta,B.R. and Martin,T.F.J. (1996) N–ethylmaleimide-sensitive factor acts at a prefusion ATP-dependent step in Ca2+-activated exocytosis. J. Biol. Chem., 271, 20223–20226. [DOI] [PubMed] [Google Scholar]
- Beckers C.J., Block, M.R., Glick, B.S., Rothman, J.E. and Balch, W.E. (1989) Vesicular transport between the endoplasmic reticulum and the Golgi stack requires the NEM-sensitive fusion protein. Nature, 339, 397–398. [DOI] [PubMed] [Google Scholar]
- Block M.R., Glick, B.S., Wilcox, C.A., Wieland, F.T. and Rothman, J.E. (1988) Purification of an N–ethylmaleimide-sensitive protein catalyzing vesicular transport. Proc. Natl Acad. Sci. USA, 85, 7852–7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M.I., Taddese, M., Whiteheart, S.W. and Stahl, P.D. (1996) A possible predocking attachment site for N–ethylmaleimide-sensitive fusion protein. Insights from in vitro endosome fusion. J. Biol. Chem., 271, 18810–18816. [DOI] [PubMed] [Google Scholar]
- Cullis P.R. and de Kruijff, B. (1978) The polymorphic phase behaviour of phosphatidylethanolamines of natural and synthetic origin. A 31P NMR study. Biochim. Biophys. Acta, 513, 31–42. [DOI] [PubMed] [Google Scholar]
- Frohlich K.U., Fries, H.W., Peters, J.M. and Mecke, D. (1995) The ATPase activity of purified CDC48p from Saccharomyces cerevisiae shows complex dependence on ATP-, ADP- and NADH-concentrations and is completely inhibited by NEM. Biochim. Biophys. Acta, 1253, 25–32. [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Otto, H., Barton, N. and Jahn, R. (1995) The N–ethylmaleimide-sensitive fusion protein and α–SNAP induce a conformational change in syntaxin. J. Biol. Chem., 270, 16955–16961. [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Roth, R., Morisaki, H., Jahn, R. and Heuser, J.E. (1997) Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell, 90, 523–535. [DOI] [PubMed] [Google Scholar]
- Hay J.C., Chao, D.S., Kuo, C.S. and Scheller, R.H. (1997) Protein interactions regulating vesicle transport between the endoplasmic reticulum and Golgi apparatus in mammalian cells. Cell, 89, 149–158. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Yamasaki, S., Nauenburg, S., Binz, T. and Niemann, H. (1995) Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J., 14, 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui S.W., Nir, S., Stewart, T.P., Boni, L.T. and Huang, S.K. (1988) Kinetic measurements of fusion of phosphatidylserine-containing vesicles by electron microscopy and fluorometry. Biochim. Biophys. Acta, 941, 130–140. [DOI] [PubMed] [Google Scholar]
- Kachar B., Fuller, N. and Rand, R.P. (1986) Morphological responses to calcium-induced interaction of phosphatidylserine-containing vesicles. Biophys. J., 50, 779–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H., Rabouille, C., Newman, R., Levine, T.P., Pappin, D., Freemont, P. and Warren, G. (1997) p47 is a cofactor for p97-mediated membrane fusion. Nature, 388, 75–78. [DOI] [PubMed] [Google Scholar]
- Lin J.W. and Sheng, M. (1998) NSF and AMPA receptors get physical. Neuron, 21, 267–270. [DOI] [PubMed] [Google Scholar]
- Littleton J.T., Chapman, E.R., Kreber, R., Garment, M.B., Carlson, S.D. and Ganetzky, B. (1998) Temperature-sensitive paralytic mutations demonstrate that synaptic exocytosis requires SNARE complex assembly and disassembly. Neuron, 21, 401–413. [DOI] [PubMed] [Google Scholar]
- Matveeva E.A., He,P. and Whiteheart,S.W. (1997) N–ethylmaleimide-sensitive fusion protein contains high and low affinity ATP-binding sites that are functionally distinct. J. Biol. Chem., 272, 26413–26418. [DOI] [PubMed] [Google Scholar]
- Mayer A., Wickner, W. and Haas, A. (1996) Sec18p (NSF)-driven release of Sec17p (α–SNAP) can precede docking and fusion of yeast vacuoles. Cell, 85, 83–94. [DOI] [PubMed] [Google Scholar]
- McDonald P.H., Cote, N.L., Lin, F.T., Premont, R.T., Pitcher, J.A. and Lefkowitz, R.J. (1999) Identification of NSF as a β-arrestin1-binding protein. Implications for β2-adrenergic receptor regulation. J. Biol. Chem., 274, 10677–10680. [DOI] [PubMed] [Google Scholar]
- Morero R.D., Vinals, A.L., Bloj, B. and Farias, R.N. (1985) Fusion of phospholipid vesicles induced by muscle glyceraldehyde-3-phosphate dehydrogenase in the absence of calcium. Biochemistry, 24, 1904–1909. [DOI] [PubMed] [Google Scholar]
- Müller J.M.M., Rabouille, C., Newman, R., Shorter, J., Freemont, P., Schiavo, G., Warren, G. and Shima, D.T. (1999) An NSF function distinct from ATPase-dependent SNARE disassembly is essential for Golgi membrane fusion. Nature Cell Biol., 1, 335–340. [DOI] [PubMed] [Google Scholar]
- Nagiec E.E., Bernstein, A. and Whiteheart, S.W. (1995) Each domain of the N–ethylmaleimide-sensitive fusion protein contributes to its transport activity. J. Biol. Chem., 270, 29182–29188. [DOI] [PubMed] [Google Scholar]
- Nichols B.J., Ungermann, C., Pelham, H.R., Wickner, W.T. and Haas, A. (1997) Homotypic vacuolar fusion mediated by t- and v–SNAREs. Nature, 387, 199–202. [DOI] [PubMed] [Google Scholar]
- Osten P. and Ziff, E.B. (1999) AMPA receptor forms a biochemically functional complex with NSF and α- and β–SNAPs. Ann. NY Acad. Sci., 868, 558–560. [DOI] [PubMed] [Google Scholar]
- Otter-Nilsson M., Hendriks, R., Pecheur-Huet, E.I., Hoekstra, D. and Nilsson, T. (1999) Cytosolic ATPases, p97 and NSF, are sufficient to mediate rapid membrane fusion. EMBO J., 18, 2074–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto H., Hanson, P.I. and Jahn, R. (1997) Assembly and disassembly of a ternary complex of synaptobrevin, syntaxin and SNAP-25 in the membrane of synaptic vesicles. Proc. Natl Acad. Sci. USA, 94, 6197–6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlati F., Weber, T., McNew, J.A., Westermann, B., Söllner, T.H. and Rothman, J.E. (1999) Rapid and efficient fusion of phospholipid vesicles by the α-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc. Natl Acad. Sci. USA, 96, 12565–12570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabouille C., Levine, T.P., Peters, J.M. and Warren, G. (1995) An NSF-like ATPase, p97 and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell, 82, 905–914. [DOI] [PubMed] [Google Scholar]
- Rodriguez L., Stirling, C.J. and Woodman, P.G. (1994) Multiple N–ethylmaleimide-sensitive components are required for endosomal vesicle fusion. Mol. Biol. Cell, 5, 773–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirover M.A. (1999) New insights into an old protein: the functional diversity of mammalian glyceraldehyde-3-phosphate dehydrogenase. Biochim. Biophys. Acta, 1432, 159–184. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett, M.K., Whiteheart, S.W., Scheller, R.H. and Rothman, J.E. (1993a) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation and fusion. Cell, 75, 409–418. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart, S.W., Brunner, M., Erdjument, B.H., Geromanos, S., Tempst, P. and Rothman, J.E. (1993b) SNAP receptors implicated in vesicle targeting and fusion. Nature, 362, 318–324. [DOI] [PubMed] [Google Scholar]
- Struck D.K., Hoekstra, D. and Pagano, R.E. (1981) Use of resonance energy transfer to monitor membrane fusion. Biochemistry, 20, 4093–4099. [DOI] [PubMed] [Google Scholar]
- Sztul E., Colombo, M., Stahl, P. and Samanta, R. (1993) Control of protein traffic between distinct plasma membrane domains. Requirement for a novel 108,000 protein in the fusion of transcytotic vesicles with the apical plasma membrane. J. Biol. Chem., 268, 1876–1885. [PubMed] [Google Scholar]
- Ungermann C., Nichols, B.J., Pelham, H.R. and Wickner, W. (1998) A vacuolar v–t–SNARE complex, the predominant form in vivo and on isolated vacuoles, is disassembled and activated for docking and fusion. J. Cell Biol., 140, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer G. (1998) Lipids of the Golgi membrane. Trends Cell Biol., 8, 29–33. [DOI] [PubMed] [Google Scholar]
- Vidal M. and Hoekstra, D. (1995) In vitro fusion of reticulocyte endocytic vesicles with liposomes. J. Biol. Chem., 270, 17823–17829. [DOI] [PubMed] [Google Scholar]
- Weber T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T.H. and Rothman, J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Whiteheart S.W., Rossnagel,K., Buhrow,S.A., Brunner,M., Jaenicke,R. and Rothman,J.E. (1994) N–ethylmaleimide-sensitive fusion protein: a trimeric ATPase whose hydrolysis of ATP is required for membrane fusion. J. Cell Biol., 126, 945–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Ashery, U., Burgoyne, R.D. and Neher, E. (1999) Early requirement for α–SNAP and NSF in the secretory cascade in chromaffin cells. EMBO J., 18, 3293–3304. [DOI] [PMC free article] [PubMed] [Google Scholar]