Abstract
The highly conserved SNARE proteins, SNAP-25, syntaxin and synaptobrevin, form a tight ternary complex, which is essential for exocytosis. Crystallization of this complex revealed a four-helix bundle with an unusual hydrophilic layer (zero layer) in its center. In order to evaluate the role of this layer in different kinetic components of secretion, we used the Semliki Forest virus (SFV) system to infect adrenal chromaffin cells with SNAP-25 Q174L, a point mutant in the zero layer. Using combined flash photolysis of caged calcium and membrane capacitance measurements, we investigated its effect on the exocytotic burst and sustained phase of exocytosis with high time resolution. Cells expressing SNAP-25 Q174L displayed a selective reduction in the sustained phase, while the two components of the exocytotic burst remained unaffected. Furthermore, the exocytotic response to the second flash was significantly reduced, indicating a decrease in refilling kinetics. We therefore conclude that the zero layer is critical for the formation of SNARE complexes, but that it plays no role in the dynamic equilibrium between the two exocytosis-competent vesicle pools.
Keywords: capacitance measurements/exocytosis/secretion/SNAP-25/SNARE complex
Introduction
The process of exocytosis is mediated by the fusion of vesicle membrane and plasma membrane. A highly conserved set of proteins, the SNAREs [soluble NSF (N-ethylmaleimide-sensitive fusion protein) attachment protein receptors] were found to play a key role in inducing this fusion upon Ca2+ influx (Söllner et al., 1993; Südhof, 1995; Weber et al., 1998; Hilfiker et al., 1999). According to present theories, the assembly and disassembly of SNARE proteins is essential for SNARE function (Rothman, 1994; Nichols and Pelham,1998). Syntaxin and SNAP-25, two SNAREs located at the plasma membrane, and synaptobrevin, a SNARE located at the synaptic vesicle membrane, have been shown to form a highly stable, ternary complex (Fasshauer et al., 1997; Sutton et al., 1998; Weis and Scheller,1998). This complex can exist in two different conformations: a trans and a cis conformation (Hanson et al., 1997b; Lin and Scheller,1997). It is largely accepted that SNARE complexes in the trans configuration play an important role in membrane fusion (Hanson et al., 1997a; Sutton et al., 1998). The ternary complex is comprised of four α-helices, each helix containing a leucine zipper-like layer consisting of 15 hydrophobic residues. Embedded within this layer is an ionic layer formed by three glutamines and one arginine residue, the so-called zero layer (Fasshauer et al., 1998; Sutton et al., 1998). Amino acids 150–206 of SNAP-25 form one of the four helices in the SNARE complex structure (Weimbs et al., 1997; Fasshauer et al., 1998).
High time resolution measurements in adrenal chromaffin cells revealed the existence of different kinetic phases of secretion (for a recent review, see Neher, 1998). A rapid increase in intracellular Ca2+ leads to an exocytotic burst followed by a sustained phase of secretion. The burst can be further resolved into two kinetically distinct components, suggesting the presence of two separate exocytosis-competent pools of vesicles (Heinemann et al., 1994). These pools most likely correspond to trans-SNARE complexes existing in a dynamic equilibrium between a loose and a tight state (Voets et al., 1999). While both states can support Ca2+-triggered exocytosis, they can be distinguished through their sensitivity to either botulinum and tetanus toxins (Xu et al., 1998) or SNARE-specific antibodies (Xu et al., 1999). Moreover, recent experiments indicate that assembly of trans-SNARE complexes and membrane fusion are tightly linked (Chen et al., 1999).
In the current study, we investigated the effect of native and mutant SNAP-25 on secretion in adrenal chromaffin cells. Overexpression of wild-type SNAP-25 had no significant effect on secretion. Overexpression of SNAP-25 Δ9, in which the last nine C-terminal residues are missing, led to a selective reduction of the fast component of the exocytotic burst, in agreement with previous data on the cleavage of endogenous SNAP-25 by botulinum toxin A (BoNT/A) (Xu et al., 1998; Criado et al., 1999). Finally, overexpression of SNAP-25 Q174L, a point mutant in the zero layer of the SNARE complex, led to a reduction in the sustained phase, while the two components of the exocytotic burst remained unaffected. The results presented here imply that the replenishment rate of the exocytosis-competent vesicles in SNAP-25 Δ9- and SNAP-25 Q174L-overexpressing cells is reduced. Furthermore, our data indicate that the zero layer is not involved in the transition between the two states of SNARE complexes, but that it plays a critical role in the assembly of the SNARE complex.
Results
Overexpressed SNAP-25 mutants are able to assemble into SNARE complexes in vivo
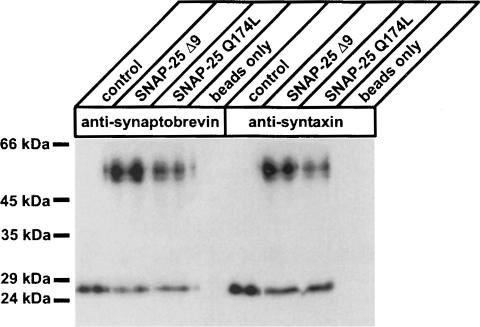
We overexpressed two Semliki Forest virus (SFV)-based mutants of SNAP-25 in bovine chromaffin cells. In SNAP–25 Δ9, the last nine C-terminal amino acids had been deleted (see below). SNAP-25 Q174L carried a mutation in the zero layer of the C-terminal α-helix of SNAP-25 (see below). To verify expression, green fluorescent protein (GFP) was attached to the N-terminus of both constructs. Quantitative Western blot analysis showed that the ratio of overexpressed SNAP-25 Δ9 or SNAP-25 Q174L to endogenous SNAP-25 was ∼25:1 (data not shown). In order to examine whether the overexpressed mutants were also able to form functional SNARE complexes in vivo, we performed immunoprecipitation experiments on infected chromaffin cells with synaptobrevin (VAMP)- and syntaxin 1-specific antibodies. Following SDS–PAGE and Western blotting, endogenous SNAP-25 and overexpressed SNAP-25 mutants were detected using a SNAP-25-specific antibody. As shown in Figure 1, the endogenous SNAP-25 in uninfected chromaffin cells yielded a single band at ∼25 kDa, representing SNAP–25 molecules that had been incorporated into SNARE complexes in these cells. In both SNAP-25 Δ9- and SNAP–25 Q174L-overexpressing cells, an additional band with an apparent mol. wt of ∼55 kDa could be detected. The detected band corresponds well with the predicted molecular weight of GFP–SNAP-25 Δ9 and GFP–SNAP–25 Q174L fusion proteins (52.4 kDa). Thus, our data demonstrate that these constructs could assemble with syntaxin and synaptobrevin (VAMP) into SNARE complexes in vivo.
Fig. 1. Overexpressed SNAP-25 mutants are able to assemble into SNARE complexes in vivo. Immunoblot analysis of co-immuno– precipitations of endogenous SNAP-25 and overexpressed SNAP-25 mutants from Triton X-100-solubilized chromaffin cells. Control represents uninfected chromaffin cells (lanes 1 and 5), while in lanes 4 and 8 only the antibody-containing beads were loaded to estimate the background signal of the heavy and light chain derived from the IgGs. The infection efficiency was 12% for SNAP-25 Δ9 (lanes 2 and 6) and 15% for SNAP-25 Q174L (lanes 3 and 7), respectively. Immuno-precipitations were performed using monoclonal antibodies against synaptobrevin and syntaxin, respectively, which were covalently coupled to protein A–Sepharose.
Kinetic study of calcium-triggered secretion in SNAP-25-infected cells
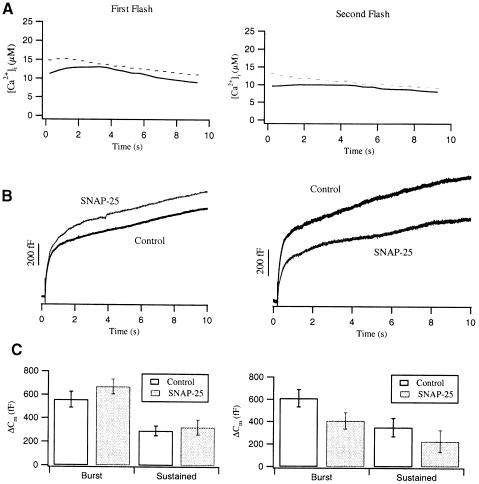
We were first interested whether overexpression of wild-type SNAP-25 had any effect on catecholamine secretion. Therefore, we infected bovine adrenal chromaffin cells with an SFV-based SNAP-25 construct (see Materials and methods). In order to verify expression and to avoid any perturbation of the SNAP-25 C-terminus, GFP was attached to the N-terminus of SNAP-25. Chromaffin cells were held in the whole-cell patch–clamp configuration in order to dialyze the caged calcium compound into the cytosol and to measure membrane capacitance (Neher and Marty, 1982; Henkel and Almers,1996). Secretion was elicited by flash photolysis of the caged calcium compound nitrophenyl-EGTA after ∼3 min of dialysis. A second flash was applied 2 min after the first one. During the first 10 s after the flashes, we employed high time resolution capacitance measurements to record the secretory response (Figure 2A). Following a flash, calcium was elevated to levels between 10 and 20 μM. This elicited in control cells a robust capacitance increase with two distinct phases (Figure 2B), which we call the exocytotic burst and the sustained phase (Neher and Zucker, 1993; Heinemann et al., 1994; Xu et al., 1998). The exocytotic burst represents the fusion of those vesicles, which are in a release-ready state and require only elevation of [Ca2+]i for exocytosis (Heinemann et al., 1993; Thomas et al., 1993; Gillis et al., 1996). It was postulated that the exocytotic burst is mediated by fusion-competent SNARE complexes (trans complexes), which may consist of either loosely or tightly assembled SNAREs. The sustained phase may represent the recruitment of additional vesicles that must undergo multiple steps of priming and maturation before they are ready for fusion (Heinemann et al., 1993; Thomas et al., 1993; Henkel and Almers, 1996; Xu et al., 1998). Therefore, in the sustained phase, the capacitance increases at a much slower rate with a time constant in the range of 5–30 s (Moser and Neher, 1997; Xu et al., 1998). In Figure 2B and C, the capacitance increase in response to the first flash was slightly larger in SNAP–25-overexpressing cells than in control cells. However, the response to a second flash given 2 min after the first one was ∼30% smaller in infected cells. Since we also observed a 30% reduction in the second flash response of cells overexpressing GFP alone (see Figure 8), this effect most likely results from the infection method and not from a specific effect of SNAP-25.
Fig. 2. Averaged, exocytotic responses to two consecutive flashes (first flash, left panel; second flash, right panel) in control and SNAP-25-overexpressing cells. (A) The averaged [Ca2+]i level resulting from flash photolysis of the caged calcium compound nitrophenyl-EGTA in control (solid line, n = 23) and SNAP-25-overexpressing cells (dashed line, n = 26). (B) Corresponding capacitance traces in response to the first and second flash from paired experiments. (C) Averaged amplitudes of the exocytotic burst and the sustained phase for control (open bar) and SNAP-25-overexpressing cells (shaded bar). The amplitudes of the exocytotic burst were measured as the Cm increase during the first 1.8 s after each flash, the sustained phase was measured as the Cm increase between 1.8 and 9.8 s after each flash. The kinetic phases in response to the first flash were not significantly different. Values are given as the mean ± SE.
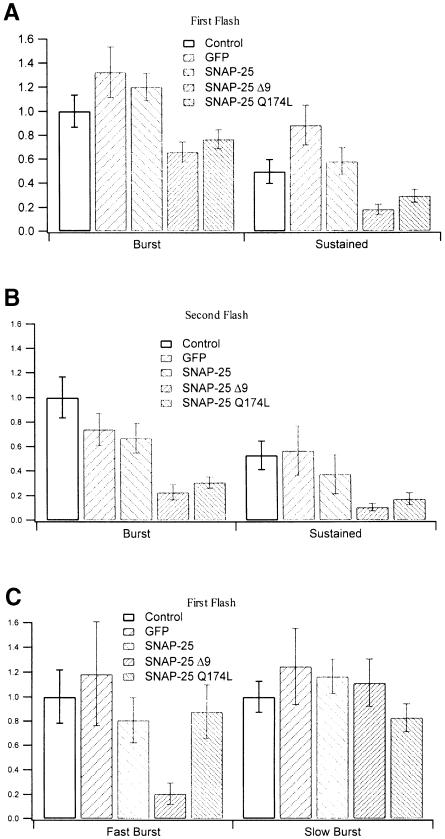
Fig. 8. Summary of the amplitudes of the different kinetic components of control and GFP-, SNAP-25-, SNAP-25 Δ9-, SNAP-25 Q174L-overexpressing cells (n = 83, 10, 26, 27 and 26, respectively). (A) The amplitudes of the exocytotic burst and the sustained phase in response to the first flash. GFP represents cells overexpressing GFP alone. Only in SNAP-25 Δ9- and SNAP-25 Q174L-overexpressing cells was the sustained phase significantly reduced (t-test, p <0.01 and p <0.05, respectively). (B) The amplitudes of the exocytotic burst and the sustained phase in response to the second flash. Although the amplitudes of the burst and sustained phase in infected cells were smaller than those of control cells, only in SNAP-25 Δ9- and SNAP–25 Q174L-overexpressing cells were both components significantly reduced (t-test, p <0.001). The data in (A) and (B) were normalized to the amplitude of the exocytotic burst in control cells. (C) Summary of the amplitudes of the fast and slow burst components in response to the first flash. Only in SNAP-25 Δ9-overexpressing cells was the fast burst reduced. The data were normalized to the amplitudes of the fast and slow burst components in control cells, respectively.
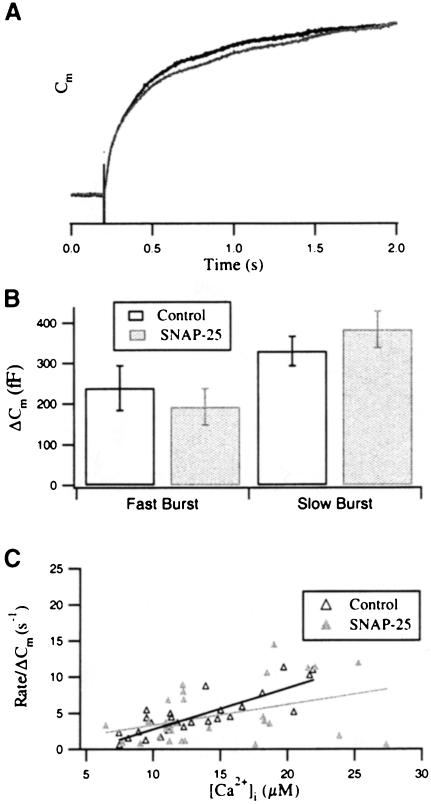
Previous work has shown that the exocytotic burst can be further resolved into two distinct components when analyzed at high time resolution (Thomas et al., 1993; Heinemann et al., 1994; Xu et al., 1998; Voets et al., 1999). We therefore performed a similar kinetic analysis in order to define which of the kinetically defined phases of the burst was affected by overexpression of SNAP–25. For this analysis, we fitted Cm traces from cells overexpressing SNAP-25 and from control cells with a triple exponential. The first exponential component represented the fast burst, the second component represented the slow burst (Figure 3). In response to the first flash, the amplitudes and time constants of the two components in control cells were similar to those of infected cells. The time constants of the fast and slow burst components were 40.5 ± 6.5 ms (n = 19) and 360.4 ± 52.5 ms (n = 23) for control cells, and 40.0 ± 5.3 ms (n = 19) and 425.4 ± 68.2 ms (n = 23) for SNAP-25-overexpressing cells, respectively. Detailed comparison of the normalized Cm responses (Figure 3A), the two components of the burst (Figure 3B) and the initial rate of Cm increase versus [Ca2+]i in the burst (Figure 3C) revealed no significant difference between SNAP-25-overexpressing cells and control cells. It demonstrated that overexpression of SNAP-25 did not change the kinetic properties of the exocytotic burst in response to the first flash. We therefore conclude that overexpression of SNAP-25 does not significantly modify the secretion behavior in adrenal chromaffin cells, in agreement with a recent report by Criado et al. (1999). It should be noted, though, that a reduction of the autaptic response occurred in cultured rat hippocampal neurons infected with SNAP–25 (Owe-Larsson et al., 1999).
Fig. 3. The exocytotic burst in response to the first flash is not changed in SNAP-25-overexpressing cells. (A) Normalized Cm traces of control (dark line) and SNAP-25-overexpressing cells (light line) are very similar during the time course of the exocytotic burst (the first 2 s after the flash). (B) Comparison of the amplitudes of the fast and the slow burst components for the first flash. The values for control (open bar) and SNAP-25-overexpressing cells (shaded bar) are not significantly different. (C) The initial rate of Cm increase is plotted against [Ca2+]i for control (▵) and SNAP-25-overexpressing cells (▴). Data were normalized with respect to the Cm increase (ΔCm) during the first 2 s after the flash. The solid lines represent the computerized fit for each data set. No significant change was observed in the [Ca2+] dependence of the initial rate constants.
Overexpression of SNAP-25 Δ9 leads to a selective reduction of the fast burst component
Based on the structure of the SNARE complex, amino acids 150–206 of the C-terminus of SNAP-25 form one of the four helices in the SNARE complex structure. In previous studies, cleavage of the last nine amino acids from the C-terminus of SNAP-25 by BoNT/A reduced both the fast component of the exocytotic burst and the sustained phase (Xu et al., 1998). Also, BoNT/A-cleaved SNAP-25 is unable to form an SDS-resistant SNARE complex (Hayashi et al., 1995; Chen et al., 1999), demonstrating that the C-terminus of SNAP-25 functions to stabilize the SNARE complex.
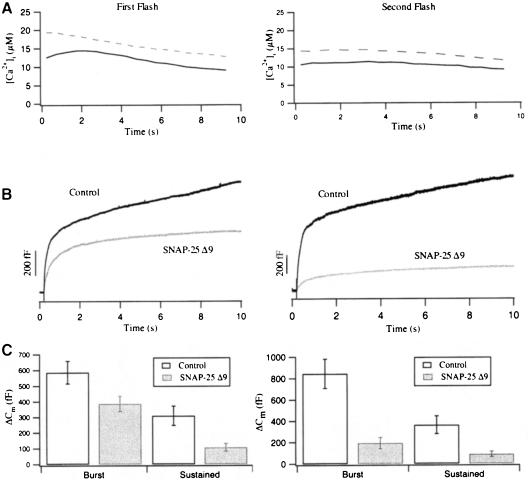
We investigated the effect of the SNAP-25 C-terminus on exocytosis by overexpressing SNAP-25 Δ9 in chromaffin cells. As for wild-type SNAP-25, GFP was attached as a fluorescent marker to the N-terminus of SNAP-25 Δ9. A similar truncation construct was used by Criado et al. (1999) and was shown to reduce the rate of secretion, as measured by amperometry. As shown in Figure 4, the amplitudes of the exocytotic burst and the sustained phase in response to the first flash were 586.5 ± 71 and 309.9 ± 61.5 fF for control cells (n = 28), and 397.1 ± 50 and 109 ± 25.9 fF for SNAP–25 Δ9-overexpressing cells (n = 27). The reductions of the sustained phase in the first flash and of both phases in the second flash were statistically significant. (t-test, p <0.01).
Fig. 4. SNAP-25 Δ9-overexpressing cells display a reduction in the exocytotic burst and the sustained phase. (A) The averaged [Ca2+]i level in control (solid line, n = 28) and SNAP-25 Δ9-overexpressing cells (dashed line, n = 27) in response to the first (left panel) and second flash (right panel). (B) Corresponding capacitance traces in response to the first and second flash from paired experiments. (C) Averaged amplitudes of the exocytotic burst and the sustained phase for control (open bar) and SNAP-25 Δ9-overexpressing cells (shaded bar). The reductions of the sustained phase in the first flash and of both phases in the second flash were statistically significant (t-test, p <0.01).
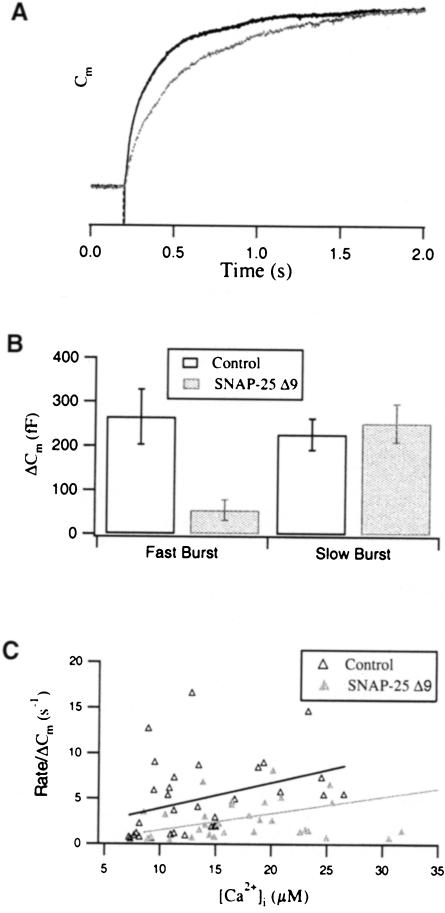
Kinetic analysis of the exocytotic burst in response to the first flash revealed a prominent slow-down of secretion in SNAP-25 Δ9-overexpressing cells (Figure 5A). When we fitted the Cm traces of control cells with a triple exponential, 20 out of 28 flash responses could be adequately fitted. In contrast, 18 out of 27 SNAP-25 Δ9–overexpressing cells could be fitted by a double exponential, indicating a selective loss of one exocytotic component. The amplitude of the fast and slow burst components was 265.4 ± 62.3 and 227.1 ± 35.3 fF in control cells, and 54.6 ± 23 and 252.8 ± 43 fF in SNAP–25 Δ9-overexpressing cells, respectively (Figure 5, see also Table I). From these data, it was evident that the fast burst component was reduced by 80% in SNAP-25 Δ9–overexpressing cells, whereas the slow burst component remained unaffected (Figure 5B). As a consequence, the initial rate of capacitance increase was slower in SNAP–25 Δ9-overexpressing cells compared with control cells (Figure 5C). Our results are consistent with the effect reported for BoNT/A, which impairs exocytosis without affecting assembly of SNARE complexes (Hayashi et al., 1995; Otto et al., 1995; Bruns et al., 1997). Since this retardation was not connected to a reduction of the intracellular Ca2+ concentration (Figure 4A), we suggest that the truncated form of SNAP–25 is compromised in mediating a particularly rapid interaction between calcium sensor and release machinery for catecholamine release. We speculate that the formation of tight trans complexes is inhibited in SNAP-25 Δ9–overexpressing cells (see Discussion). The reduction of the sustained phase, which represents the refilling and subsequent fusion of vesicles of the release-ready pool during the time of high intracellular calcium (10 s), implies that the rate of this refilling process is decreased too. This assumption is supported by the largely reduced amplitudes of both exocytotic burst and sustained phase in the second flash response of SNAP-25 Δ9-overexpressing cells (Figure 4C). Finally, the similarity to results reported on BoNT/A-treated chromaffin cells in which the endogenous SNAP-25 is cleaved (Xu et al., 1998) provides evidence that the virus-based overexpression strategy yields physiologically functional SNAP-25 Δ9 molecules.
Fig. 5. The fast burst component is selectively reduced in SNAP-25 Δ9-overexpressing cells. (A) Normalized Cm traces of control (dark line) and SNAP-25 Δ9-overexpressing cells (light line) display a different time course within the first 2 s after the flash. (B) Comparison of the amplitudes of the fast and the slow burst components for the first flash. The fast burst component was reduced by ∼80% in SNAP-25 Δ9-overexpressing cells (t-test; p <0.01), while the slow burst component was unaffected. (C) The initial rate of Cm increase is plotted against [Ca2+]i for control (▵) and SNAP-25 Δ9-overexpressing cells (▴). Data were normalized with respect to the Cm increase (ΔCm) during the first 2 s after the flash. The lines represent the computerized fit for each data set. The initial rate in SNAP-25 Δ9-overexpressing cells was slightly lower than in control cells.
Table I. Amplitudes of fast and slow burst in paired experiments.
| Fast burst (fF) |
Slow burst (fF) |
|||
|---|---|---|---|---|
| Control | Infected | Control | Infected | |
| SNAP-25 wt | 238.7 ± 54.7 (19) | 192.8 ± 44.5 (26) | 329.2 ± 36.2 (23) | 383.7 ± 45.0 (26) |
| SNAP-25 Δ9 | 265.4 ± 62.3 (28) | 54.6 ± 23.0 (27) | 227.1 ± 35.3 (28) | 252.8 ± 43.0 (27) |
| SNAP-25 Q174L | 156.8 ± 29.4 (23) | 137.6 ± 34.6 (26) | 432.2 ± 49.3 (23) | 357.3 ± 49.9 (26) |
Values given are the mean ± SE. The values in parentheses represent the number of cells.
The zero layer of the SNARE complex is critical for complex formation, but not for the last step of membrane fusion
Crystallization of the SNARE complex revealed a hydrophilic layer (‘zero layer’) embedded in the center of a four-helix bundle (Sutton et al., 1998). It was shown recently that a point mutation of one of the hydrophilic residues, SNAP-25 Q174A, decreased the thermal stability of the SNARE complexes (Chen et al., 1999).
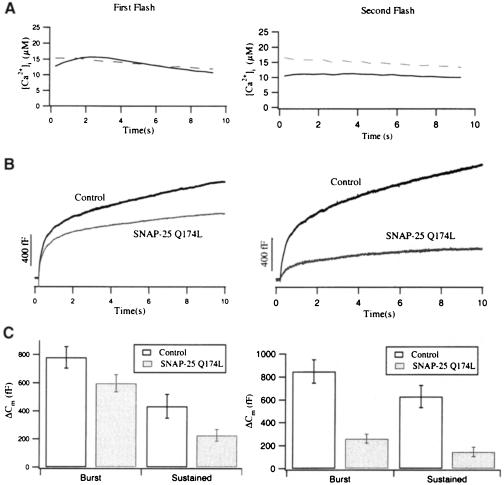
In order to understand the role of the zero layer in exocytosis, we overexpressed SNAP-25 Q174L, a zero layer mutant in the C-terminal helix of SNAP-25, in chromaffin cells. In response to the first flash, only a small reduction in the exocytotic burst was detected in these cells (779 ± 75 fF for control; 597 ± 61 fF for SNAP-25 Q174L), while the sustained phase was significantly reduced by 48% (434 ± 85 fF for control; 226 ± 40 fF for SNAP-25 Q174L) (Figure 6B, left panel). The reduction in the sustained phase already indicated a decrease in the refilling kinetics. This potential decrease became more evident in the Cm response to a second flash given 2 min after the first one (Figure 6B and C, right panel). While in control cells the amplitudes of the exocytotic burst and the sustained phase were comparable between the two flashes (848 ± 101 and 629 ± 97 fF for the second flash), SNAP-25 Q174L-overexpressing cells displayed a significant reduction by 56% for the exocytotic burst and by 35% for the sustained phase (260 ± 39 and 147 ± 41 fF for the second flash). If one assumes that physiological refilling of release-competent pools requires the formation of new SNARE complexes, these data imply that the zero layer plays an important role in the formation of these complexes.
Fig. 6. SNAP-25 Q174L-overexpressing cells display a reduction of the sustained phase in response to the first flash (left panel) and a reduction of both burst and sustained phase in response to the second flash (right panel). (A) Average [Ca2+]i level in control (solid line, n = 23) and SNAP-25 Q174L-overexpressing cells (dashed line, n = 26). (B) Corresponding capacitance traces in response to the first and second flash from paired experiments. (C) Averaged amplitudes of the exocytotic burst and the sustained phase for control (open bar) and SNAP-25 Q174L-overexpressing cells (shaded bar). In the first flash response (left panel), only the sustained phase was significantly reduced (t-test, p <0.05), while in the second flash response (right panel) both exocytotic burst and sustained phase were significantly reduced (t-test, p <0.01).
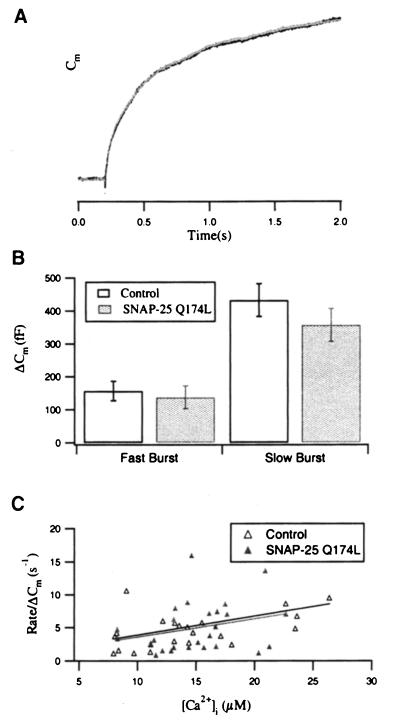
The kinetic analysis of the exocytotic burst in response to the first flash confirmed that the normalized Cm responses of control and SNAP-25 Q174L-overexpressing cells are virtually indistinguishable (Figure 7A). Furthermore, the amplitudes of both exocytotic burst components and the initial rate constant of the exocytotic burst on [Ca2+]i were not altered (Figure 7B and C). We can therefore conclude that the zero layer, in contrast to the last nine amino acids at the C-terminus of SNAP-25, plays no role in the dynamic equilibrium between the two exocytotic burst components.
Fig. 7. The equilibrium between fast and slow burst component is unaffected in SNAP-25 Q174L-overexpressing cells. (A) Normalized Cm traces of control (dark line) and SNAP-25 Q174L-overexpressing cells (light line) are indistinguishable within the time course of the exocytotic burst (the first 2 s after the flash). (B) Comparison of the amplitudes of the fast and the slow burst components for the first flash. The values for control (open bar) and SNAP-25 Q174L-overexpressing cells (shaded bar) are not significantly different. (C) The initial rate of the Cm increase is plotted against [Ca2+]i for control (▵) and SNAP-25 Q174L-overexpressing cells (▴). Data were normalized with respect to the Cm increase (ΔCm) during the first 2 s after the flash. The solid lines represent the computerized fit for each data set. The [Ca2+] dependence of the initial rate constant in SNAP–25 Q174L-overexpressing cells was almost identical to that in control cells.
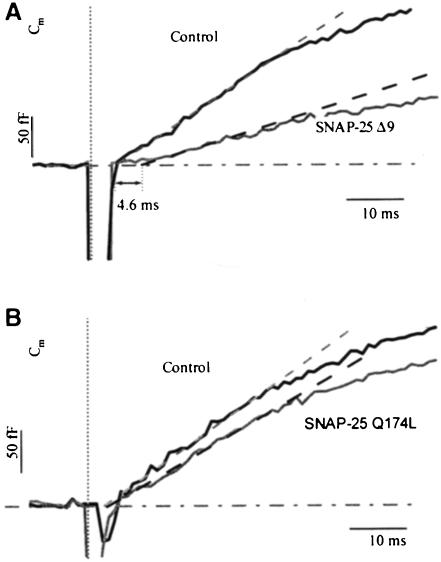
Figure 8 summarizes the amplitudes of the kinetic components for all proteins used in this study. The overexpression data of GFP alone were added to demonstrate that it did not cause a significant alteration in any of the kinetic components. This finding is in agreement with recent reports, which demonstrated that the morphology and the basic physiological features of chromaffin cells were unaffected by the infection with SFV-based constructs (Ashery et al., 1999; Duncan et al., 1999). Also, Figure 8 shows that although the sustained phases in all types of infected cells were smaller than their corresponding exocytotic burst, only in SNAP-25 Δ9- and SNAP-25 Q174L-overexpressing cells were the sustained phases significantly reduced when compared with those of control cells (t-test, p <0.01 and p <0.05, respectively) (Figure 8A). In the second flash, both phases were reduced significantly in SNAP-25 Δ9- and SNAP-25 Q174L-overexpressing cells (t-test, p <0.001), but not in GFP- and wild-type SNAP-25-overexpressing cells (Figure 8B). If we focus on the two exocytotic burst components and normalize the data with their own control values of fast and slow burst, respectively, we find that only in SNAP-25 Δ9-overexpressing cells was the fast burst component significantly reduced, whereas the slow burst component was unaffected for all types of infected cells (Figure 8C). Even higher time resolution of the Cm responses of the first flash showed that in SNAP-25 Δ9-overexpressing cells there was a 4.6 ms delay of the exocytotic burst (Figure 9A), while there was no delay in SNAP-25 Q174L-overexpressing cells (Figure 9B). This further demonstrates that the mutant of the zero layer did not affect the ability of SNAREs to form tight trans complexes.
Fig. 9. The Cm responses in SNAP-25 Δ9-overexpressing cells display a delayed onset of the exocytotic burst in response to the first flash. (A) Cm responses of control (solid line, n = 28) and SNAP-25 Δ9-overexpressing cells (gray line, n = 29). The initial slopes are displayed in dashed lines, a 4.6 ms delay of the exocytotic burst in the SNAP-25 Δ9-infected condition was observed. (B) Cm responses of control (solid line, n = 23) and SNAP-25 Q174L-overexpressing cells (gray line, n = 26). No delay was found, which indicates that the mutant of the zero layer does not affect the formation of tight trans complexes.
Discussion
The formation and stability of the ternary SNARE complex consisting of synaptobrevin, syntaxin and SNAP-25 are believed to play a central role in the molecular mechanism underlying exocytosis. The C-terminus of SNAP-25, which contributes one helix to the four-helix bundle, has been implicated in the final step of calcium-triggered exocytosis (Chen et al., 1999). However, the biochemical assay that was used in this study could not resolve different kinetic components of exocytosis. For this purpose, we used high time resolution capacitance measurements and molecular biological methods to study the functional effect of SNAP-25 C-terminal mutants on the very final membrane fusion stages of exocytosis and to establish links between the distinct kinetic components and molecular processes.
Both loosely and tightly assembled SNARE complexes are fusion competent
Before a vesicle can fuse with the plasma membrane, it has to translocate to the plasma membrane (‘docking’) and then undergo one or more maturation steps (‘priming’). Although the molecular definition of docking remains unclear, it has been shown that when neurotoxins were injected into squid nerve terminals, vesicles remained tethered or even accumulated further at the active zone (Hunt et al., 1994). Thus, SNARE complex formation does not seem to be required for morphological docking. However, since neurotoxin treatment abolishes neurotransmitter release, the SNARE complex is required at a later, post-docking step in neuronal and neuroendocrine systems.
Previous work suggested that only SNARE complexes in the trans conformation, but not in the cis conformation, are fusion competent (Ryan, 1998; Ungermann et al., 1998). Since the formation of a SNAP-25–syntaxin complex greatly increases their individual affinity for synaptobrevin (Fasshauer et al., 1997; Sutton et al., 1998; Fiebig et al., 1999), it was suggested that trans complex formation is preceded by the formation of a syntaxin–SNAP-25 dimer. While uncomplexed or loosely assembled SNARE proteins are sensitive to proteolysis by neurotoxins, the fully assembled SNARE complex is resistant to proteolysis. Therefore, it has been postulated that the trans complex oscillates between two states: a loose, toxin-sensitive state and a tight, SDS-resistant state (Rizo and Südhof, 1998; Sutton et al., 1998; Fiebig et al., 1999). Furthermore, these states can be distinguished by biophysical methods (Xu et al., 1998, 1999).
Model of secretion in neuroendocrine cells and its corresponding kinetics
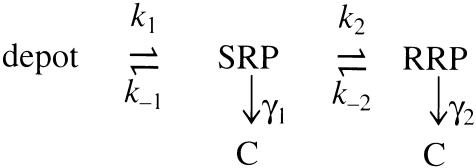
The results obtained in this study can be explained by hypothesizing an equilibrium between the releasable vesicle pools and a depot vesicle pool. They are highly compatible with a refined model (Voets et al., 1999) based on the original two-step model suggested by Heinemann et al. (1993).
Scheme 1
In this scheme, the depot pool is considered to be a large reserve pool of vesicles that can prime into the slowly releasable pool (SRP), which represents SNARE proteins that have formed loose trans complexes. Vesicles in the SRP either return to the depot pool or go to the readily releasable pool (RRP), which represents SNARE proteins that have formed tight trans complexes. Finally, vesicle fusion, which is represented by a transition to pool C, contributes to the increase of membrane capacitance. Fusion-competent SNARE complexes appear to be in a dynamic equilibrium between the two distinct states, which correspond in physiological terms to the two kinetic components of the exocytotic burst upon elevation of [Ca2+]. Vesicles in the SRP fuse with a typical rate constant of ∼3 s–1 (γ1 in the model) while vesicles in the RRP fuse with a typical rate constant of ∼30 s–1 (γ2 in the model). During Ca2+-evoked secretion, the rate of replenishment of vesicles from the depot pool to the SRP will be increased (Heinemann et al., 1993).
Our experiments revealed that the fast burst component was selectively reduced in the SNAP-25 Δ9-infected cells while the size and the rate constant of the SRP remained unaffected (see Figure 5). According to our model, such a change can be explained by a destabilization and resulting reduction of the RRP. It is important to note that our data obtained with the SFV-based overexpression of exogenous SNAP-25 Δ9 are in excellent agreement with previous studies using BoNT/A to cleave endogenous SNAP-25 (Xu et al., 1998). Since only 4% of the SNARE complexes in infected cells might contain native SNAP-25 (see Results), a contamination of our kinetic analysis by such a small fraction of native SNARE complexes would be hidden in the scatter between different cells. Thus, we conclude that the presence of endogenous SNAP-25 most likely did not influence our experiments and conclusions. Our data on the overexpression of SNAP-25 Δ9 are also in agreement with a recent report by Criado et al. (1999) using C-terminal deletion mutants of SNAP-25 and studying secretion by amperometry. Furthermore, recent work using a SNAP-25-specific antibody also showed a block of the fast exocytotic burst component, providing additional evidence for the existence of loose and tight SNARE complexes (Xu et al., 1999).
A potential role for the zero layer in Ca2+-dependent exocytosis
Since crystallization of the SNARE complex revealed the existence of a hydrophilic layer in the center of the four-helix bundle, there have been several hypotheses about the physiological function of this zero layer. One current hypothesis emerged from the observation that the leucine zipper layers act as a water-tight seal to shield the ionic interactions from the surrounding solvent. This seal might then further stabilize the four-helix oligomeric state and register of the complex by decreasing the local dielectric, thus enhancing electrostatic interactions within the ionic layer. Alternatively, when another protein such as α-SNAP or NSF might be able to puncture this seal, the ionic layer would be exposed to solvent and thereby facilitate disassembly of the complex (Sutton et al., 1998). However, biochemical data using SNARE complexes in which all four residues of the hydrophilic zero layer have been mutated to leucines showed that the sensitivity to NSF is increased (U.Matti and T.Binz, unpublished experiments), arguing against this hypothesis. On the other hand, it has been shown that mutations at the zero layer position of the C-terminal end of SNAP-25 decrease the melting point of the ternary complex (Chen et al., 1999; U.Matti and T.Binz, unpublished experiments), which is indicative of a decreased stability. In support of this hypothesis, a SNAP-25 Q53,174L double mutant displayed an even lower melting point and resulted in no detectable physiological effect (S.Wei, U.Matti and E.Neher, unpublished experiments). Our physiological measurements now show that the zero layer mutant in the C-terminus of SNAP–25, SNAP-25 Q174L, does not influence the dynamic equilibrium between loose and tight states (see Figure 7). However, it does decrease k1, the replenishment rate of the release-ready vesicle pools (see Figures 6 and 8). We therefore conclude that the zero layer plays an important role in making vesicles available for fusion, probably by keeping the leucine zipper in register during the formation of trans-SNARE complexes. In addition, our data show that this decrease in stability has no physiological significance for the transition between loose and tight trans-SNARE complexes.
Materials and methods
Generation of SNAP-25 constructs
The viral vector pSCA1 (DiCiommo and Bremner,1998) was modified by the introduction of an oligonucleotide cassette into its XmaI site to generate singular ClaI and BssHII restriction sites. The gene coding for GFP-5 (cDNA kindly provided by G.Hobom, Giessen) was amplified by PCR to generate a 5′ BglI and a 3′ BamHI site, and inserted into the BamHI site of the modified pSCA1, yielding pSCA1-GFP. The coding sequence for murine SNAP-25A was cloned into the BamHI–ClaI-cleaved pSCA1-GFP using PCR. The C-terminal truncated mutant SNAP-25 Δ9 was made in the same manner, introducing a stop codon at amino acid position 198 of SNAP-25. The zero layer mutant SNAP–25 Q174L was generated by site-directed mutagenesis. The sequence of all constructs was verified by DNA sequencing.
Cell preparation and solutions
Chromaffin cells from bovine adrenal glands were prepared and cultured as described (Ashery et al., 1999). Cells were used 1–2 days after preparation. The external bathing solution for experiments contained (in mM): 150 NaCl, 2.8 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES and 2 mg/ml glucose pH 7.2 (320 mOsm). Pipet solution was prepared as described by Xu et al. (1997) and contained (in mM): 110 Cs-glutamate, 5 nitrophenyl-EGTA, 4 CaCl2, 2 MgATP, 0.3 GTP, 0.5 furaptra, 35 HEPES; osmolarity was adjusted to 310 mOsm. Chemicals used were: nitrophenyl-EGTA (Molecular Probes, Eugene, OR), fura-2 (Texas Fluorescence Labs, Austin, TX), furaptra (Molecular Probes, Eugene, OR), CaCl2 (Sigma, St Louis, MO) and ATP (Boehringer Mannheim, Germany). The basal [Ca2+]i was measured as 100–300 nM in separate experiments in which furaptra was replaced by fura-2. All experiments were performed at 32°C.
Infection and GFP detection
Virus production was performed as described (Ashery et al., 1999). An aliquot of frozen virus (450 μl) was thawed and 450 μl of Dulbecco's modified Eagle's medium without ITS-X were added. To activate the virus, 100 μl of chymotrypsin (2 mg/ml; Boehringer Mannheim, Germany) were added and incubated for 30–50 min at room temperature. Then, 110 μl of aprotinin (6 mg/ml; Boehringer Mannheim, Germany) were added to inactivate chymotrypsin and incubated for 3–5 min at room temperature. Infection was performed on cultured cells 3–24 h after preparation. Medium volume was reduced to 0.25–0.5 ml per 35 mm plate and 0.5–1 ml of the activated virus was added. After incubation for 1–2 h at 37°C, the virus-containing medium was replaced with 2 ml of conditioned medium. Initial GFP detection was performed with an IX70 inverted microscope (Olympus Optical Co., Japan) using a GFP filter set (AHF Analysentechnik, Tübingen, Germany).
Immunoprecipitation experiments
Monoclonal antibodies against synaptobrevin (Cl 69.1; Edelmann et al., 1995) or syntaxin (HPC-1; kindly provided by Dr C.Barnstable, NH) were covalently coupled to protein A–Sepharose (Pharmacia, Sweden) using the cross-linker dimethylpimelimidate (Pierce). Unbound antibodies were removed by alternating washes with 100 mM NaHCO3, 500 mM NaCl pH 8.3 and 100 mM glycine, 500 mM NaCl pH 2.5. Uninfected and infected chromaffin cells were harvested and solubilized in 400 μl of extraction buffer [50 mM Tris–HCl pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100 and protease inhibitors (10 μg/ml soybean trypsin inhibitor, 1 μg/ml pepstatin, 11 μg/ml benzamidine, 1 μg/ml antipain, 1 μg/ml leupeptin and 0.1 mM phenylmethylsulfonyl fluoride)] for 30 min at 4°C. Lysates were clarified by centrifugation at 200 000 g for 10 min. Supernatants were split into two aliquots and incubated for 2 h with the protein A–Sepharose containing the covalently cross-linked antibodies against synaptobrevin and syntaxin, respectively. After incubation, the Sepharose was washed eight times with extraction buffer. The immunoprecipitates were analyzed by 12% SDS–PAGE and immunoblotting using a polyclonal antibody against SNAP-25 (Blasi et al., 1993).
Photolysis of caged Ca2+ and [Ca2+]i measurements
Flashes of UV light were generated as described by Heinemann et al. (1994) and coupled to the epifluorescence port of an inverted Zeiss IM35 microscope with a 100× oil immersion objective (NA 1.3) (Zeiss, Oberkochem, Germany). The cell under study was located in the center of the illuminated area. The fluorescence detection area was adjusted to cover only the diameter of the cell, and the fluorescence was measured with a photomultiplier tube. [Ca2+]i was calculated from the fluorescence ratio R according to the method of Grynkiewicz et al. (1985).
Whole-cell patch–clamp and capacitance measurements
Conventional whole-cell recordings were performed with sylgard-coated 2–3 MΩ pipets. Series resistance ranged from 4 to 12 MΩ. An EPC-9 patch–clamp amplifier was used together with the Pulse software package (HEKA Electronics, Lambrecht, Germany). Capacitance (Cm) measurements were performed using the Lindau–Neher technique implemented as the ‘sine+dc’ mode (Gillis,1995) of the software lock-in extension of Pulse, which allowed long duration Cm measurements in a single sweep. A 1000 Hz, 50 mV peak-to-peak sinusoid voltage stimulus was superimposed onto a DC holding potential of –70 mV. The capacitance traces were imported to IGOR Pro (WaveMetrics, Inc., Lake Oswego, OR). The analyses were conducted on a PC using IGOR Pro. Unless stated otherwise, the data are given as the mean ± SE.
Acknowledgments
Acknowledgements
This paper is dedicated to the memory of Professor Heiner Niemann who died on 5 September 1999. We thank Anke Bührmann and Frauke Friedlein for the preparation of chromaffin cells and virus production. This work was supported by grants from the Deutsche Forschungsgemeinschaft (SFB 523 to E.N. and Re1092/3-2 to J.R.) and a Feodor Lynen-Minerva fellowship to U.A.
References
- Ashery U., Betz, A., Xu, T., Brose, N. and Rettig, J. (1999) An efficient method for infection of adrenal chromaffin cells using the Semliki Forest virus gene expression system. Eur. J. Cell Biol., 78, 525–532. [DOI] [PubMed] [Google Scholar]
- Blasi J., Chapman, E.R., Link, E., Binz, T., Yamasaki, S., DeCamilli, P., Südhof, T.C., Niemann, H. and Jahn, R. (1993) Botulinum neurotoxin A selectively cleaves the synaptic protein SNAP-25. Nature, 365, 160–163. [DOI] [PubMed] [Google Scholar]
- Bruns D., Engers, S., Yang, C., Ossig, R., Jeromin, A. and Jahn, R. (1997) Inhibition of transmitter release correlates with the proteolytic activity of tetanus toxin and botulinus toxin A in individual cultured synapses of Hirudo medicinalis. J. Neurosci., 17, 1898–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.A., Scales, S.J., Patel, S.M., Doung, Y.-C. and Scheller, R.H. (1999) SNARE complex formation is triggered by Ca2+ and drives membrane fusion. Cell, 97, 165–174. [DOI] [PubMed] [Google Scholar]
- Criado M., Gil, A., Viniegra, S. and Gutierrez, L. (1999) A single amino acid near the C terminus of the synaptosome-associated protein of 25 kDa (SNAP-25) is essential for exocytosis in chromaffin cells. Proc. Natl Acad. Sci. USA, 96, 7256–7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCiommo D.P. and Bremner, R. (1998) Rapid, high level protein production using DNA-based Semliki Forest virus vectors. J. Biol. Chem., 273, 18060–18066. [DOI] [PubMed] [Google Scholar]
- Duncan R.R., Don-Wauchope, A.C., Tapechum, S., Shipston, M.J., Chow, R.H. and Estibeiro, P. (1999) High-efficiency Semliki Forest virus-mediated transduction in bovine adrenal chromaffin cells. Biochem. J., 342, 497–501. [PMC free article] [PubMed] [Google Scholar]
- Edelmann L., Hanson, P.I., Chapman, E.R. and Jahn, R. (1995) Synaptobrevin binding to synaptophysin: a potential mechanism for controlling the exocytotic fusion machine. EMBO J., 14, 224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D., Bruns, D., Shen, B., Jahn, R. and Brünger, A.T. (1997) A structural change occurs upon binding of syntaxin to SNAP-25. J. Biol. Chem., 272, 4582–4590. [DOI] [PubMed] [Google Scholar]
- Fasshauer D., Sutton, R.B., Brünger, A.T. and Jahn, R. (1998) Conserved structural features of the synaptic fusion complex: SNARE proteins reclassified as Q- and R-SNAREs. Proc. Natl Acad. Sci. USA, 95, 15781–15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiebig K.M., Rice, L.M., Pollock, E. and Brünger, A.T. (1999) Folding intermediates of SNARE complex assembly. Nature Struct. Biol., 6, 117–123. [DOI] [PubMed] [Google Scholar]
- Gillis K.D. (1995) Techniques for membrane capacitance measurements. In Sakmann,B. and Neher,E. (eds), Single-Channel Recording, 2nd edn. Plenum Press, New York, NY, pp. 155–198. [Google Scholar]
- Gillis K.D., Möβner, R. and Neher, E. (1996) Protein kinase C enhances exocytosis from chromaffin cells by increasing the size of the readily releasable pool of secretory granules. Neuron, 16, 1209–1220. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie, M. and Tsien, R.Y. (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem., 260, 3440–3450. [PubMed] [Google Scholar]
- Hanson P.I., Heuser, J.E. and Jahn, R. (1997a) Neurotransmitter release—four years of SNARE complexes. Curr. Opin. Neurobiol., 7, 310–315. [DOI] [PubMed] [Google Scholar]
- Hanson P.I., Roth, R., Morisaki, H., Jahn, R. and Heuser, J.E. (1997b) Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell, 90, 523–535. [DOI] [PubMed] [Google Scholar]
- Hayashi T., Yamasaki, S., Nauenburg, S., Binz, T. and Niemann, H. (1995) Disassembly of the reconstituted synaptic vesicle membrane fusion complex in vitro. EMBO J., 14, 2317–2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann C., von Rüden, L., Chow, R.H. and Neher, E. (1993) A two-step model of secretion control in neuroendocrine cells. Pflügers Arch., 424, 105–112. [DOI] [PubMed] [Google Scholar]
- Heinemann C., Chow, R.H., Neher, E. and Zucker, R.S. (1994) Kinetics of the secretory response in bovine chromaffin cells following flash photolysis of caged Ca2+. Biophys. J., 67, 2546–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel A.W. and Almers, W. (1996) Fast steps in exocytosis and endocytosis studied by capacitance measurements in endocrine cells. Curr. Opin. Neurobiol., 6, 350–357. [DOI] [PubMed] [Google Scholar]
- Hilfiker S., Greengard, P. and Augustine, G.J. (1999) Coupling calcium to SNARE-mediated synaptic vesicle fusion. Nature Neurosci., 2, 104–106. [DOI] [PubMed] [Google Scholar]
- Hunt J.M., Bommert, K., Charlton, M.P., Kistner, A., Habermann, E., Augustine, G.J. and Betz, H. (1994) A post-docking role for synaptobrevin in synaptic vesicle fusion. Neuron, 12, 1269–1279. [DOI] [PubMed] [Google Scholar]
- Lin R.J. and Scheller, R.H. (1997) Structural organisation of the synaptic exocytosis core complex. Neuron, 19, 1087–1094. [DOI] [PubMed] [Google Scholar]
- Moser T. and Neher, E. (1997) Rapid exocytosis in single chromaffin cells recorded from mouse adrenal slices. J. Neurosci., 17, 2314–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. (1998) Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron, 20, 389–399. [DOI] [PubMed] [Google Scholar]
- Neher E. and Marty, A. (1982) Discrete changes in cell membrane capacitance observed under conditions of enhanced secretion in bovine adrenal chromaffin cells. Proc. Natl Acad. Sci. USA, 79, 6712–6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. and Zucker, R.S. (1993) Multiple calcium-dependent processes related to secretion in bovine chromaffin cells. Neuron, 10, 21–30. [DOI] [PubMed] [Google Scholar]
- Nichols B.J. and Pelham, H.R.B. (1998) SNAREs and membrane fusion in the Golgi apparatus. Biochim. Biophys. Acta, 1404, 9–31. [DOI] [PubMed] [Google Scholar]
- Otto H., Hanson, P.I., Chapman, E.R., Blasi, J. and Jahn, R. (1995) Poisoning by botulinum neurotoxin A does not inhibit formation or disassembly of the synaptosomal fusion complex. Biochem. Biophys. Res. Commun., 212, 945–952. [DOI] [PubMed] [Google Scholar]
- Owe-Larsson B., Berglund, M.M., Kristensson, K., Garoff, H., Larhammar, D., Brodin, L. and Löw, P. (1999) Perturbation of the synaptic release machinery in hippocampal neurons by overexpression of SNAP-25 with the Semliki Forest virus vector. Eur. J. Neurosci., 11, 1981–1987. [DOI] [PubMed] [Google Scholar]
- Rizo J. and Südhof, T.C. (1998) Mechanics of membrane fusion. Nature Struct. Biol., 5, 839–842. [DOI] [PubMed] [Google Scholar]
- Rothman J.E. (1994) Mechanisms of intracellular protein transport. Nature, 372, 55–63. [DOI] [PubMed] [Google Scholar]
- Ryan T.A. (1998) Probing a complex question: when are SNARE proteins ensnared?Nature Neurosci., 1, 175–177. [DOI] [PubMed] [Google Scholar]
- Söllner T., Bennett, M.K., Whiteheart, S.W., Scheller, R.H. and Rothman, J.E. (1993) A protein assembly–disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell, 75, 409–418. [DOI] [PubMed] [Google Scholar]
- Südhof T.C. (1995) The synaptic vesicle cycle: a cascade of protein–protein interactions. Nature, 375, 645–653. [DOI] [PubMed] [Google Scholar]
- Sutton R.B., Fasshauer, D., Jahn, R. and Brünger, A.T. (1998) Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 Å resolution. Nature, 395, 347–353. [DOI] [PubMed] [Google Scholar]
- Thomas P., Wong, J.G. and Almers, W. (1993) Millisecond studies of secretion in single rat pituitary cells stimulated by flash photolysis of caged Ca2+. EMBO J., 12, 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C., Sato, K. and Wickner, W. (1998) Defining the functions of trans-SNARE pairs. Nature, 396, 543–548. [DOI] [PubMed] [Google Scholar]
- Voets T., Neher, E. and Moser, T. (1999) Mechanisms underlying phasic and sustained secretion in chromaffin cells from mouse adrenal slices. Neuron, 23, 607–615. [DOI] [PubMed] [Google Scholar]
- Weber T., Zemelman, B.V., McNew, J.A., Westermann, B., Gmachl, M., Parlati, F., Söllner, T.H. and Rothman, J.E. (1998) SNAREpins: minimal machinery for membrane fusion. Cell, 92, 759–772. [DOI] [PubMed] [Google Scholar]
- Weimbs T., Low, S.H., Chapin, S.J., Mostov, K.E., Bucher, P. and Hofmann, K. (1997) A conserved domain is present in different families of vesicular fusion proteins—a new superfamily. Proc. Natl Acad. Sci. USA, 94, 3046–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis W.I. and Scheller, R.H. (1998) SNARE the rod, coil the complex. Nature, 395, 328–329. [DOI] [PubMed] [Google Scholar]
- Xu T., Naraghi, M., Kang, H. and Neher, E. (1997) Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophys. J., 73, 532–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Binz, T., Niemann, H. and Neher, E. (1998) Multiple kinetic components of exocytosis distinguished by neurotoxin sensitivity. Nature Neurosci., 1, 192–200. [DOI] [PubMed] [Google Scholar]
- Xu T., Rammner, B., Margittai, M., Artalejo, A.R., Neher, E. and Jahn, R. (1999) Inhibition of SNARE complex assembly differentially affects kinetic components of exocytosis. Cell, 99, 713–722. [DOI] [PubMed] [Google Scholar]