Abstract
Brain-derived neurotrophic factor (BDNF) was studied initially for its role in sensory neuron development. Ablation of this gene in mice leads to death shortly after birth, and abnormalities have been found in both the peripheral and central nervous systems. BDNF and its tyrosine kinase receptor, TrkB, are expressed in hypothalamic nuclei associated with satiety and locomotor activity. In heterozygous mice, BDNF gene expression is reduced and we find that all heterozygous mice exhibit abnormalities in eating behavior or locomotor activity. We also observe this phenotype in independently derived inbred and hybrid BDNF mutant strains. Infusion with BDNF or NT4/5 can transiently reverse the eating behavior and obesity. Thus, we identify a novel non-neurotrophic function for neurotrophins and indicate a role in behavior that is remarkably sensitive to alterations in BDNF activity.
Keywords: brain-derived neurotrophic factor (BDNF)/hypothalamus/neurotrophins/obesity/TrkB
Introduction
In the last decade, many genes responsible for the signals underlying eating behavior and obesity have been identified. These factors include well described peripheral mediators such as insulin, glucocorticoids and leptin, as well as central mediators that are less well understood (Rosenbaum et al., 1997). The current animal models of obesity focus upon the hypothalamus as the anatomical area within the brain that controls eating behavior. In the 1940s, it was shown that hypothalamic lesions cause hyperphagia and obesity (Hetherington and Ranson, 1942). Today, several genetic models give further insight into the hypothalamus and weight regulation. In the ob and db mouse models, either leptin (ob) or its hypothalamic receptor (db) are mutated and rendered non-functional, resulting in severe early-onset obesity (Coleman, 1978; Caro et al., 1996; Chen et al., 1996; Lee et al., 1996). Overexpression of the agouti peptide or gene-targeted deletion of the hypothalamic receptor it antagonizes, the melanocortin-4 receptor, also result in obesity (Lu et al., 1994; Huszar et al., 1997). Each of these models demonstrates the importance of various specific nuclei within the hypothalamus including the arcuate, dorsomedial, ventromedial and paraventricular nuclei (Chua et al., 1996; Fan et al., 1997). Additionally, at least two new families of peptides, the orexins and cocaine- and amphetamine-related transcript (CART), have been localized to the hypothalamus. These factors are thought to mediate feeding behavior in response to neural cues that act upon the satiety centers of the hypothalamus (Kristensen et al., 1998; Sakurai et al., 1998).
The neurotrophin (NTF) family is composed of four structurally related proteins with similar functions in promoting neuronal survival within the peripheral nervous system (PNS) (Barde, 1994; Snider, 1998). NTF-deficient mice have been particularly useful in studying nervous system development, as nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT3) null mice exhibit diverse neural abnormalities and die as neonates (Snider, 1994). Signaling is mediated for each NTF through a specific Trk family receptor tyrosine kinase (TrkA, B and C) (Kaplan et al., 1991a,b; Klein et al., 1991a,b; Soppet et al., 1991; Parada et al., 1992).
The functions of neurotrophins in the central nervous system (CNS) appear more diverse than the target-derived paradigm that serves as a useful model in the PNS. BDNF protein is widely distributed in the CNS, beginning early in development and extending throughout the organism's life span. BDNF is known to play key roles early in embryogenesis by enhancing the differentiation of CNS stem cell neuronal precursors as well as being a critical component of early neural tube development in the chick (Ahmed et al., 1995; Jungbluth et al., 1997). In later stages of development, BDNF has been shown to regulate cortical dendritic growth, optic nerve branching and remodeling, development of dopaminergic networks and differentiation of hippocampal neurons (Cohen-Cory and Fraser, 1995; Vicario-Abejon et al., 1995; McAllister et al., 1997; Cellerino et al., 1998).
In the mature CNS, BDNF protein is most abundant in the hippocampus and hypothalamus (Nawa et al., 1995). The hippocampus contains areas critical to synaptic plasticity and relies on activity-dependent changes to function. BDNF and TrkB have been shown to function in the plasticity of synaptic connections involved with learning and memory, and mice heterozygous for the BDNF gene have impaired long-term potentiation (Korte et al., 1995, 1998; Patterson et al., 1996). Activity-dependent variations in neuronal BDNF synthesis within sympathetic neurons have also been shown to result in long-term alterations in synaptic input (Causing et al., 1997). In the hypothalamus, BDNF function is less well defined. Its expression is known to increase in response to osmotic or immobilization stress, though little is known about the physiological relevance that this increase may have (Castren et al., 1995; Smith et al., 1995).
The present study arose from our observation that mice with only one functional BDNF allele exhibited a tendency toward obesity. We confirm that variation of BDNF levels in the CNS has profound behavioral effects on mice. One hundred percent of the BDNF heterozygous mice exhibit a behavioral phenotype. These data may have correlates in humans where it has been noted that individuals with WAGR (Wilms' tumor, aniridia, genitourinary anomalies and mental retardation) contiguous gene syndrome whose deletions extend into the BDNF locus are obese.
Results
Weight and appetite
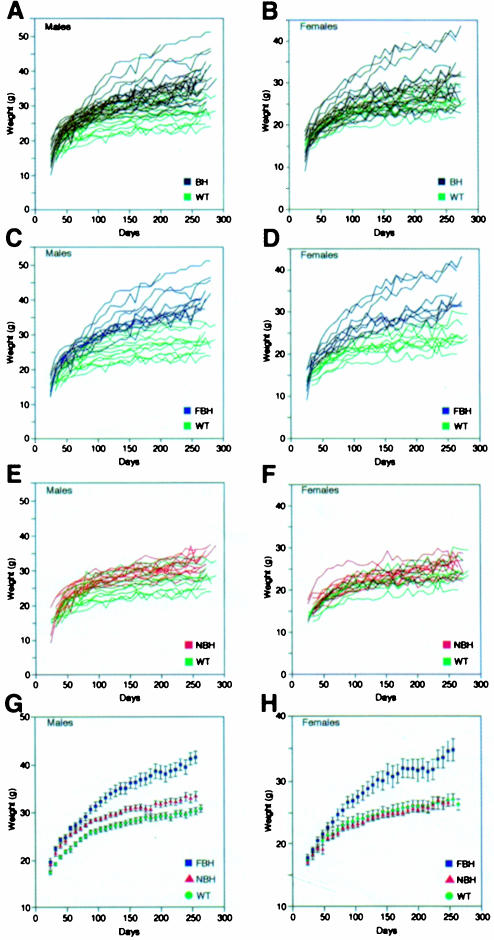
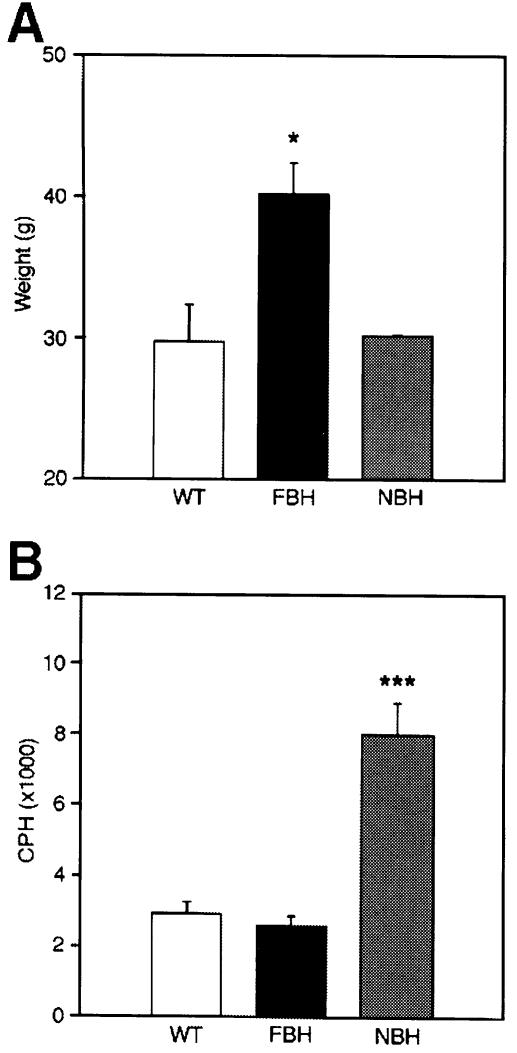
Mice heterozygous for targeted disruption of BDNF, NT4/5, NT3, TrkC and TrkA (Snider, 1994; Liebl et al., 1997, 2000; Tessarollo, 1997) were weighed every 8 days between the ages of 28 and 270 days and compared with wild-type littermates. Of these mutant strains, generated in our laboratory and maintained under identical housing and feeding conditions, only the BDNF mutants (BH) showed a significant variance in weight from wild-type. Figure 1A and B shows the individual weight charts for male and female wild-type (green) and BDNF heterozygous (black) mice. Two features are evident from examination of these panels. First, the range of weight is considerably increased for the BDNF heterozygous population; and secondly, a significant weight increase was present in a subset of BDNF mutant males (50%) and females (27%). Figure 1C–F shows a subgrouping of the BDNF heterozygous population into fat BDNF heterozygous mutant (FBH) mice (Figure 1C and D; blue) defined as weighing greater than two standard deviations above the mean weight of wild-type mice, and non-fat BDNF heterozygous mutant (NBH) mice. Over time, more mutant animals in our population became obese, with only 12% obese at 3 months, 23% at 6 months and 50% at 9 months. Figure 1E and F shows the overlapping values of NBH (red) and wild-type mice, and Figure 1G and H shows the average weights of each of the three populations. At 6 months of age, the average weights of male and female FBH mice were increased 44 and 33%, respectively. Feeding behavior reflected the increased FBH weight, where, on average, FBH mice consumed 47% more food per day (6.6 g) than NBH and wild-type mice (not shown). Similar increases in weight have been seen in heterozygous knockout mice for the melanocortin-4 receptor (Huszar et al., 1997).
Fig. 1. BDNF heterozygous (BH) mice exhibit increased weight variation with increased age (A and B). Mutant mice are subdivided based on weight into fat heterozygous (FBH; blue), non-fat heterozygous (NBH; red) and wild-type (WT; green) groups for both males (C, E and G) and females (D, F and H). FBH mice weigh greater than two standard deviations above the mean weight of wild-type mice and fall outside the wild-type range (C and D), while NBH mice fall within the wild-type weight distribution (E and F). The growth curve for males (G) represents 13 FBH, 13 NBH and 40 wild-type mice, whereas that for females (H) includes nine FBH, 21 NBH and 17 wild type. Values represent the mean ± SEM.
Increased locomotor activity
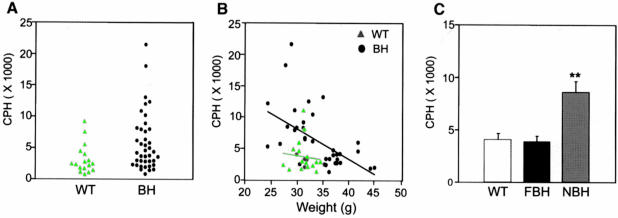
Locomotor activity tends to be depressed in obese mice, presumably due to abnormal neuroendocrine metabolism (Erickson et al., 1996). Neither FBH nor NBH mice appeared lethargic. Direct observation of BDNF heterozygous mice revealed a hyperactive behavior as compared with wild-type littermates (Figure 2). Activity monitors were employed to evaluate activity quantitatively at 6 months of age. These monitors consisted of 15 photoelectric beams spaced 3 cm apart encompassing the length of the cage. Activity was measured as the total number of beam disruptions during a nocturnal 4 h monitoring period (see Materials and methods). As for weight, an increased variance in the distribution of locomotor activity of the BDNF mutant mice was observed (Figure 2A). To examine whether a relationship exists between weight increase and locomotor activity, the two values for individual mice were compared. Figure 2B shows that while wild-type animals show little correlation between weight and activity, BDNF mutants show an inverse relationship between increasing locomotor activity and decreasing weight. This negative correlation showed a coefficient of –0.58 with a p-value <0.001. When the BDNF heterozygotes are separated into two populations by weight (Figure 1C–F), mice that fall within the NBH population exhibit more than twice the locomotor activity of wild-type and FBH mice (Figure 2C). Thus, BDNF heterozygous mice exhibit at least one of two behavioral phenotypes. While NBH mice have a tendency for increased locomotor activity, FBH mice exhibit wild-type values. This is in contrast to other obese mouse models such as ob and agouti, which tend to exhibit lethargic behavior and decreased activity (Erickson et al., 1996). This difference in the obese BDNF heterozygotes therefore suggests a relative increased locomotor activity and, furthermore, may indicate that all BDNF heterozygotes have increased activity.
Fig. 2. BDNF heterozygous mice (BH) show increased variance of activity when compared with wild-type (WT) (A). A correlation coefficient of –0.58 for activity plotted against weight (p <0.001) was obtained using a Spearman rank order test for correlation of BH mice (B). Photoelectric beam disruptions for wild-type, NBH and FBH mice are quantified in (C) (see Materials and methods). Values represent the mean ± SEM. **p <0.01. N = 9 for wild-type, and 10 each for FBH and NBH.
Fat content
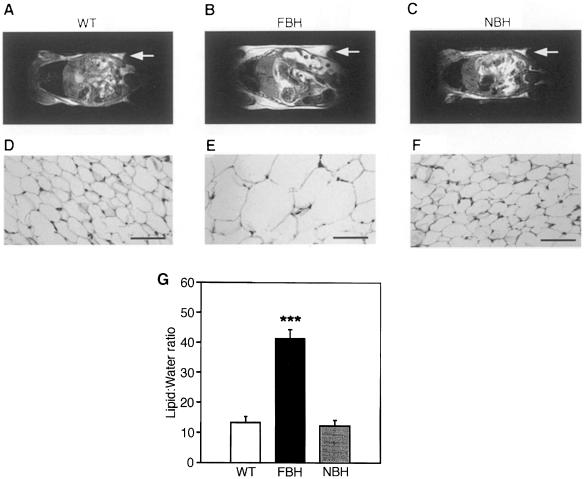
To determine whether the increased weight of FBH mice reflected increased adiposity, FBH, NBH and wild-type mice were examined using proton spectroscopy, nuclear magnetic resonance (NMR) imaging and histological analysis (see Materials and methods; Figure 3). Figure 3A–C shows representative NMR sections of the wild-type, FBH and NBH animals in a mid-horizontal plane and illustrates the size gain of the retroperitoneal fat pad in the FBH animal. Increased fat content can result from either an increase in adipocyte number (hyperplasia) or an increase in cell size (hypertrophy). Histological analysis of the inguinal fat pads indicates that the obese BDNF heterozygotes have adipose cellular hypertrophy (Figure 2D–F). Inguinal fat pad measurements of 6-month-old mice confirmed that FBH mice have adipocyte diameters ∼3-fold greater (144.3 ± 21 μm) than NBH (55.1 ± 7.0 μm) or wild-type (51.7 ± 7.6 μm) fat cells. Adipose cell hypertrophy is characteristic of human obesity. Finally, proton spectroscopy was used to quantify the percentage of fat by measuring the ratio of total lipid to water content (Stein et al., 1995). Figure 3G shows that FBH mice have a 4-fold increase in the percentage of lipid to water, while NBH mice are not different from wild-type littermate controls. This translates to 8.5% body fat content in the wild-type and NBH mice, while FBH mice have >30% body fat.
Fig. 3. FBH mice are obese and show adipocyte hypertrophy. NMR images (A–C) of whole-body horizontal sections are taken at the approximate midline. White density denotes fat, and arrows point to areas of significant fat accumulation in retroperitoneal fat pads. (D–F) Fat histology of inguinal fat pads with the bar representing 70 μm. Quantification of lipid is based on spectroscopy of lipid and water peaks (G) (see Materials and methods). Values represent the mean ± SEM. Wild-type (n = 15), FBH (n = 11) and NBH (n = 8) are labeled accordingly.
Serum levels
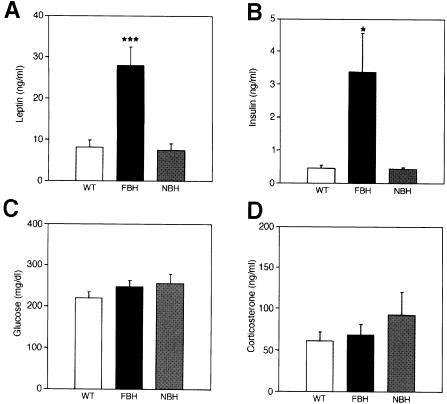
Changes in the serum levels of leptin, insulin, glucose and corticosterone have been associated with obesity (Spiegelman and Flier, 1996). To determine whether FBH mice have alterations in any of these substances, the serum of FBH, NBH and wild-type mice was analyzed (Figure 4). Six-month-old FBH mice were found to have 4- to 5-fold more serum leptin as compared with NBH and wild-type littermates (Figure 4A). This increase in leptin is consistent with the increase in adipocyte cell size. Both human and animal genetic and dietary models of obesity associate high levels of leptin with increasing adiposity (Caro et al., 1996; Friedman, 1997). Insulin is the primary modulator of glucose metabolism (Rosenbaum et al., 1997) and FBH mice have an 8-fold increase in insulin levels when compared with wild-type and NBH mice (Figure 4B). However, examination of serum glucose levels showed no significant difference between the three genotypes (Figure 4C). This suggests that FBH animals maintain normal serum glucose levels by compensating with increased insulin levels.
Fig. 4. FBH mice are leptin and insulin resistant. Plasma concentrations of leptin (A), insulin (B), glucose (C) and corticosterone (D) were measured from wild-type, FBH and NBH mice. Blood was drawn and processed as described in Materials and methods. Each value represents the mean ± SEM of 9–23 (wild-type), 12–24 (FBH) and six (NBH) mice. ***p <0.001; *p <0.05.
Corticosterone is an adrenal hormone produced in response to stress, and its levels can be abnormal in some models of obesity (Spiegelman and Flier, 1996; Rosenbaum et al., 1997). FBH, NBH and wild-type mice showed no significant difference in serum corticosterone levels (Figure 4D). Thus, FBH mice are similar to obese humans and to mouse obesity models showing increased levels of leptin and insulin (Lu et al., 1994; Huszar et al., 1997). Unlike ob and db mice (Coleman, 1978), FBH mice can still regulate glucose and corticosterone levels. Abnormalities in thyroid gland activity can also affect eating behavior and activity. To determine whether BDNF heterozygotes had altered thyroid activity, thyroid hormone measurements were performed on cohorts of wild-type, FBH and NBH mice. No differences in thyroid hormone levels were detected in the three populations (not shown).
Independent BDNF knockouts exhibit similar phenotypes
To rule out the possibility that the observed phenotypes resulted from the inadvertent alteration of a second gene in our original BDNF homologous recombinant embryonic stem (ES) cell line, we examined an independently derived BDNF knockout mouse strain (Ernfors et al., 1994). This second BDNF mutant line, maintained in a pure C57Bl/6 background, has confirmed the presence of altered eating and motor activity behavior. Males from three litters were evaluated at 7–8 months of age for weight and activity. Figure 5A illustrates that the average weight of the wild-type and NBH mice is similar, whereas the FBH mice have a 30% average weight increase. As shown in Figure 5B, locomotor activity levels for the wild-type and FBH mice are also similar, while the NBH mice show a >3-fold increase in activity. Thus, the behavioral alterations seen in BDNF heterozygotes result from the mutation of one BDNF allele and not of inadvertently mutated nearby genes. Moreover, the preservation of phenotype in the 129Sv×C57Bl/6 hybrid or the inbred C57Bl/6 background suggests that changes in these genetic backgrounds do not significantly affect the two phenotypes described here.
Fig. 5. Independently derived BDNF mutant mice in a pure C57Bl/6 background are obese and hyperactive (Ernfors et al., 1994). (A) Representative weights at 7–8 months of age; (B) activity levels in the same animals. Data represent male animals (n = 3 for each group) from two litters. Values represent the mean ± SEM.
Hypothalamic expression of BDNF and TrkB
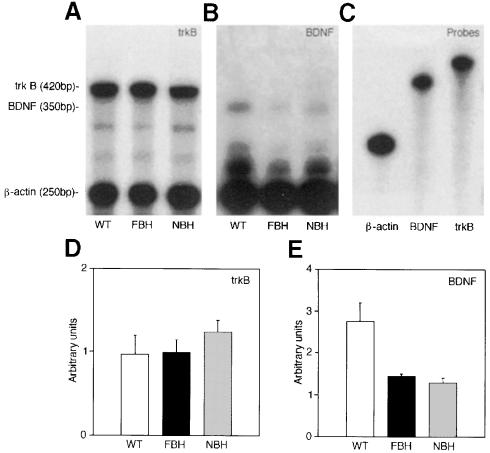
The hypothalamus is the CNS regulatory center for feeding behavior and weight control. We examined changes in BDNF or TrkB mRNA expression in BDNF heterozygous mice. RNase protection was employed to examine quantitative differences in BDNF and TrkB transcripts, while in situ hybridization evaluated regional distribution. Densitometric readings demonstrated that both FBH and NBH mice have ∼50% reduction in BDNF transcripts compared with wild-type controls (Figure 6B and E). Quantitative values were normalized to a β-actin control. No differences were observed in TrkB transcripts from the hypothalamus of wild-type, FBH and NBH mice (Figure 6A and D).
Fig. 6. BDNF heterozygotes have decreased BDNF but not TrkB transcripts in the hypothalamus. RNase protection in (A) demonstrates equivalent TrkB-protected fragments (420 bp), whereas (B) shows decreased intensity of BDNF-protected fragments (350 bp) in the FBH and NBH mutant populations. Unprotected probes are shown in (C). Densitometric quantitation is shown for TrkB (D) and BDNF (E).
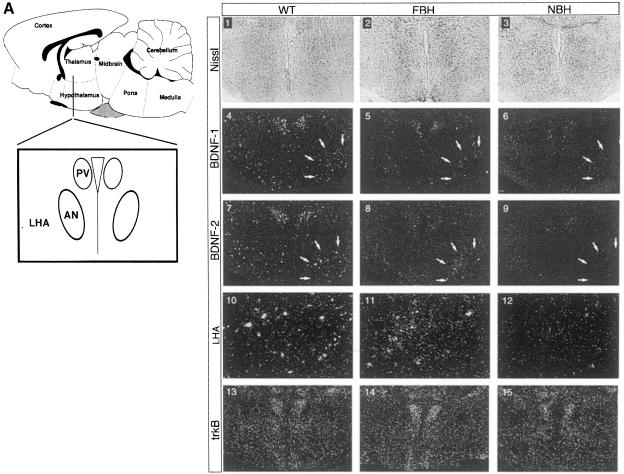
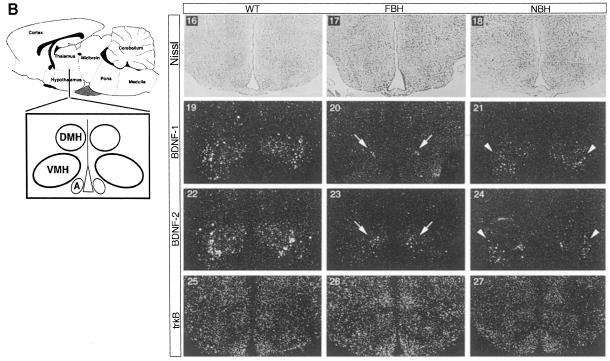
In situ hybridization was employed next to examine expression of BDNF and TrkB in specific hypothalamic nuclei. All studies were performed on four brains each of wild-type, FBH and NBH mice. The FBH and NBH mice represent mean values for obesity in the FBH group and locomotor activity in the NBH group. The areas of the hypothalamus associated with weight and feeding behavior include the paraventricular (PV), the arcuate (A), the dorsomedial (DMH) and the ventromedial (VMH) nuclei. As shown in representative sections from two independent brains (Figure 7A), BDNF is normally expressed in the PV and lateral hypothalamic area (LHA) of the anterior hypothalamus (panels 1, 4, 7 and 10). Consistent with the RNase protection data, FBH and NBH littermates appear to have reduced BDNF expression at these two nuclei (panels 2, 5, 8 and 11, and 3, 6, 9 and 12, respectively). However, comparison of LHA (Figure 7A, arrows) consistently indicates considerably lower BDNF signal in NBH sections. While TrkB hybridization was equivalent in adjacent sections (panels 13–15) controlling for hybridization efficiency, the NBH sections have reduced BDNF signal (panels 6, 9 and 12). BDNF is also expressed normally at high levels in the VMH (Figure 7B, panels 16, 19, 22 and 25). FBH and NBH mice showed decreased BDNF signal in these nuclei. We also noted an asymmetry in BDNF expression of the heterozygote VMH. FBH mice preferentially retained the dorsomedial expression (panels 20 and 23; arrows) seen in wild-type, whereas NBH (hyperactive heterozygotes) retained the lateral but not the medial expression (panels 21 and 24; arrowheads). Hybridization of adjacent sections with a TrkB probe showed widespread expression and no discernible qualitative changes in expression between the three genotypes (wild-type, NBH and FBH) (Figure 7B, panels 25–27). Thus, regional reductions in BDNF expression within the hypothalamic nuclei implicated in feeding behavior and locomotor activity may account for the phenotypes seen in BDNF heterozygous mice. Asymmetry in the reduction in BDNF expression correlates with the distinct phenotypes and may underlie the behavioral differences.
Fig. 7. TrkB and BDNF transcripts are expressed in satiety and locomotor hypothalamic nuclei. (A) Schematic of the anterior hypothalamus including the paraventricular (PV) nucleus, lateral hypothalamic area (LHA) and anterior nucleus (AN). Nissl-stained sections (1–3) represent each population. The series labeled BDNF-1 and BDNF-2 indicate representative sections from two of four total serially sectioned wild-type, FBH and NBH brains probed with BDNF. TrkB indicates an adjacent section to the section in BDNF-2. BDNF (4–9) expression is seen in the PV and LHA of wild-type, FBH and NBH mice. Arrows (4–9) encompass the right LHA region. LHA indicates panels 10–12 showing magnification of the LHA in panels 7–9. TrkB transcripts are present throughout the anterior hypothalamus (13–15). (B) The schematic to the left of the panel depicts a more posterior section than (A) of the hypothalamus including the dorsomedial (DMH), ventromedial (VMH) and arcuate (A) nuclei. BDNF (19–24) is expressed asymmetrically in the VMH of wild-type, FBH (arrows) and NBH (arrowheads) mice. TrkB transcripts are found throughout (25–27). n = 4 each for wild-type, FBH and NBH animals.
Hypothalamic expression of NPY, CART and leptin receptor
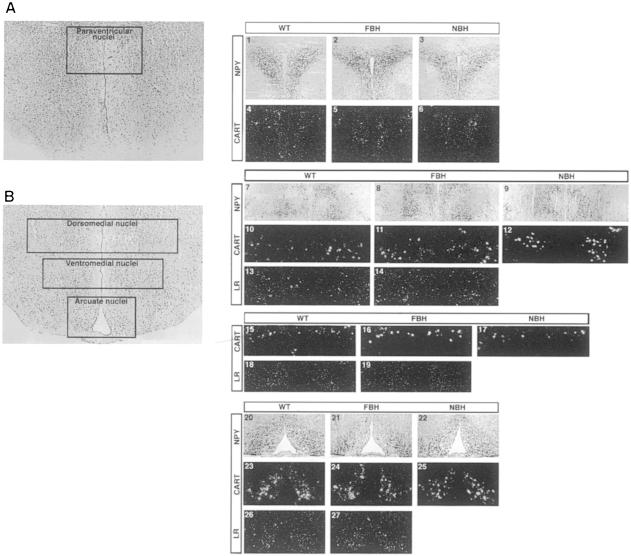
Neurotransmitters regulate neural activity in satiety centers of the hypothalamus, where alteration in neuropeptides results in abnormal feeding behavior (for a review, see Elmquist et al., 1999). Neuropeptide Y (NPY)-expressing neurons are located in several hypothalamic nuclei, including the A nucleus, which projects NPY-expressing neurons to the PV nucleus to regulate feeding behavior. Immunohistochemical examination of NPY afferents in the PV (Figure 8A, panels 1–3), DMH (Figure 8B, panels 7–9) and A (Figure 8B, panels 20–22) nuclei of wild-type, FBH and NBH mice showed no significant alteration in expression. CART is also highly expressed in the DMH (Figure 8B, panels 10–12), VMH (Figure 8B, panels 15–17) and A (Figure 8B, panels 23–25) nuclei, and to a lesser extent in the PV (Figure 8A, panels 4–6) nucleus. In situ hybridization of CART mRNA was employed, and no differences were observed in CART expression between wild-type, FBH and NBH mice.
Fig. 8. NPY, CART and leptin receptor expression in hypothalamic nuclei. (A) Coronal section of the anterior hypothalamus at the level of the paraventricular nuclei. Panels 1–6 represent bilateral paraventricular nuclei immunostained for neuropeptide Y (NPY) (1–3) and hybridized with CART mRNA transcripts (4–6) in wild-type, FBH and NBH mice, respectively. (B) Coronal section of the posterior hypothalamus at the level of the dorsomedial, ventromedial and arcuate nuclei. Panels 7–9 and 20–22 represent bilateral NPY immunoreactivity in the DMH (7–9) and A (20–22) nuclei of wild-type, FBH and NBH mice, respectively. Panels 10–12, 15–17 and 23–25 represent bilateral hybridization for CART mRNA in the DMH (10–12), VMH (15–17) and A (23–25) nuclei of wild-type, FBH and NBH mice, respectively. Panels 13, 14, 18, 19, 26 and 27 represent bilateral hybridization for leptin receptor (LR) mRNA in DMH (13 and 14), VMH (18 and 19) and A (26 and 27) nuclei of wild-type and FBH mice, respectively.
We next examined whether the observed leptin resistance was the direct result of changes in expression of the leptin receptor (LR). We found no significant difference in LR expression in the DMH (Figure 8B, panels 13 and 14), VMH (Figure 8B, panels 18 and 19) or A (Figure 8B, panels 26 and 27) nuclei of wild-type and FBH mice. The results indicate that while BDNF transcripts are differentially regulated in hypothalamic nuclei that control feeding and locomotor behavior, the expression of other mediators (i.e. NPY, CART and LR) in the same nuclei is not altered. Thus, either BDNF functions downstream or independently of the leptin/NPY axis.
FBH phenotype is reversible
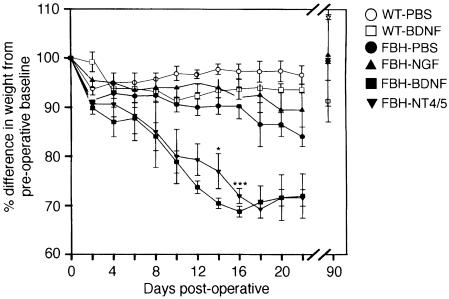
The above data are consistent with the interpretation that reduction of BDNF in the hypothalamus causes increased locomotor activity and eating behavior. This outcome could result via two alternative models. Either reduction of BDNF levels in the heterozygous mutants results in the developmental atrophy of hypothalamic cells required for the regulation of eating behavior and activity, or reduction in endogenous BDNF levels modifies the physiological function of otherwise intact hypothalamic cells. In the former case, it would not be anticipated that infusion of exogenous BDNF would reverse the phenotype since the cells in question would be absent or incompetent. In the latter model, addition of exogenous BDNF would be expected to compensate for the reduced endogenous levels and thus lead to phenotypic reversal. To discriminate between the alternative models, BDNF, NT4/5, NGF or phosphate-buffered saline (PBS) vehicle were infused into the third ventricle of FBH mice for 14 days via cannulation and application of a subcutaneous mini-pump (Figure 9). Infusion of NGF or PBS vehicle resulted in a small (10–15%), but significant, decrease in weight as compared with pre-infusion weight. We attribute this reduction to the aftermath of intracranial surgery and pump implantation. The infusion of either TrkB ligand, BDNF or NT4/5 had significantly greater effects. Weight loss in the obese (FBH) mice averaged 30% by 16 days post-infusion. This represents a decrease from an average of 42 g (pre-infusion baseline) to an average of 29 g, and approximates the average wild-type weight at a similar age. The loss in weight reflects a decrease in adipose cell size and not systemic illness or protein wasting (not shown). By day 21 post-infusion, each group of infused mice demonstrated evidence of regaining weight. After 90 days, all groups of mice had regained weight back to the pre-operative baseline (Figure 9). When wild-type mice were infused with BDNF under identical conditions, <5% loss in weight was observed (Figure 9). Food consumption showed a similar profile to weight in BDNF-infused mice. There was a 40% decrease in eating by day 14, which was followed by a post-infusion increase in feeding that reached pre-infusion levels 4 days after the infusions were completed (not shown).
Fig. 9. Intra-cranial infusion of BDNF or NT4/5 into the third ventricle of FBH mice causes a reversion in weight. PBS (•, n = 7), NGF (▴, n = 2), BDNF (▪, n = 8) or NT4/5 (▾, n = 3) were infused into the third ventricle for 14 days. After 3 months, the animals' weight returned to baseline in all groups. Infusion of PBS (○, n = 5) or BDNF (□, n = 5) in the wild-type produced no significant change in weight. Values represent the mean ± SEM.
These results give strong support to the model that reduction of endogenous BDNF levels in the hypothalamus of BDNF heterozygotes can result in eating behavior disorders. BDNF most probably functions through its receptor tyrosine kinase, TrkB, since similar results were obtained with NT4/5 infusion, but not NGF, although each of these NTFs can interact with the p75 low affinity receptor.
Discussion
The gene family of NGF-related neurotrophins is widely recognized to have important neurotrophic functions in the sculpting of the PNS. The widespread expression of these molecules and their receptors in the CNS has prompted the search for additional functions in this tissue. Numerous reports have implicated BDNF in long-term potentiation (LTP) (Korte et al., 1995, 1996; Chen et al., 1996; Patterson et al., 1996; Kang et al., 1997; Korte and Bonhoeffer, 1997) and synaptic plasticity (Levine et al., 1998). An assessment of the potential function of neurotrophins in behavior has been more difficult due to the fact that complete ablation of gene function for NGF, BDNF, NT3 or their cognate Trk family receptors is inconsistent with survival past early infancy. Nonetheless, it has been reported that mice carrying only one functional BDNF allele exhibit deficiencies in learning behavior that can be correlated to altered LTP responses (Patterson et al., 1996; Linnarsson et al., 1997). Thus, BDNF emerges as a molecule with broad and important roles beyond those implicated in sensory neuronal development, and probably functions to maintain mature neurons in brain areas other than the hippocampus.
In the present study, we identify novel behavioral abnormalities in mice with only one functional BDNF allele. In addition to learning deficiencies (Linnarsson et al., 1997), these animals appear to have the propensity towards increased motor activity. About half of BDNF heterozygous mice develop an eating behavior disorder leading to obesity, as reflected by a 300% increase in fat content by 9 months of age. These mice are apparently resistant to leptin as their endogenous levels are increased, in addition to having abnormally high insulin levels. These features parallel the human condition of obesity. Comparison of locomotor activity between the obese BDNF heterozygous mice and their wild-type littermates has not revealed differences. The fact that the obese BDNF heterozygotes are not lethargic suggests that these fat mutant mice are relatively hyperactive but, for reasons not understood, become more obese than their hyperactive and normal sized littermates.
Leptin and its receptor are essential mediators of feeding behavior at the level of the hypothalamus (Friedman, 1997; Elmquist et al., 1998). The increased leptin in BDNF heterozygotes and apparent leptin resistance cannot be attributed to reductions in the leptin receptor (Chen et al., 1996; Lee et al., 1996). Hypothalamic peptides are also expressed in satiety nuclei and have been thought to mediate feeding behavior in response to neural cues. In vitro, NPY is regulated by BDNF in hippocampal interneurons (Marty et al., 1996) and, in vivo, NPY immunoreactivity is decreased in BDNF null mice (Jones et al., 1994). In BDNF heterozygous mice, we detect no difference in NPY immunoreactivity. We also did not find differences in expression of CART in the hypothalamus. These negative results suggest that the behavioral changes induced in mice by genetic reduction of endogenous BDNF are either downstream of these eating behavior mediators or function in parallel pathways that impinge on eating behavior and locomotor activity.
The coding domain for the BDNF gene, like that of all neurotrophins, is contained in one exon. Thus, our mutant mice completely ablate the function of the recombinant allele by insertion of a neo cassette within the coding region (Liebl et al., 1997). The phenotypic similarity in two independent BDNF knockouts confirms that the observed behavioral phenotypes are attributable to BDNF function. To gain an understanding of how mutation of one BDNF allele could lead to such altered behavior, we examined the expression in the hypothalamus where the nuclei that regulate satiety and locomotor activity are localized. We did not detect overt morphological differences in this region between the wild-type and BDNF mutant mice, suggesting that no dramatic changes in cell number had occurred. However, BDNF and TrkB are expressed in the hypothalamus, and heterozygotes have reduced levels of BDNF transcripts. Thus, the BDNF/TrkB signaling system is present in the regions of the CNS that regulate satiety and activity. In situ hybridization is not a quantitative technique, but permitted us to focus regionally on nuclei within the hypothalamus that have been identified as key in regulating eating behavior. The LHA is important as a negative regulator of activity. When specific receptors in the LHA are antagonized, locomotor hyperactivity ensues (Morutto and Phillips, 1997). While the fat heterozygotes showed a reduction by about half in the LHA, we were unable to detect BDNF expression in the LHA of the four non-fat hyperactive heterozygotes examined. Similarly, asymmetric reduction of BDNF was observed in the VMH. Ablation of one germline BDNF allele may lead to the selective down-regulation or exclusion of the other allele in selective centers of the brain, resulting in a functional knockout in the nuclei that regulate activity or satiety.
Neurotrophins have been implicated indirectly in regulating appetite by infusion of either NGF or BDNF in non-obese rats (Williams, 1991; Lapchak and Hefti, 1992; Pelleymounter et al., 1995). At the highest doses, BDNF infusion in rats has been shown to cause a weight loss of 10–15% from pre-infusion baseline (Pelleymounter et al., 1995). In our studies, infusion of BDNF into wild-type mice produces a weight loss of <10%, which accounts for all of the fat stores, as we have shown that under our housing conditions wild-type and NBH mice have an average fat content of 8.5%. Infusion of vehicle or NGF in obese BDNF heterozygous mice similarly produces a modest weight loss of ∼10%. This is in contrast to infusions of BDNF or NT4/5 in obese BDNF mutant mice, which produced a 30% weight loss. These results indicate that regional cellular physiology can be corrected by the re-infusion of BDNF. Thus, the observed behavioral change in the heterozygous mice is not due to cell death caused by regional reduction of BDNF during development.
Another issue arising from our study concerns the genetic configuration of our mice and the possibility that segregating loci may modify the BDNF locus in determining whether the mice will gain weight or become hyperactive. The present studies were originated in heterozygous mice that were hybrid for the C57Bl/6 and 129Sv genetic backgrounds. Upon our initial observation that a significant number of the heterozygotes were obese, we initiated genetic crosses of the null BDNF allele to the CD1, 129Sv or C57Bl/6 strains. After six generations, we do not yet observe a segregation of the two traits. In addition, we have crossed every possible combination of mutant animals (FBH with NBH, NBH with NBH, FBH with wild-type, etc.) and have observed both fat and non-fat offspring from every combination. Thus, we are unable at this time to provide evidence for modifier loci that may contribute to the two differing phenotypes.
In mice and humans, the BDNF gene is linked to the Wilms' tumor (WT-1) and Pax-6 genes. In humans, the WAGR contiguous gene syndrome results from deficiencies in chromosome 11p13 that affect some or all of these gene functions. Two rare patients with WAGR syndrome have been reported to be obese, leading to speculation that an obesity-related gene may be present at one extreme of this locus (Marlin et al., 1994; McGaughran et al., 1995). We have inspected the data for these two patients and noted that both harbor deletions that encompass the BDNF gene (Marlin et al., 1994; McGaughran et al., 1995). This observation in humans correlates with our results in mice and raises the possibility that mutation of the BDNF gene may account for obesity in these patients.
The underlying perturbations caused by reduction of endogenous BDNF leading to behavioral modification remain unclear. The absence of changes in the leptin, NPY and CART pathways does not rule out the possibility that reduced TrkB signaling may modify one of the pathways. However, emerging data implicating BDNF and TrkB in synaptic plasticity and its modulation may provide the eventual explanation for the present results (Bonhoeffer, 1996; Chen et al., 1999). Reduction of local BDNF levels in hypothalamic nuclei may affect the strength of synaptic connections or dendritic spine density leading to altered behaviors. Modulation of plasticity in the hippocampus as monitored by LTP has been implicated in spatial learning and behavior (Malenka and Nicoll, 1999). Alterations of BDNF expression in this region have been reported to affect LTP and learning (Korte et al., 1998), thus implicating synaptic interactions as the cellular basis for these effects. It is possible that similar mechanisms may be at play in the hypothalamus. A corollary of this model is satisfied by the observation that re-introduction of BDNF restores a normal behavioral response. Development and application of sensitive electrophysiological assays that currently are applied to the hippocampus will permit more detailed examination of this model.
Materials and methods
Weight measurements and food intake
BDNF-deficient mice were generated and genotyped as previously described (Liebl et al., 1997). BDNF, NT3, NT4/5 and TrkA mutant mice were maintained in a C57Bl/6 and 129Sv mixed F2 background. All mice were kept in a pathogen-free environment on a 12 h light–dark schedule. They were housed 2–3 per cage and given water and mouse chow ad libitum. Weight was measured every eighth day between 4 and 36 weeks of age. Five- to six-month-old mice were isolated for at least 1 week prior to any measurements. Food was monitored for spillage, which was negligible. Daily intake was determined by averaging a 5–7 day total. Mean and standard errors were evaluated between wild-type and mutant groups, and the significance was determined by a Student–Newman–Keuls test.
MRI and fat histology
Magnetic resonance imaging (MRI) data were generated as previously described (Stein et al., 1995). Wild-type and BDNF mutant animals were taken at 5–6 months of age, anesthetized with 0.1 ml of ketamine:xylazine (100:0.2 mg/ml) diluted 1:1 with sterile water per 25 g of mouse weight, and magnetic spectroscopy was performed. To examine fat histology, mice were anesthetized (as described above) and perfused with 0.1 M PBS pH 7.4, and 4% paraformaldehyde (PFA) in PBS. Inguinal fat pads were dissected, post-fixed with 4% PFA for 24 h, embedded in paraffin, and 10 μm sections stained with hematoxylin and eosin. Significance in the lipid:water ratio was determined using a Kruskal–Wallis one-way analysis of variance on ranks. Fat cell diameters were determined by measuring all the intact cells in a high power field (n = 100 for each) and using the longest diameter as the final measurement.
Serum levels
Mice were terminally bled under anesthesia via cardiac puncture, collected in tubes with 50 mM EDTA, and the serum was isolated and instantly frozen in liquid nitrogen. To obtain glucose levels, 50 μl of serum were analyzed utilizing a hexokinase enzymatic reaction (Hitachi 747). Insulin and leptin levels were determined in duplicate using 50 μl of serum with radioimmunoassay kits to insulin and leptin (Linco Research). Basal corticosterone levels were determined in a separate set of animals, which were handled daily 2 h into their light cycle for 2 weeks prior to bleeding. Assays were performed in duplicate using 10 μl of serum with a rat radioimmunoassay kit (ICN). Statistical significance was determined using a Student–Newman–Keuls test for the leptin, insulin and corticosterone assays, and a one-way analysis of variance for the glucose assay.
RNase protection
Hypothalamic RNA was obtained from wild-type and BDNF mutant mice by killing animals with CO2 and removing the entire brain. Individual hypothalami were dissected by making a rostral cut at the optic chiasm, a caudal cut after the mamillary bodies and a dorsal cut at the thalamic–hypothalamic interface. Samples were then snap-frozen in liquid nitrogen and stored at –80°C. Total RNA was removed using Trizol reagent, and 25–35 μg of total RNA were obtained from each hypothalamus. TrkB- (420 bp) and BDNF- (370 bp) specific [32P]UTP-labeled probes (gift of Carlos Ibanez) were hybridized with 1 and 2 μg of total RNA, respectively, using a Hybspeed RPA kit (Ambion). Protected fragments were run and visualized on a 5% acrylamide–8 M urea denaturing gel. Each sample was run with a 250 bp β-actin control. Protected fragments were quantified by densitometry readings utilizing a Bio-Rad GS-525 phosphoimager and Molecular Analyst Software. Band intensity was standardized to the internal β-actin control.
In situ hybridization/immunohistochemistry
Six-month-old wild-type and BDNF mutant animals were anesthetized and perfused with 4% PFA in PBS pH 7.4. Brains were post-fixed with 4% PFA in PBS for 1–2 days then cryoprotected in 25% sucrose for 24 h, embedded in OCT compound, and 10 μm sections cut on a cryostat. In situ hybridization was performed on every tenth serial section encompassing the entire hypothalamus from four brains each for wild-type, FBH and NBH with TrkB- and BDNF-specific probes of 420 and 370 bp, respectively, and labeled with [35S]UTP as per previously described protocols (Tessarollo and Parada, 1995). CART probe was generated from a single cell PCR-generated E13.5 mouse DRG cDNA library and corresponds to ∼270 bp at the C-terminal end of CART cDNA. The LR probe specific to the Ob-Rb isoform was RT–PCR generated from hypothalamic RNA as previously described (Fei et al., 1997). NPY antibody was purchased from Biogenesis and used as per the manufacturer's protocol.
Activity
Six-month-old wild-type and BDNF mutant mice were monitored for total activity over a period of 4 h during the active cycle using an Opto-Varimex Mini activity monitor (Columbus Instruments). Activity monitors consisted of 15 photoelectric beams spaced 3 cm apart that encompass the length of the cage. Activity was measured as the total number of beam disruptions during the 4 h monitoring period. Each animal was monitored on two consecutive nights and the average value used for analysis. Statistical significance was determined using a Student–Newman–Keuls test. Nine wild-type and 10 each of the FBH and NBH animals were studied.
Neurotrophin infusions
Obese BDNF mutant and wild-type control mice were isolated at least 24 h prior to pump implantation. Osmotic pumps (Alza) designed to deliver 0.25 μl/h for 14 days were each filled with 100 μl of sterile PBS or human recombinant neurotrophin (BDNF and NT5 from Regeneron, NGF from Sigma) in PBS at a concentration of 200 ng/μl. The pumps were then attached to an Alzet brain infusion kit and the entire apparatus was primed overnight in sterile saline at 37°C. Mice were anesthetized with ketamine:xylazine and placed in a stereotactic apparatus. The tip of the infusion catheter was positioned stereotactically in the third ventricle –1.8 mm relative to Bregma in the midline and 5 mm deep. The cannula was secured with Loctisite 454 (Loctite) and the pump was placed subcutaneously on the animal's dorsal side. Weights and food intake were measured as described previously every other day for the subsequent 4 weeks. Animals were excluded from analysis if they either died or appeared systemically ill after <3 days post-pump implantation. Systemic illness was determined if animals showed at least two of the following: became ataxic, showed poor grooming manifested by hair sticking out on end, or became lethargic, which was defined as activity of <1000 counts during the 4 h monitoring period. No bias was observed between control and treatment groups. Upon completion of the monitoring period, 1% methylene blue was injected to confirm placement of the cannula tip into the third ventricle, and the pumps were aspirated to ensure that all vehicle/neurotrophin had been depleted. Statistical analyses were performed using a Student–Newman–Keuls test.
Acknowledgments
Acknowledgements
We thank Sadeq Kharzai for technical assistance in histology. We are grateful to Evelyn Babcock for assistance with MRI imaging (supported by NIH NCCR Biomedical Research Technology Program P41-RR02584). We thank Ward Wakeland for help with statistical analysis, and Joe Goldstein, Masashi Yanagizawa and Roger Unger for critical reading of the manuscript. This work was supported by NINDS R01 NS33199-05 (L.F.P.) and by the Excellence in Education Support for Developmental Biology (L.F.P.).
References
- Ahmed S., Reynolds, B.A. and Weiss, S. (1995) BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J. Neurosci., 15, 5765–5778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barde Y.A. (1994) Neurotrophins: a family of proteins supporting the survival of neurons. Prog. Clin. Biol. Res., 390, 45–56. [PubMed] [Google Scholar]
- Bonhoeffer T. (1996) Neurotrophins and activity-dependent development of the neocortex. Curr. Opin. Neurobiol., 6, 119–126. [DOI] [PubMed] [Google Scholar]
- Caro J.F., Sinha, M.K., Kolaczynski, J.W., Zhang, P.L. and Considine, R.V. (1996) Leptin: the tale of an obesity gene. Diabetes, 45, 1455–1462. [DOI] [PubMed] [Google Scholar]
- Castren E., Thoenen, H. and Lindholm, D. (1995) Brain-derived neurotrophic factor messenger RNA is expressed in the septum, hypothalamus and in adrenergic brain stem nuclei of adult rat brain and is increased by osmotic stimulation in the paraventricular nucleus. Neuroscience, 64, 71–80. [DOI] [PubMed] [Google Scholar]
- Causing C.G., Gloster, A., Aloyz, R., Bamji, S.X., Chang, E., Fawcett, J., Kuchel, G. and Miller, F.D. (1997) Synaptic innervation density is regulated by neuron-derived BDNF. Neuron, 18, 257–267. [DOI] [PubMed] [Google Scholar]
- Cellerino A., Pinzon-Duarte, G., Carroll, P. and Kohler, K. (1998) Brain-derived neurotrophic factor modulates the development of the dopaminergic network in the rodent retina. J. Neurosci., 18, 3351–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Kolbeck, R., Barde, Y.A., Bonhoeffer, T. and Kossel, A. (1999) Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J. Neurosci., 19, 7983–7990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., et al. (1996)Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell, 84, 491–495. [DOI] [PubMed] [Google Scholar]
- Chua S.C. Jr, Chung, W.K., Wu-Peng, X.S., Zhang, Y., Liu, S.M., Tartaglia, L. and Leibel, R.L. (1996) Phenotypes of mouse diabetes and rat fatty due to mutations in the OB (leptin) receptor. Science, 271, 994–996. [DOI] [PubMed] [Google Scholar]
- Cohen-Cory S. and Fraser, S.E. (1995) Effects of brain-derived neurotrophic factor on optic axon branching and remodelling in vivo.Nature, 378, 192–196. [DOI] [PubMed] [Google Scholar]
- Coleman D.L. (1978) obese and diabetes: two mutant genes causing diabetes–obesity syndromes in mice. Diabetologia, 14, 141–148. [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Maratos-Flier, E., Saper, C.B. and Flier, J.S. (1998) Unraveling the central nervous system pathways underlying responses to leptin. Nature Neurosci., 1, 445–450. [DOI] [PubMed] [Google Scholar]
- Elmquist J.K., Elias, C.F. and Saper, C.B. (1999) From lesions to leptin: hypothalamic control of food intake and body weight. Neuron, 22, 221–232. [DOI] [PubMed] [Google Scholar]
- Erickson J.C., Hollopeter, G. and Palmiter, R.D. (1996) Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science, 274, 1704–1707. [DOI] [PubMed] [Google Scholar]
- Ernfors P., Lee, K.F. and Jaenisch, R. (1994) Mice lacking brain-derived neurotrophic factor develop with sensory deficits. Nature, 368, 147–150. [DOI] [PubMed] [Google Scholar]
- Fan W., Boston, B.A., Kesterson, R.A., Hruby, V.J. and Cone, R.D. (1997) Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature, 385, 165–168. [DOI] [PubMed] [Google Scholar]
- Fei H., Okano, H.J., Li, C., Lee, G.H., Zhao, C., Darnell, R. and Friedman, J.M. (1997) Anatomic localization of alternatively spliced leptin receptors (Ob-R) in mouse brain and other tissues. Proc. Natl Acad. Sci. USA, 94, 7001–7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J.M. (1997) The alphabet of weight control. Nature, 385, 119–120. [DOI] [PubMed] [Google Scholar]
- Hetherington A.W. and Ranson, S.W. (1942) The spontaneous activity and food intake of rats with hypothalamic lesions. Am. J. Physiol., 136, 609–617. [Google Scholar]
- Huszar D., et al. (1997)Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell, 88, 131–141. [DOI] [PubMed] [Google Scholar]
- Jones K.R., Farinas, I., Backus, C. and Reichardt, L.F. (1994) Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell, 76, 989–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungbluth S., Koentges, G. and Lumsden, A. (1997) Coordination of early neural tube development by BDNF/trkB. Development, 124, 1877–1885. [DOI] [PubMed] [Google Scholar]
- Kang H., Welcher, A.A., Shelton, D. and Schuman, E.M. (1997) Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron, 19, 653–664. [DOI] [PubMed] [Google Scholar]
- Kaplan D.R., Hempstead, B.L., Martin-Zanca, D., Chao, M.V. and Parada, L.F. (1991a) The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science, 252, 554–558. [DOI] [PubMed] [Google Scholar]
- Kaplan D.R., Martin-Zanca, D. and Parada, L.F. (1991b) Tyrosine phosphorylation and tyrosine kinase activity of the trk proto-oncogene product induced by NGF. Nature, 350, 158–160. [DOI] [PubMed] [Google Scholar]
- Klein R., Jing, S.Q., Nanduri, V., O'Rourke, E. and Barbacid, M. (1991a) The trk proto-oncogene encodes a receptor for nerve growth factor. Cell, 65, 189–197. [DOI] [PubMed] [Google Scholar]
- Klein R., et al. (1991b)The trkB tyrosine protein kinase is a receptor for brain-derived neurotrophic factor and neurotrophin-3. Cell, 66, 395–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M. and Bonhoeffer, T. (1997) Activity-dependent synaptic plasticity: a new face of action for neurotrophins. Mol. Psychiatry, 2, 197–199. [DOI] [PubMed] [Google Scholar]
- Korte M., Carroll, P., Wolf, E., Brem, G., Thoenen, H. and Bonhoeffer, T. (1995) Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl Acad. Sci. USA, 92, 8856–8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte M., Staiger, V., Griesbeck, O., Thoenen, H. and Bonhoeffer, T. (1996) The involvement of brain-derived neurotrophic factor in hippocampal long-term potentiation revealed by gene targeting experiments. J. Physiol. Paris, 90, 157–164. [DOI] [PubMed] [Google Scholar]
- Korte M., Kang, H., Bonhoeffer, T. and Schuman, E. (1998) A role for BDNF in the late-phase of hippocampal long-term potentiation. Neuropharmacology, 37, 553–559. [DOI] [PubMed] [Google Scholar]
- Kristensen P., et al. (1998)Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature, 393, 72–76. [DOI] [PubMed] [Google Scholar]
- Lapchak P.A. and Hefti, F. (1992) BDNF and NGF treatment in lesioned rats: effects on cholinergic function and weight gain. Neuroreport, 3, 405–408. [DOI] [PubMed] [Google Scholar]
- Lee G.H., Proenca, R., Montez, J.M., Carroll, K.M., Darvishzadeh, J.G., Lee, J.I. and Friedman, J.M. (1996) Abnormal splicing of the leptin receptor in diabetic mice. Nature, 379, 632–635. [DOI] [PubMed] [Google Scholar]
- Levine E.S., Black, I.B. and Plummer, M.R. (1998) Neurotrophin modulation of hippocampal synaptic transmission. Adv. Pharmacol., 42, 921–924. [DOI] [PubMed] [Google Scholar]
- Liebl D.J., Tessarollo, L., Palko, M.E. and Parada, L.F. (1997) Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3- and TrkC-deficient embryonic mice. J. Neurosci., 17, 9113–9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebl D.J., Kleese,L.J., Tessarollo,L., Woldham,T. and Parada,L.F. (2000) Loss of BDNF-dependent neural crest derived sensory neurons in NT-4 mutant mice. Proc. Natl Acad. Sci. USA, 97, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnarsson S., Bjorklund, A. and Ernfors, P. (1997) Learning deficit in BDNF mutant mice. Eur. J. Neurosci., 9, 2581–2587. [DOI] [PubMed] [Google Scholar]
- Lu D., et al. (1994) Agouti protein is an antagonist of the melanocyte-stimulating-hormone receptor. Nature, 371, 799–802. [DOI] [PubMed] [Google Scholar]
- Malenka R.C. and Nicoll, R.A. (1999) Long-term potentiation—a decade of progress? Science, 285, 1870–1874. [DOI] [PubMed] [Google Scholar]
- Marlin S., Couet, D., Lacombe, D., Cessans, C. and Bonneau, D. (1994) Obesity: a new feature of WAGR (del 11p). Clin. Dysmorphol., 3, 255–257. [PubMed] [Google Scholar]
- Marty S., Berninger, B., Carroll, P. and Thoenen, H. (1996) GABAergic stimulation regulates the phenotype of hippocampal interneurons through the regulation of brain-derived neurotrophic factor. Neuron, 16, 565–570. [DOI] [PubMed] [Google Scholar]
- McAllister A.K., Katz, L.C. and Lo, D.C. (1997) Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron, 18, 767–778. [DOI] [PubMed] [Google Scholar]
- McGaughran J., Ward, H. and Evans, D. (1995) WAGR syndrome and multiple exostoses in a patient with del (11) (p11.2p14.2). J. Med. Genet., 32, 823–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morutto S.L. and Phillips, G.D. (1997) Cross-sensitisation with d-amphetamine following repeated intra-perifornical sulpiride infusions. Psychopharmacology, 133, 179–187. [DOI] [PubMed] [Google Scholar]
- Nawa H., Carnahan, J. and Gall, C. (1995) BDNF protein measured by a novel enzyme immunoassay in normal brain and after seizure: partial disagreement with mRNA levels. Eur. J. Neurosci., 7, 1527–1535. [DOI] [PubMed] [Google Scholar]
- Parada L.F., Tsoulfas, P., Tessarollo, L., Blair, J., Reid, S.W. and Soppet, D. (1992) The Trk family of tyrosine kinases: receptors for NGF-related neurotrophins. Cold Spring Harbor Symp. Quant. Biol., 57, 43–51. [DOI] [PubMed] [Google Scholar]
- Patterson S.L., Abel, T., Deuel, T.A., Martin, K.C., Rose, J.C. and Kandel, E.R. (1996) Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron, 16, 1137–1145. [DOI] [PubMed] [Google Scholar]
- Pelleymounter M.A., Cullen, M.J. and Wellman, C.L. (1995) Characteristics of BDNF-induced weight loss. Exp. Neurol., 131, 229–238. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M., Leibel, R.L. and Hirsch, J. (1997) Obesity. N. Engl. J. Med., 337, 396–407. [DOI] [PubMed] [Google Scholar]
- Sakurai T., et al. (1998)Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92, 573–585. [DOI] [PubMed] [Google Scholar]
- Smith M.A., Makino, S., Kim, S.Y. and Kvetnansky, R. (1995) Stress increases brain-derived neurotropic factor messenger ribonucleic acid in the hypothalamus and pituitary. Endocrinology, 136, 3743–3750. [DOI] [PubMed] [Google Scholar]
- Snider W.D. (1994) Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell, 77, 627–638. [DOI] [PubMed] [Google Scholar]
- Snider W.D. (1998) How do you feel? Neurotrophins and mechanotransduction. Nature Neurosci., 1, 5–6. [DOI] [PubMed] [Google Scholar]
- Soppet D., et al. (1991)The neurotrophic factors brain-derived neurotrophic factor and neurotrophin-3 are ligands for the trkB tyrosine kinase receptor. Cell, 65, 895–903. [DOI] [PubMed] [Google Scholar]
- Spiegelman B.M. and Flier, J.S. (1996) Adipogenesis and obesity: rounding out the big picture. Cell, 87, 377–389. [DOI] [PubMed] [Google Scholar]
- Stein D.T., Babcock, E.E., Malloy, C.R. and McGarry, J.D. (1995) Use of proton spectroscopy for detection of homozygous fatty ZDF-drt rats before weaning. Int. J. Obes. Relat. Metab. Disord., 19, 804–810. [PubMed] [Google Scholar]
- Tessarollo L. and Parada,L.F. (1995) In situ hybridization. Methods Enzymol., 254, 419–430. [DOI] [PubMed] [Google Scholar]
- Tessarollo L., Tsoulfas, P., Donovan, M.J., Palko, M.E., Blair-Flynn, J., Hempstead, B.L. and Parada, L.F. (1997) Targeted deletion of all isoforms of the trkC gene suggests the use of alternate receptors by its ligand neurotrophin-3 in neuronal development and implicates trkC in normal cardiogenesis. Proc. Natl Acad. Sci. USA, 94, 14776–14781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejon C., Johe, K.K., Hazel, T.G., Collazo, D. and McKay, R.D. (1995) Functions of basic fibroblast growth factor and neurotrophins in the differentiation of hippocampal neurons. Neuron, 15, 105–114. [DOI] [PubMed] [Google Scholar]
- Williams L.R. (1991) Hypophagia is induced by intracerebroventricular administration of nerve growth factor. Exp. Neurol., 113, 31–37. [DOI] [PubMed] [Google Scholar]