Abstract
Introduction
Experiments were designed to investigate the effects of ethyl pyruvate (EP) in a murine model of hind-limb ischemia-reperfusion (IR) injury.
Methods
C57BL6 mice underwent 90 minutes of unilateral ischemia followed by 24 hours of reperfusion using two treatment protocols. For the preischemic treatment (pre-I) protocol, mice (n = 6) were given 300 mg/kg EP before ischemia, followed by 150 mg/kg of EP just before reperfusion and at 6 hours and 12 hours after reperfusion. In a postischemic treatment (post-I) protocol, mice (n = 7) were treated with 300 mg/kg EP at the end of the ischemic period, then 15 minutes later, and 2 hours after reperfusion and 150 mg/kg of EP at 4 hours, 6 hours, 10 hours, 16 hours, and 22 hours after reperfusion. Controls mice for both protocols were treated with lactated Ringers alone at time intervals identical to EP. Skeletal muscle levels of adenosine triphosphate (ATP), interleukin-1β, keratinocyte chemoattractant protein, and thrombin antithrombin-3 complex were measured. Skeletal muscle architectural integrity was assessed microscopically.
Results
ATP levels were higher in mice treated with EP compared with controls under the both treatment protocols (p = 0.02). Interleukin-1β, keratinocyte chemoattractant protein, thrombin antithrombin-3 complex (p < 0.05), and the percentage of injured fibers (p < 0.0001) were significantly decreased in treated versus control mice under the both protocols.
Conclusion
Muscle fiber injury and markers of tissue thrombosis and inflammation were reduced, and ATP was preserved with EP in pre-I and post-I protocols. Further investigation of the efficacy of EP to modulate IR injury in a larger animal model of IR injury is warranted.
Keywords: Ischemia-reperfusion, Inflammation, Skeletal muscle, cytokines
Acute ischemia of the lower extremities, which affects > 200,000 patients annually in the United States, is a devastating event that is associated with a mortality of 25% and a major amputation rate of 20% in survivors.1 Local and systemic consequences of ischemia-reperfusion (IR) injury in skeletal muscle are similar to conditions observed in burns, sepsis, and other organ systems subjected to IR. The sequence of events that lead to widespread injury during reperfusion can be traced back to events that occur during the ischemic period. During ischemia, adenosine triphosphate (ATP) that is needed to maintain essential cellular functions and normally produced by the oxidation of free fatty acids, the breakdown of glycogen, and using creatinine phosphate is exhausted.2 The ensuing skeletal muscle necrosis is a consequence and directly correlates with the extent of ATP depletion.3 With the onset of reperfusion, the production of toxic free radicals (O2−, OH, and H2O2), also called reactive oxygen species (ROS), is also greatly enhanced. ROS are directly injurious to DNA, protein, and membranes. Furthermore, ROS are directly implicated in the activation of a leukocyte-mediated inflammatory reaction that exacerbates tissue injury during the reperfusion. Through the activation of transcription factors such as nuclear factor (NF)-κ B, ROS can direct the expression of molecules involved in the recruitment of more neutrophils to the reperfused area.4 NF-κB, in turn, controls the gene expression of proinflammatory cytokines, chemokines, and enzymes that produce secondary inflammatory mediators and cell adhesion molecules.5 Ethyl pyruvate (EP) ameliorates injury in various inflammatory conditions, including burns, shock, sepsis, necrotizing pancreatitis, and multiple models of IR injury, such as those involving the gut, heart, brain, and liver.6 EP is a more lipophilic derivative of the metabolic intermediate pyruvate. In earlier studies, pyruvate was recognized to be an antioxidant, a metabolic rescue agent, and a free radical scavenger.7 The current experiments were designed to exploit the pleiotropic characteristics of EP to treat the complex multifaceted components of skeletal muscle IR with specific evaluation of the metabolic, anti-inflammatory, antithrombotic, and structural effects of EP administration in a murine model of unilateral hind-limb IR injury. Our goal was to test EP first in a prehoc treatment (pre-I) protocol as a proof of concept method to justify further investigation and also because of its relevance in elective surgical settings. This initial pre-I approach involved assessment of the ability of EP to modulate NF-κB activation and lactate production when administered before the onset of IR. We hypothesized that if EP treatment is beneficial in the pre-I scenario, then it should function effectively in a more clinically relevant post hoc scenario.
MATERIALS AND METHODS
Animal Protocol
All experimental procedures were approved by the Massachusetts General Hospital’s institutional review board and was in accordance with Principles of Laboratory Animal Care. C57BL6 mice (Jackson Laboratory, Bar Harbor, ME) were anesthetized using intraperitoneal administration of 50 mg/kg of pentobarbital.
Induction of Ischemia
Reproducible levels of ischemia were obtained with the use of a 4.5-ounze orthodontic rubber bands (ORBs; American Orthodontics, Sheboygan, WI). Briefly, after anesthesia, the mice were placed on a preheated table to maintain the mouse’s monitored temperature at 37°C. The mouse limbs were lifted, blood was allowed to drain for 30 seconds, and the ORB was delivered to the proximal limb for 90 minutes using a standard McGivney Hemorrhoidal Ligator (Militex Corporation, Bethpage, NY). Ischemia was confirmed using laser Doppler imaging (Moor Instruments, Wilmington, DE) as previously described.8 Reperfusion was initiated by cutting the rubber band from the mouse limb.
Pre-I Protocol
Treated animals (EP, n = 6) received EP (Sigma-Aldrich, St Louis, MO), 300 mg/kg, in 0.4 mL of lactated Ringers (LR) solution intraperitoneally 15 minutes before ischemia. This was followed by 150 mg/kg of EP just before and at 6 hours and 12 hours after reperfusion. Untreated pre-I mice (LR, n = 6) were given 0.4 mL of LR intraperitoneally using the same dosing schedule as mice receiving EP. Fifteen minutes after the induction of anesthesia, the ORBs were used to induce unilateral hind-limb ischemia for 90 minutes followed by 24 hours of reperfusion. Mice (EP, n = 6, and LR, n = 6) remained anesthetized throughout the duration of ischemia and the initial early reperfusion (1-hour) interval. Mice studied for 24-hour reperfusion were allowed to recover from anesthesia after the initial 1 hour of reperfusion and were returned to their cages. After 24 hours of reperfusion, the animals were killed (isoflurane). IR and contralateral limbs were then harvested and the posterior hind-limb calf muscle was isolated and immediately frozen in liquid nitrogen for biochemical analysis.
Postischemic Treatment Protocol
Treated animals (EP, n = 7) received EP, 300 mg/kg, in 0.4 mL of LR intraperitoneally 15 minutes before, 15 minutes after, and 2 hours into the reperfusion period. Additional doses of 150 mg/kg of EP were given at 4 hours, 6 hours, 10 hours, 16 hours, and 22 hours reperfusion. Untreated post-ischemic treatment (post-I) protocol mice (LR, n = 7) were given 0.4 mL of LR intraperitoneally using the same dosing schedule as mice receiving EP. Limb harvesting was done the same way as for the pre-I protocol.
Short Reperfusion Experiments
These experiments were designed to assess the activation of NF-κβ after acute hind-limb IR. For these experiments, treated animals (EP, n = 5) received EP, 300 mg/kg, in 0.4 mL of LR intraperitoneally 15 minutes before ischemia. This was followed by 300 mg/kg of EP just before and 15 minutes into the reperfusion period. Untreated mice (LR, n = 5) were given 0.4 mL of LR intraperitoneally using the same dosing schedule. To provide a negative control group for the short reperfusion experiments, mice (n = 6) were anesthetized for 90 minutes without ischemia and allowed to recover for 45 minutes (sham reperfusion). These negative control mice received 0.4 mL of LR intraperitoneally using the same dosing schedule as untreated mice. All mice were killed at 45 minutes into the reperfusion or sham reperfusion period. Nuclear extracts were immediately prepared from the muscle tissue.
Determination of NF-κB Activation
Skeletal muscle NF-κB activation was assessed only in mice subjected to the short reperfusion period (described above). Total nuclear protein extract was prepared using a nuclear extraction kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. In brief, 200 mg of fresh muscle was removed from the posterior calf and rinsed briefly in ice-cold phosphate-buffered saline (PBS), then homogenized immediately in hypotonic buffer supplemented with 1 μL detergent mix and 1 μL/mL 1 mol/L dithiothreitol. The homogenate was placed on ice for 30 minutes and then centrifuged for 10 minutes at 850g at 4°C. After resuspending the pellet in hypotonic buffer, 50 μL/mL detergent was added to each milliliter total volume, then mixed and centrifuged for 30 seconds at 14,000g. The supernatant was decanted, and the pellet was resuspended in complete lysis buffer. Samples were rocked on ice for 30 minutes and cleared by centrifugation at 14,000g for 10 minutes at 4°C. Supernatants were stored in aliquots at −80°C until analysis. Protein concentrations in the nuclear extracts were determined using a bicin-choninic acid assay with bovine serum albumin as standard (Pierce Biotechnology, Rockford, IL). To estimate NF-κB activation in tissues, we used a Trans-AM NF-κB p65 Transcription Factor Assay Kit (Active Motif). A 96-well plate was loaded with 20 μg of total nuclear protein. Wells have been coated with oligonucleotide containing the NF-κB consensus sequence (5′-GGGACTTTCC-3′). The active form of p65 NF-κB in the nuclear extract specifically binds to this consensus site and is recognized by a primary antibody. A horseradish peroxidase-conjugated IgG antibody and chromagen substrate are used to detect the absorbance at 450 nm (A450) using spectrophotometric plate reader (Molecular Devices, Sunnyvale, CA). Results are represented as mean ± standard error of the mean.
L-Lactate
The L-lactate levels were measured in mice receiving only the pre-I protocol. Briefly, 200 mg skeletal muscle tissues were homogenized using a polytron tissue disruptor (Kinematica, Bohemia, NY) in 10% trichloroacetic acid. Samples were centrifuged at 10,000g for 10 minutes at 4°C. Diluted aliquots from each sample were added to a reagent mix containing a final concentration of 453 mmol/L glycine buffer (pH = 9), 362 mmol/L hydrazine, 2.47 mmol/L β-nicotine adenine dinucleotide (NAD), and 5 units/mL type III L-lactic dehydrogenase from bovine heart (Sigma-Aldrich). A serial dilution of L-(+)-lactic acid was used to generate a standard curve. Samples and standard were incubated for 60 minutes at room temperature in the dark. Aliquots (200 μL per reaction) were removed into a quartz microplate in duplicate and read at 340 nm wavelength using a microplate reader (Molecular Devices). The concentrations of the unknown samples were extrapolated from the standard curve. Data were expressed as micrograms per milligram of tissue weight.
Skeletal Muscle ATP Content
Two hundred milligrams of frozen skeletal muscle samples from the injured and noninjured contralateral hind-limbs were homogenized on ice in 10% trichloroacetic acid using a polytron tissue disruptor (Kinematica). Samples were cleared by centrifuging for 10 minutes 10,000g at 4°C. ATP levels were measured after dilution in Dulbecco’s-modified PBS (with Ca++ and Mg++, pH = 7.4) using ATPlite luminescence assay according to the manufacturer’s protocol (Perkin Elmer Life, Boston, MA). Top counts were read in 1450 Micro beta plate reader (PerkinElmer Life). Concentrations of the unknowns were extrapolated from purified ATP standards and expressed as micromoles per milligram of tissue weight. ATP from six replicate assays per group was analyzed statistically.
Tissue Cytokines and Myeloperoxidase Levels
Hind-limb posterior calf skeletal muscle samples only from ischemia reperfused limbs were snap frozen in liquid nitrogen immediately after harvest and stored at −80°C until analysis. Two hundred milligrams of muscle samples was homogenized with a polytron homogenizer (Kinematica) in a test tube containing radioimmunoprecipitation assay buffer supplemented with protease inhibitor cocktail (Sigma P-8340) and placed on ice for 30 minutes. The homogenized sample was transferred to a microcentrifuge tube, and the supernatant was cleared at 10,000g for 10 minutes and frozen in aliquots at −80°C until analysis. The levels of proinflammatory skeletal muscle cytokines keratinocyte chemoattractant (KC) protein and inter-leukin (IL)-1β were measured using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN). The tissue levels of myeloperoxidase (MPO), a surrogate for inflammatory cell infiltration, were also measured with ELISA (Cell Sciences, Canton, MA). Thrombin/antithrombin-III complex (TAT-III), a marker for activation of thrombin and local tissue thrombosis, was also measured with an ELISA (DAD Behring, Newark, DE). The ELISA plates were read with a Spectromax-250 plate reader (Molecular Devices). Values were extrapolated off the standard curve and normalized to the total protein concentration, which was determined with the BCA Protein Assay Reagent Kit (Pierce Biotechnology). Muscle from ischemia reperfused limbs of LR- and EP-treated mice with pre-I and post-I protocols mice were compared.
Histologic Assessment
EP-treated (n = 5) and LR-treated (n = 5) mice were prepared using both the pre-I and post-I protocols in the same manner as described above. At the end of reperfusion, mice were killed using isoflurane, and the skin was removed from the lower torso. Hind-limbs were harvested and fixed in 4% paraformaldehyde overnight. Gastrocnemius muscles were isolated and rinsed in PBS for 1 hour followed by serial dehydration in graded acetone. Trimmed muscles were embedded under vacuum conditions using a glycol methacrylate monomer based embedding kit (JB-4; Polysciences, Warrington, PA) according to the manufacturer’s protocol. Hardened molds were mounted on a motorized microtome (Leica, Nussloch, Germany). Two-micrometer thin sections were obtained using glass knifes. Tissue sections were stained with Masson’s trichrome stain and observed under 200× power light microscope; 16× images were acquired from each muscle section and assigned a serial number using SPOT Insight microscope camera (Diagnostic Instruments, Sterling Heights, MI). A random number generator was used to obtain a unique set of field numbers. Images were retrieved with Adobe Photoshop software (Adobe, San Jose, CA). A minimum of 600 total fibers were counted per tissue section by a blinded observer, and the numbers of injured and uninjured fibers were sorted. Summary data were expressed as percent of all fibers showing signs of morphologic injury.9
Statistical Analyses
Statistical analyses was performed with Instat (Graph-Pad, San Diego, CA). Skeletal muscle tissue cytokine and MPO levels, and histologic assessment in treated and untreated mice after 24 hours of reperfusion were compared with each other using unpaired t tests. Analysis of variance was used to analyze NF-κB experiments, and posttests were performed using Mann Whitney analysis.
RESULTS
Short Reperfusion Using the Pre-I Protocol
NF-κβ Activation
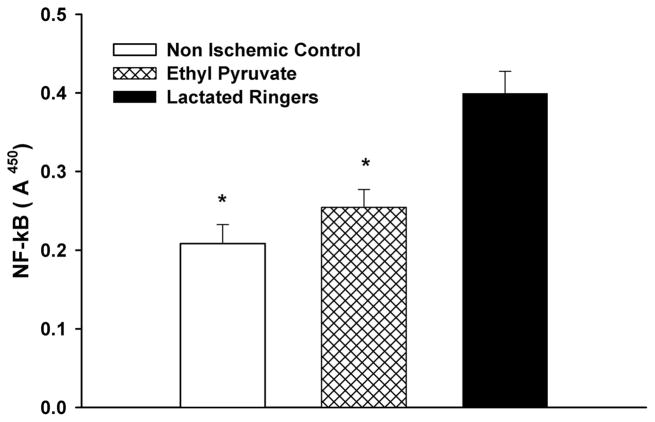
Levels of NF-κB in the skeletal muscle of EP-treated, LR-treated mice after 90 minutes of ischemia followed by 45 minutes of reperfusion, and nonischemic controls are shown in Figure 1. NF-κB activation was significantly higher in LR-treated limbs when compared with both EP-treated and nonischemic control samples (nonischemic control A450, 0.21 ± 0.024; EP A450, 0.25 ± 0.023; LR A450, 0.40 ± 0.028; p < 0.01). There was no significant difference between the nonischemic control and EP-treated samples.
Figure 1.
NF-κβ expression following IR: EP treatment before ischemia significantly reduced NF-κβ expression in IR muscle compared with LR-treated mice (*p < 0.01) but not different from nonischemic control mice (anesthesia alone, no hind-limb ischemia).
Tissue Lactate Levels
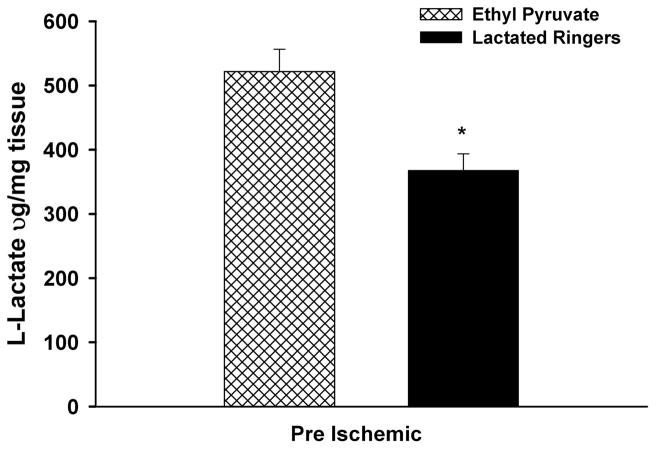
The levels of L-lactate in skeletal muscle of mice receiving the pre-I protocol after 90 minutes of ischemia and 24 hours of reperfusion are shown in Figure 2. The L-lactate levels were significantly increased in the limbs of EP- versus LR-treated mice (EP, 521.7 μ g/mg protein ± 34.9 μ g/mg protein versus LR, 367.9 μ g/mg protein ± 25.8 μg/mg protein; p = 0.005).
Figure 2.
Skeletal muscle levels of L-lactate: after 90 minutes of unilateral ischemia and 24 hours of reperfusion in EP significantly increased muscle lactate levels compared with LR-treated controls (p = 0.005).
Pre-I Versus Post-I Treatment Protocol
Skeletal Muscle ATP Content
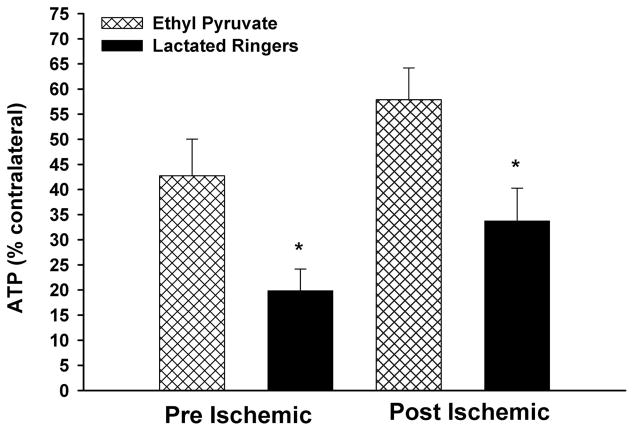
The percent contralateral muscle ATP levels after 90 minutes of unilateral ischemia followed by 24 hours of reperfusion for the pre-I and post-I protocol groups are displayed in Figure 3. Higher levels of ATP were detected in the limbs of the pre-I-treated animals (EP, 42.7% ± 7.3% vs. LR, 19.9% ± 4.3%; p = 0.02) and the post-I-treated mice (EP, 57.9% ± 6.3% vs. LR, 33.8% ± 6.5%; p = 0.02).
Figure 3.
Skeletal muscle ATP levels: after 90 minutes of unilateral ischemia followed by 24 hours of reperfusion, EP treatment with both the pre-I and post-I protocols effectively preserved skeletal muscle ATP levels (*p = 0.02 vs. LR-treated mice).
Interleukin-1β
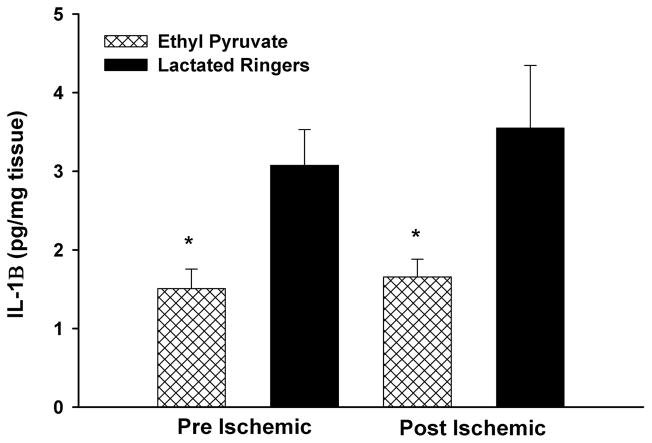
IL-1β levels were decreased in limbs of EP- versus LR-treated mice subjected to the pre-I (EP, 1.51 pg/mg protein ± 0.25 pg/mg protein vs. LR, 3.1 pg/mg protein ± 4.6 pg/mg protein; p = 0.01) and the post-I (EP, 1.65 pg/mg protein ± 0.23 pg/mg protein vs. LR, 3.55 pg/mg protein ± 0.79 pg/mg protein; p = 0.004) protocols (Fig. 4).
Figure 4.
Skeletal muscle IL-1β levels. EP treatment in the pre-I and post-I protocols significantly reduced IL-1β levels compared with LR-treated mice (p < 0.001).
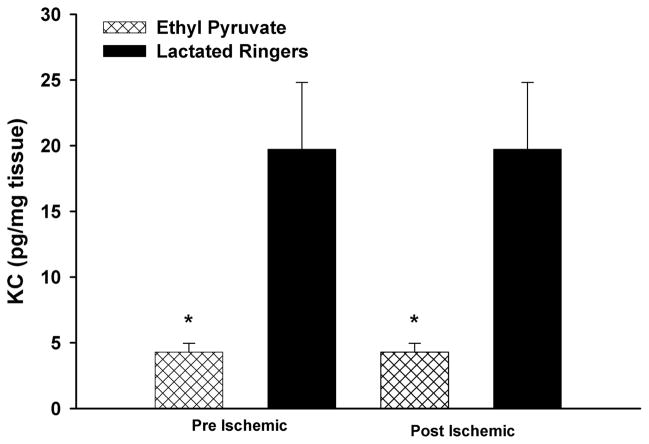
KC Protein
KC levels were decreased in limbs of EP- versus LR-treated mice subjected to the pre-I (EP, 6.7 pg/mg protein ± 1.6 pg/mg protein vs. 14.3 pg/mg protein ± 2.3 pg/mg protein; p = 0.02) and the post-I (4.28 pg/mg protein ± 0.68 pg/mg protein vs. 19.74 pg/mg protein ± 5.07 pg/mg protein, respectively; p = 0.002) protocols (Fig. 5).
Figure 5.
Skeletal muscle KC levels: after 90 minutes of ischemia and 24 hours of reperfusion, KC levels were reduced after EP treatment using the pre-I and post-I protocols (p = 0.02) of KC.
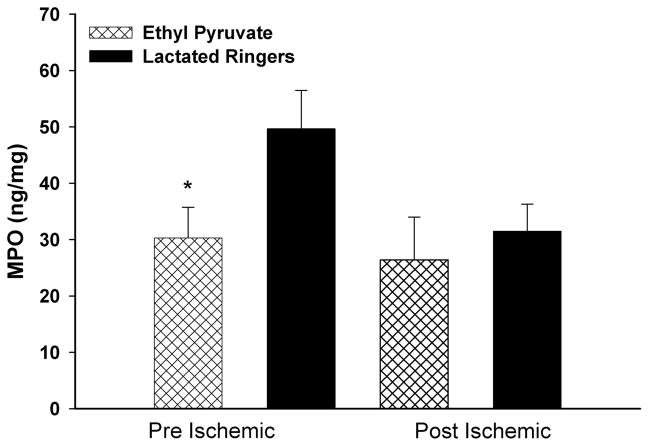
Myeloperoxidase
MPO levels were also decreased in EP- versus LR-treated animals subjected to pre-I (30.3 ng/mg protein ± 5.4 ng/mg protein vs. 49.7 ng/mg protein ± 6.8 ng/mg protein, respectively; p = 0.026) but not the post-I (26.4 ng/mg protein ± 7.6 ng/mg protein vs. 31.5 ng/mg protein ± 4.8 ng/mg protein, respectively; p = 0.58) protocols (Fig. 6).
Figure 6.
Skeletal muscle levels of MPO: EP treatment with the pre-I (p < 0.03) but not the post-I protocol reduced MPO levels by 24 hours reperfusion.
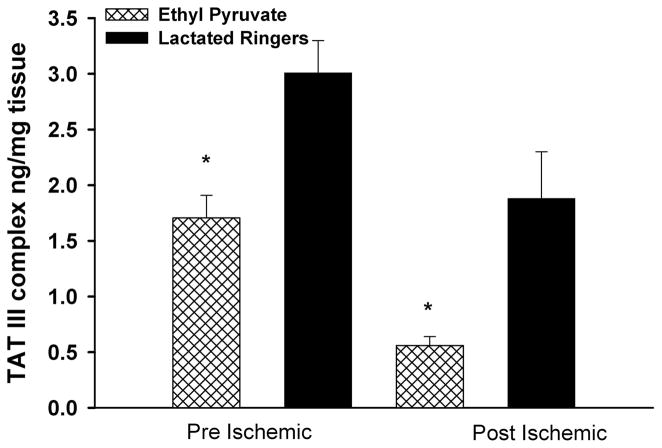
Markers of Thrombosis
TAT-III complexes were significantly decreased in EP-versus LR-treated hind-limbs subjected to the pre-I (1.71 ng/mg protein ± 0.20 ng/mg protein vs. 3.01 ng/mg protein ± 0.29 ng/mg protein, respectively; p = 0.02) and the post-I (0.56 ng/mg protein ± 0.08 ng/mg protein vs. 1.88 ng/mg protein ± 0.42 ng/mg protein, respectively; p = 0.003) protocols (Fig. 7).
Figure 7.
TAT-III complexes in reperfused skeletal muscle. Mice treated with EP in the pre-I and post-I protocols markedly (p < 0.008) reduced tissue levels of TAT III.
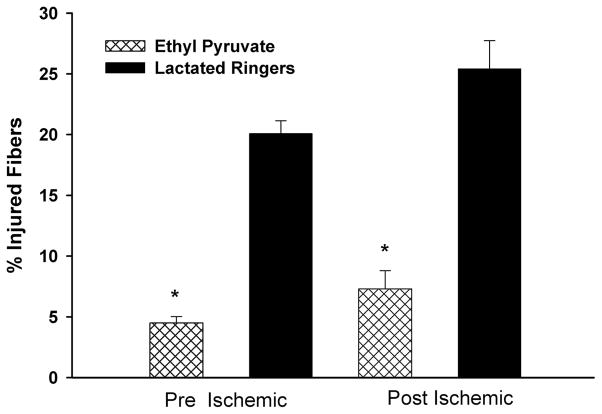
Histologic Assessment
The percent of total fibers that were injured were significantly lower in EP- versus LR-treated animals subjected to the pre-I (4.49% ± 0.54% vs. 20.1% ± 1.05%, respectively; p < 0.0001) and the post-I (7.31% ± 1.50% vs. 25.42% ± 2.31%, respectively; p < 0.0001) protocols (Fig. 8).
Figure 8.
Percentage of injured fibers in EP- versus LR-treated animals: EP treatment in the pre-I and post-I protocols resulted in significant reduction of injured fibers after IR (p < 0.0001).
DISCUSSION
In this model of acute hind-limb IR, EP preserved skeletal muscle energy levels, decreased fiber injury, and decreased the levels of inflammatory and prothrombotic markers. This protection was observed when EP was administered before and 15 minutes after the onset of ischemia. These results are of clinical importance because of two reasons: (1) morbidity and mortality associated with acute lower limb IR in humans remains high;10,11 and (2) EP was equally effective when administered in a clinically relevant post-I protocol. The experimental approach to provide proof of principle that EP might be protective involved administering this drug to mice before and after the onset of ischemia. This treatment strategy is helpful when screening for potentially useful drugs for the treatment of IR after limb ischemia12,13 and also in the setting of elective surgical procedures. Unfortunately, when limb ischemia affects humans, there is always a significant lag time between the onset of limb ischemia, the diagnosis of the problem, and the therapeutic intervention. Thus, a clinically relevant drug must protect tissue from injury even when administered in a postischemic scenario as well or better than when given before the onset of ischemia.
Because NF-κB is known to modulate the physiologic responses to IR in a variety of tissue models, initial experiments were performed to evaluate the ability of EP to decrease its activation after a brief period of reperfusion. EP administered using the pre-I protocol significantly decreased NF-κB activation (Fig. 1). These findings are consistent with literature that shows that EP inhibits the activation of NF-κB after injury.14
A consequence of IR is the depletion of NAD+, an important electron acceptor necessary for the glyceraldehyde-3-phosphate dehydrogenase reaction of glycolysis leading to ATP synthesis. Its been hypothesized that EP might protect tissue from injury by providing a source of pyruvate to cells for conversion to lactate and generation of NAD+ during periods of ischemia. NAD+ generated from pyruvate can then be used to make ATP via substrate level phosphorylation. Therefore, a series of experiments were performed to determine whether administration of EP to mice using the pre-I protocol would results in an increase in skeletal muscle lactate 24 hours after reperfusion. EP administered to mice using the pre-I protocol increased skeletal muscle lactate levels significantly by 24 hours (Fig. 2). The finding of increased muscle lactate levels associated with EP administration provided rationale to perform additional experiments to determine whether the increase in tissue lactate was coincident with increased levels of ATP, along with decreased fiber injury, cytokines, inflammatory, and prothrombotic markers. In these and all further experiments, the effect of the pre-I and post-I protocols on these variables were directly compared.
EP administered using the pre-I and post-I protocols increased skeletal muscle ATP levels after IR in a manner similar to the observed increase in lactate (Fig. 3). These findings are consistent with literature that showed that EP can preserve myocardial ATP levels after 10 minutes of ischemia.15 Although ATP levels did not return to preischemic baseline after treatment with EP, these results are consistent with previous studies with canine gracilis muscle where recovery of energy stores was incomplete even after 2 days of reperfusion.2 The observed failure to return to baseline pre-ischemic ATP levels might reflect a temporal delay in muscle recovery or ongoing oxidative stress. Although we have not directly measured tissue levels of NAD+, it is unlikely that significant levels of ATP could be maintained after IR without regeneration of intracellular NAD+.
Alternatively, EP might preserve ATP levels through pathways dependent on the enzyme pyruvate dehydrogenase. This enzyme is responsible for the shuttling of carbons to the tricarboxylic acid cycle through the conversion of pyruvate to acetyl-CoA.16 Pyruvate dehydrogenase becomes dysfunctional during IR,17 which can lead to limited substrate availability for the tricarboxylic acid cycle and, hence, decreased production of ATP. EP can directly provide increased substrate for the pyruvate dehydrogenase reaction and might help to overcome the dysfunction that results from IR.18 Providing substrate directly for the tricarboxylic acid cycle may be important, because one of the known consequences of IR is the inability of cells to import and break down the glucose necessary for energy generation in the cell. Precise understanding of how EP may modulate the energy metabolic pathway in skeletal muscle will require detailed analysis of mitochondrial and cytosolic components using techniques that use subcellular fractionation.
It is also possible that the higher levels of tissue ATP associated with EP administration may be a consequence of preserved tissue viability because of a decrease in the inflammatory and thrombotic components of IR injury. To test this hypothesis, skeletal muscle tissue levels of markers of inflammation and thrombosis were evaluated after administration of EP to skeletal muscle in the pre-I and post-I protocols. IL-1β is an early cytokine hat has been shown to be relevant in multiple models of IR.19 Levels of IL-1β increase during hypoxia and the early part of reperfusion and are implicated in the activation of other cytokines.20 Release of IL-1β was markedly reduced at 24 hours reperfusion after both EP administrations using the pre-I and post-I protocols (Fig. 4). Although EP has not been shown to directly inhibit IL-1β in other models of IR, the observations in this report are consistent with the effect of EP in models of in vitro21 or in vivo inflammation.22 Our experiments showed that EP treatment decreased the accumulation of KC in reperfused skeletal muscle (Fig. 5). KC is the murine analog of human IL-8, a CXC chemokine that is involved in neutrophil chemotaxis and recruitment to the area of injury.23 Antagonists of the CXC chemokines have been shown to attenuate IR injury in liver and spinal cord tissue.24 KC expression has been shown to be under the specific control of the transcription factor NF-κB;25 thus the decrease in tissue KC content after treatment with EP is consistent with our earlier findings of decreased NF-κB expression (Fig. 1).
Further analysis of the anti-inflammatory cascade showed that EP administered using the pre-I but not the post-I protocol significantly decreased the levels of MPO compared with LR-treated samples (Fig. 6). MPO has been used as a marker of neutrophil infiltration in various models of IR.26 MPO contributes to the enzymatic destruction of tissues and the generation of the potent oxidant, hypochlorous acid. It is not clear whether EP directly or indirectly inhibited the activation of neutrophils, and hence the release of MPO, or alternatively that EP’s action on the expression of cytokines in reperfused skeletal muscle resulted in decreased numbers of neutrophils entering the injured area. Failure of the post-I protocol to reduce MPO levels during reperfusion may be related to the variability of MPO levels in mice. It is also possible that EP administered during the post-I protocol altered MPO levels at an earlier or later time period than the 24-hour interval where these measurements were performed.
Thrombosis is a well-recognized consequence of IR.27,28 The “no-reflow” phenomenon, a consequence of capillary plugging by platelet aggregates, neutrophils, and thrombus, has been described extensively in tissue subjected to IR. This thrombotic component of IR effectively reduces tissue perfusion and exacerbates tissue injury during reperfusion.29 TAT-III levels have been detected in skeletal muscle subjected to IR injury30 and in in vitro models of vascular injury.31 EP decreased the levels of TAT-III complexes when compared with treatment with LR alone in both the pre-I and post-I protocols (Fig. 7). These findings are consistent with a report that EP has an antithrombotic effect by inhibiting tissue factor synthesis by stimulated human monocytes.32 This is a clinically relevant observation because many of the complications of reperfusion injury are related to thrombosis, and considerable resources are directed toward antithrombotic therapeutic interventions. EP might be an attractive alternative to replace or reduce the use of the antithrombotics (e.g., heparin) usually administered after emergency vascular interventions. These antithrombotic agents may cause bleeding complications and do not have the anti-inflammatory30 and metabolic rescue profile that EP has demonstrated in our model.
In concert with the biochemical analyses of skeletal muscle, the structural evaluation of this tissue revealed a marked reduction in fiber disruption in mice treated with EP using the pre-I and post-I protocols (Fig. 8). This finding of preserved skeletal muscle architecture is consistent with the cumulative effects of decreased inflammation, thrombosis, and preserved intracellular energy stores.
The results of a recent clinical trial of 102 high-risk patients undergoing coronary artery bypass grafting treated with placebo or EP failed to reveal differences in clinical parameters or markers of systemic inflammation.33 The findings from this small clinical trial have dampened some enthusiasm for the use of EP in clinical trials of shock or sepsis. The authors of this report speculate that the proinflammatory response of patients undergoing cardiopulmonary bypass may be substantially different from patients suffering from sepsis or trauma, thereby accounting from the lack of efficacy of EP to have an effect in this patient population. Furthermore, the negative findings of one small trial are dwarfed by the large amount of positive data from the use of EP in models with multiple injuries.
CONCLUSION
EP preserved ATP and decreased levels of inflammatory and thrombotic markers during hind-limb IR in a mouse model. When EP was administered before ischemia (pre-I) or during ischemia before reperfusion (post-I), there was an equivalent cytoprotective effect against skeletal muscle structural injury, inflammation, and tissue thrombosis. The metabolic, anti-inflammatory, and anti-thrombotic properties of EP ameliorate the multicomponent nature of IR in skeletal muscle. The biochemical analysis was confirmed by histologic assessment of skeletal muscle structural integrity. The pleiotropic properties of EP make it a potential therapeutic agent for the treatment of hind-limb IR injury.
Acknowledgments
Supported by the National Institutes of Health grant 1R01AR055843, the American Diabetes Association, the Pacific Vascular Research Foundation, and the Department of Surgery, Division of Vascular and Endovascular Surgery, Massachusetts General Hospital. Dr. Watkins is the Isenberg Scholar in Academic Surgery at the Massachusetts General Hospital.
References
- 1.Henke PK, Stanley JC. The treatment of acute embolic lower limb ischemia. Adv Surg. 2004;38:281–291. [PubMed] [Google Scholar]
- 2.Rubin BB, Liauw S, Tittley J, Romaschin AD, Walker PM. Prolonged adenine nucleotide resynthesis and reperfusion injury in postischemic skeletal muscle. Am J Physiol. 1992;262:H1538–H1547. doi: 10.1152/ajpheart.1992.262.5.H1538. [DOI] [PubMed] [Google Scholar]
- 3.Hickey MJ, Knight KR, Hurley JV, Lepore DA. Phosphoenolpyruvate/adenosine triphosphate enhances post-ischemic survival of skeletal muscle. J Reconstr Microsurg. 1995;11:415–422. doi: 10.1055/s-2007-1006555. [DOI] [PubMed] [Google Scholar]
- 4.Carden DL, Granger ND. Pathophysiology of ischemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Nichols TC. NF-κβ and reperfusion injury. Drug News Perspect. 2004;17:99 –104. doi: 10.1358/dnp.2004.17.2.829042. [DOI] [PubMed] [Google Scholar]
- 6.Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. J Intern Med. 2007;261:349 –362. doi: 10.1111/j.1365-2796.2007.01789.x. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell-Tormey J, Nathan CF, Lanks K, DeBoer CJ, de la Harpe J. Secretion of pyruvate. An antioxidant defense of mammalian cells. J Exp Med. 1987;165:500 –514. doi: 10.1084/jem.165.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonheur JA, Albadawi H, Patton GM, et al. A noninvasive murine model of hind limb ischemia-reperfusion injury. J Surg Res. 2004;116:55– 63. doi: 10.1016/s0022-4804(03)00232-4. [DOI] [PubMed] [Google Scholar]
- 9.McCormack MC, Kwon E, Eberlin KR, Watkins MT. Development of reproducible histologic injury severity scores: skeletal muscle reperfusion injury. Surgery. 2008;143:126 –133. doi: 10.1016/j.surg.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Ouriel K, Veith F, Sasahara A. Thrombolysis or peripheral arterial surgery: phase I results. TOPAS Investigators. J Vasc Surg. 1996;23:64–73. doi: 10.1016/s0741-5214(05)80036-9. [DOI] [PubMed] [Google Scholar]
- 11.Walker PM, Romaschin AD, Davis S, Piovesan J. Lower limb ischemia: phase 1 results of salvage perfusion. J Surg Res. 1999;84:193–198. doi: 10.1006/jsre.1999.5641. [DOI] [PubMed] [Google Scholar]
- 12.Chan RK, Ding G, Verna N, et al. IgM binding to injured tissue precedes complement activation during skeletal muscle ischemia-reperfusion. J Surg Res. 2004;122:29 –35. doi: 10.1016/j.jss.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Wakai A, Winter DC, Street JT, O’Sullivan RG, Wang JH, Redmond HP. Inosine attenuates tourniquet-induced skeletal muscle reperfusion injury. J Surg Res. 2001;99:311–315. doi: 10.1006/jsre.2001.6192. [DOI] [PubMed] [Google Scholar]
- 14.Han Y, Englert JA, Yang R, Delude RL, Fink MP. Ethyl pyruvate inhibits nuclear factor-kappaB-dependent signaling by directly targeting p65. J Pharmacol Exp Ther. 2005;312:1097–1105. doi: 10.1124/jpet.104.079707. [DOI] [PubMed] [Google Scholar]
- 15.Woo YJ, Taylor MD, Cohen JE, et al. Ethyl pyruvate preserves cardiac function and attenuates oxidative injury after prolonged myocardial ischemia. J Thorac Cardiovasc Surg. 2004;127:1262–1269. doi: 10.1016/j.jtcvs.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 16.Zeng J, Liu J, Yang GY, Kelly MJ, James TL, Litt L. Exogenous ethyl pyruvate versus pyruvate during metabolic recovery after oxidative stress in neonatal rat cerebrocortical slices. Anesthesiology. 2007;107:630–640. doi: 10.1097/01.anes.0000281898.01966.1e. [DOI] [PubMed] [Google Scholar]
- 17.Martin E, Rosenthal RE, Fiskum G. Pyruvate dehydrogenase complex: metabolic link to ischemic brain injury and target of oxidative stress. J Neurosci Res. 2005;79:240 –247. doi: 10.1002/jnr.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karabeyoglu M, Unal B, Bozkurt B, et al. The effect of ethyl pyruvate on oxidative stress in intestine and bacterial translocation after thermal injury. J Surg Res. 2008;144:59 – 63. doi: 10.1016/j.jss.2007.02.050. [DOI] [PubMed] [Google Scholar]
- 19.Akuzawa S, Kazui T, Shi E, Yamashita K, Bashar AH, Terada H. Interleukin-1 receptor antagonist attenuates the severity of spinal cord ischemic injury in rabbits. J Vasc Surg. 2008;48:694 –700. doi: 10.1016/j.jvs.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 20.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995;146:419 – 428. [PMC free article] [PubMed] [Google Scholar]
- 21.Sappington PL, Fink ME, Yang R, Delude RL, Fink MP. Ethyl pyruvate provides durable protection against inflammation-induced intestinal epithelial barrier dysfunction. Shock. 2003;20:521–528. doi: 10.1097/01.shk.0000092697.10326.8b. [DOI] [PubMed] [Google Scholar]
- 22.Di Paola R, Mazzon E, Genovese T, et al. Ethyl pyruvate reduces the development of zymosan-induced generalized inflammation in mice. Crit Care Med. 2009;37:270 –282. doi: 10.1097/CCM.0b013e318192fa63. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi Y. Neutrophil infiltration and chemokines. Crit Rev Immunol. 2006;26:307–316. doi: 10.1615/critrevimmunol.v26.i4.20. [DOI] [PubMed] [Google Scholar]
- 24.Zhao X, Town JR, Yang A, et al. A novel ELR-CXC chemokine antagonist reduces intestinal ischemia reperfusion-induced mortality, and local and remote organ injury. J Surg Res. 2010;162:264 –273. doi: 10.1016/j.jss.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 25.Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007;6:1302–1312. doi: 10.4161/cbt.6.8.4506. [DOI] [PubMed] [Google Scholar]
- 26.Cobelens PM, van Putte BP, Kavelaars A, Heijnen CJ, Kesecioglu J. Inflammatory consequences of lung ischemia-reperfusion injury and low-pressure ventilation. J Surg Res. 2009;153:295–301. doi: 10.1016/j.jss.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 27.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620 – 630. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 28.Messina LM. In vivo assessment of acute microvascular injury after reperfusion of ischemic tibialis anterior muscle of the hamster. J Surg Res. 1990;48:615– 621. doi: 10.1016/0022-4804(90)90241-s. [DOI] [PubMed] [Google Scholar]
- 29.May JW, Jr, Chait LA, O’Brien BM, Hurley JV. The no-reflow phenomenon in experimental free flaps. Plast Reconstr Surg. 1978;61:256 –267. doi: 10.1097/00006534-197802000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Abbruzzese TA, Albadawi H, Kang J, et al. Enoxaparin does not ameliorate limb ischemia-reperfusion injury. J Surg Res. 2008;147:260 –266. doi: 10.1016/j.jss.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 31.Ku PT, D’Amore PA. Regulation of basic fibroblast growth factor (bFGF) gene and protein expression following its release from sublethally injured endothelial cells. J Cell Biochem. 1995;58:328 –343. doi: 10.1002/jcb.240580307. [DOI] [PubMed] [Google Scholar]
- 32.van Zoelen MA, Bakhtiari K, Dessing MC, et al. Ethyl pyruvate exerts combined anti-inflammatory and anticoagulant effects on human monocytic cells. Thromb Haemost. 2006;96:789 –793. [PubMed] [Google Scholar]
- 33.Bennett-Guerrero E, Swaminathan M, Grigore AM, et al. A phase II multicenter double-blind placebo-controlled study of ethyl pyruvate in high-risk patients undergoing cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 2009;23:324–329. doi: 10.1053/j.jvca.2008.08.005. [DOI] [PubMed] [Google Scholar]