Abstract
Citrus flavonoids have been shown to decrease plasma lipid levels, improve glucose tolerance, and attenuate obesity. One possible mechanism underlying these physiological effects is reduction of hepatic levels of the mRNA for stearoyl-CoA desaturase-1 (SCD1), since repression of this enzyme reduces hyperlipidemia and adiposity. Here, we show that citrus flavonoids of two structural classes reduce SCD1 mRNA concentrations in a dose-dependent manner in rat primary hepatocytes. This is the first demonstration of repression of SCD1 by citrus flavonoids, either in vivo or in cultured cells. Furthermore, it is the first use of freshly-isolated hepatocytes from any animal to examine citrus flavonoid action at the mRNA level. This study demonstrates that regulation of SCD1 gene expression may play a role in control of obesity by citrus flavonoids and that rat primary hepatocytes are a physiologically-relevant model system for analyzing the molecular mechanisms of flavonoid action in the liver.
Background
Understanding the molecular mechanisms that regulate lipid synthesis and deposition is of paramount importance, since obesity increases the risk of prevalent, life-threatening diseases such as diabetes and atherosclerosis. An intriguing model proposes that obesity is attenuated by lowering the amount of hepatic and/or adipose stearoyl-CoA desaturase-1 (SCD1), the rate-limiting enzyme in biosynthesis of monounsaturated fatty acids, which are preferred for triglyceride assembly [1]. This model is supported by gene knockout or knockdown studies, in which reduction of SCD1 mRNA levels restricted adiposity, insulin resistance, and hepatic lipid accumulation in rodents [2-5]. Conversely, elevated SCD1 levels in humans were associated with high plasma lipid concentrations, elevated hepatic lipid synthesis, obesity, or familial combined hyperlipidemia [6-9].
In the quest for therapies to alleviate obesity and associated illnesses, citrus flavonoids (Figure 1) are particularly promising, since a large body of research in humans and animals has shown hypolipidemic and/or antidiabetic effects of citrus fruits and juices [10-12], as well as purified flavonoids [12-20]. To examine the molecular mechanisms of citrus flavonoid action in more detail than is possible in vivo, the human hepatoma HepG2 cell line has been used extensively to establish that citrus flavonoids act through multiple pathways to reduce hepatic lipid secretion, and that the effects are consistent with physiological responses to these compounds in humans and animals [21-26]. Our previous work showed that citrus flavonoids regulated transcription of the low-density lipoprotein receptor (LDLR) gene in HepG2 cells, and that the DNA binding site for the transcription factor, sterol regulatory element binding protein (SREBP), was necessary for the regulation [27]. This work was the first direct demonstration that citrus flavonoids act at the level of hepatic gene transcription. Although the experimental manipulability of HepG2 cells has facilitated the analysis of underlying molecular mechanisms, it is desirable to use primary hepatocytes, since they more closely represent the physiology of intact liver. However, we are aware of only one published experiment in which citrus flavonoid action, specifically inhibition of apolipoprotein B secretion, was demonstrated in primary liver cells [21]. Therefore, the present study developed the use of isolated hepatocytes for examining hepatic effects of citrus flavonoids at the mRNA level. We chose to examine regulation of SCD1 mRNA because of the hypothesis that repression of SCD1 plays a key role in control of obesity and diabetes [1], and because of the recent report of citrus flavonoid attenuation of adiposity and insulin resistance in mice fed a high-fat diet [20].
Figure 1.
Structures of two classes of citrus flavonoids.
Methods
Animals, primary hepatocyte isolation, and flavonoid treatment in culture
Male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) were used at 12-17 weeks of age, following protocols that conform with NIH guidelines and were approved by the University of Missouri Animal Care and Use Committee. Hepatocytes were isolated by collagenase perfusion [28] and cultured as described in Additional file 1-Detailed methods. Hesperetin (≥ 95% pure) was from Sigma. Nobiletin was purified from tangerine peel and recrystallized twice to yield a purity of >99% [29]. Flavonoid stock solutions (50 mM) were prepared in dimethyl sulfoxide, the final concentration of which was 0.3% (v/v) in flavonoid-treated and control cells.
RNA purification and analysis by molecular hybridization or quantitative real-time polymerase chain reaction (qRT-PCR)
RNA purification and molecular hybridization were conducted as described in Additional file 1-Detailed methods. Total RNA (20 μg/sample) was size fractionated on a formaldehyde gel and transferred to GeneScreen. Single-stranded cDNA probes for SCD1 and eukaryotic initiation factor 3H (EIF3H) mRNAs (Integrated DNA Technologies, Coralville, IA) (Table 1) were labeled, hybridized to the membrane, and detected by phosphorimaging. SCD1 mRNA was normalized to EIF3H mRNA, to correct for variable gel loading and any general flavonoid toxicity at higher flavonoid concentrations. The normalized results for treated samples are expressed as percent of the untreated control. qRT-PCR was carried out with SYBR-Green-based methodology (see Additional file 1-Detailed methods), using primer pairs for SCD1 or EIF3H (Table 1).
Table 1.
Sequences of hybridization probes and qRT-PCR primers
| Name | DNA Sequence |
|---|---|
| Hybridization Probes | |
| 5' 3' | |
| SCD1 (AS)1 | 1007 GTGGTGAAGTTGATGTGCCAGCGGTACTCACTG 975 |
| EIF3H (AS)2 | 1034 GGCAGTGAACTCCTTGATGTTCTGGCAGTAAGTGTT 999 |
| qRT-PCR Primers | |
| 5' 3' | |
| SCD1 (S)1 | 26 GAAGCGAGCAACCGACAGCCAC 47 |
| SCD1 (AS)1 | 180 GTCTTCTTCCAGATAGAGGGGCAC 157 |
| EIF3H (S)2 | 850 AACACCAGTATCAGCAGCGTCG 871 |
| EIF3H (AS)2 | 1027 AACTCCTTGATGTTCTGGCAGTAAGTG 1001 |
1 Sequence and numbering based on rat SCD1 (GenBank ID: NM_139192.2)
2 Sequence and numbering based on rat EIF3H (GenBank ID: NM_198751.1)
SCD1 and EIF3H hybridization probes are located within the protein-coding regions. The PCR-amplified sequence from EIF3H mRNA includes most of the 33-mer used as the EIF3H hybridization probe. The PCR-amplified sequence from SCD1 mRNA does not overlap with the SCD1 hybridization probe, because of the necessity to avoid potential cross reactivity with SCD2 mRNA, but it does produce an amplicon that is mostly within the protein-coding region. The SCD1 primer set does not match the SCD2 mRNA sequence (GenBank ID: NM_031841.1), and cloning and sequencing of the product generated by qRT-PCR confirmed that the amplified sequence was SCD1.
Results
Verification of hybridization probes for SCD1 and EIF3H mRNAs
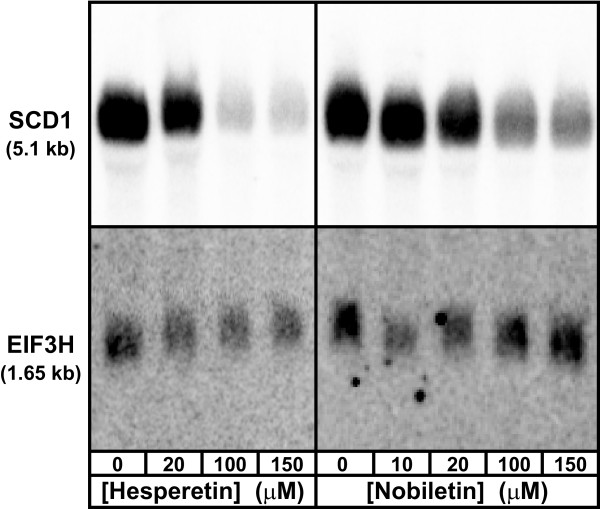
Rats have two SCD genes, SCD1 and SCD2 (sometimes called SCD). Hybridization of size-fractionated rat hepatocyte RNA with the SCD1 probe yielded a single RNA band of ~5,100 bases (Figure 2), similar to the previously-described ~5,900 bases [30]. These sizes are larger than the reported 4475 bases (GenBank ID: NM_139192.2), but that sequence is not necessarily full length. Although our hybridization probe matches SCD2 mRNA (GenBank ID: NM_031841.1), it is unlikely that the detected RNA is SCD2, since that isoform was completely undetectable in rat liver tissue [30]. qRT-PCR experiments below confirmed that the SCD isoform expressed in rat hepatocytes was SCD1. For normalization we used mRNA for the housekeeping protein, EIF3H. The EIF3H probe hybridized with a single RNA species of ~1,650 bases (Figure 2), which is compatible with the reported 1,243 bases (Genbank ID#: NM_198751.1).
Figure 2.
Specificity of hybridization probes for SCD1 or EIF3H mRNA in rat hepatocyte RNA. Rat hepatocytes were treated with vehicle, 20-150 μM hesperetin, or 10-150 μM nobiletin for 20 h. Total RNA was hybridized with cDNA probes for SCD1 mRNA or the normalizer, EIF3H mRNA. Apparent sizes of the RNAs are denoted on the left in kilobases (kb).
Dose-dependent repression of SCD1 mRNA levels by hesperetin or nobiletin in rat hepatocytes
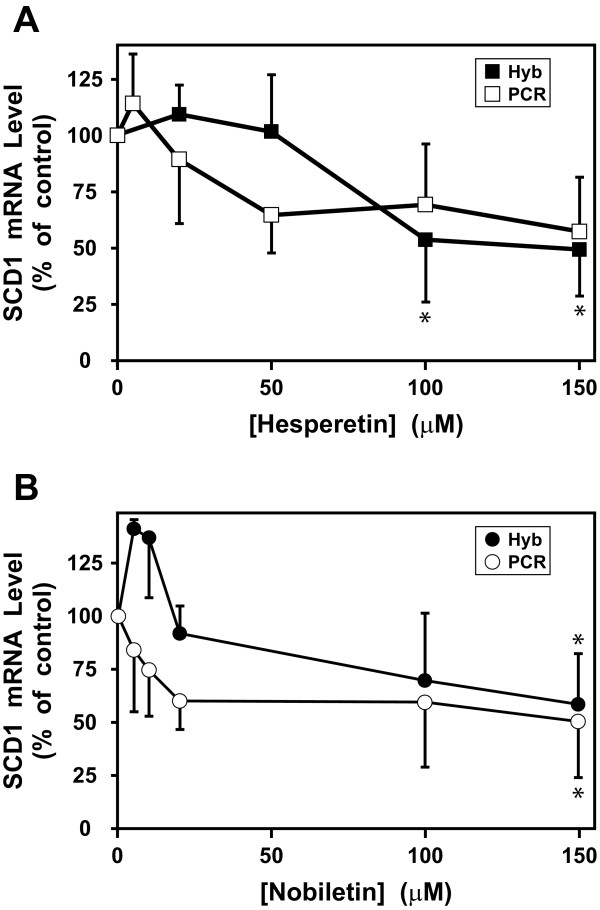
To represent the flavanone class, we used hesperetin (Figure 1), since it was more effective than naringenin in HepG2 cells [22]. For the polymethoxylated flavone class, which has been shown to be more potent (i.e. effective at lower doses) than flavanones in vivo [19] and in HepG2 cells [23,27], we chose nobiletin, since it was more effective than tangeretin in HepG2 cells (our unpublished data). For quantitative analysis, mRNA concentrations were assayed both by hybridization, which allowed assessment of RNA integrity and correct size (as in Figure 2), and by qRT-PCR, which allowed more rapid quantitation and exclusive detection of the SCD1 isoform. For 150 μM hesperetin, repression of SCD1 mRNA reached 49% (by hybridization) or 57% (by qRT-PCR) compared to the untreated control (Figure 3A). The inhibition was statistically significant (P ≤ 0.05) at 100 and 150 μM hesperetin by the hybridization assay. The qRT-PCR data did not quite reach statistical significance, but the results were very similar to those in the hybridization assay. For 150 μM nobiletin, the inhibitory effect was 58% (by hybridization) or 50% (by qRT-PCR), which was statistically significant (P ≤ 0.05) by both assays (Figure 3B). At low concentrations of nobiletin (5-10 μM), there is some difference in the pattern of the response by the two assays, but none of the effects in this concentration range were significantly different from the control. Despite the differences at low doses, the overall trend is a decrease in SCD1 mRNA with increasing concentrations of nobiletin, similar to that of hesperetin.
Figure 3.
Dose-dependent repression of SCD1 mRNA levels in rat hepatocytes by hesperetin or nobiletin. Rat hepatocytes were treated with vehicle or 5-150 μM hesperetin or nobiletin for 18-20 h in four independent experiments. mRNAs were quantitated by hybridization (closed symbols) or qRT-PCR (open symbols). Effects of increasing doses of (A) hesperetin or (B) nobiletin on normalized SCD1 mRNA levels are expressed as percent relative to the untreated control. At each flavonoid concentration, n = 3 or 4, and the error bars represent SD. Each experimental condition was compared back to the control by one-way ANOVA with Dunnett's post test using InStat (GraphPad, La Jolla, CA). An asterisk indicates a statistically-significant difference from the untreated control (P ≤ 0.05).
Discussion
The citrus flavonoid repression of SCD1 mRNA levels described here is compatible with the recent report that naringenin reduced adiposity and weight gain in mice after 4 weeks [20], based on the model that SCD1 plays an important role in obesity control [1]. The in vivo effects of flavonoids were proposed to be due to a reduction in the amount of SREBP1 [20]. However, previous work in HepG2 cells indicated that citrus flavonoids stimulate, rather than repress, SREBP levels after short term treatments [21,27]. This apparent discrepancy may be explained by well-established mechanisms whereby SREBPs stimulate many genes that elevate lipids and cholesterol production [31]. Cholesterol then sequesters SREBPs in an inactive form, which leads, in the long term, to decreased expression of genes that were initially induced, including the SREBP genes themselves [31-33]. Thus, SREBP effects on hepatic lipid handling in vivo are a complex balance between opposing actions and feedback mechanisms [31].
Because citrus flavonoids elevate SREBPs in HepG2 cells, the simplest prediction is that these compounds stimulate SREBP activity in primary rat hepatocytes. However, our observation of the repression of SCD1 mRNA is not compatible with this prediction, since the SCD1 gene is a positive target for both SREBP1 and SREBP2 [32,33]. Thus, our results suggest that, in rat liver cells, either the flavonoids reduce SREBPs or repression of SCD1 mRNA occurs by SREBP-independent mechanisms. A study with a different flavonoid, the soy isoflavone genistein, also showed repression of SCD1 mRNA levels in HepG2 cells [34]. This repression correlated with a 50% decrease in nuclear SREBP1 and a 5-fold increase in nuclear SREBP2, but these conclusions are not definite since the particular antibody used should not recognize the mature N-terminal portion of SREBP2 in the nucleus, and data from multiple experiments were not reported [34]. Another group found that soy isoflavones increased the amount of the C-terminal mature portion of SREBP2 in whole cell extracts of HepG2 cells after 24 h, but SREBP1 levels did not change [35]. Because of this variability regarding flavonoid effects on SREBP levels in HepG2 cells, the rat primary hepatocytes will be invaluable for deciphering the mechanisms underlying the complexities of regulation of both isoforms of SREBP, as well as the role of SREBP in flavonoid repression of the SCD1 gene.
Freshly-isolated hepatocytes allow a more thorough mechanistic analysis of flavonoid action than is possible in vivo and are more physiologically-relevant than tumor-derived HepG2 cells. A detailed molecular understanding is essential for evaluating the potency and efficacy of flavonoids of different structural classes and metabolic forms, so that ultimately the most effective flavonoid-based treatments can be used for combating atherosclerosis, diabetes, and obesity.
Abbreviations
EIF3H: eukaryotic initiation factor 3H; LDLR: low-density lipoprotein receptor; qRT-PCR: quantitative real-time polymerase chain reaction; SCD: stearoyl-CoA desaturase; SREBP: sterol regulatory element binding protein.
Competing interests
JAM receives a small portion of the annual payment to the United States Department of Agriculture for licensing of U.S. patents 6,184,246 and 6,987,125, which deal with the cardiovascular and inflammation protection actions of citrus polymethoxylated flavones. The other authors declare that they have no competing interests.
Authors' contributions
LAN participated in experimental design and carried out experiments. DEJ carried out experiments. JAM supplied research expertise and carried out flavonoid purification. SDS supplied research expertise and experimental materials. LJH conceived of the study, participated in experimental design, carried out experiments, and drafted the manuscript. All authors edited the draft manuscript, and read and approved the final manuscript.
Authors' information
*Department of Medical Pharmacology and Physiology, MA 415 Medical Sciences Building, University of Missouri School of Medicine, One Hospital Drive, Columbia, Missouri 65212, USA. Telephone: 1-573-882-5373. FAX: 1-573-884-4276.
Supplementary Material
Detailed methods. Methodological details for hepatocyte isolation and culture, RNA purification, molecular hybridization, and qRT-PCR.
Contributor Information
LaNita A Nichols, Email: NicholsLA@health.missouri.edu.
Daniel E Jackson, Email: JacksonDan@health.missouri.edu.
John A Manthey, Email: John.Manthey@ARS.USDA.GOV.
Shivendra D Shukla, Email: ShuklaSD@health.missouri.edu.
Lené J Holland, Email: HollandL@health.missouri.edu.
Acknowledgements and funding
We thank B. Morin and A. McClellan for helpful comments on the manuscript. This work was funded in part by grants from the National Institutes of Health (#R01 AA016347 to SDS) and the University of Missouri Research Council (to LJH).
References
- Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase as a new drug target for obesity treatment. Obesity Rev. 2005;6:169–174. doi: 10.1111/j.1467-789X.2005.00177.x. [DOI] [PubMed] [Google Scholar]
- Sampath H, Miyazaki M, Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase-1 mediates the pro-lipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- Jiang G, Li Z, Liu F, Ellsworth K, Dallas-Yang Q, Wu M, Ronan J, Esau C, Murphy C, Szalkowski D, Bergeron R, Doebber T, Zhang BB. Prevention of obesity in mice by antisense oligonucleotide inhibitors of stearoyl-CoA desaturase-1. J Clin Invest. 2005;115:1030–1038. doi: 10.1172/JCI23962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Juárez R, Pocai A, Mulas C, Ono H, Bhanot S, Monia BP, Rossetti L. Critical role of stearoyl-CoA desaturase-1 (SCD1) in the onset of diet-induced hepatic insulin resistance. J Clin Invest. 2006;116:1686–1695. doi: 10.1172/JCI26991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Flowers MT, Sampath H, Chu K, Otzelberger C, Liu X, Ntambi JM. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 2007;6:484–496. doi: 10.1016/j.cmet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791:85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attie AD, Krauss RM, Gray-Keller MP, Brownlie A, Miyazaki M, Kastelein JJ, Lusis AJ, Stalenhoef AFH, Stoehr JP, Hayden MR, Ntambi JM. Relationship between stearoyl-CoA desaturase activity and plasma triglycerides in human and mouse hypertriglyceridemia. J Lipid Res. 2002;43:1899–1907. doi: 10.1194/jlr.M200189-JLR200. [DOI] [PubMed] [Google Scholar]
- Mar-Heyming R, Miyazaki M, Weissglas-Volkov D, Kolaitis NA, Sadaat N, Plaisier C, Pajukanta P, Cantor RM, de Bruin TWA, Ntambi JM, Lusis AJ. Association of stearoyl-CoA desaturase 1 activity with familial combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 2008;28:1193–1199. doi: 10.1161/ATVBAHA.107.160150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong MF-F, Hodson L, Bickerton AS, Roberts R, Neville M, Karpe F, Frayn KN, Fielding BA. Parallel activation of de novo lipogenesis and stearoyl-CoA desaturase activity after 3 d of high-carbohydrate feeding. Am J Clin Nutr. 2008;87:817–823. doi: 10.1093/ajcn/87.4.817. [DOI] [PubMed] [Google Scholar]
- Gorinstein S, Caspi A, Libman I, Katrich E, Lerner HT, Trakhtenberg S. Preventive effects of diets supplemented with sweetie fruits in hypercholesterolemic patients suffering from coronary artery disease. Preventive Med. 2004;38:841–847. doi: 10.1016/j.ypmed.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Gorinstein S, Caspi A, Libman I, Lerner HT, Huang D, Leontowicz H, Leontowicz M, Tashma Z, Katrich E, Feng S, Trakhtenberg S. Red grapefruit positively influences serum triglyceride level in patients suffering from coronary atherosclerosis: studies in vitro and in humans. J Agric Food Chem. 2006;54:1887–1892. doi: 10.1021/jf058171g. [DOI] [PubMed] [Google Scholar]
- Gorinstein S, Leontowicz H, Leontowicz M, Krzeminski R, Gralak M, Delgado-Licon E, Martinez Ayala AL, Katrich E, Trakhtenberg S. Changes in plasma lipid and antioxidant activity in rats as a result of naringin and red grapefruit supplementation. J Agric Food Chem. 2005;53:3223–3228. doi: 10.1021/jf058014h. [DOI] [PubMed] [Google Scholar]
- Jung UJ, Kim HJ, Lee JS, Lee MK, Kim HO, Park EJ, Kim HK, Jeong TS, Choi MS. Naringin supplementation lowers plasma lipids and enhances erythrocyte antioxidant enzyme activities in hypercholesterolemic subjects. Clin Nutr. 2003;22:561–568. doi: 10.1016/S0261-5614(03)00059-1. [DOI] [PubMed] [Google Scholar]
- Roza JM, Xian-Liu Z, Guthrie N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern Ther Health Med. 2007;13:44–48. [PubMed] [Google Scholar]
- Judy W, Stogsdill W, Judy D, Judy J, Sharma P, Evans M, Guthrie N. Efficacy of Diabetinol on glycemic control in insulin resistant hamsters and subjects with impaired fasting glucose - a pilot study. J Functional Foods. 2010;2:171–178. doi: 10.1016/j.jff.2010.04.005. [DOI] [Google Scholar]
- Jung UJ, Lee M-K, Park YB, Kang MA, Choi M-S. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Intl J Biochem Cell Biol. 2006;38:1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Li RW, Theriault AG, Au K, Douglas TD, Casaschi A, Kurowska EM, Mukherjee R. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006;79:365–373. doi: 10.1016/j.lfs.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Bok S-H, Lee S-H, Park Y-B, Bae K-H, Son K-H, Jeong T-S, Choi M-S. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and acyl CoA:cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J Nutr. 1999;129:1182–1185. doi: 10.1093/jn/129.6.1182. [DOI] [PubMed] [Google Scholar]
- Kurowska EM, Manthey JA. Hypolipidemic effects and absorption of citrus polymethoxylated flavones in hamsters with diet-induced hypercholesterolemia. J Agric Food Chem. 2004;52:2879–2886. doi: 10.1021/jf035354z. [DOI] [PubMed] [Google Scholar]
- Mulvihill EE, Allister EM, Sutherland BG, Telford DE, Sawyez CG, Edwards JY, Markle JM, Hegele RA, Huff MW. Naringenin prevents dyslipidemia, apolipoprotein B overproduction, and hyperinsulinemia in LDL receptor-null mice with diet-induced insulin resistance. Diabetes. 2009;58:2198–2210. doi: 10.2337/db09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, de Dreu LE, Huff MW. Inhibition of net HepG2 cell apolipoprotein B secretion by the citrus flavonoid naringenin involves activation of phosphatidylinositol 3-kinase, independent of insulin receptor substrate-1 phosphorylation. Diabetes. 2003;52:2554–2561. doi: 10.2337/diabetes.52.10.2554. [DOI] [PubMed] [Google Scholar]
- Wilcox LJ, Borradaile NM, de Dreu LE, Huff MW. Secretion of hepatocyte apoB is inhibited by the flavonoids, naringenin and hesperetin, via reduced activity and expression of ACAT2 and MTP. J Lipid Res. 2001;42:725–734. [PubMed] [Google Scholar]
- Kurowska EM, Manthey JA. In: Flavonoids in Cell Function. Buslig B, Manthey J, editor. New York: Kluwer Academic/Plenum Publishers; 2002. Regulation of lipoprotein metabolism in HepG2 cells by citrus flavonoids; pp. 173–179. [DOI] [PubMed] [Google Scholar]
- Kurowska EM, Manthey JA, Casaschi A, Theriault AG. Modulation of HepG2 cell net apolipoprotein B secretion by the citrus polymethoxyflavone, tangeretin. Lipids. 2004;39:143–151. doi: 10.1007/s11745-004-1212-8. [DOI] [PubMed] [Google Scholar]
- Borradaile NM, Carroll KK, Kurowska EM. Regulation of HepG2 cell apolipoprotein B metabolism by the citrus flavanones hesperetin and naringenin. Lipids. 1999;34:591–598. doi: 10.1007/s11745-999-0403-7. [DOI] [PubMed] [Google Scholar]
- Allister EM, Borradaile NM, Edwards JY, Huff MW. Inhibition of microsomal triglyceride transfer protein expression and apolipoprotein B100 secretion by the citrus flavonoid naringenin and by insulin involves activation of the mitogen-activated protein kinase pathway in hepatocytes. Diabetes. 2005;54:1676–1683. doi: 10.2337/diabetes.54.6.1676. [DOI] [PubMed] [Google Scholar]
- Morin B, Nichols LA, Zalasky KM, Davis JW, Manthey JA, Holland LJ. The citrus flavonoids hesperetin and nobiletin differentially regulate low density lipoprotein receptor gene transcription in HepG2 liver cells. J Nutr. 2008;138:1274–1281. doi: 10.1093/jn/138.7.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng Y-I, Shukla SD. Ethanol alters angiotensin II stimulated mitogen activated protein kinase in hepatocytes: agonist selectivity and ethanol metabolic independence. Eur J Pharmacol. 2000;398:323–331. doi: 10.1016/S0014-2999(00)00313-7. [DOI] [PubMed] [Google Scholar]
- Swift LJ. Flavones of the neutral fraction of the benzene extractables of an orange peel juice. J Agric Food Chem. 1965;13:431–433. doi: 10.1021/jf60141a013. [DOI] [Google Scholar]
- Moreau C, Froment P, Tosca L, Moreau V, Dupont J. Expression and regulation of the SCD2 desaturase in the rat ovary. Biol Reprod. 2006;74:75–87. doi: 10.1095/biolreprod.105.044545. [DOI] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. J Clin Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shah NA, Warrington JA, Anderson NN, Park SW, Brown MS, Goldstein JL. Combined analysis of oligonucleotide microarray data from transgenic and knockout mice identifies direct SREBP target genes. Proc Natl Acad Sci USA. 2003;100:12027–12032. doi: 10.1073/pnas.1534923100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin ES, Lee HH, Cho SY, Park HW, Lee SJ, Lee TR. Genistein downregulates SREBP-1 regulated gene expression by inhibiting site-1 protease expression in HepG2 cells. J Nutr. 2007;137:1127–1131. doi: 10.1093/jn/137.5.1127. [DOI] [PubMed] [Google Scholar]
- Mullen E, Brown RM, Osborne TF, Shay NF. Soy isoflavones affect sterol regulatory element binding proteins (SREBPs) and SREBP-regulated genes in HepG2 cells. J Nutr. 2004;134:2942–2947. doi: 10.1093/jn/134.11.2942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed methods. Methodological details for hepatocyte isolation and culture, RNA purification, molecular hybridization, and qRT-PCR.