Abstract
Backround
The aim of the present study was to assess the effects of zinc or copper and polyphenolic compounds on the 8-isoprostaglandin F2α concentration in the serum and urine of rats with mammary cancer (adenocarcinoma) induced with 7,12-dimethylbenz[a]antracene. The research focused on the kinetics of alterations in urinary 8-isoPGF2α at the early stage of carcinogenesis as well as the influence of dietary factors on the process. The impact of selected compounds on the intensity of DMBA - induced carcinogenesis was also assessed.
Result and conclusions
Administration of DMBA, a compound that inducers mammary tumors in experimental animals, increased the serum and urinary 8-isoPGF2α levels in study rats. In the rat model, diet supplementation with zinc, combined with selected polyphenolic compounds (resveratrol or genistein) yielded a statistically significant decrease in the rat serum and urinary biomarker concentration with a simultaneously significant stimulation of carcinogenesis.
The results indicate that there is an inverse correlation between the intensity of DMBA-induced carcinogenicity and the level of 8-isoPGF2α in urine and serum of rats.
Introduction
Breast cancer represents the most common neoplastic disease in females, accounting for up to one third of new diagnoses of women's cancer in certain region of the world. The fundamental issue in breast cancer control is prevention, which depends on detection of the determinants of the disease, in terms of initiation and promotion. It is worth mentioning that despite numerous research conducted in different areas, currently there is no one ideal, specific biomarker of the early phase of cancer development, including breast cancer. The possibility of using biomarkers of breast carcinogenesis, such as 8-isoprostaglandin F2α, and dietary factors is very promising [1,2].
F2-isoprostanes are the isomers of F-prostaglandin, produced by cyclooxygenase-independent free radical peroxidation of arachidonic acid. The formation of 8-isoPGF2α causes damage to biomembranes and to oxidative modification of plasma lipoproteins. Carcinogenesis may be initiated by lipid peroxidation, which ultimately produces damage to proteins and DNA [3].
Another problem is the influence of dietary factor on biomarkers and the mechanisms of the disease development process. It is estimated that approximately one-third of human cancers are associated with inappropriate dietary habits and lifestyle. Therefore, dietary intake of trace elements and polyphenols becomes an important factor in controlling oxidative stress and the pathologies that ensue [4-8].
The aim of the present study was to assess the effect of zinc or copper and polyphenolic compounds (resveratrol or genistein) on the concentration of 8-isoprostaglandin F2α in the serum and urine of rats with mammary cancer (adenocarcinoma) induced with 7,12-dimethylbenz[a]antracene. No reports have shown kinetic alterations in the urinary 8-isoPGF2α quantity excreted at the early stage of carcinogenesis or the impact of dietary factors on the process. The impact of selected compounds on the intensity of DMBA - induced carcinogenesis was also assessed.
It is still not clear what kind of combinations and doses of trace elements and polyphenolic compounds should be given in order to achieve the most beneficial health effects in human cancer prevention or for improvement of pharmacological treatment.
Materials and methods
Laboratory animals
Female Sprague-Dawley rats were obtained from the Animal Laboratory, Department of General and Experimental Pathology, Medical University of Warsaw. The study was approved by the Ethics Committee, Medical University of Warsaw. The animals were divided into 12 groups (Table 1). Those in groups 1 to 6 (n = 60) were treated twice with of 80 mg/kg of body weight DMBA (Sigma-Aldrich, St. Louis, MO, USA) in rapeseed oil (via gavage) to induce mammary cancer (adenocarcinoma); the first treatment was given at 50 days, followed by the same dose (a repeat dose of 80 mg/kg body weight) at 80 days of rat age. Rats from groups 7 to 12 (control groups) (n = 40) were accommodated under the same conditions as those from groups 1 to 6, and received the same diet, but no DMBA treatment. Tumors were evaluated histopathologically to confirm malignancy and to prove that they were adenocarcinomas (II and III degree). In DMBA-untreated groups spontaneous cancers were not observed.
Table 1.
Composition of diets administered of different groups
| Diets | groups/carcinogens | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMBA + | DMBA - | |||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 1. Standard diet | + | + | + | + | + | + | + | + | + | + | + | + |
| 2. Zinc 6.9 mg/mL (231 mg Zn/kg diet) | - | + | - | - | - | - | - | + | - | - | - | - |
| 3. Zinc 6.9 mg/mL (231 mg Zn/kg diet) and resveratrol 0.1 mg/mL (0.2 mg/kg bw) | - | - | + | - | - | - | - | - | + | - | - | - |
| 4. Zinc (6.9 mg/mL) (231 mg Zn/kg diet) and genistein 0.1 mg/mL (0.2 mg/kg bw) | - | - | - | + | - | - | - | - | - | + | - | - |
| 5. Copper 1.3 mg/mL (42.6 mg Cu/kg diet) | - | - | - | - | + | - | - | - | - | - | + | - |
| 6. Copper 1.3 mg/mL (42.6 mg Cu/kg diet) and resveratrol 0.1 mg/mL (0.2 mg/kg bw) | - | - | - | - | - | + | - | - | - | - | - | + |
Abbreviations: DMBA - 7,12-dimethylbenz[a]anthracene; DMBA (+) corresponds to DMBA-treated groups; DMBA (-) corresponds to DMBA-untreated groups;
All animals had a free access to water and food (standard diet: Labofeed H, Żurawia 19, 89-240 Kcynia, Poland). Table 1 shows additional different types of bioelements or polyphenolic compounds also given to the animals. The rats were fed extra supplements suspended in water, (0.4 ml daily via gavage), from 40 days until 20 weeks of age (sacrifice time by decapitation). The animals were fed only the standard diet (without supplementation) received 0.4 ml of water. The polyphenol dose levels were selected based on human average daily consumption (extrapolating on the rats' body weight) [9,10]. The doses of bioelements were established based on the values used in the Labofeed H diet (standard diet) (77 mg Zn/kg diet; 21.3 mg Cu/kg diet) [11]. The zinc and copper content in the standard diet were determined following wet microwave sample mineralization using atomic absorption spectrophotometry (AAS). The dose levels of each agent were selected for projected drug levels.
In order to obtain rat urine samples, the animals were placed in individual metabolic cages for 24 hours. Urine was collected at 10 and 12 weeks of age, at an early, nonpalpable stage of carcinogenesis. The animals were sacrificed by decapitation at 20 weeks of age, and serum were collected. The biomaterial samples were stored at a temperature of - 70C until the test time.
The concentration of 8-isoPGF2α in serum and urine was determined by a competitive enzyme-linked immunosorbent assay (ELISA) (8-isoprostane EIA kit, Cayman Chemical Company (Ann Arbor. MF, USA).
Determination of creatinine level
The 8-isoPGF2α level was standardised by conversion to the creatinine level. The latter was examined in urine samples with creatinine test (Hydrex, Warsaw, Poland) based on Jaffe's reaction (in an alkaline environment creatinine forms a colored complex with picric acid).
Superoxide dismutase (SOD) activity
Superoxide dismutase activity in serum was determined with the Superoxide Dismutase Assay kit, Cayman Chemical Company (Ann Arbor, MI). The assay kit utilizes a tetrazolium salt for detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical. The SOD assay measures all three types of SOD (Cu/Zn, Mn and FeSOD).
The animals were examined by palpation during the study to characterize the time course of tumor development. At sacrifice (at 20 weeks of rats's age) tumors were removed, weighed and histopathological examinaton was performed.
The results obtained were statistically assessed using the Kolmogorow-Smirnow test, ANOVA with POST-HOC NIR tests (SPSS 12 programme).
Results and discussion
Mammary glands of several rat strains, mainly Spraque-Dawley, are susceptible to transformation induced by chemical carcinogens. One widely used active chemical inductors of mammary carcinogenesis is 7,12-dimethylbenz[a]anthracene [1]. The results of this study indicated that the amount of 8-isoPGF2α in serum and urines from rats treated with cancerogenic factor and receiving a standard diet was higher than in the control group receiving analogous diet but with no DMBA treatment, however, the differences were not statistically significant for the reason of high values of standard deviation (Figures 1, 2 and 3). Similar trend regarding amount of 8-isoPGF2α in serum of rats treated with carbon tetrachloride was acquired by Kadiiska et al. [12]. Administration of DMBA resulted in an increase of biomarkers indicating damage of DNA (8-hydroxy-2'-deoxyguanosine), proteins (carbonyl groups of amino acids) and lipids (8-isoPGF2α) [13]. These findings may confirm the relationship between DMBA and stimulation of the free radical processes.
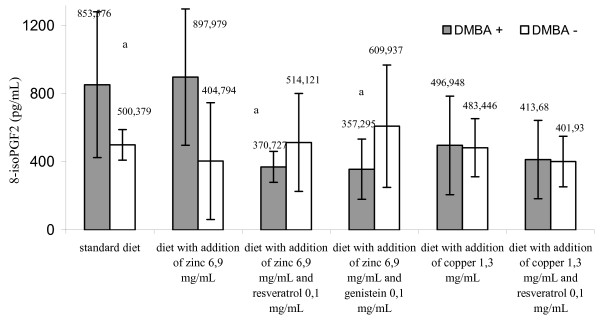
Figure 1.
The content of 8-isoPGF2α (pg/mL) (means ± standard deviation) in serum of DMBA treated and untreated animals in relation to diet. a - p < 0.05 group treated DMBA, fed standard diet versus groups treated DMBA, fed diet with addition of zinc or copper separately or in combination with resveratrol and genistein. The date refer to 20 weeks of the animals' age (decapitation time). Abbreviations: DMBA - 7,12-dimethylbenz[a]anthracene; DMBA (+) corresponds to DMBA-treated groups; DMBA (-) corresponds to DMBA-untreated groups; 8-isoPGF2α - 8-isoprostaglandin F2α.
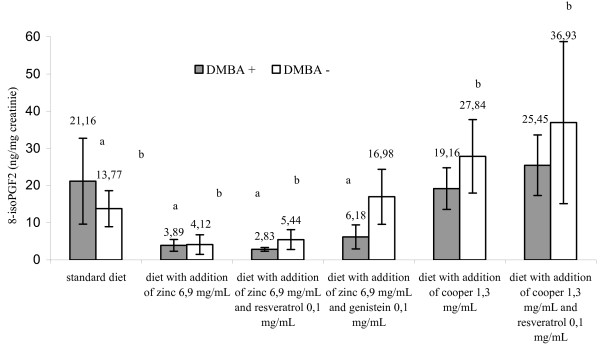
Figure 2.
Urinary 8-isoPGF2α (ng/mg creatinine) in rats fed different diets in early-onset breast cancer - 10 week of the rodent's age. a - p < 0.05 group treated DMBA, fed standard diet versus groups treated DMBA, fed diet with addition of zinc or copper separately or in combination with resveratrol and genistein. b - p < 0.05 group untreated DMBA, fed standard diet versus groups untreated DMBA, fed diet with addition of zinc or copper separately or in combination with resveratrol and genistein. Abbreviations: DMBA - 7,12-dimethylbenz[a]anthracene; DMBA (+) corresponds to DMBA-treated groups; DMBA (-) corresponds to DMBA-untreated groups; 8-isoPGF2α - 8-isoprostaglandin F2α.
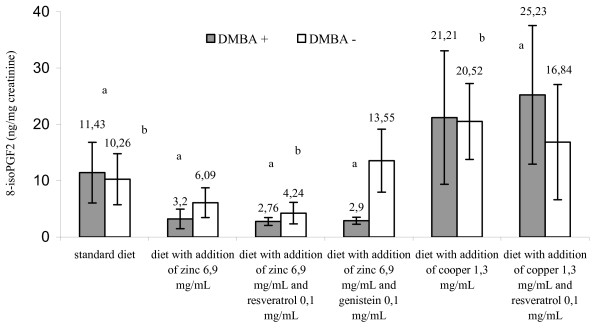
Figure 3.
Urinary 8-isoPGF2α (ng/mg creatinine) in rats fed different diets in early-onset breast cancer - 12 week of the rodent's age. a - p < 0.05 group treated DMBA, fed standard diet versus groups treated DMBA, fed diet with addition of zinc or copper separately or in combination with resveratrol and genistein. b - p < 0.05 group untreated DMBA, fed standard diet versus groups untreated DMBA, fed diet with addition of zinc or copper separately or in combination with resveratrol and genistein. Abbreviations: DMBA - 7,12-dimethylbenz[a]anthracene; DMBA (+) corresponds to DMBA-treated groups; DMBA (-) corresponds to DMBA-untreated groups; 8-isoPGF2α - 8-isoprostaglandin F2α.
It was found that zinc administration combined with selected polyphenolic compounds (resveratrol or genistein) statistically decreased the biomarker content level in the rat serum and urine compared with the animals receiving exclusively a standard diet (p < 0,05) (Figures 1, 2 and 3). It was demonstrated that diet supplementation with zinc did not decrease the content level of the studied biomarker in serum of DMBA-treated rats. On the contrary, it stimulated the process (853,576 ± 428,232 versus 897,979 ± 400,809 pg/ml), although, the response was not statistically significant (p < 0,05). In this case, synergistic reactions between Zn and polyphenols was observed. Evidence shows an ability of polyphnols to scavange free radicals, build complexes with metal ions catalysing formation of free radicals, and also inhibiting certain enzymes participating in oxidation [6-8].
Zn plays an essential role in cell membrane integrity and is a component of more than 300 different enzymes that function in many aspects of cellular metabolism. In numerous systems Zn can antagonize the catalytic properties of the redox-active transition metals (Fe and Cu) with respect to their abilities to promote formation of hydroxyl radical (.OH) from H2O2 and superoxide [14]. Surprisingly, the results of our work indicated no connection between superoxide dismutase activity and Zn or Cu supplemented diets (Table 2). Therefore, the decision was made to discontinue the search for mutual correlations of 8-isoPGF2α concentration, dependent on the diet and the enzyme activity, especially, that the rat group receiving additional zinc exhibited a stimulated lipid peroxidation (Table 2, Figures 1, 2 and 3). There is no univocal evidence in the literature to confirm that dietary zinc supplementation in applied dose exerts an impact on the activity of superoxide dismutase. Reports seem to indicate that diets supplemented with zinc in different quantities did not cause significant changes in SOD activity, what was also confirmed by our study [15,16].
Table 2.
Superoxide dismutase activity in rat's serum in relation to diet
| diet | Cancerogenic factor | Superoxide dismutase (U/mL) activity in serum |
|---|---|---|
| 1. Standard diet | DMBA + | 19.73 ± 2.82 |
| DMBA - | 22.34 ± 1.65 | |
| 2. Zinc 6.9 mg/mL (231 mg Zn/kg diet) | DMBA + | 19.52 ± 1.83 |
| DMBA - | 19,11 ± 3,77 | |
| 3. Zinc 6.9 mg/mL (231 mg Zn/kg diet) and resveratrol 0.1 mg/mL (0.2 mg/kg bw) | DMBA + | 20.81 ± 2.02 |
| DMBA - | 21.96 ± 2.31 | |
| 4. Zinc (6.9 mg/mL) (231 mg Zn/kg diet) and genistein 0.1 mg/mL (0.2 mg/kg bw) | DMBA + | 19.69 ± 2.67 |
| DMBA - | 21.93 ± 2.27 | |
| 5. Copper 1.3 mg/mL (42.6 mg Cu/kg diet) | DMBA + | 20.64 ± 2.24 |
| DMBA - | 22.29 ± 3.99 | |
| 6. Copper 1.3 mg/mL (42.6 mg Cu/kg diet) and resveratrol 0.1 mg/mL (0.2 mg/kg bw) | DMBA + | 19.74 ± 3.20 |
| DMBA - | 22.07 ± 4.01 |
The date refer to serum evaluated at 20 weeks of the animals' age (decapitation time)
Abbreviations: DMBA - 7,12-dimethylbenz[a]anthracene; DMBA (+) corresponds to DMBA-treated groups; DMBA (-) corresponds to DMBA-untreated groups;
Many reasearchers have investigated the dietary supplementation effects on the levels of urinary isoprostane or isoprostane metabolites. Diets rich in fruit and vegetables diminish the excretion of urinary 8-isoPGF2α [2]. The streptozotocin model of diabetes used in the rats showed reduced biomarker levels following the diet supplementation with vitamin E [17]. Urinary and plasma F2-isoprostanes were significantly decreased with alcohol-free red wine [18].
Cooper is generally considered as a pro-oxidant metal that participates in Fenon-like reactions. One of the mechanisms of procancerous Cu activity involves its ability to produce a strongly reactive hydroxyl radical responsible for oxidative damage to DNA strands and also peroxidation of cell membrane lipids [14]. As shown in our paper, the animals receiving diet supplemented with copper or copper and resveratrol had significantly higher amount of 8-isoPGF2α in urine compared with the animals receiving a standard or zinc supplemented diet (p < 0,05) (Figure 2, 3). This implies a pro-oxidant effect of cooper.
Another relevant issue in our study was to assess the severity of carcinogenesis expressed by tumor weight and number in particular rat groups as well as the onset of the initial tumors (Table 3). The data refer to tumors evaluated in 20th week of the animals age (decapitation time). The largest and most numerous tumors per animal were found in the group receiving zinc and resveratrol supplemented diet, which might have been due to the fact that in that group the first tumors appeared at 13 weeks of rat age, i.e. two weeks earlier than in the group fed the standard diet. Zinc compounds are considered to be chemopreventive agents chiefly due to their antioxidative properties. However, reports show that excessive Zn in the animal body may exacerbate tumorigenesis [14]. The harmful role of Zn in tumorigenesis could depend on the dose of zinc and synergism between various antioxidant used in the study. What is interesting there was an inverse correlation between the intensity of cancer development process and the level of 8-isoPGF2α in urine and serum of rats. A plausible explanation of this fact could be that Zn acts to improve health through a wide variety of biological mechanisms and not antioxidant activity alone. Thomson et al [19] reported a moderately significant inverse association of 8-isoPGF2α within plasma carotenoid levels, a protective association not seen with dietary carotenoid levels.
Table 3.
Tumor induction in 7.12-dimethylbenz[a]antracene treated groups in relation to diet
| Diet | Tumor incidence (%)1 |
Number of tumors in one rat | Maximum tumor weight in one rat in group (g) | First week at onset |
|---|---|---|---|---|
| 1. Standard diet | 9/9 (100) | 1-5 | 12 | 15 |
| 2. Zinc 6.9 mg/mL (231 mg Zn/kg diet) | 9/9 (100) | 1-5 | 20 | 15 |
| 3. Zinc 6.9 mg/mL (231 mg Zn/kg diet) and resveratrol 0.1 mg/mL (0.2 mg/kg bw) | 10/10 (100) | 2-10 | 30 | 13 |
| 4. Zinc (6.9 mg/mL) (231 mg Zn/kg diet) and genistein 0.1 mg/mL (0.2 mg/kg bw) | 10/11 (91) | 1-6 | 15 | 15 |
| 5. Copper 1.3 mg/mL (42.6 mg Cu/kg diet) | 9/9 (100) | 1-5 | 7 | 14 |
| 6. Copper 1.3 mg/mL (42.6 mg Cu/kg diet) and resveratrol 0.1 mg/mL (0.2 mg/kg bw) | 12/12 (100) | 2-6 | 11 | 14 |
The date refer to tumors evaluated at 20 weeks of the animals' age (decapitation time).
1 Tumor incidence is expressed as the number of tumor-bearing rats over total number of rats in the group.
Conclusions
Administration of DMBA, a compound that induces mammary cancer, increased the level of 8-isoPGF2α in serum and urine of tested rats. In this model, a diet supplementation with zinc combined with selected polyphenolic compounds (resveratrol or genistein) caused statistically significant decrease in concentration of the studied biomarker (8-isoPGF2α) in rat serum and urine with simultaneous significant stimulation of carcinogenesis. Finally, the level of 8-isoPGF2α seen in the serum and urine in the rat model did not indicate a positive correlation with the risk of carcinogenesis, and this response, to some extent negates the value of 8-isoPGF2α as a potential determinant of early pathogenesis of cancer.
List of abbreviations
AAS: atomic absorption spectrophotometry, Cu: copper, DMBA: 7,12-dimethylbenz[a]anthracene, ELISA: enzyme-linked immunosorbent assay, SOD: superoxide dismutase, Zn: zinc, 8-isoPGF2α: 8-isoprostaglandin F2α.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
BB planed, designed and carried out the expeiment and performed the statistical analysis. AT coordinated the study. SB and MS carried out the ELISA analysis. All authors read and approved the final manuscript.
Contributor Information
Barbara Bobrowska, Email: barbara.bobrowska@wum.edu.pl.
Andrzej Tokarz, Email: andrzej.tokarz@wum.edu.pl.
Sławomir Białek, Email: slawomir.bialek@wum.edu.pl.
Małgorzata Seweryn, Email: m_seweryn@wp.pl.
Acknowledgements and Funding
This work was supported by the Ministry of Science and Higher Education grant NN405 358339
References
- Barros ACSD, Muranaka ENK, Mori LJ, Pelizon CHT, Iriya K, Giocondo G, Pinotti JA. Induction of experimental mammary carcinogenesis in rats with 7,12-dimethylbenz(a)anthracene. Rev Hosp Clin Fac Med S Paulo. 2004;59:257–261. doi: 10.1590/s0041-87812004000500006. [DOI] [PubMed] [Google Scholar]
- Griffiths RH, Møller L, Bartosz G, Bast A, Bertoni-Freddari C, Collins A, Cooke M, Coolen S, Haenen G, Hoberg AM, Loft S, Lunec J, Oliński R, Parry J, Pompella A, Poulsen H, Verhagen H, Astley SB. Biomarkers. Mol Aspects Med. 2002;23:101–208. doi: 10.1016/S0098-2997(02)00017-1. [DOI] [PubMed] [Google Scholar]
- Ozdol NC, Ates A, Aydintug OT, Melli M. Urinary 8-isoprostaglandin F2α level in Behcet's disease. Prostag Oth Lipid M. 2005;78:96–106. doi: 10.1016/j.prostaglandins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Franklin RB, Costello LC. Zinc as an anti-tumor agent in prostate cancer and in other cancers. Arch Biochem Biophys. 2007;463:211–217. doi: 10.1016/j.abb.2007.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabovsky AB, Komarov AM, Ivie JS, Buettner GR. Minimization of Free Radical Damage by Metal Catalysis of Multivitamin/Multimineral Supplements. Nutr J. 2010;9:61. doi: 10.1186/1475-2891-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar FH, Li Y. Mechanisms of cancer chemoprevention by soy isoflavone genistein. Cancer Metast Rev. 2002;21:265–280. doi: 10.1023/A:1021210910821. [DOI] [PubMed] [Google Scholar]
- Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Therapeut. 2001;90:157–177. doi: 10.1016/S0163-7258(01)00137-1. [DOI] [PubMed] [Google Scholar]
- Soobrattee MA, Neergheen VS, Luximon-Ramma A, Aruoma OI, Bahorun T. Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res. 2005;579:200–213. doi: 10.1016/j.mrfmmm.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Fremont L. Biological effects of resveratrol. Life Sci. 2000;66:663–673. doi: 10.1016/S0024-3205(99)00410-5. [DOI] [PubMed] [Google Scholar]
- Wakai K, Egami I, Kato K, Kawamura T, Tamakoshi A, Lin Y, Nakayama T, Wada M, Ohno Y. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;47:141–147. doi: 10.1207/S15327914NC330204. [DOI] [PubMed] [Google Scholar]
- Reeves PG, Nielsen FH, Fahey GC. AIN-93 purified diets for laboratory rodents: final raport of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- Kadiiska MB, Gladen BC, Baird DD, Graham LB, Parker CE, Ames BN, Basu S, FitzGerald GA, Lawson JA, Marnett LJ, Morrow JD, Murray DM, Plastaras J, Roberts LJ, Rokach J, Shigenaga MK, Sun J, Walter PB, Tomer KB, Barrett JC, Mason RP. Biomarkers of oxidative stress study: III. Effects of the nonsteroidal anti-inflammatory agents indomethacin and meclofenamic acid on measurements of oxidative products of lipids in CCl4 poisoning. Free Radical Bio Med. 2005;38:711–718. doi: 10.1016/j.freeradbiomed.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Bobrowska B, Tokarz A, Grynkiewicz G, Bialek S, Matysiak M, Bat-Erdene T. The effect of polyphenols on markers of oxidative damage and DMBA induced carcinogenesis in rats. J Food Lipids. 2009;16:103–112. doi: 10.1111/j.1745-4522.2009.01135.x. [DOI] [Google Scholar]
- Formigari A, Irato P, Santon A. Zinc, antioxidant system and metallothionein in metal mediated-apoptosis: biochemical and cytochemical aspects. Comp Biochem Phys C. 2007;146:443–459. doi: 10.1016/j.cbpc.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Tamura T, Olin KL, Goldenberg RL, Johnston KE, Dubard MB, Keen CL. Plasma extracellular superoxide dismutase activity in healthy pregnant women is not influenced by zinc supplementation. Biol Trace Elem Res. 2001;80:107–113. doi: 10.1385/BTER:80:2:107. [DOI] [PubMed] [Google Scholar]
- Subramanian P, Sivabalan S, Menon VP, Vasudevan K. Influence of chronic zinc supplementation on biochemical variables and circadian rhythms in wistar rats. Nutr Res. 2000;20:413–425. doi: 10.1016/S0271-5317(00)00134-2. [DOI] [Google Scholar]
- Montero A, Munger KA, Khan RZ, Valdivielso JM, Morrow JD, Guasch A, Ziyadeh FN, Badr KF. F2-isoprostanes mediate high glucose-induced TGF-ß synthesis and glomerular proteinuria in experimental type I diabetes. Kidney Int. 2000;58:1963–1972. doi: 10.1111/j.1523-1755.2000.00368.x. [DOI] [PubMed] [Google Scholar]
- Caccetta RA, Burke V, Mori TA, Beilin LJ, Puddey IB, Croft KD. Red wine polyphenols, in the absence of alcohol, reduce lipid peroxidative stress in smoking subjects. Free Radical Bio Med. 2001;30:636–642. doi: 10.1016/S0891-5849(00)00497-4. [DOI] [PubMed] [Google Scholar]
- Thomson CA, Stendell-Hollis NR, Rock CL, Cussler EC, Flatt SW, Pierce JP. Plasma and dietary carotenoids are associated with reduced oxidative stress in women previously treated for breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(10):2008–2015. doi: 10.1158/1055-9965.EPI-07-0350. [DOI] [PMC free article] [PubMed] [Google Scholar]