Abstract
The small GTPase Rac regulates cytoskeletal organization, cell cycle progression, gene expression and oncogenic transformation, processes that depend upon both soluble growth factors and adhesion to the extracellular matrix (ECM). We now show that growth factors and adhesion to the ECM both contribute independently and approximately equally to Rac activation. However, activated Rac in non-adherent cells failed to stimulate the Rac effector PAK. V12 Rac or Rac activated by serum translocated to the membrane fraction of adherent cells but remained mainly cytoplasmic in suspended cells. An activated Rac mutant lacking a membrane-targeting sequence did not activate PAK in adherent cells, while mutations that forced membrane targeting restored PAK activation in suspended cells. In vitro, V12 Rac showed greater binding to membranes from adherent relative to suspended cells, indicating that cell adhesion regulated membrane binding sites for Rac. These results show that ECM regulates the ability of Rac to couple with PAK via an effect on membrane binding sites that facilitate their interaction.
Keywords: integrin/membrane translocation/p21-activated kinase/Rac/Rho family GTPase
Introduction
Both soluble growth factors and integrin-mediated adhesion to the extracellular matrix (ECM) are required for many cellular processes such as growth, survival and migration (Klemke et al., 1994; Jones et al., 1995; Schwartz, 1997; Schwartz and Baron, 1999). Consistent with the idea that signals from these receptors must therefore be integrated properly, instances have been identified where induction of specific signaling events depends on both adhesion and growth factors. Examples where growth factors require cell adhesion to trigger optimal induction of signaling pathways include stimulation of inositol lipid hydrolysis and activation of the protein kinases Erk1, Erk2, JNK, Akt and phosphatidylinositol (PI) 3-kinase (McNamee et al., 1993; Khwaja et al., 1997; Lin et al, 1997; Renshaw et al., 1997; Short et al., 1998). However, the mechanisms by which signals from ECM receptors and growth factor receptors are integrated to exert this control are currently unclear.
It has been proposed that growth factor-induced signaling can occur within integrin-containing adhesion complexes (Plopper et al., 1995; Miyamoto et al., 1996), formation of which is regulated by Rho-family GTPases (Ridley and Hall, 1992; Nobes and Hall, 1995). The Rho-family GTPase Rac mediates a number of adhesion- and growth factor-dependent responses including formation of focal complexes and lamellipodia that function in the advancement of the leading edge of migrating cells and that can be stimulated with platelet-derived growth factor (PDGF) or by plating cells on ECM proteins (Ridley et al., 1992; Nobes and Hall, 1995; Clark et al., 1998; Price et al., 1998). Furthermore, adhesion to fibronectin strongly induces the kinase activity of the Rac and Cdc42 effector PAK, suggesting that integrin-mediated adhesion activates these GTPases (Price et al., 1998). Rac has also been shown to regulate G1 cell cycle progression (Olson et al., 1995; Westwick et al., 1997) and to participate in cellular transformation by Ras (Qiu et al., 1995). The growth of most untransformed cells is dependent on adhesion to the ECM, and detachment from the ECM leads to cell cycle arrest at G1 (Stoker et al., 1968). The notion that adhesion-dependent regulation of Rho-family GTPases could contribute to anchorage dependence of growth is therefore an intriguing possibility. Mechanisms for precise spatial and temporal integration of signals from both integrins and growth factor receptors may also be critical for coordination of cell movement (Klemke et al., 1994; Anand-Apte et al., 1997).
A number of distinct pathways downstream of Rac have been identified, including activation of JNK, formation of lamellipodia and focal complexes, cell migration, formation of cell–cell adhesions and endocytosis (reviewed in Hall, 1998; Mackay and Hall, 1998). Many potential effectors have been identified that bind preferentially to GTP-loaded Rac and that may mediate these effects. The best characterized effector proteins comprise the PAK family of 62–68 kDa serine/threonine kinases (reviewed in Knaus and Bokoch, 1998). Studies performed both in vitro and in vivo show that PAK1 binds active Rac or Cdc42, resulting in PAK autophosphorylation and increased kinase activity. While the downstream functions of PAK proteins are complex and somewhat controversial, PAK remains the only Rac effector whose activation state can be readily assayed in response to physiological stimuli. It may therefore provide a useful model for understanding mechanisms of GTPase coupling to effectors.
While performing studies designed to elucidate the role of integrins and growth factors in the activation of Rac, we made the unexpected observation that cell adhesion strongly influenced the ability of GTP-bound Rac to activate PAK1. Further experimentation revealed a role for subcellular compartmentalization in these effects. The data therefore define a novel mechanism of cooperation between cell adhesion and growth factors that may account for their interdependent effects on cell growth, gene expression and cell motility.
Results
Rac GTP loading
Previous work using a variety of indirect methods suggested that integrin-mediated cell adhesion could activate Rac (Clark et al., 1998; Price et al., 1998). To test this idea directly, pull-down assays were carried out in which Rac GTP-loading was determined by specific binding of the active GTPase to the p21-binding domain of PAK1 fused to glutathione S-transferase (GST–PBD) (Glaven et al., 1999). Serum-starved NIH 3T3 cells were detached with trypsin and after 3 h in suspension were replated on fibronectin-coated tissue culture dishes. Lysates were prepared, and the amount of Rac precipitated with the GST–PBD determined by Western blotting. These results showed that adhesion to fibronectin stimulated a transient increase in the levels of activated Rac (Figure 1A). These data are consistent with our published work demonstrating that membrane ruffling and PAK activity are stimulated transiently under these conditions (Price et al., 1998).
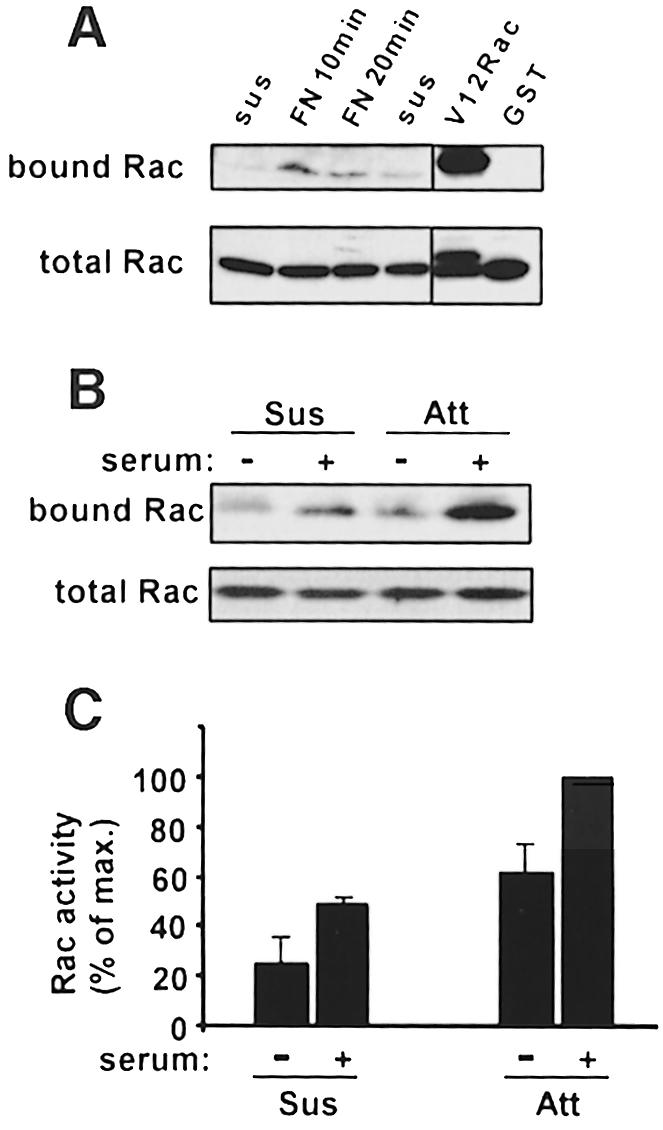
Fig. 1. Rac activation by cell adhesion and serum. (A) Serum-starved NIH 3T3 cells were detached and held in suspension for 3 h, then plated on fibronectin-coated dishes. Cell lysates were incubated with GST–PBD beads or GST-only beads, and bound GTP-Rac was detected by Western blotting. Right panel: cells transiently transfected with V12 Rac were used as a positive control to demonstrate specific binding to GST–PBD but not to GST. Lower panel: total cell lysates probed for Rac demonstrate equal amounts of total Rac. Data are representative of four independent experiments. (B) Attached (Att) or suspended (Sus) serum-starved cells were stimulated with 10% calf serum for 10 min, and Rac activity was assayed as described. Data are representative of four experiments. (C) Densitometric quantification of (B). Relative Rac activity was calculated from the amount of PBD-bound Rac normalized to the amount of Rac in whole-cell lysates, and then expressed as a percentage of the maximum in each experiment. Bar graphs represent means ± SEM of four experiments.
To explore the relationship between integrins and growth factors in the activation of Rac, we examined the effects of serum on the activation of Rac in adherent and suspended cells. Serum-starved cells were either kept adherent or detached and incubated in suspension for 3 h. Unstimulated adherent cells showed higher levels of Rac activity compared with unstimulated suspended cells (Figure 1B and C). Addition of 10% calf serum triggered activation of Rac under both conditions. Activation was detectable by 1 min after addition of serum, was maximal at ∼10 min and remained stable for >30 min in both adherent and suspended cells (not shown). Analysis at 10 min after serum stimulation in multiple experiments consistently showed that effects of adhesion and serum were additive (Figure 1C). Notably, levels of Rac activity in serum-stimulated suspended cells were similar to those in adherent cells without serum. We conclude that adhesion and growth factors in serum contribute independently and approximately equally to Rac GTP loading.
PAK kinase assays
PAK1 activity can be assayed readily by immune precipitation followed by an in-gel kinase assay using myelin basic protein (MBP) as a substrate. In contrast to Rac activity, PAK kinase activity was strongly induced by serum in adherent cells but was nearly undetectable in suspended cells with or without serum (Figure 2A). PAK activity in suspended cells was often too low to quantitate accurately, but was <2% of the signal in adherent cells. Time course experiments revealed little induction of PAK activity in suspended cells at any time between 2 and 60 min after addition of serum (not shown). PAK activity in suspended Rat-1 and Cos7 cells was slightly higher than in 3T3 cells but was still below 10% of the signal in adherent cells (data not shown). Experiments using purified PDGF instead of serum yielded essentially identical results (not shown). Additional experiments showed that replating cells on an antibody to the integrin β1 subunit restored PAK activation in response to serum, whereas adhesion to anti-CD44 did not, indicating that the effect is mediated specifically by integrins (Figure 2B).
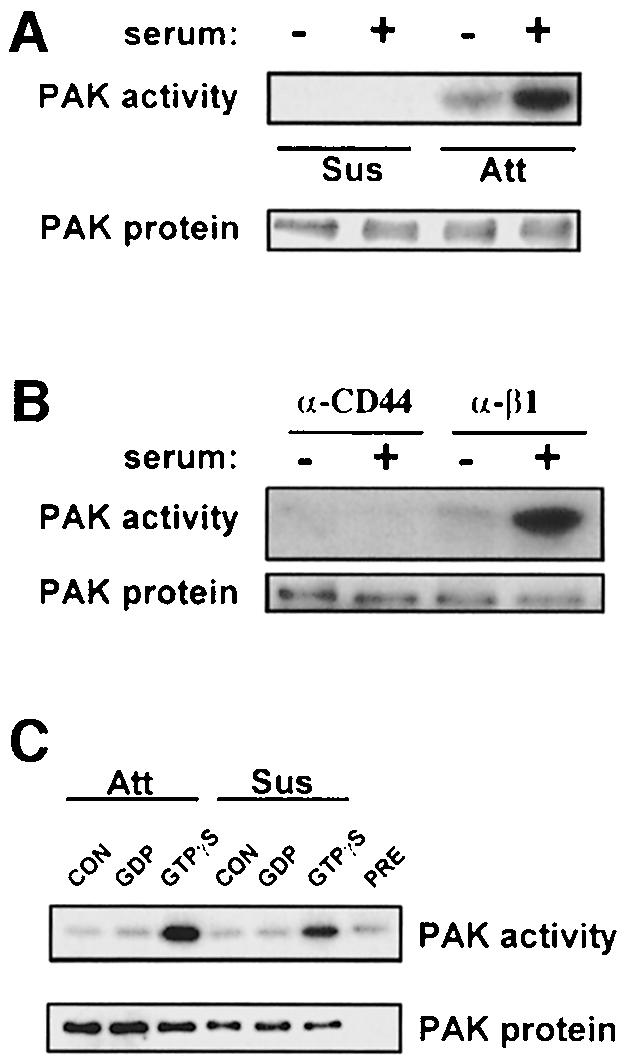
Fig. 2. PAK activation requires integrin-mediated cell adhesion. (A) Stably adherent serum-starved cells or cells in suspension for 3 h were stimulated with 10% serum for 10 min. PAK protein was immunoprecipitated and its kinase activity was assayed. Data are representative of six independent experiments. (B) Cells were maintained in suspension for 3 h and then replated on plastic coated with anti-mouse CD44 IgG or anti-mouse β1 integrin IgG. After 2 h, cells were stimulated with 10% serum for 10 min and PAK kinase activity assayed. Data are representative of two experiments. (C) PAK immunoprecipitates from serum-starved attached (Att) or suspended (Sus) cells were incubated without (CON) or with recombinant GST–Cdc42 loaded with GDP or GTPγS as indicated. PAK in vitro kinase activity was then assayed using MBP as substrate. Kinase activity from adherent cells immunoprecipitated with pre-immune serum (PRE) and incubated with GTPγS-loaded GST–Cdc42 is shown as a control. Data are representative of three experiments.
We next tested whether PAK in non-adherent cells is inherently insensitive to stimulation by active GTPases. PAK was immunoprecipitated from serum-starved adherent or suspended cells and incubated with recombinant Cdc42 loaded with GDP or GTPγS. Subsequent kinase assays showed that Cdc42-GTPγS stimulated activity of PAK from attached or unattached cells equally well (Figure 2C). Thus, PAK from suspended cells can be stimulated normally by an active GTPase in vitro.
Mutationally activated Rac
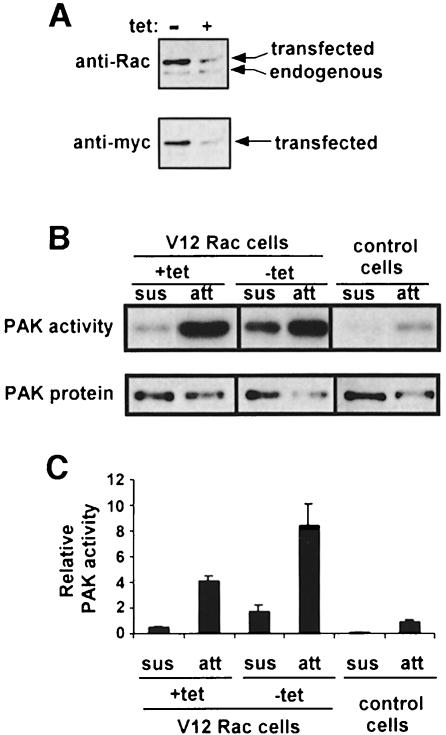
It is apparent from these data that adherent cells without serum had much higher PAK activity than suspended cells with serum, whereas Rac activity was similar under these two conditions (Figure 1C). Thus, activities of Rac and PAK in suspended and adherent cells show a significant discrepancy. To confirm that regulation of PAK by ECM was distinct from Rac activation, Rat-1 cells expressing V12 Rac under the control of a tetracycline (tet)-repressible promoter were examined. In the presence of tet, expression of Myc-tagged V12 Rac was similar to that of endogenous Rac, whereas in the absence of tet, V12 Rac expression was ∼5-fold higher (Figure 3A). In the presence of tet, V12 Rac strongly induced PAK activity in adherent cells; however, PAK activity was still down-regulated in suspended cells (Figure 3B and C). When the V12 Rac protein level was increased by withdrawal of tet, PAK activity became partially adhesion independent (Figure 3B and C). When Cos7 cells were transiently transfected with V12 Rac to obtain still higher expression, the activation of PAK was completely adhesion independent (not shown). These results clearly demonstrate that adhesion regulates PAK activation at a step that is distinct from GTP loading, and that this effect can be overcome by high expression of active Rac.
Fig. 3. V12 Rac is adhesion dependent. Rat-1 cells stably expressing Myc-tagged V12 Rac were cultured for 48 h in the presence or absence of tetracycline (tet) and extracts prepared. (A) Lysates were analyzed by Western blotting with anti-Rac and anti-myc mAbs to assess cellular levels of the V12 and endogenous Rac. (B) Control Rat-1 cells or V12 Rac cells were cultured with or without tet in low serum, then placed in suspension for 2 h or kept stably adherent. PAK was then immunoprecipitated and its kinase activity determined using the in-gel kinase assay. Data are representative of three independent experiments. (C) Densitometric quantitation of PAK kinase activity normalized for PAK protein levels; values are means ± SD from three experiments.
Rac membrane localization
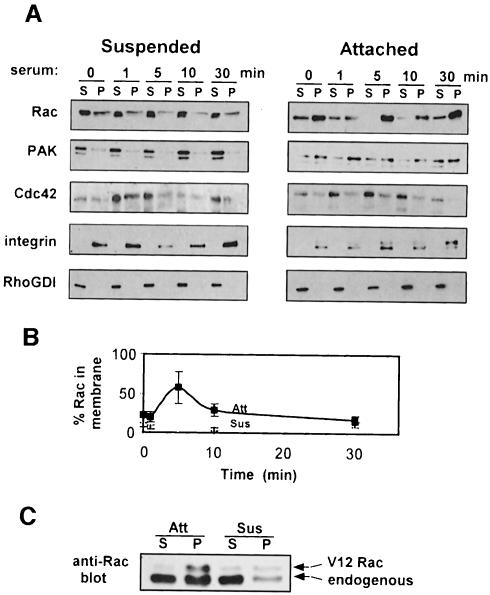
The above results are suggestive of differential compartmentalization of Rac and PAK in suspended cells, so that they interact poorly even when biochemically competent to do so. To test the effects of adhesion on protein compartmentalization, particulate and cytosolic fractions were prepared from adherent and suspended cells. Western blotting showed that Rac translocated to the membrane fraction in adherent cells following serum stimulation but completely failed to translocate to the membrane fraction in suspended cells (Figure 4A and B). Controls showed that the β1 integrin subunit was restricted to the particulate fraction, whereas RhoGDI was exclusively cytoplasmic (Figure 4A). PAK membrane localization was insensitive to serum but consistently showed a slight shift toward the membrane compartment in adherent cells (Figure 4A). However, quantitation of multiple experiments showed that this difference was only ∼2-fold. Cdc42, in contrast, showed a low level of membrane localization and no translocation in response to serum under any conditions (Figure 4A).
Fig. 4. Rac translocation to the particulate fraction. (A) Serum-starved attached (Att) or suspended (Sus) cells were stimulated with 10% serum and subjected to hypotonic lysis. The particulate (P) and soluble (S) fractions were isolated and samples with 10 µg of protein were analyzed. These amounts typically represent 1–2% of the cytoplasmic fraction and 10–20% of the membrane fraction. Samples were subjected to SDS–PAGE and Western blotting with anti-Rac, anti-PAK or anti-Cdc42. The anti-integrin β1 subunit and anti-RhoGDI were used as markers for the membrane and cytosol, respectively. Results are representative of three independent experiments. The shift in mobility of the β1 subunit is consistent with previously described changes in integrin processing in suspended cells (Dalton et al., 1995). (B) Densitometric quantification of Rac translocation. The amount of Rac was corrected for total protein and the percentage in the membrane fraction calculated. Values are means ± SEM from three separate experiments. (C) Soluble and particulate fractions from attached or suspended Rat-1-V12 Rac cells in the presence of tetracycline (10 µg each) were analyzed by Western blotting. The upper band co-migrates with the Myc-positive V12 Rac while the lower band co-migrates with endogenous Rac in untransfected cells. As in (A), 10 µg of the particulate and soluble fractions represents 10–20% and 1–2% of the total, respectively.
To test whether the effects of adhesion on compartmentalization were distinct from effects on GTP loading of Rac, the Rat-1 cells expressing activated Rac were also examined. V12 Rac showed enhanced localization to the membrane fraction in adherent cells compared with suspended cells (Figure 4C), demonstrating that the effect of cell adhesion on membrane localization of Rac is not due to differences in GTP loading.
Functional importance of membrane targeting
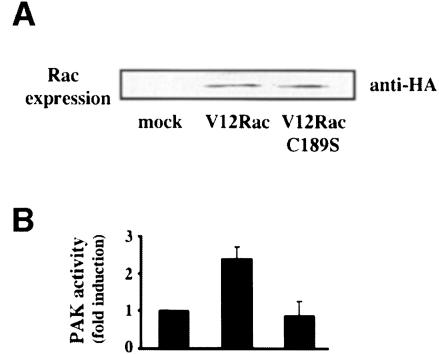
Previous studies have indicated that coupling of small GTPases to their effector pathways in cells generally requires membrane association (Mohr et al., 1990; Qiu et al., 1991), but this principle has not been tested specifically for Rac and PAK. To determine whether membrane localization was required for Rac activation of PAK, 3T3 cells were transiently transfected with normal V12 Rac or V12 Rac containing a point mutation in the CAAX membrane-targeting sequence (C189S) using a low copy mammalian expression vector to obtain moderate expression. At 48 h, adherent cells were extracted and endogenous PAK immunoprecipitated. Kinase assays showed that the membrane-targeted V12 Rac but not the mutant Rac increased PAK activity (Figure 5). This result demonstrates that membrane association of Rac is required for PAK activation.
Fig. 5. Membrane targeting is required for PAK activation. Normal V12 Rac or V12 Rac with a mutated CAAX sequence in the vector pBK-CMV were transiently transfected into NIH 3T3 cells. Transfection efficiency was ∼30%. (A) Western blots of lysate from transfected cells demonstrate equivalent expression of the Rac constructs. (B) At 24 h, cells were transferred to medium with 0.2% serum. At 48 h, the adherent cells were harvested and total endogenous PAK was immunoprecipitated. Both PAK protein and kinase activity were assayed and specific activity (kinase activity normalized for PAK protein) calculated. Values are means ± SD (n = 3).
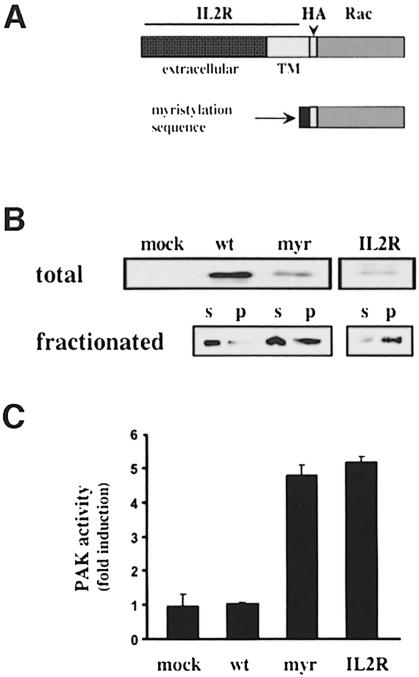
We also wished to test the importance of membrane targeting by determining whether localization of Rac to membranes in suspended cells would be sufficient to restore activation of PAK. We therefore constructed two mutants of Rac with stronger membrane-targeting sequences. In one, the myristylation sequence of src was added to the N-terminus of wild-type Rac to create a protein with two membrane-targeting sequences. In the second, wild-type Rac was brought to the inner surface of the plasma membrane by fusing it to the transmembrane region of the interleukin-2 (IL-2) receptor Tac subunit (Figure 6A). As expected, these constructs showed enhanced membrane localization in suspended 3T3 cells (Figure 6B). Cells were then transiently transfected with wild-type Rac or the membrane-targeted variants and placed in suspension at 48 h. After 3 h, cells were stimulated with serum and total endogenous PAK immunoprecipitated. Analysis of its kinase activity (normalized to levels of PAK protein) showed that the highly membrane-targeted constructs induced 5-fold activation of PAK in suspended cells (Figure 6C). Correcting for the 30% transfection efficiency suggests that stimulation was ∼15-fold. This increase occurred despite the low expression of the membrane-targeted mutants compared with wild-type Rac (Figure 6B). In contrast, the membrane-targeted constructs had no effect on PAK activity in adherent cells (not shown). These results demonstrate that membrane localization of Rac is sufficient to restore PAK activation in non-adherent cells.
Fig. 6. Restoring PAK activity in suspended cells. (A) Diagram of membrane-targeted Rac mutants. HA-tagged wild-type Rac was fused to the transmembrane domain of the Tac subunit of the IL-2 receptor or the myristylation sequence from c-src. (B) Upper panel: a Western blot with anti-HA antibody of total cell lysate from transfected cells. The myristylated and IL2R–Rac chimeras were expressed at substantially lower levels than wild-type HA-Rac. Inclusion of cDNA for green fluorescent protein showed that transfection efficiency was ∼30%. Lower panel: the transfected Rac proteins in the soluble and particulate fractions of non-adherent cells subjected to homogenization and fractionation. Wild-type Rac was primarily cytosolic whereas both mutants show enhanced localization to the particulate fraction. (C) Serum-starved cells were placed in suspension for 3 h, stimulated with serum for 10 min and total endogenous PAK immunoprecipitated. PAK protein and kinase activity were determined and the specific activity calculated. Values are means ± SD (n = 4).
Rac membrane binding sites
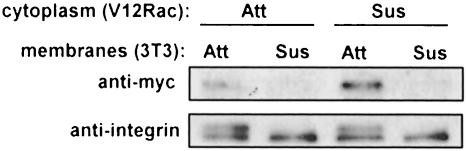
Effects on membrane binding of Rac might be due to post-translational modifications of Rac, to changes in interactions with RhoGDI that sequester Rac in the cytoplasm, or to differences in membrane binding sites. To distinguish these possibilities, cytosol from adherent or suspended V12 Rac-expressing cells was prepared, while the membrane fraction was isolated from adherent or suspended normal 3T3 cells. The V12 Rac-containing cytosol was then incubated with 3T3 membranes, the membranes sedimented and the presence of Rac in the membrane pellets assayed by Western blotting with anti-Myc antibody. V12 Rac from suspended cells showed somewhat higher binding to membranes than did Rac from adherent cells, consistent with the higher level of V12 Rac in the cytoplasm under these conditions (Figure 7). In both cases, however, binding to membranes from attached cells was substantially higher compared with membranes from suspended cells. Quantitation revealed that binding to membranes from adherent cells was consistently 3- to 7-fold higher than to membranes from suspended cells (n = 3). These results show that Rac from suspended cells is unimpaired in its ability to associate with membranes; instead, adhesion regulates Rac translocation via an effect on its membrane binding sites.
Fig. 7. In vitro membrane binding of V12 Rac. Cytosol from attached or suspended V12 Rac cells was incubated with equal amounts of membranes from attached or suspended NIH 3T3 cells. Membranes were collected by centrifugation and V12 Rac detected by Western blotting with anti-Myc. Western blotting for the integrin β1 subunit demonstrates equivalent levels of membrane protein in each sample. Data are representative of three independent experiments.
Discussion
These data, in addition to directly demonstrating regulation of Rac GTP loading by cell adhesion to ECM, reveal that adhesion regulates the ability of active Rac to stimulate PAK kinase activity. This effect is highly potent as PAK activity in suspended cells is at most a few percent of the level in adherent cells. These effects occur under conditions where activity of endogenous Rac varies by only ∼2-fold and were confirmed using constitutively active V12 Rac.
The results also show that differences in compartmentalization are responsible for these effects. Serum stimulation triggers membrane translocation of Rac in adherent cells, whereas virtually no membrane translocation was detected in suspended cells. V12 Rac also showed markedly higher membrane association in adherent compared with non-adherent cells. That membrane localization is functionally important was demonstrated by the observation that mutation of the membrane-targeting sequence abrogated PAK stimulation by V12 Rac in adherent cells, while forcing membrane association of wild-type Rac in suspended cells restored PAK activity.
PAK is also an effector for Cdc42 (Knaus and Bokoch, 1998) and, while the major growth factors in serum are not thought to activate Cdc42, we cannot exclude the possibility that Cdc42 may also contribute to PAK activation in serum-stimulated adherent cells. However, no serum-induced translocation of Cdc42 was detected even in adherent cells. Thus, whether Cdc42 is adhesion dependent in a manner similar to Rac remains to be investigated. In any case, experiments with V12 Rac unambiguously demonstrated that Rac localization and coupling to PAK are regulated by cell adhesion.
We also considered the possibility that adhesion may regulate PAK localization and function directly, as PAK distribution also showed some shift toward the cytosol in suspended cells. However, the effect of adhesion on localization of PAK was relatively weak compared with Rac, while the highly membrane-targeted Rac mutants were effective at restoring PAK activity in suspended cells. We therefore suggest that Rac is the major target for regulation by adhesion and that effects on PAK localization are secondary to changes in Rac. Consistent with this idea, expression of the highly membrane-localized Rac variants caused an increase in the membrane association of PAK in suspended cells (not shown).
Finally, to explore the mechanism by which cell adhesion influences Rac association with membranes, in vitro binding assays with V12 Rac and isolated membrane were carried out. These assays showed that Rac from non-adherent cells was fully competent to bind to membranes, whereas Rac from either adherent or suspended cells bound well only to membranes from adherent cells. Thus, adhesion regulates the membrane binding sites for Rac.
Membrane localization is critical for function of Ras-family proteins, even when mutationally activated (Mohr et al., 1990; Qiu et al., 1991). Recent studies have indicated that membrane localization of Ras is more complex than previously suspected, involving transit through the endoplasmic reticulum and Golgi (Choy et al., 1999). However, Rac and other Rho-family GTPases differ from Ras in that, despite the presence of a hydrophobic prenyl group at their C-termini, they rapidly translocate between membrane and cytosolic compartments (Abo et al., 1994; Fleming et al., 1996). The factors that regulate association of Rho-family GTPases with membranes are incompletely understood, but RhoGDI clearly plays a role in this process since it promotes dissociation of GTPases from membranes and sequesters them in the cytosol (Abo et al., 1994; Nomanbhoy et al., 1999). Nucleotide exchange factors have also been proposed to be involved, since they are often membrane bound and are believed to interact with GTPases at the plasma membrane (Bokoch et al., 1994; Michaels et al., 1997). Our data, however, argue against a critical role for GEFs in the adhesion-dependent localization, as the effect was clearly separable from GTP loading.
It has been reported that forced membrane localization of PAK also strongly enhances its activation (Lu et al., 1997). Thus, available evidence argues that interaction of Rac with its effectors occurs preferentially in an adhesion-dependent membrane compartment that enhances the efficiency of their interaction. Overexpression of Rac probably overcomes the requirement for adhesion by mass action, so that high levels of Rac can promote the activation of PAK even if the association is less efficient. The observation that this occurs by regulating membrane binding sites also raises the interesting possibility that adhesion may control the subcellular localization of Rac–effector interactions within cells. Thus, growth factors could globally activate Rac but the locations at which cytoskeletal structures form could be determined by adhesive interactions.
The major biological functions of Rac, including cell growth, migration and gene expression, are strongly dependent on cell interactions with ECM. For example, stimulation of JNK by cytokines or c-fos expression by growth factors is minimal in non-adherent endothelial cells or fibroblasts (Wary et al., 1996; Short et al., 1998). In the case of migration, subcellular control of Rac interaction with effectors would allow cells to determine where to extend lamellipodia on the basis of contacts with ECM, which would facilitate efficient cell movement. Elucidating how Rac-dependent pathways integrate information from integrins and growth factors is therefore essential to understanding their functions in the context of intact tissues. Our data show that Rac effector functions can be regulated by adhesion to ECM independently of GTP loading via an effect on compartmentalization. Further investigation of the mechanisms governing membrane targeting of Rac is clearly warranted.
Materials and methods
Cell culture
NIH 3T3 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% calf serum, penicillin and streptomycin (Gibco-BRL, Gaithersberg, MD). Cells were starved for 24 h in 0.2% serum prior to stimulation and assays of Rac or PAK. Rat-1 cells expressing V12 Rac were cultured routinely in DMEM with 10% fetal bovine serum in the presence of 0.4 mg/ml G418, 2.5 µg/ml puromycin and 2 µg/ml tetracycline; where indicated, tet was withdrawn 24 h before the experiment. For suspension, 2 × 106 cells in DMEM with 0.2% bovine serum albumin (BSA) were plated in 15 cm bacterial plastic dishes coated with 10 mg/ml heat-denatured BSA. For replating on antibodies, bacterial plastic dishes were coated with either 25 µg/ml rabbit anti-hamster IgG (Sigma Chemicals, St Louis, MO) followed by 10 µg/ml HMβ1-1 hamster anti-mouse β1 integrin IgG (Pharmingen, San Diego, CA), or 25 µg/ml goat anti-rat IgG (Sigma) followed by 10 µg/ml 5D2-27 rat anti-mouse CD44 IgG (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA). Subsequently, dishes were blocked with 10 mg/ml heat-denatured BSA. For transfection experiments, cells in 60 mm tissue culture dishes at ∼50% confluence were transfected with 2.0 µg of total DNA using the Effectene reagent according to the manufacturer’s instructions (Qiagen, San Diego, CA); 0.2 µg of vector for green fluorescent protein was included routinely to estimate transfection efficiency, which was consistently ∼30%. Cell lysis and analysis were performed 48 h after transfection as described for each experiment.
Membrane-targeted Rac constructs
The cDNA for human wild-type Rac containing a hemagglutinin (HA) tag at the 5′ end was subcloned into the HindIII site of the pcDNA3 IL2R construct lacking a cytoplasmic domain (Miyamoto et al., 1996). The resulting fusion protein has the extracellular and transmembrane domains of the Tac subunit of the IL-2 receptor with HA-Rac replacing the cytoplasmic domain. For the myristylated variant, the coding sequence for the 12 N-terminal residues of c-src (MGSSKSKPKDPS) was fused to the 5′ end of the HA-Rac sequence and subcloned into pcDNA3. Both constructs were confirmed by DNA sequencing.
GTPase assays
NIH 3T3 cells were serum starved overnight then detached and kept in suspension in DMEM with 0.2% BSA for 3 h or maintained attached in the same medium. Cells were then stimulated with serum or plated on dishes coated with 25 µg/ml fibronectin. At the times indicated, cells were chilled on ice, washed with ice-cold phosphate-buffered saline (PBS) and lysed in buffer containing 0.5% NP-40, 50 mM Tris pH 7.4, 150 mM NaCl, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 µg/ml aprotinin, 1 µg/ml leupeptin and 20 µg of recombinant GST–PBD. Lysates were then incubated with glutathione–agarose beads (Pharmacia) for 30 min at 4°C, washed with lysis buffer and eluted with SDS sample buffer. Bound Rac was analyzed by Western blotting using a monoclonal anti-Rac antibody (Upstate Biotechnology, Lake Placid, NY). Whole-cell lysates were also analyzed for the presence of Rac for normalization.
PAK kinase assays
PAK was precipitated from cell lysates with anti-PAK1 antibodies (polyclonal anti-PAK1 R626; Dharmawardhane et al., 1997) and kinase activity determined using an in-gel kinase assay with MBP as substrate, as previously described (Price et al., 1998). PAK protein in the precipitates was determined by Western blotting with the same antibody. In some cases, autoradiographs were quantitated by scanning densitometry and the specific activity (kinase activity normalized to PAK protein) calculated.
In vitro PAK stimulation
PAK immunoprecipitations were rinsed once and suspended in 20 µl of kinase buffer containing 20 mM HEPES pH 7.6, 20 µM β-glycerophosphate, 20 mM p-nitrophenylphosphate, 10 mM MgCl2, 1 mM dithiothreitol (DTT) and 0.5 mM sodium orthovanadate. Recombinant GST–Cdc42 was loaded with GDP or GTPγS as described (Chong et al., 1994), and 10 µl of kinase buffer containing 0.5 µg of GST–Cdc42-GDP or GST–Cdc42-GTPγS was added. The reaction was initiated by addition of 5 µCi of [γ-32P]ATP and 20 µM cold ATP. Samples were incubated for 20 min at 30°C. The reaction was stopped by placing the samples on ice and adding 1 mM cold ATP. Supernatants and beads were separated, and the supernatants containing the phosphorylated MBP were analyzed by SDS–PAGE on 15% gels and by autoradiography. The beads containing the immunoprecipitated PAK were analyzed by SDS–PAGE and Western blotting with anti-PAK to normalize for PAK protein levels. Results were quantitated by densitometry.
Subcellular fractionation
Adherent and suspended cells (∼1 × 107) were washed, drained and treated with ice-cold hypotonic lysis buffer (10 mM Tris pH 7.4, 1.5 mM MgCl2, 5 mM KCl, 1 mM DTT, 0.2 mM sodium vanadate, 1 mM PMSF, 1 µg/ml aprotinin, 1 µg/ml leupeptin) for 5 min. Adherent cells were scraped and cell lysates were homogenized with 15 strokes of a Dounce homogenizer. Homogenates were centrifuged at 2000 r.p.m. for 3 min to pellet nuclei and intact cells, and supernatants were then spun at 15 000 r.p.m. at 4°C for 30 min in a refrigerated microcentrifuge to sediment plasma membranes. The cytosol-containing supernatant was removed and the crude membrane pellet gently washed with hypotonic lysis buffer. Membrane and cytosol fractions were then assayed for total protein, and equal amounts were analyzed by Western blotting and quantitated by scanning densitometry.
Acknowledgments
Acknowledgements
We thank Dr Marc Symons (Picower Institute, Manhasset, NY) for providing Rat-1 cells that express V12 Rac, Tim Reed for providing the C189S Rac mutant, and Gary Bokoch for providing anti-PAK serum. We thank Bette Cessna for excellent secretarial assistance. This work was supported by Human Frontier Science Program #LT0019/1998-M (to M.A.d.P.) and USPHS grant RO1 GM47214 (to M.A.S).
References
- Abo A., Webb,M.R., Grogan,A. and Segal,A.W. (1994) Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochem. J., 298, 585–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand-Apte B., Zetter,B.R., Viswanathan,A., Qui,R.G., Chen,J., Ruggieri,R. and Symons,M. (1997) Platelet-derived growth factor and fibronectin stimulated migration are differentially regulated by the Rac and extracellular signal regulated kinase pathways. J. Biol. Chem., 272, 30688–30692. [DOI] [PubMed] [Google Scholar]
- Bokoch G.M., Bohl,B.P. and Chuang,T.H. (1994) Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J. Biol. Chem., 269, 31674–31679. [PubMed] [Google Scholar]
- Chong L.D., Traynor-Kaplan,A., Bokoch,G.M. and Schwartz,M.A. (1994) The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell, 79, 507–513. [DOI] [PubMed] [Google Scholar]
- Choy E., Chiu,V.K., Siletti,J., Feoktisov,M., Morimoto,T., Michaelson,D., Ivanov,I.E. and Phillips,M.R. (1999) Endomembrane trafficking of Ras: the CAAX motif targets proteins to the ER and Golgi. Cell, 98, 69–80. [DOI] [PubMed] [Google Scholar]
- Clark E.A., King,W.G., Brugge,J.S. Symons,M. and Hynes,R.O. (1998) Integrin mediated signals regulatd by members of the Rho family of GTPases. J. Cell Biol., 142, 573–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton S.L., Scharf,E., Briesewitz,R., Marcantonio,E.E. and Assoian,R.K. (1995) Cell adhesion to extracellular matrix regulates the life cycle of integrins. Mol. Biol. Cell, 6, 1781–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhane S., Sanders,L.C., Martin,S.S., Daniels,R.H. and Bokoch,G.M. (1997) Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J. Cell Biol., 138, 1265–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming I.N., Elliot,C.M. and Exton,J.H. (1996) Differential translocation of Rho family GTPases by lysophosphatidic acid, endothelin-1 and platelet derived growth factor. J. Biol. Chem., 271, 33067–33073. [DOI] [PubMed] [Google Scholar]
- Glaven J.A., Whitehead,I., Bagrodia,S., Kay,R. and Cerione,R.A. (1999) The Dbl-related protein Lfc localizes to microtubules and mediates the activation of Rac signaling pathways in cells. J. Biol. Chem., 274, 2279–2285. [DOI] [PubMed] [Google Scholar]
- Hall A. (1998) Rho GTPases and the actin cytoskeleton. Science, 279, 509–514. [DOI] [PubMed] [Google Scholar]
- Jones J.I., Doerr,M.E. and Clemmons,D.R. (1995) Cell migration: interactions among integrins, IGFs and IGFBPs. Prog. Growth Factor Res., 6, 319–327. [DOI] [PubMed] [Google Scholar]
- Khwaja A., Rodriguez-Viciana,P., Wennstrom,S., Warne,P.H. and Downward,J. (1997) Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J., 16, 2783–2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke R.L., Yebra,M., Bayna,E.M. and Cheresh,D.A. (1994) Receptor tyrosine kinase signaling required for integrin αvβ5-directed cell motility but not adhesion on vitronectin. J. Cell Biol., 127, 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus U.G. and Bokoch,G.M. (1998) The p21Rac/Cdc42-activated kinases (PAKs). Int. J. Biochem. Cell Biol., 30, 857–862. [DOI] [PubMed] [Google Scholar]
- Lin T.H., Chen,Q., Howe,A. and Juliano,R.L. (1997) Cell anchorage permits efficient signal transduction between ras and its downstream kinases. J. Biol. Chem., 272, 8849–8852. [PubMed] [Google Scholar]
- Lu W., Katz,S., Gupta,R. and Mayer,B.J. (1997) Activation of Pak by membrane localization mediated by an SH3 domain from the adapter protein Nck. Curr. Biol., 7, 85–94. [DOI] [PubMed] [Google Scholar]
- Mackay D.J. and Hall,A. (1998) Rho GTPases. J. Biol. Chem., 273, 20685–20688. [DOI] [PubMed] [Google Scholar]
- McNamee H.M., Ingber,D.E. and Schwartz,M.A. (1993) Adhesion to fibronectin stimulates inositol lipid synthesis and enhances PDGF-induced inositol lipid breakdown. J. Cell Biol., 121, 673–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels F., Stam,J.C., Hordijk,P.L., VanderKammen,R.A., Ruuls-VanStalle,L., Feltkamp,C.A. and Collard,J.G. (1997) Regulated membane localization of Tiam-1, mediated by the NH2-terminal pleckstrin homology domain, is required for Rac-dependent membrane ruffling and c-jun NH2-terminal kinase activity. J. Cell Biol., 137, 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto,H., Gutkind,J.S. and Yamada,K.M. (1996) Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol., 135, 1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr C., Just,I., Hall,A. and Aktories,K. (1990) Morphological alterations of Xenopus oocytes induced by valine-14 p21Rho depend on isoprenylation and are inhibited by Clostridium botulinum C3 ADP-ribosyltransferase. FEBS Lett., 275, 168–172. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1995) Rho, Rac and Cdc42 GTPases regulate the assembly of multi-molecular focal complexes associated with actin stress fibers, lamellipodia and filopodia. Cell, 81, 53–62. [DOI] [PubMed] [Google Scholar]
- Nobes C.D. and Hall,A. (1999) Rho GTPases control polarity, protrusion and adhesion during cell movement. J. Cell Biol., 144, 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomanbhoy T.K., Erickson,J.W. and Cerione,R.A. (1999) Kinetics of Cdc42 membrane extraction by Rho-GDI monitored by real-time fluorescence resonance energy transfer. Biochemistry, 38, 1744–1750. [DOI] [PubMed] [Google Scholar]
- Olson M.F., Ashworth,A. and Hall,A. (1995) An essential role for rho, rac and cdc42 GTPases in cell cycle progression through G1. Science, 269, 1270–1272. [DOI] [PubMed] [Google Scholar]
- Plopper G.E., McNamee,H.P., Dike,L.E., Bojanowski,K. and Ingber,D.E. (1995) Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol. Biol. Cell, 6, 1349–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L.S., Leng,J., Schwartz,M.A. and Bokoch,G.M. (1998) Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol. Biol. Cell, 9, 1863–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu M.-S., Pitts,A.F., Winter,T.R. and Green,S.H. (1991) Ras isoprenylation is required for ras-induced but not for NGF-induced neuronal differentiation in PC12 cells. J. Cell Biol., 115, 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu R.G., Chen,J., Kirn,D., McCormick,F. and Symons,M. (1995) An essential role for Rac in Ras transformation. Nature, 374, 457–459. [DOI] [PubMed] [Google Scholar]
- Renshaw M.W., Ren,X.D. and Schwartz,M.A. (1997) Activation of the MAP kinase pathway by growth factors requires integrin-mediated cell adhesion. EMBO J., 16, 5592–5599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley A.J. and Hall,A. (1992) The small GTP binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell, 70, 389–399. [DOI] [PubMed] [Google Scholar]
- Ridley A.J., Paterson,H.F., Johnston,C.L., Diekmann,D. and Hall,A. (1992) The small GTP-binding protein rac regulates growth factor induced membrane ruffling. Cell, 70, 401–410. [DOI] [PubMed] [Google Scholar]
- Schwartz M.A. (1997) Integrins, oncogenes and anchorage independence. J. Cell Biol., 139, 575–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.A. and Baron,V. (1999) Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol., 11, 197–202. [DOI] [PubMed] [Google Scholar]
- Short S.M., Talbott,G.A. and Juliano,R.L. (1998) Integrin-mediated signaling events in human endothelial cells. Mol. Biol. Cell, 9, 1969–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., O’Neill,C., Berryman,S. and Waxman,V. (1968) Anchorage and growth regulation in normal and virus-transformed cells. Int. J. Cancer, 3, 683–693. [DOI] [PubMed] [Google Scholar]
- Wary K.K., Maneiro,F., Isakoff,S.J., Marcantonio,E.E. and Giancotti,F.G. (1996) The adapter protein Shc couples a class of integrins to the control of cell cycle progression. Cell, 87, 733–743. [DOI] [PubMed] [Google Scholar]
- Westwick J.K., Lambert,Q.T., Clark,G.J., Symons,M., VanAelst,L., Pestell,R.G. and Der,C.J. (1997) Rac regulation of transformation, gene expression and actin organization by multiple PAK-independent pathways. Mol. Cell. Biol., 17, 1324–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]