Abstract
Expression and purification of recombinant human paraoxonase-1 (rHuPON1) from bacterial systems have proven elusive. Most systems for successful production of recombinant PON1 have relied on either eukaryotic expression in baculovirus or prokaryotic expression of synthetic, gene-shuffled rabbit-mouse-human PON1 hybrid molecules. We review here methods and protocols for the production of pure, native rHuPON1 using an E. coli expression system followed by conventional column chromatographic purification. The resulting rHuPON1 is stable, active, and capable of protecting PON1 knockout mice (PON1-/-) from exposure to high levels of the organophosphorus (OP) compound diazoxon. Bacterially-derived rHuPON1 can be produced in large quantities and lacks the glycosylation of eukaryotic systems that produces immunogenic complications when used as a therapeutic. The rHuPON1 should be useful for treating insecticide OP exposures and reducing risks of other diseases resulting from low PON1 status. The ease of mutagenesis in bacterial systems will also allow for the generation and screening of rHuPON1 variants with enhanced catalytic efficiencies against nerve agents and other OP compounds.
Introduction
Each year there are approximately 2.5 million insecticide poisonings resulting in 250,000 deaths (WHO 1986, 1990). Many of these poisonings result from exposure to organophosphorus (OP) insecticides. In addition to accidental exposure to insecticides, the possibility exists of deliberate exposure to OP nerve agents or toxic industrial OPs. One approach for treating exposure to OP compounds has been the injection of stoichiometric scavengers such as butyrylcholinesterase (Ashani et al. 1991, Broomfield et al. 1991). The advantage of using butyrylcholinesterase (BChE) as a therapeutic is that it will bind most toxic OP compounds; however, the disadvantage of using a stoichiometric scavenger is that it binds only a single molecule of an OP per large protein molecule. An attractive therapeutic alternative is the use of catalytic scavengers that hydrolyze many OP molecules per each injected protein molecule (Li et al. 2000, Lenz et al. 2007).
Paraoxonase-1 (PON1) is a leading candidate for use as a catalytic scavenger of toxic OP compounds. PON1 is capable of hydrolyzing a wide range of substrates including the OP pesticide products paraoxon (PO), diazoxon (DZO) and chlorpyrifos oxon (CPO), as well as nerve agents such as VX, VR, sarin and soman (Davies et al. 1996, Lenz et al. 2007). One requirement for a useful catalytic scavenger will be minimal immune reaction to the injected protein. The most promising approach to achieve this goal will be the use of engineered proteins of human origin. Other enzymes, such as bacterial phosphotriesterases, are capable of inactivating organophosphate toxins, but these are not of human origin (reviewed in Raushel 2002). As PON1 is a native human protein, it will be much less likely to elicit an immune response compared to OP hydrolases from other organisms. OP hydrolysis is generally viewed as a promiscuous function of PON1 - its actual biological role is still debated (James 2006) though it appears to be important for protecting against cardiovascular disease (Jarvik et al. 2000) and Pseudomonad infection (Ozer et al. 2005, Stoltz et al. 2008). PON1’s antioxidant capability and association with HDL suggests that injectable, therapeutic rHuPON1 will have other possible applications such as prevention of blood vessel re-occlusion following intervention. The Q192R polymorphism in human PON1 affects the catalytic efficiency of hydrolysis for some OP substrates. Rabbit PON1 with lysine (K) at position 192 has a very high hydrolysis of chlorpyrifos oxon (Furlong et al. 1989, Hassett et al. 1991).
An effective catalytic scavenger must have a high catalytic efficiency in order to detoxify and protect against specific compounds (Li et al. 2000). For many years, it was thought that high levels of PON1 would protect against exposure to PO, from which PON1 derives its name. However, after the knockout mouse model was developed, this was shown to be incorrect. The PON1-/- mice were significantly more susceptive to CPO (Shih et al. 1998) and DZO (Li et al. 2000); however, the PON1-/- mice did not differ from wild-type mice in their susceptibility to PO. Similar results were found when mice were injected with purified human PON1 enzyme to test protection in vivo. The PON -/- mice were protected from inhibition of brain acetylcholinesterase when given PONR192 or PONQ192 and then dosed with either CPO or DZO. Neither purified PON alloform was able to protect mice against exposure to PO. The catalytic efficiency was determined for hydrolysis of CPO, DZO and PO for both PONR192 and PONQ192. Both PON1s had nearly equivalent catalytic efficiency for DZO, reflected in the equal protection provided when injected. PONR192 had higher catalytic efficiency for hydrolysis of CPO, and provided better protection than PONQ192 against CPO exposure. The PON1R192 alloform was nearly nine times more efficient than PONQ192 at hydrolyzing PO, but the overall catalytic efficiency was so low for both alloforms that neither could protect against PO exposure (Li et al. 2000). These experiments provided convincing evidence that engineered increases in catalytic efficiency will be required for protection against PO (and most likely nerve agents as well). We estimate that a ten-fold increase in catalytic efficiency of PON1R192 will be sufficient to provide in vivo protection against PO exposure (Li et al. 2000).
Based on this previous work, the three important requirements for the development of a catalytic scavenger are minimal immunogenicity, a catalytic efficiency sufficient to protect against the specific compound and a good half-life following injection. With PON1, the development of rapid screening protocols for identifying variants with improved catalytic efficiency of hydrolysis is necessary. To prevent decoration of the scavenger with immunogenic carbohydrate chains, PON1 will be best expressed in a non-eukaryotic system. If active recombinant human PON1 (rHuPON1) could be expressed in an E. coli system, two of these goals would be realized. This system would also provide a more convenient system for scale-up production and for rapid screening of variants with increased catalytic efficiencies.
The difficulty of using E. coli as a host has been noted previously (Brushia et al. 2001). In attempts to produce a more soluble version of PON1, Aharoni et al. (2004) used a gene-shuffling protocol to express a soluble variant of PON1 that was used to determine the first crystal structure of a PON1 enzyme (Harel et al. 2004). The resulting PON1 sequence was closer to rabbit PON1 than human PON1, with 91% identity to wild-type rabbit PON1. This hybrid protein would most likely not be ideal for use as a prophylactic or therapeutic drug in humans; to avoid immunological complications it would be desirable to have a protein therapeutic that is as close as possible to the native human sequence, with minimal changes necessary for increasing catalytic efficiency.
The purification of PON1, a highly hydrophobic protein, has also proved to be an obstacle in the development of a catalytic scavenger. Much of the early PON1 purification efforts used human serum as starting material. These early efforts produced quite pure PON1 following several chromatographic steps (Gan et al. 1991, Furlong et al. 1991). Recent improvements have reduced the number of steps required to purify human plasma PON1. Sinan et al. (2006) purified plasma PON1 with two steps, precipitation and hydrophobic interaction chromatography.
Expression and Purification of rHuPON1 from E. coli
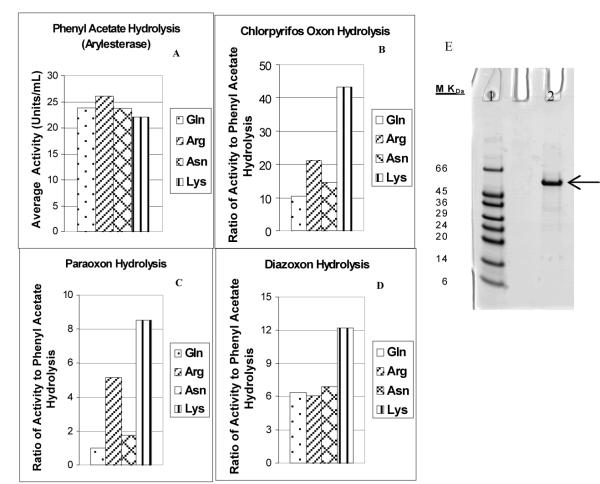
To work out conditions for producing active human PON1 in an E. coli expression system, we expressed a GST-tagged rHuPON1 protein and demonstrated that active rHuPON1 fusion protein could be expressed and purified from E. coli. This expression system also allowed us to examine the effects of amino acid substitutions on the catalytic efficiency of the rHuPON1. Rabbit PON1 protein sequence (Hassett et al. 1991) suggested the possibility that substituting the 192 amino acid with lysine would lead to increased catalytic efficiency against OPs. We knew that a single amino acid substitution could produce a large change in catalytic rates based on the variation seen in humans with the two natural PON1192 alloforms. The first E. coli system used the popular pET vectors and produced active rHuPON1 with a GST tag for ease of purification. Both native human PON1192 alloforms as well as the K192 variant were generated and characterized. Since the Q192 alloform of plasma PON1 is more catalytically active against nerve agents than the R192 alloform, a variant with asparagine (N) at position 192 was also expressed and characterized. Because the PON1192 polymorphism has little effect on the activity of the enzyme towards phenyl acetate, we used the ratio of OP hydrolytic substrate activity to phenyl acetate hydrolytic activity for all four constructs. The PON1K192 alloform did indeed show an increased activity against the three OPs tested (CPO, PO and DZO) relative to phenyl acetate (Figure 1).
Figure 1.
GST-PON1 produced in E. coli. (A) Equivalent rates of arylesterase activity, (B, C, D) ratios of rates of hydrolysis of chlorpyrifos oxon, paraoxon and diazoxon, respectively, to rates of phenyl acetate hydrolysis in rHuPON1 variants with Q, R, N or K at position 192. (E) SDS gel electrophoretic analysis of purified rHuPON1Q192 fusion protein (arrow). Lane 1, molecular weight markers; Lane 2, purified rHuPON1Q192 fusion protein.
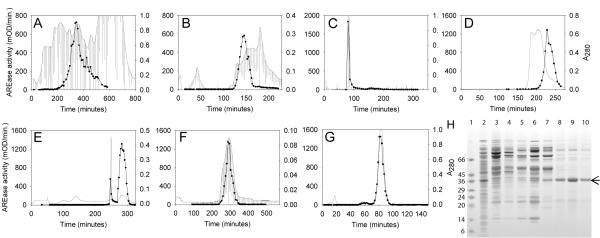
We have recently purified and characterized E. coli-generated native, wild-type human rHuPON1Q192 and rHuPON1R192 alloforms, as well as the rHuPON1K192 variant. These rHuPON1192 proteins were purified to greater than 90% homogeneity (Stevens et al. 2008) A new expression system was developed to produce the untagged native rHuPON1 which would be ideal for a therapeutic protein. We used the Eurogentec Staby system, that employs a toxin-antitoxin method to force the expressing cells to retain the plasmid (Couturier et al. 1992). Another plasmid, pRARE, that encodes tRNAs common in mammalian proteins but rare in bacterial proteins, was co-transformed to increase yields (Invitrogen). This E. coli expression system produced active protein. Cells were grown in a 14 L fermenter, harvested, then lysed with glass beads in a detergent-containing buffer and purified using column chromatography. The final series of columns utilized multiple ion exchange DEAE columns with and without detergent at different pH values and a hydrophobic interaction column with a detergent-containing buffer elution (Figure 2).
Figure 2.
Purification of the untagged rHuPON1K192 variant. Column steps were: A, DEAE 1; B, DEAE 2; C, Hydroxyapatite (HA); D, DEAE 3; E, Hydrophobic interaction column (HIC); F, Gel filtration (GF); and G, DEAE 4. The activity traces are shown as black lines with data points and the A280 traces are shown as continuous grey lines with fraction ticks. H, shows an SDS-PAGE analysis of the pooled fractions from each step: Lane 1, molecular weight markers; Lane 2, cell extract; Lane 3, DEAE 1; Lane 4, Desalting column; Lane 5, DEAE 2; Lane 6, HA; Lane 7, DEAE 3; Lane 8, HIC; Lane 9, GF and Lane 10, DEAE 4. Arrow represents the mobility of rHuPON1. Figure reproduced from Stevens et al. 2008.
Kinetic Properties of rHuPON1192 Variants
Kinetic analysis was performed using all three variants of PON1. Notably, PON1K192 had a catalytic activity approximately twice that of PON1R192 for several OP substrates (CPO, DZO, PO). However, the increased efficiency towards PO is most likely not yet high enough to protect against a PO exposure in the PON1-/- mouse model. The Km values for PON1K192 were higher relative to PON1R192; however, the Vmax was also much higher for the PON1K192 variant. Catalytic efficiencies for the two natural rHuPON1 human alloforms were similar to those of PON1 purified from serum (Li et al. 2000) (Table 1). Initial turnover values for the rHuPON1 variants were carried out with VX and VR nerve agents (Table 2). None of the rHuPON1 variants could be saturated at 1.4 mM VX and only rHuPON1Q192 could be saturated at 1.4 mM VR. The Km of rHuPON1Q192 for VR hydrolysis was 0.72 mM and the Kcat was 29.8 min−1.
Table 1.
Kinetic analysis of substrate hydrolysis for insecticide OPs
| Substrate | PON1 varianta | Km (mM) | Vmax (U/mg) | Vmax/Km |
|---|---|---|---|---|
| Paraoxon | rHuPON1K192 | 0.925 ± 0.029 | 11.66 ± 0.06 | 12.61 ± 0.32 |
| rHuPON1R192 | 0.868 ± 0.016 | 6.61 ± 0.18 | 7.62 ± 0.23 | |
| plasma HuPON1R192 | 0.52 | 3.26 | 6.27 | |
|
| ||||
| Diazoxon | rHuPON1K192 | 2.57 ± 0.62 | 301 ± 51 | 118 ± 10 |
| rHuPON1R192 | 1.33 ± 0.08 | 119 ± 5 | 89.8 ± 2.3 | |
| plasma HuPON1R192 | 1.02 | 79 | 77 | |
|
| ||||
| Chlorpyrifos-oxon | rHuPON1K192 | 0.317 ± 0.02 | 245 ± 3 | 777 ± 66 |
| rHuPON1R192 | 0.131 ± 0.005 | 34.7 ± 0.3 | 266 ± 12 | |
| plasma HuPON1R192 | 0.25 | 64 | 256 | |
|
| ||||
| Phenyl acetate | rHuPON1K192 | 3.22 ± 0.79 | 3020 ± 300 | 966 ± 166 |
| rHuPON1R192 | 0.957 ± 0.02 | 680 ± 0 | 711 ± 16 | |
Data for plasma PON1are from Li et al. 2000; data for rHuPON1 are from Stevens et al. 2008.
Table 2.
Turnover numbers for substrate hydrolysis of nerve agent OPs
| Substrate | PON1 variant | Turnover (min-1) |
|---|---|---|
| VR | rHuPON1K192 | 5.2 |
| rHuPON1Q192 | 19.4 | |
| rHuPON1R192 | 6.8 | |
|
| ||
| VX | rHuPON1K192 | 5.74 |
| rHuPON1Q192 | 33.4 | |
| rHuPON1R192 | 10.6 | |
Testing rHuPON1K192 as a therapeutic
To test the in vivo efficacy of using rHuPON1 as a catalytic scavenger, experiments were carried out using the PON1-/- knockout mice. These mice were chosen for tests of the rHuPON1 since there is no DZOase activity in these mice. Mice were housed in either barrier or modified SPF (specific pathogen free) facilities with 12 h dark-light cycles and free access to food and water. All experiments were carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health. Animal use protocols were approved by the Institutional Animal Care and Use Committee at the University of Washington.
To determine if the E. coli-produced rHuPON1 was nontoxic, 1.12 DZOase units of PON1K192 were injected intraperitoneally (ip) into each of three of the PON1-/- mice. The mice showed no signs of physical symptoms. The increase in serum PON1 DZOase levels was about half that of wild-type levels. This increase in levels peaked about 8 hours after injection. Antibodies to PON1 could not be detected in mouse serum by ELISA 4 months after injection. Mice were still alive with no symptoms 12 months following injection.
The next question was to determine if injected rHuPON1 could protect PON1-/- mice from OP exposure. Administration of 192 ug of rHuPON1K192 (3.91 units of total DZOase activity) ip and intramuscularly (im) to 2 mice was followed by dermal exposure to 1 mg/kg DZO 48 hours following injection. Six hours following exposure, the brain cholinesterase levels of the mice were determined. Mice that were injected with the rHuPON1K192 had no observable inhibition of ChE while mice exposed to DZO and not receiving rHuPON1K192 had an approximate 50% reduction in brain ChE. The next question to be addressed was whether rHuPON1 could be injected 10 min after dermal exposure to high levels of DZO (i.e. >2x LD50). One mouse was exposed to 3 mg/kg and the other to 7 mg/kg. However, the mice not only survived, but showed fewer cholinergic symptoms than control mice that were exposed to a lower dose (1.5 mg/kg).
Conclusions
We have previously proposed the use of PON1 as a catalytic scavenger for treating OP exposures based on experiments where purified plasma PON1 provided protection against OP exposure in wild-type mice (Li et al. 2000). This series of experiments has provided evidence of the suitability of using rHuPON1 for therapeutic or prophylactic protection against OP exposure. These experiments have demonstrated that native human PON1 can be produced in bacterial cells and processed to sufficient purity for injection without side effects. This E. coli expression system will allow for high throughput screening for mutants with improved catalytic efficiency and for scale-up production of therapeutic PON1.
Acknowledgements
This work was supported by National Institutes of Health Grants ES09883, ES04696, ES07033, and ES09601/EPA: RD-83170901, and a grant from the University of Washington Center for Process Analytical Chemistry (CFO1). This work was supported in part by the Defense Threat Reduction Agency(DEL, DMC and TCO) Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army.
References
- Aharoni A, Gaidukov L, Yagur S, Toker L, Silman I, Tawfik DS. Directed evolution of mammalian paraoxonases PON1 and PON3 for bacterial expression and catalytic specialization. Proc Natl Acad Sci USA. 2004;101:482–487. doi: 10.1073/pnas.2536901100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashani Y, Shapira S, Levy D, Wolfe AD, Doctor BP, Raveh L. Butyrylcholinesterase and acetylcholinesterase prophylaxis against soman poisoning in mice. Biochem Pharmacol. 1991;41:37–41. doi: 10.1016/0006-2952(91)90008-s. [DOI] [PubMed] [Google Scholar]
- Bernard P, Couturier M. Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. J Mol Biol. 1992;226:735–745. doi: 10.1016/0022-2836(92)90629-x. [DOI] [PubMed] [Google Scholar]
- Broomfield CA, Maxwell DM, Solana RP, Castro CA, Finger AV, Lenz DE. Protection by butyrylcholinesterase against organophosphorus poisoning in nonhuman primates. J Pharmacol Exp Ther. 1991;259(2):633–8. [PubMed] [Google Scholar]
- Brushia RJ, Forte TM, Oda MN, La Du BN, Bielicki JK. Baculovirus-mediated expression and purification of human serum paraoxonase 1A. J Lipid Res. 2001;42:951–958. [PubMed] [Google Scholar]
- Davies HG, Richter RJ, Keifer M, Broomfield CA, Sowalla J, Furlong CE. The effect of the human serum paraoxonase polymorphism is reversed with diazoxon, soman and sarin. Nat Genet. 1996;14:334–336. doi: 10.1038/ng1196-334. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Richter RJ, Seidel SL, Costa LG, Motulsky AG. Spectrophotometric assays for the enzymatic hydrolysis of the active metabolites of chlorpyrifos and parathion by plasma paraoxonase/arylesterase. Anal Biochem. 1989;180:242–247. doi: 10.1016/0003-2697(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Furlong CE, Richter RJ, Chapline C, Crabb JW. Purification of rabbit and human serum paraoxonase. Biochemistry. 1991;30:10133–10140. doi: 10.1021/bi00106a009. [DOI] [PubMed] [Google Scholar]
- Gan KN, Smolen A, Eckerson HW, La Du BN. Purification of human serum paraoxonase/arylesterase: Evidence for one esterase catalyzing both activities. Drug Metabolism and Dispos. 1991;19:100–106. [PubMed] [Google Scholar]
- Harel M, Aharoni A, Gaidukov L, Brumshtein B, Khersonsky O, Meged R, Dvir H, Ravelli RBG, McCarthy A, Toker L, Silman I, Sussman JL, Tawfik DS. Structure and evolution of the serum paraoxonase family of detoxifying and anti-atherosclerotic enzymes. Nat Struct Mol Biol. 2004;11:412–419. doi: 10.1038/nsmb767. [DOI] [PubMed] [Google Scholar]
- Hassett C, Richter RJ, Humbert R, Chapline C, Crabb JW, Omiecinski CJ, Furlong CE. Characterization of cDNA clones encoding rabbit and human serum paraoxonase: The mature protein retains its signal sequence. Biochemistry. 1991;30:10141–10149. doi: 10.1021/bi00106a010. [DOI] [PubMed] [Google Scholar]
- James RW. A long and winding road: defining the biological role and clinical importance of paraoxonases. Clin Chem Lab Med. 2006;44:1052–1059. doi: 10.1515/CCLM.2006.207. [DOI] [PubMed] [Google Scholar]
- Jarvik GP, Rozek LS, Brophy VH, Hatsukami TS, Richter RJ, Schellenberg GD, Furlong CE. Paraoxonase (PON1) phenotype is a better predictor of vascular disease than is PON1(192) or PON1(55) genotype. Arterioscler Thromb Vasc Biol. 2000;20(11):2441–2447. doi: 10.1161/01.atv.20.11.2441. [DOI] [PubMed] [Google Scholar]
- Lenz DE, Yeung D, Smith JR, Sweeney RE, Lumley LA, Cerasoli D. Stoichiometric and catalytic scavengers as protection against nerve agent toxicity: a mini review. Toxicology. 2007;233:31–39. doi: 10.1016/j.tox.2006.11.066. [DOI] [PubMed] [Google Scholar]
- Li WF, Costa LG, Richter RJ, Hagen T, Shih DM, Tward A, Lusis AJ, Furlong CE. Catalytic efficiency determines the in-vivo efficacy of PON1 for detoxifying organophosphorus compounds. Pharmacogenetics. 2000;10:767–779. doi: 10.1097/00008571-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Ozer EA, Pezzulo A, Shih DM, Chun C, Furlong C, Lusis AJ, Greenberg EP, Zabner J. Human and murine paraoxonase 1 are host modulators of Pseudomonas aeruginosa quorum-sensing. Fems Microbiol Lett. 2005;253:29–37. doi: 10.1016/j.femsle.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Raushel FM. Bacterial detoxification of organophosphate nerve agents. Current Opinion in Microbiology. 2002;5(3):288–295. doi: 10.1016/s1369-5274(02)00314-4. [DOI] [PubMed] [Google Scholar]
- Shih DM, Gu LJ, Xia YR, Navab M, Li WF, Hama S, Castellani LW, Furlong CE, Costa LG, Fogelman AM, Lusis AJ. Mice lacking serum paraoxonase are susceptible to organophosphate toxicity and atherosclerosis. Nature. 1998;394:284–287. doi: 10.1038/28406. [DOI] [PubMed] [Google Scholar]
- Sinan S, Kockar F, Arslan O. Novel purification strategy for human PON1 and inhibition of the activity by cephalosporin and aminoglikozide derived antibiotics. Biochimie. 2006;88:565–574. doi: 10.1016/j.biochi.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Stevens RC, Suzuki SM, Cole TB, Park SS, Richter RJ, Furlong CE. Engineered recombinant human paraoxonase 1 (rHuPON1) purified from Escherichia coli protects against organophosphate poisoning. Proc Natl Acad Sci USA. 2008;105(35):12780–12784. doi: 10.1073/pnas.0805865105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltz DA, Ozer EA, Taft PJ, Barry M, Liu L, Kiss PJ, Moninger TO, Parsek MR, Zabner J. Drosophila are protected from Pseudomonas aeruginosa lethality by transgenic expression of paraoxonase-1. J Clin Invest. 2008;118:3123–3131. doi: 10.1172/JCI35147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization [25-29 November 1985];WHO; Geneva: Informal consultation on planning strategy for the prevention of pesticide poisoning. 1986 Geneva. WHO/VBC/86.926.
- World Health Organization WHO; Geneva: Public health impact of pesticides used in agriculture. 1990