Abstract
An initial administration of 60% nitrous oxide (N2O) evokes hypothermia in rats and if the administration continues for more than 1 – 2 hours, acute tolerance typically develops such that the initial reduction in core temperature (Tc) reverses and Tc recovers toward control values. Calorimeter studies at normal ambient temperature indicate that hypothermia results from a transient reduction in heat production (HP) combined with an elevation in heat loss. Acute tolerance develops primarily due to progressive increases in HP. Our aim was to determine whether rats provided a choice of ambient temperatures would behaviorally facilitate or oppose N2O -induced hypothermia. A gas-tight thermally-graded alleyway (range, 6.7 – 37.0°C) enabled male Long-Evans rats (n=12) to select a preferred ambient temperature during a 5-hour steady-state administration of 60% N2O and a separate paired control gas exposure (order counterbalanced). Tc was measured telemetrically from a sensor surgically implanted into the peritoneal cavity > 7 days before testing. Internal LED lighting maintained the accustomed day:night cycle (light cycle 0700 – 1900 h) during sessions lasting 45.5 hours. Rats entered the temperature gradient at 1100 h, and the 5-h N2O or control gas period did not start until 23 hours later to provide a long habituation / training period. Food and water were provided ad libitum at the center of the alleyway. The maximum decrease of mean Tc during N2O administration occurred at 0.9 h and was −2.05 ± 0.25°C; this differed significantly (p<0.0001) from the corresponding Tc change at 0.9 h during control gas administration (0.01 ± 0.14°C). The maximum decrease of mean selected ambient temperature during N2O administration occurred at 0.7 h and was −13.58 ± 1.61°C; this differed significantly (p < 0.0001) from the corresponding mean change in selected ambient temperature at 0.7 h during control gas administration (0.30 ± 1.49°C). N2O appears to induce a regulated hypothermia because the selection of a cool ambient temperature facilitates the reduction in Tc. The recovery of Tc during N2O administration (i.e., acute tolerance development) could have been facilitated by selection of ambient temperatures that were warmer than those chosen during control administrations, but interestingly, this did not occur.
Keywords: Thermoregulation, acute tolerance, intrasessional tolerance, behavioral thermoregulation, allostasis
1. Introduction
Drugs cause changes in measured variables and these changes are casually referred to as a “drug effect” or sometimes as a “drug response.” Although such terms are in common usage, they obscure the complexity underlying events that are typically assessed and quantified in drug studies. Ramsay and Woods [1] argued that the typical whole-animal outcome variables that are measured following a drug administration represent a mixture of a drug’s pharmacological action on a physiological system(s) and the individual’s response(s) to the drug-induced disturbance, as well as multiple other factors that influence the measured variable(s). It is the integrated contribution of all of these components that determines the value of what is measured following drug administration. Furthermore, the relative contribution of each of these components to the measured variable can change over time, both within a single drug administration as well as over repeated drug administrations, and these changing relationships can explain the development of phenomena such as acute and chronic drug tolerance [1]. By studying drug-induced disturbances in body temperature, our lab is able to investigate the interrelationship among the underlying components that are typically unmeasured yet that contribute importantly to the overall measure of body temperature.
Thermoregulation is often described as the archetype of a physiologically regulated system. There is broad agreement that deep body temperature is regulated because actual or anticipated thermal disturbances elicit effector responses that defend core temperature (e.g., the recruitment of thermal reflexes in response to changes of ambient temperature). For the thermoregulatory system, core temperature (Tc) is usually considered the regulated variable [2, 3] and thermoeffectors control or modulate its value [4, 5]. The multiple thermoregulatory effectors include vasomotor tone (e.g., peripheral vasoconstriction / vasodilation), metabolic heat production (e.g., shivering, non-shivering thermogenesis) and evaporative heat loss (e.g., sweating, panting, grooming saliva) as well as a myriad of behavioral strategies that modulate Tc [6]. In comparison to autonomic thermoeffectors, behavioral effectors often provide quicker and more energetically efficient ways to influence Tc [7].
Thermoregulation has important strengths as an experimental paradigm for understanding drug-related phenomena such as initial sensitivity and tolerance development [8]. Changes in Tc are determined by the net difference between metabolic HP and HL. Incorporating telemetric measurement of Tc with simultaneous total calorimetry (i.e., combining indirect and direct calorimetric methods) allows for the non-invasive and continuous measurement of HP and HL in undisturbed test subjects [8 – 11]. Non-invasive measurement of Tc is critical because handling experimental rodents (e.g., to collect data or administer a drug) influences the dependent measures of interest (i.e., Tc, HP, HL) [12 – 14]. In addition, behavioral thermoregulation can be assessed in undisturbed rodents by allowing them to select their preferred ambient temperature (Tsel) in a temperature gradient using a method that has been well described and accepted [15 – 18]. Gordon [16] (p. 45) cites several reasons to choose the thermal gradient method over other operant approaches for assessing behavioral thermoregulation, including that thermoregulatory behavior in the temperature gradient: 1) is less influenced by motoric drug effects, 2) represents a naturalistic response that requires much less training for a rat than other operant methods, and 3) does not require the rat to make a near continuous operant response.
Nitrous oxide (N2O) is a pharmacologically active gas that is administered via inhalation and causes hypothermia in rats [19 – 21]. Continuously administered N2O rapidly reaches a steady-state concentration in the body that can be maintained for prolonged periods of time, and it undergoes little, if any, metabolism [22 – 24]. These characteristics are important because a steady-state drug concentration removes variation in the dependent measures that typically occurs when a drug concentration changes over time.
In rats tested using total calorimetry, an initial administration of 60% N2O results in a rapid but transient reduction in HP and elevation in HL resulting in a state of negative heat balance and a marked drop in Tc [8]. Notably, the effect of N2O to initially increase HL is observed at concentrations as low as 30%, but at concentrations from 30% to 50% this influence on Tc is promptly countered by increases in HP that maintain normothermia. This illustrates the value of measuring the underlying determinants because it clarifies whether initial insensitivity (i.e., a lack of change on the summated outcome variable during an initial drug administration) occurs because the drug has no effect on the variable’s underlying determinants or whether the drug-induces changes in the determinants that are offsetting and result in no net change [10]. Similarly, acute tolerance development is explained by progressive increases in HP over the course of an initial N2O exposure such that the initially negative heat balance reverses and the rat shifts into positive heat balance enabling recovery from hypothermia despite the continued presence of N2O [8 – 10]. Chronic tolerance develops to the hypothermic effect of 60% N2O over repeated administrations [25], and this appears to result primarily from the growth of HP over repeated drug administrations [9].
By enabling measurement of HP and HL, total calorimetry provides insight into the role that primarily autonomic effectors have on determining Tc during N2O administration. However, little is known about the behavioral thermoregulatory effectors that are activated during N2O administration. Pertwee and colleagues [26, 27] studied behavioral thermoregulation in mice administered sub-anesthetic concentrations of N2O. When given a choice between a warmer (27.5°C) or cooler (21.6°C) environment, mice receiving N2O spent significantly more time in the cooler area, suggesting that thermoregulatory behavior was not disabled by N2O but actually participated in the regulation of Tc [26]. That is, the mice could have selected warmer temperatures and slowed or reversed the fall in Tc; however, by selecting cooler temperatures they exacerbated the N2O-induced hypothermia.
Gordon [16, 28, 29] has suggested that changes in Tc following drug administration (or other physiological challenges such as hypoxia [30] and hemorrhage [31]) may be categorized on the basis of Tc and thermoeffector action. Specifically, if behavioral and autonomic effectors influence Tc to move it in the same direction as the observed change in Tc, then the drug-induced change in Tc is considered to be “regulated.” For example, if Tc is reduced following a drug administration and this change is accompanied by a decrease in the activity of heat-generating / conserving effectors and an increase in heat-dissipating effectors, then the drug would be considered to cause a regulated hypothermia (sometimes called anapyrexia [32]). The converse situation of regulated hyperthermia, as exemplified by a fever, occurs when an elevation in Tc is preceded by increased activation of heat-generating / conserving effectors and an inhibition of heat-dissipating effectors. In the situation when the observed change in Tc is accompanied by autonomic and behavioral effector actions that resist or oppose the change in Tc, then the measured change in Tc is described as being “forced” (i.e., the drug causes a forced hypothermia or a forced hyperthermia). For example, a forced hypothermia would involve a reduction in Tc accompanied by thermoeffector actions that increase HP and minimize HL, such as occurs when a subject is placed in a very cold environment.
Gordon [16] (p. 32) contends that “Categorizing thermoregulatory responses into forced versus regulated responses is essential for understanding the mechanism of action of a toxic chemical or drug.” As emphasized by Ramsay and Woods [1], the consequences of a drug administration are predicated on understanding the effectors that are activated. The purpose of the present study was to extend our research on the thermoeffectors that influence Tc during N2O administration. A gas-tight thermal gradient was constructed to assess how behavioral effectors operate during N2O administration. The gradient includes a radiotelemetric system for measuring Tc along with preferred ambient temperature and motor activity. The goal was to determine whether the hypothermia observed during a 60% N2O administration is forced or regulated.
2. Material and methods
2.2 Subjects
Twelve (N = 12) young adult male Long-Evans rats (Charles River) with a mean weight of 265g (SEM = 9) were housed in groups of 2–3 rats per cage with free access to pelleted chow (5053 PicoLab Diet 20, LabDiet, Brentwood, MO) and water. The housing room had a 12 h:12 h light/dark cycle (0700–1900 h) and an ambient temperature of 22 ± 1°C. The animal procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
2.2 Apparatus
2.2.1 Thermal Gradient
The thermal gradient apparatus was constructed from a rectangular copper “pipe” with sealed ends. The copper pipe was divided lengthwise into two pieces so that it could be opened and closed. A thermal gradient was created by warming one end of the pipe and cooling the other with temperature-controlled circulating water. An alleyway, suspended within the gradient, allowed a rat to position itself along the thermal continuum at its preferred ambient temperature. This device was based on Gordon’s design [16] (p. 46) with modifications to make it gas-tight.
The gradient’s copper shell (6.35 mm thickness) was fabricated by the Alaskan Copper Works (Seattle, WA, USA) as two pieces (i.e., an upper and lower shell). A wide (50.8 mm) lip extended around the perimeter on both upper and lower halves of the gradient. A continuous gasket (6.35 mm thickness) made from compressible closed-cell neoprene rubber (G-231-N, Rubatex International, Bedford, VA, USA) was placed on the lip of the lower shell with pressure-sensitive adhesive. The gasket formed a gas-tight seal when the gradient’s upper shell was lowered onto the lower shell. The gradient was supported on a custom-built superstructure (80/20 Inc., Columbia City, IN, USA). The upper half of the gradient was lowered into place on the lower shell using pulleys and a linear actuator (actuator model LAS4-1-1-300-24G, actuator controller model LAK2-AA-110-G, Hiwin Corporation, Elgin, IL, USA) that were fixed to the superstructure. With the upper and lower halves of the gradient together, the internal rectangular space within the gradient was 190.5 cm long, 17.14 cm high and 17.14 cm wide.
Both ends of the upper and lower copper shells were fabricated with integral water jackets that surrounded the last 10.16 cm of the end of each shell. Two recirculating, temperature-controlled water baths (NesLab Model RTE7, Thermo Fisher Scientific, Newington, NH, USA) were used to pump water through the water jackets. The temperatures of the warm and cool water baths could be adjusted independently to establish the desired temperature range across the gradient. The external surface of the gradient and all tubes circulating water to and from the water jackets were insulated.
A removable acrylic alleyway (182.9 cm long, 12.06 cm high, 12.06 cm wide) was suspended from a support structure (80/20 Inc., Columbia City, IN, USA) that fit within the thermal gradient. The full-length alleyway was made by abutting four 45.72 cm-long alleyway segments. The lateral walls of the alleyway were slitted and the alleyway floor was made of acrylic rods that allowed feces and urine to fall into bedding-filled waste trays placed on the floor of the gradient. The internal support structure also had a hinged-acrylic lid that, when closed, formed the top of the suspended alleyway. The internal support structure held 24 infrared beam pairs (model OPB100Z, Optek, Carrollton, TX, USA) that detected the rat’s location within the gradient. The infrared beam pairs were positioned 19 mm above the alleyway floor and were placed 76.2 mm apart along the length of the alleyway ending 38.1 mm from either end. A thermistor with 0.1°C accuracy (model KS222J2, U.S. Sensor Corporation, Orange, CA, USA) was positioned 10 mm above each of the 24 infrared detectors. External to the gradient were three, 8-channel USB-Temp devices (Measurement Computing Corporation, Norton, MA, USA) that were wired to the thermistors as well as to the infra-red beams which used the 8 digital I/O lines of each USB-Temp. The telemetric Tc signal was received by an antenna wire that was strung around the side walls of the alleyway using plastic mounting brackets placed on the internal support structure. To provide uniform illumination for a 24-h light/dark cycle (0700 lights on, 1900 lights off), 128 evenly-spaced white light LEDs (Osram, LINEARlight Model #OS-LM01A-W2-854, Munich, Germany) ran the length of the alleyway and projected their light upward from top edge of the internal support structure. Water and pelleted chow were freely available in the center of alleyway via a water spout and a food hopper attached to the alleyway wall. A USB digital camera was mounted on the internal support structure at one end of the gradient which allowed observation of the rat inside the alleyway. All wires exiting the gradient passed through conveniently placed, small, gas-tight access ports located on the lower copper shell.
2.2.2 Gas Delivery
Medical grade oxygen (O2) and nitrogen (N2) were combined to produce a control gas with an outflow consisting of 21% oxygen and 79% nitrogen. The 60% N2O gas condition was created by blending 21% O2, 19% N2, and 60% medical grade N2O. Gas entered the gradient through an inlet port located in the top center of the upper copper shell and was dispersed by the acrylic lid covering the alleyway. Gas exited through two outlet ports centered at each end of the lower half of the gradient. Concentrations of N2O, O2, and CO2 were measured using an infrared gas analyzer (Normocapoxy, Datex Instruments Corp., Helsinki, Finland) that drew gas samples via a t-connector placed in the influent and effluent gas lines connected to the copper shell.
2.2.3 Measurement of Tc, data acquisition and instrument control
Telemetric measurement of Tc was accomplished using Mini-Mitter’s (Bend, OR, USA) TR 3000 Receiver, Data Port 24, VitalVIEW software, and an implantable battery-operated temperature sensor (Model VM-FH Disc). These transmitters had a sensitivity of ±0.1°C and were calibrated for accuracy by the manufacturer each time the battery was replaced. [At the completion of this study, Mini-Mitter discontinued their battery-operated temperature sensors and so the thermal gradient’s telemetric Tc measurement equipment was replaced by a commercial system from Data Sciences International (Saint Paul, MN, USA).] All other instrument control and data acquisition were performed using custom programs N2O written in LabVIEW 6.1 (National Instruments, Austin, Texas).
2.3 Surgical placement of the telemetric temperature sensor
One week prior to the start of testing, a battery-operated telemetric temperature sensor was implanted into the rat’s peritoneal cavity to measure Tc. Each rat was anesthetized with isoflurane (3–5% for induction and 1–3% for maintenance) for the surgical procedure. Surgery was performed with the rat on a ~39°C heating pad. Meloxicam (an NSAID) was provided in the drinking water (0.02 mg/ml H2O) from 1 day before to 2 days after surgery.
2.4 Experimental Design and Procedures
A counterbalanced, cross-over design was used so that each rat received both a control gas exposure and a 60% N2O exposure in the thermal gradient. With a single thermal gradient, two rats could be tested in a 2-week period. The order of the two gas exposures was counterbalanced such that when a rat was randomly assigned to receive control gas in the first week and 60% N2O in the second week, the other rat of the pair was assigned to the opposite order. Each rat’s two gas exposure sessions took place one week apart. After the first 6 rats had completed the study, the cool and warm ends of the gradient were switched for testing the next 6 rats.
All rats had two 45.5-hour test sessions that were separated by one week. Each rat was placed individually in the thermal gradient at 1100 h with control gas entering at a flow rate of 6 L/min. [Gordon and colleagues [13] place a rat in the thermal gradient the day before a test session to allow the rat to adapt over night to its surroundings and exhibit normal thermoregulatory behavior.] Each rat remained in the thermal gradient breathing control gas until 1000 h the next day. At 1000 h the gas flow rate increased to 12 L/min for 20 minutes while either control gas or 60% N2O was administered. The gas flow rate was increased so that the targeted 60% N2O concentration could be reached more quickly yielding an effluent N2O concentration of 55% by 12.3 minutes after the start of N2O delivery and 59–60% by 19.3 minutes. At 1020 h, the flow rate of the assigned gas reverted to 6 L/min and the gas exposure continued until 1500 h. At 1500 h, the 5-h assigned gas administration was finished and control gas was delivered at a flow rate of 12 L/min for 20 minutes. At 1520 h, the flow rate of control gas was reduced to 6 L/min and this was maintained until the session ended the next morning at 0830 h and the rat was removed. Thus, each testing session lasted 45.5 hours (i.e., 23 h of control gas followed by 5 h of control gas or 60% N2O, and then 17.5 h of control gas).
2.5 Data Analysis
Temperature and position data were recorded throughout the 45.5 h trials. The rat’s position in the alleyway was determined every 6 sec by recording infrared beam breaks from the 24, sequentially-numbered, infrared beam locations. The position of the rat was considered the average value of the location numbers of the broken infrared beam signals. The distance traveled by the rat was computed based on the 7.62 cm separation between consecutive infrared beam locations. The absolute value of the difference between successive time-stamped rat position values was multiplied by 7.62 cm to calculate the distance traveled per logged value. These distances were summed over the total duration of a specified time bin interval to result in the distance traveled during each time bin. The ambient temperature corresponding to the rat’s position within the gradient (Tsel) was assessed every 6 s. Tsel was calculated as the mean temperature from the thermistor(s) that corresponded with the broken infrared beam location(s). Tc data were recorded every 15 s. Individual Tc data were smoothed using locally-weighted least squares (LOWESS; 1% data windows). Gas concentration data were recorded every 20 s. The Tc minus Tsel difference was computed for each rat, because this is an important determinant of heat loss [33]. For each rat, Tc, Tsel and the Tc minus Tsel difference were averaged, while distance was summed, across 12 min bins. Statistical and graphical analyses utilized the individual binned averages and sums.
Statistical testing was conducted using generalized estimating equations (GEE) [34]. Statistical analyses of Tc, Tsel and the Tc minus Tsel difference were adjusted for their respective baseline values. Baseline values were calculated based on the average of the 12 minute bin just prior to the experimental period. Significance was established at alpha = 0.05, two-tailed. Data are presented as mean ± SEM unless otherwise noted.
3. Results
The thermal gradient established a temperature range of approximately 30°C with a mean low temperature of 6.7°C (SD = 0.85) and a mean high temperature of 37.0°C, (SD = 0.27). Figure 1 depicts mean temperature and position-related outcomes recorded during 25 h of the 45.5 h session in the thermally-graded alleyway. GEE analysis identified significant main effects for condition (control vs. N2O; p < 0.0001) and significant condition by time interactions (p < 0.0001) for all outcomes recorded during the 5-h experimental period. In comparison to control exposures, N2O evoked highly significant (p < 0.0001) decreases in both Tc (−0.96 ± 0.08°C; Fig. 1A) and Tsel (−7.17 ± 1.17°C; Fig. 1B) over the 5-h experimental period averages. During N2O administration, these variables exhibited temporal excursions having patterns consistent with the development of acute tolerance, such that by the end of the 5-h 60% N2O administration, their values differed only slightly from control levels. The maximum decrease of mean Tc during N2O administration occurred at 0.9 h and was −2.05 ± 0.25°C; this differed significantly (p<0.0001) from the corresponding Tc change at 0.9 h during control gas administration (0.01 ± 0.14°C). The maximum decrease of mean Tsel during N2O administration occurred at 0.7 h and was −13.58 ± 1.61°C; this differed significantly (p < 0.0001) from the corresponding mean Tsel at 0.7 h during control gas administration (0.30 ± 1.49°C). In the last 1 h of N2O administration, Tc differed from baseline by −0.22 ± 0.15°C (p = 0.17), while Tsel differed by −2.88 ± 2.22°C (p = 0.22). Corresponding values during control gas administration were slightly above baseline: 0.14 ± 0.12°C (p = 0.007) for Tc and 1.80 ± 1.28°C (p = 0.19) for Tsel. The within-subject comparisons (N2O minus control gas) were significantly different for both Tc (control minus N2O = 0.35 ± 0.17°C; p = 0.038) and Tsel (control minus N2O = 4.68 ± 2.18°C; p = 0.031) during the last 1-h of the experimental period. However, mean Tc returned towards the control value with a consistently steep slope in comparison to the return of Tsel, which exhibited a prolonged plateau at ~10°C below control values (Fig. 1B). Notably, during the initial steep increase of Tc, the difference between Tc and Tsel (Fig. 1C) remained favorable to increased heat loss (p < 0.0001 in comparison to the Tc – Tsel difference during control gas). This pattern suggests a compensatory increase of metabolic heat production, a compensatory decrease of heat loss, or both. One possible mechanism for increased heat production is suggested by a ~2-fold increase of locomotion during N2O administration shown in Fig. 1D (92.6 ± 12.5 cm per 12-min bin during N2O vs. 44.4 ± 6.3 cm during control gas, p < 0.0001), since heat production increases in proportion to locomotion.
Figure 1.
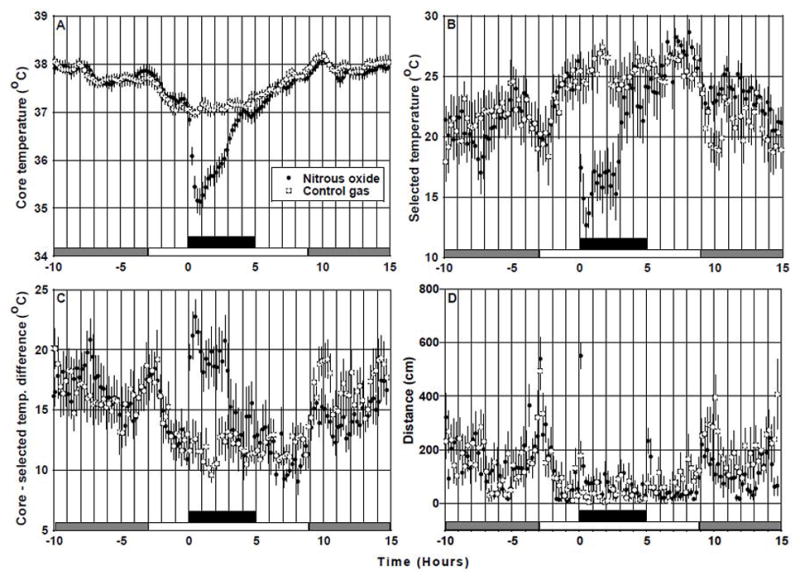
Effects of N2O inhalation on temperature and activity outcomes in Long-Evans rats (n = 12) housed in a gas-tight, thermally-graded alleyway. Black bar indicates 5-h period of steady-state 60% N2O or paired control gas exposures. Day and night cycles correspond to white and gray bars, respectively. Temperature data are means (± SEM) of averaged individual data recorded in successive 12-min bins; distance data are means (± SEM) of binned individual sums. GEE analysis revealed highly significant main effects for condition (control vs. N2O) (p < 0.0001) and highly significant condition by time interactions (p < 0.0001) during the period of N2O administration. Baseline Tc was 37.12 ± 0.06°C, baseline Tsel was 25.16 ± 0.62°C, baseline Tc minus Tsel was 11.96 ± 0.60°C, and baseline distance was 41.53 ± 14.00 cm. Baseline values of these dependent variables did not differ between conditions (p > 0.05). Note that the steep recovery of Tc (A) occurs despite the persistence of conditions that favor increased heat loss [decreased Tsel (B) and increased Tc – Tsel temperature difference (C)] which suggests compensation by increased heat production.
During the first 6 hours of the dark cycle following N2O inhalation, rats selected warmer Tsel values compared to the same period following the paired control gas administration (23.83 ± 0.44 °C vs. 20.65 ± 0.74 °C; p = 0.0002; Fig. 1B) and this preference was associated with a lower level of locomotor activity (151.1 ± 15.9 cm vs. 247.7 ± 17.7 cm per 12-min bin; p = 0.0001; Fig. 1D). Figure 1A indicates that during the first 6 hours of the post- N2O administration dark cycle, Tc was similar between conditions (N2O vs. control = 37.88 ± 0.05 vs. 37.97 ± 0.07; p = 0.06). One interpretation of this pattern of data is that a potential effect of lower activity-related heat production would be to favor reduced Tc but this was countered by elevated Tsel preference. Future studies would be required to investigate this unanticipated observation.
4. Discussion
Rats developed a regulated hypothermia when initially administered 60% N2O. Their greatest mean preference for cooler ambient temperatures occurred prior to reaching their lowest mean Tc (Figure 1A and 1B). Thus, the N2O -induced lowering of Tc was facilitated by a behavioral effector (i.e., moving to a cooler ambient location). Previous calorimetric research [8] suggests that the initial reduction of Tc caused by 60% N2O can also reflect a transient reduction of HP in addition to the dominant pharmacological effect of N2O that augments the conductance of body heat from the core to the environment. These autonomic effectors combined with behavioral preference for cooler ambient temperature work together to mediate a marked N2O -induced hypothermia.
Following the first hour of a 5-h, steady-state administration of 60% N2O, Tc began to steadily return toward near-baseline values (Fig. 1A) which indicates the development of acute tolerance to N2O -induced hypothermia [1, 8, 20, 21, 25]. Interestingly, this recovery in Tc occurred without an obvious complementary increase in behavioral preference for warmer ambient temperatures as Tsel remained relatively stable during the second and third hours of the N2O exposure (Fig 1B). This argues for the activation of a compensatory increase of metabolic heat production and / or a compensatory decrease of heat loss to account for the recovery of Tc considering that the difference between Tc and Tsel (Fig. 1C) remained favorable to increased heat loss. Previous calorimetric research [8] suggested that increases in HP over the course of the steady-state N2O administration contribute to the development of acute tolerance. The increase in motor activity in the thermal gradient during N2O administration could contribute to increasing Tc, although it is possible that an increase of convective HL could offset this effect. A similar hypothesis proposed that increases in body heat content resulting from elevated motor activity might explain the nocturnal rise in Tc observed in rodents [12, 35].
Of particular note, during the development of acute tolerance, rats did not facilitate the recovery of their Tc by moving to a warmer ambient temperature. Indeed, Tsel during N2O administration was always significantly lower than during control gas administration. Yet, after the first hour of N2O administration, rats achieved a state of positive heat balance, most likely via an endogenous heat production source involving increased activity and / or unmeasured autonomic-based facultative thermogenesis (shivering or nonshivering thermogenesis). Thus, during acute tolerance development, some effector mechanisms must have influenced Tc in an opposite direction to the behavioral thermoregulatory effector measured by Tsel. This apparent effector opposition complicates the interpretation of whether the hypothermia observed during the acute tolerance development phase of the N2O exposure should be considered regulated or forced because the effector actions are not coordinated to oppose or facilitate the recovery of Tc. This conundrum illustrates a limitation of applying a homeostatic set-point model in all situations [36] and especially in non-naturalistic experimental ones such as when pharmacological agents are administered [1, 5, 37]. This situation may occur because the effector systems for regulating Tc are relatively independent of one another [2, 3, 38, 39] although it is well-appreciated that under most naturalistic circumstances the effectors function in a coordinated manner [5]. The independence of the autonomic and behavioral thermoregulatory effectors emphasized by Satinoff [36] is apparent during the acute tolerance development phase of the current study. Indeed, the dissociation of normally coordinated effector function when confronted with evolutionarily aberrant challenges may be an underlying cause of what is currently described as allostasis [37]. For example, Goldstein [40] describes the costs of allostasis (e.g., being energetically inefficient) with the analogy of regulating a home’s temperature with opposing effectors concurrently active (a home’s furnace and air conditioner) which could be viewed analogously to the HP and behavioral thermoeffector action during the development of acute tolerance. HP increases both within an initial N2O exposure and over repeated N2O exposures, which contributes to the development of acute and chronic tolerance, respectively, to N2O -induced hypothermia [8, 9]. This increase in HP over repeated N2O exposures can lead to an allostatic overcorrection of Tc [9] although this phenomenon must be investigated using the thermal gradient to determine whether the overcorrection is facilitated or opposed by behavioral thermoeffectors.
In considering the development of an apparent dissociation between autonomic and behavioral thermoregulatory responses following the initial period of N2O administration in the thermal gradient, it is important to consider the effects of N2O on systems other than thermoregulation [41, 42] such as pain, stress and anxiety. It is possible, even likely, that this multiplicity of effects can produce uncoordinated autonomic and behavioral responses during a prolonged N2O administration. Notably, N2O has antinociceptive effects that involve activation of central corticotrophin releasing factor and noradrenergic pathways [43, 44] that are also more generally involved in arousal and stress responding [45]. We [46] and others [44] reported marked c-Fos staining in the hypothalamic paraventricular nucleus, a brain region critically involved in stress responding, following N2O administration in rats. Consistent with these observations, existing data indicate that N2O administration evokes elevated circulating corticosterone levels [47]. Taken together, these observations involving the recruitment of CNS substrates involved in stress responding during N2O inhalation raise the possibility that the increased HP observed during N2O represents a non-thermal stress response because hyperthermia does occur in response to various stressors [12]. Alternatively, it is possible that the persistence of cool seeking behavior, even as HP increases, might reflect a persistent activation of a behavioral mechanism that blunts potentially toxic drug effects by promoting lower body temperature (or restraining the rise in body temperature), consistent with the view of Gordon [29]. This discussion highlights the limitations of assuming homeostatically coordinated behavioral and autonomic effector actions during drug administrations, or in other settings in which evolutionarily-shaped regulatory systems conflict or are faced with “unanticipated” perturbations [35, 37, 48, 49]. For example, Refinetti [50] contends that the circadian system’s oscillatory influence on Tc via HP and HL thermoeffectors is opposed by the thermoregulatory system’s behavioral effectors which results in a dampened circadian pattern of measured Tc reflecting “the integrated output of the two systems” (p. 574). Refinetti [50] suggests that the “violation of homeothermy” (p. 575) represented by the opposition between the thermoregulatory and circadian systems can be explained by their different evolutionary histories.
Using a similar research approach, Gordon and colleagues [13] investigated the causes of hypothermia induced by the neurotensin analog, NT77, using telemetric Tc measurement, direct and indirect calorimetry and a thermal gradient. NT77 caused a preference for cooler ambient temperatures indicative of a regulated hypothermia and also caused a reduction in HP with no change in HL. The nature of the hypothermia induced by N2O appears to be different than NT77, as N2O causes a reliable initial increase in HL and whole-body conductance [9, 10]. It is impossible to compare these agents with respect to the development of acute tolerance because waning NT77 concentrations following the initial peak after ip injection may have accounted for the eventual recovery of Tc and Tsel toward baseline levels, whereas N2O was administered at a steady-state concentration.
Tc represents the integral of many factors, but only a subset of these constitutes biobehavioral control system responses made by thermoregulatory effectors. The scientific challenge is to sort out the influence of these CNS-controlled effectors from the myriad of other influences on the measured variable [1]. Research to identify the contribution of each of these sub-components to the resultant value of Tc will inform our understanding of regulation. For example, the CNS is only able to influence the value of Tc through the effector responses that it controls. Thus, understanding how the CNS responds during an initial challenge may be useful for understanding how it learns to regulate critical variables in anticipation of a potentially disruptive challenge and thereby reduce the impact of the disturbance on the regulated variable.
The broad importance of understanding CNS-control over effectors is one of the fundamental issues in regulatory physiology [1, 37, 51 – 57]. The methods discussed in the present study address this question in the context of drug-induced disturbances although the principles may be more generally applied across regulatory systems.
Acknowledgments
This investigation was supported by the National Institutes of Health (NIDA grant DA023484 and NIDCR grant T32 DE07132) and the Alcohol and Drug Abuse Institute at the University of Washington. We gratefully acknowledge Christopher W. Prall, Danny Georgiev and Shehzad Butt for their technical contributions to this study. We are indebted to Christopher Gordon and Steve Woods for their valuable advice and suggestions in the preparation of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ramsay DS, Woods SC. Biological consequences of a drug administration: implications for acute and chronic tolerance. Psychol Rev. 1997;104:170–93. doi: 10.1037/0033-295x.104.1.170. [DOI] [PubMed] [Google Scholar]
- 2.Kanosue K, Crawshaw LI, Nagashima K, Yoda T. Concepts to utilize in describing thermoregulation and neurophysiological evidence for how the system works. Eur J Appl Physiol. 2010;109:5–11. doi: 10.1007/s00421-009-1256-6. [DOI] [PubMed] [Google Scholar]
- 3.Werner J. System properties, feedback control and effector coordination of human temperature regulation. Eur J Appl Physiol. 2010;109:13–25. doi: 10.1007/s00421-009-1216-1. [DOI] [PubMed] [Google Scholar]
- 4.Brobeck JR. Exchange, control and regulation. In: Yamamoto WW, Brobeck JR, editors. Physiological Controls and Regulations. Philadelphia: Saunders; 1965. pp. 1–14. [Google Scholar]
- 5.Cabanac M. Adjustable set point: to honor Harold T. Hammel J Appl Physiol. 2006;100:1338–46. doi: 10.1152/japplphysiol.01021.2005. [DOI] [PubMed] [Google Scholar]
- 6.Gordon CJ. Autonomic Nervous System: Central Thermoregulatory Control. In: Squire LR, editor. Encyclopedia of Neuroscience. Vol. 1. Oxford: Academic Press; 2009. pp. 891–8. [Google Scholar]
- 7.Satinoff E. Behavioral thermoregulation in the cold. In: Fregly MJ, Blatteis CM, editors. Handbook of Physiology, Vol. 1, Section 4: Environmental Physiology. New York: Oxford University Press; 1996. pp. 481–505. [Google Scholar]
- 8.Kaiyala KJ, Ramsay DS. Assessment of heat production, heat loss, and core temperature during nitrous oxide exposure: a new paradigm for studying drug effects and opponent responses. Am J Physiol Regul Integr Comp Physiol. 2005;288:R692–701. doi: 10.1152/ajpregu.00412.2004. [DOI] [PubMed] [Google Scholar]
- 9.Kaiyala KJ, Butt S, Ramsay DS. Systems-level adaptations explain chronic tolerance development to nitrous oxide hypothermia in young and mature rats. Psychopharmacology (Berl) 2007a;191:233–42. doi: 10.1007/s00213-006-0655-1. [DOI] [PubMed] [Google Scholar]
- 10.Kaiyala KJ, Butt S, Ramsay DS. Direct evidence for systems-level modulation of initial drug (in)sensitivity in rats. Psychopharmacology (Berl) 2007b;191:243–51. doi: 10.1007/s00213-006-0657-z. [DOI] [PubMed] [Google Scholar]
- 11.Kaiyala KJ, Ramsay DS. Direct Animal Calorimetry, the Underused Gold Standard for Quantifying the Fire of Life. Comp Biochem Physiol A Mol Integr Physiol. 2010 doi: 10.1016/j.cbpa.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briese E. Normal body temperature of rats: the setpoint controversy. Neurosci Biobehav Rev. 1998;22:427–36. doi: 10.1016/s0149-7634(97)00051-1. [DOI] [PubMed] [Google Scholar]
- 13.Gordon CJ, McMahon B, Richelson E, Padnos B, Katz L. Neurotensin analog NT77 induces regulated hypothermia in the rat. Life Sci. 2003;73:2611–23. doi: 10.1016/s0024-3205(03)00663-5. [DOI] [PubMed] [Google Scholar]
- 14.Peris J, Cunningham CL. Stress enhances the development of tolerance to the hypothermic effect of ethanol. Alcohol Drug Res. 1987;7:187–93. [PubMed] [Google Scholar]
- 15.Gordon CJ, Lee KL, Chen TL, Killough P, Ali JS. Dynamics of behavioral thermoregulation in the rat. Am J Physiol. 1991;261:R705–711. doi: 10.1152/ajpregu.1991.261.3.R705. [DOI] [PubMed] [Google Scholar]
- 16.Gordon CJ. Temperature and toxicology: an integrative, comparative and environmental approach. Boca Raton: CRC Press; 2005. [Google Scholar]
- 17.Gordon CJ, Refinetti R. Measurement of behavioral thermoregulation. In: Conn PM, editor. Methods in Neurosciences: Paradigms for the Study of Behavior. Vol. 14. San Diego: Academic Press, Inc; 1993. pp. 266–80. [Google Scholar]
- 18.Satinoff E. Thermoregulation. In: Whishaw IQ, Kolb B, editors. The Behavior of the Laboratory Rat: A Handbook with Tests. Oxford: Oxford University Press; 2005. pp. 226–35. [Google Scholar]
- 19.Quock RM, Panek RW, Kouchich FJ, Rosenthal MA. Nitrous oxide-induced hypothermia in the rat. Life Sci. 1987;10(41):683–90. doi: 10.1016/0024-3205(87)90447-4. [DOI] [PubMed] [Google Scholar]
- 20.Kaiyala KJ, Leroux BG, Watson CH, Prall CW, Coldwell SE, Woods SC, Ramsay DS. Reliability of individual differences in initial sensitivity and acute tolerance to nitrous oxide hypothermia. Pharmacol Biochem Behav. 2001;68:691–9. doi: 10.1016/s0091-3057(01)00488-9. [DOI] [PubMed] [Google Scholar]
- 21.Ramsay DS, Omachi K, Leroux B, Seeley RJ, Prall CW, Woods SC. Nitrous oxide induced hypothermia in the rat: acute and chronic tolerance. Pharmacol Biochem Behav. 1999;62:189–96. doi: 10.1016/s0091-3057(98)00156-7. [DOI] [PubMed] [Google Scholar]
- 22.Eger EI., II . MAC. In: Eger EI II, editor. Nitrous oxide/ N2O. New York: Elsevier Science Publishing Company, Inc; 1985. pp. 57–67. [Google Scholar]
- 23.Stenqvist O. Nitrous oxide kinetics. Acta Anaesthesiol Scand. 1994;38:757–60. doi: 10.1111/j.1399-6576.1994.tb03997.x. [DOI] [PubMed] [Google Scholar]
- 24.Trudell JR. Metabolism of nitrous oxide. In: Eger EI II, editor. Nitrous oxide / N2O. New York: Elsevier Science Publishing Company, Inc; 1985. pp. 203–10. [Google Scholar]
- 25.Ramsay DS, Kaiyala KJ, Leroux BG, Woods SC. Individual differences in initial sensitivity and acute tolerance predict patterns of chronic drug tolerance to nitrous-oxide-induced hypothermia in rats. Psychopharmacology (Berl) 2005;181:48–59. doi: 10.1007/s00213-005-2219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pertwee RG, Marshall NR, Macdonald AG. Effects of subanesthetic doses of inert gases on behavioral thermoregulation in mice. J Appl Physiol. 1986;61:1623–33. doi: 10.1152/jappl.1986.61.5.1623. [DOI] [PubMed] [Google Scholar]
- 27.Pertwee RG, Marshall NR, Macdonald AG. Behavioural thermoregulation in mice: Effects of low doses of general anaesthetics of different potency. Exp Physiol. 1990;75:629–37. doi: 10.1113/expphysiol.1990.sp003441. [DOI] [PubMed] [Google Scholar]
- 28.Gordon CJ. A review of terms for regulated vs. forced, neurochemical-induced changes in body temperature. Life Sci. 1983;32:1285–95. doi: 10.1016/0024-3205(83)90802-0. [DOI] [PubMed] [Google Scholar]
- 29.Gordon CJ. The therapeutic potential of regulated hypothermia. Emerg Med J. 2001;18:81–9. doi: 10.1136/emj.18.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon CJ. The role of behavioral thermoregulation as a thermoeffector during prolonged hypoxia in the rat. J Therm Biol. 1997;22:315–24. [Google Scholar]
- 31.Brown JW, Whitehurst ME, Gordon CJ, Carroll RG. Thermoregulatory set point decreases after hemorrhage in rats. Shock. 2005;23:239–42. [PubMed] [Google Scholar]
- 32.IUPS. Glossary of terms for thermal physiology [revised by Committee on Thermal Physiology, International Union of Physiological Sciences (IUPS)] Pflugers Arch. 1987;410:567–87. [PubMed] [Google Scholar]
- 33.Gordon CJ. Temperature regulation in laboratory rodents. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- 34.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 35.Gordon CJ. 24-hour control of body temperature in rats. I. Integration of behavioral and autonomic effectors. Am J Physiol. 1994;267:R71–7. doi: 10.1152/ajpregu.1994.267.1.R71. [DOI] [PubMed] [Google Scholar]
- 36.Refinetti R. Homeostasis and circadian rhythmicity in the control of body temperature. Ann N Y Acad Sci. 1997;813:63–70. doi: 10.1111/j.1749-6632.1997.tb51673.x. [DOI] [PubMed] [Google Scholar]
- 37.Woods SC, Ramsay DS. Homeostasis: from Curt Richter to the Present. Appetite. 2007;49:388–98. doi: 10.1016/j.appet.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Satinoff E. Neural organization and evolution of thermal regulation in mammals. Science. 1978;201:16–22. doi: 10.1126/science.351802. [DOI] [PubMed] [Google Scholar]
- 39.McAllen RM, Tanaka M, Ootsuka Y, McKinley MJ. Multiple thermoregulatory effectors with independent central controls. Eur J Appl Physiol. 2010;109:27–33. doi: 10.1007/s00421-009-1295-z. [DOI] [PubMed] [Google Scholar]
- 40.Goldstein DS. Merging of the homeostat theory with the concept of allostatic load. In: Schulkin J, editor. Allostasis, Homeostasis, and the Costs of Physiological Adaptation. Cambridge: Cambridge University Press; 2004. pp. 99–112. [Google Scholar]
- 41.Emmanouil DE, Quock RM. Advances in understanding the actions of nitrous oxide. Anesth Prog. 2007;54:9–18. doi: 10.2344/0003-3006(2007)54[9:AIUTAO]2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fujinaga M, Maze M. Neurobiology of nitrous oxide-induced antinociceptive effects. Molecular Neurobiology. 2002;25:167–89. doi: 10.1385/MN:25:2:167. [DOI] [PubMed] [Google Scholar]
- 43.Sawamura S, Kingery WS, Davies MF, Agashe GS, Clark JD, Kobilka BK, Hashimoto T, Maze M. Antinociceptive action of nitrous oxide is mediated by stimulation of noradrenergic neurons in the brainstem and activation of [alpha]2B adrenoceptors. J Neurosci. 2000;20:9242–51. doi: 10.1523/JNEUROSCI.20-24-09242.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawamura S, Obara M, Takeda K, Maze M, Hanaoka K. Corticotropin-releasing factor mediates the antinociceptive action of nitrous oxide in rats. Anesthesiology. 2003;99:708–15. doi: 10.1097/00000542-200309000-00028. [DOI] [PubMed] [Google Scholar]
- 45.Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaiyala KJ, Thiele TE, Watson CH, Ramsay DS. Nitrous oxide-induced c-Fos expression in the rat brain. Brain Res. 2003;967:73–80. doi: 10.1016/s0006-8993(02)04219-1. [DOI] [PubMed] [Google Scholar]
- 47.Fischer GJ, Gilman SC, Blank C. Corticosterone response underlying tolerance to stress induced by a gaseous (nitrous oxide) environment. Percept Mot Skills. 1987;64:799–808. doi: 10.2466/pms.1987.64.3.799. [DOI] [PubMed] [Google Scholar]
- 48.Mrosovsky N. Rheostasis: the physiology of change. New York: Oxford University Press; 1990. [Google Scholar]
- 49.Refinetti R. Metabolic heat production, heat loss and the circadian rhythm of body temperature in the rat. Exp Physiol. 2003;88:423–9. doi: 10.1113/eph8802521. [DOI] [PubMed] [Google Scholar]
- 50.Refinetti R. The circadian rhythm of body temperature. Front Biosci. 2010;15:564–94. doi: 10.2741/3634. [DOI] [PubMed] [Google Scholar]
- 51.Domjan M. Pavlovian conditioning: a functional perspective. Annu Rev Psychol. 2005;56:179–206. doi: 10.1146/annurev.psych.55.090902.141409. [DOI] [PubMed] [Google Scholar]
- 52.Dworkin BR. Learning and physiological regulation. Chicago: The University of Chicago Press; 1993. [Google Scholar]
- 53.Hammel HT. Anesthetics and body temperature regulation. Anesthesiology. 1988;68:833–5. [PubMed] [Google Scholar]
- 54.Ramsay DS, Seeley RJ, Bolles RC, Woods SC. Ingestive homeostasis: the primacy of learning. In: Capaldi ED, editor. Why we eat what we eat. Washington DC: American Psychological Association; 1996. pp. 11–27. [Google Scholar]
- 55.Siegel S. Learning and the wisdom of the body. Learn Behav. 2008;36:242–52. doi: 10.3758/lb.36.3.242. [DOI] [PubMed] [Google Scholar]
- 56.Somjen GG. The missing error signal-regulation beyond negative feedback. News Physiol Sci. 1992;7:184–5. [Google Scholar]
- 57.Woods SC. The eating paradox: how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]


