Abstract
Intake of saturated fats and simple carbohydrates, two of the primary components of a modern Western diet, is linked with the development of obesity and Alzheimer's Disease. The present paper summarizes research showing that Western diet intake is associated with cognitive impairment, with a specific emphasis on learning and memory functions that are dependent on the integrity of the hippocampus. The paper then considers evidence that saturated fat and simple carbohydrate intake is correlated with neurobiological changes in the hippocampus that may be related to the ability of these dietary components to impair cognitive function. Finally, a model is described proposing that Western diet consumption contributes to the development of excessive food intake and obesity, in part, by interfering with a type of hippocampal-dependent memory inhibition that is critical in the ability of animals to refrain from responding to environmental cues associated with food, and ultimately from consuming energy intake in excess of that driven solely by caloric need.
Obesity and Alzheimer's Disease (AD) are two of the most serious and costly health challenges facing Western cultures. Since 1980, the prevalence of obesity in the United States has increased by 75%, with fully one third of men and women now classified as obese [1]. Obesity is a main component of what is termed the “metabolic syndrome”, which also includes glucose intolerance, insulin resistance, high triglyceride levels, low levels of high density lipoprotein (HDL) density, and hypertension as primary characteristics [2]. There is currently little agreement about the causes of the continuing rise in obesity, and treatments that can stem or reverse this trend have not yet been developed. Similarly, the incidence of AD in the global population is projected to increase four-fold over the next 40 years [3], afflicting as many as 14 million people in the United States alone by 2050 [4]. AD is characterized by a severe, age-related decline in memory and cognitive functioning. At present, there is no cure for AD and its root causes remain elusive. In addition to the high cost in terms of quality of life, the estimated annual U.S. healthcare costs for the victims of obesity and AD presently exceeds $140 billion [5] and $170 billion [6], respectively. Thus, identifying risk factors and strategies for preventing or delaying the onset and progression of both of these disorders is of paramount importance.
Traditionally, investigators have viewed the problems of obesity and related metabolic disorders on one hand (e.g., Type ll Diabetes Mellitus, hypertension) and AD and cognitive dementias on the other, as involving distinct etiologies, which target different underlying behavioral and biological functions that rely on largely separate brain structures and circuits. For example, it is abundantly clear that manipulations of the hypothalamus (e.g., surgical, genetic, hormonal) can have profound effects on eating and body weight gain for experimental animals and that increases in energy intake and body weight regulation in humans are accompanied by marked changes in hypothalamic neurohormonal signaling pathways [7]. In contrast, the hippocampus is the site of structural abnormalities associated with early stages of AD and other cognitive dementias [8, 9]. In fact, the hippocampus is preferentially susceptible compared to other brain regions to a variety of insults (e.g., environmental toxicants, cardiovascular and metabolic perturbations) that have cognitive dysfunction as their signature symptoms [10]. In addition, findings that selective removal of the hippocampus is accompanied by specific types of learning and memory impairment have also focused much research attention on the hippocampus as a substrate for amnesias and other forms of cognitive decline [11].
In spite of these differences, evidence is beginning to accumulate for important commonalities in the etiologies of both energy dysregulation and cognitive impairment. Specifically, saturated fats and refined carbohydrates are the principal components of a “Western diet” that are believed to promote excess energy intake and body weight gain [12]. Several recent studies have also linked elevated intake of saturated fat and simple sugars to increased incidence of AD [13–15] and milder forms of cognitive dysfunction (e.g., [16–19]). In addition, several recent reports indicate that selective hippocampal damage in rodents and pathologies that are largely confined to the hippocampus in humans are associated with increased energy intake [20, 21] and meal frequency (e.g., [22]). Thus, there is evidence suggesting that dietary factors are associated with the emergence of hippocampal pathology and that hippocampal pathology is associated with the emergence of increased food intake and body weight gain.
This paper has two main objectives: Our primary aim is to review evidence that Western diets impair cognitive functioning, with special emphasis on the functions of the hippocampus. To achieve this objective, we will consider findings from studies that link consumption of saturated fats and simple carbohydrates to the development of cognitive dementias, including AD and mild cognitive impairment (MCI), a diagnosis given to individuals that exhibit deficits in memory, language, or other mental functions that exceed what is expected as part of normal aging, but that do not interfere significantly with their daily activities [23]. We will also assess the nature of learning and memory processes that may be altered by these diets and we will consider the possibility that such alterations are based on interference with the function of the hippocampus. Our second goal is to consider the hypothesis that the disruptive effects of Western diets on learning and memory function also contribute to the ability of these diets to promote excess food intake and body weight gain. Although diets high in saturated fats and sugars tend to be energy dense, this fact in itself does not explain why animals overeat them. That is, given that energy regulation depends on matching energy intake with energy expenditure, intake of Western diets in excess of energy needs reflects a failure of energy regulation. We will consider the possibility that this failure is based, in part, on disruption of hippocampal-dependent learning and memory processes that underlie control of appetitive behavior.
Components of Western Diets Associated with Cognitive Dysfunction
Although Western diets contain various combinations and concentrations of different sources of macro- and micronutrients, research has focused primarily on cognitive impairment produced by consumption of two of the primary components of that diet, namely saturated fats and simple sugars.
Saturated Fats
Fatty acids are categorized into saturated fatty acids (SFA) and unsaturated fatty acids, including mono (MUFA) and polyunsaturated (PUFA) fatty acids. While intake of PUFAs, particularly Omega-3 fatty acids, is considered to be protective against cognitive decline (see [24] for review), intake of SFA appears to have the opposite effect. For example, data from cross-sectional (e.g., [25]) and longitudinal population-based studies (e.g., [26]) have shown that intake of SFA is correlated with impaired cognitive function. Morris and colleagues have evaluated the relationship between intake of dietary fats and the development of cognitive decline and dementia in humans in a series of population-based prospective studies that focused on age-related cognitive change. They reported that, for subjects aged 65 years and older, high intakes of SFA, but not total fat, over four- and six-year periods led to a greater risk for the development of AD and MCI [27, 28]. In a more recent longitudinal prospective study, Eskelinen and colleagues [17] examined the relationship between SFA intake by humans and the development of clinical MCI 21 years later. The authors found that abundant dietary SFA was associated with an increased risk of developing MCI. However, cognitive impairment was not global, but tended to be more pronounced in specific types of learning and memory tasks. More specifically, SFA was associated with impaired performance in a test of prospective memory, which involves memory based on performing an intended action at some future time [29]. On the other hand, performance in other mnemonic domains, such as immediate verbal memory and semantic memory, was not linked with SFA intake.
One limitation in the studies described above is that differences in body mass index (BMI) or adiposity were not accounted for, thus making it difficult to dissociate the effects of SFAs on cognitive function from their effects on the development of body weight gain and obesity. However, relationships between SFA intake and cognitive decline have been found after adjusting for measures of hypertension (blood pressure, etc.) and the presence of adult-onset (i.e., Type II) diabetes [17, 19], both of which are strongly correlated with BMI in humans [30].
Evidence from studies employing rodent models is also consistent with the hypothesis that SFA intake can lead to cognitive impairment. Greenwood and Winocur have shown that consumption of a high SFA diet with a complex carbohydrate source can impair learning and memory performance in rats (e.g.,[31, 32]). One study [33] evaluated the effects of three months exposure to a diets high in either SFA, polyunsaturated fatty acids (PUFAs), or monounsaturated fatty acids (MUFAs) on the ability of rats to learn an appetitive operant conditioning task. They found that the rats receiving the SFA diet were impaired in learning the task, whereas intake of PUFAs or MUFAs had little impact on performance relative to a low fat control diet. Thus, diets high in SFA appear to have a larger disruptive effect on cognitive function in rodents relative to diets high in unsaturated fats or low in total fat.
Simple Carbohydrates
Simple carbohydrates (mono and disaccharides, e.g., glucose, sucrose) are considered a major part of Western diets [34]. They can be differentiated from complex carbohydrates (polysaccharides, starch) based on a higher glycemic index, which is a measure of the effect that ingested substances have on postprandial blood glucose levels. Evidence shows that consumption of a meal containing simple carbohydrates can impair postprandial memory function relative to intake of complex carbohydrates. For instance, the effects of consuming a meal with a high or a low glycemic index on postprandial memory performance was examined in adult subjects with well controlled Type II Diabetes Mellitus (T2DM) [35]. It was found that the high glycemic meal led to poorer performance in memory tests that were given between 1–2 hours after eating. Similarly, other studies have found that a higher relative to a lower glycemic load for a meal (i.e., higher glycemic index) can lead to poorer memory performance in nondiabetic subjects, including children [16] and normal weight undergraduate female subjects ([36], but see [37]). These studies suggest that consumption of simple relative to complex carbohydrates can impair postprandial memory performance in humans. Epidemiological and experimental research is needed to examine the possibility that longer-term intake of simple carbohydrates can also have a detrimental impact on cognitive function in human populations.
Perhaps more compelling evidence for a role of simple carbohydrate intake in cognitive impairment comes from a recent study that examined the effects of sucrose intake on performance in a novel object recognition task in rats [38]. This task is based on the tendency of rats to prefer the exploration of novel compared to familiar objects. Rats are familiarized with an object in a first phase, and are then presented with the familiar and a novel object in a second phase. Failure to explore the novel object more than the familiar object in the second phase can be interpreted as a memory deficit [18, 38]. Rats given chronic daily access to a sucrose solution (32% sucrose solution) in addition to standard chow were impaired in learning and memory performance compared to rats that only received chow access, whereas performance was not affected in rats given chow supplemented with access to Crisco (≈25% SFA, 75% MUFA/PUFA). Importantly, body weights were not significantly different between the sucrose and Crisco-fed groups, although both groups were heavier than the control group. These results suggest that dietary sucrose may be affecting cognitive function independent of its effects on the development of obesity, as the two diets had differential effects on learning performance but not on body weight gain.
Other evidence also suggests that glycemic index may be one important factor mediating the effects of simple carbohydrates on learning and memory function [39]. Rats were given one of three diets: high in fat and sucrose (HFS), high in fat and glucose (HFG), or a standard chow control diet. While body weight gain did not differ between rats maintained on the HFG or HFS diets, rats receiving the HFG diet were impaired in learning a Pavlovian conditioning task relative to the control group, whereas the HFS-fed rats were not impaired. This observed learning impairment following HFG but not HFS exposure is potentially based on the higher glycemic load of the HFG relative to the HFS diet, given that glucose is a more potent stimulator of insulin secretion than sucrose [40].
It also appears that the effects of consuming simple compared to complex carbohydrates on cognitive function may differ depending on factors such as the type of learning or memory assessment that is used. For example, intake of simple relative to complex carbohydrate in adult subjects with well-controlled T2DM is associated with impairments in several measures of delayed verbal memory (e.g., paragraph and word list recalls), whereas only marginal impairments are found in a digit span working memory task [35]. Contrary to these findings, however, a study using healthy children as subjects demonstrated impaired immediate, but not delayed verbal memory following intake of simple relative to complex carbohydrates [16]. These studies suggest that simple carbohydrates can impair postprandial memory performance for both immediate and delayed information, and that the effects of simple carbohydrates on memory performance may depend upon the age and/or diabetic status of the subjects. However, variations in task measures and experimental designs limits direct comparison across these studies.
Western Diets and Hippocampal-dependent Learning and Memory
Impaired learning and memory for spatial relations among objects in the environment has long been considered the benchmark test for hippocampal dysfunction in animals. A widely-used task for assessing spatial learning and memory in rodents is the Morris Water Maze (MWM). In this task, a rodent is placed into a circular pool of water where they learn to swim to an escape platform hidden a few millimeters below the surface. Visual cues are placed outside of the pool to serve as spatial landmarks. Subjects are given trials that differ with respect to the spatial starting location at the edge of the pool. The animals learn to find the escape platform with increasing efficiency across trials, presumably by using spatial reference cues located outside of the maze. Rats with damage confined to the hippocampus are severely impaired in learning and remembering the location of the platform in the MWM task (see [41] for review).
Several studies have shown that consuming components of a Western diet can also disrupt learning and memory performance in the MWM task. Rodents receiving several months of ad libitum exposure to diets with high levels of both SFA and sucrose [42–45], diets that contain high levels of SFA with more complex carbohydrates [46, 47], as well as diets that contain high levels of sucrose without elevated levels of fat [18] are impaired in learning the location of the hidden platform relative to control animals receiving a low-fat, complex carbohydrate-based diet. Furthermore, intake of a diet high in SFA and sucrose produces disruptions in memory retention tests [48], in which the platform is removed and the percentage of time spent in the correct quadrant of the maze is recorded.
Intake of Western diets is also associated with impairments in other spatial memory paradigms, such as a stone T-maze task [49] and a water radial maze [50, 51], that like the MWM, require animals to learn to swim to the location of a hidden escape platform. Diet-induced impairments have also been reported in paradigms that involve learning the spatial location of aversive reinforcement, such as a foot shock [47].
A number of studies have used the eight-arm radial maze to assess the effects of diets high in SFA and simple sugars on hippocampal-dependent spatial learning and memory function based on appetitive reinforcers (i.e., food or sucrose pellets; [31, 52, 53]. The radial maze task can be structured so that across trials a certain number of the arms of the maze are always baited with a food pellet and other arms are never baited. The ability of the rats to enter only the baited arms and to refrain from entering the unbaited arms is considered to be an index of reference memory, and entering an arm on a given trial that is never baited would be recorded as a reference memory error. The radial arm maze can also be used to assess working memory, which involves remembering which arms have already been visited on a given trial and which have not. Working memory errors occur when rats return to an arm (previously baited or nonbaited) that they have already visited on the current trial. Both reference memory and working memory with spatial cues are impaired as a consequence of selective damage to the hippocampus [54]. Similarly, deficits in both spatial reference and spatial working memory have been reported in rats that have been maintained on a Western diet (e.g., [50, 55]).
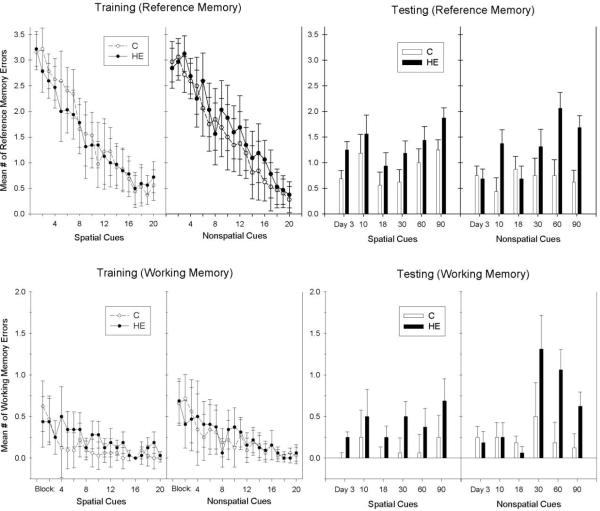
Recent studies have also examined the effects of very short-term periods of Western-diet maintenance on learning and memory function. For instance, Murray et al. [53] demonstrated that rats fed a high fat diet for only 9-days showed significantly more spatial working memory errors in an appetitive radial arm maze paradigm compared to controls receiving a low fat diet. In addition, in a variation of the radial maze task that allowed assessment of learning and memory with nonspatial and well as spatial cues, Kanoski and Davidson [52] found that after only 72-hr consumption of a Western-diet (aka “High Energy”, or “HE” diet), rats made significantly more working and reference memory errors than did chow-fed controls when the task required learning about spatial cues, but were not different from controls when performance was based on nonspatial cues. Deficits in reference and working memory performance based on nonspatial cues emerged only after 60 days on the Western diet (see Figure 1). Because rats with the hippocampus removed are not reported to show deficits in nonspatial reference memory, the results suggest that longer-term exposure to a Western diet may interfere with hippocampal-independent as well as hippocampal-dependent memory processes. Furthermore, both Kanoski and Davidson and Murray et al. reported that spatial reference and working memory deficits occurred prior to the emergence of significantly greater body weight gain by rats fed the Western diet compared to chow-fed controls. This suggests that the short-term detrimental cognitive effects of consuming a Western diet can occur in the absence of differences in body weight.
Figure 1.
Rats maintained on a high energy diet (HE Diet) were impaired relative to controls receiving a standard chow diet (C Diet) in reference and working memory problems based on spatial cues after 3 days of consumption, whereas reference and working memory based on nonspatial cues were impaired only after 60 days of Western diet maintenance (from [52]). Note that the ranges of the Y-axes differ for mean # of working and reference memory errors.
There are also examples of hippocampal-dependent nonspatial learning and memory problems that are sensitive to disruption induced by intake of Western diets. In general, these problems require rats to resolve what Morris [56] described as “predictable ambiguity”, which can involve learning when to respond or to refrain from responding to a nonspatial cue (e.g., an auditory or visual CS) that signals reinforcement under some conditions, but is not reinforced at other times. Both hippocampal damage (see [57] for review) and consumption of diets high in SFA and processed sugars (e.g., [39, 58]) or diets high in SFA with complex carbohydrates (e.g., [33]) have been reported to disrupt performance on these types of problems by reducing the ability to learn to inhibit responding on nonreinforced trials.
Diet-induced Neurophysiological Changes and Hippocampal Dysfunction
Excessive consumption of Western diets can produce a number of neurophysiological changes that can directly or indirectly impact the hippocampus. These changes include impaired glucoregulation, reduced levels of neurotrophins, neuroinflammation, and alterations in the structural integrity of the blood-brain barrier. The results of a number of studies link these changes to impairments in several types of hippocampal-dependent learning and memory operations.
Glucoregulation
Intake of saturated fat and refined carbohydrates can contribute to the development of metabolic syndrome [12], which includes insulin resistance and glucose intolerance as primary characteristics. Several recent studies point to peripheral insulin resistance as an important contributor to the decline in cognitive function that occurs in individuals with metabolic syndrome. Insulin resistance is found in approximately 80% of obese individuals, and is characterized by poor glycemic control resulting from persistent extreme elevations in, and decreased activity of, peripheral insulin [59]. High dietary levels of simple carbohydrates and saturated fat are thought to contribute strongly to the development of insulin resistance in humans [60]. In otherwise healthy elderly subjects who do not have T2DM, declining insulin sensitivity is associated with impaired cognitive performance [61–63], whereas cognitive function is improved for T2DM subjects following treatments that improve glucose regulation [64, 65].
The most consistently reported cognitive deficits associated with insulin resistance and poor glycemic control in humans are found in memory tests that are also impaired in individuals with damage to the hippocampus, such as tests of delayed verbal memory [66, 67]. This suggests that hippocampal function may be related to glycemic regulation. Consistent with this idea, Gold and colleagues [68] recently demonstrated in middle-aged subjects with well-controlled T2DM that tests of delayed verbal memory (paragraph recall) were impaired, whereas performance on tasks that are not considered to be sensitive to hippocampal damage (e.g., immediate recall, attention) was not different from age-matched controls. Interestingly, MRI scans revealed that subjects who were impaired in the memory task demonstrated a reduction in brain volume that was specific to the hippocampal region. Thus, poor glycemic control contributed to both the memory impairment and hippocampal atrophy. On the other hand, other variables commonly associated with T2DM and metabolic syndrome, including elevated BMI, hypertension, and dyslipidemia, did not contribute independently to either the memory impairment or the hippocampal atrophy.
Evidence from rodents also suggests that insulin resistance may be a key player in the relationship between Western diets and at least some types of hippocampal-dependent memory disruption. In a recent study, rats were fed either a high fat (HF) diet or a standard chow diet for one month [69]. One week prior to behavioral testing in a Morris water maze, half of the rats from each dietary treatment were given daily administration of a vehicle control or of rosiglitazone, an insulin sensitizer that increases the effectiveness of peripheral insulin signaling. The rats given the HF-diet were impaired in the spatial Morris water maze task when receiving control injections; however, the HF-diet group receiving the insulin sensitizer performed at a level similar to control rats fed a standard diet. In addition to improving spatial learning performance, rosiglitazone also lowered plasma glucose, triglycerides, cholesterol, and insulin relative to HF-rats receiving vehicle. These findings are consistent with the results of a study that reported improved cognitive performance and insulin sensitivity in human T2DM subjects that received rosiglitazone treatment for six weeks [65]. Thus, it appears that for both humans and rodents, ameliorating peripheral insulin resistance can reduce the effects of Western diets on some types of cognitive performance.
Peripheral insulin regulation may affect memory function by influencing insulin signaling in the hippocampus directly. Insulin and its receptor (IR) are abundant in the hippocampus, and administration of insulin has been shown to enhance memory at optimal doses in both rodents (intracerebroventricular administration, [70]) and humans (administered intranasally [71]). Evidence suggests that levels of CNS insulin depend upon transport of peripheral insulin into the brain, as little or no insulin is produced in the CNS (see [72]). A potential link between these results and dietary fat intake was provided by Woods and colleagues [73], who showed that high fat diet-induced obesity in dogs resulted in reduced insulin transport into the CNS relative to normal weight dogs.
The possibility that diet-induced peripheral insulin resistance is related to impaired CNS insulin signaling is supported by the results of a recent study [74]. Mice that were given a diet high in SFA and sucrose for one year were impaired in learning an appetitive operant conditioning task. The authors measured insulin-mediated signaling in the hippocampus by assessing the ability of insulin to stimulate phosphorylation of its downstream signaling kinase Akt/PKB. The results showed that relative to chow-fed controls, mice given the Western diet exhibited peripheral insulin resistance, as well as reduced insulin signaling in the hippocampus. Another study further supports the hypothesis that diets high in SFA can disrupt insulin signaling in the hippocampus [75]. Rats given a high SFA diet for several months were impaired relative to chow-fed controls in a spatial working memory task. Performance in the memory task was enhanced in control rats following direct hippocampal administration of insulin, whereas hippocampal insulin-induced enhancement was not observed in rats maintained on the high SFA diet. Collectively, these studies suggest that dietary SFA may have detrimental consequences on both peripheral and hippocampal insulin signaling. An interesting question is whether or not maintenance on a Western diet can impair central insulin signaling prior to the development of peripheral insulin resistance.
Brain-derived neurotrophic factor (BDNF)
BDNF is a neurotrophin that plays an important role in the survival, maintenance, and growth of many types of neurons [76]. BDNF is abundantly expressed in the hippocampus, hypothalamus, and cerebral cortex [77]. Interference with BDNF or its receptor can reduce synaptic plasticity [78] and neurogenesis [79] in the hippocampus. Both synaptic plasticity and neurogenesis have been postulated to be neuronal processes that underlie hippocampal-dependent learning and memory [80].
Diets high in SFA and simple sugars have been shown to reduce BDNF levels and to interfere with both synaptic plasticity and neurogenesis in the hippocampus of rodents [42]. In addition, diet-induced reductions in levels of hippocampal BDNF levels are accompanied by performance deficits in several hippocampal-dependent learning and memory problems. For example, Molteni et al. [48] reported that rats that had been maintained for 90 days on a diet high in SFA and sucrose exhibited both decreased hippocampal BDNF and impaired performance in the water maze. In addition, Molteni et al. [43] showed that both of these effects could be prevented by giving rats access to a running wheel during the period when the Western diet was available. That is, the detrimental effects of a Western diet on spatial learning and memory was abolished by a manipulation that also blocked the ability of that diet to reduce hippocampal BDNF.
Kanoski et al. [39] assessed the effects of consuming a diet high in SFA and glucose on BDNF levels in the dorsal and ventral regions of the hippocampus and in the medial prefrontal cortex (mPFC). Rats maintained on this diet for 90+ days exhibited reduced BDNF in the mPFC and ventral hippocampus, but not in the dorsal hippocampus. Anatomical studies have shown that the ventral hippocampal CA1 cell fields project directly to the mPFC [81]. Thus, the ventral hippocampal / medial prefrontocortical circuit may be more sensitive to diet-induced effects on BDNF than the dorsal area of the hippocampus. The dorsal hippocampus has been reported to have a more prominent role in learning and retaining spatial relations compared to the ventral hippocampus, which has been linked more closely to nonspatial processes (see [82] for review). One implication of these results is that because this diet had little effect on BDNF levels in the dorsal hippocampus, dietary influences on spatial learning and memory may not depend critically on levels of hippocampal BDNF.
On the other hand, the above findings also imply that intake of Western diets should impair performance on nonspatial learning and memory tasks to the extent that performance on those problems depends upon levels of BDNF in the ventral hippocampus or mPFC. Consistent with this hypothesis, Kanoski et al. [39] also found that rats that exhibited reduced levels of BDNF in the ventral hippocampus and the mPFC were also impaired in a Pavlovian discrimination-reversal task. That is, rats maintained on a diet high in SFA and glucose were not impaired in learning a simple discrimination problem in which the brief presentation of one stimulus (e.g., a light) was followed by delivery of sucrose pellets and the brief presentation of another cue (e.g., a tone) was not followed by sucrose. However, when the discriminative contingency was reversed (i.e., the tone was followed by sucrose pellets and the light was not) the rats maintained on this diet were slower than chow-fed controls at learning to respond appropriately. This pattern of results is like that shown by animals with hippocampal damage or with mPFC lesions in that the performance of these animals is not different from controls during the acquisition of a simple discrimination, but is impaired after the original discriminative contingency has been reversed [83, 84]. Also noteworthy, Kanoski et al. [39] found neither discrimination reversal performance nor reduced BDNF levels in the hippocampus or mPFC for rats that had been maintained on a Western diet that was high in saturated fat and sucrose. Thus, as with learning and memory function, the ability of Western diets to influence levels of BDNF in the brain may depend upon the glycemic nature of the carbohydrate source in the diet.
In summary, the possibility that central BDNF reduction mediates, in part, the effects of Western diet consumption on cognitive function is supported by the fact that dietary-induced alterations in BDNF occur in areas of the brain, namely the hippocampus and mPFC, that are functionally compromised by the same type of dietary manipulation. Furthermore, a Western diet can interfere with neural processes that are mediated by BDNF and considered to be related to learning and memory function, including synaptic plasticity and neurogenesis. Research that has demonstrated diet-induced alterations in BDNF employed dietary manipulations that included high levels of both SFA and simple sugars. More research would be useful in evaluating the relative contributions of each of these dietary components to alterations in BDNF levels in the brain.
Neuroinflammation
Inflammation in the brain is linked with cognitive dysfunction and AD (see [85] for review) and increased levels of inflammatory cytokines (e.g., IL-1β, IL-6) can disrupt hippocampal synaptic plasticity [86]. Recent studies show that rodents maintained on diets high in SFA have elevated markers of brain inflammation relative to standard diet-fed controls. In one study [49] mice were fed a high SFA diet for 16 weeks prior to being tested in spatial memory task. Consistent with previous studies, SFA intake was associated with impaired spatial memory and reduced BDNF. The authors also showed that intake of the SFA-based diet was linked with elevations in various markers of neuroinflammation, including increased expression of TNF-α, IL-6, and the chemokine MCP-1.
Other studies suggest a contribution of maternal diet to the neuroinflammation caused by SFA intake. It was recently found that SFA-induced impairment in spatial memory in the MWM and elevations in neuroinflammatory markers (e.g., IL-6) were exacerbated in rats born to dams fed a high SFA diet relative to those born to chow-fed dams [87]. Another study examined the effects of a maternal SFA intake on neuroinflammation in rats that were given a standard chow diet at weaning [88]. They demonstrated that adult rats from SFA-fed dams had elevated levels of the inflammatory marker IL-1β in the hippocampus relative to rats from chow-fed dams, despite being maintained on standard chow postweaning. Collectively, these studies suggest that one mechanism through which SFA intake can potentially alter cognitive function is by elevating brain inflammation, particularly when high levels of SFA are present in both the maternal and progeny diet.
Blood-Brain Barrier Integrity
The blood-brain barrier (BBB) is a specialized system comprising a microvascular endothelium that limits the entry of many blood components into the brain [89]. Damage to the BBB in humans has been shown to be strongly correlated with the development of MCI [90] and AD [91], and in some cases has been shown to precede the development clinical symptoms in both human AD patients [92] and transgenic mouse models of AD [93]. A number of recent findings suggest that dietary and metabolic factors are related to disrupted BBB integrity. For example, a recent longitudinal study found that adiposity in midlife female subjects was strongly linked with BBB integrity 24 years later, which was measured from the CSF/serum albumin ratio [94]. Dietary and metabolic factors have also been linked with BBB disruption in rodents as well. Banks and colleagues have shown that diet-induced obesity in rodents can interfere with the active transport mechanisms of various energy balance-relevant hormones across the BBB, including leptin [95] and ghrelin [96]. In addition, increased BBB permeability has been demonstrated in the cholesterol-fed New Zealand white rabbit, which is used as an animal model for AD. Several studies have shown that when these rabbits are fed a diet enriched with cholesterol (1–2%) for several weeks, increases in BBB permeability to normally excluded Evans Blue dye develops, primarily in the hippocampal region and the frontal cortex (see [97]).
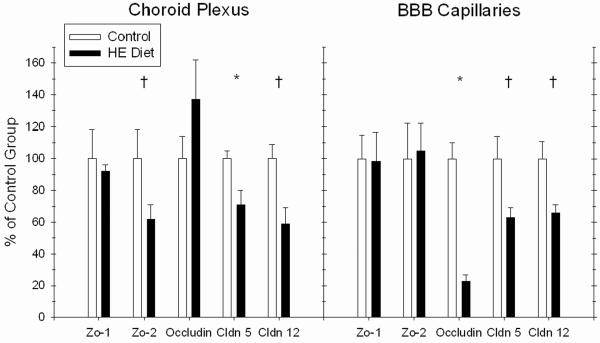
Kanoski et al. [58] recently assessed the effects of Western diet (i.e., HE diet) consumption on the integrity of the BBB and the choroid plexus, which comprises the primary structure of the blood-csf barrier (BcsfB) in rats. BBB and BcsfB integrity was examined by measuring: 1) mRNA expression of proteins that comprise the tight junctions of the BBB and BcsfB, including occludin, claudin 5, claudin 12, Zo-1, and Zo-1, and 2) permeability to sodium fluorescein (NaFl), a tracer that is normally precluded across an intact BBB [98]. Results showed that Western diet maintenance reduced expression of the tight junction proteins occludin, claudin 5, and claudin 12 (see Figure 2a), which are considered important in permeability restriction [99]. Furthermore, maintenance on the Western diet was accompanied by increased NaFl permeability in the hippocampus. In contrast, no permeability differences based on diet were found in the striatum and the prefrontal cortex (see Figure 2b). These findings point to disruption of BBB integrity as a novel mechanism that may underlie the detrimental effects of Western diet consumption on cognitive function, and they further highlight the vulnerability of the hippocampus to dietary and metabolic factors.
Figure 2.
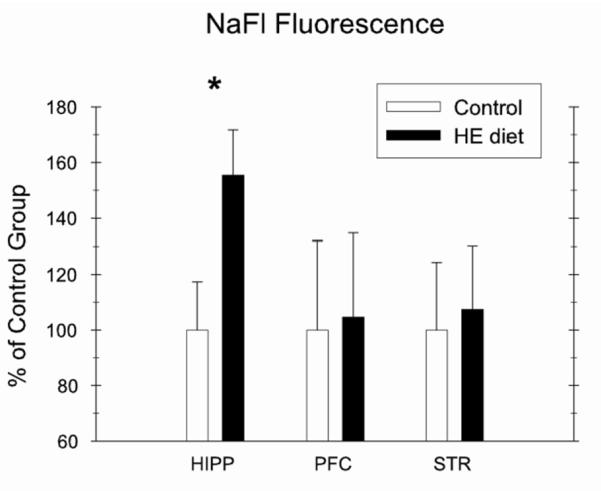
Maintenance on an HE Diet for over 4 months impaired blood-brain barrier integrity relative to rats maintained on standard chow (C) by reducing mRNA expression of tight junction proteins (Fig. 2a) and by increasing blood-to-brain permeability of the tracer sodium fluorescein (NaFl) in the hippocampus (HIPP), but not in the prefrontal cortex (PFC) and striatum (STR) (Fig. 2b; from [58]).
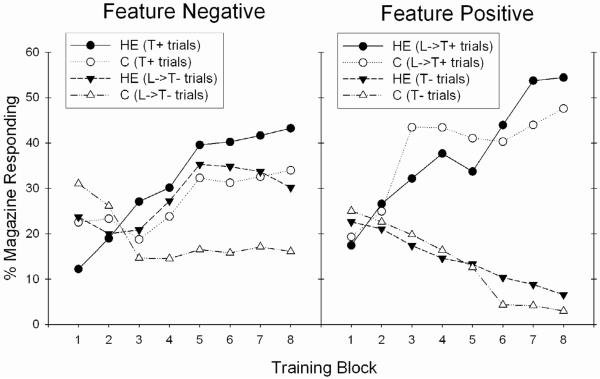
The Western diet-fed rats with impaired BBB integrity in the study described above were also evaluated for learning performance in three types of discrimination problems. In the serial feature negative discrimination problem, a discrete “target” stimulus (A; e.g., a tone) is followed by food reinforcement when presented alone (A+ trials) but not when preceded by the presentation of another “feature” stimulus (X; e.g., a light). In this problem, the feature stimulus can be thought of as “setting the occasion” for when the target stimulus is not followed by reinforcement. Thus, in the serial FN task, rats must learn to refrain from responding to a cue when it is preceded by a feature stimulus, and not to refrain from responding when that cue is presented alone. Holland and colleagues [100] demonstrated that rats with selective lesions of the hippocampus were impaired in learning a serial feature negative discrimination, displaying increased appetitive responding on nonrewarded trials (X→A− trials) compared to control rats with an intact hippocampus. Kanoski et al. [58] also tested the rats on a simple discrimination (B+,C−) problem and on a serial feature positive discrimination task, where a target stimulus is reinforced when preceded by a feature stimulus (X→A+ trials), but not when presented alone (A− trials); both the simple discrimination and the serial feature positive discrimination tasks are not impaired in rats with selective hippocampal lesions [100, 101]. Rats fed the Western diet performed like rats with hippocampal lesions in that they were impaired, relative to chow-fed controls, at solving the serial feature negative discrimination problem, but were unimpaired at solving the serial feature positive or the simple discrimination (see Figure 3). Furthermore, for rats fed the Western diet, impairment on the serial feature negative tasks took the form of elevated appetitive responding on nonrewarded trials relative to controls, the same response profile that has been observed in rats with hippocampal lesions[100] (see [84, 102] for review).
Figure 3.
Rats maintained on an HE Diet for over three months were impaired in learning a hippocampal-dependent serial feature negative problem (T+, L−>T−), but not a serial feature positive problem (L−>T+, T−) relative to controls maintained on standard chow (C) (from [58]).
The pattern of results described above suggests that consuming a Western diet may alter BBB permeability in a manner that produces selective deficits in learning and memory processes that rely on the hippocampus, while sparing, at least initially, learning and memory functions that depend primarily on other brain structures or circuits. However, precisely how changes in BBB permeability might alter hippocampal-dependent learning and memory functions is an open question. It is possible that a leaky BBB allows toxins, metals such as copper, lead, and iron, and other harmful blood-borne substances to enter the CNS. Some metals are thought to contribute towards the development of neurodegenerative diseases via BBB influx (see [103] for review). It is also possible that BBB disruption contributed to cognitive impairment by facilitating blood to brain entry of β-amyloid, the primary constituent of amyloid plaques in AD. Blood-borne β-amyloid peptides are effectively blocked from access to the brain in healthy humans and rodents with an intact BBB [104, 105]. However, when the BBB is compromised pharmacologically, peripherally-derived β-amyloid peptides can enter the CNS in both humans and rodents [106]. Furthermore, it has recently been shown that maintenance on a diet high in SFA in mice led to increased Aβ plaque load in the hippocampal dentate gyrus relative to controls given a low fat control diet, whereas no diet-based Aβ differences were found in the cerebral cortex and the ACC [107].
It also remains to be determined whether or not diet-induced changes in BBB permeability and any alterations in brain structure or function that these changes produce can be reversed by dietary or other means. It is conceivable that even if the integrity of the BBB can be restored, irreversible damage to the hippocampus might be produced, for example, by the accumulation of heavy metals. On the other hand, increased BBB permeability could be accompanied by more or less reversible physiological changes in access to or clearance of hormones (e.g., insulin), metabolites or their by-products (e.g., fatty acids, cholesterol, triglycerides), in levels of BDNF, or in neuroinflammation. Furthermore, questions about the amount and duration of exposure to Western diets that induce BBB changes and hippocampal dysfunction also need to be specified. Similarly, the possibility that there may be critical periods of development that differ in terms of sensitivity to the detrimental effects of Western diets is relatively unexplored.
Vicious Cycle Model: Linking Western Diet Consumption to Obesity and Cognitive Disorders
It may be that the ability of a Western diet to interfere with hippocampal-dependent learning and memory processes is causally linked to its capacity to promote excessive energy intake and obesity [21, 57]. Research from our laboratory [55] and elsewhere [53, 108] has shown that Western diet-induced learning and memory impairments can precede the development of diet-induced obesity. It may be the case that impairments in hippocampal-dependent processes promote intake and body weight gain by interfering with learned control of energy regulation.
As noted previously, Morris [56] proposed that the hippocampus is critically involved with resolving “predictable ambiguity”, which can be said to exist when the relationship between a particular stimulus and an outcome (e.g., food) varies dependent on the presence or absence of another stimulus or set of stimuli. The serial feature negative discrimination problem discussed previously involves this type of relationship because a target stimulus is reinforced in the absence (on A+ trials) and nonreinforced in the presence of the negative feature stimulus (X). Thus, the negative feature stimulus can be said to resolve predictable ambiguity by signaling that the target stimulus will not be reinforced on X→A- trials when the feature is present. According to prevailing views of animal learning and memory, the ability of the target cue to elicit appetitive and eating responses is directly related to how strongly it can activate the memorial representation of the reinforcing postingestive consequences of eating [109]. Behavior is suppressed on X→A- trials because the presence of the negative feature stimulus inhibits the excitement of that memory by the target cue.
A similar set of stimulus-event relations may also be part of the physiological control of energy and body weight regulation (see Figure 4). Woods has proposed that bouts of eating are initiated primarily by environmental cues associated with food [110]. Physiological or “hunger” signals arising from energy deficit normally playing little, if any, role in initiating intake. Instead, energy balance regulation depends on the generation of physiological “satiety” signals that act to terminate meals and to suppress the capacity of environmental cues to stimulate additional appetitive and eating behavior. However, this model, and indeed, most models of energy regulation have little to say about the mechanisms that enable physiological satiety signals to exert inhibitory control over appetitive and consummatory responses. According to our view, this type of inhibitory control could occur because satiety cues function as negative feature stimuli. That is, energy regulation depends on the ability of animals to solve a serial feature negative discrimination problem in which interoceptive satiety cues (X) signal that environmental food-related target cues (A) will not be followed by an appetitive postingestive outcome (e.g., X→A-).
Figure 4.
Woods (2004) model of energy regulation interpreted as a serial feature negative discrimination problem.
As noted above, animals with hippocampal lesions [100] and animals maintained on Western diets [58] are impaired in solving serial feature negative discriminations as well as other appetitive conditioning problems that involve learning when a given cue will and will not be followed by food (see [84, 102] for reviews). The data suggest that impaired performance following these surgical and dietary manipulations occurs because animals have difficulty inhibiting activation of the memory of food reinforcement on X→A- trials, rather than, for example, having difficulty exciting the activation of that memory. Similarly, if energy regulation depends, in part, on using satiety signals to determine when food-related cues will and will not be followed by appetitive postingestive reinforcement, manipulations that compromise hippocampal function should disrupt the regulation of energy intake by interfering with the ability of animals to solve this discrimination. As a consequence of this interference, satiety cues would be less able to suppress the ability of food-related cues to activate the memory of the reinforcing postingestive consequences of eating, and thus less able to prevent that memory from exciting appetitive and consummatory behaviors.
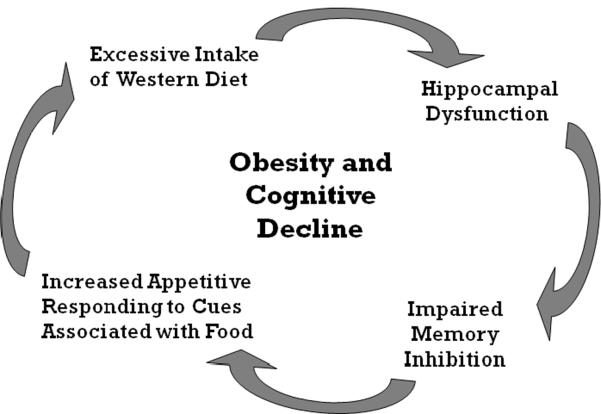
In support of this possibility, rats with selective lesions to the hippocampus show elevated food-sated appetitive responding (e.g., lever pressing, food cup approach) relative to intact controls [111–113]. Furthermore, Davidson et al. [20] recently showed that selective lesions to either the complete or the ventral hippocampus produced an increase in food intake and body weight gain in rats relative to intact and sham-operated controls. In addition, Davidson et al. [114] showed that rats without a hippocampus are less able to use their interoceptive energy state cues to inhibit appetitive conditioned responding compared to controls with an intact hippocampus. If the memory impairment produced by consumption of SFA and simple carbohydrates is like that produced by hippocampal lesions, this could account, at least in part, for why Western diets promote excessive intake and body weight gain. Moreover, if the degree of interference with hippocampal function increases with the duration of exposure or the amount of Western diet consumed, this could lead to a “vicious cycle” (see Figure 5) in which continued eating leads to greater impairment of hippocampal-dependent memory function which further weakens the ability to inhibit intake of the Western diet that promoted hippocampal dysfunction in the first place.
Figure 5.
“Vicious Circle” model proposing that Western Diet intake contributes to obesity development, in part, by impairing hippocampal-dependent memory inhibition, thereby interfering with the ability to refrain from appetitive responding to environmental cues associated with food.
Implications
This review shows that evidence is accumulating for the main components of a “vicious cycle” model that links intake of Western diets with hippocampal dysfunction. Namely that (a) Western diet intake interferes with hippocampal functioning; (b) interference with hippocampal functioning can impair memory inhibition along with other cognitive functions and; (c) impaired memory inhibition can promote the elicitation of appetitive behavior by food-related environmental cues. However, this model also generates many questions. For example, it is not known whether overweight or obese people exhibit the type of inhibitory memory impairment that the model proposes. If these impairments are observed it will also be important to determine when they first begin to emerge. It is also unclear whether the impact on the hippocampus of consuming Western diet is reversible when the intake of the diet is discontinued or reduced. Furthermore, although evidence has emerged linking Western diets and obesity in mid-life to AD and other types of cognitive decline much later (e.g., [115, 116]), the mechanisms underlying this potential connection have not yet been established. In particular, if hippocampal dysfunction promotes body weight gain, why does body weight gain often emerge years before serious cognitive decline? One possibility is that the degree of cognitive impairment produced by consuming Western diets gradually increases over time and it may take many years before the diagnostic criteria for full-blown cognitive pathology is achieved. In this regard, our working model suggests that it may be possible to identify impairments in specific types of memory processes, particularly hippocampal-dependent inhibitory memory, as early markers for much more serious disorder later on. In rodents and humans, there also seem to be differences in sensitivity to the capacity of Western diets to promote excessive caloric intake and weight gain. An additional question of interest is whether or not these differences in sensitivity are also correlated with differences in the effects of Western diets on hippocampal-dependent learning and memory processes. Given the serious negative health consequences of both obesity and cognitive decline, providing answers to these and other questions related to Western diet consumption and hippocampal function should be an important goal of researchers interested in disorders of energy regulation and cognition.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology. 2007;132:2087–102. doi: 10.1053/j.gastro.2007.03.052. [DOI] [PubMed] [Google Scholar]
- [2].Abete I, Astrup A, Martinez JA, Thorsdottir I, Zulet MA. Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev. 2010;68:214–31. doi: 10.1111/j.1753-4887.2010.00280.x. [DOI] [PubMed] [Google Scholar]
- [3].Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–7. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Kelley BJ, Petersen RC. Alzheimer's disease and mild cognitive impairment. Neurol Clin. 2007;25:577–609. v. doi: 10.1016/j.ncl.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood) 2009;28:w822–31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- [6].Alzheimer's Disease Facts and Figures 2007. Alzheimer's Association; 2010. [Google Scholar]
- [7].Benoit SC, Kemp CJ, Elias CF, Abplanalp W, Herman JP, Migrenne S, et al. Palmitic acid mediates hypothalamic insulin resistance by altering PKC-theta subcellular localization in rodents. J Clin Invest. 2009;119:2577–89. doi: 10.1172/JCI36714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, et al. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer's disease. Neurology. 2001;57:2229–35. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- [10].Walsh TJ, Emerich DF. The hippocampus as a common target of neurotoxic agents. Toxicology. 1988;49:137–40. doi: 10.1016/0300-483x(88)90185-0. [DOI] [PubMed] [Google Scholar]
- [11].Rothman SM, Mattson MP. Adverse stress, hippocampal networks, and Alzheimer's disease. Neuromolecular Med. 2010;12:56–70. doi: 10.1007/s12017-009-8107-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hu F, van Dam R, Liu S. Diet and risk of Type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44:805–17. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- [13].Berrino F. Western diet and Alzheimer's disease. Epidemiol Prev. 2002;26:107–15. [PubMed] [Google Scholar]
- [14].Grant WB, Campbell A, Itzhaki RF, Savory J. The significance of environmental factors in the etiology of Alzheimer's disease. J Alzheimers Dis. 2002;4:179–89. doi: 10.3233/jad-2002-4308. [DOI] [PubMed] [Google Scholar]
- [15].Pasinetti G, Eberstein J. Metabolic syndrome and the role of dietary lifestyles in Alzheimer's disease. J Neurochem. 2008;106:1503–14. doi: 10.1111/j.1471-4159.2008.05454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Benton D, Maconie A, Williams C. The influence of the glycaemic load of breakfast on the behaviour of children in school. Physiol Behav. 2007;92:717–24. doi: 10.1016/j.physbeh.2007.05.065. [DOI] [PubMed] [Google Scholar]
- [17].Eskelinen M, Ngandu T, Helkala E, Tuomilehto J, Nissinen A, Soininen H, et al. Fat intake at midlife and cognitive impairment later in life: a population-based CAIDE study. Int J Geriatr Psychiatry. 2008;23:741–7. doi: 10.1002/gps.1969. [DOI] [PubMed] [Google Scholar]
- [18].Jurdak N, Lichtenstein A, Kanarek R. Diet-induced obesity and spatial cognition in young male rats. Nutr Neurosci. 2008;11:48–54. doi: 10.1179/147683008X301333. [DOI] [PubMed] [Google Scholar]
- [19].Morris MC, Evans DA, Bienias JL, Tangney CC, Wilson RS. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62:1573–9. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- [20].Davidson TL, Chan K, Jarrard LE, Kanoski SE, Clegg DJ, Benoit SC. Contributions of the hippocampus and medial prefrontal cortex to energy and body weight regulation. Hippocampus. 2009;19:235–52. doi: 10.1002/hipo.20499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Davidson TL, Kanoski SE, Walls EK, Jarrard LE. Memory inhibition and energy regulation. Physiol Behav. 2005;86:731–46. doi: 10.1016/j.physbeh.2005.09.004. [DOI] [PubMed] [Google Scholar]
- [22].Rozin P, Dow S, Moscovitch M, Rajaram S. What causes humans to begin and end a meal? A role for memory for what has been eaten, as evidenced by a study of multiple meal eating in amnesic patients. Psychological Science. 1998;9:392–6. [Google Scholar]
- [23].Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- [24].Carrie I, Abellan Van Kan G, Rolland Y, Gillette-Guyonnet S, Vellas B. PUFA for prevention and treatment of dementia? Curr Pharm Des. 2009;15:4173–85. doi: 10.2174/138161209789909764. [DOI] [PubMed] [Google Scholar]
- [25].Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–80. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- [26].Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- [27].Morris M, Evans D, Tangney C, Bienias J, Schneider J, Wilson R, et al. Dietary copper and high saturated and trans fat intakes associated with cognitive decline. Arch Neurol. 2006;63:1085–8. doi: 10.1001/archneur.63.8.1085. [DOI] [PubMed] [Google Scholar]
- [28].Morris MC, Evans DA, Bienias JL, Tangney CC, Bennett DA, Aggarwal N, et al. Dietary fats and the risk of incident Alzheimer disease. Arch Neurol. 2003;60:194–200. doi: 10.1001/archneur.60.2.194. [DOI] [PubMed] [Google Scholar]
- [29].Einstein GO, Smith RE, McDaniel MA, Shaw P. Aging and prospective memory: the influence of increased task demands at encoding and retrieval. Psychol Aging. 1997;12:479–88. doi: 10.1037//0882-7974.12.3.479. [DOI] [PubMed] [Google Scholar]
- [30].Tesfaye F, Nawi N, Van Minh H, Byass P, Berhane Y, Bonita R, et al. Association between body mass index and blood pressure across three populations in Africa and Asia. J Hum Hypertens. 2007;21:28–37. doi: 10.1038/sj.jhh.1002104. [DOI] [PubMed] [Google Scholar]
- [31].Greenwood CE, Winocur G. Learning and memory impairment in rats fed a high saturated fat diet. Behav Neural Biol. 1990;53:74–87. doi: 10.1016/0163-1047(90)90831-p. [DOI] [PubMed] [Google Scholar]
- [32].Greenwood CE, Winocur G. Glucose treatment reduces memory deficits in young adult rats fed high-fat diets. Neurobiology of Learning & Memory. 2001;75:179–89. doi: 10.1006/nlme.2000.3964. [DOI] [PubMed] [Google Scholar]
- [33].Greenwood CE, Winocur G. Cognitive impairment in rats fed high-fat diets: a specific effect of saturated fatty-acid intake. Behavioral Neuroscience. 1996;110:451–9. doi: 10.1037//0735-7044.110.3.451. [DOI] [PubMed] [Google Scholar]
- [34].Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, et al. Origins and evolution of the Western diet: health implications for the 21st century.[see comment] American Journal of Clinical Nutrition. 2005;81:341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- [35].Papanikolaou Y, Palmer H, Binns MA, Jenkins DJ, Greenwood CE. Better cognitive performance following a low-glycaemic-index compared with a high-glycaemic-index carbohydrate meal in adults with type 2 diabetes. Diabetologia. 2006;49:855–62. doi: 10.1007/s00125-006-0183-x. [DOI] [PubMed] [Google Scholar]
- [36].Nabb S, Benton D. The influence on cognition of the interaction between the macro-nutrient content of breakfast and glucose tolerance. Physiol Behav. 2006;87:16–23. doi: 10.1016/j.physbeh.2005.08.034. [DOI] [PubMed] [Google Scholar]
- [37].Smith M, Foster J. The impact of a high versus a low glycaemic index breakfast cereal meal on verbal episodic memory in healthy adolescents. Nutr Neurosci. 2008;11:219–27. doi: 10.1179/147683008X344110. [DOI] [PubMed] [Google Scholar]
- [38].Jurdak N, Kanarek R. Sucrose-induced obesity impairs novel object recognition learning in young rats. Physiol Behav. 2009;96:1–5. doi: 10.1016/j.physbeh.2008.07.023. [DOI] [PubMed] [Google Scholar]
- [39].Kanoski SE, Meisel RL, Mullins AJ, Davidson TL. The effects of energy-rich diets on discrimination reversal learning and on BDNF in the hippocampus and prefrontal cortex of the rat. Behav Brain Res. 2007;182:57–66. doi: 10.1016/j.bbr.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Trout DL, Hallfrisch J, Behall KM. Atypically high insulin responses to some foods relate to sugars and satiety. International Journal of Food Sciences & Nutrition. 2004;55:577–88. doi: 10.1080/09637480400029308. [DOI] [PubMed] [Google Scholar]
- [41].D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- [42].Stranahan A, Norman E, Lee K, Cutler R, Telljohann R, Egan J, et al. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–8. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gomez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–40. doi: 10.1016/j.neuroscience.2003.09.020. [DOI] [PubMed] [Google Scholar]
- [44].Goldbart A, Row B, Kheirandish-Gozal L, Cheng Y, Brittian K, Gozal D. High fat/refined carbohydrate diet enhances the susceptibility to spatial learning deficits in rats exposed to intermittent hypoxia. Brain Res. 2006;1090:190–6. doi: 10.1016/j.brainres.2006.03.046. [DOI] [PubMed] [Google Scholar]
- [45].Wu A, Molteni R, Ying Z, Gomez-Pinilla F. A saturated-fat diet aggravates the outcome of traumatic brain injury on hippocampal plasticity and cognitive function by reducing brain-derived neurotrophic factor. Neuroscience. 2003;119:365–75. doi: 10.1016/s0306-4522(03)00154-4. [DOI] [PubMed] [Google Scholar]
- [46].Yu H, Bi Y, Ma W, He L, Yuan L, Feng J, et al. Long-term effects of high lipid and high energy diet on serum lipid, brain fatty acid composition, and memory and learning ability in mice. Int J Dev Neurosci. 2009 doi: 10.1016/j.ijdevneu.2009.12.001. [DOI] [PubMed] [Google Scholar]
- [47].Farr S, Yamada K, Butterfield D, Abdul H, Xu L, Miller N, et al. Obesity and hypertriglyceridemia produce cognitive impairment. Endocrinology. 2008;149:2628–36. doi: 10.1210/en.2007-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Molteni R, Barnard RJ, Ying Z, Roberts CK, Gomez-Pinilla F. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience. 2002;112:803–14. doi: 10.1016/s0306-4522(02)00123-9. [DOI] [PubMed] [Google Scholar]
- [49].Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Granholm A, Bimonte-Nelson H, Moore A, Nelson M, Freeman L, Sambamurti K. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J Alzheimers Dis. 2008;14:133–45. doi: 10.3233/jad-2008-14202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Thirumangalakudi L, Prakasam A, Zhang R, Bimonte-Nelson H, Sambamurti K, Kindy MS, et al. High cholesterol-induced neuroinflammation and amyloid precursor protein processing correlate with loss of working memory in mice. J Neurochem. 2008;106:475–85. doi: 10.1111/j.1471-4159.2008.05415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. J Exp Psychol Anim Behav Process. 2010;36:313–9. doi: 10.1037/a0017228. [DOI] [PubMed] [Google Scholar]
- [53].Murray AJ, Knight NS, Cochlin LE, McAleese S, Deacon RM, Rawlins JN, et al. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009 doi: 10.1096/fj.09-139691. [DOI] [PubMed] [Google Scholar]
- [54].Jarrard LE, Davidson TL, Bowring B. Functional differentiation within the medial temporal lobe in the rat. Hippocampus. 2004;14:434–49. doi: 10.1002/hipo.10194. [DOI] [PubMed] [Google Scholar]
- [55].Kanoski SE, Davidson TL. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. Journal of Experimental Psychology: Animal Behavior Processes. 2010 doi: 10.1037/a0017228. in press. [DOI] [PubMed] [Google Scholar]
- [56].Morris R. In: The Hippocampus Book. Anderson P, Morris R, Amaral D, Bliss T, O'Keefe J, editors. Oxford University Press; New York, NY: 2006. p. 872. [Google Scholar]
- [57].Davidson TL, Kanoski SE, Schier LA, Clegg DJ, Benoit SC. A potential role for the hippocampus in energy intake and body weight regulation. Curr Opin Pharmacol. 2007;7:613–6. doi: 10.1016/j.coph.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Kanoski SE, Zhang Y, Zheng W, Davidson TL. The Effects of a High-Energy Diet on Hippocampal Function and Blood-Brain Barrier Integrity in the Rat. J Alzheimers Dis. 2010;21:207–19. doi: 10.3233/JAD-2010-091414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Martyn J, Kaneki M, Yasuhara S. Obesity-induced insulin resistance and hyperglycemia: etiologic factors and molecular mechanisms. Anesthesiology. 2008;109:137–48. doi: 10.1097/ALN.0b013e3181799d45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Gross L, Li L, Ford E, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr. 2004;79:774–9. doi: 10.1093/ajcn/79.5.774. [DOI] [PubMed] [Google Scholar]
- [61].Vanhanen M, Koivisto K, Kuusisto J, Mykkanen L, Helkala E, Hanninen T, et al. Cognitive function in an elderly population with persistent impaired glucose tolerance. Diabetes Care. 1998;21:398–402. doi: 10.2337/diacare.21.3.398. [DOI] [PubMed] [Google Scholar]
- [62].Kaplan R, Greenwood C, Winocur G, Wolever T. Cognitive performance is associated with glucose regulation in healthy elderly persons and can be enhanced with glucose and dietary carbohydrates. Am J Clin Nutr. 2000;72:825–36. doi: 10.1093/ajcn/72.3.825. [DOI] [PubMed] [Google Scholar]
- [63].Kalmijn S, Feskens E, Launer L, Stijnen T, Kromhout D. Glucose intolerance, hyperinsulinaemia and cognitive function in a general population of elderly men. Diabetologia. 1995;38:1096–102. doi: 10.1007/BF00402181. [DOI] [PubMed] [Google Scholar]
- [64].Meneilly G, Cheung E, Tessier D, Yakura C, Tuokko H. The effect of improved glycemic control on cognitive functions in the elderly patient with diabetes. J Gerontol. 1993;48:M117–21. doi: 10.1093/geronj/48.4.m117. [DOI] [PubMed] [Google Scholar]
- [65].Ryan C, Freed M, Rood J, Cobitz A, Waterhouse B, Strachan M. Improving metabolic control leads to better working memory in adults with type 2 diabetes. Diabetes Care. 2006;29:345–51. doi: 10.2337/diacare.29.02.06.dc05-1626. [DOI] [PubMed] [Google Scholar]
- [66].Greenwood CE, Kaplan RJ, Hebblethwaite S, Jenkins DJ. Carbohydrate-induced memory impairment in adults with type 2 diabetes.[see comment] Diabetes Care. 2003;26:1961–6. doi: 10.2337/diacare.26.7.1961. [DOI] [PubMed] [Google Scholar]
- [67].Stewart R, Liolitsa D. Type 2 diabetes mellitus, cognitive impairment and dementia. Diabetic Medicine. 1999;16:93–112. doi: 10.1046/j.1464-5491.1999.00027.x. [DOI] [PubMed] [Google Scholar]
- [68].Gold S, Dziobek I, Sweat V, Tirsi A, Rogers K, Bruehl H, et al. Hippocampal damage and memory impairments as possible early brain complications of type 2 diabetes. Diabetologia. 2007;50:711–9. doi: 10.1007/s00125-007-0602-7. [DOI] [PubMed] [Google Scholar]
- [69].Pathan A, Gaikwad A, Viswanad B, Ramarao P. Rosiglitazone attenuates the cognitive deficits induced by high fat diet feeding in rats. Eur J Pharmacol. 2008;589:176–9. doi: 10.1016/j.ejphar.2008.06.016. [DOI] [PubMed] [Google Scholar]
- [70].Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiology & Behavior. 2000;68:509–14. doi: 10.1016/s0031-9384(99)00220-6. [DOI] [PubMed] [Google Scholar]
- [71].Reger M, Watson G, Green P, Baker L, Cholerton B, Fishel M, et al. Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J Alzheimers Dis. 2008;13:323–31. doi: 10.3233/jad-2008-13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Woods S, Seeley R, Baskin D, Schwartz M. Insulin and the blood-brain barrier. Curr Pharm Des. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- [73].Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW. Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes. 2000;49:1525–33. doi: 10.2337/diabetes.49.9.1525. [DOI] [PubMed] [Google Scholar]
- [74].Mielke J, Nicolitch K, Avellaneda V, Earlam K, Ahuja T, Mealing G, et al. Longitudinal study of the effects of a high-fat diet on glucose regulation, hippocampal function, and cerebral insulin sensitivity in C57BL/6 mice. Behav Brain Res. 2006;175:374–82. doi: 10.1016/j.bbr.2006.09.010. [DOI] [PubMed] [Google Scholar]
- [75].McNay EC, Ong CT, McCrimmon RJ, Cresswell J, Bogan JS, Sherwin RS. Hippocampal memory processes are modulated by insulin and high-fat-induced insulin resistance. Neurobiol Learn Mem. 2010;93:546–53. doi: 10.1016/j.nlm.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. Embo J. 1982;1:549–53. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Leibrock J, Lottspeich F, Hohn A, Hofer M, Hengerer B, Masiakowski P, et al. Molecular cloning and expression of brain-derived neurotrophic factor. Nature. 1989;341:149–52. doi: 10.1038/341149a0. [DOI] [PubMed] [Google Scholar]
- [78].Danzer S, Kotloski R, Walter C, Hughes M, McNamara J. Altered morphology of hippocampal dentate granule cell presynaptic and postsynaptic terminals following conditional deletion of TrkB. Hippocampus. 2008;18:668–78. doi: 10.1002/hipo.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Bergami M, Rimondini R, Santi S, Blum R, Gotz M, Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc Natl Acad Sci U S A. 2008;105:15570–5. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–24. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- [81].Swanson LW. A direct projection from Ammon's horn to prefrontal cortex in the rat. Brain Res. 1981;217:150–4. doi: 10.1016/0006-8993(81)90192-x. [DOI] [PubMed] [Google Scholar]
- [82].Fanselow MS, Dong HW. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron. 2010;65:7–19. doi: 10.1016/j.neuron.2009.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Berger TW, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behavioural Brain Research. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- [84].Davidson TL, Jarrard LE. The hippocampus and inhibitory learning: a `Gray' area? Neurosci Biobehav Rev. 2004;28:261–71. doi: 10.1016/j.neubiorev.2004.02.001. [DOI] [PubMed] [Google Scholar]
- [85].Moore AH, O'Banion MK. Neuroinflammation and anti-inflammatory therapy for Alzheimer's disease. Adv Drug Deliv Rev. 2002;54:1627–56. doi: 10.1016/s0169-409x(02)00162-x. [DOI] [PubMed] [Google Scholar]
- [86].Jankowsky JL, Patterson PH. Cytokine and growth factor involvement in long-term potentiation. Mol Cell Neurosci. 1999;14:273–86. [PubMed] [Google Scholar]
- [87].White CL, Pistell PJ, Purpera MN, Gupta S, Fernandez-Kim SO, Hise TL, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35:3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bilbo SD, Tsang V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010 doi: 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- [89].Persidsky Y, Ramirez S, Haorah J, Kanmogne G. Blood-brain barrier: structural components and function under physiologic and pathologic conditions. J Neuroimmune Pharmacol. 2006;1:223–36. doi: 10.1007/s11481-006-9025-3. [DOI] [PubMed] [Google Scholar]
- [90].Wang H, Golob E, Su M. Vascular volume and blood-brain barrier permeability measured by dynamic contrast enhanced MRI in hippocampus and cerebellum of patients with MCI and normal controls. J Magn Reson Imaging. 2006;24:695–700. doi: 10.1002/jmri.20669. [DOI] [PubMed] [Google Scholar]
- [91].Bowman G, Kaye J, Moore M, Waichunas D, Carlson N, Quinn J. Blood-brain barrier impairment in Alzheimer disease: stability and functional significance. Neurology. 2007;68:1809–14. doi: 10.1212/01.wnl.0000262031.18018.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Skoog I, Wallin A, Fredman P, Hesse C, Aevarsson O, Karlsson I, et al. A population study on blood-brain barrier function in 85-year-olds: relation to Alzheimer's disease and vascular dementia. Neurology. 1998;50:966–71. doi: 10.1212/wnl.50.4.966. [DOI] [PubMed] [Google Scholar]
- [93].Ujiie M, Dickstein D, Carlow D, Jefferies W. Blood-brain barrier permeability precedes senile plaque formation in an Alzheimer disease model. Microcirculation. 2003;10:463–70. doi: 10.1038/sj.mn.7800212. [DOI] [PubMed] [Google Scholar]
- [94].Gustafson D, Karlsson C, Skoog I, Rosengren L, Lissner L, Blennow K. Mid-life adiposity factors relate to blood-brain barrier integrity in late life. J Intern Med. 2007;262:643–50. doi: 10.1111/j.1365-2796.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- [95].Banks W, Coon A, Robinson S, Moinuddin A, Shultz J, Nakaoke R, et al. Triglycerides induce leptin resistance at the blood-brain barrier. Diabetes. 2004;53:1253–60. doi: 10.2337/diabetes.53.5.1253. [DOI] [PubMed] [Google Scholar]
- [96].Banks W, Burney B, Robinson S. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides. 2008;29:2061–5. doi: 10.1016/j.peptides.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Sparks D. The early and ongoing experience with the cholesterol-fed rabbit as a model of Alzheimer's disease: the old, the new and the pilot. J Alzheimers Dis. 2008;15:641–56. doi: 10.3233/jad-2008-15410. [DOI] [PubMed] [Google Scholar]
- [98].Kozler P, Pokorny J. Altered blood-brain barrier permeability and its effect on the distribution of Evans blue and sodium fluorescein in the rat brain applied by intracarotid injection. Physiol Res. 2003;52:607–14. [PubMed] [Google Scholar]
- [99].Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–37. doi: 10.1016/s1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- [100].Holland PC, Lamoureux JA, Han J-S, Gallagher M. Hippocampal lesions interfere with Pavlovian negative occasion setting. Hippocampus. 1999;9:143–57. doi: 10.1002/(SICI)1098-1063(1999)9:2<143::AID-HIPO6>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- [101].Jarrard LE, Davidson TL. On the hippocampus and learned conditional responding: effects of aspiration versus ibotenate lesions. Hippocampus. 1991;1:107–17. doi: 10.1002/hipo.450010110. [DOI] [PubMed] [Google Scholar]
- [102].Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav Brain Res. 2001;119:111–30. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- [103].Yokel RA. Blood-brain barrier flux of aluminum, manganese, iron and other metals suspected to contribute to metal-induced neurodegeneration. J Alzheimers Dis. 2006;10:223–53. doi: 10.3233/jad-2006-102-309. [DOI] [PubMed] [Google Scholar]
- [104].Kandimalla K, Curran G, Holasek S, Gilles E, Wengenack T, Poduslo J. Pharmacokinetic analysis of the blood-brain barrier transport of 125I-amyloid beta protein 40 in wild-type and Alzheimer's disease transgenic mice (APP,PS1) and its implications for amyloid plaque formation. J Pharmacol Exp Ther. 2005;313:1370–8. doi: 10.1124/jpet.104.081901. [DOI] [PubMed] [Google Scholar]
- [105].Poduslo J, Curran G, Sanyal B, Selkoe D. Receptor-mediated transport of human amyloid beta-protein 1–40 and 1–42 at the blood-brain barrier. Neurobiol Dis. 1999;6:190–9. doi: 10.1006/nbdi.1999.0238. [DOI] [PubMed] [Google Scholar]
- [106].Clifford P, Zarrabi S, Siu G, Kinsler K, Kosciuk M, Venkataraman V, et al. Abeta peptides can enter the brain through a defective blood-brain barrier and bind selectively to neurons. Brain Res. 2007;1142:223–36. doi: 10.1016/j.brainres.2007.01.070. [DOI] [PubMed] [Google Scholar]
- [107].Hooijmans CR, Rutters F, Dederen PJ, Gambarota G, Veltien A, van Groen T, et al. Changes in cerebral blood volume and amyloid pathology in aged Alzheimer APP/PS1 mice on a docosahexaenoic acid (DHA) diet or cholesterol enriched Typical Western Diet (TWD) Neurobiol Dis. 2007;28:16–29. doi: 10.1016/j.nbd.2007.06.007. [DOI] [PubMed] [Google Scholar]
- [108].Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, et al. High-fat diet impairs hippocampal neurogenesis in male rats. European Journal of Neurology. 2006;13:1385–8. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- [109].Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu Rev Neurosci. 1988;11:329–52. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- [110].Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. American Journal of Physiology - Gastrointestinal & Liver Physiology. 2004;286:G7–13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- [111].Clifton PG, Vickers SP, Somerville EM. Little and often: Ingestive behavior patterns following hippocampal lesions in rats. Behavioral Neuroscience. 1998;112:502–11. doi: 10.1037//0735-7044.112.3.502. [DOI] [PubMed] [Google Scholar]
- [112].Davidson TL, Jarrard LE. A role for hippocampus in the utilization of hunger signals. Behav Neural Biol. 1993;59:167–71. doi: 10.1016/0163-1047(93)90925-8. [DOI] [PubMed] [Google Scholar]
- [113].Schmelzeis MC, Mittleman G. The hippocampus and reward: effects of hippocampal lesions on progressive-ratio responding. Behavioral Neuroscience. 1996;110:1049–66. doi: 10.1037//0735-7044.110.5.1049. [DOI] [PubMed] [Google Scholar]
- [114].Davidson TL, Kanoski SE, Chan K, Clegg DJ, Benoit SC, Jarrard LE. Hippocampal lesions impair retention of discriminative responding based on energy state cues. Behav Neurosci. 2010;124:97–105. doi: 10.1037/a0018402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gustafson D. A life course of adiposity and dementia. Eur J Pharmacol. 2008;585:163–75. doi: 10.1016/j.ejphar.2008.01.052. [DOI] [PubMed] [Google Scholar]
- [116].Whitmer R, Gustafson D, Barrett-Connor E, Haan M, Gunderson E, Yaffe K. Central obesity and increased risk of dementia more than three decades later. Neurology. 2008;71:1057–64. doi: 10.1212/01.wnl.0000306313.89165.ef. [DOI] [PubMed] [Google Scholar]