Abstract
The inner ear consists of the cochlea (the organ of hearing) and the vestibular system (the organs of balance). Within the vestibular system, linear acceleration and gravity are detected by the saccule and utricle. Resting above the neurosensory epithelia of these organs are otoconia, minute proteinaceous and crystalline (calcite) inertial masses that shift under the physical forces imparted by linear movements and gravity. It is the transduction and sensation of these movements and their integration with vision and proprioceptive inputs that contribute to the sensation of balance. It has been proposed that a reactive oxygen species- (ROS-) generating NADPH oxidase comprising the gene products of the Nox3, Noxo1, and Cyba genes plays a critical and constructive role in the process of inner-ear development, specifically, the deposition of otoconia. Inactivation in mouse of any of the NADPH oxidase components encoded by the Nox3, Noxo1, or Cyba gene results in the complete congenital absence of otoconia and profound vestibular dysfunction. Here we describe our use of PCR, reverse transcription-PCR (RT-PCR), and rapid amplification of cDNA ends (RACE) with traditional and high-throughput (HTP) sequencing technologies to extend and complete the molecular characterization of an allelic series of seven mutations in the Nox3 gene. Collectively, the mutation spectrum includes an endogenous retrovirus insertion, two missense mutations, a splice donor mutation, a splice acceptor mutation, premature translational termination, and a small duplication. Together, these alleles provide tools to investigate the mechanisms of otoconial deposition over development, throughout aging, and in various disease states.
Introduction
The mouse Nox3 gene encodes an NADPH oxidase that is expressed primarily in the inner ear and is known to be required for the proper development and function of the vestibular system (Banfi et al. 2004; Cheng et al. 2001; Paffenholz et al. 2004). Specifically, the six-transmembrane NADPH-binding protein encoded by Nox3 is believed to interact with a two-transmembrane protein encoded by Cyba and a cytosolic protein encoded by Noxo1 to form part, or all, of a functional NADPH oxidase complex that is capable of transporting electrons across biological membranes to oxygen (O2) forming superoxide (O2•−) and a cascade of subsequent reactive oxygen species (ROS).
Mutation of any of these three components has a profound effect on the ability of mice to detect linear acceleration or gravity, deficits manifested overtly by abnormalities in behavior (Bergstrom et al. 2004; Kiss et al. 2006; Nakano et al. 2008; Paffenholz et al. 2004). Aberrant behavior may include oblique posturing of the head and, to varying degrees, hyperactivity and circling. Additional behavioral abnormalities are apparent when the animals are challenged and include impaired air and ground righting reflexes, poor performance on rotating rod and stationary balance beam tests, and an inability to swim.
Although the saccule and utricle (the linear acceleration-and gravity-detecting organs within the vestibule) exhibit no apparent cellular abnormalities, in mutant mice they nonetheless remain inactive due to a complete congenital absence of otoconia, crystalline inertial masses that normally rest atop these organs (Jones et al. 1999; Nakano et al. 2008). In normal mice, in response to physical forces, the otoconia shift imparting shearing forces on the underlying stereocilia of the saccule and utricle. These mechanical signals are transduced by the saccule and utricle into action potentials that propagate along the eighth cranial (vestibulocochlear, VIII) nerve to the brain.
Jones et al. have developed a noninvasive method for detecting these vestibular-evoked potentials (VsEPs) (Jones and Jones 1999; Jones et al. 1999, 2002, 2003a, b, 2004, 2004, 2006), and it has now been shown that homozygosity for loss-of-function mutations in each of Nox3, Cyba, and Noxo1 leads to the complete loss of VsEPs (Jones et al. 1999; Nakano et al. 2008). Thus, it seems quite clear that the production of ROS plays an important and constructive role in the development and function of the vestibular system.
In the cochlea, the organ of hearing, the situation may be quite the opposite. Due to their reactive nature, ROS interact with a variety of small inorganic molecules and biological macromolecules and can cause irreversible oxidative damage to these targets specifically, as well as to cells and organisms more generally (Bedard and Krause 2007). In fact, ROS have been widely implicated as factors in age-, noise-, and drug-induced hearing loss (Darrat et al. 2007; Guthrie 2008; Henderson et al. 1999, 2006; Hosokawa 2002; Kopke et al. 1999; Rybak et al. 2007; van den Berg et al. 2006). Moreover, the discovery of Nox3 in the inner ear has raised the possibility of the Nox3-dependent NADPH oxidase as a source of potentially harmful ROS in these settings. A recent study by Mukherjea et al. (2010) suggests that this may indeed be the case. In their study, the authors were able to inhibit cisplatin-induced hearing loss in the chinchilla by the transtympanic administration of a siRNA directed against Nox3. In other studies the complete loss of function at the Nox3 locus may provide protection against hearing loss in some susceptible strains (K.N. Mills and S.M. Jones, personal communication). Accordingly, whether it be to understand a constructive role in the structure and function of the vestibular system, or possible deleterious roles in age-, noise-, and drug-related hearing loss, a complete understanding of the function of inner-ear NADPH oxidase components and their mutant alleles is required.
Studies of such allelic series (e.g., Lmna and associated laminopathies, for reviews see Burke and Stewart 2006; Capell and Collins 2006; Scharner et al. 2010; Stewart et al. 2007; Worman et al. 2009) are informative by identifying structure/function relationships across the various domains of multidomain proteins and by displaying the broad range of phenotypic consequences potentially engendered by a wide spectrum of mutation types and locations. Moreover, by identifying mutant alleles with various characters (hypermorphic, hypomorphic, neomorphic, antimorphic), the geneticist is often empowered to conduct a detailed functional dissection of mechanism. In addition, when mutant alleles arise spontaneously on strains not commonly employed in large gene-trapping and gene-targeting projects, mutant phenotypes can be altered by genetic modifiers present in the novel strain. This provides deeper insight into the role that genetic background can have on the manifestation of phenotype, can lead to the identification of modifying genes, and offers inroads to other interacting factors and pathways.
In regard to alleles of the inner-ear NADPH oxidase, a full molecular characterization of mutation types may prove necessary for understanding the range of clinical presentation among patients at vestibular testing facilities and the range of phenotypes observed among mutation-bearing strain sets in the basic sciences laboratory. In this regard, a survey of the Mouse Genome Informatics website identifies mutant mouse strains representing a single mutant allele of Noxo1 (known as hslt), a single mutant allele of Cyba (known as nmf333) and six mutant alleles of Nox3 (known as het, het-2J, het-3J, het-4J, R96, and R542) (Bergstrom et al. 2004; Kiss et al. 2006; Nakano et al. 2008; Paffenholz et al. 2004). With the addition of a newly identified allele of Nox3 (het-5J) described in this report, the number of Nox3 mutant alleles represented by mouse strains is now seven.
In this study we describe our use of PCR, reverse transcription-PCR (RT-PCR), and rapid amplification of cDNA ends (RACE), as well as traditional Sanger sequencing methods and more recent high-throughput (HTP) sequencing technologies, to extend and complete the molecular characterization of all of the known mutant alleles at Nox3. In turn, we hope to gain a greater understanding of the genotype/phenotype correlations in Nox3 function and the important roles Nox3 plays in development, physiology and disease.
Materials and methods
Mice
All mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and were housed on a bedding of white pine shavings and fed NIH-31 5K54 (4% fat) or NIH-31 5K52 (6% fat) diet and acidified water ad libitum. All experiments were performed with the approval of The Jackson Laboratory Institutional Animal Care and Use Committee (IACUC) and in compliance with all applicable laws and guidelines.
Nomenclature
All mouse alleles, genes, and strains are referred to by official nomenclature. The Nox3het allele arose spontaneously on the GL/Le strain (JAX #000255) but has since been made congenic (#002557) onto the C57BL/6JEiJ strain (#000924). The Nox3het–2J allele arose spontaneously on the B10.D1-H2q/SgJ strain (#002024) and has been cryopreserved as an F1 hybrid (#003483) with the C57BL/6J strain (#000664). The Nox3het–3J allele arose during ethyl methane sulfonate- (EMS-) induced mutagenesis of CJ7 ES cells (strain 129S1/SvImJ, #002448) and has since been made congenic onto the C57BL/6J strain (#000664). The Nox3het–4J allele (#005014) arose during N-ethyl-N-nitrosourea- (ENU-) induced mutagenesis of C57BL/6J mice (#000664). The Nox3het–5J allele arose spontaneously on the CBySmn.CB17-Prkdcscid/J strain (#001803) but has since been crossed to the BALB/cByJ strain (#001026). Two remaining mutant alleles, the Nox3R542 and Nox3R96 alleles, arose during ENU-induced mutagenesis of the C3HeB/FeJ strain (#000658) but were not used in this study. Genomic coordinates are those from Build 37 of the mouse genome sequence. The numbering of the 13 exons of Nox3 is according to the UCSC gene prediction in the mouse reference genome sequence (NCBI37/mm9) and differs from that shown in Fig. 4 of Paffenholz et al. (2004) by the addition of a single exon between exons 4 and 5, and the renumbering of all following exons.
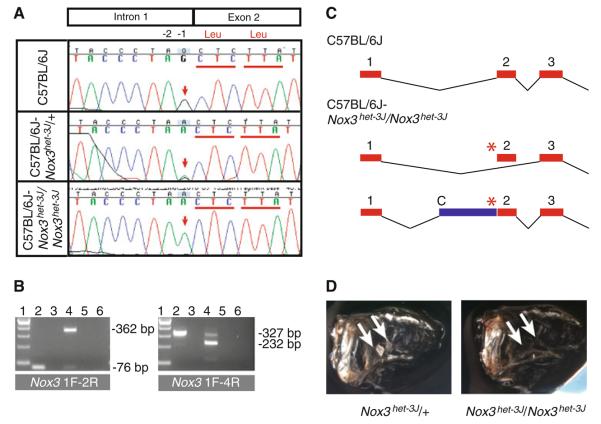
Fig. 4.
Analysis of the Nox3het–3J allele. a Sanger sequencing-based confirmation of the Nox3het–3J mutation identified by HTP sequencing. The mutation consists of a G > A transition at the −1 position of the splice acceptor site just prior to exon 2. b RT-PCR analysis of C57BL/6J and Nox3het–3J/Nox3het–3J inner-ear RNA with 1F/2R and 1F/4R primer pairs. Lane 1, 100-bp ladder; lane 2, wild-type RNA; lane 3, negative reverse transcriptase (−RT) control; lane 4, Nox3het–3J/Nox3het–3J RNA; lane 5, −RT control; lane 6, no template control. c Models of wild-type (C57BL/6J) and mutant (C57BL/6J-Nox3het–3J/Nox3het–3J) Nox3 transcripts. Red numbered rectangles, exons; asterisk, mutation; C, cryptic splice acceptor; blue rectangle, intronic sequence incorporated into the mutant transcript. d Whole-mount preparation of cleared temporal bones from adult Nox3het–3J/+ and Nox3het–3J/Nox3het–3J mice. Note the presence of otoconia (arrows) in the heterozygotes and their absence in mutant homozygotes
Oligonucleotides
Oligonucleotides (Integrated DNA Technologies, Coralville, IA) used for genotyping, amplification of Nox3 sequences from genomic DNA, RACE, or RT-PCR analysis of Nox3 cDNAs are given in Table 1. Oligonucleotides were designed using the Primer 3.0 algorithm alone (Rozen and Skaletsky 2000) or in conjunction with the ExonPrimer function (Institute of Human Genetics, Helmholtz Center Munich, Munich, Germany) of the UCSC Genome Browser (Kent et al. 2002).
Table 1.
Oligonucleotides
| Nox3het | |
| Genotyping | |
| Nox3-int12F | GTTCTGGAGCACCACCTTGT (use at 200 nM) |
| Nox3-int12R | CCCATAGGGAGCCAAGAAAT (use at 300 nM) |
| ERV-R | TGTCAAGCTGACTCCACCAG (use at 100 nM) |
| RACE | |
| 3′RACE outer primer | GCGAGCACAGAATTAATACGACT |
| 3′RACE inner primer | CGCGGATCCGAATTAATACGACTCACTATAGG |
| 3′NoxInH | CGCAAGCTTTATTTCACTACCCCGTGAGC |
| 3′NoxOut | CATGCCGAGGCTAGCAGT |
| 3′RaceInX (3′ RACE adapter) | CGCCTCGAGGAATTAAATACGACTCACTATAGGT12VN |
| Nox3het–2J | |
| Genotyping | |
| Nox3-10F | GGCAGAGGAGAGATTTGAGG |
| Nox3-10R | TGGGATGTCTTTGTGTCCAA |
| Nox3het–3J | |
| Genotyping | |
| Nox3-673F | TGACAACTTCTGCATTTCATCTG |
| Nox3-939R | GGAGGATGATGAAAACATTGAAG |
| RT-PCR | |
| Nox3ex-1F | TGTCATGCCGGTGTGCTGGA |
| Nox3ex-2R | AGGCGTTTACTGCCAGCCAT |
| Nox3ex-4R | CCCGTAGGCAACGAGTTTGTGGA |
| Nox3het–4J | |
| Genotyping | |
| Nox3-10F | GGCAGAGGAGAGATTTGAGG |
| Nox3-10R | TGGGATGTCTTTGTGTCCAA |
| RT-PCR | |
| Ap3d1ex-1F | TCCGCAACCACAAGGAGGACGA |
| Ap3d1ex-8R | TCGATCAGCTTCTTGCCCAGCC |
| Nox3ex-1F | TGTCATGCCGGTGTGCTGGA |
| Nox3ex-4R | CCCGTAGGCAACGAGTTTGTGGA |
| Nox3ex-9F | TCAGGAGACTGGACAGAGGCGT |
| Nox3ex-10F | ACCCCGTGAGCGTGTGCATT |
| Nox3ex-10R | AATGCACACGCTCACGGGGT |
| Nox3ex-11F | CTCACTGGCTGGGATGAAAACCA |
| Nox3ex-11R | CGGCATCCCGGCAGATCCAATA |
| Nox3ex-12R | TTCGTCGTTCCAGTTGGGTCGC |
| Nox3het–5J | |
| Amplification of duplication breakpoint | |
| Nox3-46393F | CCGAGGATCTCCATTACTGC |
| Nox3-44516R | GCAGATACCAAATTGCACTCTG |
| het-5JR | AAGACGTCTCCCATGTTCCA |
| Nox3R96 | |
| Genotyping | |
| Nox3R96-F | GCATTTGGCAGAAACCTCTT |
| Nox3R96-R | CACTGGGAGAGATGCACAAA |
| Nox3R542 | |
| Genotyping | |
| Nox3R542-F | AATAAAGCATTTCAAGCCGTG |
| Nox3R542-R | TTGTCTGGACTTCAGGGAGG |
The oligonucleotides shown here are those used for the genotyping, RACE, RT-PCR, and duplication breakpoint analyses of Nox3het, Nox3het–3J, Nox3het–4J, and Nox3het–5J alleles described in the text. Although the molecular lesions of the Nox3het–2J, Nox3R96, and Nox3R542 alleles have been described elsewhere, genotyping primers are provided here for the detection of those alleles. A HindIII site (in the 3′NoxInH oligonucleotide) and an XhoI site (in the 3′RaceInX oligonucleotide) that were used for cloning the 3′ RACE product from the Nox3het allele are underlined. Primer names and numbering are arbitrary
PCR
Unless otherwise stated, all PCR reactions were performed using the following cycling conditions: preheating at 95°C for 3 min; 40 cycles of amplification at 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s; and a final extension of 72°C for 9 min 30 s.
RT-PCR
Total RNA was isolated from individual adult wild-type, heterozygous, and homozygous mutant temporal bone explants (containing the inner ear) using an RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer's directions. First-strand synthesis reactions (generally 8 μl RNA, 200–300 ng) were run in accordance with the SuperScript III (Invitrogen, Carlsbad, CA) random hexamer or similar protocols. PCR reactions (30 μl) were performed on cDNA and on no-reverse transcriptase controls to amplify segments of the Nox3 transcript. Each reaction used 2 μl cDNA, 5 Prime Master Mix (5 Prime, Gaithersburg, MD; final concentration 1X), and the appropriate primers (200 nM) or similar reaction conditions. Amplification was performed on a MJ Research (Waltham, MA) PTC-200 Thermocycler (Bio-Rad, Hercules, CA) and involved a 2:30-min denaturing step at 95°C followed by 40 cycles at 95°C for 30 s, 55°C for 30 s, and a 72°C extension step for 90 s. Products were resolved on 1–2% SeaKem LE agarose gels (Lonza, Basel, Switzerland).
RACE
Total RNA was isolated from individual adult wild-type, heterozygous, and homozygous mutant temporal bone explants (containing the inner ear) using an RNeasy Mini Kit (Qiagen) according to the manufacturer's directions. RACE reactions were performed using a First Choice RLM-RACE Kit (Ambion, Austin, TX) according to the manufacturer's directions. Products were resolved on 1–2% SeaKem LE agarose gels (Lonza).
Sequencing
Sanger sequencing
Amplification products were purified using a Qiagen QIAquick or a MinElute PCR Purification Kit according to manufacturer's directions. Cycle sequencing reactions were performed on PCR products (5–20-ng product, 20 pmol sequencing primer) using the ABI BigDye Terminator Chemistry v3.1 (Applied Biosystems, Carlsbad, CA). Finished reactions were purified using the Agencourt CleanSeq system (Agencourt, Bioscience Corp., Beverly, MA) and resolved on an ABI 3730xl DNA sequencer. Sequence reads were analyzed and aligned using Sequencher software (Gene Codes Corp., Ann Arbor, MI).
Next-generation sequencing
Sequencing libraries (v4, Illumina, Inc., San Diego, CA) were prepared from B6Ei.GL-Nox3het/Nox3het and B6.129S1-Nox3het–3J/Nox3het–3J DNA, bar-coded, and hybridized to a sequence capture array (Agilent, Inc., Santa Clara, CA) containing 358,713 probes (3-bp offset) arrayed in duplicate and designed from unique Chromosome 17 sequences in and around Nox3 (Chr 17: nt 3367120–5220663). Eluted DNA was sequenced by the 75-nucleotide paired-end method using an Illumina GAIIx Genome Analyzer. Sequence reads were downloaded and then processed and mapped to the C57BL/6J (Build 37) reference sequence using the Burrows–Wheeler Aligner (Li and Durbin 2010). Sequences were then viewed graphically using the Integrative Genomics Viewer (IGV, Broad Institute, Cambridge, MA).
Observation of otoconia in cleared temporal bones
Temporal bones were dissected from euthanized adult mice. Specimens were fixed by flushing neutral buffered formalin through a hole in the cochlear apex prior to immersion in neutral buffered formalin for 1–12 h. Specimens were then briefly washed and dehydrated through hourly or overnight washes in an ascending ethanol series (70–100%). Finally, specimens were cleared overnight in methyl salicylate and observed/photographed under polarized light on a dissecting microscope.
Results
The Nox3het allele
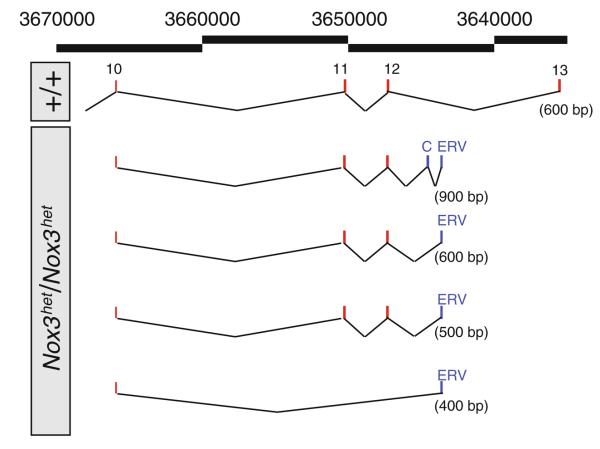
The Nox3het allele arose on the GL/Le strain of mice in 1976 (Sweet 1980) and has since been made congenic onto the C57BL/6JEiJ strain. We conducted RT-PCR and RACE analyses of total RNA purified from adult temporal bone/inner-ear explants from B6Ei.GL-Nox3het/Nox3het mice. Amplification products from the 5′ and central regions of the mutant cDNA were of sizes consistent with those expected from the predicted intron/exon structure of the Nox3 gene. In contrast, however, 3′ RACE amplification products from exon 10 to the 3′ end of the mutant cDNA were not only the expected size of 600 bp, but also the unexpected sizes of approximately 400, 500, and 900 bp (Fig. 1). Purification and sequencing of the mutant amplification products revealed aberrant splicing causing three phenomena—the skipping of exons 11 and 12, the normal inclusion of exons 11 and 12, and the normal inclusion of exons 11 and 12 along with a cryptic exon downstream of exon 12—but in all cases invariably splicing into a novel (not Nox3-derived) terminal sequence prior to exon 13. The results of a BLAST search of the short terminal sequence most closely matched database entries representing the family of mouse endogenous retroviral elements (ERVs); however, due to the small size of the insertion captured within the mutant cDNA, a definitive identification was not possible. From these results we hypothesized that a retroviral element had inserted into intron 12 and that a portion of the retroviral sequence was being inappropriately spliced into Nox3 mRNAs. To test this hypothesis we set up a series of amplification reactions across intron 12 of the Nox3 gene using both C57BL/6JEiJ and B6Ei.GL-Nox3het/Nox3het genomic DNAs as the amplification template. All segments of intron 12 amplified as expected from control DNA. In contrast, for mutant DNA, two overlapping regions failed to amplify, suggesting an insertion in mutant DNA within the 61-bp region of overlap. Long PCR reactions designed to span the putative insertion failed, suggesting an insertion of several kilobases or more, or otherwise resistant to amplification. To further substantiate these findings and obtain sequence from within the putative insertion, we took a sequence-by-synthesis approach. Sequencing libraries were prepared from B6Ei.GL-Nox3het/Nox3het DNA and hybridized to a sequence capture array designed from 1,185,475 bp of unique Chromosome 17 sequences (representing 64.0% of the overall 1,853,543-bp region) in and around Nox3. Eluted DNA was sequenced using an Illumina GAIIx Genome Analyzer. Sequence reads were downloaded and then processed and mapped to the C57BL/6J (mm9) reference sequence using the Burrows–Wheeler Aligner (Li and Durbin 2010). Analysis showed that 99.73% of the probed sequences were covered by the Nox3het sequence data. Sequences were then viewed graphically using the Integrative Genome Viewer.
Fig. 1.
3′ RACE analysis of Nox3het transcripts. 3′ RACE analysis of Nox3het/Nox3het inner-ear total RNA reveals the aberrant splicing products shown here that terminate in a sequence showing similarity to the sequences of mouse endogenous retroviruses. Alternating black lines with numbers, genome coordinates on Chromosome 17 (build 37); top schematic, 3′ RACE products from wild-type RNA; remaining schematics, 3′ RACE products from mutant RNA that splice into an exon derived from the endogenous retroviral sequence (ERV), may include a cryptic exon (C) or may skip exons 11 and 12. The size difference observed between the second and third mutants' 3′ RACE products is due to a difference in the size of included ERV sequence
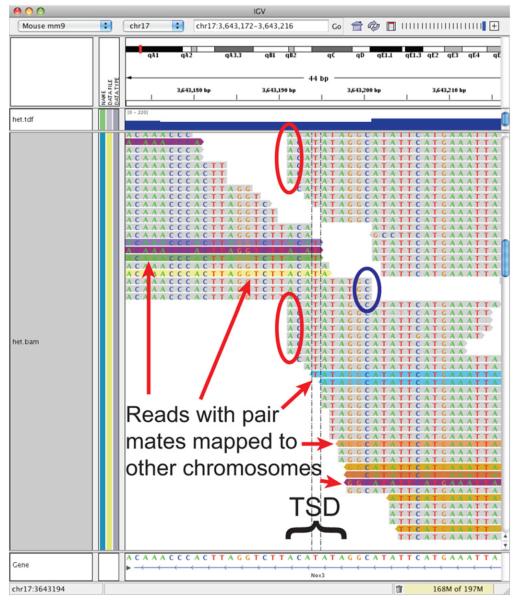
Within the immediate region of the suspected insertion, several interesting features were apparent on the IGV display (Fig. 2). First, several reads within the area of the insertion were denoted as having paired ends that mapped to a chromosome other than Chromosome 17. Next, while most reads outside of the area of the suspected insertion were generally of uniform length and offset from each other by one or a few nucleotides on each end, those in the immediate area of the suspected insertion appeared truncated by varying lengths such that many of the reads aligned at their truncated ends. Reads leading from the area of the suspected insertion toward the proximal end of the chromosome and those leading toward the distal end overlapped by a 7-nt sequence (ACATATA). We reasoned that sequence reads overlapping the insertion site at each end were truncated by the sequence-processing algorithm to eliminate portions of the read derived from insertion sequences not present in the reference genome. With regard to distally pointing reads, this process seemed imperfect, leaving a few nucleotides of poorly aligning sequence still present on the otherwise truncated read. To further analyze the region, we obtained all sequence reads that mapped completely or at least on one end to this area of Chromosome 17. The sequences were then used to construct local alignments de novo. When aligned, two interesting contigs emerged (shown in part in Fig. 3a). One contig consisted of sequences immediately proximal to the area of the suspected insertion; next, contained a 7-nt TATATGT sequence, i.e., the reverse complement of the ACATATA sequence of read overlap observed in the IGV display; and, finally, ended with a sequence that when analyzed by the CENSOR function of the GIRI REPBASE Update (Jurka et al. 2005) showed strong similarity to the mouse endogenous retrovirus class 13B (similarity score = 0.8161, BLAST alignment score = 502). Likewise, the second contig consisted of sequences immediately distal to the area of the suspected insertion; again, contained the 7-nt TATATGT sequence of read overlap observed in the IGV display; and, finally, ended with a sequence that showed strong similarity to the mouse endogenous retrovirus class 13A2 (similarity score = 0.8651, BLAST alignment score = 814). The results of the RACE, PCR, and HTP sequencing analyses are entirely consistent with the insertion of an ERV element immediately after nucleotide 3643197 of Chromosome 17 and causing a 7-nt target site duplication of nucleotides 3643191–3643197. It is this sequence that is present within both of the local alignment contigs and displayed as a region of overlap in the IGV display. Furthermore, mapping the insertion point among the intron 12 amplicons easily explains the basis of the two failed assays, as the integration site of the ERV interrupts the 3′ more (chromosome-proximal) assay and disrupts one of the primer binding sites of the 5′ more (chromosome-distal) assay.
Fig. 2.
Integrative genome viewer (IGV) display of the endogenous retroviral insertion site in the Nox3het allele. The IGV display of the endogenous retroviral insertion site reveals signatures indicative of the insertion, including truncation of reads directed distally (red ovals), truncation of reads directed proximally (blue oval), reads with pair mates mapped to other chromosomes (variously colored reads), and overlap of truncated reads in the area of an ACATATA target site duplication (TSD)
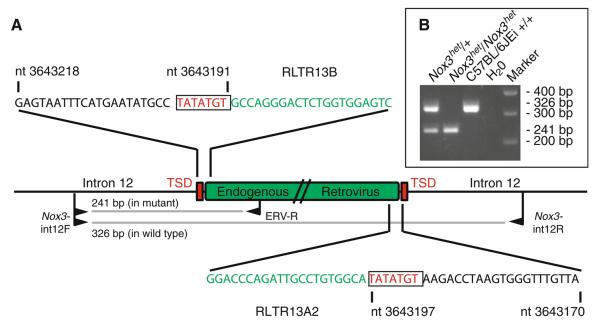
Fig. 3.
Detailed description of the endogenous retroviral insertion site in the Nox3het allele. a Schematic describing the endogenous retroviral (ERV) insertion in the Nox3het allele. Central horizontal line, intron 12 of the Nox3 gene; green rectangle, ERV; red rectangles, target site duplications (TSD); black sequences, partial sequence from intron 12; red sequences, target site duplication; green sequences, partial ERV sequences showing similarity to ERV classes RLTR13B and RLTR13A2; nucleotide (nt) numbers, Chromosome 17 genome positions (build 37); black flags, PCR primers; gray lines, amplification products from the wild-type and mutant alleles. b (inset) The Nox3het genotyping assay detects a 326-bp fragment in wild-type mice, a 241-bp fragment in Nox3het mutant mice, and both fragments in heterozygous mice
Using this new knowledge of the Nox3het causative mutation, we designed a direct PCR assay for the Nox3het allele by selecting a pair of PCR primers that flank and a third primer within the ERV insertion (Fig. 3b). This assay clearly distinguishes among Nox3het/Nox3het, Nox3het/+, and +/+ genotypes.
The Nox3het–3J allele
The Nox3het–3J allele arose as part of an ethyl methane sulfonate (EMS) mutagenesis project of CJ7 embryonic stem cells (Munroe et al. 2000) and has been shown to be allelic to Nox3 by complementation test (Paffenholz et al. 2004). Although capable of producing a variety of mutation types, EMS most often results in guanine-to-adenine transitions (Klungland et al. 1995; Munroe et al. 2000). We prepared sequencing libraries from B6.129-Nox3het–3J/Nox3het–3J mutant DNA, captured regions of DNA in and around Nox3 using the same custom capture array described above, and sequenced the DNA using an Illumina GAIIx Genome Analyzer. Analysis showed that 99.73% of the probed sequences were covered by the Nox3het–3J sequence data. Sequence data were uploaded to the Computational Sciences Statistics and Analysis Group of The Jackson Laboratory and compared to C57BL/6J reference sequence, resulting in an annotation file of nearly 6000 lines of annotated differences from reference. Most variants appeared at extremely low allele frequencies and were interpreted as spurious sequencing artifacts. Of the remaining variants, comparison to mouse genomic sequence of inbred strains (Wellcome Trust Sanger Institute, Hinxton, UK) revealed that most of the sequence variants represented single nucleotide polymorphisms (SNPs) within the 129S1/SvImJ-derived congenic region surrounding the het-3J allele. One variant, however, was observed within the highly conserved splice acceptor site at the 3′ end of intron 1 that was not present in 129S1/SvImJ genomic sequence (Chromosome 17, position 3695584). This sequence variant was confirmed by the Sanger dideoxy method (Fig. 4a). RT-PCR analysis clearly demonstrates the effect this mutation has on Nox3 splicing (Fig. 4b). Although splicing of adult temporal bone/inner-ear RNA in wild-type (+/+) C57BL/6J mice is as expected, in B6.129-Nox3het-3J/Nox3het–3J homozygotes a sequenced 362-bp RT-PCR product amplified with exon 1 (forward) and exon 2 (reverse) primers demonstrates the use of a cryptic splice acceptor in intron 1 (Fig. 4b, left panel). When assessed with exon 1 (forward) and exon 4 (reverse) primers, the predominant RT-PCR product is consistent in size with transcripts omitting exon 2 (Fig. 4b, right panel). These models are shown schematically in Fig. 4c. The predicted effects on the NOX3 protein are the introduction of six novel amino acids prior to a premature stop codon, and deletion of amino acids 37–68 with the remainder of the protein remaining in frame. Also of note, the mutation, a guanine-to-adenine transition, is entirely consistent with the mutagenic profile typical of EMS. Combined, the complementation test, HTP sequencing, Sanger sequencing, and RT-PCR analysis clearly demonstrate that a guanine-to-adenine transition on Chromosome 17 at position 3695584 is the causative mutation underlying the vestibular dysfunction observed in Nox3het-3J/Nox3het–3J mice.
The Nox3het–4J allele
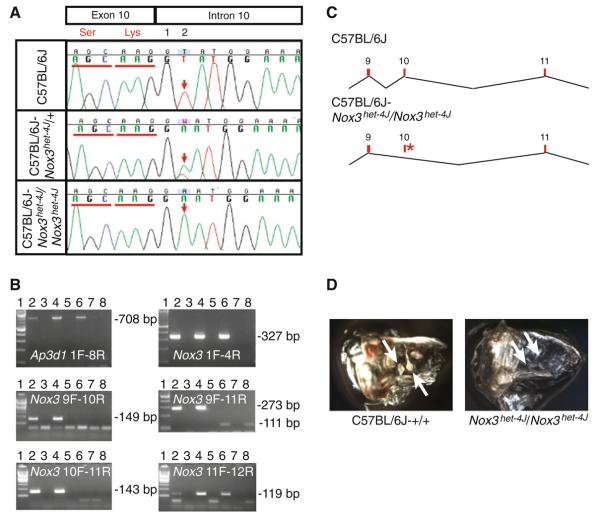
The Nox3het–4J allele (also known as nmf250) arose as part of an N-ethyl-N-nitrosourea (ENU) mutagenesis program of C57BL/6J mice (Goldowitz et al. 2004). Complementation tests between nmf250 mice and Nox3het mice confirm that nmf250 is allelic with Nox3. Thus, the nmf250 allele has been redesignated as het-4J. We looked for the mutation underlying the Nox3het–4J allele by amplification and Sanger-based sequence analysis of genomic DNA in and around Nox3 exons, that was amplified with paired primers positioned within flanking intronic sequence. The results of this analysis uncovered a thymine-to-adenine transversion at position 2 of the highly conserved splice donor sequence immediately following exon 10 (Chromosome 17, position 3665819, Fig. 5a). RT-PCR analysis of total RNA from adult temporal bone/inner-ear explants confirms aberrant splicing of Nox3 transcripts in the mutant strain (consistent with omission of exon 10 and a resulting frameshift) that is not seen in wild-type control mice (Fig. 5b, c). Together, the complementation test, Sanger sequencing, and RT-PCR analysis clearly demonstrate that a thymine-to-adenine transversion on Chromosome 17 at position 3665819 is the causative mutation underlying the vestibular dysfunction observed in Nox3het–4J/Nox3het–4J mice.
Fig. 5.
Analysis of the Nox3het–4J allele. a Sanger sequencing-based identification of the Nox3het–4J mutation. The mutation consists of a T > A transversion at the +2 position of the splice donor site just after exon 10. b RT-PCR analysis of C57BL/6J and Nox3het–4J/Nox3het–4J inner-ear RNA with Ap3d1 1F/8R (positive control) primers as well as the Nox3 1F/4R, 9F/10R, 9F/11R, 10F/11R, and 11F/12R primer pairs. Lane 1, 100-bp ladder; lane 2, wild-type RNA; lane 3, negative reverse transcriptase (−RT) control; lane 4, Nox3het–4J/+ RNA; lane 5, −RT control; lane 6, Nox3het–4J/Nox3het–4J RNA; lane 7, −RT control; lane 8, no template control. c Models of wild-type (C57BL/6J) and mutant (C57BL/6J-Nox3het–4J/Nox3het–4J) Nox3 transcripts. Red numbered rectangles, exons; asterisk, mutation. d Whole-mount preparation of cleared temporal bones from adult C57BL/6J-+/+and Nox3het–4J/Nox3het–4J mice. Note the presence of otoconia (arrows) in the wild-type preparation and their absence in mutant homozygotes
The Nox3het–5J allele
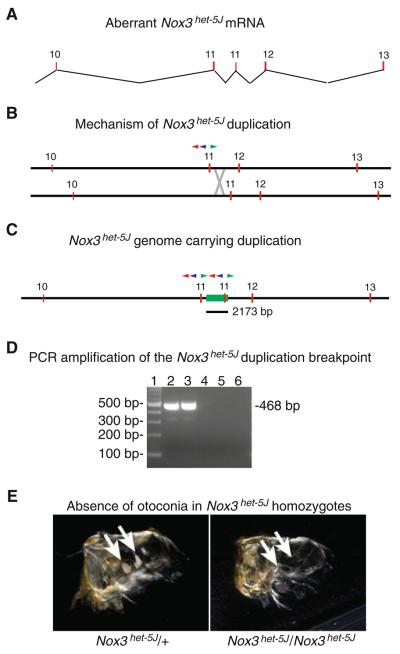
The Nox3het–5J allele (Fig. 6) arose spontaneously on the CBySmn.CB17-Prkdcscid/J strain at The Jackson Laboratory, where it was identified by the Phenodeviant Search Program of the laboratory's Mouse Mutant Resource (Mouse Mutant Resource Web Site 2010). It has since been maintained by crossing to BALB/cByJ mice. Allelism with Nox3 was confirmed by complementation test. To explore the molecular basis of the Nox3het–5J mutation, we conducted an RT-PCR analysis of total RNA purified from adult temporal bone/inner-ear explants from both Nox3het–5J/Nox3het–5J homozygotes and BALB/cByJ control mice. Amplification products from the wild-type cDNA and most regions of the mutant cDNA were of sizes consistent with those expected from the predicted intron/ exon structure of the Nox3 gene. In contrast, however, one RT-PCR amplification product from the 3′ region of mutant cDNA was of an unexpected size, approximately 150 bp larger than the wild-type product. Purification and sequencing of the mutant amplification product revealed a tandem duplication of exon 11 (Fig. 6a), suggesting a similar duplication of genomic DNA in and around the region of the duplicated exon (Fig. 6b, c). To test this hypothesis, we designed a series of upstream-pointing PCR primers within intron 10 (e.g., red and blue primers in Fig. 6b) and a series of downstream-pointing primers in intron 11 (e.g., green primer in Fig. 6b), reasoning that in the event of a duplication encompassing exon 11, some of these otherwise divergently pointing primers would now be pointed toward each other (Fig. 6c) and of small enough size for amplification. Amplification reactions with mutant template DNA consisting of the forward primer in conjunction with each of the two reverse primers did result in strong amplification from each reaction that was not observed in the corresponding BALB/cByJ (+/+) control reaction (e.g., Fig. 6d). Purification and sequencing of the mutant amplification products allowed us to determine the extent of the tandem duplication on Chromosome 17 from position 3649673 to 3651846. Together, these results demonstrate that a 2173-bp tandem duplication on Chromosome 17 is the molecular lesion underlying the Nox3het–5J mutant allele. It should also be noted that the duplication of exon 11 (147 bp) in Nox3 RNA is not predicted to result in a frameshift but, instead, to encode the duplication of 49 in-frame amino acids (amino acids 457–505) prior the translation of exons 12 and 13 and translational termination at the native stop codon.
Fig. 6.
Analysis of the Nox3het–5J allele. a RT-PCR analysis and Sanger sequencing identified a duplication of exon 11 in Nox3het–5J cDNA. Numbered red rectangles, exons. b Proposed mechanism for the Nox3het–5J duplication by uneven crossing over. Numbered red rectangles, exons; red triangle, Nox3-44516R primer; blue triangle, het-5JR primer; green triangle, Nox3-46393F primer; gray X, crossover event. c Schematic of the Nox3het–5J allele. Red numbered rectangles, exons; green rectangle, duplicated region of 2173 bp encompassing exon 11; red, blue, and green triangles are as in b. Note that in the duplication-bearing allele, the three primers located between exons 11 are now pointed toward each and are able to support amplification. d Amplification across the duplication in the Nox3het–5J allele with the Nox3-46393F and het-5JR primers. Lane 1, 100-bp ladder; lanes 2–3, Nox3het–5J/Nox3het–5J DNA; lanes 4–5, wild-type DNA; lane 6, no template control. e Whole-mount preparation of cleared temporal bones from adult Nox3het–5J/+ and Nox3het–5J/Nox3het–5J mice. Note the presence of otoconia (arrows) in the heterozygotes and their absence in mutant homozygotes
Discussion
Allelic series are important for identifying structure/function relationships within proteins, for displaying the phenotypic consequences of specific mutations, and for identifying mutant alleles with various characteristics (e.g., hypermorphic, antimorphic). In addition, mutant alleles arising in various inbred strains provide insight into the phenotypic effects of genetic background and can lead to the identification of modifying genes and interacting pathways. In this report we considered the single mutant alleles of Cyba and Noxo1 and extended previous studies to provide a complete molecular characterization of the seven known mutant alleles of the NADPH oxidase Nox3 (Table 2, Fig. 7). In this section we discuss the effects of inactivating mutations, in-frame insertions/deletions, and missense mutations on NOX3 protein structure and function. A fuller understanding of Nox3 mutant alleles may offer clues into the precise function of Nox3 in the mammalian inner ear.
Table 2.
Mutant allelesa of the inner ear's NADPH oxidase components Cyba, Noxol, and Nox3
| Gene | Allele | Strain of origin (current strain) |
Mutation | Documented phenotypes |
Effect to transcript/protein | Reference |
|---|---|---|---|---|---|---|
| Cyba | nmf333 | A.B6->Tyr+/J (same) | ENU-induced T > C transition in exon 5 | B, O, V | Tyr > His; nonconservative substitution; no protein expression; no haploinsufficiency observed | Nakano et al. 2008 |
| Noxol | hslt | SJL/l-Thy1a/J (SJL/J) | Spontaneous A insertion in exon 1 | B, O, V | Frameshift; premature stop; transcript is detectable; haploinsufficiency observed | Bergstrom et al. 2004; Kiss et al. 2006; K.N. Mills and S.M. Jones, personal communication |
| Nox3 | het | GL/Le (B6ELGL congenic) | Spontaneous ERV insertion in intron 12 (see text) | B, O, V | Splicing abnormalities; transcript is detectable | Paffenholz et al. 2004; and this report |
| het-2J | B10.D1-H2q/SgJ (B6 × B10.D1-H2q/SgJ hybrid) | Spontaneous G > A transition in exon 10 | B, O, Vb | Gly > Arg; nonconservative substitution; not hypermorphic or antimorphic | Paffenholz et al. 2004; K.N. Mills and S.M. Jones, personal communication | |
| het-3J | 129S1/SvImJ (B6.129S1 congenic) | EMS-induced G > A transition at splice acceptor in intron 1 (see text) | B, O | Skipping of exon 2 with no frameshift; deletion of first transmembrane domain; use of cryptic splice acceptor in intron 1 with frameshift and premature stop | This report | |
| het-4J | C57BL/6J (same) | ENU-induced T > A transversion at splice donor in intron 10 (see text) | B, O, V | Skipping of exon 10 with frameshift and premature stop | This report and K.N. Mills and S.M. Jones, personal communication | |
| het-5J | CBySmn.CB17-Prkdcscid/J (BALB/cByJ) | Spontaneous tandem duplication encompassing exon 11 (see text) | B, O | Duplication of amino acids 457–505 of the NADPH binding domain | This report | |
| R96 | C3HeB/FeJ (same) | ENU-induced T > A transversion in exon 5 | B, O | Tyr > premature stop | Paffenholz et al. 2004 | |
| R542 | C3HeB/FeJ (same) | ENU-induced A > G transition in exon 12 | B, O | Lys > Glu; nonconservative substitution | Paffenholz et al. 2004 |
The known spontaneously arising and mutagen-induced mutant alleles of Cyba, Noxo1, and Nox3 are shown along with strain information, the nature of the mutation, documented phenotypes, and the known or predicted effect on transcript/protein expression
B behavioral, O absence of otoconia, V absence of VsEPs
In addition to the alleles shown, there are a number of curated gene-trapped and gene-targeted alleles of Nox3, Noxo1, and Cyba represented only as cell lines. At the time of this writing, none of these lines has been used to create mice and therefore no vestibular phenotype information is available for these lines. Furthermore, the characterization of these gene-trapped and genetargeted cell lines is well understood as they have been designed, a priori, to create null alleles of the altered genes
VsEPs are absent in Nox3het/Nox3het–2J compound heterozygotes
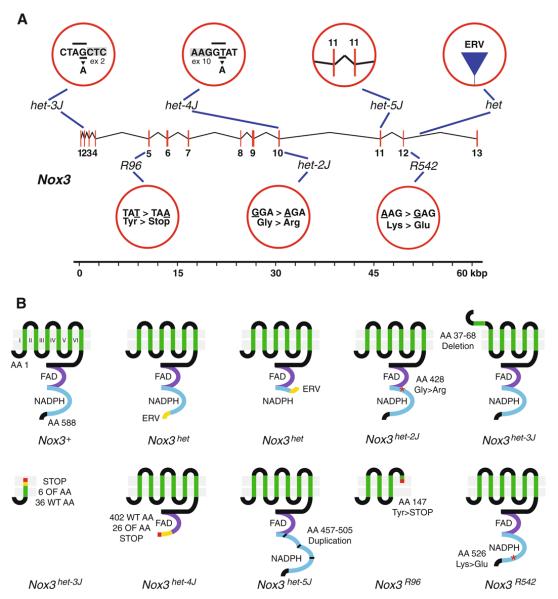
Fig. 7.
A summary of the mutations in Nox3 and their effects on the NOX3 protein. a Shown are the Nox3 transcription unit (numbered vertical red lines, exons) as well as the mutations occurring in the het, het-2J, het-3J, het-4J, het-5J, R96, and R542 alleles of Nox3. For the het allele: ERV endogenous retrovirus. For the het-2J, R96, and R542 alleles: shown are the wild-type (left) and mutant (right) codons and amino acids. The nucleotide that underwent mutation is underlined. For the het-3J and het-4J alleles: shown are seven nucleotides at the intron/exon boundary. Gray labeled rectangle, exon; underlined, wild-type nucleotide with mutant nucleotide shown below; overlined, AG/GT splicing acceptor/donor dinucleotide sequence. For the het-5J allele: shown are the duplicated exons 11. Also shown is a 60-kbp scale. b Shown schematically are the predicted effects of Nox3 mutant alleles on the NOX3 protein. Numbered green vertical lines, transmembrane domains; purple lines, FAD binding domains; blue lines, NADPH binding domains; yellow lines, novel amino acids encoded by the endogenous retrovirus (het) or due to frameshift mutations (het-3J and het-4J); red asterisks, missense mutations; shortened first transmembrane domain in het-3J, in-frame deletion; red lines, premature stop codons; segmented blue line in het-5J, in-frame duplication of the second region of the NADPH binding domain; AA amino acid, ERV endogenous retrovirus, FAD, FAD binding domain; NADPH, NADPH binding domain; OF out-of-frame, WT wild type. This figure is a schematic representation of the effects of Nox3 mutant alleles on translation and is not meant to imply that mutant proteins are present or properly targeted to the cell membrane
Inactivating mutations
Our interest in NADPH oxidase components is related to their primary role in otoconial deposition and, subsequently, the requirement for otoconia in the transduction of the mechanical shearing forces of linear acceleration into action potentials transmitted along the vestibulocochlear nerve. Thus, the presence/absence of otoconia and VsEPs can provide anatomical and physiological correlates of protein function. With regard to VsEPs, Nox3het and Nox3het–4J homozygotes have been assessed, with both showing a complete absence of response (Jones et al. 1999; K.N. Mills and S.M. Jones, personal communication). Moreover, it has been described that these strains as well as Nox3R96 and Noxo1hslt homozygotes exhibit a complete absence of otoconia (Bergstrom et al. 2004; Kiss et al. 2006; Paffenholz et al. 2004). In addition, the frameshift mutation in the Noxo1hslt allele and the aberrant splicing in the Nox3het, Nox3het–3J, and Nox3het–4J alleles are predicted to lead to the introduction of premature stop codons. In sum, it is likely that Nox3het, Nox3het–3J, Nox3het–4J, Nox3R96, and Noxo1hslt are all functional null alleles.
In-frame insertions/deletions
In addition to those alleles and splice forms leading to the premature termination of translation, the Nox3het–5J allele and at least one splice form of the Nox3het–3J allele have the potential to significantly alter NOX3 protein structure without altering the NOX3 reading frame. The aberrant Nox3het–3J transcript that omits exon 2 is predicted to result in an in-frame deletion of amino acids 37–67, including the first transmembrane domain. In vitro studies utilizing HEK (human embryonic kidney)-293 cells and CHO (Chinese hamster ovary)-K1 cells have highlighted key interactions among wild-type NOX3, CYBA, and NOXO1 proteins required for proper maturation and function of the Nox3-based NADPH oxidase complex (Nakano et al. 2007), including interactions apart from the first transmembrane domain that may remain intact in the Nox3het–3J context. Likewise, the aberrant Nox3het–5J transcript that duplicates exon 11 is predicted to result in an in-frame duplication of amino acids 457–505, including a significant portion of the NADPH binding domain. Thus, while a case could be made for residual activity of the Nox3het–3J and Nox3het–5J alleles, no direct biochemical or physiological studies have investigated this possibility. Nonetheless, given the extensive alterations to the NOX3 protein, any physiologically significant activity seems unlikely. Indeed, the only measure of functional activity existing for the Noxhet–3J and Nox3het–5J alleles, the assessment of otoconial density, suggests no functional activity as otoconia are consistently absent in Nox3het–3J and Nox3het–5J homozygotes.
Missense mutations
In addition to the protein-truncating mutations and extensive in-frame protein alterations described above, three mutant alleles of inner-ear NADPH oxidase components encode single-amino-acid missense mutations.
The Nox3het–2J allele encodes a nonconservative glycine-to-arginine (Gly > Arg) substitution of amino acid 428 of the NOX3 protein (Paffenholz et al. 2004; D. Bergstrom, unpublished results). Likewise, the Nox3R542 allele encodes a nonconservative lysine-to-glutamic acid (Lys > Glu) substitution of amino acid 526 of the NOX3 protein. Although no functional studies have been conducted with the Nox3R542 allele, recently electrophysio-logical studies have been conducted comparing the Nox3het–2J allele with the original Nox3het allele (K.N. Mills and S.M. Jones, personal communication). In these studies, Nox3het/+, Nox3het–2J/+, Nox3het/Nox3het–2J, and +/+ genotypes on a fixed genetic background were assessed for VsEP threshold. Nox3het/Nox3het–2J compound heterozygotes displayed no VsEP response, suggesting no residual function encoded by the Nox3het–2J allele. Moreover, Nox3het–2J/+ mice retained the same VsEP threshold as their +/+ littermates, demonstrating that the Nox3het–2J allele has no antimorphic character acting to inhibit the wild-type allele.
With regard to the NOX3-interacting protein CYBA, the Cybanmf333 allele encodes a nonconservative tyrosine-to-histidine substitution of amino acid 121 of the CYBA protein. Cybanmf333/Cybanmf333 mutants display profound vestibular defects and have absent VsEP responses, and CYBA protein is not expressed in these mutants. Within phagocytic cells the absence of CYBA abolishes ROS generation, with the corresponding mice exhibiting a chronic granulomatous disease-like susceptibility to opportunistic infection (Nakano et al. 2008).
Summary
In sum, a number of mutant alleles exist for the NADPH oxidase components Nox3, Noxo1, and Cyba, with the corresponding mutant mouse strains showing profound vestibular dysfunction. The NOX3 protein, in particular, appears to be under strong constraint to conserve primary amino acid sequence as a variety of mutation types and locations tend to lead to a common loss-of-function phenotype. Identifying less severe alleles may require VsEP screening techniques to identify mice with elevated VsEP thresholds that may not show overt behavioral deficits.
Acknowledgments
The authors acknowledge the support of the Mouse Mutant Resource (NIH/NCRR grant RR001183) and the Computational Sciences Statistics and Analysis Group, Allele Typing & Sequencing Service, Fine Mapping Laboratory, DNA Resource, and Cryopreservation Service of The Jackson Laboratory. The authors thank Ms. Leslie Haynes and The Jackson Laboratory Research Animal Facility staff for animal husbandry. The authors especially thank Dr. Yueming Ding for analysis of HTP sequence and Dr. Leah Rae Donahue, Director of the Genetic Resource Science group, for facilities and support. This work was supported by NIH/NIDCD grant DC007431 to DEB and NIH/NCI CORE grant CA34196 to JAX.
References
- Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, et al. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bergstrom DE, Bergstrom RA, Munroe RJ, Schimenti JC, Gagnon LH, et al. The Nox3 and Noxo1 genes, encoding presumptive members of an NADPH oxidase complex, are required for normal vestibular function and development; 18th international mammalian genome conference; Seattle, WA. 17–22 Oct 2004.2004. [Google Scholar]
- Burke B, Stewart CL. The laminopathies: the functional architecture of the nucleus and its contribution to disease. Annu Rev Genomics Hum Genet. 2006;7:369–405. doi: 10.1146/annurev.genom.7.080505.115732. [DOI] [PubMed] [Google Scholar]
- Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–952. doi: 10.1038/nrg1906. [DOI] [PubMed] [Google Scholar]
- Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- Darrat I, Ahmad N, Seidman K, Seidman MD. Auditory research involving antioxidants. Curr Opin Otolaryngol Head Neck Surg. 2007;15:358–363. doi: 10.1097/MOO.0b013e3282efa641. [DOI] [PubMed] [Google Scholar]
- Goldowitz D, Frankel WN, Takahashi JS, Holtz-Vitaterna M, Bult C, et al. Large-scale mutagenesis of the mouse to understand the genetic bases of nervous system structure and function. Brain Res Mol Brain Res. 2004;132:105–115. doi: 10.1016/j.molbrainres.2004.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie OW. Aminoglycoside induced ototoxicity. Toxicology. 2008;249:91–96. doi: 10.1016/j.tox.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Henderson D, McFadden SL, Liu CC, Hight N, Zheng XY. The role of antioxidants in protection from impulse noise. Ann N Y Acad Sci. 1999;884:368–380. doi: 10.1111/j.1749-6632.1999.tb08655.x. [DOI] [PubMed] [Google Scholar]
- Henderson D, Bielefeld EC, Harris KC, Hu BH. The role of oxidative stress in noise-induced hearing loss. Ear Hear. 2006;27:1–19. doi: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- Hosokawa M. A higher oxidative status accelerates senescence and aggravates age-dependent disorders in SAMP strains of mice. Mech Ageing Dev. 2002;123:1553–1561. doi: 10.1016/s0047-6374(02)00091-x. [DOI] [PubMed] [Google Scholar]
- Jones TA, Jones SM. Short latency compound action potentials from mammalian gravity receptor organs. Hear Res. 1999;136:75–85. doi: 10.1016/s0378-5955(99)00110-0. [DOI] [PubMed] [Google Scholar]
- Jones SM, Erway LC, Bergstrom RA, Schimenti JC, Jones TA. Vestibular responses to linear acceleration are absent in otoconia-deficient C57BL/6JEi-het mice. Hear Res. 1999;135:56–60. doi: 10.1016/s0378-5955(99)00090-8. [DOI] [PubMed] [Google Scholar]
- Jones SM, Subramanian G, Avniel W, Guo Y, Burkard RF, et al. Stimulus and recording variables and their effects on mammalian vestibular evoked potentials. J Neurosci Methods. 2002;118:23–31. doi: 10.1016/s0165-0270(02)00125-5. [DOI] [PubMed] [Google Scholar]
- Jones SM, Johnson KR, Yu H, Erway LC, Alagramam KN, et al. A survey of vestibular function in mutant mouse strains. Assoc Res Otolaryngol. 2003a doi: 10.1007/s10162-005-0009-4. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Johnson KR, Yu H, Zheng QY, Erway LC, et al. A comparison of vestibular and auditory function in inbred mouse strains. Assoc Res Otolaryngol. 2003b doi: 10.1016/j.brainres.2006.01.066. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Erway LC, Johnson KR, Yu H, Jones TA. Gravity receptor function in mice with graded otoconial deficiencies. Hear Res. 2004;191:34–40. doi: 10.1016/j.heares.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Jones SM, Johnson KR, Yu H, Erway LC, Alagramam KN, et al. A quantitative survey of gravity receptor function in mutant mouse strains. J Assoc Res Otolaryngol. 2005;6:297–310. doi: 10.1007/s10162-005-0009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SM, Jones TA, Johnson KR, Yu H, Erway LC, et al. A comparison of vestibular and auditory phenotypes in inbred mouse strains. Brain Res. 2006;1091:40–46. doi: 10.1016/j.brainres.2006.01.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurka J, Kapitonov VV, Pavlicek A, Klonowski P, Kohany O, et al. Repbase update, a database of eukaryotic repetitive elements. Cytogenet Genome Res. 2005;110:462–467. doi: 10.1159/000084979. [DOI] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, et al. The human genome browser at UCSC. Genome Res. 2002;12:996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss PJ, Knisz J, Zhang Y, Baltrusaitis J, Sigmund CD, et al. Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Curr Biol. 2006;16:208–213. doi: 10.1016/j.cub.2005.12.025. [DOI] [PubMed] [Google Scholar]
- Klungland A, Laake K, Hoff E, Seeberg E. Spectrum of mutations induced by methyl and ethyl methanesulfonate at the hprt locus of normal and tag expressing Chinese hamster fibroblasts. Carcinogenesis. 1995;16:1281–1285. doi: 10.1093/carcin/16.6.1281. [DOI] [PubMed] [Google Scholar]
- Kopke R, Allen KA, Henderson D, Hoffer M, Frenz D, et al. A radical demise. Toxins and trauma share common pathways in hair cell death. Ann N Y Acad Sci. 1999;884:171–191. doi: 10.1111/j.1749-6632.1999.tb08641.x. [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics. 2010;26:589–595. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouse Mutant Resource Web Site . Mouse mutant resource web site. The Jackson Laboratory; Bar Harbor, ME: 2010. [Google Scholar]
- Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, et al. Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal. 2010;13:589–598. doi: 10.1089/ars.2010.3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munroe RJ, Bergstrom RA, Zheng QY, Libby B, Smith R, et al. Mouse mutants from chemically mutagenized embryonic stem cells. Nat Genet. 2000;24:318–321. doi: 10.1038/73563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, et al. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem J. 2007;403:97–108. doi: 10.1042/BJ20060819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Longo-Guess CM, Bergstrom DE, Nauseef WM, Jones SM, et al. Mutation of the Cyba gene encoding p22phox causes vestibular and immune defects in mice. J Clin Invest. 2008;118:1176–1185. doi: 10.1172/JCI33835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev. 2004;18:486–491. doi: 10.1101/gad.1172504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Whitworth CA, Mukherjea D, Ramkumar V. Mechanisms of cisplatin-induced ototoxicity and prevention. Hear Res. 2007;226:157–167. doi: 10.1016/j.heares.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Scharner J, Gnocchi VF, Ellis JA, Zammit PS. Genotype–phenotype correlations in laminopathies: how does fate translate? Biochem Soc Trans. 2010;38:257–262. doi: 10.1042/BST0380257. [DOI] [PubMed] [Google Scholar]
- Stewart CL, Kozlov S, Fong LG, Young SG. Mouse models of the laminopathies. Exp Cell Res. 2007;313:2144–2156. doi: 10.1016/j.yexcr.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet H. Head tilt. Mouse News Lett. 1980;63:19. [Google Scholar]
- van den Berg JH, Beijnen JH, Balm AJ, Schellens JH. Future opportunities in preventing cisplatin induced ototoxicity. Cancer Treat Rev. 2006;32:390–397. doi: 10.1016/j.ctrv.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Worman HJ, Fong LG, Muchir A, Young SG. Laminopathies and the long strange trip from basic cell biology to therapy. J Clin Invest. 2009;119:1825–1836. doi: 10.1172/JCI37679. [DOI] [PMC free article] [PubMed] [Google Scholar]