Abstract
Learned anticipatory and compensatory responses allow the animal and human to maintain metabolic homeostasis during periods of nutritional challenges, either acutely within each meal or chronically during periods of overnutrition. This paper discusses the role of neurally-mediated anticipatory responses in humans and their role in glucoregulation, focusing on cephalic phase insulin and pancreatic polypeptide release as well as compensatory insulin release during the etiology of insulin resistance. The necessary stimuli required to elicit CPIR and vagal activation are discussed and the role of CPIR and vagal efferent activation in intra-meal metabolic homeostasis and during chronic nutritional challenges are reviewed.
Keywords: Vagus nerve, cephalic phase insulin, pancreatic polypeptide, insulin resistance
1. Introduction
In the paper entitled “The Eating Paradox: How We Tolerate Food”(1), the author Stephen C. Woods proposes the analogy between food ingestion and drug consumption and argues that physiological and behavioral tolerance to the daily consumption of nutrients must develop to limit metabolic disruptions and maintain homeostasis. Learned anticipatory responses are hypothesized to “minimize the impact of meals” and cephalic phase insulin release (CPIR) is used as an example of a conditioned physiological response which adapts to changing levels of food intake and contributes to glucoregulation. In the present article, I will review what is known about anticipatory responses and discuss the hypothesis of their contribution to metabolic homeostasis, focusing primarily on hormonal responses in humans. Furthermore, I will extend the hypothesis by postulating how impairments in vagally-mediated adaptive responses may be involved in the etiology of insulin resistance.
1.1 Anticipatory Responses: Historical Overview
Anticipatory responses were first identified by a series of elegant experiments conducted by Ivan Pavlov at the turn of the century (2). Pavlov demonstrated that the secretion of a variety of physiological responses including saliva, gastric acid and pancreatic enzymes could all be entrained by repeated pairing of external signals such as a bell or the sight of a meal, to meal ingestion. He further demonstrated that the mere taste of food in the oral cavity, independent of nutrient digestion and absorption, could elicit these same responses. To ensure that no nutrients were being absorbed, the dogs were implanted with esophageal and gastric fistulae preventing the nutrients in the oral cavity from reaching the stomach and intestine. In another set of experiments, gastric and pancreatic enzyme secretion were inhibited by severing the vagus nerve, thereby demonstrating mediation by the parasympathetic branch of the autonomic nervous system (2). The involvement of the brain in the mediation of these responses resulted in the coinage of the term “cephalic phase responses” referring to coming from the head. Pavlov’s studies provided the key criteria of the current definition of an anticipatory or cephalic phase response; neurally-mediated, anticipatory responses occurring prior to nutrient absorption.
Identification of anticipatory hormonal release only came many decades later with the development of the radioimmunoassay which facilitated measurement of hormones, such as insulin and glucagon. Early experiments examined the role of learning in controlling blood glucose levels. Insulin was administered in a Pavlovian conditioning paradigm to cause conditioned changes in blood glucose levels. In these experiments, humans, dogs or rats were injected with insulin, inducing hypoglycemia and subsequent physiological perturbations (3–8). After repeated administration of insulin, saline was then injected. Blood glucose levels typically dropped and increases in plasma insulin were hypothesized to mediate the decline in blood glucose levels. Some studies utilizing more physiological (i.e. lower) doses of insulin reported increases in plasma glucose. Thus, the directionality of the conditioned responses was controversial (9). Neural mediation of the conditioned hypoglycemia was confirmed by vagotomy as well as administration of the muscarinic antagonist, atropine which inhibits the binding of acetylcholine to receptors on the pancreas (10). The importance of dose and the temporal relationship between the unconditioned stimuli and response and a general review of these studies is provided in the paper by Woods and Kulkosky (8). Over the next couple of decades, research in this area moved away from these non-physiological paradigms and migrated towards studies that addressed the relationship of the conditioned insulin response to food intake and how the conditioned insulin response contributed to glucose homeostasis (8;11–15).
2. Anticipatory or Cephalic Phase Insulin Release (CPIR): Definition and Identification
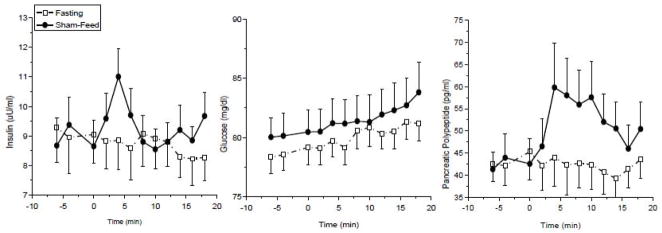
Currently, anticipatory or cephalic phase insulin release (CPIR) is defined as insulin release which occurs prior to nutrient absorption in response to sensory stimulation of the oral cavity by the taste of food or food ingestion. In humans, the response is typically characterized by a rise in plasma insulin levels that occurs independently of increases in blood glucose, peaking within 4 minutes after sensory stimulation and returning to baseline by 8–10 minutes post stimulation (Fig. 1, left and middle graph). As the response occurs rapidly, is of small magnitude (approximately 1% of normal post prandial insulin release) and exhibits a large variability, identification of CPIR requires careful and rapid blood sampling, adequate baseline sampling and an appropriate control condition (16;17;17). Furthermore, for nutrients which are rapidly absorbed such as liquids, distinguishing between neural and nutrient-mediated insulin release can be problematic. To differentiate between neural and nutrient stimulation, animals can be implanted with oral or gastric fistulas to uncouple the effects of oral stimulation from absorption, creating a sham-feeding condition (15;18). In humans, subjects are often requested to perform a modified sham-feed in which food is tasted, chewed and then expectorated (17). Neural mediation of hormonal release has been verified by studies showing inhibition of the response by the muscarinic antagonist atropine (18) as well as vagotomy in animals (13;19–21). Supporting evidence for the neural mediation of CPIR, is provided by the lack of CPIR in rats (22) and humans who have undergone pancreas-kidney transplantation and have no neural innervation of the transplanted pancreas (23).
Figure 1.
Cephalic phase insulin release (left graph), plasma glucose levels (middle graph) and cephalic phase pancreatic polypeptide release (right graph) during a sham-feed (solid circles) compared to fasting (open squares) in normal weight humans (mean±s.e., n=10)
3. Cephalic Phase Insulin and Pancreatic Polypeptide Release: Window into Vagal Activation
At the onset of food ingestion, activated vagal efferent fibers terminating on the pancreas release acetylcholine which binds to muscarinic receptors on the pancreatic islet. Muscarinic receptor activation stimulates the release of insulin as well as other hormones stored in the pancreatic islet (24–26). Glucagon localized in the α-cell and pancreatic polypeptide (PP) found in the delta cells are both released in response to acetylcholine. Insulin and glucagon are under complex regulatory control responding to activation or inhibition by multiple nutritional and neural signals including circulating plasma glucose levels, amino acids and neurotransmitters from both branches of the autonomic nervous system. In contrast, PP is released only in response to vagal activation (i.e. not to increases in glucose levels) and is not inhibited by the sympathetic nervous system (26). As PP release is dependent on vagal efferent activation, the hormone can be used as a marker of vagal activity. This provides an important biological tool for the measurement of vagal activation since acetylcholine released by the vagus nerve cannot be directly measured in plasma due to its rapid degradation in the synaptic cleft, rendering all measurements of vagal efferent activity in humans indirect. Furthermore, as the magnitude of PP release to sham-feeding is much larger than CPIR (Fig. 1, right graph), this allows for the measurement of graded responses to stimuli that is not possible with CPIR.
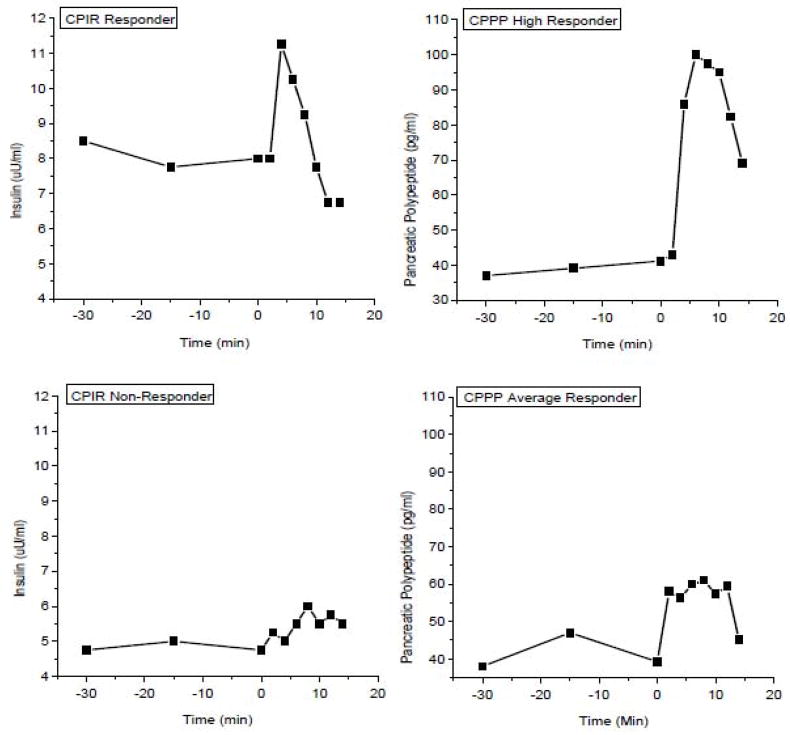
While increases in cephalic phase insulin release would be expected to occur coincident with cephalic phase pancreatic polypeptide (CPPP) since both are dependent on vagal activation, we (27) and others (28) have reported increases in CPPP independent of CPIR. This can occur on an individual level as illustrated in Figure 2 where one individual (upper graphs) exhibits robust insulin and PP responses to a sham-feed while the individual in the lower graphs, does not exhibit a CPIR and the PP response is modest. Alternatively, we have also seen group responses where PP is increased to a sham-feed but CPIR does not occur. The lack of an increase in anticipatory insulin release in the presence of vagal activation may be due to a simultaneous activation of the sympathetic nervous system resulting in adrenergic inhibition of insulin release (1;29). Coincident activation of both branches of the autonomic nervous system are observed during both hyper- and hypo-glycemia (30;31) and in fact, cephalic phase noradrenaline release has been reported in animals (29;32;33). Alternatively, if the mean group response to a stimulus shows an increase in both cephalic phase insulin and PP but on an individual level, some subjects do not exhibit a CPIR in the presence of a PP response, it could be interpreted that this individual did experience vagal activation to the particular stimulus but that a stress response inhibited insulin release. In contrast, an absence of both cephalic phase PP and CPIR provides convincing evidence that vagal efferent activation at the pancreas did not take place at all. Thus, in humans, monitoring both hormones can provide insight into how experimental stimuli and paradigms influence anticipatory responses. Unfortunately, the PP assay for rat and mouse pancreatic polypeptide is not reliable as this would provide another tool for assessment of vagal activation in rodent models.
Figure 2.
Differential individual responses to sham-feeding. Upper left graph illustrates a cephalic phase insulin response (CPIR) responder who exhibits a large cephalic phase pancreatic polypeptide response CPPP: upper right graph). Bottom left graph illustrates a CPIR non-responder who exhibits an average CPPP response.
4. Cephalic Phase Insulin and Pancreatic Polypeptide: Necessary Stimuli for Elicitation
Understanding the stimuli required to elicit CPIR and CPPP provides insight into the environmental and physiological conditions which elicit the release of anticipatory hormones as well as vagal efferent activation, both of which can have profound effects on how nutrients are metabolized. Based on the Pavlovian studies, cephalic phase insulin and PP release could be expected to be a purely learned phenomenon, i.e. occurring coincident with the expectation of food or with a learned paired stimulus and in fact, numerous experiments in rodents have demonstrated conditioned hormonal release. In laboratory animals, one can design experiments to differentiate between conditioned and unconditioned hormonal responses by manipulating the sensory stimuli or the timing of food availability. Novel stimuli which have not previously been experienced by the animal can be presented and hormonal responses as the unconditioned responses measured. Individual taste stimuli including sweet, sour, salty and umami taste have been evaluated as well as palatable and unpalatable foods (15;34–38). Overall, a fairly large body of work suggests that CPIR can be both a conditioned and unconditioned response that occurs most robustly to sweet tasting palatable stimuli.
The data in humans are less clear. For example, while sweet tasting solutions consistently and reliably elicit CPIR in animals, in humans, neither sucrose, aspartame nor saccharine solutions have been shown to be effective stimuli (39) nor have sweetened tablets (40). A recent study in which both CPIR and CPPP were evaluated confirm that sweet nutritive and non-nutritive solutions do not elicit either response, suggesting a lack of vagal efferent activation to clear liquids(41). With regards to the importance of food palatability as a required component for CPIR elicitation in humans, we did not find an effect of palatability on CPIR using two different experimental approaches. In one study, we allowed subjects to select foods that they designated as either highly liked or highly disliked (42) and in the other study, we rendered a food stimulus (cracker and cream cheese) that was initially rated as highly palatable, as unpalatable by the addition of either too much sweetener or salt (41). In the latter experiment, despite the lack of effect of palatability on CPIR, we did find that PP release was attenuated in the unpalatable conditions. Therefore, the magnitude of CPPP does appear to be sensitive to an individual’s perception of the food stimulus. Macronutrient content may also be an important essential stimulus for vagal activation as we found that cake containing both carbohydrate and fat elicited greater CPPP than the same cake composed only of carbohydrate (27). Overall, these studies underscore a number of important concepts with respect to anticipatory hormonal responses in humans: 1) CPIR in humans is a fragile response which may be inhibited by sympathetic responses or require greater vagal activation than CPPP: 2) CPPP provides a more sensitive index of vagal activation than CPIR; 3) in humans, sweet liquids do not elicit vagal efferent activity and 4) fat may be an important sensory component for initiating vagal activation from the oral cavity.
The question with regards to the role of learning or conditioning in eliciting the cephalic phase responses is one which is very difficult to address in humans. Identifying foods or the sensory qualities of food which have not previously been associated or experienced with nutrients is practically impossible. For example, if since birth, sweet taste, the purportedly optimal elicitor of CPIR, has been associated with the metabolic consequences of nutrient ingestion such as would first occur during ingestion of milk as an infant, then the presentation of any sweet tasting food, could elicit conditioned cephalic phase responses that could not be differentiated from unconditioned responses. Thus, a completely novel stimulus must be presented which could then be “learned” to be associated with a metabolic consequence. Using this approach, we developed two uniquely flavored solutions (eucalyptus/lavender and cardamom) that were paired with either a nutritive carbohydrate (polycose) or a carbohydrate which contained no absorbable nutrients (cellulose) (Teff and Breslin, unpublished data). Neither solution was sweet, and pilot testing confirmed that the two solutions had equivalent sensory and hedonic ratings. Healthy, normal weight control subjects (n=10) underwent metabolic testing involving tasting and then ingesting each of the two solutions over a 4 day period during which we measured both cephalic phase (response to taste alone) and post prandial hormonal responses to first time exposure. For the next 6 weeks, subjects then alternated daily ingestion of either the nutritive or non-nutritive solutions, each paired with one distinctive flavor. Hedonic responses were measured weekly. At the end of the 6 weeks, hormonal responses to the two solutions were measured as conducted prior to repeated exposure. We found that upon initial exposure to the novel solutions, neither the nutritive or non-nutritive solution elicited a cephalic phase pancreatic polypeptide or insulin response (Fig 3: insets). Furthermore, the nutritive solution containing polycose did not result in an elevation in post prandial PP levels during the initial ingestion (Fig 3: left graph, solid squares). The initial absence of a post prandial PP response to what is essentially a glucose load (polycose) is controversial as some studies have reported increases in PP following glucose ingestion (43) while others have not (44). Intravenous glucose infusion is known to suppress PP release by a direct effect on pancreatic delta cells (43) so it is possible that the elevated glucose levels in response to glucose ingestion attenuates PP release even in the presence of vagal activation. However, if ingestion of a sweet nutritive liquid does not elicit vagal efferent activation, this would have important implications for how humans store and recognize nutrients derived from beverages. Increased intake of sweetened beverages, particularly sodas have been associated with increased body adiposity and obesity (45) although this is a highly controversial topic (46). Some investigators have suggested that beverages are not “recognized” by the normal regulatory mechanisms involved in detecting caloric intake and thus contribute to over-eating (47;48). A lack of vagal activation may contribute to an inability to detect calories ingested in a clear, liquid form.
Figure 3.
Pancreatic polypeptide (PP) levels prior to (open squares) and following (solid circles) 6 weeks of drinking a flavored polycose solution every other day (left graph, mean±s.e., n=10; treatment effect, P<0.01). Inset: cephalic phase PP in response to the taste of flavored polycose solution prior to ingesting the solution, before and after 6 weeks of exposure. Plasma insulin levels (mean±s.e.) in the same individuals under the same conditions (right graph). Inset: CPIR in response to the taste of flavored polycose prior to ingesting the solution, before and after 6 weeks of exposure. No significant effects of the treatment on cephalic phase or post-prandial insulin were found. (Unpublished data, Teff and Breslin).
After drinking the novel flavored nutritive liquid every other day for 6 weeks, a significant increase in post prandial pancreatic polypeptide was observed (Figure 3: left graph). The increases in post prandial PP levels occurred independent of increases in cephalic phase or post prandial insulin (Fig. 3, right graph) and glucose (data not shown). These data suggested that vagally-mediated conditioning was taking place resulting in an increase in PP release to a stimulus that was previously ineffective. The lack of significant effect on indices of glucose metabolism may have been due to the relatively subtle flavor manipulations.
5. CPIR and Vagal Activation: Role in Intra-Meal Homeostasis
A variety of approaches have been utilized to address the contribution of CPIR to postprandial glucose regulation (49–53). In humans, some studies examine the effect of bypassing stimulation of CPIR or inhibit the response by pharmacological agents, while others enhance the presence of insulin during the preabsorptive period in populations postulated to have impaired or absent CPIR such as Type 2 diabetics (T2DM). For example, if intragastric glucose administration which would typically bypass stimulation of CPIR is coupled with a sham-feed, post-prandial glucose levels are 30% lower compared to intragastric glucose administration without a sham-feed (54). Similar results were reported when a sham-feed was coupled with intravenous glucose infusion(55). Conversely, the infusion of somatostatin during the beginning of meal onset to inhibit early insulin release has been shown to impair glucose tolerance in normal healthy subjects (56) as has the infusion of trimethaphan, a ganglionic antagonist which also inhibits early insulin release (57). In T2DM subjects, infusion of insulin during the first 30 min of meal ingestion lowers post-prandial glucose but if the exact same amount of insulin is delayed by 30 min and then infused, post-prandial glucose levels are significantly higher (58). More recently, nateglinide, an insulinotropic agent with a rapid onset of action, similar to but more prolonged than CPIR has been shown to increase glucose tolerance compared to other agents which enhance later phase insulin release (59).
In light of the relatively small magnitude of CPIR, it is unlikely that the observed effects of CPIR on post-prandial glucose levels are due to muscle tissue uptake of glucose. Instead, the rapid increase in insulin immediately prior to meal ingestion is most likely exerting effects on hepatic glucose and fat metabolism. It is worth noting that due to hepatic degradation, the peripheral levels of circulating insulin are 50% less than the amount released by the pancreas. Thus, the concentration of insulin in the portal vein draining into the liver is significantly higher than the insulin measured peripherally. The increase in insulin during the preabsorptive period may have multiple effects. First, it can prevent a rapid rise in plasma glucose levels that would require more prolonged and extensive post-prandial insulin release. Second, the increase in insulin has been shown to inhibit gluconeogenesis, thereby reducing hepatic glucose production (60;61). Thirdly, CPIR can inhibit lipolysis by increasing lipoprotein lipase activity in adipose tissue (62) thereby lowering free fatty acid levels (58) Combined, these three mechanisms contribute to the optimization of post-prandial glucose homeostasis. Neural mediation of insulin release during this time period increases the adaptability of this response to changes in the external environment and facilitates optimal metabolic flexibility to maintain homeostasis during ingestion of food (53;63). If vagal activation does not occur in response to a particular food or during meal ingestion, then CPIR will not occur and post-prandial glucose levels will be less well regulated. These human data support the Woods hypothesis by providing evidence for the physiological importance of this anticipatory response.
6. CPIR and Vagal Activity: Role in Glucose Homeostasis During Chronic Metabolic Challenges
One of the key arguments of the Eating Paradox paper centers on the concept that compensatory physiological mechanisms are learned to maintain metabolic homeostasis in the face of nutritional challenges. The increased magnitude of CPIR reported in animal models of obesity is provided as evidence of an adaptive response, facilitating increased disposition of ingested food as the quantity of food increases (51;64). As both the cephalic phase and post-prandial hyperinsulinemia in rodent models of obesity can be attenuated by vagotomy (65;66), it has been assumed that there is an adaptive increase in vagal efferent activity in rodent obesity. Human studies provide some supporting evidence of increased CPIR in obese subjects (67–71). Our laboratory, however, has demonstrated that only obese individuals with elevated fasting plasma insulin levels, an indication of insulin resistance, exhibit CPIR of greater magnitude than lean subjects (72). Furthermore, when the response is expressed as a percentage of baseline levels, the optimal approach when comparing populations of varying baselines (73), then CPIR is actually attenuated in the obese. We concluded from these data that 1) the increased magnitude of CPIR in obese humans is a consequence of insulin resistance and 2) that there is inadequate CPIR to compensate for the impaired glucose homeostasis in obese humans. To address this last hypothesis, we conducted a study in which we infused a tiny amount of insulin during the first 10 min of food ingestion, mimicking CPIR, in lean and obese subjects (74). The total amount of insulin infused was equivalent to the amount of insulin released peripherally during the cephalic phase period. In the lean subjects, the insulin infusion had no effect on post-prandial insulin or glucose levels. In contrast, in the obese subjects, the insulin infusion lowered post-prandial glucose area under the curve by approximately 27%; a surprising effect in an insulin-resistant population. Thus, supplementation of insulin during this critical period was able to optimize glucose homeostasis, providing more evidence for the importance of early insulin release.
While our human data argued against an increased magnitude of CPIR in obesity independent of insulin resistance, comparing obese humans, who have been obese for an extended amount of time, to rodents who have been obese for a relatively short time, may not be valid. Many years ago, the concept of dynamic and static stages of obesity was introduced. During the dynamic stage of obesity, the animal would be considered in a non-homeostatic state as physiological mechanisms are recruited to try and maintain homeostasis as food intake and body adiposity increase. In contrast, when animals reach their steady state or metabolic set point, whether at normal levels of adiposity for lean animals or elevated levels for obese animals, the static stage is achieved and they are in metabolic balance. Most obese humans by their second or third decade of life have been metabolically obese for many years, and could be considered in steady state. Metabolic and physiological responses to dietary challenges would be expected to differ depending on whether the human (or animal) is in a dynamic or steady stage of obesity and this could account for the lack of consistency between animal or human studies.
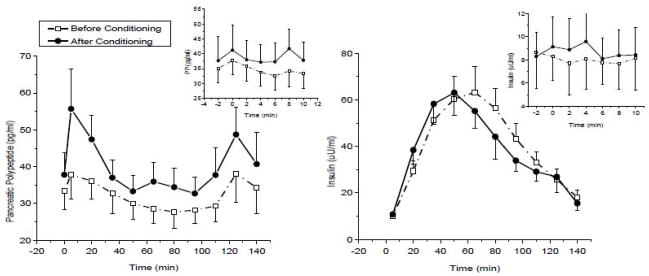
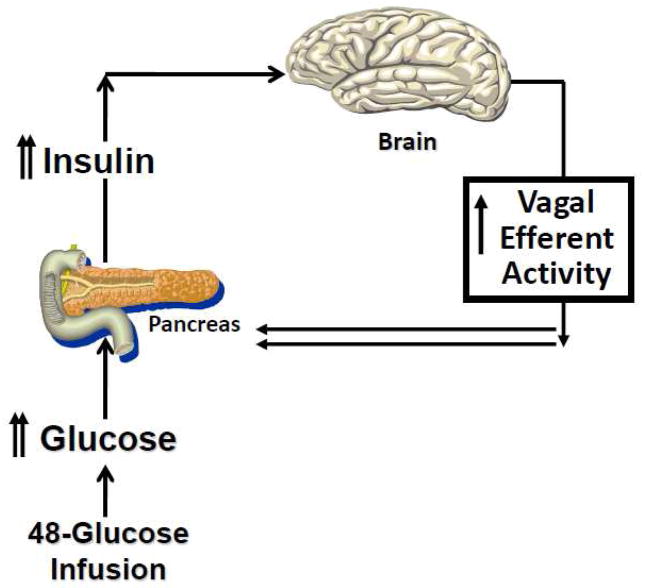
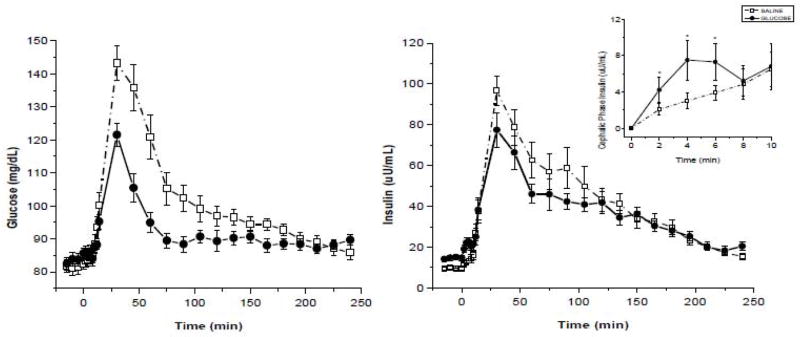
Identifying and evaluating an individual during the dynamic stage of obesity would be very difficult as one may have to conduct the metabolic assessment in childhood or early adolescence. To determine the vagal contribution to insulin release during the dynamic stage of obesity, we created a physiological paradigm that replicates a hypercaloric state such as would occur during the onset of overeating. In a series of experiments (75–77), we infused glucose intravenously (IV) over a 48-h period and monitored hormonal, cardiovascular and behavioral responses during the infusion period, and to acute meal and IV glucose challenges conducted 3-hours after termination of the infusion. Responses were compared to a saline control condition. Subjects were permitted to ingest food normally but received intravenously, the caloric equivalent of their daily metabolic requirements. This manipulation resulted in mildly elevated blood glucose levels (around 110 mg/dl) and compensatory hyperinsulinemia similar to that observed in a glucose intolerant individual. We hypothesized that the increase in plasma glucose and insulin would be recognized by the brain and induce an increase in vagal efferent activity (Fig. 4). which in turn would elicit an increase in insulin release to our challenges (both IV and meal). Using atropine as a pharmacological tool, we were able to demonstrate the neural contribution to the compensatory insulin response during an IV glucose challenge at the end of the infusion which was not observed during the 48-h saline control arm. These data confirmed studies in rodents demonstrating that hyperglycemia elicits vagal mediation of insulin release. We also examined cephalic phase and post-prandial insulin release after the 48-h glucose challenge and found that the magnitude of CPIR was increased compared to the saline control and that this was also associated with a lower post-prandial insulin response (Figure 5). Therefore, in response to a rapid onset of a hypercaloric state, during which the b-cell is chronically challenged, there is an induction of vagal efferent activity which contributes to an increase in neurally-mediated insulin release. The increase in CPIR helps to maintain post-prandial insulin and glucose levels within the limits of normal glucose tolerance.
Figure 4.
Hypothesized effect of a chronic metabolic challenge on vagal efferent activity: During a 48-h glucose infusion in humans, increases in circulating glucose and insulin are recognized by the brain resulting in an induction of vagal efferent activity, thereby contributing to an increase in cephalic phase and compensatory insulin release.
Figure 5.
Plasma glucose (left graph) and insulin levels (right graph) (mean±s.e., n=16) following a 48-h saline infusion (dotted line, open squares) or glucose infusion (solid line, solid circles). Post-prandial glucose (P<0.01) and insulin levels (P<0.01) were significantly lower following the glucose solution compared to the saline solution. Inset: cephalic phase insulin release during the saline and glucose infusions. CPIR was significantly greater after the glucose infusion compared to saline control (P<0.05). See (Ref 77).
7. Conclusions
Overall, the human data support the Woods hypothesis that the magnitude of the cephalic phase insulin response adapts to the metabolic state of the animal and contributes to metabolic homeostasis. Neurally mediated responses such as CPIR and compensatory insulin release allow the animal to adapt to disruptions in metabolic status. The critical mediator of these adaptive responses is the vagus nerve, which connects the central nervous system to peripheral target tissues. I would extend the overall hypothesis by postulating that during prolonged periods of hypernutrition, such as occurs during the etiology of human obesity, the neurally-mediated adaptive responses become limited or inadequate, due to either a down-regulation of centrally-mediated vagal efferent activity or a decreased sensitivity of muscarinic or nicotinic postsynaptic receptors on peripheral target tissues. An additional mechanism may be that a maximal threshold of vagal efferent activity is achieved and further activation is not possible. The decreased or ineffective vagal efferent activity would result in decreased metabolic flexibility or adaptability of the animal or person to nutritional challenges, ultimately contributing to impaired glucose metabolism through decreased CPIR, inadequate compensatory insulin release and lack of suppression of gluconeogenesis. In light of the increasing evidence of the importance of the role of the vagus in glucose and lipid metabolism (78–81) including the regulation of hepatic glucose production, decreases in vagal efferent activity could be a major component contributing to the etiology of insulin resistance in human obesity.
Acknowledgments
Support: NIH DK58003-07 (K.T.), DK-19525, MO1-RR00042 as well as unrestricted funds from the Monell Chemical Senses Center and a small grant from Kellog’s which supported the conditioning study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Woods SC. The eating paradox:how we tolerate food. Psychol Rev. 1991;98:488–505. doi: 10.1037/0033-295x.98.4.488. [DOI] [PubMed] [Google Scholar]
- 2.Pavlov I. The Work of the Digestive Glands. London: Charles Griffin; 1902. [Google Scholar]
- 3.Woods SC, Shogren RE., Jr Glycemic responses following conditioning with different doses of insulin in rats. J Comp Physiol Psychol. 1972;81:220–225. doi: 10.1037/h0033529. [DOI] [PubMed] [Google Scholar]
- 4.Woods SC, Alexander KR, Porte D., Jr Conditioned insulin secretion and hypoglycemia following repeated injections of tolbutamide in rats. Endocrinology. 1972;90:227–231. doi: 10.1210/endo-90-1-227. [DOI] [PubMed] [Google Scholar]
- 5.Woods SC, Hutton RA, Makous W. Conditioned insulin secretion in the albino rat. Proc Soc Exp Biol Med. 1970;133:964–968. doi: 10.3181/00379727-133-34605. [DOI] [PubMed] [Google Scholar]
- 6.Eikelboom R, Stewart J. Conditioning of drug-induced physiological responses. Psychol Rev. 1982;89:507–528. [PubMed] [Google Scholar]
- 7.Ramsay DS, Woods SC. Biological consequences of drug administration: implications for acute and chronic tolerance. Psychol Rev. 1997;104:170–193. doi: 10.1037/0033-295x.104.1.170. [DOI] [PubMed] [Google Scholar]
- 8.Woods SC, Kulkosky PJ. Classically conditioned changes of blood glucose level. Psychosomatic Medicine. 1976;38:201–219. doi: 10.1097/00006842-197605000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Matysiak J, Green L. On the directionality of classically-conditioned glycemic responses. Physiol Behav. 1984;32:5–9. doi: 10.1016/0031-9384(84)90061-1. [DOI] [PubMed] [Google Scholar]
- 10.Woods SC. Conditioned hypoglycemia: effect of vagotomy and pharmacological blockade. Am J Physiol. 1972;223:1424–1427. doi: 10.1152/ajplegacy.1972.223.6.1424. [DOI] [PubMed] [Google Scholar]
- 11.Woods SC, Vasselli JR, Kaestner E, Szakmary GA, Milburn P, Vitiello MV. Conditioned insulin secretion and meal feeding in rats. J Comp Physiol Psychol. 1977;91:128–133. doi: 10.1037/h0077307. [DOI] [PubMed] [Google Scholar]
- 12.Strubbe JH, Bouman PR. Plasma insulin patterns in the unanesthetized rat during intracardial infusion and spontaneous ingestion of graded loads of glucose. Metabolism. 1978;27:341–351. doi: 10.1016/0026-0495(78)90114-2. [DOI] [PubMed] [Google Scholar]
- 13.Strubbe JH. Parasympathetic involvement in rapid meal-associated conditioned insulin secretion in the rat. Am J Physiol. 1992;263:R615–R618. doi: 10.1152/ajpregu.1992.263.3.R615. [DOI] [PubMed] [Google Scholar]
- 14.Strubbe JH, Steffens AB. Rapid insulin release after ingestion of a meal in the unanesthetized rat. Am J Physiol. 1975;229:1019–1022. doi: 10.1152/ajplegacy.1975.229.4.1019. [DOI] [PubMed] [Google Scholar]
- 15.Grill HJ, Berridge KC, Ganster DJ. Oral glucose is the prime elicitor of preabsorptive insulin secretion. Am J Physiol. 1984;246:R88–R95. doi: 10.1152/ajpregu.1984.246.1.R88. [DOI] [PubMed] [Google Scholar]
- 16.Teff KL, Mattes RD, Engelman K. Cephalic phase insulin release in normal weight males: verification and reliability. Am J Physiol. 1991;261:E430–E436. doi: 10.1152/ajpendo.1991.261.4.E430. [DOI] [PubMed] [Google Scholar]
- 17.Teff KL, Levin BL, Engelman K. Oral sensory stimulation in men: effects on insulin, C-peptide, and catecholamines. Am J Physiol. 1993;265:R1223–R1230. doi: 10.1152/ajpregu.1993.265.6.R1223. [DOI] [PubMed] [Google Scholar]
- 18.Berthoud HR, Jeanrenaud B. Shamfeeding-induced cephalic phase insulin release in the rat. Am J Physiol. 1982;242:E280–E285. doi: 10.1152/ajpendo.1982.242.4.E280. [DOI] [PubMed] [Google Scholar]
- 19.Woods SC, Bernstein IL. Cephalic insulin response as a test for completeness of vagotomy to the pancreas. Physiol Behav. 1980;24:485–488. doi: 10.1016/0031-9384(80)90241-3. [DOI] [PubMed] [Google Scholar]
- 20.Berthoud H-R, Powley TL. Identification of vagal preganglionics that mediate cephalic phase insulin response. Am J Physiol. 1990;258:R523–R530. doi: 10.1152/ajpregu.1990.258.2.R523. [DOI] [PubMed] [Google Scholar]
- 21.Cox JE, Powley T. Prior vagotomy blocks VMH obesity in pair fed rats. Am J Physiol. 1981;240:E573–E583. doi: 10.1152/ajpendo.1981.240.5.E573. [DOI] [PubMed] [Google Scholar]
- 22.Berthoud HR, Trimble ER, Siegel EG, Bereiter DA. Cephalic-phase insulin secretion in normal and pancreatic islet- transplanted rats. Am J Physiol. 1980;238:E336–E340. doi: 10.1152/ajpendo.1980.238.4.E336. [DOI] [PubMed] [Google Scholar]
- 23.Secchi A, Caldara R, Caumo A, et al. Cephalic-phase insulin and glucagon release in normal subjects and in patients receiving pancreas transplantation. Metabolism. 1995;44:1153–1158. doi: 10.1016/0026-0495(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 24.Ahren B, Taborsky GJ. The mechanism of vagal nerve stimulation of glucagon and insulin secretion in the dog. Endocrinology. 1986;118:1551–1557. doi: 10.1210/endo-118-4-1551. [DOI] [PubMed] [Google Scholar]
- 25.Ahren B. Autonomic regulation of islet hormone secretion--implications for health and disease. Diabetologia. 2000;43:393–410. doi: 10.1007/s001250051322. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz T, Holst J, Fahrenkrug J, et al. Vagal, cholinergic regulation of pancreatic polypeptide secretion. J Clin Invest. 1978;61:781–789. doi: 10.1172/JCI108992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crystal S, Teff KL. Tasting fat: cephalic phase hormonal responses and food intake in restrained and unrestrained eaters. Physiol Behav. 2006;89:213–220. doi: 10.1016/j.physbeh.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Morricone L, Bombonato M, Cattaneo A, et al. Food-related sensory stimuli are able to promote pancreatic polypeptide elevation without evident cephalic phase insulin secretion in human obesity. Horm Metab Res. 2000;32:240–245. doi: 10.1055/s-2007-978628. [DOI] [PubMed] [Google Scholar]
- 29.Steffens AB, Van der Gugten J, Godeke J, Luiten PGM, Strubbe JH. Meal-induced increases in parasympathetic and sympathetic activity elict simultaneous rises in plasma insulin and free fatty acids. Physiol Behav. 1986;37:119–122. doi: 10.1016/0031-9384(86)90393-8. [DOI] [PubMed] [Google Scholar]
- 30.Havel P, Parry S, Curry D, Stern j. Autonomic nervous system mediation of the pancreatic polypeptide response to insulin-induced hypoglycemia in conscious rats. Endocrinology. 1992;130:2225–2229. doi: 10.1210/endo.130.4.1347741. [DOI] [PubMed] [Google Scholar]
- 31.Havel P, Akpan J, Curry D, Stern j, Gingerich R, Ahren B. Autonomic control of pancreatic polypeptide and glucagon secretion during neuroglucopenia and hypoglycemia in mice. Am J Physiol. 1993;265:R246–R254. doi: 10.1152/ajpregu.1993.265.1.R246. [DOI] [PubMed] [Google Scholar]
- 32.Ahren B, Taborsky GJ, Porte D., Jr Neuropeptidergic versus cholinergic and adrenergic regulation of islet hormone secretion. Diabetologia. 1997:827–836. doi: 10.1007/BF00870137. [DOI] [PubMed] [Google Scholar]
- 33.Ahren B, Holst J. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 34.Niijima A. Effects of taste stimulation on the efferent activity of the pancreatic vagus nerve in the rat. Brain Res Bull. 1991;26:161–164. doi: 10.1016/0361-9230(91)90202-u. [DOI] [PubMed] [Google Scholar]
- 35.Niijima A, Togiyama T, Adachi A. Cephalic-phase insulin release induced by taste stimulus of monosodium glutamate (umami taste) Physiol Behav. 1990;48:905–908. doi: 10.1016/0031-9384(90)90247-2. [DOI] [PubMed] [Google Scholar]
- 36.Berthoud HR, Bereiter DA, Trimble ER, Siegel EG, Jeanrenaud B. Cephalic Phase, Reflex Insulin Secretion. Diabetologia. 1981;20:393–401. doi: 10.1007/BF00254508. [DOI] [PubMed] [Google Scholar]
- 37.Berridge K, Grill HJ, Norgren R. Relation of consummatory responses and preabsorptive insulin release to palatability and learned taste aversions. J Comp Physiol Psychol. 1981;95:363–381. doi: 10.1037/h0077782. [DOI] [PubMed] [Google Scholar]
- 38.Louis-Sylvestre J. Palatability and preabsorptive insulin release in the rat. Neurosci Behav Rev. 1980;4:43–46. doi: 10.1016/0149-7634(80)90047-0. [DOI] [PubMed] [Google Scholar]
- 39.Teff KL, Devine J, Engelman K. Sweet taste: effect on cephalic phase insulin release in men. Physiol Behav. 1995;57:1089–1095. doi: 10.1016/0031-9384(94)00373-d. [DOI] [PubMed] [Google Scholar]
- 40.Abdallah L, Chabert M, Louis-Sylvestre J. Cephalic phase responses to sweet taste. Am J Clin Nutri. 1997;65:737–743. doi: 10.1093/ajcn/65.3.737. [DOI] [PubMed] [Google Scholar]
- 41.Teff KL. Cephalic phase pancreatic polypeptide responses to liquid and solid stimuli in humans. Physiol Behav. 2010;99:317–323. doi: 10.1016/j.physbeh.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teff KL, Engelman K. Palatability and dietary restraint: effect on cephalic phase insulin release in woman. Physiol Behav. 1996;60:567–573. doi: 10.1016/s0031-9384(96)80033-3. [DOI] [PubMed] [Google Scholar]
- 43.Sive AA, Vinik AI, van Tonder SV. Pancreatic polypeptide (PP) responses to oral and intravenous glucose in man. Am J Gastroenterology. 1979;71:183–185. [PubMed] [Google Scholar]
- 44.Floyd J, Fajans S, Pek S, Chance R. A newly recognized pancreatic polypeptide: plasma levels in health disease. Recent Prog Horm Res. 1976:519–570. doi: 10.1016/b978-0-12-571133-3.50019-2. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig D, Peterson K, Gortmaker S. Relation between consumption of sugar-sweetened drinks and childhood obestiy: a prospective, observational analysis. Lancet. 2001;357:505–508. doi: 10.1016/S0140-6736(00)04041-1. [DOI] [PubMed] [Google Scholar]
- 46.Allison DB, Mattes RD. Nutritively sweetened beverage consumption and obesity: the need for solid evidence on a fluid issue. JAMA. 2009;301:318–320. doi: 10.1001/jama.2008.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattes R. Fluid calories and energy balance: the good, the bad, and the uncertain. Physiol Behav. 2006;89:66–70. doi: 10.1016/j.physbeh.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 48.Mattes RD. Fluid energy--Where’s the problem? J Am Diet Assoc. 2006;106:1956–1961. doi: 10.1016/j.jada.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 49.Berthoud HR. The relative contribution of the nervous system, hormones and metabolites to the total insulin response during a meal in a rat. Metabolism. 1984;33:18–24. doi: 10.1016/0026-0495(84)90157-4. [DOI] [PubMed] [Google Scholar]
- 50.Siegel EG, Trimble ER, Renold AE, Berthoud HR. Importance of preabsorptive insulin release on oral glucose tolerance: studies in pancreatic islet transplanted rats. Gut. 1980;21:1002–1009. doi: 10.1136/gut.21.11.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Powley TL. The ventromedial hypothalamic syndrome, satiety and a cephalic phase hypothesis. Psychol Rev. 1977;84:89–126. [PubMed] [Google Scholar]
- 52.Strubbe JH, Steffens AB. Rapid insulin release after ingestion of a meal in the unanesthetized rat. Am J Physiol. 1975;229:1019–1022. doi: 10.1152/ajplegacy.1975.229.4.1019. [DOI] [PubMed] [Google Scholar]
- 53.Storlien LH, Bruce DG. Mind over metabolism: the cephalic phase in relation to non-insulin- dependent diabetes and obesity. Biological Psychology. 1989;28:3–23. doi: 10.1016/0301-0511(89)90108-7. [DOI] [PubMed] [Google Scholar]
- 54.Teff KL, Engelman K. Oral sensory stimulation improves glucose tolerance: effects on post-prandial glucose, insulin, C-peptide and glucagon. Am J Physiol. 1996;270:R1371–R1379. doi: 10.1152/ajpregu.1996.270.6.R1371. [DOI] [PubMed] [Google Scholar]
- 55.Lorentzen M, Madsbad S, Kehlet H, Tronier B. Effect of sham-feeding on glucose tolerance and insulin secretion. Acta Endocrinologica (Copenh) 1987;115:84–86. doi: 10.1530/acta.0.1150084. [DOI] [PubMed] [Google Scholar]
- 56.Calles-Escandon J, Robbins DC. Loss of early phase of insulin release in humans impairs glucose tolerance and blunts thermic effect of glucose. Diabetes. 1987;36:1167–1172. doi: 10.2337/diab.36.10.1167. [DOI] [PubMed] [Google Scholar]
- 57.Ahren B, Holst J. The cephalic insulin response to meal ingestion in humans is dependent on both cholinergic and noncholinergic mechanisms and is important for postprandial glycemia. Diabetes. 2001;50:1030–1038. doi: 10.2337/diabetes.50.5.1030. [DOI] [PubMed] [Google Scholar]
- 58.Bruce DG, Chisholm DJ, Storlien LH, Kraegen EW. Physiological importance of deficiency in early prandial insulin secretion in non-insulin dependent diabetics. Diabetes. 1988;37:736–744. doi: 10.2337/diab.37.6.736. [DOI] [PubMed] [Google Scholar]
- 59.Kahn SE, Montgomery B, Howell W, et al. Importance of early phase insulin secretion to intravenous glucose tolerance in subjects with type 2 diabetes mellitus. J Clin Endo Metab. 2001;86:5824–5829. doi: 10.1210/jcem.86.12.8105. [DOI] [PubMed] [Google Scholar]
- 60.Cherrington AD, Marshall BA, Moore MC, Pagliosotti MJ, Shiota M. A role for the autonomic nervous system in regulation of glucose uptake by the liver. In: Shimazu T, editor. Liver Innervation. London: John Libber & Co. Ltd; 1996. [Google Scholar]
- 61.Shimazu T. Central nervous system regulation of liver and adipose tissue metabolism. Diabetologia. 1981;20 (Suppl):343–356. [PubMed] [Google Scholar]
- 62.Picard F, Nalmi N, Richard D, Deshaies Y. Response of adipose tissue lipoprotein lipase to the cephalic phase of insulin secretion. Diabetes. 1999;48:452–459. doi: 10.2337/diabetes.48.3.452. [DOI] [PubMed] [Google Scholar]
- 63.Storlien L, Oakes ND, Kelley DE. Metabolic flexibility. Proc Nutr Soc. 2004;63:363–368. doi: 10.1079/PNS2004349. [DOI] [PubMed] [Google Scholar]
- 64.Berthoud H, Powley T. Altered plasma insulin and glucose after obesity-producing bipiperidyl brain lesions. Am J Physiol. 1985;248:R46–R53. doi: 10.1152/ajpregu.1985.248.1.R46. [DOI] [PubMed] [Google Scholar]
- 65.Rohner-Jeanrenaud F, Jeanrenaud B. Involvement of the Cholinergic System in insulin and glucagon oversecretion of genetic preobesity. Endocrinology. 1997:830–834. doi: 10.1210/endo-116-2-830. [DOI] [PubMed] [Google Scholar]
- 66.Ionescu E, Jeanrenaud-Rohner F, Proietto J, Rivest RW, Jeanrenaud B. Taste-induced changes in plasma insulin and glucose turnover in lean and genetically obese rats. Diabetes, Vol. 1988;37:773–779. doi: 10.2337/diab.37.6.773. [DOI] [PubMed] [Google Scholar]
- 67.Simon C, Schlienger JL, Sapin R, Imler M. Cephalic phase insulin secretion in relation to food presentation in normal and overweight subjects. Physiol Behav. 1986;36:465–469. doi: 10.1016/0031-9384(86)90316-1. [DOI] [PubMed] [Google Scholar]
- 68.Sjostrom L, Garellick G, Krotkiewski M, Luyckx A. Peripheral insulin in response to the sight and smell of a food. Metabolism. 1980;29:901–909. doi: 10.1016/0026-0495(80)90031-1. [DOI] [PubMed] [Google Scholar]
- 69.Rodin J. Has the distinction between internal versus external control of feeding outlived its usefulness? In: Bray GA, editor. Recent advances in obesity. London: Neuman; 1978. [Google Scholar]
- 70.Parra-Covarrubias A, Rivera-Rodriguez I, Almaraz-Ugalde A. Cephalic phase of insulin secretion in obese adolescents. Diabetes. 1971;20:800–802. doi: 10.2337/diab.20.12.800. [DOI] [PubMed] [Google Scholar]
- 71.Karhunen LJ, Lappalainen RI, Niskanen LK, Turpeinen AK, Uusitupa MI. Determinants of the cephalic-phase insulin response in obese nondiabetic subjects. Metabolism. 1996;45:168–173. doi: 10.1016/s0026-0495(96)90048-7. [DOI] [PubMed] [Google Scholar]
- 72.Teff KL, Mattes RD, Engelman K, Mattern J. Cephalic-phase insulin in obese and normal-weight men: relation to postprandial insulin. Metabolism. 1993;42:1600–1608. doi: 10.1016/0026-0495(93)90157-j. [DOI] [PubMed] [Google Scholar]
- 73.Porte D, Jr, Bagdade JD, Bierman EL. The critical role of obesity in the interpretation of serum insulin levels. Adv Metab Disord. 1970;1(Suppl) doi: 10.1016/b978-0-12-027361-4.50027-2. [DOI] [PubMed] [Google Scholar]
- 74.Teff KL, Townsend RR. Early phase insulin infusion and muscarinic blockade in obese and lean subjects. Am J Physiol. 1999;277:R198–R208. doi: 10.1152/ajpregu.1999.277.1.R198. [DOI] [PubMed] [Google Scholar]
- 75.Teff KL, Townsend RR. Prolonged mild hyperglycemia induces vagally mediaed compensatory increase in C-peptide secretion in humans. J Clin Endo Metab. 2004;89:5606–5613. doi: 10.1210/jc.2003-032094. [DOI] [PubMed] [Google Scholar]
- 76.Petrova M, Townsend RR, Teff K. Prolonged (48-h) modest hyperinsulinemia decreases nocturnal heart rate variability and attenuates the nocturnal decline in blood pressure. J Clin Endo Metab. 2006;91:851–859. doi: 10.1210/jc.2005-1752. [DOI] [PubMed] [Google Scholar]
- 77.Teff KL, Petrova M, Havel PJ, Townsend RR. 48-h glucose infusion in humans: effect on hormonal responses, hunger and food intake. Physiol Behav. 2007;90:733–743. doi: 10.1016/j.physbeh.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metabolism. 2005;1:53–61. doi: 10.1016/j.cmet.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 79.Obici S, Feng S, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 80.Lam T, Pocai A, Gutierrez-Juarez R, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 81.Pocai A, Lam TK, Gutierrez-Juarez R, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]