Summary
Microglia, the primary resident immune cells of the CNS, exhibit dynamic behavior involving rapid process motility and cellular migration that is thought to underlie key functions of immune surveillance and tissue repair. Although age-related changes in microglial activation have been implicated in the pathogenesis of neurodegenerative diseases of aging, how dynamic behavior in microglia is influenced by aging is not fully understood. In this study, we employed live imaging of retinal microglia in situ to compare microglial morphology and behavioral dynamics in young and aged animals. We found that aged microglia in the resting state have significantly smaller and less branched dendritic arbors, and also slower process motilities, which likely compromise their ability to continuously survey and interact with their environment. We also found that dynamic microglial responses to injury were age-dependent. While young microglia responded to extracellular ATP, an injury-associated signal, by increasing their motility and becoming more ramified, aged microglia exhibited a contrary response, becoming less dynamic and ramified. In response to laser-induced focal tissue injury, aged microglia demonstrated slower acute responses with lower rates of process motility and cellular migration compared to young microglia. Interestingly, the longer term response of disaggregation from the injury site was retarded in aged microglia, indicating that senescent microglial responses, while slower to initiate, are more sustained. Together, these altered features of microglial behavior at rest and following injury reveal an age-dependent dysregulation of immune response in the CNS that may illuminate microglial contributions to age-related neuroinflammatory degeneration.
Keywords: microglia, aging, retina, age-related macular degeneration, imaging, laser
Introduction
Microglia, the predominant resident immune cells of the central nervous system (CNS), have been implicated in the pathogenesis of age-related neurodegenerative diseases including Alzheimer’s disease and age-related macular degeneration (Xu et al. 2009; Perry et al. 2010). Under physiologic conditions in the intact, healthy CNS, microglia continuously survey their environment with dynamic movements of their processes, allowing the neural parenchyma to be completely sampled every few hours with very little movement of the microglial somata (Davalos et al. 2005; Nimmerjahn et al. 2005). The physical extent of this immune surveillance is dependent on the distribution of microglia, the ramified morphology of individual cells, and the rate of remodeling of microglial processes. Given their basal behavior, microglia are also able to respond dynamically to tissue injury by transitioning to a migratory phenotype and aggregating at sites of tissue injury (Dailey & Waite 1999; Davalos et al. 2005). The functional implications of these forms of dynamic behavior, though incompletely understood, include synapse maintenance and elimination (Wake et al. 2009), clearance of apoptotic cells (Petersen & Dailey 2004), phagocytosis of cellular debris (Nimmerjahn et al. 2005) and changes in immune cell distribution (Raivich et al. 1999). Given the important roles that microglia are capable of performing, microglial dynamic behaviors likely underlie key roles in constitutive CNS immune functioning (Raivich 2005).
Alterations in microglial function have been linked to the development of neurodegenerative diseases (Amor et al. 2010). As advanced age is the largest risk factor for many diseases in this category, aging changes in microglia have been hypothesized to drive the pathogenic progression through a diminution of neuroprotective functions, direct increases in neurotoxicity, and dysregulated responses to signals and perturbations (Flanary et al. 2007; Streit et al. 2008; Luo et al. 2010). Age-related alterations previously characterized in microglia include changes in cytokine production (Inamizu et al. 1985; Sheng et al. 1998; Ye & Johnson 2001; Sierra et al. 2007; Njie et al. 2010), increased expression of activation markers (Perry et al. 1993; Ogura et al. 1994; Sheffield & Berman 1998; Kullberg et al. 2001; Chan-Ling et al. 2007), and the emergence of dystrophic morphologies (Streit et al. 2004). However, specific age-related alterations in the dynamic behavior of microglia have not been previously examined. Since dynamic microglial behavior may underlie important constitutive functions and responses to injury, age-related alterations in this aspect of microglial physiology may illuminate pathogenic mechanisms in age-related neurodegenerative disease.
We chose to explore age-related changes in microglia of the retina, which is a highly accessible part of the CNS with a well-described microglial distribution. The retina is a multi-laminar sheet of neural tissue containing both neurons and microglia in organized layers. Retinal microglia in the resting state are highly ramified and have a characteristic regular mosaic distribution in which microglial somata tile the horizontal plane of the retina with their processes distributed in a non-overlapping pattern, primarily in the inner and outer plexiform layers (Santos et al. 2008). We have previously documented that retinal microglia, like those in the brain, exhibit extensive dynamic behavior in ex vivo whole-mount retinal preparations (Lee et al. 2008; Liang et al. 2009), which are also observed in in vivo retinal recordings (Eter et al. 2008; Paques et al. 2010). These features enabled us to employ ex vivo imaging of the explanted retina to investigate microglial dynamic behavior and its alterations with age. In this study, we examined young and aged animals for parameters that influence the ability of microglia in the resting state to survey their extracellular milieu, specifically: 1) the density of microglial distribution, 2) the morphological parameters of a ramified cell, and 3) the dynamics of surveying movements of the ramified processes. In addition, we examined the age-dependence of dynamic responses of microglia to ATP, a signaling molecule produced in tissue injury, and also in response to direct tissue injury. Our results indicate that aging retinal microglia show quantitative changes in their ramified morphology and process dynamics that could decrease the extent of immune surveillance they are capable of performing under resting conditions. In addition, dynamic microglial responses to extracellular signals and tissue injury undergo age-dependent dysregulation that decreases their overall rate and responsiveness. These results constitute novel observations of how microglia undergo senescent alterations in their morphologic and behavioral phenotypes that may contribute to the pathogenesis of age-related neurodegenerative disease.
Results
Age-dependent changes in microglial morphology and distribution in the mouse retina
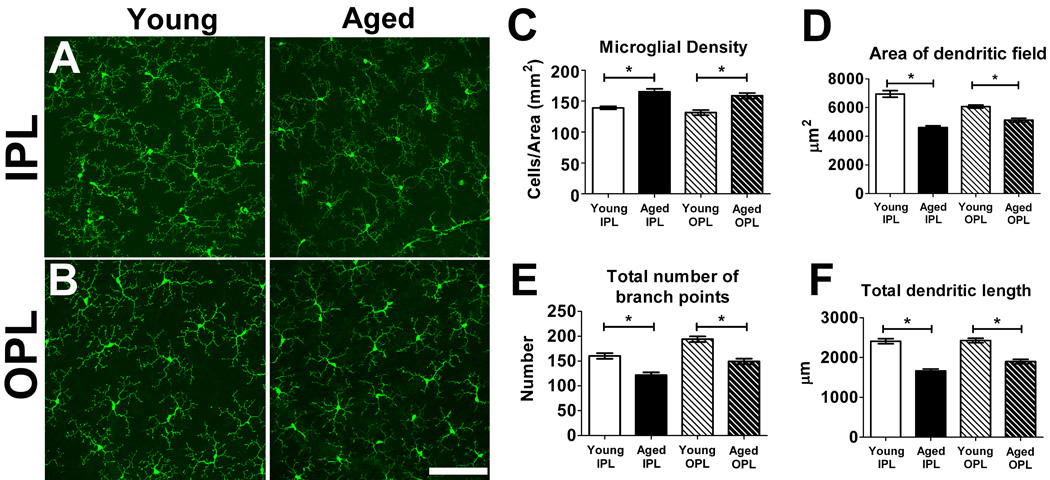
Microglia in the mature retina have a highly ramified morphology, extending their processes primarily in the inner plexiform layer (IPL) and outer plexiform layer (OPL) in a territorial and non-overlapping manner (Lee et al. 2008; Santos et al. 2008). We used confocal microscopy to image these horizontal arrays of ramified microglia in the retina of young (2–3 months old) and aged (18–24 months old) animals in flat-mounted retinal preparations. Grossly, microglial morphology was qualitatively similar between young and aged animals in both the IPL and OPL; dystrophic microglia with aberrant cytoplasmic structures and fragmentation, such as those described in aged human brains with Alzheimer’s disease (Streit et al. 2004; Streit et al. 2009), were observed very rarely in either age group (Fig. 1A, B).
Figure 1. Morphology and distribution of retinal microglia in young (2–3 months old) and aged (18–24 months old) CX3CR1+/GFP mice.
Resting retinal microglia, as visualized in retinal flat-mount preparations, possess small somata and ramified morphologies that were regularly distributed in the vitreal half of the retina with processes located in the inner plexiform layer (IPL) (A) and the outer plexiform layer (OPL) (B). Qualitatively similar microglial distributions and morphologies were observed in both young (left panels) and aged (right panels) retinas. Scale bar = 100 µm. (C) Densities of microglial somata in both IPL and OPL layers of young and aged retinas were measured in the mid-peripheral retina, showing increased microglial cell densities in the IPL and the OPL in aged compared to young retina (n = 24 retina from 6 young and 6 aged animals). Analyses of morphological parameters of area of dendritic field (D), total number of branch points (E), and total dendritic length of individual microglia (F), revealed that aged microglia have a smaller and less branched dendritic morphologies compared to young microglia in both the IPL and the OPL layers (n = 255 cells from 6 young animals and 205 cells from 6 aged animals). (Asterisks indicate comparisons for which p<0.05)
Quantitative analysis of horizontal density of ramified retinal microglia was performed in the IPL and OPL for both age groups in comparable mid-peripheral retinal areas. Within both age groups horizontal densities of microglial somata in the IPL versus the OPL and were found to be similar, however between age groups microglial densities in both the IPL and the OPL were slightly greater in the aged retina compared to the young retina (Fig. 1C). Quantitative analysis of the morphology of individual cells revealed that microglia in the aged retina had dendritic arbors that on average covered smaller horizontal areas (Fig. 1D), possessed a lower degree of branching (Fig. 1E), and had lower total dendritic lengths (Fig. 1F) compared to microglia in young retina.
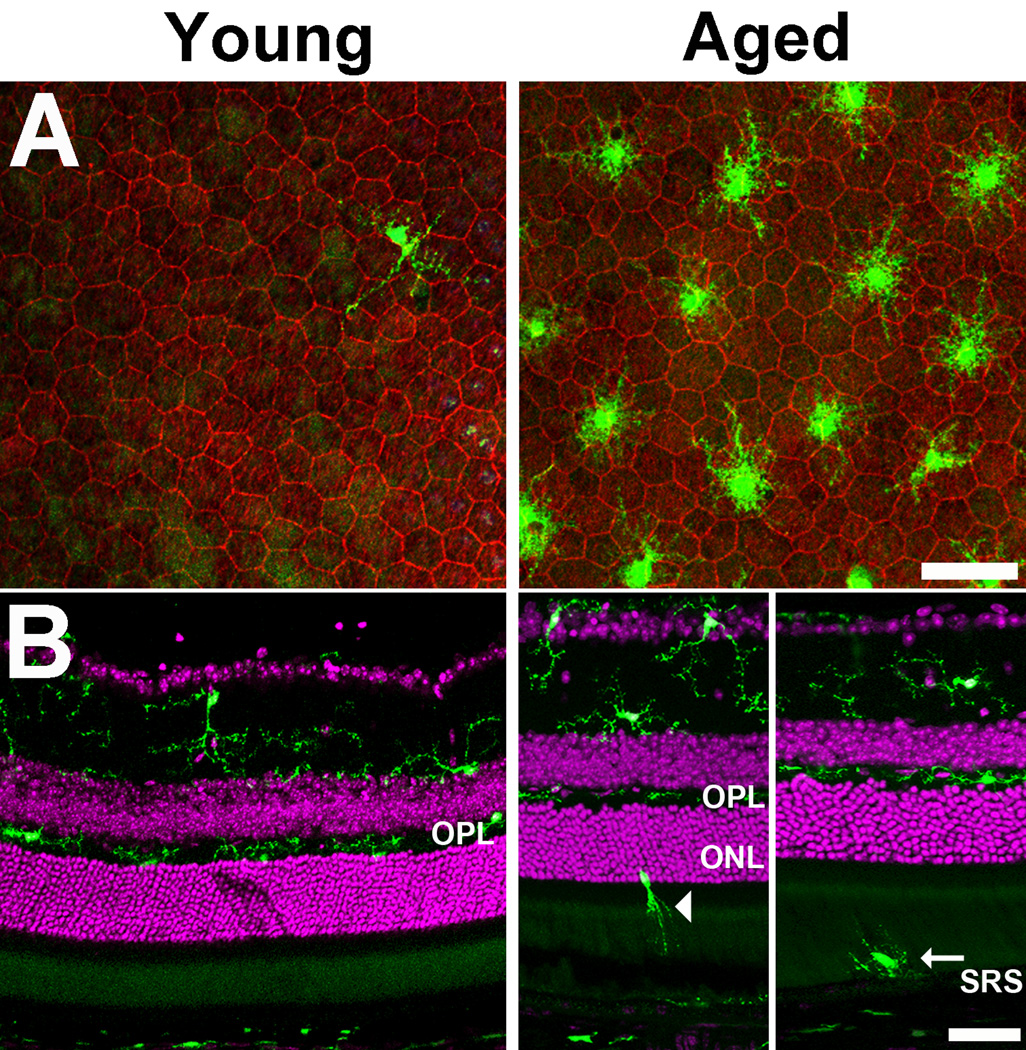
The distribution of microglia in the different lamina of mouse retina was also significantly altered between the two age groups. Consistent with previous reports (Santos et al. 2008), we found that few or no microglia were located in the outer retina external to the OPL in young animals (Fig. 2A,B; left). However, in aged animals, microglia with shorter and less branched processes were found in significantly increased numbers in the subretinal space (Fig. 2A,B; right). This accumulation likely resulted in part from the migration of microglia from the inner to the outer retina during aging; isolated microglia with elongated morphologies were detected in the outer retina with processes oriented towards the subretinal space in aged animals (Fig. 2B right), suggestive of a directed migration from the inner to the outer retina.
Figure 2. Translocation of microglia to the outer retina with increasing age.
(A) The distribution of retinal microglia in the subretinal space, as visualized in RPE-sclerochoroidal wholemounts, varied between young (left) and aged (right) animals. In young animals, only an occasional microglia cell (green) could be located on the apical surface of RPE cells in the subretinal space (RPE cellular outlines stained with phalloidin, red). In aged animals, significantly greater numbers of microglia accumulated in the subretinal space above RPE cells. These subretinal microglia in the aged retina also had a less ramified morphology with shorter, less branched processes. Scale bar = 50 µm. (B) Changes in microglial distribution with age were also evident in vibratome cross-sections. Retinal microglia in young animals (left) were predominantly confined to the inner retina up to and including the outer plexiform layer (OPL), while in aged animals (right) , retinal microglia could be observed traversing the outer retina in the outer nuclear layer (ONL) (arrowhead) and the subretinal space (SRS)(arrow). Scale bar = 50 µm.
Age-dependent changes in microglial process motility
Time-lapse live-cell confocal imaging in ex vivo retinal explants was employed to document and quantify process motility in individual young and aged microglia. Microglia in young retina demonstrated dynamic and extensive movements of their processes that were random in direction but balanced in terms of extensions and retractions to maintain overall dendritic symmetry and arbor size (Fig. 3A and B). Microglia in aged retina exhibited process dynamics that were similar to those seen in young animals in terms of extent and randomness, also maintaining balance across the dendritic arbor (Fig. 3C and D) (Supplementary movies 1 and 2). Overt cellular migration and translocation of soma position in resting microglia was absent in either age group over the time-frame of the recordings (approximately 10 minutes). Quantification of process motilities for individual microglia in the IPL and OPL of young and aged mice showed that mean process motility was significantly greater in IPL microglia than OPL microglia in both young and aged retina. Significantly, age-dependent changes were also detected; mean process motility of IPL microglia was slightly though not significantly lower in aged compared to young microglia (−7.9%, p = 0.4438), and mean process motility in OPL microglia was significantly lower in aged compared to young microglia (−14.0%, p = 0.007) (Fig. 3E).
Figure 3. Dynamic process motility in retinal microglia in young and aged CX3CR1+/GFP mice.
(A) Representative time-lapse recording of a retinal microglial cell from a young animal, demonstrating dynamic structural plasticity in ramified process. Higher magnification images (inset) of an individual process, taken 80s apart, demonstrate continuous process extensions, retractions, additions, and eliminations. A colorized subtraction image of time-lapse images highlights simultaneous process additions and extensions (in green) and process retractions and eliminations (in red) across a 10-min interval in a representative young retinal microglia cell (B). Positive and negative structural changes were balanced across the dendritic arbor to maintain stable dendritic size, complexity, and symmetry. In aged animals, a qualitatively similar pattern of structural changes in the processes of retinal microglia were found (C, D). (E) Quantitative comparison of process motility in individual retinal microglia located in the inner plexiform layer (IPL) and outer plexiform layer (OPL) of young (n = 42 cells from 4 animals) and aged (n = 49 cells from 4 animals) mice however revealed that mean process motilities were significantly greater in microglia located in the IPL compared to those located in the OPL for both young and aged retina. Comparing young with aged microglia, process motility in aged microglia in the IPL was slightly but not significantly lower, while those in the OPL were significantly lower than their counterparts in the young retina. Scale bars = 50 µm.
Age-dependent changes in microglial response to exogenous ATP
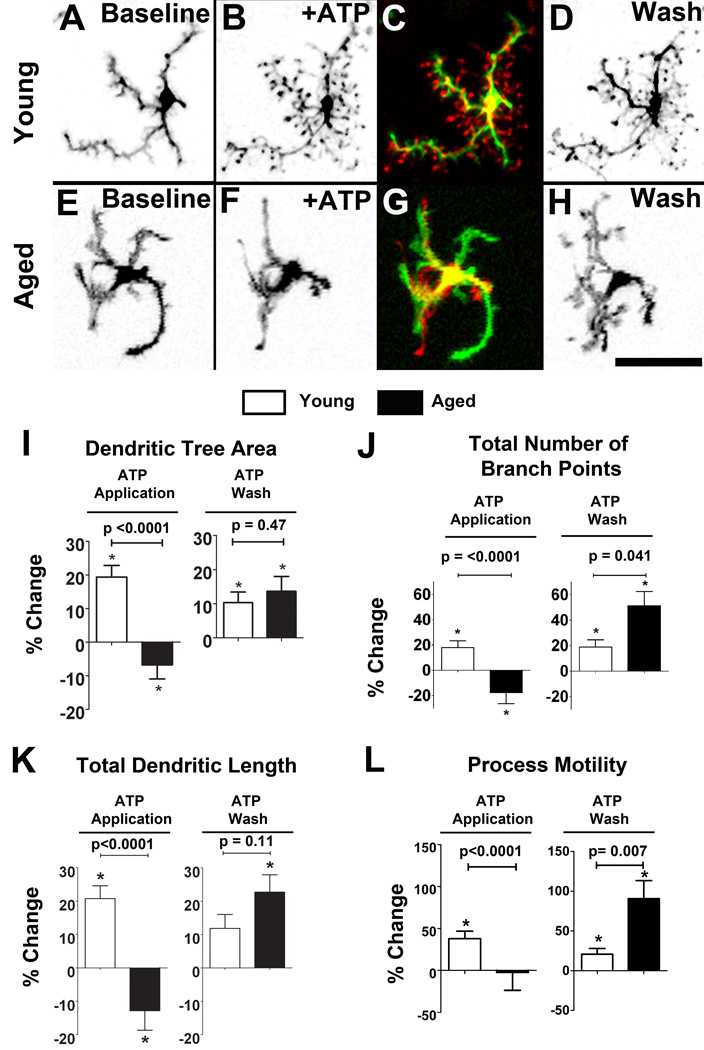
Using a similar experimental explant preparation, we also examined microglial dynamic behavior in response to exogenous signals. Extracellular ATP is a prominent signaling molecule involved in multiple forms of intercellular communications (Ralevic & Burnstock 1998), and can be released at high concentrations during tissue injury (Zimmermann 1994). Here we evaluated if microglial responses to extracellular ATP demonstrate age-related differences. In time-lapse recordings of ex vivo microglia in retinal explants, we found that microglia in young retina responded to the bath application of 1 mM ATP with a rapid extension of existing processes and the formation of new processes (Fig. 4A–C). Upon ATP washout, these young microglia interestingly demonstrated a further increase in process extension (Fig. 4D) (Supplementary movie 3). In contrast, aged microglia responded predominantly to ATP application by retraction and elimination of processes (Fig. 4E–G). Upon washout of ATP, they demonstrated a prominent extension of processes and increase in overall dendritic structure (Fig. 4H), similar to that seen in young microglia (Supplementary movie 4). We analyzed these structural changes in individual cells by quantifying the morphological parameters of dendritic tree area, total number of branch points, and total dendritic length. Young microglia exhibited increases in all 3 morphological parameters in response to ATP, while aged microglia exhibited decreases in the same parameters (Fig. 4I, J, K, left). On ATP washout, both young and aged microglia showed increases in these morphological parameters, with a trend towards a greater increase in aged compared to young microglia (Fig. 4I, J, K, right). In addition, we analyzed and compared the rate of dynamic motility of microglial processes in young and aged retina during and after ATP application. While young microglia increased mean process motility with ATP application, this parameter remained relatively unchanged in aged microglia. With ATP washout, however, both young and old microglia increased in process motility, with a significantly larger increase occurring in aged microglia (Fig. 4L). Dynamic microglial responses to ATP have been previously shown to be regulated by P2 purinergic receptors on the surface of microglia, particularly via metabotropic P2Y12 receptors, which are specifically expressed in microglia of the CNS (Haynes et al., 2006). We evaluated whether differences in P2Y12 expression in young versus aged microglia may correlate with their differences in responses to exogenous ATP. Analysis of P2Y12 expression on an mRNA level demonstrated a statistically higher (137%) expression of P2Y12 in aged microglia (p<0.05), indicating that signaling via P2Y12 receptors, and possibly its downstream mediators (Ohsawa et al.) may undergo age-dependent alterations.
Figure 4. Age-dependent changes in microglial morphology and process motility in response to exogenous ATP.
Microglia from young animals responded rapidly to bath application of ATP (1 mM) by increasing from their baseline morphological structure (A) by the extension of existing processes and the addition of new processes (B). A colorized subtraction image of time-lapse images across a 10-minute interval in a representative retinal microglial cell demonstrates a predominant addition of new processes and elongation of existing processes (coded in red) (C). Upon washout of ATP, microglia from young retina exhibited further increases in their morphological structure (D). Conversely, microglia from aged animals responded to bath application of ATP (1 mM) by a reducing their morphological structure (E to F), so that a colorized subtraction image primarily demonstrates process retraction (coded in green) (G). Upon washout of ATP, aged microglia, like young microglia, further increased their morphological structure (H). Scale bars = 50 µm. Quantitative analysis of changes in morphological parameters upon the addition and wash-out of ATP are compared in young (white bars) and aged microglia (black bars). Percentage changes in parameters during ATP application were relative to those under baseline conditions, while changes during ATP wash were relative to those during ATP application. Morphological parameters include: dendritic tree area (I), total number of branching points in a single cell (J), and total dendritic length (K). (L) Quantitative analysis of process motility (i.e. rate of movement of processes of individual cells) revealed that while young microglia increased their process motility in response to ATP application, aged microglia demonstrated no significant change. On ATP washout, both young and aged microglia increased their process motility, with aged microglia demonstrating a significantly larger increase (asterisks * indicate significant (p<0.05) differences from baseline values). The number of animals analyzed for (I)–(L) were: young , n = 58 cells from 6 animals; aged, n = 50 cells from 5 animals.
Age-dependent changes in dynamic microglial responses to focal tissue injury
Retinal microglia have been demonstrated to respond to focal tissue injury by increasing their mean process motility and also acquiring a migratory phenotype (Eter et al. 2008; Lee et al. 2008). To create a model for focal tissue injury in the retina, we employed a slit-lamp mounted photocoagulation laser to deliver a precisely controlled retinal burn (50 µm diameter) to young and aged ex-vivo retinal explants (Fig. 5A). Dynamic responses to tissue injury were examined in young and aged retina to elucidate any age-dependence of dynamic microglial responses. Analysis of microglial process motility before and after laser injury revealed that while young microglia significantly upregulated their process motility after laser injury (p = 0.03), aged microglia did not show any significant changes in motility (p = 0.35) (Fig. 5B). Following laser injury, retinal microglia in both young and aged age-groups were observed to migrate through retinal tissue (Fig. 5C, D). Comparisons of mean migratory velocity and maximum migratory velocity between age-groups showed lower values for aged microglia compared to young microglia (−18.1% for mean migratory velocity, (p = 0.005), and −21.0% for maximum migratory velocity, (p<0.02)) (Fig. 5E, F), reflecting an age-dependent decrease in migratory capacity.
Figure 5. Age-dependent changes in microglial process motility and migratory velocity in response to focal laser injury ex vivo.
(A) Migration and polarization of retinal microglia 1.5 hours after the application of focal laser injury (indicated by red spot) in a retinal explant from a young mouse. Microglia polarized their processes toward the site of laser injury and migrated to cluster around the injury site. Scale bar = 100 µm. (B) Quantification of microglial process motility in young and aged retina under baseline condition and following application of focal laser injury. Whereas microglia in young retina significantly increased process motility after laser injury, microglia from aged retina failed to change process motility. (C, D) Microglia in young and aged retina after laser injury acquired a migratory capacity, extending their processes towards the site of injury and directing their migration in that same direction. Time-lapse images taken 10 minutes apart show a similar nature of microglia migration. Superposition of colored images (right) demonstrate displacement of the cell from the start of the recording (red, at t=0) to the end of the recording (green, t=40 min). Scale bar = 50 µm. Migration velocities following laser injury were compared between microglia from young and aged animals. For both mean migratory velocity (E) and maximum instantaneous velocity (F), young microglia exhibited significantly higher values than aged microglia, indicating an age-dependence in post-injury migratory response. Asterisks * indicate significant (p<0.05) differences from baseline values, N.S indicates comparisons for which p >0.05. The number of animals analyzed were: young , n = 74 cells from 7 animals; aged, n = 50 cells from 5 animals.
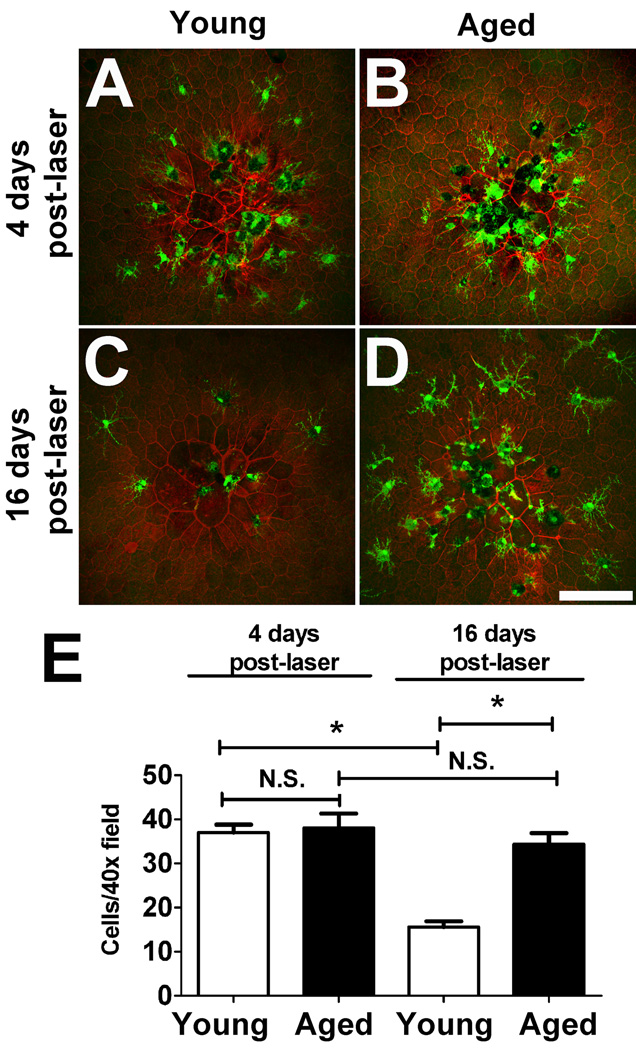
We also examined the consequences of altered microglial migration over longer time periods by examining microglial aggregation and disaggregation following tissue injury in vivo. A model of focal laser injury delivered to the retina of anesthesized animals was used. Previous in vivo studies using this model have indicated two phases of microglia response to laser injury, an earlier aggregation phase where microglia migrate towards and accumulate around the site of the laser injury and a later disaggregation phase when the tissue effects of injury wane and microglia accumulation at the injury site decreases (Eter et al. 2008). We monitored microglia cell numbers in the region of focal laser injury at 4 days and at 16 days following injury in sclerochoroidal-RPE whole-mounts. We chose to examine microglial aggregation at the level of the pigmented RPE as it is the locus of absorption of laser energy. At 4 days post-injury, both young and aged retina showed qualitatively similar degree of microglia aggregation at the site of injury (Fig. 6A,B). However, at 16 days post-injury, while microglia in the young retina demonstrated a decreased number of microglia at the injury site, those in the aged retina continued to be concentrated at the injury site without any obvious disaggregation (Fig. 6C, D). Quantitatively, the density of microglia in a 40× magnification field centered on the laser burn were similar between young and aged retina at 4 days post-injury (p = 0.67) (Fig. 6E). At 16 days, the density of aggregated microglia was significantly lower in young retina (−57.9% compared to that at 4 days; p < 0.0001), while that in aged retina was not significantly changed, indicating an age-related dependent decline in the ability of microglia to disaggregate following their response to injury. No consistent differences in microglial morphology between age groups or between post-injury times were noted.
Figure 6. Age-dependent changes in microglia aggregation and disaggregation following focal laser injury in vivo.
Four days following focal laser retinal injury in vivo, microglia in both young (A) and aged (B) animals were found to aggregate at an increased density at the site of the laser injury. At 16 days post-injury, microglia in young retina were more dispersed from the laser lesion site (C) while microglia in aged retina maintained their aggregation at the laser site (D). Quantification of microglial accumulation at the laser lesion site revealed that while microglia aggregation density 4 days post-laser was similar between young and aged retina, microglial disaggregation and dispersal occurred at 16 days post-laser in young animals but not as readily in aged retina. Asterisks * indicate significant (p<0.05) differences from baseline values, N.S. indicates comparisons for which p >0.05. Scale bar = 100 µm. The number of animals analyzed at the 4 day time point: young, n = 35 lesions from 9 animals, aged, n = 18 lesions from 5 animals. The number of animals analyzed at the 16 day time point: young, n = 32 lesions from 8 animals, aged, n = 20 lesions from 5 animals.
Discussion
Age-related changes in resting microglial distribution, morphology and behavior
Studies of aging in microglia raise the question of whether microglia are long-lived cells in the CNS. The long-term maintenance and tenure of microglia within the CNS have been evaluated in a number of studies. In both the mouse brain (Lawson et al. 1992) and the mouse retina (Xu et al. 2007), in situ microglial proliferation in the uninjured adult neural parenchyma has been found to occur at low levels. Previous studies have also attempted to estimate how quickly, if at all, microglia in the CNS are turned over by the entry of bone-marrow derived precursors into the CNS. Studies that employed transplantation of GFP-marked bone-marrow cells into lethally-irradiated recipient mice have produced mixed results; some have reported prominent rates of turnover (Xu et al. 2007; Kezic et al. 2008), while another has reported very low rates over a 12 month-period (Kaneko et al. 2008). On the other hand, studies that employ parabiosis to create chimerism in circulating bone-marrow derived precursors (which avoid confounding effects associated with irradiation or transplantation procedures) convincingly demonstrate that CNS microglia are rarely replaced by bone marrow cells in uninjured animals (Ajami et al. 2007; Mildner et al. 2007). The combination of low proliferation and low turnover rates indicates that the majority of microglia are long-lived cells with prolonged residence times in the CNS and are therefore susceptible to in situ aging effects occurring over an animal’s normal lifespan.
In our studies, we observed that retinal microglia in aged mice maintained ramified morphologies and demonstrated a “tiled” distribution in the IPL and OPL of the inner retina resembling those in young animals. However, in our analysis of microglial distributions in young and aged retina at matched eccentricities (i.e. at comparable retinal areas along the central-to-peripheral axis), we detected a small but significant age-related increase in microglia cell density in both the IPL and the OPL. The factors giving rise to this slight increase in density are unknown and suggest the existence of low levels of local proliferation and/or recruitment of circulating microglial precursors that exceed the rates of egress or death of local microglia over the normal lifespan of the animal. Whether this increment occurs gradually as a function of age, or is induced by multiple experiences of environmental influences (e.g. infections, injuries, inflammatory insults) over a lifetime is a question of interest in our future investigations. Conversely, in a separate study of the mouse brain, microglial densities in most, but not all, areas in the brain have been noted to decrease with time (Ma et al. 2003). However, this study compared mice aged 7 weeks to those aged 14.5–16 weeks (i.e. over the first quarter of the animal’s lifespan) and may have measured changes more related to maturation than to senescence. Also, we observed in the aged retina that microglia have an altered laminar distribution, accumulating in the subretinal space of the outer retina, a zone which in the young retina is normally devoid of microglia. This mislocation of microglia to the outer retina was also observed in mice deficient in chemokines and chemokines receptors (Combadiere et al. 2007; Tuo et al. 2007; Luhmann et al. 2009), suggesting that age-dependent changes in microglial responsiveness to extracellular trophic and adhesion signals may be responsible for their changed laminar distribution (Wynne et al. 2010).
We did not observe any dystrophic morphologies (i.e. deramified microglia with tortuous processes and fragmented cytoplasm) in the aged mouse retina that have been noted to occur sporadically in aged human brains (Streit et al. 2004). However, our analysis of morphologies of young versus aged microglia revealed that despite appearing grossly similar, aged microglia had ramified morphologies that were less branched, had shorter overall process lengths, and covered less dendritic “territory” than those of young microglia. Consistent with our findings, examination of brain microglia in aged mice has also not uncovered dystrophic morphologies, only morphologies with “reduced arborization” (Sierra et al. 2007). The age-related morphological differences that we did observe are likely to influence a microglia cell’s ability to detect and survey signals in its direct vicinity, as well as to influence neighboring cells. While the conversion of a resting microglia to a fully activated one has been associated with a polar transition from a ramified to an amoeboid morphology, it is unclear if different levels of graded activation, characterized molecularly (Stout & Suttles 2005; Mosser & Edwards 2008), may also correspond with more intermediate transitions in cellular morphology. It is possible that the quantitative age-related morphological differences observed here in aged microglia (i.e. less ramified, less branching) may also correspond to graded increases in activation states, reflecting a gradual polarization towards an activated, proinflammatory, aging phenotype (Lee et al. 2000; Lu et al. 2004).
We noted that while the nature of surveillance movements of microglia in the resting state was qualitatively similar between young and aged animals (i.e. rapid protrusion and retraction of processes that are randomly directed and balanced between positive and negative change), their dynamics were quantitatively diminished in aged animals, particularly in the OPL. This slowed behavior, combined with smaller and less extensive dendritic arbors, may further decrease an aged microglia’s ability to detect extracellular signals and influence nearby cells. The functional consequences of this diminution may represent one facet of the well-documented decline in immune system function in aging (Dorshkind & Swain 2009). Also, to the extent that dynamic microglial processes are involved in ongoing synapse maintenance (Wake et al. 2009), their decreased rates of movement within the retina may possibly be related to previously documented age-related declines in electrophysiological function and synaptic transmission (Birch & Anderson 1992; Gresh et al. 2003). In this view, age-related neurodegeneration occurring on a synaptic level may contributed to by the failure of aged microglial to support and maintain active synapses.
We speculate that the mechanisms underlying these age-related differences in resting microglial morphology and dynamism may involve both intrinsic cell-autonomous changes and/or alterations in the extracellular milieu. It is interesting to note that in both young and aged animals, microglial process motilities were higher in the IPL than in the OPL, suggesting that the synaptic environment (synapse type or neurotransmitter preponderance) may play an extracellular role in influencing microglial morphology and behavior. As such, aging changes in neurons that alter neurotransmission or synaptic communication in the retina may be a mechanism influencing microglial morphology and motility. However, cell-autonomous age-related alterations within microglia can also be a cause of observed changes, such as alterations in the expression of receptors on the microglial cell surface, as in the case of P2Y12. Future studies will be directed at discovering age-related changes in gene expression in retinal microglia in order to understand the molecular bases for the cell-autonomous alterations described here.
Age-related changes in dynamic microglial behavior in response to tissue injury and injury-associated signals
It has been previously noted that microglial responses to tissue injury undergo age-dependent change. Studies employing models of CNS injury or disease have demonstrated that aged microglia respond with a higher and more sustained level of activation, and are associated with increased neuronal death and delayed functional recovery. In a rat model of intracerebral hemorrhage, aged animals, relative to young animals, had lower levels of microglial activation one day post-injury but higher levels at 3 days post-injury (Wasserman et al. 2008). Eventual axonal damage and functional loss were also comparatively greater in aged animals in this model (Wasserman & Schlichter 2008). Studies of intracerebral hemorrhage in senescence-accelerated prone (SAMP8) mice also demonstrated analogous differences between senescence-prone versus senescence-resistant mice (Lee et al. 2006). Similar trends were also found in mouse models of MPTP-induced degeneration (Sugama et al. 2003) and traumatic brain injury (Sandhir et al. 2008). These studies have largely relied on molecular analyses (i.e. the expression of activation markers and inflammatory cytokines associated with activation) to illustrate the aging-related alterations in the magnitude and temporal course of microglial activation in response to injury. Our findings here present a correlate to those altered responses on the level of changes in microglial morphology and dynamic behavior, and provide a corresponding cellular description for the nature of senescent immune responses to CNS injury.
Dynamic microglial responses to tissue injury have been shown to be mediated by multiple extracellular signals (Biber et al. 2007). ATP is one prominent example of a signal that is released at elevated concentrations in neural tissue following injury (Zimmermann 1994; Fields & Stevens-Graham 2002), and acts directly on microglia (Inoue 2002). ATP gradients in tissue provide extracellular cues to increase the motility of microglia and to guide the migration; in tissue injury, these chemokine gradients contribute to their dynamic and polarized responses to injury sites (Inoue 2002; Davalos et al. 2005; Haynes et al. 2006). We therefore chose to examine ATP-induced dynamic responses in young and aged microglia in situ to discover potential relevant age-related differences in microglial signaling. Our results demonstrate that the effect of ATP application on microglia morphology was qualitatively different between young and aged microglia, with young microglia becoming more ramified in response to ATP and aged microglia becoming less so. Young microglia also increased their process motility in response to ATP application while aged microglia did not alter their motility significantly. These differences are more likely to arise from age-related differences in ATP signaling than from general decreases in cellular motility or metabolism; upon ATP washout, aged microglia displayed a prominent but opposite increase in motility and morphological parameters, which in relative terms constituted larger increases than those seen in young microglia. The mechanisms underlying these differences to ATP signaling remain undetermined. Interestingly, we found that P2Y12, a Gi-adenylyl cyclase coupled metabotropic purinergic receptor that is expressed specifically in microglia (Sasaki et al. 2003) and a primary site at which ATP acts to induce dynamic microglial responses (Haynes et al. 2006), was expressed at higher levels in aged compared to the young retina. This result suggests that the age-dependent responses to ATP in microglia may be regulated in a cell-autonomous manner on the level of the purinergic receptor signaling on the microglial cell surface or by other downstream effector molecules.
We also analyzed aging-dependent differences in microglial dynamic responses to tissue injury in a focal laser injury model. We found that while young microglia were able to upregulate process motility in response to laser injury, aged microglia failed to do so, remaining at their mean baseline levels. Also, while aged microglia were able to acquire a migratory capacity post-injury similar to young microglia, their migratory rates were significantly reduced. These differences suggest a slower and less dynamic short-term post-injury response in aged microglia that may relate to a diminished mobilization of microglia to the injury site and a slower upregulation of injury-induced responses. It has been proposed that the rapid dynamic microglial process movements following injury may serve to restore tissue homeostasis by clearing the parenchyma of released intracellular products and tissue debris and also by physically shielding the surrounding tissue from the area of injury (Nimmerjahn et al. 2005; Shaked et al. 2005). The slower dynamics of this short-term response in aged microglia may thus translate to a decreased ability to restore and maintain tissue homeostasis in the acute aftermath of injury.
In our longer term experiments involving an in vivo model of laser retinal injury, we observed that microglial accumulation at the laser injury site during the earlier aggregation phase was similar in young and aged retinas at 4 days post-injury, despite the short-term differences in dynamic motility and migration noted above. However, disaggregation from the injury site, observed in the longer post-injury term of 16 days, was significantly slowed in aged retinas. The size and nature of the lesions (number and disposition of retinal pigment epithelial cells) at this time point were not distinct between the age groups, but microglia with deramified morphologies were accumulated at the lesion sites in significantly greater numbers in aged animals. These results are consistent with findings in which microglial activation in aged mice in response to injury, assayed on a molecular level, was not only more prominent but also more sustained (Sugama et al. 2003; Sandhir et al. 2008; Wynne et al. 2010). The altered and delayed dynamics of deactivation and dispersal of aged microglia following injury may prevent normal tissue homeostasis from being restored and induce chronic states of neuroinflammation implicated in the pathogenesis of age-related neurodegeneration(Medzhitov 2008). While the deleterious effects of sustained aggregation of microglia in the subretinal space were not apparent in our laser injury model at 16 days post-injury, it is possible that sustained accumulations of activated microglia over longer periods may exert pathogenic alterations in the retina. Indeed, in mouse models of age-related macular degeneration (AMD), animals that exhibit abnormally elevated accumulations of activated microglia in the outer retina also demonstrate surrounding photoreceptor degeneration (Combadiere et al. 2007; Tuo et al. 2007). Human histopathological specimens of AMD were also found to contain activated microglia in the outer retina (Gupta et al. 2003). In glaucoma, another age-related retinal degeneration, accumulations of activated microglia have similarly been observed in loci of neural degeneration in both mouse models and human pathological specimens (Neufeld 1999; Yuan & Neufeld 2001; Bosco et al. 2008). These data suggest that the age-related alterations in microglial deactivation and dispersal may potentially relate to mechanisms of neural degeneration of these retinal pathologies.
It is possible that age-related alterations in microglial responses to injury, just as in the case of resting microglia behavior, may also be impacted by by age-related changes arising from the extracellular retinal millieu and from neighboring cells. While our experiments demonstrate that microglial responses to exogenous ATP, an injury-related signal, were clearly different between young and aged microglia, it is possible that age-related differences in the release and composition of injury-related signals from surrounding retinal cells may also exist in vivo and thus contribute to a differential microglial response. Studying responses of microglia isolated from young and aged retinas to identical applications of injury-related signals in vitro may be helpful in dissecting the relative contributions of cell-autonomous versus cell-non-autonomous factors to the differences observed here.
In summary, we report here that the aging phenotype of microglia involves alterations in their morphology and dynamic behavior. These alterations were evident under both resting conditions and in response to injury and injury-associated signals. These differences indicate a decreased capacity of microglia in aged animals to survey neural parenchyma with their ramified processes under resting conditions. Also, they reflect a dysfunctional response to injury that is in general less prominent and rapid in the acute post-injury phase, but is more prolonged and sustained in the chronic phase. These alterations in dynamic microglial behavior reveal aspects of the microglial aging phenotype that in combination with age-related molecular changes illuminate mechanisms by which senescent microglia may drive features of disease progression in aging nervous systems. Dynamic behavior in microglia, a key aspect of microglial physiology, therefore represents a phenotypic trait that may be useful in elucidating microglial dysfunction in age-related neurodegeneration and for assaying the cellular effects of potential therapeutic interventions.
Experimental Procedures
Experimental Animals
Heterozygous CX3CR1+/GFP transgenic animals, created by breeding CX3CR1GFP/GFP mice obtained from The Jackson Laboratory (Bar Harbor, ME) (Jung et al. 2000) with wild type C57BL/6 mice, were used for both live-cell imaging and morphological analysis in fixed tissue. Microglia in CX3CR1+/GFP animals were rendered visible by their expression of green fluorescent protein (GFP). Two groups of animals were defined for age-dependent comparisons: (1) young adult animals (2 to 3 months of age) and (2) aged animals (18 to 24 months of age). All animals were bred and housed in a National Institutes of Health animal facility. Experiments were conducted according to protocols approved by the local Institutional Animal Care and Use Committee and adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Ex-Vivo Time-Lapse Confocal Microscopy
CX3CR1+/GFP mice were euthanized and their eyes were immediately enucleated and placed in a solution of oxygenated Ringer’s: 125 mM NaCl, 5 mM KCl, 1.5 mM CaCl2, 0.75 mM MgCl2/6 H2O, 1.25 mM NaH2PO4, 10 mM D-glucose, 20 mM HEPES (pH 7.35–7.45). Retinal explants were acutely isolated from the eye cups and flat-mounted with the ganglion cell layer uppermost on Millipore filter paper (HABP045; Millipore, Billerica, MA, USA). For live imaging experiments, retinal explants were transferred to a 32 °C temperature-controlled stage (Bioptechs, Butler, PA) through which oxygenated Ringer’s solution was continuously superfused. GFP-labeled microglia were imaged using a confocal microscope (SP2; Leica, Exton, PA, USA) and a 40× (0.80 numerical aperture) immersion objective. Z-series stacks of ramified retinal microglia located in both the outer and the inner plexiform retinal layers were captured at a resolution of 512×512 pixels at 0.5–1.0 µm intervals. Each Z-series image stack included the entire dendritic arbor of most microglia in the imaging field; the few microglia for which some processes were incompletely captured in the image stack were omitted from analysis. Time-lapse series of Z-image stacks were captured at a rate of 1 stack per 10 seconds. Maximum projections of the image stacks in the Z-direction were then created for morphological and motility analyses.
Time-lapse recordings were performed to evaluate the effects of exogenous ATP on microglial morphology and dynamic behavior. During continuous recording segments consisting of three 8.3 minute intervals, 50 image-stacks per interval were captured of microglia: 1) at baseline, 2) during superfusion of the recording chamber with 1 mM ATP and 3) after washout with Ringer’s solution.
Changes in retinal microglia migration and process motility in response to tissue injury were also monitored by time-lapse confocal imaging. Using a model of laser-induced focal injury, retinal explants mounted on a filter paper support were placed perpendicular to the light path of a slit-lamp-mounted 532-nm photocoagulation laser (Iridex, Mountain View, CA, USA). Isolated laser burns were created in retinal tissue using the following settings: power, 130 mW; duration, 140 ms; spot size, 50 µm. Following laser injury, retinal explants were returned to a 32 °C oxygenated incubation chamber for 1.5 hours before being imaged.
Image Processing and Analysis
Images were processed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) as previously described (Lee et al. 2008; Liang et al. 2009). Briefly, two-dimensional time-lapse movies were created from maximum intensity projections in the z-dimension and aligned in the x-y plane. Focus drift was minimized during recordings and motion analysis was limited only to the microglia whose morphologies fully contained in the imaging space, avoiding focus change artifacts.
Measurements of microglial density in the inner plexiform layer (IPL) and outer plexiform layer (OPL) were performed by counting microglial somata in confocal images of flat-mounted retina (20× objective fields, measuring 0.75 × 0.75 mm) in the mid-peripheral retina at a standard radial distance of 375 µm from the optic nerve. Two to four fields were sampled from each flat-mounted retina. Morphological analysis of microglial dendritic structure was performed on z-projections of confocal images of individual microglia. Three morphological parameters were calculated: (1) dendritic tree area, (2) total branch point number, and (3) total dendritic length. Dendritic tree area was quantified by circumscribing the area outlined by the terminals of dendritic processes using the smooth polygon tool in NIH Image J. Total dendritic length and total branch point number were computed after a binarized image of the microglia cell was converted to a topological skeleton depiction using the “skeletonize” function. Microglial process motility was computed for individual cells by performing image subtractions between time-consecutive aligned images. The computed area of the cell that was added or lost between images was used as a measure of the instantaneous rate of structural change in the motile processes. A mean rate across a recording segment was calculated as the average of instantaneous rates across that segment. Microglia migration was determined by tracking the displacement of soma position of individual microglia durig a time-lapse recording using the plugin MTrackJ. Instantaneous and mean migratory velocities were computed for each microglia in the recording field from the recorded tracks.
Statistical Analysis
Statistical analyses were performed using statistical software (Graphpad, San Diego, CA, USA). A normality test (D’Agostino and Pearson) was used to analyze the distribution of all data sets. For 2-way comparisons, the data sets were analyzed with an unpaired two-tailed t-test when they followed a Gaussian distribution (comparison of dendritic tree area of young versus aged microglia in response to ATP application) and with the nonparametric Mann-Whitney test if they did not follow a normal distribution (comparison of branch point number, total dendritic length, and process motility of young versus aged microglia in response to ATP application; comparison between young versus aged migratory velocity). Comparisons between 3 or more data sets were performed using one-way analyses of variance (ANOVA) with the Bonferroni ‘s multiple comparison test if the distributions were Gaussian (comparisons of baseline microglial density), and with the Kruskal-Wallis test with the Dunn’s multiple comparison test if the distributions were non-Gaussian (comparisons of baseline dendritic field area, branch point number, total dendritic length and process motility; comparisons of young and aged post-laser process motility; comparisons of in vivo post-laser microglial aggregation) . A P value <0.05 was set as the basis for rejecting the null hypothesis. In all graphical representations, the error bars indicate standard error (SE).
In Vivo Laser Injury Model
An in vivo retinal laser injury model was used to evaluate changes in microglial aggregation following focal retinal injury. Experimental animals were anesthetized with an intraperitoneal injection of ketamine (90 mg/kg) and xylazine (8 mg/kg) and their pupils were dilated with 1% tropicamide (Akorn Inc, Buffalo Grove, IL, USA) and 2.5% phenylephrine (Alcon Laboratories Inc, Forth Worth, TX). Corneal anesthesia was provided using topical 0.5% proparacaine (Alcon Laboratories Inc, Fort Worth, TX, USA). Laser injury was applied to the retina using a slit-lamp-mounted, 532 nm wavelength, photocoagulation laser (Iridex, Mountain View, CA, USA) and a handheld focusing lens. Using laser settings to create burns that did not rupture Bruch’s membrane (power, 50 mW; duration, 100 ms; spot size, 100 µm), 4 well-spaced laser burns were placed circumferentially approximately 375 µm from the optic nerve. Throughout the procedure, corneal hydration was maintained with 2.5% hypromellose ophthalmic solution (Gonak; Akron Inc, Buffalo Grove, IL, USA). Animals were euthanized at 4 days or 16 days post-injury, enucleated, and flat mount preparations of retinal and RPE tissue prepared and imaged by confocal microscopy.
Immunohistochemistry
Eyes from enucleated CX3CR1+/GFP mice were fixed in 4% paraformaldehyde in 1× phosphate-buffered saline (PBS). For flat-mount preparations, retinas were dissected from the eyecups in a single sheet and mounted on glass slides in mounting medium (Fluoromount, Sigma Aldrich, St. Louis, MO, USA) with the ganglion cell layer face-up. Sclerochoroidal flat-mounts which had been stained overnight with phalloidin (1:80; Invitrogen, Carlsbad, CA, USA) were similarly prepared with the retinal pigment epithelium (RPE) layer uppermost. Vibratome sections were prepared by embedding fixed eyecups in 7% agarose and cut into 100µm sections with a vibrating microtome (VT1000S; Leica, Wetzlar, Germany). Sections were stained with 4’,6-diamidino-2-phenylindole (DAPI; 1:1000; Invitrogen, Carlsbad, CA, USA) to mark the retinal nuclear layers. Tissue preparations were imaged using confocal microscopy with either a 20× dry objective (0.70 NA) or a 40× oil objective (0.75–1.25 NA) at a resolution of 1024×1024 pixels.
RT-PCR and Enzyme-Linked Immunosorbent Assay
For RT-PCR analysis of P2Y12 expression levels, acutely isolated retinal tissue (6 animals, 3 from each age group) was transferred into a RNA stabilization solution (RNAlater; Ambion, Austin, TX, USA). Total RNA was isolated using a commercial kit (RNeasy Mini kit; Qiagen, Valencia, CA, USA) and a cDNA synthesis kit (Ambion) was used to create first-strand cDNA. 1µL of cDNA diluted with Tris-EDTA was added to each 20 µL PCR reaction. CX3CL1-specific cDNA amplification was performed as previously described (Liang et al. 2009). Glyceraldehyde 3’-phosphate dehydrogenase (GAPDH) was used as an internal control to normalize the mRNA concentration of CX3CL1 across samples. Primers used in the amplification reaction were: 5’ ggcctctgagaaccttggtg 3’ (forward) and 5’ cattggggtctcttcgc 3’ (reverse).
Supplementary Material
Acknowledgments
Funding: This study was supported by the National Eye Institute Intramural Research Program and a grant from the American Health Assistance Foundation (AHAF). Acknowledgement is made to the donors of Macular Degeneration Research (MDR), a program of the American Health Assistance Foundation, for support of this research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Author Contributions:
Conceived and designed the experiments: MD, WW. Performed the experiments: MD, LZ, AF, JA, RF. Analyzed the data: MD, WW. Contributed reagents/materials/analysis tools: RF, WW. Wrote the paper: MD, JA, RF, WW.
Contributor Information
Mausam R. Damani, Email: mdamani@gmail.com.
Lian Zhao, Email: zhaolia@nei.nih.gov.
Aurora M. Fontainhas, Email: fontainhasa@niddk.nih.gov.
Juan Amaral, Email: amaralj@nei.nih.gov.
Robert N. Fariss, Email: farissr@nei.nih.gov.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, van der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129:154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Birch DG, Anderson JL. Standardized full-field electroretinography. Normal values and their variation with age. Arch Ophthalmol. 1992;110:1571–1576. doi: 10.1001/archopht.1992.01080230071024. [DOI] [PubMed] [Google Scholar]
- Bosco A, Inman DM, Steele MR, Wu G, Soto I, Marsh-Armstrong N, Hubbard WC, Calkins DJ, Horner PJ, Vetter ML. Reduced retina microglial activation and improved optic nerve integrity with minocycline treatment in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2008;49:1437–1446. doi: 10.1167/iovs.07-1337. [DOI] [PubMed] [Google Scholar]
- Chan-Ling T, Hughes S, Baxter L, Rosinova E, McGregor I, Morcos Y, van Nieuwenhuyzen P, Hu P. Inflammation and breakdown of the blood-retinal barrier during "physiological aging" in the rat retina: a model for CNS aging. Microcirculation. 2007;14:63–76. doi: 10.1080/10739680601073451. [DOI] [PubMed] [Google Scholar]
- Combadiere C, Feumi C, Raoul W, Keller N, Rodero M, Pezard A, Lavalette S, Houssier M, Jonet L, Picard E, Debre P, Sirinyan M, Deterre P, Ferroukhi T, Cohen SY, Chauvaud D, Jeanny JC, Chemtob S, Behar-Cohen F, Sennlaub F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–2928. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dailey ME, Waite M. Confocal imaging of microglial cell dynamics in hippocampal slice cultures. Methods. 1999;18:222–230. 177. doi: 10.1006/meth.1999.0775. [DOI] [PubMed] [Google Scholar]
- Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Swain S. Age-associated declines in immune system development and function: causes, consequences, and reversal. Curr Opin Immunol. 2009;21:404–407. doi: 10.1016/j.coi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eter N, Engel DR, Meyer L, Helb HM, Roth F, Maurer J, Holz FG, Kurts C. In vivo visualization of dendritic cells, macrophages, and microglial cells responding to laser-induced damage in the fundus of the eye. Invest Ophthalmol Vis Sci. 2008;49:3649–3658. doi: 10.1167/iovs.07-1322. [DOI] [PubMed] [Google Scholar]
- Fields RD, Stevens-Graham B. New insights into neuron-glia communication. Science. 2002;298:556–562. doi: 10.1126/science.298.5593.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, Streit WJ. Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Res. 2007;10:61–74. doi: 10.1089/rej.2006.9096. [DOI] [PubMed] [Google Scholar]
- Gresh J, Goletz PW, Crouch RK, Rohrer B. Structure-function analysis of rods and cones in juvenile, adult, and aged C57bl/6 and Balb/c mice. Vis Neurosci. 2003;20:211–220. doi: 10.1017/s0952523803202108. [DOI] [PubMed] [Google Scholar]
- Gupta N, Brown KE, Milam AH. Activated microglia in human retinitis pigmentosa, late-onset retinal degeneration, and age-related macular degeneration. Exp Eye Res. 2003;76:463–471. doi: 10.1016/s0014-4835(02)00332-9. [DOI] [PubMed] [Google Scholar]
- Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- Inamizu T, Chang MP, Makinodan T. Influence of age on the production and regulation of interleukin-1 in mice. Immunology. 1985;55:447–455. [PMC free article] [PubMed] [Google Scholar]
- Inoue K. Microglial activation by purines and pyrimidines. Glia. 2002;40:156–163. doi: 10.1002/glia.10150. [DOI] [PubMed] [Google Scholar]
- Jung S, Aliberti J, Graemmel P, Sunshine MJ, Kreutzberg GW, Sher A, Littman DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–4114. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Nishiguchi KM, Nakamura M, Kachi S, Terasaki H. Characteristics of bone marrow-derived microglia in the normal and injured retina. Invest Ophthalmol Vis Sci. 2008;49:4162–4168. doi: 10.1167/iovs.08-1738. [DOI] [PubMed] [Google Scholar]
- Kezic J, Xu H, Chinnery HR, Murphy CC, McMenamin PG. Retinal microglia and uveal tract dendritic cells and macrophages are not CX3CR1 dependent in their recruitment and distribution in the young mouse eye. Invest Ophthalmol Vis Sci. 2008;49:1599–1608. doi: 10.1167/iovs.07-0953. [DOI] [PubMed] [Google Scholar]
- Kullberg S, Aldskogius H, Ulfhake B. Microglial activation, emergence of ED1-expressing cells and clusterin upregulation in the aging rat CNS, with special reference to the spinal cord. Brain Res. 2001;899:169–186. doi: 10.1016/s0006-8993(01)02222-3. [DOI] [PubMed] [Google Scholar]
- Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48:405–415. doi: 10.1016/0306-4522(92)90500-2. [DOI] [PubMed] [Google Scholar]
- Lee CK, Weindruch R, Prolla TA. Gene-expression profile of the ageing brain in mice. Nat Genet. 2000;25:294–297. doi: 10.1038/77046. [DOI] [PubMed] [Google Scholar]
- Lee JC, Cho GS, Choi BO, Kim HC, Kim YS, Kim WK. Intracerebral hemorrhage-induced brain injury is aggravated in senescence-accelerated prone mice. Stroke. 2006;37:216–222. doi: 10.1161/01.STR.0000195151.46926.7b. [DOI] [PubMed] [Google Scholar]
- Lee JE, Liang KJ, Fariss RN, Wong WT. Ex vivo dynamic imaging of retinal microglia using time-lapse confocal microscopy. Invest Ophthalmol Vis Sci. 2008;49:4169–4176. doi: 10.1167/iovs.08-2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang KJ, Lee JE, Wang YD, Ma W, Fontainhas AM, Fariss RN, Wong WT. Regulation of dynamic behavior of retinal microglia by CX3CR1 signaling. Invest Ophthalmol Vis Sci. 2009;50:4444–4451. doi: 10.1167/iovs.08-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429:883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Luhmann UF, Robbie S, Munro PM, Barker SE, Duran Y, Luong V, Fitzke FW, Bainbridge JW, Ali RR, MacLaren RE. The drusenlike phenotype in aging Ccl2-knockout mice is caused by an accelerated accumulation of swollen autofluorescent subretinal macrophages. Invest Ophthalmol Vis Sci. 2009;50:5934–5943. doi: 10.1167/iovs.09-3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Morton AJ, Nicholson LF. Microglia density decreases with age in a mouse model of Huntington's disease. Glia. 2003;43:274–280. doi: 10.1002/glia.10261. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Mildner A, Schmidt H, Nitsche M, Merkler D, Hanisch UK, Mack M, Heikenwalder M, Bruck W, Priller J, Prinz M. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld AH. Microglia in the optic nerve head and the region of parapapillary chorioretinal atrophy in glaucoma. Arch Ophthalmol. 1999;117:1050–1056. doi: 10.1001/archopht.117.8.1050. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura K, Ogawa M, Yoshida M. Effects of ageing on microglia in the normal rat brain: immunohistochemical observations. Neuroreport. 1994;5:1224–1226. doi: 10.1097/00001756-199406020-00016. [DOI] [PubMed] [Google Scholar]
- Ohsawa K, Irino Y, Sanagi T, Nakamura Y, Suzuki E, Inoue K, Kohsaka S. P2Y12 receptor-mediated integrin-beta1 activation regulates microglial process extension induced by ATP. Glia. 58:790–801. doi: 10.1002/glia.20963. [DOI] [PubMed] [Google Scholar]
- Paques M, Simonutti M, Augustin S, Goupille O, El Mathari B, Sahel JA. In vivo observation of the locomotion of microglial cells in the retina. Glia. 2010 doi: 10.1002/glia.21037. [DOI] [PubMed] [Google Scholar]
- Perry VH, Matyszak MK, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- Petersen MA, Dailey ME. Diverse microglial motility behaviors during clearance of dead cells in hippocampal slices. Glia. 2004;46:195–206. doi: 10.1002/glia.10362. [DOI] [PubMed] [Google Scholar]
- Raivich G. Like cops on the beat: the active role of resting microglia. Trends Neurosci. 2005;28:571–573. doi: 10.1016/j.tins.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Werner A, Bluthmann H, Doetschmann T, Kreutzberg GW. Molecular signals for glial activation: pro- and anti-inflammatory cytokines in the injured brain. Acta Neurochir Suppl. 1999;73:21–30. doi: 10.1007/978-3-7091-6391-7_4. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos AM, Calvente R, Tassi M, Carrasco MC, Martin-Oliva D, Marin-Teva JL, Navascues J, Cuadros MA. Embryonic and postnatal development of microglial cells in the mouse retina. J Comp Neurol. 2008;506:224–239. doi: 10.1002/cne.21538. [DOI] [PubMed] [Google Scholar]
- Sasaki Y, Hoshi M, Akazawa C, Nakamura Y, Tsuzuki H, Inoue K, Kohsaka S. Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia. 2003;44:242–250. doi: 10.1002/glia.10293. [DOI] [PubMed] [Google Scholar]
- Shaked I, Tchoresh D, Gersner R, Meiri G, Mordechai S, Xiao X, Hart RP, Schwartz M. Protective autoimmunity: interferon-gamma enables microglia to remove glutamate without evoking inflammatory mediators. J Neurochem. 2005;92:997–1009. doi: 10.1111/j.1471-4159.2004.02954.x. [DOI] [PubMed] [Google Scholar]
- Sheffield LG, Berman NE. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Sheng JG, Mrak RE, Griffin WS. Enlarged and phagocytic, but not primed, interleukin-1 alpha-immunoreactive microglia increase with age in normal human brain. Acta Neuropathol. 1998;95:229–234. doi: 10.1007/s004010050792. [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55:412–424. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Braak H, Xue QS, Bechmann I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:475–485. doi: 10.1007/s00401-009-0556-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Miller KR, Lopes KO, Njie E. Microglial degeneration in the aging brain--bad news for neurons? Front Biosci. 2008;13:3423–3438. doi: 10.2741/2937. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45:208–212. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Sugama S, Yang L, Cho BP, DeGiorgio LA, Lorenzl S, Albers DS, Beal MF, Volpe BT, Joh TH. Age-related microglial activation in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced dopaminergic neurodegeneration in C57BL/6 mice. Brain Res. 2003;964:288–294. doi: 10.1016/s0006-8993(02)04085-4. [DOI] [PubMed] [Google Scholar]
- Tuo J, Bojanowski CM, Zhou M, Shen D, Ross RJ, Rosenberg KI, Cameron DJ, Yin C, Kowalak JA, Zhuang Z, Zhang K, Chan CC. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–3836. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman JK, Schlichter LC. White matter injury in young and aged rats after intracerebral hemorrhage. Exp Neurol. 2008;214:266–275. doi: 10.1016/j.expneurol.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Wasserman JK, Yang H, Schlichter LC. Glial responses, neuron death and lesion resolution after intracerebral hemorrhage in young vs. aged rats. Eur J Neurosci. 2008;28:1316–1328. doi: 10.1111/j.1460-9568.2008.06442.x. [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog Retin Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Xu H, Chen M, Mayer EJ, Forrester JV, Dick AD. Turnover of resident retinal microglia in the normal adult mouse. Glia. 2007;55:1189–1198. doi: 10.1002/glia.20535. [DOI] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. An age-related decline in interleukin-10 may contribute to the increased expression of interleukin-6 in brain of aged mice. Neuroimmunomodulation. 2001;9:183–192. doi: 10.1159/000049025. [DOI] [PubMed] [Google Scholar]
- Yuan L, Neufeld AH. Activated microglia in the human glaucomatous optic nerve head. J Neurosci Res. 2001;64:523–532. doi: 10.1002/jnr.1104. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends Neurosci. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.