Abstract
There are now several murine models of autoimmune cholangitis that have features both similar and distinct from human PBC. One such model, the NOD.c3c4 mouse, manifests portal cell infiltrates, anti-mitochondrial antibodies but also biliary cysts. The biliary cysts are not a component of PBC and not found in the other murine models. To address the immunopathology in these mice, we generated genetically B cell deficient Igμ−/− NOD.c3c4 mice and compared the immunopathology of these animals to control B cell sufficient NOD.c3c4 mice. B cell deficient mice demonstrated decreased number of non-B cells in the liver accompanied by reduced numbers of activated natural killer cells. The degree of granuloma formation and bile duct damage were comparable to NOD.c3c4 mice. In contrast, liver inflammation, biliary cyst formation and salivary gland inflammation was significantly attenuated in these B cell deficient mice. In conclusion, B cells play a critical role in promoting liver inflammation and also contribute to cyst formation as well as salivary gland pathology in autoimmune NOD.c3c4 mice, illustrating a critical role of B cells in modulating specific organ pathology and, in particular, in exacerbating both the biliary disease and the sialadenitis.
Keywords: B cells, autoimmune cholangitis, Mu deficient mice, anti-mitochondrial antibodies
Introduction
A major impediment to our understanding of the mechanisms of human primary biliary cirrhosis (PBC) has been the absence of animal models. Recently, however, several rodent models have been described [1–6]; each of these presents both similarities but also some differences with human PBC. In both patients and mice, the hallmark of disease remains the presence of serum anti-mitochondrial antibodies and portal cell infiltrates. The first murine model described is that of the NOD.c3c4 mouse, which manifests anti-mitochondrial antibodies, portal inflammation, the ability to transfer disease to naïve recipients, but also the presence of biliary cysts [1]. The mechanism for cyst development is unclear and the histopathology of these cysts is distinct from any comparable lesion found in humans with PBC.
In both patients and mice, the role of B cells in the pathogenesis of PBC has also been a controversial issue. There is, for example, no relationship between the titer of AMA and the severity of clinical disease [7] and the titers of AMA following therapeutic orthotopic liver transplantation in PBC patients does not correlate with recurrence of disease [8]. Further, recent work on another murine model of autoimmune cholangitis, the dnTGFβRII mice, suggests that regulatory B cells may actually be important contributors to disease and the absence of B cells in this model appears to exacerbate liver pathology [9]. We have taken advantage of the availability of both dnTGFβRII mice and NOD.c3c4 mice, (NOD.B6 Idd3/17/10/Idd18 B10 Idd9.1/9.2/9.3 mice, hereafter referred to NOD.c3c4 mice), to address the role of B cells [1, 10]. In particular, we generated genetically B cell deficient (Igμ−/−) NOD.c3c4 mice and compared the immunopathology of these mice to control B cell sufficient (Igμ+/+) NOD.c3c4 mice. As expected, the Igμ−/− NOD.c3c4 mice had no B cells but interestingly not only had an amelioration of salivary gland inflammation, but also reduced numbers of inflammatory liver infiltrates, ameliorated liver inflammation, and a significantly lower prevalence of biliary cyst formation. We suggest that this data is a further indicator that B cells play a promoting role in the immunopathology of autoimmune biliary disease as well as sialadenitis in this strain.
Materials and Methods
B cell deficient Igμ−/− NOD.c3c4 mice
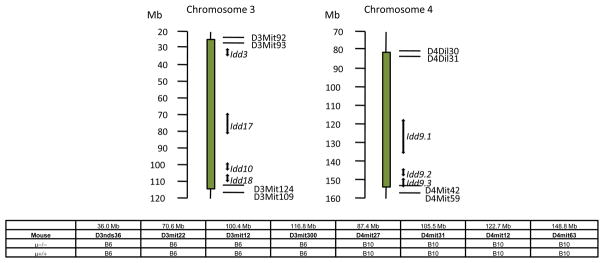
NOD.c3c4 mice (NOD. B6 Idd3/17/10/Idd18 B10 Idd9.1/9.2/9.3 mice) were obtained from Taconic, Inc. (Germantown, NY). The genetic origin of these mice has been previously described in detail [1, 10], including the presence of NOD alleles at seven insulin-dependent diabetes (Idd) genes, Idd3, Idd17. Idd 10 and Idd18 on chromosome 3, and, Idd9.1. Idd9.2 and Idd9.3 on chromosome 4 which are replaced by B6 and B10 derived resistance alleles respectively (Figure 1). Hence, these animals are fully protected from diabetes but develop autoimmune biliary disease. Progressive biliary cyst formation and peribiliary tract lymphocytic infiltration lead to hepatic failure and a moribund state in around 50 % of females and 25% of males at 9–11 months of age [10]. To generate B cell deficient NOD.c3c4 mice, female NOD.c3c4 mice were bred onto male B6.129S2-Igh-6tm1Cgn/J mice (hereafter referred to as B6.Igμ−/− mice) on the C57BL/6 (B6) strain background (The Jackson Laboratory, Bar Harbor, Maine) at the University of California animal facility (Davis, CA). Female Igμ+/− F1 mice were back-crossed onto male NOD.c3c4 mouse. After repeated crossing of off-spring female onto male NOD.c3c4 mouse, male and female Igμ+/−F7 mice were obtained, which were genotyped to confirm introgressed B6/B10 derived resistance alleles [1, 10]. Just one mating pair of Igμ+/− F7 mice was utilized to develop B cell deficient Igμ−/− and sufficient Igμ+/+ NOD.c3c4 littermates, which were further genotyped to confirm the presence of c3 and c4 introgressed alleles (Figure 1) and the chromosome one regions (not shown). The resulting mice were used for the studies reported herein. All mice were maintained in individually ventilated cages under specific pathogen-free conditions. Serum samples were serially collected from groups of eight B cell deficient and 8 sufficient mice at 4, 8, 14 and 24 weeks of age. Also, peripheral blood mononuclear cells (PBMC) were prepared for flow cytometric analysis at 8 and 24 weeks of age. Fifteen mice of each group were sacrificed at 24 weeks of age and examined for liver and salivary gland pathology. Livers and spleens from seven mice of each group at 24 weeks of age were utilized to isolate mononuclear cells for flow cytometric analysis. Eight mice of each group were also sacrificed for liver and salivary gland pathology at 8 weeks of age.
Figure 1.
Genetic map of NOD.c3c4 B cell-deficient (μ−/−) mice. PCR confirmed the presence of introgressed genome derived from B6/B10 mice in chromosomes 3 and 4 of B cell- deficient (μ−/−) and sufficient (μ+/+) NOD.c3c4 mice.
Humoral Immunity
Serum samples were stored at −70°C until assayed. Serum levels of total IgG, IgA, and IgM were quantified using optimal dilutions with a mouse Ig ELISA kit (BETHYL, Montgomery, TX). Known standards were used throughout. Serum levels of anti-PDC-E2 were quantified using an ELISA as described elsewhere [11]. Previously defined and titered known positive and negative sera standards were included with each assay.
Flow Cytometry
Peripheral blood mononuclear cells (PBMC) were prepared from Igμ−/− and Igμ+/+ NOD.c3c4 mice at 8 and 24 weeks of age. Briefly, 50 μL of peripheral blood was collected into heparinized tube and PBMC was isolated using Accu-Paque Lymphocytes (Accurate Chemical & Scientific Corp., Westbury, NY). Cells were pre-incubated with anti-mouse FcR blocking reagent and then incubated at 4°C with a combination of fluorochrome-conjugated antibodies, including PE-Cy5 conjugated anti-CD8 (eBioscience), PE-CD19 (BioLegend), FITC conjugated anti-CD69 (BioLegend), APC-Cy7 conjugated anti-CD3 (BD biosciences, San Jose, CA), APC conjugated anti-CD4 (eBioscience). Mononuclear cells from liver and spleen were isolated as previously described [2]. Cells were pre-incubated with anti-mouse FcR (clone 2.4G2) blocking reagent and then incubated at 4°C with various combination of fluorochrome-conjugated Abs to facilitate the identification of various subsets. The reagents utilized included FITC conjugated anti-Gr-1 (BioLegend), PE-Cy5 conjugated anti-CD19 (BioLegend), Alexa Fluor 750 conjugated anti-CD11b (eBioscience, San Jose, CA), APC-Cy7 conjugated anti-CD3 (BD biosciences, San Jose, CA), Alexa Fluor 647 conjugated anti-CD5 (BioLegend), APC conjugated anti-CD4 (BioLegend), PE conjugated anti-CD49b (DX5) (BioLegend), and FITC conjugated anti-CD69 (BioLegend). Multi-color flow analyses were performed using a FACScan flow cytometer (BD Immunocytometry Systems, San Jose, CA) upgraded by Cytec Development (Fremont, CA) to allow for 5-color analysis. Acquired data were analyzed with CELLQUEST Software (BD Biosciences).
Liver, Spleen and Salivary gland Preparation
Portions of the liver, spleen, and salivary glands were excised and immediately fixed with 10% buffered formalin solution for 2 days at room temperature (RT). Paraffin-embedded tissue sections were then cut into 4-μm slices for routine hematoxylin (DakoCytomation, Carpinteria, CA) and eosin (American Master Tech Scientific, Lodi, CA) (H&E) staining.
Scoring of liver inflammation was performed on H&E stained sections of liver using a set of 6 indices by a “blinded” pathologist (KT), including levels of liver inflammation, portal inflammation, parenchymal inflammation, granuloma formation, bile duct damage, and biliary cyst formation. Based on the levels of pathology, the indices were scored as 0, nil; 1, minimal; 2, mild; 3, moderate; 4, severe pathology. For the evaluation of salivary gland samples, the “blinded” pathologist (KT) evaluated the existence of lymphoid cell aggregation around the ducts as a sign of Sjögren’s syndrome, and classified it into three levels of severity, i.e., 0, nil; 1, mild; 2, severe (0, absence or less than 50 lymphocytes around a duct; 1, > 50 lymphocytes around a duct; 2, > 50 lymphocytes around a duct with ductal damage or more frequent inflammatory foci).
Cytokine Analysis
The inflammatory cytokines, TNF-α, IL-6, IL-10, IL-12p70, MCP-1 and IFN-γ , were quantitated in total liver protein extracts using a mouse inflammatory cytometric bead array (BD Biosciences). To extract total liver protein, 100–200 mg frozen liver tissue was homogenized in TNE buffer (1% NP 40, 10 mM Tris-Hcl pH 7.5, 150 mM NaCl, 1 mM EDTA) containing a protease inhibitor and phosphatase inhibitor cocktail (Roche, Indianapolis, IN), as described [12]. The suspension was centrifuged at 12000 rpm for 20 min at 4 °C and stored at −80 °C. The protein concentration was measured using the BCA kit (Pierce Biotechnology, Rockford, IL). Thirty μg of total protein was applied for the assay using a mouse inflammatory cytometric bead array kit (BD Biosciences).
Statistical Analysis
Values were expressed as the mean ± standard error of the mean (SEM) and evaluated using a two-tailed unpaired Mann-Whitney test. Frequency of portal inflammation and bile duct damage were evaluated using the Fisher’s exact test. P < 0.05 was considered statistically significant.
Results
Sera immunoglobulins and anti-mitochondrial antibodies
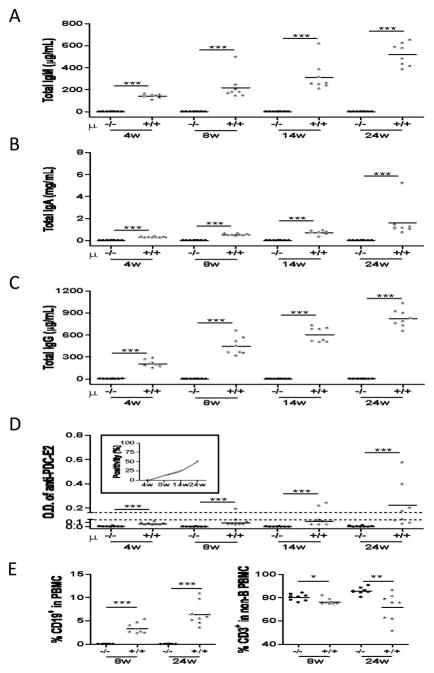
As expected, serum levels of IgM, IgG and IgA were reduced in Igμ−/− NOD.c3c4 mice compared to Igμ+/+ NOD.c3c4 mice (Figures 2A–C). Furthermore, while we readily detected AMA in Igμ+/+ NOD.c3c4 mice, such reactivity was undetectable in Igμ−/− NOD.c3c4 mice (Figure 2D). We confirmed the absence of peripheral CD19+ B cells in Igμ−/− NOD.c3c4 mice at 8 and 24 weeks of age (Figure 2E). The frequency of CD3+ cells was also greater in the PBMC’s of Igμ−/− mice compared to Igμ+/+ mice at these time points (Figure 2E).
Figure 2.
Serum reactivity of AMA, total IgM, A, and G in B cell deficient and sufficient mice. A–C. Serum levels of total IgM, A and G increased in time-dependent manner in B cell sufficient mice. D. AMA positivity was defined as the value of OD > 2 times SD above the mean of the OD of normal B6 female mice (24 weeks old, n=3). Approximately 50% of serum samples were positive for AMA in B cell-sufficient mice at 24 weeks of age. Sera from B cell deficient mice did not develop any reaction in these assays. E. Peripheral frequency of CD19+ B cells and CD3+ cells was examined in B cell deficient and sufficient mice. (n=8 respectively. *: p < 0.05, **: p < 0.01, ***: p < 0.001 in Mann-Whitney Test)
Liver immunopathology
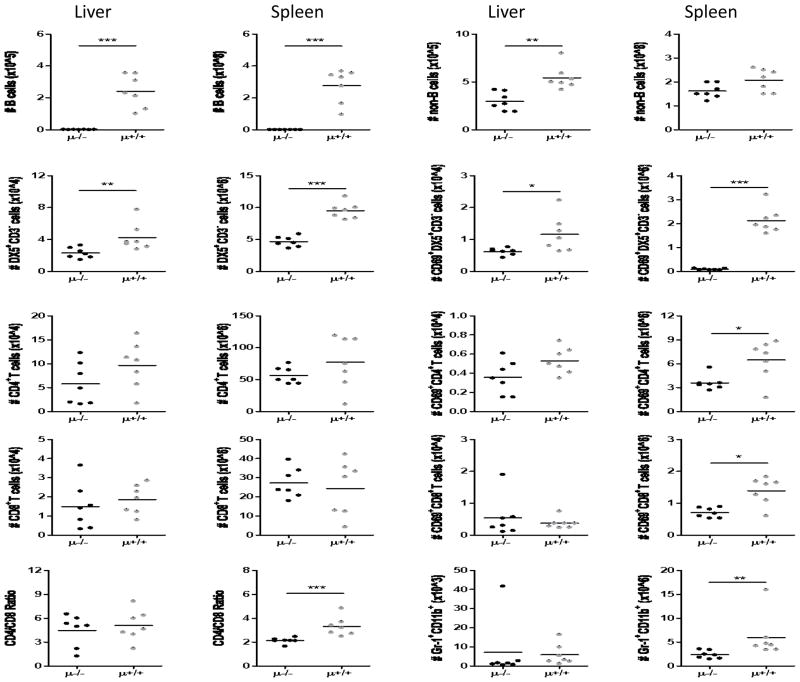
Flow cytometric analysis confirmed the absence of CD19+ B cells both in the livers and in the spleens of Igμ−/− NOD.c3c4 mice (Figure 3). The absence of B cells reduced the numbers of hepatic, but not splenic non-B cell populations in NOD.c3c4 mice (Figure 3). In the non B cell fractions, both non-activated and activated (upregulated CD69) DX5+CD3− cells (NK cells) were significantly decreased in the liver and spleens of the Igμ−/− NOD.c3c4 mice (Figure 3). Whereas the numbers of activated CD4+ and CD8+ T cells were diminished in the spleens of B cell depleted mice, the intra-hepatic numbers of DX5−CD3+CD4+ nor DX5−CD3+CD8+ cells were similar regardless of B cell absence (Figure 3). Of note, splenic Gr-1+ CD11b+ granulocytes demonstrated significant reduction in the absence of B cells in this strain.
Figure 3.
Mononuclear cell profiles in liver and spleen. Absolute numbers of non-B cells were lower in liver and spleen of B cell deficient (μ−/−) mice compared to B cell sufficient controls. DX5+CD3− cells (designated NK cells here) were decreased in liver and spleen of μ−/− mice, and the number of activated NK cells decreased. The other cell populations did not demonstrate significant differences in liver whereas some did in spleen. (The numbers shown here were normalized per 1 gram of liver and spleen tissue.) (n=7. *p < 0.05, ***p < 0.001 in Mann-Whitney Test)
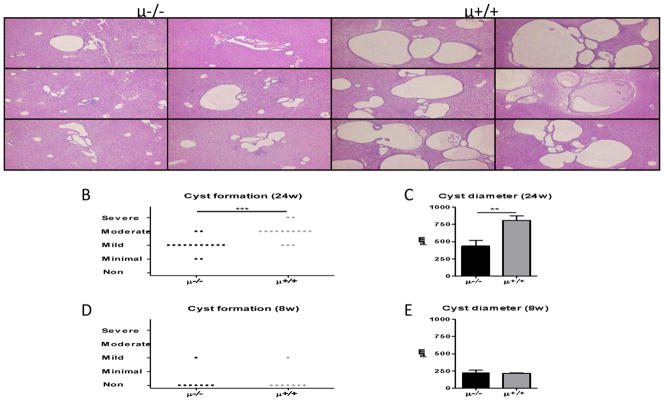
H&E stained liver sections demonstrated that intrahepatic biliary cysts were increased in B cell sufficient NOD.c3c4 mice contrasting to B cell deficient NOD.c3c4 mice (Figure 4A). Whereas, the lack of B cells significantly reduced biliary cyst formation in NOD.c3c4 mice at 24 weeks of age (Figure 4B,C), biliary cystogenesis was not prominent in either strain at 8 weeks of age (Figure 4D,E). Of note, similar levels of common bile duct dilatation was observed in both B cell deficient and sufficient mice (data not shown).
Figure 4.
Biliary cyst formation. A. Panels show 6 representative liver histopathology sections of B cell deficient (μ−/−) and sufficient (μ+/+) NOD.c3c4 mice. A. Mildly dilated bile ducts were observed scatteredly in B cell deficient mice whereas livers in B cell sufficient mice demonstrated marked ductal dilatation. B. Liver cyst formation was partly suppressed in B cell deficient mice at 24 weeks of age (μ−/−, n=15; μ+/+, n=15). C. The average diameter of three maximal bile cysts in the examined section was significant lower in 24 week old B cell deprived mice. D,E. No difference was observed in 8 week-old mice. (H&E staining. Black scale bars indicate 100 μm in A. *: p < 0.05, **: p < 0.01, ***: p < 0.001 in Mann-Whitney Test)
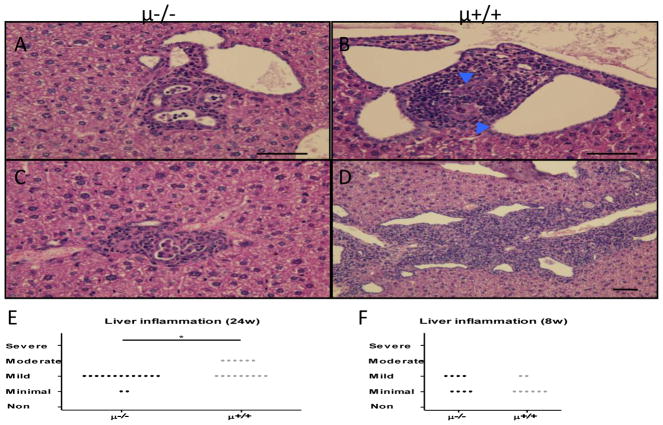
Hepatic peri-biliary inflammatory cell infiltrates were increased in B cell sufficient compared to B cell deficient NOD.c3c4 mice (Figure 5A–D). On the other hand, formation of granulomas, and bile duct damage, was observed in 30 to 40 % and 70 to 80 % respectively of examined liver samples in both strains. The degree of liver inflammation appeared to be age related. Thus, whereas the relative levels of liver inflammation were comparable in both strains at 8 weeks of age, there was a marked reduction at 24 weeks of age but only in the B cell deficient NOD.c3c4 mice (Figure 5E,F).
Figure 5.
Hepatic inflammation. Panels A, C and B, D showed representative histopathology liver inflammation of B cell deficient (μ−/−) and sufficient (μ+/+) NOD.c3c4 mice respectively. A. Mild lymphoid cell infiltration and slight ductal dilatation was observed in several portal tracts in μ−/− mice. Inflammatory cells were seen in the ductal lumen. B. Lymphocytic aggregation was observed around damaged interlobular bile ducts (arrowhead) in μ+/+ mice. C. Histiocytes aggregated in several portal tracts of μ−/− mice. D. Moderate portal inflammation and dilated bile ducts observed in enlarged portal tracts of μ+/+ mice. E. Liver inflammation was partly suppressed in B cell deficient mice at 24 weeks of age (μ−/−, n=15; μ+/+, n=15). F. No difference was observed in 8 week-old mice. (H&E staining. Black scale bars indicate 100 μm in A–F. *: p < 0.05, **: p < 0.01, ***: p < 0.001 in Mann-Whitney Test)
Salivary immunopathology
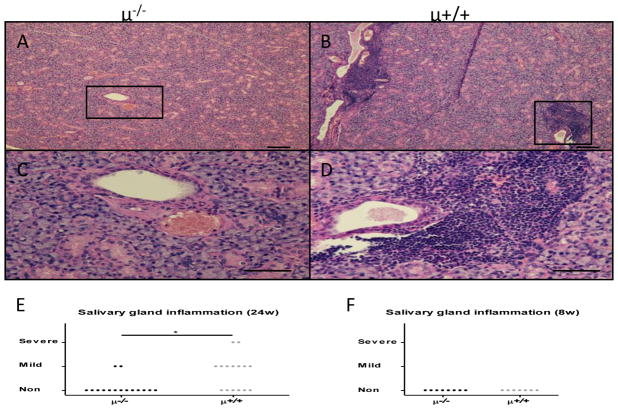
Studies of H&E stained salivary gland sections demonstrated a similar age related differences in the pathology as seen with the liver tissues. Thus while tissues from the B cell sufficient NOD.c3c4 mice showed mild to severe inflammation there was virtually undetectable inflammation in similar sections from the B cell deficient mice (Figure 6A–D). The degree of inflammation was significantly lower in Igμ−/− NOD.c3c4 mice at 24 weeks, but not at 8 weeks of age, compared to that of control mice (Figure 6E,F).
Figure 6.
Salivary gland inflammation. A–D. Representative histology showed inflammatory cell infiltrates around the salivary gland ducts in B cell competent mice whereas no inflammation was observed in B cell deficient mice. E. Degree of salivary gland inflammation was significantly lower in B cell deficient mice at 24 weeks of age (μ−/−, n=15; μ+/+, n=15). F. Salivary gland inflammation was not observed in either group at 8 weeks of age (μ−/−, n=8; μ+/+, n=7). (H&E staining. Scale bar 100 μm. *: p < 0.05 in Mann-Whitney Test)
Discussion
We demonstrate herein that genetically B cell deficient NOD.c3c4 mice showed decreased hepatic inflammation and biliary cyst formation compared to B cell competent NOD.c3c4 mice. Hepatic mononuclear cell infiltrates in B cell deficient NOD.c3c4 mice consisted of reduced numbers of activated and non-activated NK cells. In addition, markedly attenuated inflammation was observed in salivary glands of B cell deficient NOD.c3c4 mice. Thus B cells promote hepatic inflammation and biliary cystogenesis, in NOD.c3c4 mice, and B lymphocytes, (including those with specificity for PDC-E2), may enhance progression of inflammatory responses around bile ducts and in salivary glands.
Results from our recent studies provide evidence for the existence of a B cell subset with regulatory function i.e., Breg cells in dnTGF-βRII mice [9], a concept that is supported by studies of such Breg cells in a number of other autoimmune diseases[13, 14]. dnTGF-βRII mice display features of colitis, in addition to cholangitis [15], and since genetic B-cell depletion in dnTGF-βRII mice augments inflammation in the colon as well as the liver, Bregs are reasoned to be functional from the initiation to the progression of both colitis and autoimmune cholangitis in this strain. However, the existence of B cell subsets with opposing activities, i.e. promoting versus suppressing disease-progression, have been described in the murine EAE and lupus models [16, 17]. In this regard it is of interest to note that treatment of young dnTGF-βRII mice with anti-CD20 mAb led to the amelioration of liver inflammation, but exacerbated colon inflammation, suggesting that B cells may promote liver inflammation during the early disease phase in this strain [11]. These observations suggest the presence of B cell subsets with opposing function in autoimmune diseases including liver. IL-10 producing B cells have been reported as a primary suppressor B cell subset in EAE and murine lupus We suggest that an imbalance of IL-10 modulates not only suppressor but also other effector B cell pathways, and further that this modulation will vary according to the time and disease phase of the the autoimmune disease [16, 17]. Of note, our data herein demonstrate an amelioration of liver inflammation around bile ducts and salivary glands, suggesting a predominant proinflammatory effect of B cells in NOD.c3c4 mice from neonates through 24 weeks of age.
As described above, B cell deprivation in NOD.c3c4 mice demonstrated the amelioration of liver inflammation and biliary cyst formation (Figure 4). Since our recent report suggests that the early (3 week) development of an inflammatory response associated with common bile duct (CBD) dilatation was associated with the development of autoimmune biliary disease, [1], we examined the common bile duct but could not detect any difference in the levels of the CBD dilatation between these strains at either 8 or 24 weeks of age. Further, there was no biliary cyst formation in either strain at 8 weeks (Figure 4D,E), suggesting B cells might impact biliary cystogenesis after this time point. Flow cytometric analysis showed a significant reduction of the absolute numbers of activated (CD69-expressing) and non-activated NK cells in livers of B cell deprived NOD.c3c4 mice at 24 weeks of age (Figure 3). These findings suggest that increased number of activated NK cells is correlated with biliary cystogenesis in the B cell competent NOD.c3c4 mice. Similar to these findings, we have demonstrated increased number of hepatic NK cells in human PBC [18]. These findings suggest that the biliary cyst phenotype (i.e. the extensive proliferation of biliary epithelial cells) in these mice is at least partially a result of inflammation mediated by B, NK and other lymphocyte populations.
B cell deficient mice lack anti-PDC-E2 antibodies (Figure 2D), likely leading to an amelioration of bile duct damage due to reduction of PDC-E2 primed CD8+ T cells and NK cells that induce antibody-dependent cell-mediated cytotoxicity (ADCC) on biliary epithelia using anti-PDC-E2 [18–20]. B cell deprivation may reduce expression of CX3CL1 (Fractalkine) on biliary epithelia and attenuate adhesion of NK cells to those in NOD.c3c4 mice [21, 22] since CX3CL3 expression is upregulated on biliary epithelia in human PBC [23, 24]. However, the Fas-ligand Fas axis would rarely contribute to amelioration of biliary damage in B cell depleted NOD.c3c4 mice due to attenuated expression of Fas on intrahepatic bile ducts in this strain [25]. Further, proinflammatory cytokines would have a minor role in reduction of bile duct damage since hepatic levels of inflammatory cytokines including TNF-α and IFN-γ were very low or undetectable. Interestingly, IL-12p40 has been suggested as a therapeutic target in another mouse model for human PBC [26]. We note that in humans a unique triad comprised of monocyte-derived macrophages from human PBC patients, biliary epithelial apotopes, and AMA upregulate the production of inflammatory cytokines [27], however, B cell deprivation ablates serum AMA (Figure 2), and sequentially eliminates this possible mechanism of biliary duct damage in this strain. Further specific discussion regarding the specificity of the AMA and data regarding the breaking of tolerance have been extensively discussed elsewhere [28–33].
We have also demonstrated herein marked improvement of sialadenitis, suggesting B cells positively contribute to the progression of inflammatory responses in salivary glands in this model (Figure 6). Thus, these data suggest that B cells promote disease progression both in liver and in salivary glands of NOD.c3c4 mice. PBC patients frequently demonstrate sicca syndromes [34], and PBC sera react to apoptotic blebs and bodies of human epithelia of intrahepatic bile duct as well as salivary gland, but not to a variety of other cell lineages [35]. Immune complexes binding to apoptotic bodies may enhance priming of bile duct and salivary gland reactive CD8+ T cells through antigen presentation [19]. Also, NK cells may ligate immune complexes of PDC-E2/anti-PDC-E2 on the salivary epithelial cells, and damage them utilizing an ADCC effector mechanism.
In summary, these studies demonstrate that genetically determined B cell deprivation ameliorates hepatic inflammation and biliary cyst formation, accompanied with a reduced number of hepatic NK cells, and attenuates salivary gland inflammation in NOD.c3c4 mice. These data suggest that therapeutic B cell depletion may be a potent regulator of autoimmune biliary disease and salivary gland inflammation. However, since genetic B cell deprivation has distinct effects on the autoimmune liver diseases seen in NOD.c3c4 mice and dnTGF-βRII mice respectively [9], the findings suggest a need for further studies to establish the effects of the treatment of human autoimmune biliary disease by therapeutic B cell depletion on specific B cell subsets.
Acknowledgments
Financial Support: This work was supported by National Institute of Health grants (DK39588 and DK074768, M.E.G.; DK077961, Z.X.L.), UCD Center for Health and Nutrition Research Pilot Grant (Z.X.L.).
Abbreviations
- AMA
anti-mitochondrial autoantibodies
- CBD
common bile duct
- Idd
insulin dependent diabetes
- NK
natural killer
- NOD
nonobese diabetes
- PBC
primary biliary cirrhosis
- PDC
pyruvate dehydrogenase complex
- TGF-β
transforming growth factor-β
Footnotes
No conflicts of interest exist
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, Peterson LB, Leung PS, Cheng C, Mackay IR, Gershwin ME, Ridgway WM. NOD.c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203:1209–1219. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, Ridgway WM, Ansari AA, Coppel RL, Li MO, Flavell RA, Kronenberg M, Mackay IR, Gershwin ME. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. Journal of Immunology. 2006;177:1655–1660. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 3.Salas JT, Banales JM, Sarvide S, Recalde S, Ferrer A, Uriarte I, Oude Elferink RP, Prieto J, Medina JF. Ae2a,b-deficient mice develop antimitochondrial antibodies and other features resembling primary biliary cirrhosis. Gastroenterology. 2008;134:1482–1493. doi: 10.1053/j.gastro.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 4.Ueno Y, Ambrosini YM, Moritoki Y, Ridgway WM, Gershwin ME. Murine models of autoimmune cholangitis. Curr Opin Gastroenterol. 2010;26:274–279. doi: 10.1097/MOG.0b013e32833755aa. [DOI] [PubMed] [Google Scholar]
- 5.Ueno Y, Moritoki Y, Shimosegawa T, Gershwin ME. Primary biliary cirrhosis: what we know and what we want to know about human PBC and spontaneous PBC mouse models. J Gastroenterol. 2007;42:189–195. doi: 10.1007/s00535-007-2019-y. [DOI] [PubMed] [Google Scholar]
- 6.Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, Yang GX, Ridgway W, Ueno Y, Ansari AA, Coppel RL, Mackay IR, Gershwin ME. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44:1240–1249. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 7.Joshi S, Cauch-Dudek K, Heathcote EJ, Lindor K, Jorgensen R, Klein R. Antimitochondrial antibody profiles: are they valid prognostic indicators in primary biliary cirrhosis? Am J Gastroenterol. 2002;97:999–1002. doi: 10.1111/j.1572-0241.2002.05620.x. [DOI] [PubMed] [Google Scholar]
- 8.Neuberger J. Liver transplantation for primary biliary cirrhosis: Indications and risk of recurrence. Journal of Hepatology. 2003;39:142–148. doi: 10.1016/s0168-8278(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 9.Moritoki Y, Zhang W, Tsuneyama K, Yoshida K, Wakabayashi K, Yang GX, Bowlus C, Ridgway WM, Ueno Y, Ansari AA, Coppel RL, Mackay IR, Flavell RA, Gershwin ME, Lian ZX. B Cells Suppress the Inflammatory Response in a Mouse Model of Primary Biliary Cirrhosis. Gastroenterology. 2008 doi: 10.1053/j.gastro.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Koarada S, Wu Y, Fertig N, Sass DA, Nalesnik M, Todd JA, Lyons PA, Fenyk-Melody J, Rainbow DB, Wicker LS, Peterson LB, Ridgway WM. Genetic control of autoimmunity: protection from diabetes, but spontaneous autoimmune biliary disease in a nonobese diabetic congenic strain. J Immunol. 2004;173:2315–2323. doi: 10.4049/jimmunol.173.4.2315. [DOI] [PubMed] [Google Scholar]
- 11.Moritoki Y, Lian ZX, Lindor K, Tuscano J, Tsuneyama K, Zhang W, Ueno Y, Dunn R, Kehry M, Coppel RL, Mackay IR, Gershwin ME. B-cell depletion with anti-CD20 ameliorates autoimmune cholangitis but exacerbates colitis in transforming growth factor-beta receptor II dominant negative mice. Hepatology. 2009;50:1893–1903. doi: 10.1002/hep.23238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Sharma R, Ju ST, He XS, Tao Y, Tsuneyama K, Tian Z, Lian ZX, Fu SM, Gershwin ME. Deficiency in regulatory T cells results in development of antimitochondrial antibodies and autoimmune cholangitis. Hepatology. 2008;49:545–552. doi: 10.1002/hep.22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- 14.Mizoguchi A, Bhan AK. A case for regulatory B cells. Journal of Immunology. 2006;176:705–710. doi: 10.4049/jimmunol.176.2.705. [DOI] [PubMed] [Google Scholar]
- 15.Gorelik L, Flavell RA. Abrogation of TGFβ signaling in T cells leads to spontaneous T cell differentiation and autoimmune disease. Immunity. 2000;12:171–181. doi: 10.1016/s1074-7613(00)80170-3. [DOI] [PubMed] [Google Scholar]
- 16.Haas KM, Watanabe R, Matsushita T, Nakashima H, Ishiura N, Okochi H, Fujimoto M, Tedder TF. Protective and pathogenic roles for B cells during systemic autoimmunity in NZB/W F1 mice. J Immunol. 2010;184:4789–4800. doi: 10.4049/jimmunol.0902391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, Gershwin ME. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26:232–240. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Kita H, Lian ZX, Van de Water J, He XS, Matsumura S, Kaplan M, Luketic V, Coppel RL, Ansari AA, Gershwin ME. Identification of HLA-A2-restricted CD8(+) cytotoxic T cell responses in primary biliary cirrhosis: T cell activation is augmented by immune complexes cross-presented by dendritic cells. J Exp Med. 2002;195:113–123. doi: 10.1084/jem.20010956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takai T. Multiple loss of effector cell functions in FcR gamma-deficient mice. Int Rev Immunol. 1996;13:369–381. doi: 10.3109/08830189609061759. [DOI] [PubMed] [Google Scholar]
- 21.Goda S, Imai T, Yoshie O, Yoneda O, Inoue H, Nagano Y, Okazaki T, Imai H, Bloom ET, Domae N, Umehara H. CX3C-chemokine, fractalkine-enhanced adhesion of THP-1 cells to endothelial cells through integrin-dependent and -independent mechanisms. J Immunol. 2000;164:4313–4320. doi: 10.4049/jimmunol.164.8.4313. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T, Imai H, Yoshie O, Bloom ET, Domae N, Umehara H. Fractalkine-mediated endothelial cell injury by NK cells. J Immunol. 2000;164:4055–4062. doi: 10.4049/jimmunol.164.8.4055. [DOI] [PubMed] [Google Scholar]
- 23.Shimoda S, Harada K, Niiro H, Taketomi A, Maehara Y, Tsuneyama K, Kikuchi K, Nakanuma Y, Mackay IR, Gershwin ME, Akashi K. CX3CL1 (fractalkine): a signpost for biliary inflammation in primary biliary cirrhosis. Hepatology. 2010;51:567–575. doi: 10.1002/hep.23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Isse K, Harada K, Zen Y, Kamihira T, Shimoda S, Harada M, Nakanuma Y. Fractalkine and CX3CR1 are involved in the recruitment of intraepithelial lymphocytes of intrahepatic bile ducts. Hepatology. 2005;41:506–516. doi: 10.1002/hep.20582. [DOI] [PubMed] [Google Scholar]
- 25.Nakagome Y, Ueno Y, Kogure T, Fukushima K, Moritoki Y, Ridgway WM, Eric Gershwin M, Shimosegawa T. Autoimmune cholangitis in NOD.c3c4 mice is associated with cholangiocyte-specific Fas antigen deficiency. J Autoimmun. 2007;29:20–29. doi: 10.1016/j.jaut.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, Ansari AA, Okazaki K, Lian ZX, Coppel RL, Mackay IR, Gershwin ME. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, Ansari AA, Coppel RL, Worman HJ, Gores GJ, Gershwin ME. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010 doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung PS, Chuang DT, Wynn RM, Cha S, Danner DJ, Ansari A, Coppel RL, Gershwin ME. Autoantibodies to BCOADC-E2 in patients with primary biliary cirrhosis recognize a conformational epitope. Hepatology. 1995;22:505–513. [PubMed] [Google Scholar]
- 29.Moteki S, Leung PS, Dickson ER, Van Thiel DH, Galperin C, Buch T, Alarcon-Segovia D, Kershenobich D, Kawano K, Coppel RL, et al. Epitope mapping and reactivity of autoantibodies to the E2 component of 2-oxoglutarate dehydrogenase complex in primary biliary cirrhosis using recombinant 2-oxoglutarate dehydrogenase complex. Hepatology. 1996;23:436–444. doi: 10.1002/hep.510230307. [DOI] [PubMed] [Google Scholar]
- 30.Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, Barsky D, Ansari AA, Coppel RL, Mackay IR, Gershwin ME. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Liu H, Norman GL, Shums Z, Worman HJ, Krawitt EL, Bizzaro N, Vergani D, Bogdanos DP, Dalekos GN, Milkiewicz P, Czaja AJ, Heathcote EJ, Hirschfield GM, Tan EM, Miyachi K, Bignotto M, Battezzati PM, Lleo A, Leung PS, Podda M, Gershwin ME, Invernizzi P. PBC screen: an IgG/IgA dual isotype ELISA detecting multiple mitochondrial and nuclear autoantibodies specific for primary biliary cirrhosis. J Autoimmun. 2010;35:436–442. doi: 10.1016/j.jaut.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Tsuda M, Torgerson TR, Selmi C, Gambineri E, Carneiro-Sampaio M, Mannurita SC, Leung PS, Norman GL, Gershwin ME. The spectrum of autoantibodies in IPEX syndrome is broad and includes anti-mitochondrial autoantibodies. J Autoimmun. 2010;35:265–268. doi: 10.1016/j.jaut.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Juran BD, Lazaridis KN. Update on the genetics and genomics of PBC. J Autoimmun. 2010;35:181–187. doi: 10.1016/j.jaut.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leuschner U. Primary biliary cirrhosis--presentation and diagnosis. Clin Liver Dis. 2003;7:741–758. doi: 10.1016/s1089-3261(03)00101-6. [DOI] [PubMed] [Google Scholar]
- 35.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, Gores GJ, Ansari AA, Van de Water J, Gershwin ME. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49:871–879. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]