Abstract
Background
Both long and short sleep duration have been associated with obesity, cardiovascular disease, and diabetes. However, there have been no previous studies investigating the potential relationship between altered sleep duration and allergen sensitization.
Objective
To explore the association between sleep duration and sensitization to food and aeroallergens.
Methods
This study includes 1534 rural Chinese adolescent twins aged 12 to 21 years who completed standard sleep questionnaires and skin prick tests (SPTs) to 9 food and 5 aeroallergens. Total sleep time was defined as the interval from bedtime to wake-up time minus sleep latency. Sensitization was defined as having at least one positive SPT.
Results
Compared to individuals with the highest (3rd) tertile of sleep duration, those who slept less were more likely to be sensitized to any food allergen with odds ratios (ORs) of 1.9 (95% confidence interval(CI):1.3–2.7) and 1.4 (95%CI:1.0–1.9) for the 1st and 2nd tertiles (trend test Ptrend=3×10−4), respectively. The corresponding ORs for sensitization to any aeroallergen were 1.5 (95%CI: 1.1–2.0) and 1.3 (95%CI:1.0–1.7) (Ptrend=8×10−3). These associations were independent of percent body fat. In addition, we observed a significant dose-response association between the number of positive SPTs and percentage of shortest sleep duration (1st tertile) (Ptrend=1×10−3).
Conclusions and Clinical Relevance
In this sample of relatively lean rural Chinese adolescents, we found that short sleep duration was associated with increased risk of sensitization to food and aeroallergens, independent of percent body fat. Longitudinal studies are needed to further determine the temporal and causal relationships. If short sleep duration indeed is one of the risk factors for allergic sensitization, the global burden of allergic diseases could be dramatically reduced by providing appropriate guidance on sleep duration for youth.
Keywords: sleep duration, skin prick test, allergen, sensitization, adolescent
INTRODUCTION
Allergic diseases, including atopic dermatitis, food allergy, allergic rhinitis, and asthma are common causes of morbidity worldwide [1–4], and have become more prevalent in the past decades [4–8]. Allergic sensitization is considered a harbinger of, or a prerequisite for, many allergic disorders and is usually defined as having detectable allergen specific IgE either by way of in vivo (skin prick tests [SPTs]) or in vitro methods. The etiology of allergic sensitization to food and aeroallergens is not fully understood. In light of the rapid rise of allergic diseases, identification of modifiable risk factors for allergic sensitization and diseases is of great clinical and public health importance.
One such potentially modifiable risk factor is short sleep duration. The mean sleep time among American adults has declined from 8.5 to 6.9 hours per night over the last 40 years [9, 10]. Similarly, sleep duration in schoolchildren (especially adolescents) also has decreased [11, 12]. This change appears in parallel with an increased prevalence of westernized diseases, such as obesity, cardiovascular disease, diabetes, and cancer [13–17]. Numerous epidemiologic studies have indicated that altered sleep duration (especially inadequate sleep) plays an important role in overall human health and disease, with an impact on metabolism[18,19], diabetes[20], immune function[21,22], and mortality[23]. Possible mechanisms may be due to the detrimental effects of sleep deprivation on metabolic [24] and immune regulation [25], both of which may influence the development of allergic sensitization and/or diseases, although no studies to date have been conducted to investigate the potential association between sleep duration and allergic sensitization.
Most previous studies on the relationship between short sleep duration and health outcomes were conducted in adults. Few such studies were conducted in adolescents [26] and none in relatively lean rural Chinese adolescents. Adolescents are at a particularly high risk for short sleep duration due to biological factors such as developmentally-adjusting circadian rhythms, as well as increased social and educational demands [27]. It is well-recognized that the prevalence of allergic disease is higher in adolescents than in adults [28, 29]. Collectively, this provides a compelling rationale to examine the effect of short sleep duration on allergic sensitization in adolescents.
Using a large rural Chinese adolescent cohort, the central focus of our study was to investigate the potential relationship of short sleep duration and allergic sensitization to common food allergens and aeroallergens among adolescents. Additionally, since previous studies have suggested that both sleep duration and allergic sensitization are associated with adiposity [16, 30], we also examined whether the relationship of short sleep duration and allergic sensitization was partially mediated by percentage body fat.
METHODS
Study Sample
This study used data obtained from an ongoing NIH-funded prospective study on precursors of metabolic syndrome in children in a large Chinese twin cohort. The baseline study was carried out in 8 counties of the Anqing region of China from 1998 to 2000, and detailed information for the baseline recruitment has been reported previously [31]. The follow-up study was conducted from 2005 to 2008. Participants were invited to a central office to complete the questionnaire interview (which included items about sleep, home environment, smoking and history of breastfeeding). In addition, participants underwent physical examinations, SPTs, anthropometric measurements, and DEXA scans. This study focused on participants aged 12 to 21 years with complete information on sleep questionnaires and SPTs at the follow-up survey. All subjects enrolled in this study provided informed consent. The study protocol was approved by the Institutional Review Boards at Children's Memorial Hospital and the Institute of Biomedicine, Anhui Medical University in Hefei, China.
Anthropometry and Puberty
Height and weight were measured using standard protocols. Height was measured to the nearest 0.1 cm on a portable stadiometer. Weight was measured to the nearest 0.1 kg with the subjects standing motionless on a scale. Each anthropometric measure was repeated three times, and the mean value was used for subsequent analyses.
Dual Energy X-ray Absorptiometry Measurements
Standard whole-body scans were performed by dual energy X-ray absorptiometry densitometer (DEXA, GE-Lunar Prodigy, USA) to measure total body fat and three regional fat and lean compositions, including the trunk (chest, abdomen and pelvis), arms, and legs. A standard software calculation [32] was used to calculate total body fat, which was measured with a Lunar DPXL instrument (Madison, WI, USA). Percent body fat was calculated as (total body fat/ body weight) × 100.
Skin Prick Tests (SPTs)
Detailed descriptions of SPTs for this twin cohort have been reported previously [33]. Briefly, the SPT was performed on the volar surfaces of the arms on normal skin using the Multi-Test II (Lincoln Diagnostics, Decatur, IL). Subjects were tested to 14 allergens, including 9 foods (cow milk, egg white, soybean, wheat, peanut, black walnut, sesame seed, fish mix [cod, flounder, halibut, mackerel, tuna], shellfish mix [clam, crab, oyster, scallops, shrimp]), 5 aeroallergens (Alternaria tenuis, cat hair, dog epithelia, house dust mite mix [equal parts mixture of Dermatophagoides farinae and Dermatophagoides pteronyssinus], and cockroach mix [American and German cockroach]), positive control (histamine, 1.0 mg/mL) (Greer, Lenoir, NC) and negative control (50% glycerinated saline). The largest wheal diameter and perpendicular wheal diameter were measured 15 minutes after application. The average values computed from the largest wheal diameter and perpendicular wheal diameter were used for the subsequent analyses. SPT was considered invalid and excluded if the wheal diameter of the negative control was greater than 3 mm, or the difference of histamine minus saline was 3 mm or less. Among valid SPTs, a positive SPT was defined if the mean wheal diameter was 3 mm or greater than the saline control. “Any sensitization”, “any sensitization to food”, and “any sensitization to aeroallergens” was defined as a positive SPT to at least one of the 14 allergens; at least one of the 9 food allergens; and one of the 5 aeroallergens tested, respectively.
Sleep Parameter Measurements
Sleep duration was assessed by standard sleep questionnaires and diaries. A Chinese translation of the Pittsburgh Sleep Quality Index (PSQI) questionnaire, which has been proven to have excellent sensitivity and reliability [34], was used to obtain average sleep characteristics over the month prior to the interview. The major questions in the PSQI about sleep duration were as follows:
“During the last month, what time did you usually go to bed for sleep?”
“During the last month, how long did it take for you to fall asleep (after going to bed) each night?” (i.e. sleep latency)
“During the last month, what time did you usually wake up in the morning?”
“During the last month, how many hours did you actually sleep during the night?”
Children under 18 years old were asked to answer these questions first. Their parents were then asked to confirm and verify their children’s’ answers during a subsequent joint interview. Sleep diaries were obtained for 4–7 consecutive days from the date of the interview.
In the sleep diary, all subjects were asked to answer the following questions:
“What time did you wake up today?”
“What time did you go to bed for sleep last night?”
“How long did it take for you to fall asleep (after going to bed) last night?”
“How many hours did you sleep last night?”
In addition, pre-sleep activities, defined as anything done within one hour before bedtime, were also recorded by participants.
In this report, total sleep time was defined using sleep parameters including bedtime at night, wake-up time in the morning, and sleep latency (i.e. total sleep time (hours) = wake-up time in the morning - bedtime at night - sleep latency time) obtained via sleep questionnaires.
Statistical Analysis
The major outcomes included sensitization to any food allergen and any aeroallergen, as well as sensitization to a specific allergen if its prevalence was 10% and above. We presented the mean anthropometric measures and the frequencies of epidemiological factors by sensitization status (yes vs. no). We then compared the differences by fitting generalized estimating equations (GEE) to adjust for the correlation within each twin pair.
Sleep duration status was grouped into general tertiles or age group, gender-specific tertiles; the lowest tertile was defined as having short sleep duration. We applied GEE models to examine the associations between tertiled sleep duration and allergen sensitization. We first fitted Model 1 to adjust for covariates that are potential risk factors for allergic sensitization or allergic disease [35], including age, gender, education (primary school or lower, junior high (middle school, high school or higher), occupation (student/non-student), visible cockroaches in the home (none, occasional, some, or many), tobacco exposure (active/passive), and birth order. To evaluate whether the relationship of sleep duration and allergic sensitization was partially mediated by body fat, we fitted Model 2 by including percent body fat (age, gender-specific tertiles) and the covariates in Model 1. Finally, we examined the effect of short sleep duration on the number of allergen sensitizations (i.e. number of positive SPTs: sensitization to zero, one, two, three, and four or more allergens). All analyses were performed using SAS software, version 9.0 (SAS Institute, Cary, North Carolina).
RESULTS
Characteristics of the study subjects
A total of 1,534 participants aged 12 to 21 years were included in the present study. The number of subjects who were sensitized to any food allergen, any aeroallergen, and none of the 14 common allergens was 379 (24.7%), 651 (42.4%), and 759 (49.5%), respectively. Among these common allergens, sensitization to four specific allergens (shellfish, peanut, house dust mite, and cockroach) each had a prevalence of 10% and above (Supplemental Table I), and thus were tested for the associations with sleep duration indicated below. Table 1 shows the general characteristics of the subjects by sensitization status. Compared with twins who had no allergen sensitization, those sensitized to any food allergen were younger (16.3 vs. 16.7 years) and also had a lower BMI (19.0 vs. 19.4 Kg/m2) (p<0.05). This difference was not seen for the sensitization to aeroallergens; instead, twins who were sensitized to any aeroallergen were taller (158.0 vs. 156.1 cm) (p<0.01) and heavier (48.7 vs. 47.4 Kg) (p<0.001) than non-sensitized twins. The prevalence of any aeroallergen sensitization was higher in boys than in girls (p<0.01). There were no significant differences between the non-sensitized group and the groups with any food sensitization and any aeroallergen sensitization with regard to percent body fat, smoking status, breastfeeding, and the presence of pets, mice, farm animals, or cockroaches in the home. In addition, the subgroup of subjects sensitized to both food and aeroallergen had demographic characteristics that were similar to the non-sensitized controls. We also performed analyses to examine the associations of those factors with sleep duration (Supplemental Table II). Compared with subjects who had longer sleep duration, those with short sleep duration and medium sleep duration had higher weight, height, education level, and were more likely to be students. Additionally, the subjects with short sleep duration were slightly older than those with longer sleep duration.
Table I.
Characteristics of Chinese Adolescents.
| Variables | Any food allergen sensitization | Any aeroallergen sensitization | Both Food & aeroallergen sensitization | No sensitization |
|---|---|---|---|---|
| N=379 | N=651 | N=261 | N=759 | |
| Mean±SD | ||||
| Age (Yr) | 16.3 ± 2.1* | 16.8 ±2.1 | 16.6 ±2.1 | 16.7 ±2.1 |
| Weight (kg) | 46.8±8.3 | 48.7 ± .1** | 47.9 ± 8.4 | 47.4 ± 7.5 |
| Height (cm) | 156.6 ± 8.1 | 158.0 ± 8.2*** | 157.3 ±8.5 | 156.1 ± 7.9 |
| BMI (kg/m2) | 19.0± 2.5* | 19.4 ± 2.4 | 19.3 ± 2.5 | 19.4 ± 2.3 |
| Percent body fat | 18.7±9.3 | 18.2 ±9.4 | 19.1±9.3 | 19.2±9.7 |
| N (%) | ||||
| Sex | ||||
| Male | 212(55.9) | 389(59.8)** | 146(55.9) | 383(50.5) |
| Female | 167(44.1) | 262(40.2) | 115(44.1) | 376(49.5) |
| Education | ||||
| Primary school and lower | 44(11.6) | 51(7.8) | 27(10.3) | 71(9.4) |
| Junior High/middle school | 229(60.7) | 392(60.2) | 152(58.2) | 467(61.5) |
| High school and higher | 105(27.7) | 208(32.0) | 82(31.4) | 221(29.1) |
| Occupation | ||||
| Students | 276(72.8)* | 443(68.1) | 185(70.9) | 499(65.7) |
| Others | 103(27.2) | 208(31.9) | 76(29.1) | 260(34.3) |
| Smoking Status | ||||
| Non-Smoking | 91 (24.0) | 132 (20.3) | 61(23.4) | 155(20.4) |
| Current or passive Smoking | 288 (76.0) | 519 (79.7) | 200(76.6) | 604 (79.6) |
| Breastfeeding | ||||
| No | 157(44.4) | 276(42.4) | 110(42.1) | 322(42.4) |
| Yes | 222 (58.6) | 375 (57.6) | 151(57.9) | 437 (57.6) |
| Pet at home | ||||
| No | 163(43.0) | 308(47.3) | 118(45.2) | 334(44.0) |
| Yes | 216 (57.0) | 343(52.7) | 143(54.8) | 425 (56.0) |
| Farm animal | ||||
| No | 95(25.1) | 165(25.3) | 62(23.7) | 168(22.1) |
| Yes | 284(74.9) | 486(74.7) | 199(76.3) | 591(77.9) |
| Mice at home | ||||
| No | 117 (30.9) | 218 (33.5) | 80(30.7) | 251 (33.1) |
| Yes, Occasionally | 175 (46.2) | 283 (43.5) | 123(47.1) | 347 (45.7) |
| Yes, Some or Many | 87 (23.0) | 150 (23.0) | 58(22.2) | 161 (21.2) |
| Cockroach at home | ||||
| No | 222 (58.6) | 411 (63.1) | 151(57.8) | 480 (63.2) |
| Yes, Occasionally | 119 (31.4) | 191 (29.3) | 85(32.6) | 224 (29.5) |
| Yes, Some or Many | 38 (10.0) | 49 (7.5) | 25(9.6) | 55 (7.3) |
| Birth order | ||||
| First | 196(51.7) | 332(51.0) | 135(51.7) | 373(49.1) |
| Second | 183(48.3) | 319(49.0) | 126(48.3) | 386(50.9) |
GEE model was used to test the differences between no sensitization group and any food allergen sensitization group, any aeroallergen sensitization group, both food and aeroallergen sensitization group;
ii
p<0.05;
p<0.01;
p<0.001.
Distribution of sleep duration
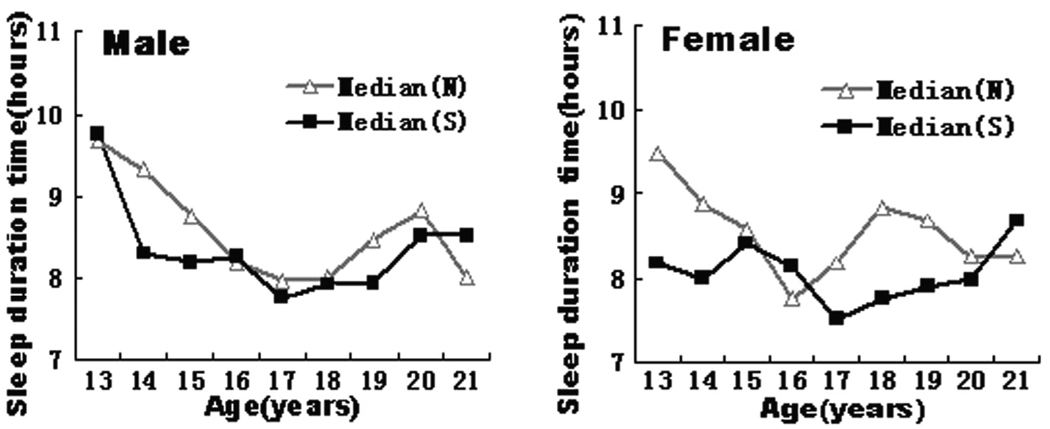
The distributions of median sleep duration vs. age by allergic sensitization status and gender were illustrated in Figure 1. Of note, there were only four subjects who were 12 years of age in this study population; these four subjects were merged to the age 13 group when we plotted Figure 1. Compared to the non-sensitization group, most of the age-specific medians of sleep duration were lower in the allergic sensitization group. Additionally, the ranges of sleep duration tertiles were 3.0 to 7.8 hours (T1:1st tertile), 7.8 to 8.9 hours (T2:2nd tertile), and 8.9 to 14.7 hours (T3:3rd tertile), respectively.
Figure.1. Age-specific median sleep duration by sensitization status in male (left) and female (right).
S denotes the allergic sensitization group; N denotes the non-allergic-sensitization group. Four subjects who were 12 years-old were grouped with those aged 13.
Relationship between sleep duration and allergen sensitization to food and aeroallergens
As shown in Tables 2 and 3, there were dose-response relationships between sleep duration (grouped by general tertiles) and allergen sensitization to any food allergen, any aeroallergen, and four common allergens. Compared with those having the highest tertile of sleep duration, twins who slept less were more likely to be sensitized to any food allergen with ORs of 1.9 (95% confidence interval(CI):1.3–2.7) and 1.4 (95%CI:1.0–1.9) for the 1st and 2nd tertiles, respectively (p-trend=3 × 10−4, model 1) (Table 2). Similarly, the risk of being sensitized to any aeroallergen increased with decreasing sleep duration (2nd tertile: 1.3(95%CI: 1.0–1.7); p-trend=8×10−3; 1st tertile: 1.5 (95%CI:1.1–2.0) (Table 3). These dose-response patterns were also observed for sensitization to peanut, shellfish, house dust mites, and cockroach. Finally, the associations persisted after adjusting for percent body fat (Model 2 in Table 2 and Table 3). Note that the results did not substantially change when we analyzed age group, gender-specific sleep duration tertiles compared to general sleep duration tertiles (Supplemental Table III and IV) or when we excluded subjects with current or prior allergic diseases or sleep disorders (Supplemental Table V and VI).
Table II.
Association of sleep duration with sensitization to food allergens.
| Sleep duration groups |
Sensitization to shellfish | Sensitization to peanut | Sensitization to any food allergen | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | OR(95% CI) | P value | N (%) | OR(95% CI) | P value | N (%) | OR(95% CI) | P value | |
| MODEL1 | |||||||||
| T3 | 68(19.6) | Reference | 45(13.9) | Reference | 105(27.3) | Reference | |||
| T2 | 82(25.3) | 1.4(1.0–2.1) | 0.083 | 69(22.2) | 1.8(1.1–2.8) | 0.012 | 123(33.7) | 1.4(1.0–1.9) | 0.060 |
| T1 | 99(29.4) | 1.9(1.3–2.9) | 0.0009 | 75(24.0) | 2.4(1.5–3.8) | 0.002 | 151(38.8) | 1.9(1.3–2.7) | 0.0003 |
| Trend | P=0.0009 | P=0.002 | P=0.0003 | ||||||
| MODEL2 | |||||||||
| T3 | 68(19.6) | Reference | 45(13.9) | Reference | 105(27.3) | Reference | |||
| T2 | 82(25.3) | 1.3(0.9–2.0) | 0.137 | 69(22.2) | 1.7(1.1–2.7) | 0.011 | 123(33.7) | 1.3(0.90–1.8) | 0.121 |
| T1 | 99(29.4) | 1.9(1.3–2.9) | 0.001 | 75(24.0) | 2.1(1.3–3.38) | 0.002 | 151(38.8) | 1.9(1.3–2.6) | 0.0004 |
| Trend | P=0.001 | P=0.002 | P=0.0004 | ||||||
GEE model was used to test the associations between sleep duration groups and sensitization to food allergens;
All subjects were divided into three groups by sleep duration tertiles (3.0 to 7.8hours ( T1: 1st tertile), 7.8 to 8.9 hours(T2:2nd tertile), and 8.9 to 14.7 hours(T3:3rd tertile));
Model 1 was adjusted with age, sex, occupation, education, cockroach at home, smoking status, and birth order;
Model 2 was further adjusted with percent body fat besides the covariates adjusted in model 1.
Table III.
Association of Sleep Duration with Sensitization to Aeroallergens.
| Sleep duration groups |
Sensitization to dust mites | Sensitization to cockroach | Sensitization to any aeroallergen | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | OR(95% CI) | P value | N (%) | OR(95% CI) | P value | N (%) | OR(95% CI) | P value | |
| MODEL1 | |||||||||
| T3 | 146(34.4) | Reference | 141(33.6) | Reference | 192(40.8) | Reference | |||
| T2 | 169(41.1) | 1.3(1.0–1.8 | 0.072 | 143(37.1) | 1.2(0.9–1.6) | 0.314 | 215(47.1) | 1.3(1.0–1.7) | 0.091 |
| T1 | 202(45.9) | 1.6(1.2–2.2) | 0.003 | 168(41.4) | 1.4(1.0–2.0) | 0.028 | 244(50.6) | 1.5(1.1–2.0) | 0.008 |
| Trend | P=0.003 | P=0.028 | P=0.008 | ||||||
| MODEL2 | |||||||||
| T3 | 146(34.4) | Reference | 141(33.6) | Reference | 192(40.8) | Reference | |||
| T2 | 169(41.1) | 1.4(1.0–1.8) | 0.105 | 143(37.1) | 1.2(0.9–1.6) | 0.356 | 215(47.1) | 1.3(1.0–1.7) | 0.106 |
| T1 | 202(45.9) | 1.7(1.3–2.4) | 0.004 | 168(41.4) | 1.6(1.2–2.3) | 0.031 | 244(50.6) | 1.6(1.2–2.2) | 0.009 |
| Trend | P=0.004 | P=0.031 | P=0.009 | ||||||
GEE model was used to test the associations between sleep duration groups and sensitization to aero allergens;
All subjects were divided into three groups by sleep duration tertiles (3.0 to 7.8 hours ( T1: 1st tertile), 7.8 to 8.9 hours(T2:2nd tertile), and 8.9 to 14.7 hours(T3:3rd tertile));
Model 1 was adjusted with age, sex, occupation, education, cockroach at home, smoking status, and birth order;
Model 2 was further adjusted with percentage body fat besides the covariates adjusted in model1.
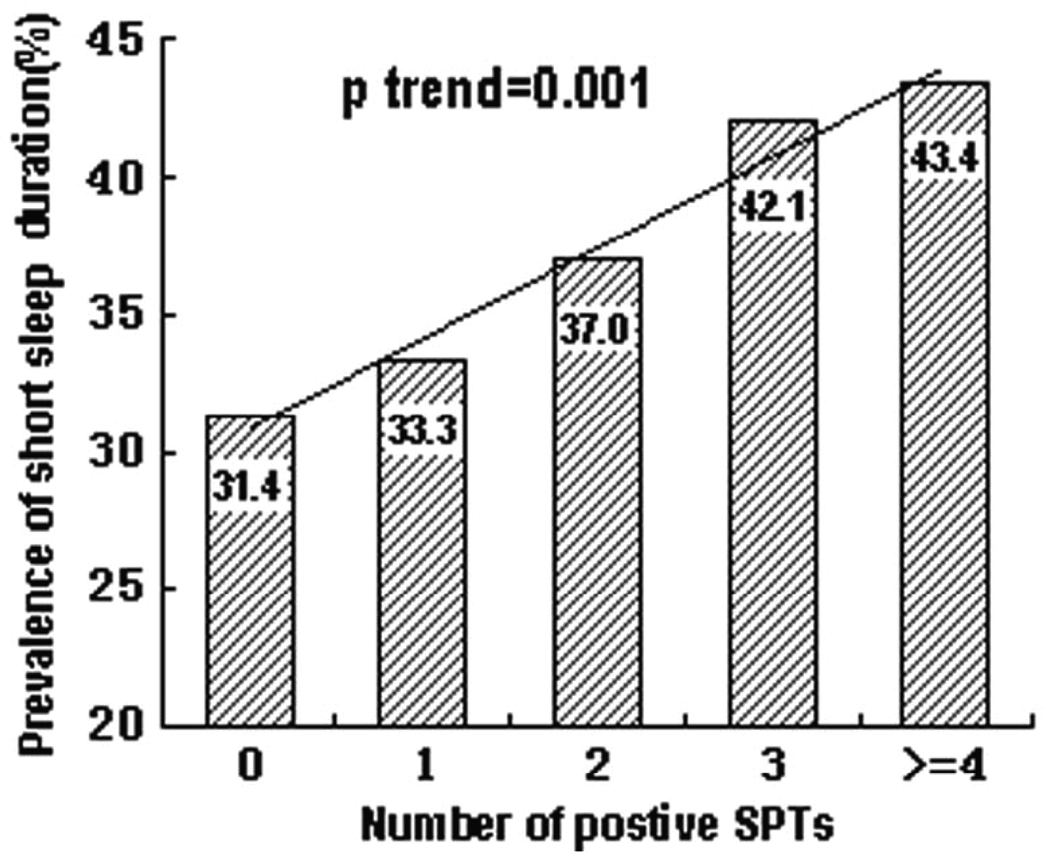
Relationship between short sleep duration and the number of sensitizations
We further investigated the relationship between short sleep duration and the number of sensitizations (i.e. number of positive SPTs) after adjusting for covariates in Model 2. As shown in Figure 2, twins with more positive SPTs were more likely to have a higher percentage of short sleep duration. This positive linear trend was statistically significant (p-trend=1×10−3).
Figure.2. Dose-response effect of allergen sensitization on prevalence of short sleep duration.
GEE model was used to test the associations of short sleep duration and allergen sensitization with adjustment for age, sex, occupation, education, cockroach at home, smoking status, birth order and percent body fat ;Short sleep duration was defined as the 1st tertile of sleep duration tertiles (I.e. bottom tertile, 3.0 to 7.8 hours).
DISCUSSION
To our knowledge, this is the first study to investigate the potential relationship between sleep duration and allergen sensitization in a large population. We demonstrated not only that short sleep duration was associated with increased sensitization to food allergens and aeroallergens, but also that such an association appeared to occur in a dose-response manner and was independent of percent body fat. As compared to adults and urban or western counterparts, this is a relatively lean rural population [30], thus, it is less likely to be confounded by obesity and obesity-associated sleep disorders, and urban factors that could adversely affect the sleep environment. Although this study is limited by its cross-sectional study design, the finding on the association between sleep duration and allergen sensitization is intriguing. It raises the possibility that short sleep duration may be a modifiable risk factor for allergic sensitization or future development of allergic diseases.
Inadequate sleep is common in modern societies [9, 10]. Evidence from the National Sleep Foundation Survey in the U.S. indicated that 45% of adolescents slept less than 8 hours on school nights, and 78% reported that they needed more than 8 hours of sleep to feel their best [36]. Compared to their U.S. counterparts, Chinese adolescents reported later bedtimes, earlier rise times, and shorter sleep duration [37]. Our previous study reported that later bedtime coupled with earlier wake-up time associated with academic demand appeared to be important contributors to sleep loss among this population, in which the majority was school-age students [12]. Both epidemiological and experimental studies have shown that short sleep duration is associated with an increased risk of developing diabetes [14, 38], obesity [39, 40], and cardiovascular disease [13, 41], with evidence of influence on immune function. To our knowledge, however, no previous study has examined the relationship between short sleep duration and IgE-mediated allergen sensitization.
In the present study, we demonstrated a dose-response relationship between short sleep duration and allergen sensitization to food and aeroallergens. Allergen sensitization was more prevalent among those in the lower sleep duration tertiles compared to those in the highest sleep duration tertile. These associations were not substantially changed when we adjusted for percentage body fat and other covariates, such as age, occupation, education, cockroach at home, smoking status, and birth order. More interestingly, we found a linear relationship between short sleep duration and the number of positive SPTs.
Although the biologic mechanisms underlying this finding remain unclear, the immune alterations due to sleep deprivation may contribute to the development of allergen sensitization. Prior studies have demonstrated that sleep deprivation affects immune function, including an increased susceptibility to infections [25, 42]. Leeuwen et al [43] reported that sleep restriction resulted in an elevated production of pro-inflammatory cytokines (e.g., IL-1β, IL-6, and IL-17), which play important roles in immune defense and tissue damage [44–46]. So far, the results related to the effect of sleep deprivation on immunoglobulin level are not well understood in humans. One study reported that immunoglobulin level increases with sleep deprivation [47], while another study suggested that sleep deprivation decreased the immunoglobulin level [48]. However, most of these studies were based on short periods of sleep loss. The effects of long-term sleep deprivation on B-cell proliferation and IgE production have not yet been elucidated. It is possible that the influence of sleep duration on immune function varies according to the degree of sleep deprivation. For example, natural killer-cell activity has been reported to decrease after partial night sleep deprivation [49, 50] and increase during prolonged sleep deprivation [51].
Our findings should be interpreted in the context of this study. Total sleep duration was calculated based on sleep questionnaires, which, we believe, reflect long-term sleep habits. Due to the cross-sectional nature of our study, we cannot determine the causal relationship between short sleep duration and allergen sensitization, which may be bi-directional. However, in this study population we speculate that short sleep duration more likely led to allergen sensitization rather than vice versa for the following reasons. Our previous report showed that there was a low prevalence of allergic diseases in this population despite a high prevalence of sensitization [25], and thus the vast majority of sensitized subjects were clinically asymptomatic. Furthermore, we also obtained similar results when the subjects with current or prior allergic diseases or sleep disorders were excluded from the analyses.
There were several limitations in our study. Firstly, although we tested the most common food and indoor allergens among our population, we may have missed some common and important food or aeroallergens, for example, the pollens. This may lead to a misclassification of the non-sensitized group. this were the case, it would have biased the sleep-sensitization association towards null. That is, we might have underestimated the true association. Secondly, in this report we treated twins as individuals, although we corrected intra-twin correlations using GEE. We did not correct multiple comparisons because two highly correlated phenotypes were tested here. Nevertheless, the robust association and highly significant p-values suggested that the associations were unlikely due to chance alone.
In summary, we demonstrated that short sleep duration was associated with sensitization to food allergens and aeroallergens in this large sample of rural Chinese adolescents. Our findings may be generalized to the general population in the Anqing area because this twin population was enrolled from the local community, had similar demographic characteristics and lifestyle, and were comparable to the local general population with regards to age- and gender-specific weight, height, and pulmonary functions as demonstrated in our previous study [31]. But caution is needed in generalizing our findings to other populations due to differences in demographic, environmental, dietary, and lifestyle factors. Additional longitudinal and experimental studies are needed to confirm our findings and elucidate the causality of the association between short sleep duration and allergen sensitization, and the underlying biological mechanisms, which may provide useful information and help in developing novel prevention and treatment strategies for reducing the global burden of allergic diseases.
Supplementary Material
Acknowledgment
This study is supported in part by grant R01 HD049059 from the National Institute of Child Health and Human Development; R01 HL0864619 from the National Heart, Lung, and Blood Institute; R01 AG032227 from the National Institute of Aging; and the Food Allergy Project. Dr. Xin Liu has been supported in part with Federal funds from the National Institutes of Health (NIH), through the Clinical and Translational Science Awards Program (CTSA), Northwestern University KL2RR025740. We acknowledge the assistance and cooperation of the faculty and staff of the Anhui Institute of Biomedicine, Anhui Medical University, and thank all study participants for their support.
Footnotes
Disclosure Statement
All authors have declared no conflict for this manuscript.
References
- 1.Falade AG, Olawuyi JF, Osinusi K, Onadeko BO. Prevalence and severity of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema in 6- to 7-year-old Nigerian primary school children: the international study of asthma and allergies in childhood. Med Princ Pract. 2004;13:20–25. doi: 10.1159/000074046. [DOI] [PubMed] [Google Scholar]
- 2.Lee SL, Wong W, Lau YL. Increasing prevalence of allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood) Pediatr Allergy Immunol. 2004;15:72–78. doi: 10.1046/j.0905-6157.2003.00109.x. [DOI] [PubMed] [Google Scholar]
- 3.Pearce N, Ait-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms: phase III of the International Study of Asthma and Allergies in Childhood (ISAAC) Thorax. 2007;62:758–766. doi: 10.1136/thx.2006.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williams H, Stewart A, von Mutius E, Cookson W, Anderson HR. Is eczema really on the increase worldwide? J Allergy Clin Immunol. 2008;121:947–954. doi: 10.1016/j.jaci.2007.11.004. e15. [DOI] [PubMed] [Google Scholar]
- 5.Bjorksten B, Clayton T, Ellwood P, Stewart A, Strachan D. Worldwide time trends for symptoms of rhinitis and conjunctivitis: Phase III of the International Study of Asthma and Allergies in Childhood. Pediatr Allergy Immunol. 2008;19:110–124. doi: 10.1111/j.1399-3038.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 6.Williams H, Robertson C, Stewart A, et al. Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol. 1999;103:125–138. doi: 10.1016/s0091-6749(99)70536-1. [DOI] [PubMed] [Google Scholar]
- 7.Macan J, Varnai VM, Maloca I, Kanceljak-Macan B. Increasing trend in atopy markers prevalence in a Croatian adult population between 1985 and 1999. Clin Exp Allergy. 2007;37:1756–1763. doi: 10.1111/j.1365-2222.2007.02836.x. [DOI] [PubMed] [Google Scholar]
- 8.Pallasaho P, Ronmark E, Haahtela T, Sovijarvi AR, Lundback B. Degree and clinical relevance of sensitization to common allergens among adults: a population study in Helsinki, Finland. Clin Exp Allergy. 2006;36:503–509. doi: 10.1111/j.1365-2222.2006.02460.x. [DOI] [PubMed] [Google Scholar]
- 9.Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36:103–116. doi: 10.1001/archpsyc.1979.01780010109014. [DOI] [PubMed] [Google Scholar]
- 10.Foundation, N.S. Sleep in America Poll. 2005. [Google Scholar]
- 11.Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115:241–249. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- 12.Ouyang FLB, Wang B, Yang J, et al. Sleep patterns among rural Chinese twin adolescents. Sleep Med. 2009;10:479–489. doi: 10.1016/j.sleep.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolk R, Gami AS, Garcia-Touchard A, Somers VK. Sleep and cardiovascular disease. Curr Probl Cardiol. 2005;30:625–662. doi: 10.1016/j.cpcardiol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Ferrie JE, Shipley MJ, Cappuccio FP, et al. A prospective study of change in sleep duration: associations with mortality in the Whitehall II cohort. Sleep. 2007;30:1659–1666. doi: 10.1093/sleep/30.12.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 16.Yu Y, Lu BS, Wang B, Wang H, et al. Short sleep duration and adiposity in Chinese adolescents. Sleep. 2007;30:1688–1697. doi: 10.1093/sleep/30.12.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krueger PM, Friedman EM. Sleep Duration in the United States: A Cross-sectional Population-based Study. Am J Epidemiol. 2009;169:1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gami AS, Somers VK. Obstructive sleep apnea, metabolic syndrome, and cardiovascular outcomes. Eur Heart J. 2004;25(9):709–711. doi: 10.1016/j.ehj.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 19.Taheri S, Lin L, Austin D, Young T, Mignot E. Short sleep duration is associated with reduced leptin, elevated ghrelin, and increased body mass index. PLoS Med. 2004;1:e62. doi: 10.1371/journal.pmed.0010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gottlieb DJ, Punjabi NM, Newman AB, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 21.Teramoto S, Yamamoto H, Yamaguchi Y, Namba R, Ouchi Y. Obstructive sleep apnea causes systemic inflammation and metabolic syndrome. Chest. 2005;127(3):1074–1075. doi: 10.1378/chest.127.3.1074. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–1316. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 23.Patel SR, Ayas NT, Malhotra MR, et al. A prospective study of sleep duration and mortality risk in women. Sleep. 2004;27:440–444. doi: 10.1093/sleep/27.3.440. [DOI] [PubMed] [Google Scholar]
- 24.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant PA, Trinder J, Curtis N. Sick and tired: Does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–467. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 26.Knutson KL. Sex differences in the association between sleep and body mass index in adolescents. J Pediatr. 2005;147:830–834. doi: 10.1016/j.jpeds.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 27.Carskadon MA, Acebo C. Regulation of sleepiness in adolescents: update, insights, and speculation. Sleep. 2002;25:606–614. doi: 10.1093/sleep/25.6.606. [DOI] [PubMed] [Google Scholar]
- 28.Huurre TM, Aro HM, Jaakkola JJ. Incidence and prevalence of asthma and allergic rhinitis: a cohort study of Finnish adolescents. J Asthma. 2004;41:311–317. doi: 10.1081/jas-120026088. [DOI] [PubMed] [Google Scholar]
- 29.Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2006;117:S470–S475. doi: 10.1016/j.jaci.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 30.Ouyang F, Kumar R, Pongracic J, et al. Adiposity, serum lipid levels, and allergic sensitization in Chinese men and women. J Allergy Clin Immunol. 2009;123:940–948. doi: 10.1016/j.jaci.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Y, Kumar R, Venners S, et al. Age and gender specific lung function predictive equations provide similar predictions for both a twin population and a general population from age 6 through adolescence. Pediatr Pulmonol. 2007;42:631–639. doi: 10.1002/ppul.20631. [DOI] [PubMed] [Google Scholar]
- 32.Pietrobelli A, Formica C, Wang Z, Heymsfield SB. Dual-energy X-ray absorptiometry body composition model: review of physical concepts. Am J Physiol. 1996;271:E941–E951. doi: 10.1152/ajpendo.1996.271.6.E941. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Ouyang F, Pongracic JA, et al. Dissociation between the prevalence of atopy and allergic disease in rural China among children and adults. J Allergy Clin Immunol. 2008;122:929–935. doi: 10.1016/j.jaci.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Liu L. Sleep habits and insomnia in a sample of elderly persons in China. Sleep. 2005;28:1579–1587. [PubMed] [Google Scholar]
- 35.Kay AB. Allergy and allergic diseases: first of two parts. N Engl J Med. 2001;344:30–37. doi: 10.1056/NEJM200101043440106. [DOI] [PubMed] [Google Scholar]
- 36.Foundation, N.S Sleep in America Poll. 2006. [Google Scholar]
- 37.Liu X, Liu L, Owens JA, Kaplan DL. Sleep patterns and sleep problems among schoolchildren in the United States and China. Pediatrics. 2005;115:241–249. doi: 10.1542/peds.2004-0815F. [DOI] [PubMed] [Google Scholar]
- 38.Chaput JP, Despres JP, Bouchard C, Astrup A, Tremblay A. Sleep duration as a risk factor for the development of type 2 diabetes or impaired glucose tolerance: Analyses of the Quebec Family Study. Sleep Med. 2009;10:919–924. doi: 10.1016/j.sleep.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 39.Park SE, Kim HM, Kim DH, et al. The association between sleep duration and general and abdominal obesity in Koreans: data from the Korean national health and nutrition examination survey, 2001 and 2005. Obesity. 2009;17:767–771. doi: 10.1038/oby.2008.586. [DOI] [PubMed] [Google Scholar]
- 40.Ozturk A, Mazicioglu M, Poyrazoglu S, Cicek B, Gunay O, Kurtoglu S. The relationship between sleep duration and obesity in Turkish children and adolescents. Acta Paediatr. 2009;98:699–702. doi: 10.1111/j.1651-2227.2008.01169.x. [DOI] [PubMed] [Google Scholar]
- 41.Ikehara S, Iso H, Date C, et al. Association of sleep duration with mortality from cardiovascular disease and other causes for Japanese men and women: the JACC study. Sleep. 2009;32:295–301. doi: 10.1093/sleep/32.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Irwin M. Effects of sleep and sleep loss on immunity and cytokines. Brain Behav Immun. 2002;16:503–512. doi: 10.1016/s0889-1591(02)00003-x. [DOI] [PubMed] [Google Scholar]
- 43.van Leeuwen WM, Lehto M, Karisola P, et al. Sleep restriction increases the risk of developing cardiovascular diseases by augmenting pro-inflammatory responses through IL-17 and CRP. PLoS ONE. 2009;4:e4589. doi: 10.1371/journal.pone.0004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Opp MR. Cytokines and sleep. Sleep Med Rev. 2005;9:355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 45.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 46.Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoimmunity. Curr Opin Immunol. 2008;20:663–668. doi: 10.1016/j.coi.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Hui L, Hua F, Diandong H, Hong Y. Effects of sleep and sleep deprivation on immunoglobulins and complement in humans. Brain Behav Immun. 2007;21:308–310. doi: 10.1016/j.bbi.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Boyum A, Wiik P, Gustavsson E, et al. The effect of strenuous exercise, calorie deficiency and sleep deprivation on white blood cells, plasma immunoglobulins and cytokines. Scand J Immuol. 1996;43:228–235. doi: 10.1046/j.1365-3083.1996.d01-32.x. [DOI] [PubMed] [Google Scholar]
- 49.Irwin M, McClintick J, Costlow C, Fortner M, White J, Gillin JC. Partial night sleep deprivation reduces natural killer and cellular immune responses in humans. FASEB J. 1996;10:643–653. doi: 10.1096/fasebj.10.5.8621064. [DOI] [PubMed] [Google Scholar]
- 50.Irwin M, Mascovich A, Gillin JC, Willoughby R, Pike J, Smith TL. Partial sleep deprivation reduces natural killer cell activity in humans. Psychosom Med. 1994;56:493–498. doi: 10.1097/00006842-199411000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Dinges DF, Douglas SD, Zaugg L, et al. Leukocytosis and natural killer cell function parallel neurobehavioral fatigue induced by 64 hours of sleep deprivation. J Clin Invest. 1994;93:1930–1939. doi: 10.1172/JCI117184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.