Abstract
Background
Models of cocaine addiction emphasize the role of disrupted frontal circuitry supporting cognitive control processes. Yet, addiction-related alterations in functional interactions among brain regions, especially between the cerebral hemispheres, are rarely examined directly. Resting state fMRI approaches, which reveal patterns of coherent spontaneous fluctuations in the fMRI signal, offer a means to directly quantify functional interactions between the hemispheres. We examined interhemispheric resting state functional connectivity (RSFC) in cocaine dependence using a recently validated approach named “voxel-mirrored homotopic connectivity.”
Methods
We compared interhemispheric RSFC between 25 adults (aged 35.0±8.8) meeting DSM-IV criteria for cocaine dependence within the past 12 months, but currently abstaining (>2 weeks) from cocaine, and 24 healthy comparisons (35.1±7.5), group-matched on age, sex, education and employment status.
Results
We observed reduced prefrontal interhemispheric RSFC in cocaine dependent participants relative to controls. Further analyses demonstrated a striking cocaine-dependence-related reduction in interhemispheric RSFC among nodes of the dorsal attention network (DAN), comprising bilateral lateral frontal, medial premotor and posterior parietal areas. Further, within the cocaine-dependent group, RSFC within the DAN was associated with self-reported lapses of attention.
Conclusions
Our findings provide further evidence of an association between chronic exposure to cocaine and disruptions within large-scale brain circuitry supporting cognitive control. We did not detect group differences in DTI measures, suggesting that alterations in the brain’s functional architecture associated with cocaine exposure can be observed in the absence of detectable abnormalities in the white matter microstructure supporting that architecture.
Keywords: cocaine, resting state functional connectivity, interhemispheric, fMRI, prefrontal, cognitive control
Introduction
Cocaine addiction profoundly alters the integrity of prefrontal brain regions supporting cognitive control processes (1–3). The addicted individual’s inability to exert volitional control over their behavior, and the consequent dominance of motivational processes that drive reflexive drug use, are commonly attributed to these disturbances in prefrontal function (1,2,4,5). Acknowledging that frontal dysfunction alone cannot explain the pathophysiology of addiction, recent work (2,4,6–13) emphasizes the role of disrupted functional circuitry. However, addiction-related alterations in functional interactions between the cerebral hemispheres are rarely examined directly.
Diffusion tensor and structural imaging studies suggest that chronic exposure to cocaine affects the integrity of white matter tracts connecting distal brain regions. Cocaine dependence is associated with decreased fractional anisotropy (FA), suggesting altered white matter microstructure, in inferior and orbital frontal regions (14–16), and anterior (17) and posterior (15,18) corpus callosum, although regions of increased FA have also been reported (16). Such white matter abnormalities correlate with cocaine use duration (15), and measures of cognitive and behavioral control (15–17). Decrements in white matter integrity, particularly in corpus callosum, may affect interhemispheric functional interactions fundamental to integrative attentional processing and cognitive control (19–21). Supporting this, EEG and fMRI studies have demonstrated altered interhemispheric EEG coherence (22–24) and abnormal bilateral task-related activation (25,26) in cocaine users.
Resting state fMRI (R-fMRI) approaches, which reveal patterns of coherent spontaneous fluctuations in the fMRI signal, offer a means to directly quantify interhemispheric functional interactions. Functional homotopy – the high degree of correlated activity between homotopic interhemispheric counterparts – is one of the most salient aspects of the brain’s intrinsic functional architecture (27). The vast majority of large-scale functional networks detected using both task-based and R-fMRI are bilateral (28,29), and strong resting state functional connectivity (RSFC) is observable between homotopic regions with few monosynaptic callosal connections (30–32), suggesting that functional homotopy reflects an essential aspect of brain function. Consistent with this conclusion, homotopic RSFC exhibits regional variation congruent with the brain’s functional hierarchy (33). Further, the developmental trajectories of homotopic RSFC show regional and hierarchical specificity across the lifespan (34), and homotopic RSFC is disrupted in autism (35).
A conspicuous feature of the brain’s functional architecture, homotopic RSFC may provide a sensitive indicator of the effects of cocaine exposure on the integrity of cognitive control circuitry. In an initial demonstration of the sensitivity of RSFC approaches to the impact of cocaine on interhemispheric connectivity (36) cocaine administration decreased RSFC within bilateral primary visual and motor cortex in cocaine addicts. Other studies suggest effects of substance dependence on RSFC within and between functional networks, including those associated with cognitive control processes (8–13). Here, we directly examined interhemispheric RSFC in cocaine dependence using a recently validated approach named “voxel-mirrored homotopic connectivity” (VMHC; 34). VMHC quantifies the RSFC between each voxel in one hemisphere and its mirrored counterpart in the opposite hemisphere. We compared VMHC between cocaine-dependent individuals (COC) and matched controls, hypothesizing that COC would show reduced interhemispheric RSFC. Given evidence for frontal lobe dysfunction associated with cocaine addiction (1,3,37,38), we expected frontal areas to be particularly affected. We also hypothesized reduced FA in COC relative to controls. Finally, we explored whether, within COC, RSFC was related to cognitive control function, using the Cognitive Failures Questionnaire (CFQ; 39). High CFQ scores indicate frequent “cognitive slips and errors,” which may reflect ineffective cognitive control and a predominance of automatic, stimulus-driven responses over “controlled behavior” (40). Such behavioral tendencies are relevant in addiction, wherein environmental cues drive reflexive behaviors (substance seeking and use), and there is a failure or inability to exert cognitive control over behavior (1,5).
Methods
Participants
Cocaine-dependent Group (COC)
Thirty-nine right-handed (per self report and the Edinburgh Inventory; 41) adults (36.6±9yrs) meeting DSM-IV criteria for cocaine dependence within the past 12 months, but currently abstaining (>2 weeks) were scanned. Ten participants were excluded from analyses: 5 tested positive for cocaine at the scan, one exhibited an incidental brain abnormality, and 4 exhibited excessive movement (displacement/rotation >2.5mm/°). Twenty-five of the remaining 29 participants, for whom healthy comparison participants group-matched on age, sex, education level, and employment-status were available, were included in the present analyses (see Table 1 for demographics; Figure S4 in Supplement for supplementary analyses including all 29 cocaine-dependent participants).
Table 1.
Participant Demographics
| Cocaine | Controls | |
|---|---|---|
| Number of Participants | 25 | 24 |
| Mean ± SD Age (years) | 35.0 (8.8) | 35.1 (7.5) |
| Number (%) Male | 23 (92%) | 20 (83%) |
| Level of Education | ||
| Some High School | 0 | 1 |
| High School Graduate or GED | 7 | 3 |
| Some College | 10 | 10 |
| College Graduate | 7 | 8 |
| Advanced Graduate/Professional Degree | 1 | 2 |
| Employment | ||
| Full-Time | 8 | 8 |
| Part-Time | 7 | 4 |
| Unemployed | 8 | 6 |
| Student | 1 | 6 |
| Disability/Retired | 1 | 0 |
| Mean ± SD years since initiation of cocaine use* | 11.43 (8.5) | — |
| Mean Age of initiation of cocaine use* | 22 | |
| Frequency of Cocaine Use | ||
| Daily | 8 | |
| 5 times per week | 2 | |
| 3–4 times per week | 13 | |
| Twice per week | 2 | |
| Mean ± SD CSSA | 12.48 (8.4) | — |
| Mean ± SD CFQ | 28.68 (13.0) | — |
GED: General Educational Development. CSSA: Cocaine Selective Severity Assessment. CFQ: Cognitive Failures Questionnaire. Age, sex distributions, educational attainment and employment status did not differ significantly (p> .20).
Data available for 21 out of 24 participants.
COC were recruited through the research program at the NYU School of Medicine and the VA NY Harbor Healthcare System (VA NYHHS). Written informed consent was obtained from all participants, who received monetary compensation for their participation. VA NYHHS, NYU and NYU School of Medicine IRBs approved study procedures.
Cocaine dependence and comorbid Axis-I diagnoses were assessed with the Structured Clinical Interview for DSM-IV (SCID). Participants were included if they met criteria for cocaine dependence within the past 12 months, but reported abstaining from cocaine use for the last 2 weeks. Ten-drug (including cocaine, amphetamines, marijuana and opiates) urine screen at assessment and scan confirmed abstinence. The majority of participants reported using cocaine intranasally, and consuming 1–2 grams per use (Table 1). Cocaine withdrawal severity was assessed using the Cocaine Selective Severity Assessment (CSSA; 42). CSSA scores over 20 indicate more severe withdrawal symptoms (43,44). The mean CSSA score was 12.8±8.4 (range: 2–28). Only 4 participants had CSSA scores over 20, suggesting that overall, our participants were not in acute cocaine withdrawal.
Current or lifetime history of neurological disorders, autism, schizophrenia, suicidality, psychosis, mania, current psychotropic use, and current substance abuse/dependence (other than cocaine and nicotine) were exclusionary. Four participants reported a lifetime history of major depressive disorder (3 in full remission, one reported mild symptoms during the past month). One participant met criteria for post-traumatic stress disorder (mild symptoms in the past month). One participant reported a history of panic disorder, and one of bulimia nervosa; neither reported current symptoms. Eleven participants had a lifetime history of alcohol dependence, and one a history of alcohol abuse; none met criteria for dependence within the past month Six participants had lifetime histories of cannabis dependence, and 4 of cannabis abuse; none met criteria for dependence within the past month. Smoking status was available for 20 of the 25 participants; 13 were smokers (see Supplement).
Control Group
We selected 24 right-handed adults from a pool of adults participating in ongoing studies in our laboratory to match the COC group on age, sex, education, employment and handedness (per the Edinburgh Inventory). Only participants with negligible movement (displacement/rotation <2.5mm/°) were included. The SCID was administered to all control participants; inclusion required absence of current or past DSM-IV Axis-I psychiatric diagnoses, including substance abuse or dependence. Smoking status was available for 16 of the 24 control participants; 3 were smokers.
Data Acquisition
Imaging data were acquired using a Siemens Allegra 3T (NYU Center for Brain Imaging). A T1-weighted image (MPRAGE, TR=2530ms; TE=3.25ms; TI=1100ms; flip angle=7°; 128 slices; FOV=256mm; voxel-size=1×1.3×1.3mm) and one 6-minute resting state scan (multi-echo echo planar imaging (EPI) sequence; 180 time points; TR=2000ms; flip angle=90°; 33 slices; voxel-size=3×3×4mm) were acquired. For most participants, a second resting state scan was acquired later in the session (see Supplement). A field map and short-TE EPI scan were also acquired to improve functional-to-anatomical co-registration.
All COC and 11 controls were scanned with eyes open. The remaining 13 controls were scanned with eyes open and closed, counterbalanced. For 6 participants, the order was “eyes-open” first, while for the remaining 7 the order was reversed.
DTI Data
Two DTI scans were acquired per participant using a twice-refocused diffusion-weighted EPI sequence(45) (TR=5200ms; TE=78ms; 50 slices; matrix 64×64; FOV=192mm; acquisition voxel size=3×3×3mm; 64 diffusion directions with b-value 1000s/mm2; 1 image with no diffusion weighting; bandwidth=3720Hz/pixel). A gradient echo field map (TR=834ms; TE=5.23/7.69ms) acquisition used the same slice positioning and resolution as DTI scans.
Resting State Functional Connectivity
Data processing was performed using AFNI (46) and FSL (47). Preprocessing comprised slice time correction; 3-D motion correction; temporal despiking; spatial smoothing (FWHM=6mm); mean-based intensity normalization; temporal bandpass filtering (0.009–0.1Hz); linear and quadratic detrending; nuisance signal removal (white matter, CSF, global signal, motion parameters) via multiple regression (see Kelly et al. (48) for details); linear registration of functional to structural images (with intermediate registration to a low-resolution image and b0 unwarping); nonlinear registration of structural images to the MNI152 template (49,50).
Voxel-Mirrored Homotopic Connectivity
To account for geometric differences between hemispheres, we refined the registration from individual anatomical to MNI152 template space using a group-specific symmetrical template. All 49 registered structural images were averaged to create a mean image, which was then averaged with its left-right mirror to generate a group-specific symmetrical template. Nonlinear registration to this symmetrical template was performed for each participant, and the resultant transformation was applied to each participant’s preprocessed functional data.
Homotopic RSFC was computed as the Pearson correlation (Fisher-Z-transformed) between every pair of symmetric interhemispheric voxels’ time-series. The resultant correlations constitute voxel-mirrored homotopic connectivity (VMHC).
We examined global and regional group differences in VMHC. Global VMHC was calculated by averaging VMHC values across all brain voxels within a unilateral hemispheric grey matter mask (there is only one correlation for each pair of homotopic voxels). The mask was created using the MNI152 grey matter tissue prior included with FSL (threshold=25% tissue-type probability). We excluded voxels medial of x=±4, to minimize artifactually increased VMHC as a result of blurring across the midline. Group comparisons of global VMHC were performed using t-tests. For this and all subsequent analyses, only “eyes-open” scans were included. To account for controls whose “eyes-open” scan was their second scan, scan order was modeled as a nuisance covariate, along with age and sex.
To test for regional group differences in VMHC, individual-level VHMC maps were entered into a group-level voxel-wise t-test analysis using a mixed-effects ordinary least squares model. Multiple comparisons corrections were performed using Gaussian Random Field theory (min Z>2.3, cluster significance: p<0.05, corrected). To address potential confounds associated with interhemispheric structural asymmetry, we repeated our analyses with (1) no smoothing and (2) exaggerated smoothing (10mm FWHM). Further, we calculated voxel mirrored homotopic morphometry (VMHM) – a measure of left-right differences in grey matter (GM) volume for each participant, using FSL’s VBM-style analysis pipeline (51,52) (see Supplement for details and for group comparisons of whole-brain VBM). In supplementary group comparisons of VMHC, left-right differences in GM volume were controlled for by including the 3-D VMHM volumes as a voxel-dependent covariate.
Finally, as COC and control groups differed significantly in smoking and psychiatric history, we performed two additional group-level analyses which controlled for whether a participant (1) smoked; and (2) had a history of any psychiatric disorder, including cannabis and/or alcohol abuse/dependence (see Supplement).
Seed-based RSFC
We examined the RSFC associated with areas exhibiting significantly different VMHC between groups. Specifically, we computed whole-brain voxel-wise correlations associated with mean time-series derived separately for two regions-of-interest (ROIs), comprising all voxels within the inferior frontal sulcus (IFS) area exhibiting greater VMHC for controls, relative to COC (Figure 1C). Fisher-Z-transformed correlation maps were entered into a group-level voxel-wise t-test analysis. Whole-brain correction for multiple comparisons was performed (min Z>2.3; cluster significance: p<0.05, corrected).
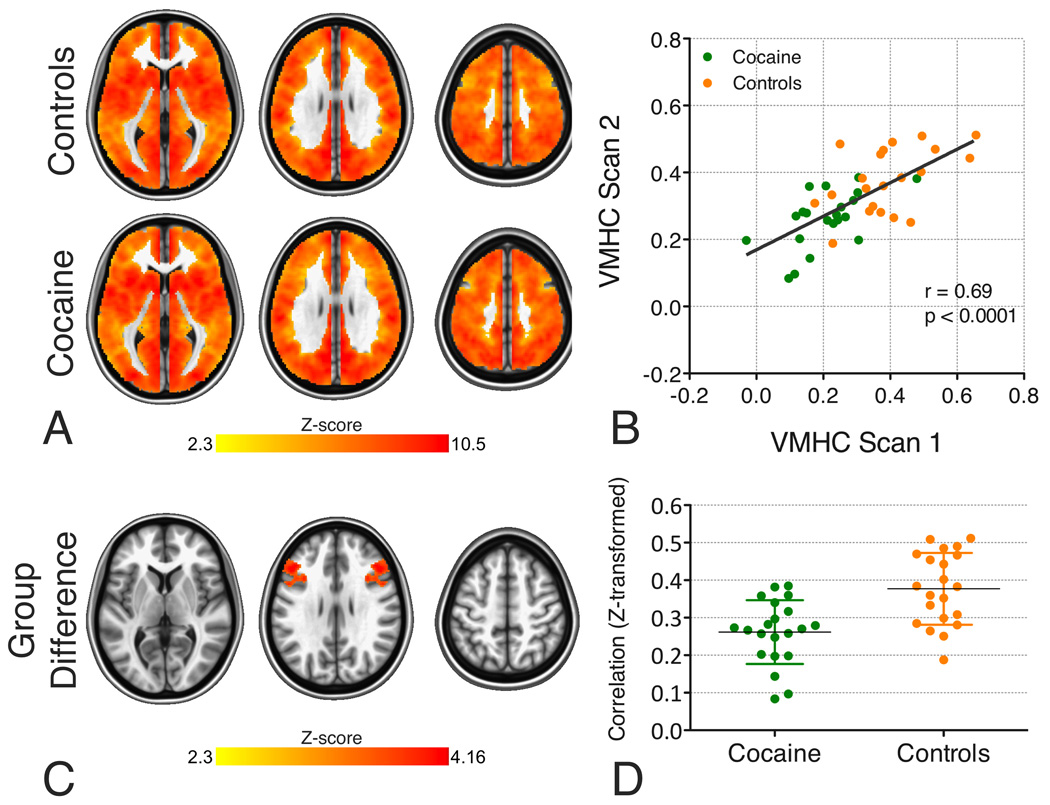
Figure 1. Voxel-Mirrored Homotopic Connectivity (VMHC).
A. Group-level VMHC for the control and cocaine-dependent groups (Z>2.3, cluster-level p<0.05, corrected; images are axial slices at z = 5; 28; and 51). Although there is only one correlation for each pair of homotopic voxels, results are projected on to both hemispheres, to minimize confusion regarding the laterality of the results.
B. Cross-scan consistency of mean VMHC values across the IFS area exhibiting significant group differences in the primary VMHC analysis (i.e., the area shown in Figure 1C; controls: r=0.495, p<0.05; cocaine: r=0.587, p<0.01; all participants: r=0.69; p<0.0001). See Supplement for full details of the within-sample replication analysis.
C. Inferior frontal sulcus (IFS) area for which the control group exhibited significantly stronger VMHC than the cocaine-dependent group (Z>2.3, cluster-level p<0.05, corrected).
D. In recognition of the fact that non-independence in voxel-wise analyses of group differences necessarily provides inflated estimates of effect sizes (89–91), the plots show mean VMHC values across the IFS area exhibiting significant group differences in the primary VMHC analysis (shown in B) computed on the basis of the secondary scan (Scan 2) data. The group difference in VMHC for the secondary scan is significant (controls mean Scan 2 VMHC=0.38±0.10, cocaine mean Scan 2 VMHC=0.26±0.09; t(2,38)=4.13, p<0.001).
Within-sample Replication
See Supplement for within-session stability analyses.
Brain/Behavior Relationships
The Cognitive Failures Questionnaire (CFQ; 39), a 25-item measure of self-reported attentional lapses with good construct validity and test-retest reliability (53,54), was provided by 23 COC. Higher scores indicate more frequent lapses of attention and are associated with poorer performance on task-based measures of attentional function (40,55). Scores on the CFQ correlate well with deficits in cognitive control (39,40) as well as anxiety symptoms (39). First, we explored the relationship between the CFQ and mean prefrontal interhemispheric RSFC within a 4mm-radius sphere centered on the peak of the group difference in VMHC, after regressing out age and sex from both the RSFC data and CFQ scores. Second, we examined the voxel-wise association between the CFQ and RSFC by entering CFQ scores as a covariate in the group-level seed-based RSFC analysis, controlling for age and sex.
DTI
DTI data analysis was performed using FSL tract-based spatial statistics (tbss; 56). Each participant’s pair of DTI scans were concatenated and corrected for eddy currents and motion. Diffusion gradients were rotated to improve consistency with motion parameters. Magnetic field inhomogeneities were accounted for by field map reconstruction, and data were averaged across scans to improve SNR. Diffusion tensors were fitted for each voxel to obtain images containing fractional anisotropy (FA), first diffusion eigenvariate (L1), mean diffusivity (MD) and radial diffusivity (RD) values, registered to the FMRIB58_FA standard space image (1mm3 resolution) using FNIRT. A group-mean FA skeleton was created, and each participant’s standard space FA data were projected onto the skeleton. Resultant skeletonised FA images were entered into voxel-wise non-parametric group comparisons, performed using randomize. The mean FA skeleton (thresholded at FA=0.2) was used as a mask, and 5000 permutations were performed, covarying for age and sex. We corrected for multiple comparisons (p<0.05) using threshold-free cluster enhancement (tfce), controlling for spatial non-stationarity (57). See Supplement for additional DTI analyses.
Results
Voxel-mirrored Homotopic Connectivity
Consistent with previous studies (27,33), homotopic RSFC was a robust global brain phenomenon, with regional differences in strength (Figure 1A). While the control and cocaine groups did not differ on global VMHC (controls=0.33±0.07; cocaine=0.34±0.07; p>0.50), group comparisons, controlling for age, sex and scan order, revealed one region in which controls exhibited stronger VMHC than COC. This region extended from deep within the IFS into the middle frontal gyrus and ventral premotor cortex, anteriorly from the inferior frontal junction, along the superior part of pars opercularis and triangularis, and into the frontal operculum (Figure 1C). This group difference remained significant (1) in the secondary scan (Figure 1D); (2) when different levels of smoothing were applied (Figure S3A&B in Supplement); and (3) when left-right differences in grey matter volume (Figures S3C and S4D in Supplement), (4) smoking (Figure S4B in Supplement), and (5) psychiatric history were taken into account (Figure S4C and Table S1 in Supplement). No regions exhibited stronger VMHC in the cocaine, relative to the control group.
Seed-based RSFC
We examined whole-brain RSFC associated with two ROIs (one per hemisphere; Figure 1C), comprising the IFS area that exhibited greater VMHC for controls, relative to COC.
Both left and right IFS exhibited RSFC with a large bilateral dorsal fronto-parietal network comprising lateral prefrontal cortex (primarily middle frontal gyrus, but also portions of inferior and superior frontal gyri), dorsal premotor cortex, including the frontal eye fields (FEF), dorsal paracingulate cortex and pre-supplementary motor areas (preSMA), posterior parietal cortex and intraparietal sulcus (IPS), posterior middle temporal cortex, and caudate (Figure S1 in Supplement). This network is commonly identified as the fronto-parietal or dorsal attention network (DAN) (58–61). Consistent with the VMHC analysis, controls exhibited stronger RSFC between the right IFS seed and left lateral prefrontal and premotor cortex. The left IFS seed was similarly consistent with the VMHC analysis, but also revealed reduced RSFC between left IFS and right posterior parietal cortex and IPS in COC, relative to controls (Table 2; see also Figure S1 in Supplement). These group differences remained significant (1) in the secondary scan (except left IFS RSFC with right IFS/MFG, which just failed to reach significance -see Figure S2 and text in Supplement); (2) when grey matter volume (Figure S4D in Supplement), (3) smoking and (Figure S4B in Supplement) (4) psychiatric history were taken into account (Figure S4C and Table S2 in Supplement). No regions exhibited stronger RSFC in COC, relative to the control group.
Table 2.
Significant Group Differences in VMHC and Seed-Based RSFC
| Cluster Location | Peak (MNI) | Cluster Size (2mm3 voxels) |
Peak Z | ||
|---|---|---|---|---|---|
| x | y | z | |||
| VMHC | |||||
| Controls>Cocaine | |||||
| IFS, MFG, IFG, ventral PCG | ±50 | 10 | 32 | 545 | 4.16 |
| Seed-Based RSFC: Left IFS Seed | |||||
| Controls>Cocaine | |||||
| Right IFS, MFG, IFG, ventral PCG | 46 | 30 | 26 | 1857 | 4.22 |
| Intraparietal Sulcus, Posterior Superior Parietal Cortex | 52 | −56 | 54 | 769 | 4.26 |
| Seed-Based RSFC: Right IFS Seed | |||||
| Controls>Cocaine | |||||
| Left IFS, MFG, IFG | −44 | 32 | 20 | 1280 | 4.09 |
Abbreviations: IFS: Inferior Frontal Sulcus; MFG: Middle Frontal Gyrus; IFG: Inferior Frontal Gyrus; PCG: Precentral Gyrus
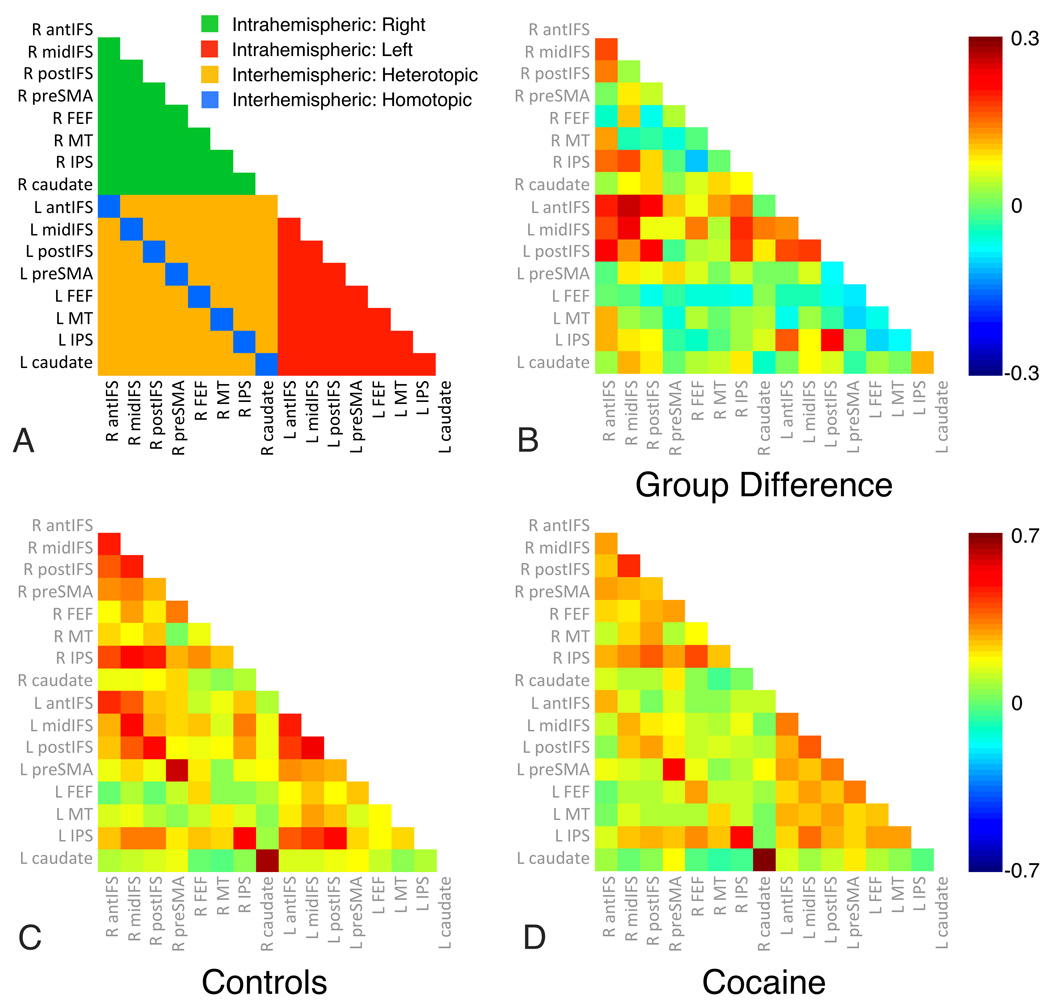
To further assess the nature of cocaine-related decrements in RSFC within the DAN, we (1) identified peak regions for right and left IFS RSFC maps, revealing 8 unique unilateral nodes; (2) created spherical ROIs (4mm radius), centered on each node and its homotopic interhemispheric counterpart (see Table S3 in Supplement for coordinates); (3) extracted the mean time-series for each ROI; and (4) computed all pairwise correlations between nodes, for each subject. We then sorted correlations according to whether they were between intrahemispheric (e.g., right IFS and right IPL), heterotopic interhemispheric (e.g., right IFS and left IPL) or homotopic interhemispheric (e.g., right and left IFS) node pairs, and tested for group differences in mean RSFC for each of these correlation types (62). Relative to controls, COC exhibited reduced homotopic (controls mean=0.45±0.11; COC=0.39±0.09; t(2,47)=2.66, p<0.05) and heterotopic (controls mean=0.16±0.07; COC=0.11±0.07; t(2,47)=2.68, p<0.05) interhemispheric RSFC, but not reduced intrahemispheric RSFC (controls left-left mean=0.25±0.07; COC=0.23±0.08; t(2,47)=0.76, p=0.45; controls right-right mean=0.25±0.1; COC=0.21±0.08; t(2,47)=1.44, p=0.16) between nodes of the DAN (Figure 2).
Figure 2. Cocaine-dependent participants exhibit reduced interhemispheric, but not intrahemispheric RSFC within the Dorsal Attention Network (DAN).
We tested for group differences in intrahemispheric (e.g., right IFS and right IPL), heterotopic interhemispheric (e.g., right IFS and left IPL) and homotopic interhemispheric (e.g., right and left IFS) RSFC between all pairs of 16 DAN nodes. Relative to controls, the cocaine-dependent group exhibited reduced homotopic (controls mean=0.45±0.11; cocaine mean=0.39±0.09; t(2,47)=2.66, p<0.05) and heterotopic (controls mean=0.16±0.07; cocaine mean=0.11±0.07; t(2,47)=2.68, p<0.05) interhemispheric RSFC, but not reduced intrahemispheric RSFC (controls left-left mean=0.25±0.07; cocaine left-left mean=0.23±0.08; t(2,47)=0.76, p=0.45; controls right-right mean=0.25±0.1; cocaine right-right mean=0.21±0.08; t(2,47)=1.44, p=0.16). Abbreviations: R: Right; L: Left; antIFS: anterior inferior frontal sulcus; midIFC: middle IFS; postIFS: posterior IFS; preSMA: presupplementary motor area; FEF: frontal eye fields; MT: middle temporal area; IPS: intraparietal sulcus.
Brain/Behavior Relationships
The DAN subserves top-down control of attention and behavior (58,59). As such, we tested whether DAN RSFC was associated with the CFQ. CFQ scores were available for 23 COC participants but not for controls; one score, >3 SD above the mean, was excluded.
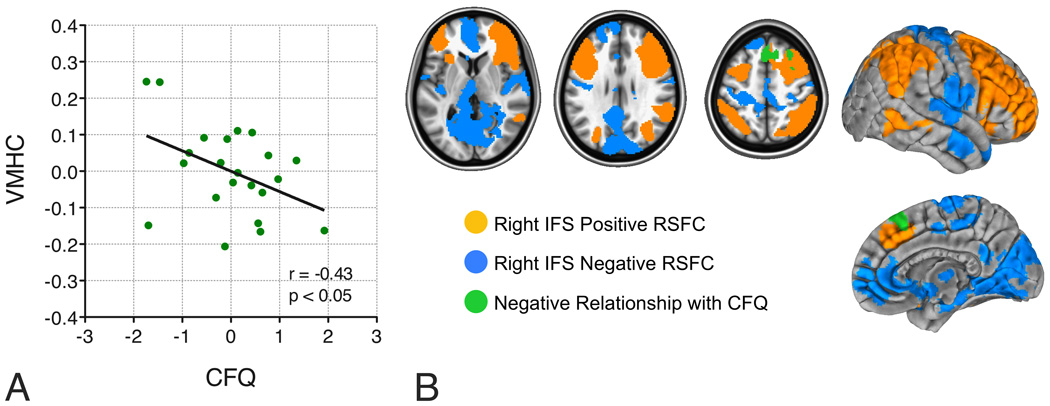
First, we examined the relationship between the CFQ and interhemispheric RSFC (VMHC) associated with the IFS region that exhibited significantly weaker RSFC in COC, relative to controls. Within COC, we observed a significant negative correlation between CFQ score and mean VMHC within a spherical 4mm-radius ROI centered on the VMHC group difference peak (reported in Table 2; Figure 3A; r=−0.43, n=22 p<0.05), suggesting that weaker prefrontal interhemispheric RSFC was associated with more frequent attentional failures. However, this finding should be interpreted with caution, as it did not remain significant when CSSA withdrawal symptoms were taken into account (r=−0.36, p=0.1), and it was absent in the second scan (r = 0.27, n=17, p=0.27).
Figure 3. ROI-based and voxel-wise brain/behavior relationships.
A. Interhemispheric RSFC (i.e., VMHC) within a 4mm-radius sphere centered on the peak of the group difference in VMHC correlated with self-reported cognitive failures, as measured by the Cognitive Failures Questionnaire (CFQ; r=−0.43, n=23, p<0.05). Cocaine-dependent participants with the weakest prefrontal interhemispheric RSFC reported experiencing more frequent attentional failures. This relationship remained significant after adjusting for cocaine withdrawal symptoms (as measured by the Cocaine Selective Severity Assessment – CSSA: r=−0.66, p<0.001).
B. Voxel-wise analyses revealed relationships between right IFS RSFC and self-reported cognitive failures. Shown in green is the medial/superior lateral premotor area whose RSFC with right IFS exhibited a negative relationship with the CFQ. Cocaine-dependent participants with the weakest RSFC between these two areas reported experiencing more frequent attentional failures. Axial slices (z = 5; 28; and 51) are displayed according to neurological convention (right is right).
The voxel-wise analysis revealed a more robust brain/behavior relationship. Higher CFQ scores were associated with weaker positive RSFC between the right IFS seed and bilateral preSMA (Figure 3B). This relationship just escaped significance in the secondary scan data (n=17; r=−0.46, p=0.056); but was significant after partialling out CSSA scores (Scan 1: r=−0.66, p<0.001; Scan 2: r=−0.50, p<0.05). No relationship was observed for the left IFS seed.
DTI
Voxel-wise non-parametric group comparisons of skeletonised FA data revealed no significant group differences, even when age and sex covariates were removed, and when a parametric voxel-wise analysis of all (non-skeletonised) white matter FA, L1, MD and RD values was performed. ROI-based analyses of FA within specific white matter tracts similarly did not reveal any group differences, nor any correlations between FA and VMHC. Additional exploratory analyses are in the Supplement.
Discussion
Homotopic functional connectivity is one of the most salient characteristics of the brain’s intrinsic functional architecture (27,33), likely reflecting the importance of interhemispheric communication to integrated brain function underlying coherent cognition and behavior. Altered interhemispheric functional interactions have been found in psychiatric and clinical disorders (35,63–66), and in normal aging (67–69). With a few exceptions (22–26), electrophysiological and neuroimaging studies have less frequently paid direct attention to interhemispheric interactions in substance dependence.
Here, we demonstrated reduced prefrontal interhemispheric RSFC in abstinent cocaine-dependent participants, relative to controls. Follow-up analyses demonstrated striking cocaine dependence-related reductions in homotopic and heterotopic interhemispheric RSFC among the nodes of a dorsal fronto-parietal network implicated in top-down control of attention and behavior by both task-based and R-fMRI studies (28,60,61), although the extensive pattern of RSFC over prefrontal areas suggests that the network also encompasses regions typically considered part of the ventral attention network (58,59). Interestingly, the IFS area that exhibited robust group differences in interhemispheric RSFC has been identified as a site of interaction between the dorsal and ventral attention networks (59,66,70), and thus has been postulated to subserve a general role in both attentional control and awareness (70).
The brain’s intrinsic functional architecture constitutes the foundation upon which momentary neuronal responses underlying cognition and behavior are built (71,72). Thus, although we detected decrements in interhemispheric RSFC among nodes of the dorsal attention network while participants were at rest, those decrements likely contribute to impaired functioning when that network is called upon to support goal-directed thought and action. Consistent with this proposal, two studies in patients with unilateral stroke demonstrated that reduced interhemispheric RSFC between homotopic and heterotopic nodes of the DAN was associated with impaired behavioral performance on a target detection task (66,73). Further, there was a correlated restoration of both interhemispheric RSFC and behavior with time (66), an observation confirmed in the rat sensorimotor system (74). Interestingly, studies with healthy controls have shown that disrupting the balance of prefrontal interhemispheric interaction with transcranial stimulation alters subsequent risk-taking behavior during gambling tasks (75,76). These findings suggest the importance of homotopic and heterotopic interaction to cognition and behavior. Although we did not directly assess attentional control, RSFC within the dorsal attention network (between right IFS and bilateral preSMA) was related to self-reported attentional lapses, and a less robust relationship was observed between self-reported attentional lapses and IFS homotopic interhemispheric RSFC. Future work is required not only to replicate the present findings and their generalizability to other substances of abuse, but also to identify the behavioral correlates of impaired interhemispheric RSFC in substance dependence.
Our cocaine-dependent participants were abstaining from cocaine use (>2 weeks), and were not in acute withdrawal (low group mean CSSA score). Accordingly, the RSFC decrements we observed cannot be attributed to recent cocaine intoxication. Nor are they likely to reflect group differences in smoking or psychiatric history (Figure S4 in Supplement). Instead, they likely reflect enduring effects of chronic cocaine exposure. Nonetheless, we cannot rule out the possibility that such decrements preceded the initiation of cocaine addiction and may constitute a vulnerability to the development of substance-related disorders (77). Studies of animals experimentally exposed to cocaine are beginning to tease apart these possibilities (78,79). In humans, this question may best be examined in longitudinal and familial/twin studies (80). Alternatively, “reversal” of decrements with maintenance of abstinence may help differentiate direct effects of cocaine use from pre-existing conditions. We plan to examine these issues in follow-up studies.
Despite robust effects of cocaine dependence on RSFC, and previous studies demonstrating significant cocaine-related alterations in FA (14–18), we observed no significant group differences in DTI measures. We did observe a significant correlation between FA in the posterior limb of the internal capsule and reported duration of cocaine dependence (see Supplement). While a complete understanding of this observation awaits further studies, Xu et al. (81) observed that FA in this area correlated with duration of abstinence in cocaine-dependent individuals.
Optimistically, preservation of structural connectivity encourages hope for recovery of function with abstinence from use, or as a result of cognitive interventions. Studies of cognitive training interventions suggest that functional deficits are sometimes reversible (82) –future studies should investigate whether cognitive training can normalize attentional function and RSFC within attentional networks. On the other hand, our failure to detect significant group differences in DTI measures may reflect either weakness in our protocol (e.g., low resolution), or in DTI FA measures more broadly –for example, crossing fibers, integral to interhemispheric connectivity, are known to affect DTI measures such as FA (83,84). A number of studies have demonstrated associations between chronic cocaine exposure and reduced cortical thickness (77) and grey matter (GM) volume (85–87). While we did not detect group differences in GM volume or GM volume asymmetries, an important future direction for the present dataset is to examine cortical thickness and its relationship with R-fMRI measures.
Several other limitations should be noted. First, the brain is not symmetric. To improve the functional correspondence between homotopic regions, we used a symmetric standard template and smoothed the functional data. We also performed supplementary analyses to ensure that potential group differences in morphometric asymmetry could not account for our findings. Nonetheless, future studies should consider data-driven approaches (e.g., clustering interhemispheric voxels on the basis of their RSFC) to define functional homotopy. Second, as is typical of studies in cocaine-dependent populations, the sample was predominantly male. R-fMRI studies have only begun to understand sex-differences, in the context of exceptionally large samples (88). The potential role of sex-differences in our findings should be examined in future studies with considerably larger samples. Third, the cocaine and control groups differed in smoking status. Although supplementary analyses suggested that the RSFC decrements we observed are unlikely to reflect group differences in smoking, future studies should match groups on smoking status and history. Finally, studies should endeavor to control for the potential effects of participants’ current state (e.g., anxiety, arousal) on R-fMRI measures.
In conclusion, the present work suggests that measures of homotopic and heterotopic interhemispheric RSFC may provide sensitive tools with which to study the large-scale circuitry supporting cognitive control and its disruption in addictive disorders.
Supplementary Material
Acknowledgements
The authors thank Dr. Souheil Inati and Dr. Pablo Valesco for their work on EPI and DTI sequence development, Saroja Bangaru and Devika Jutagir for their assistance in data analysis and study management, Dr. Maarten Mennes and Dr. Adriana Di Martino for their helpful editorial and aesthetic advice, the Stavros Niarchos Foundation, Leon Levy Foundation, and Phyllis Green and Randolph Cōwen for their generous support of the NYU Child Study Center, and all our participants for their time and cooperation. Scripts containing the processing commands employed here to compute seed-based RSFC have been released as part of the “1000 Functional Connectomes Project”(88) (http://www.nitrc.org/projects/fcon_1000). The data used in the present study have been released for unrestricted non-commercial use (http://fcon_1000.projects.nitrc.org/indi/retro/nyuCocaine.html) via the International Neuroimaging Data-sharing Initiative (INDI; http://fcon_1000.projects.nitrc.org).
Financial support for this project was provided by grants from the National Institute on Drug Abuse (R03DA024775, to C.K.; R01DA016979, to F.X.C.; 2T32DA007254-16A2 support for C.C.), the National Institute of Mental Health (R01MH083246 and R01MH081218 to F.X.C. and M.P.M.), and Autism Speaks, as well as gifts to the NYU Child Study Center from the Stavros Niarchos Foundation, Leon Levy Foundation, and an endowment provided by Phyllis Green and Randolph Cōwen.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Garavan H, Hester R. The role of cognitive control in cocaine dependence. Neuropsychol Rev. 2007;17:337–345. doi: 10.1007/s11065-007-9034-x. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aron JL, Paulus MP. Location, location: using functional magnetic resonance imaging to pinpoint brain differences relevant to stimulant use. Addiction. 2007;102 Suppl 1:33–43. doi: 10.1111/j.1360-0443.2006.01778.x. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- 5.Baler RD, Volkow ND. Drug addiction: the neurobiology of disrupted self-control. Trends Mol Med. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasi D, Volkow ND, Wang R, Carrillo JH, Maloney T, Alia-Klein N, et al. Disrupted functional connectivity with dopaminergic midbrain in cocaine abusers. PLoS One. 2010;5:e10815. doi: 10.1371/journal.pone.0010815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu H, Salmeronl BJ, Ross TJ, Geng X, Zhanl W, Stein EA, et al. Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting state functional connectivity. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan K, Qin W, Dong M, Liu J, Liu P, Zhang Y, et al. Combining spatial and temporal information to explore resting-state networks changes in abstinent heroin-dependent individuals. Neurosci Lett. 2010;475:20–24. doi: 10.1016/j.neulet.2010.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Ma N, Liu Y, Li N, Wang CX, Zhang H, Jiang XF, et al. Addiction related alteration in resting-state brain connectivity. Neuroimage. 2010;49:738–744. doi: 10.1016/j.neuroimage.2009.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole DM, Beckmann CF, Long CJ, Matthews PM, Durcan MJ, Beaver JD. Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.04.251. [DOI] [PubMed] [Google Scholar]
- 13.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of nicotine addiction and nicotine's actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry. 2009;66:431–441. doi: 10.1001/archgenpsychiatry.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- 15.Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, et al. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Res. 2010;181:57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, et al. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, et al. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Banich MT. The missing link: the role of interhemispheric interaction in attentional processing. Brain Cogn. 1998;36:128–157. doi: 10.1006/brcg.1997.0950. [DOI] [PubMed] [Google Scholar]
- 20.Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123(Pt 7):1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 21.Hoptman MJ, Davidson RJ. How and why do the two cerebral hemispheres interact? Psychol Bull. 1994;116:195–219. doi: 10.1037/0033-2909.116.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Reid MS, Flammino F, Howard B, Nilsen D, Prichep LS. Topographic imaging of quantitative EEG in response to smoked cocaine self-administration in humans. Neuropsychopharmacology. 2006;31:872–884. doi: 10.1038/sj.npp.1300888. [DOI] [PubMed] [Google Scholar]
- 23.Reid MS, Flammino F, Howard B, Nilsen D, Prichep LS. Cocaine cue versus cocaine dosing in humans: evidence for distinct neurophysiological response profiles. Pharmacol Biochem Behav. 2008;91:155–164. doi: 10.1016/j.pbb.2008.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roemer RA, Cornwell A, Dewart D, Jackson P, Ercegovac DV. Quantitative electroencephalographic analyses in cocaine-preferring polysubstance abusers during abstinence. Psychiatry Res. 1995;58:247–257. doi: 10.1016/0165-1781(95)02474-b. [DOI] [PubMed] [Google Scholar]
- 25.Hanlon CA, Wesley MJ, Roth AJ, Miller MD, Porrino LJ. Loss of laterality in chronic cocaine users: an fMRI investigation of sensorimotor control. Psychiatry Res. 2010;181:15–23. doi: 10.1016/j.pscychresns.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanlon CA, Wesley MJ, Porrino LJ. Loss of functional specificity in the dorsal striatum of chronic cocaine users. Drug Alcohol Depend. 2009;102:88–94. doi: 10.1016/j.drugalcdep.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salvador R, Suckling J, Schwarzbauer C, Bullmore E. Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philos Trans R Soc Lond B Biol Sci. 2005;360:937–946. doi: 10.1098/rstb.2005.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, et al. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci U S A. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro R, Fox PT, Paus T. Functional coactivation map of the human brain. Cereb Cortex. 2008;18:2553–2559. doi: 10.1093/cercor/bhn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, et al. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 31.Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, et al. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Martino A, Scheres A, Margulies DS, Kelly AM, Uddin LQ, Shehzad Z, et al. Functional connectivity of human striatum: a resting state FMRI study. Cereb Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- 33.Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly AM, Uddin LQ, et al. Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. J Neurosci. 2008;28:13754–13764. doi: 10.1523/JNEUROSCI.4544-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, Bangaru S, et al. Growing together and growing apart: Regional and sex differences in the lifespan developmental trajectories of functional homotopy. J Neurosci. 2010;30:15034–15043. doi: 10.1523/JNEUROSCI.2612-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson JS, Druzgal TJ, Froehlich A, Dubray MB, Lange N, Alexander AL, et al. Decreased Interhemispheric Functional Connectivity in Autism. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li SJ, Biswal B, Li Z, Risinger R, Rainey C, Cho JK, et al. Cocaine administration decreases functional connectivity in human primary visual and motor cortex as detected by functional MRI. Magn Reson Med. 2000;43:45–51. doi: 10.1002/(sici)1522-2594(200001)43:1<45::aid-mrm6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 37.Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- 38.Garavan H, Stout JC. Neurocognitive insights into substance abuse. Trends Cogn Sci. 2005;9:195–201. doi: 10.1016/j.tics.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Broadbent DE, Cooper PF, FitzGerald P, Parkes KR. The Cognitive Failures Questionnaire (CFQ) and its correlates. Br J Clin Psychol. 1982;21(Pt 1):1–16. doi: 10.1111/j.2044-8260.1982.tb01421.x. [DOI] [PubMed] [Google Scholar]
- 40.Manly T, Robertson IH, Galloway M, Hawkins K. The absent mind: further investigations of sustained attention to response. Neuropsychologia. 1999;37:661–670. doi: 10.1016/s0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- 41.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 42.Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, et al. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449–461. doi: 10.1016/s0306-4603(98)00011-2. [DOI] [PubMed] [Google Scholar]
- 43.Mulvaney FD, Alterman AI, Boardman CR, Kampman K. Cocaine abstinence symptomatology and treatment attrition. J Subst Abuse Treat. 1999;16:129–135. doi: 10.1016/s0740-5472(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 44.Kampman KM, Pettinati HM, Volpicelli JR, Oslin DM, Lipkin C, Sparkman T, et al. Cocaine dependence severity predicts outcome in outpatient detoxification from cocaine and alcohol. Am J Addict. 2004;13:74–82. doi: 10.1080/10550490490265389. [DOI] [PubMed] [Google Scholar]
- 45.Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- 46.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 47.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23 Suppl 1:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 48.Kelly C, de Zubicaray G, Di Martino A, Copland DA, Reiss PT, Klein DF, et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–7378. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andersson JLR, Jenkinson M, Smith SM. Non-linear optimisation. FMRIB technical report TR07JA1. 2007
- 50.Andersson JLR, Jenkinson M, Smith SM. Non-linear registration, aka Spatial normalisation. FMRIB technical report TR07JA2. 2007
- 51.Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- 53.Vom Hofe A, Mainemarre G, Vannier L. Sensitivity to everyday failures and cognitive inhibition: Are they related? European Journal of Applied Psychology. 1998;48:49–55. [Google Scholar]
- 54.Rast P, Zimprich D, Van Boxtel M, Jolles J. Factor structure and measurement invariance of the cognitive failures questionnaire across the adult life span. Assessment. 2009;16:145–158. doi: 10.1177/1073191108324440. [DOI] [PubMed] [Google Scholar]
- 55.Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. 'Oops!': performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- 56.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 57.Edden RA, D.K.J . International Society for Magnetic Resonance in Medicine. Stockholm, Sweden: 2010. Skeleton Thickness Biases Statistical Power in Skeleton-Based Analyses of Diffusion MRI Data. [Google Scholar]
- 58.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- 59.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A. 2006;103:10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12:99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gee DG, Biswal BB, Kelly C, Stark DE, Margulies DS, Shehzad Z, et al. Low frequency fluctuations reveal integrated and segregated processing among the cerebral hemispheres. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2010.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pettigrew JD, Miller SM. A 'sticky' interhemispheric switch in bipolar disorder? Proc Biol Sci. 1998;265:2141–2148. doi: 10.1098/rspb.1998.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Clarke AR, Barry RJ, Heaven PC, McCarthy R, Selikowitz M, Byrne MK. EEG coherence in adults with attention-deficit/hyperactivity disorder. Int J Psychophysiol. 2008;67:35–40. doi: 10.1016/j.ijpsycho.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 66.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Moore AB, Tyner C, Hu X. Asymmetric connectivity reduction and its relationship to "HAROLD" in aging brain. Brain Res. 2009;1295:149–158. doi: 10.1016/j.brainres.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging. 2002;17:85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 69.Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. 2009;60:173–196. doi: 10.1146/annurev.psych.59.103006.093656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Asplund CL, Todd JJ, Snyder AP, Marois R. A central role for the lateral prefrontal cortex in goal-directed and stimulus-driven attention. Nat Neurosci. 2010;13:507–512. doi: 10.1038/nn.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raichle ME. Two views of brain function. Trends Cogn Sci. 2010;14:180–190. doi: 10.1016/j.tics.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 72.Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, Castellanos FX, et al. Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage. 2010;50:1690–1701. doi: 10.1016/j.neuroimage.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, et al. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, et al. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;27:12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Makris N, Gasic GP, Kennedy DN, Hodge SM, Kaiser JR, Lee MJ, et al. Cortical thickness abnormalities in cocaine addiction--a reflection of both drug use and a pre-existing disposition to drug abuse? Neuron. 2008;60:174–188. doi: 10.1016/j.neuron.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 2009;171:242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Beveridge TJ, Gill KE, Hanlon CA, Porrino LJ. Review. Parallel studies of cocaine-related neural and cognitive impairment in humans and monkeys. Philos Trans R Soc Lond B Biol Sci. 2008;363:3257–3266. doi: 10.1098/rstb.2008.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Agrawal A, Lynskey MT. Are there genetic influences on addiction: evidence from family, adoption and twin studies. Addiction. 2008;103:1069–1081. doi: 10.1111/j.1360-0443.2008.02213.x. [DOI] [PubMed] [Google Scholar]
- 81.Xu J, DeVito EE, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. White matter integrity is associated with treatment outcome measures in cocaine dependence. Neuropsychopharmacology. 2010;35:1541–1549. doi: 10.1038/npp.2010.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kelly C, Foxe JJ, Garavan H. Patterns of normal human brain plasticity after practice and their implications for neurorehabilitation. Arch Phys Med Rehabil. 2006;87:S20–S29. doi: 10.1016/j.apmr.2006.08.333. [DOI] [PubMed] [Google Scholar]
- 83.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–268. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 84.Tuch DS, Reese TG, Wiegell MR, Wedeen VJ. Diffusion MRI of complex neural architecture. Neuron. 2003;40:885–895. doi: 10.1016/s0896-6273(03)00758-x. [DOI] [PubMed] [Google Scholar]
- 85.Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, et al. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- 86.Sim ME, Lyoo IK, Streeter CC, Covell J, Sarid-Segal O, Ciraulo DA, et al. Cerebellar gray matter volume correlates with duration of cocaine use in cocaine-dependent subjects. Neuropsychopharmacology. 2007;32:2229–2237. doi: 10.1038/sj.npp.1301346. [DOI] [PubMed] [Google Scholar]
- 87.Matochik JA, London ED, Eldreth DA, Cadet JL, Bolla KI. Frontal cortical tissue composition in abstinent cocaine abusers: a magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- 88.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly High Correlations in fMRI Studies of Emotion, Personality and Social Cognition. Perspectives in Psychological Science. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- 91.Poldrack RA, Mumford JA. Independence in ROI analysis: where is the voodoo? Soc Cogn Affect Neurosci. 2009;4:208–213. doi: 10.1093/scan/nsp011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.