Abstract
Severe abnormalities in brain glucose/energy metabolism and insulin signaling have been documented to take a pivotal role in early sporadic Alzheimer’s disease (sAD) pathology. Indeed, the “insulin-resistant brain state” has been hypothesized to form the core of the neurodegenerative events that occur in sAD. In this vein, intracerebroventricular administration of subdiabetogenic doses of streptozotocin (STZ) in rats can induce an insulin-resistant brain state, which is proposed as a suitable experimental model of sAD. This review highlights the involvement of disturbed brain insulin metabolism in sAD etiopathogenesis. Furthermore, current knowledge demonstrates that central STZ administration produces brain pathology and behavioral changes that resemble changes found in sAD patients. The STZ-intracerebroventricularly treated rat represents a promising experimental tool in this field by providing new insights concerning early brain alterations in sAD, which can be translated in novel etiopathogenic and therapeutic approaches in this disease.
Keywords: Alzheimer’s disease, glucose metabolism, insulin signaling, streptozotocin
Introduction
Alzheimer’s disease (AD), the most common form of dementia among older people, affects approximately 35 million people worldwide, and thus is a major health concern in our society (Querfurth and LaFerla, 2010). AD is clinically manifested by progressive memory loss and a gradual decline in cognitive function, culminating in the premature death of the individual typically 3–9 years after diagnosis (Querfurth and LaFerla, 2010). Neuropathologically, AD is characterized by a dramatic loss of neurons and synapses, especially in the hippocampus and cortex, the extracellular accumulation of neuritic plaques, containing amyloid-β (Aβ) peptide, and the presence of intracellular neurofibrillary tangles (NFT) composed of hyperphosphorylated tau protein (Goedert and Spillantini, 2006; Moreira et al., 2009; Moreira et al., 2006; Moreira et al., 2007c; Selkoe, 2001). Despite indistinguishable clinical dementia symptoms, there are two different types of origin-based AD. In a small proportion (familial early-onset AD), the disease has a genetic origin and is caused by missense mutations in three genes: amyloid-β protein precursor (AβPP), presenilin-1, and presenilin-2 (Rocchi et al., 2003). Consequently, there is an abnormal and permanent generation of Aβ fragments that deposit as plaques. However, the great majority of AD cases are sporadic in origin, with aging, type 2 diabetes and apolipoprotein E4 as the main risk factors (Hoyer, 2004a).
Over the last decades, the causes underlying AD pathology have been a “hot topic” in this field. The most prevailing, the “amyloid cascade” hypothesis, proposes that pathological assemblies of Aβ are the cause of both familial (fAD) and sporadic (sAD) forms of AD, whereas other neuropathological alterations are downstream consequences of a gradual aberrant accumulation of Aβ (Hardy and Selkoe, 2002). In accordance, compelling evidence derived from genetic models clearly demonstrates that severe amyloidosis triggers the fAD pathology (Games et al., 2006; Gimenez-Llort et al., 2007; Hardy and Selkoe, 2002). However, Aβ has not been proven to be required for the onset and progression of the sAD, thus the “amyloid cascade” hypothesis may not be applicable to sAD (Hoyer, 2004b; Joseph et al., 2001). A candidate etiological event in sAD is disturbed brain insulin metabolism (Cardoso et al., 2009). As a matter of fact, it has been reported that early abnormalities in brain glucose/energy metabolism are pronounced in structures with both high glucose demands and high insulin sensitivity, including parietotemporal and frontal areas, which suggests a role for impaired insulin signaling in the pathogenesis of sAD (Henneberg and Hoyer, 1995; Hoyer, 2002, 2004b). Moreover, AD patients had been shown to have lower cerebrospinal fluid (CSF) and higher plasma insulin levels (Watson et al., 2003) as well as decreased insulin receptor (IR) density and reduced tyrosine kinase activity (Frolich et al., 1999), reinforcing the idea that abnormalities in brain insulin function and insulin signal transduction are major factors that mechanistically influence the onset of sAD pathology. It has also been demonstrated that insulin administration improves cognitive performance in AD subjects (Craft et al., 1999; Watson and Craft, 2004).
AD has been recognized as an “insulin-resistant brain state”. In the search for a non-transgenic animal model for sAD, the intracerebroventricular (icv) injection of diabetogenic streptozotocin (STZ) in rats has emerged as an experimental model of the early pathophysiological changes in sAD (Hoyer, 2004b). Indeed, several behavioral, neurochemical and structural features that resemble those found in human AD brain have been extensively documented after the icv administration of STZ in rats (Grunblatt et al., 2007; Salkovic-Petrisic and Hoyer, 2007). Thus, the first part of this review is aimed to discuss and summarize the critical involvement of impaired insulin signal transduction and glucose metabolism in the AD etiopathogenesis. In the second part, this review highlights convincing evidences that targeting brain insulin cascade with icv administration of STZ is a suitable strategy to mimic human sAD condition.
Insulin function and signaling in the brain
For a long time, the brain was classically considered to be an insulin-insensitive organ. However, during the late 1960s, the first evidence of immunoreactive insulin in dog CSF arose (Margolis and Altszuler, 1967), indicating that circulating insulin could cross the blood-brain barrier (BBB). One decade later, insulin and IRs were found throughout the brain (Havrankova et al., 1978), initiating questions for the putative roles for insulin in brain physiology and pathophysiology. IRs are widely distributed in the brain with variable densities in different brain areas, the highest concentration being found in the olfactory bulb, cerebral cortex, hippocampus, cerebellum and hypothalamus (Unger et al., 1989). Two types of IRs have been reported in the adult mammalian brain: a peripheral type on glial cells, and brain-specific type with high concentrations on neurons (Adamo et al., 1989). Although, the presence of immunoreactive insulin in the brain is established, the origin of brain insulin is still a controversial question. In the mature brain the majority of insulin derives from the periphery, being transported by CSF into the brain after its synthesis in pancreatic β-cells (Banks, 2004; Burns et al., 2007; Erol, 2008; Salkovic-Petrisic and Hoyer, 2007). The transport of insulin across the BBB occurs mainly via a carrier-mediated, saturable, regulatable and temperature-sensitive active process (Banks, 2004; Burns et al., 2007; Erol, 2008; Salkovic-Petrisic and Hoyer, 2007). However, a smaller proportion of insulin could be synthesized de novo in the brain (Wozniak et al., 1993). This idea was confirmed by the observation of the existence of preproinsulin I and II mRNA and insulin synthesis in both immature and mature mammalian neuronal cells (Schechter and Abboud, 2001; Schechter et al., 1996; Schechter et al., 1992). Additionally, insulin mRNA was found to be dispersed in a highly specific pattern with the highest density in pyramidal cells of the hippocampus and a high density in the medial prefrontal cortex, the enthorinal cortex, perirhinal cortex, thalamus, granule cell layer of the olfactory bulb, and hypothalamus (Devaskar et al., 1994; Young, 1986). Furthermore, no evidence of insulin mRNA or synthesis was detected in glial cells (Devaskar et al., 1994). Moreover, in an attempt to explain the differing distribution patterns of IRs and insulin I, Zhao and collaborators (2004) hypothesize that IRs in different locations in the brain may use insulin from different sources for cell-to-cell communication and neuronal signal transduction. Reinforcing the ability of the brain to synthesize insulin by itself, Santos and collaborators (1999) reported a stimulation of immunoreactive insulin release by glucose in rat brain synaptosomes.
Insulin has been documented to exert pleiotropic actions in the brain (Cardoso et al., 2009). In addition to be the master regulator of brain glucose metabolism, insulin also functions as a neuromodulatory and neuroendocrine molecule, playing a significant role in neuronal growth and survival (Cardoso et al., 2009; Gasparini and Xu, 2003). Indeed, emerging evidence has suggested that insulin signaling plays a role in synaptic plasticity by modulating activities of excitatory and inhibitory receptors such as glutamate and GABA receptors, and by triggering signal transduction cascades leading to alteration of gene expression that is required for long-term memory consolidation (Zhao et al., 2004). As well as in the periphery, the insulin actions in the brain are mediated by the IRs, which belong to the tyrosine kinase receptors superfamily (Lizcano and Alessi, 2002). Briefly, insulin binds to the extracellular domain of the receptor promoting the autophosphorylation of its intracellular domain, thus triggering intrinsic tyrosine kinase activity. Activated IR is responsible for the phosphorylation of several tyrosine residues resulting in receptor autophosphorylation and phosphorylation of intracellular substrates, including the insulin receptor substrates (IRS) and the Src-homology-2-containing protein (Czech and Corvera, 1999; Paz et al., 1996; Saltiel and Pessin, 2002). Then, the phosphorylation of intracellular substrates leads to the recruitment and activation of multiple proteins and the initiation of several signaling cascades, amongst the most prominent of which are the phosphoinositide 3-kinase (PI3-K) and the mitogen-activated protein kinase (MAPK) signaling pathways (Johnston et al., 2003; Kahn and White, 1988; White and Kahn, 1994). Activation of PI3-K pathway, in turn mediates the activation of the protein kinase-B, promoting neuronal survival by directly inactivating the proapoptotic machinery (Dudek et al., 1997; van der Heide et al., 2006). PI3-K/Akt signaling cascade has been shown to trigger the translocation of the insulin-sensitive glucose transporter 4 (GLUT-4) to the membrane surface, which consequently enhances cellular glucose uptake (Bryant et al., 2002; Johnston et al., 2003). Additionally, activated PI3-K/Akt also phosphorylates (at the serine 9 residue) and therefore inhibits both α and β cytosolic forms of glycogen synthase kinase-3 (GSK-3) (Cross et al., 1995). It was demonstrated that GSK-3α regulates the formation of Aβ peptides (Phiel et al., 2003). Accordingly, it was also reported that insulin regulates soluble AβPP release via PI3-K-dependent pathway, being speculated by the authors that the PI3-K involvement in AβPP metabolism may act at the level of vesicular trafficking (Solano et al., 2000). Furthermore, Gasparini and colleagues (2001) reported that insulin reduces intraneuronal Aβ accumulation by accelerating AβPP/Aβ trafficking from the trans-Golgi network, a major cellular site for Aβ generation, to the plasma membrane. In addition, it was also found that insulin increases the extracellular Aβ level by promoting its secretion and by inhibiting its degradation via insulin-degrading enzyme (IDE) (Qiu et al., 1998; Vekrellis et al., 2000). However, it was demonstrated that insulin action on AβPP metabolism requires MAPK signaling pathway (Gasparini et al., 2001). On the other side, GSK-3β isoform is believed to play a role in tau-protein phosphorylation (Ishiguro et al., 1993). Indeed, using cultured human neuronal cells, Hong and Lee (1997) demonstrated that GSK-3 phosphorylates tau and reduces its affinity for microtubules. Conversely, insulin stimulation reduced tau phosphorylation and promotes tau binding to microtubules; these effects of insulin being mediated through the inhibition of GSK-3β via the PI3-K/Akt signaling pathway (Hong and Lee, 1997).
As previously mentioned, both insulin mRNA and IRs are highly expressed in hippocampus, a region that is recognized as an important integration center for learning and memory, suggesting a role for insulin on cognitive function (Park, 2001; Zhao et al., 2004). The precise mechanism(s) underlying the effects of insulin is still unclear; however, it has been shown that both insulin-stimulated PI3-K and MAPK signaling pathways are critically involved in learning and memory processes. For instance, extracellular-regulated kinase 1/2 (ERK1/2) has essential roles in neuronal survival (Xia et al., 1995) and synaptic plasticity related to learning and memory formation (Davis and Laroche, 2006). Furthermore, there is an absolute requirement for ERK activity in the induction of long-term potentiation (English and Sweatt, 1996) and memory consolidation (Eckel-Mahan et al., 2008). Moreover, insulin-induced MAPK signaling pathway activation after learning may lead to regulation of gene expression that is required for long-term memory storage (Zhao et al., 2004). PI3-K pathway is involved in long-term depression by promoting internalization of glutamatergic AMPA receptors (Man et al., 2000), or via recruiting of functional GABA receptors to the postsynaptic membrane (Wan et al., 1997). Previously, it was also reported that insulin-mediated PI3-K signaling cascade also stimulates nitric oxide generation through the activation of the endothelial nitric oxide synthase (Montagnani et al., 2001), which has been shown to be expressed in the hippocampus, and implicated in learning and synaptic plasticity (Doreulee et al., 2003). Additionally, it has also been proposed that the modulation of brain glucose metabolism as well as the expression and trafficking of glucose transporters (GLUTs) is another mechanism by which insulin affects cognitive function (McEwen and Reagan, 2004). Finally, it was recently reported that insulin is also able to regulate cerebral vasoreactivity (Katakam et al., 2009a; Katakam et al., 2009b). The authors found that insulin-induced vasodilation is primarily by endothelium-dependent mechanisms, nitric oxide, and endothelium-derived hyperpolarizing factor, whereas vasoconstriction is mediated by metabolites of cyclooxygenase and cytochrome P450, endothelin, and reactive oxygen species (Katakam et al., 2009a; Katakam et al., 2009b). Thus, the multiple mechanisms by which insulin regulates the tone of the cerebral arteries underscore the dynamic role of insulin in cerebrovascular regulation and the potential for cerebrovascular dysfunction in the “insulin-resistant brain state”.
In summary, insulin is undoubtedly crucial for normal brain function. Thus, it is not surprising that disturbances on brain insulin function and insulin signal transduction have been associated with pathological conditions, including AD. The next section seeks to explore the involvement of impaired brain glucose/energy metabolism as well as disturbed insulin signaling in AD pathophysiology.
Linking impaired brain glucose/energy metabolism and dysfunctional brain insulin signaling in sporadic Alzheimer’s disease
Glucose is, by far, the main brain energy substrate and it is essential to maintain cerebral energy metabolism. Since neurons are incapable to synthesize or store glucose, they are dependent on glucose transport across the BBB, which is mediated by GLUTs (Scheepers et al., 2004). The most predominate GLUT isoforms in the brain are GLUT-1 and GLUT-3 (Vannucci et al., 1997). GLUT-1 is localized in neurons, cerebrovascular endothelial cells, astrocytes, and oligodendrocytes, while GLUT-3 is specifically expressed in neurons (Vannucci et al., 1997; Yu and Ding, 1998).
Positron emission tomography imaging studies revealed impaired brain glucose in AD patients, which precedes neuropsychological impairment and atrophy (Azari et al., 1993; Silverman et al., 2001; Small et al., 1996). Additionally, Hoyer (2004a) proposed that this impairment seems to be a cause, rather than a consequence, of neurodegeneration in AD. Indeed, cerebral glucose utilization is reduced by 45%, and cerebral blood flow (CBF) by approximately 20%, in the early stages of AD. However, in the later stages of the disease, metabolic and physiological abnormalities aggravate, resulting in 55–65% reductions in CBF (Hoyer and Nitsch, 1989). Moreover, Liu and collaborators (Liu et al., 2008) demonstrated that both GLUT-1 and -3, which are responsible for glucose uptake into neurons, were decreased in AD brain, this decrease being associated with decreased O-GlcNAcylation, the hyperphosphorylation of tau, and the density of NFTs in human brains. The decrease in the cerebral glucose metabolism correlated with the altered expression and decreased activity of mitochondrial energy related proteins, including pyruvate dehydrogenase (PDH), isocitrate dehydrogenase, and α-ketoglutarate dehydrogenase, findings also documented in postmortem AD brain and fibroblasts from AD patients (Bubber et al., 2005; Huang et al., 2003). These enzymes are known to be highly susceptible to oxidative modification and are altered by exposure to a range of pro-oxidants (Tretter and Adam-Vizi, 2000). In addition, Bubber and collaborators (2005) verified that all the changes in tricarboxylic acid cycle activities (specifically that of PDH complex) were correlated with the degree of clinical disability in AD, suggesting a coordinated mitochondrial alteration. The diminished activity of PDH promotes a reduction in acetyl coenzyme A levels, which in turn decreases the acetylcholine synthesis (Sims et al., 1983). Moreover, the degeneration of the cholinergic neurons associated with disturbances in the serotoninergic, noradrenergic and dopaminergic systems have been reported to be correlated with the progression of mental impairment in AD patients (Baskin et al., 1999; Wang et al., 2007). Another decisive pathophysiological consequence of the altered brain glucose metabolism is a decrease in ATP production from glucose by around 50% in the beginning of sAD, compromising the ATP-dependent processes crucial for the normal cell functioning (Mattson et al., 2001; Moreira et al., 2007b).
Mitochondria are the central coordinators of energy metabolism. Altered mitochondrial function and mitochondrial turnover have also been critically implicated in AD pathology. Several studies demonstrated accumulation of abnormal mitochondria in dystrophic neurites of senile plaques in AD (Hirai et al., 2001; Kidd, 1964; Luse and Smith, 1964; Terry et al., 1964). Moreover, a decline in respiratory chain complexes I, III, and IV activities was found in platelets and lymphocytes from AD patients and postmortem AD brain tissue (Bosetti et al., 2002; Kish et al., 1992; Parker et al., 1994; Valla et al., 2006), suggesting that mitochondrial abnormalities are present at the earliest symptomatic stages of the disease. Data from our laboratory also revealed that AD fibroblasts present high levels of oxidative stress and apoptotic markers when compared with young and age-matched controls (Moreira et al., 2007a). Additionally, AD-type changes could be generated in control fibroblasts using N-methylprotoporphyrin to inhibit cytochrome c oxidase assembly, which indicates that the observed oxidative damage was associated with mitochondrial dysfunction (Moreira et al., 2007a). Accordingly, de la Monte and Wands (2006) examined postmortem brain tissue from AD patients with different degrees of severity and found that the severity of AD was related to impairments in mitochondrial gene expression, namely in complex IV, increased levels of p53 and molecular indexes of oxidative stress, including nitric oxide synthase and NADPH-oxidase. Thus, mitochondrial malfunction exacerbates oxidative stress, such that levels of lipid, protein, and nucleic acid oxidation are increased in vulnerable neurons in AD (Castellani et al., 2001; Nunomura et al., 2001; Nunomura et al., 1999; Smith et al., 1997; Straface et al., 2005). Vulnerable neurons in AD also accumulate mitochondrial degradation products, thus, suggesting either a greater turnover of mitochondria by autophagy or a reduction of proteolytic turnover (Hirai et al., 2001). Altogether, these findings demonstrate that cerebral hypometabolism during the early stages of AD is characterized by reduced glucose utilization, deficient energy metabolism, and mitochondria dysfunction.
Over the last decades, several hypotheses have been proposed in an attempt to explain the relationship between impaired cerebral glucose/energy metabolism and AD pathology. Rapoport (2003) proposed that in an initial stage of the disease, changes in synaptic structure and function reduce neuronal energy demand leading to potentially reversible downregulation of neuronal oxidative phosphorylation (OXPHOS). However, as disease progresses, NFTs with abnormally phosphorylated tau protein accumulate within neuronal cytoplasm, to the point that they co-opt the non-phosphorylated tau necessary for axonal transport of mitochondria between the cell nucleus and the synapse. In this stage, severe energy depletion and other pathological processes associated with irreversibly downregulated OXPHOS lead to cell death (Rapoport, 2003). Meanwhile, accumulating evidence from the literature suggests a role for insulin resistance and/or reduced insulin actions underlying impaired brain glucose/energy metabolism in the pathogenesis of the disease (Cardoso et al., 2009; Hoyer, 2004a; Rivera et al., 2005; Steen et al., 2005). The first evidence for the so-called “insulin-resistant brain state” was provided in 1998, when Frolich and collaborators (1998) found decreased insulin levels and tyrosine kinase activity, along with an increase in IRs density, in the sAD brain. Accordingly, posterior analysis of postmortem human AD brains revealed a reduction in insulin mRNA and protein levels (Lester-Coll et al., 2006; Rivera et al., 2005; Steen et al., 2005), IRs expression (Frolich et al., 1999; Moloney et al., 2010), IRS-1 and IRS-2 levels and PI3-K and ERK1/2 activities (Watson and Craft, 2004), these abnormalities being correlated with the severity and progression of dementia and neurodegeneration. Indeed, Rivera and collaborators (2005) demonstrated that increasing AD Braak stage was associated with progressively reduced levels of mRNA corresponding to insulin, insulin-like growth factor-1 (IGF-1), and IGF-2 polypeptides and their receptors. Moreover, a correlation between Akt activity/protein levels and Braak staging has been documented in human AD postmortem analysis, indicating a time-dependent and insulinstimulated PI3-K signaling dependent pattern of changes (Pei et al., 2003). Furthermore, AD patients show increased fasting plasma insulin levels, decreased CSF insulin levels, and/or decreased CSF/plasma insulin ratio, besides increased Aβ levels (Watson and Craft, 2004), suggesting a decrease in insulin clearance, which may provoke an elevation of plasma Aβ levels (Li and Holscher, 2007). Additionally, as already mentioned, IRs density is up-regulated in AD (Frolich et al., 1998), showing an impairment of the insulin signal transduction cascade similar to that seen in type 2 diabetes. Furthermore, it has been shown that type 2 diabetes is a risk factor for AD and that AD patients have a higher risk to develop type 2 diabetes (Cole and Frautschy, 2007; Moreira et al., 2009), fostering the description of sAD as the “type 2 diabetes of the brain” (Hoyer, 1998).
An intriguing question is whether the central insulin-resistant state causes all the typical neuropathological hallmarks of AD. Features of endothelial dysfunction and vascular damage have been extensively documented in AD, which point for a vascular component as a potential unifying factor linking disrupted brain insulin signaling and AD pathology (Rocchi et al., 2009; Skoog, 2000). A previous study performed in a mouse model of AD shows that the disturbed cross-talk between IGF-1 and AMP-activated protein kinase, a cell sensor that intervenes in angiogenic signaling and also interacts with insulin/IGF-1 signaling pathways, is a plausible cause of cerebrovascular dysfunction characterized by elevated levels of vascular endothelial growth factor (VEGF) and increased brain endothelial cell division associated with abnormally low brain vessel density (Lopez-Lopez et al., 2007). These findings are in accordance with other reports showing high VEGF levels and reduced brain vessel density in AD patients (Buee et al., 1994; Tarkowski et al., 2002). More recently, Takeda and collaborators (2010) demonstrated that type 2 diabetes aggravates cognitive dysfunction through cerebrovascular changes, such as vascular inflammation and cerebral amyloid angiopathy in an AD mouse model, these neuropathological changes being related with brain insulin signaling impairment. Recently, Bhat (2010) argued that the two key pathogenic processes associated with metabolic disorders, inflammation and insulin resistance may re-emerge within the brain because of cerebrovascular dysfunction characterized by increased BBB permeability and reduced transport of trophic factors, including insulin and IGF-1, which creates an insulin/IGF-deficient environment in the brain. Meanwhile, compelling evidence demonstrates that insulin might interfere directly with the onset of AD pathology through vascular-independent mechanisms. For instance, insulin was found to influence AβPP metabolism potentiating Aβ formation (Solano et al., 2000) and tau phosphorylation (Cheng et al., 2005) (Figure 1). Insulin has been proposed to increase the extracellular concentration of Aβ by the enhancing its trafficking from the endoplasmic reticulum and trans-Golgi network, the main site for Aβ generation, to the plasma membrane, which significantly reduces the intracellular concentration of Aβ derivatives (Aβ40 and Aβ42) (Gasparini et al., 2001). Moreover, abnormalities in brain insulin signaling could promote Aβ accumulation since it has been reported that insulin regulates soluble AβPP release via PI3-K-dependent pathway, and inhibition of GSK-3β attenuates AβPP metabolism (Solano et al., 2000) (Figure 1). On the other side, insulin also inhibits the extracellular degradation of Aβ by IDE (Figure 2), a metalloprotease enzyme responsible for insulin degradation and is also the main soluble Aβ degrading enzyme at neutral pH (Gasparini et al., 2001). The hypothesis that insulin modulates Aβ levels by interfering with IDE is supported by 1) a decrease in IDE activity and mRNA and protein levels in AD brain, 2) impaired brain Aβ and insulin degradation in knockout mice lacking IDE (Frolich et al., 1999; Hong and Lee, 1997; Lucas et al., 2001), 3) increased IDE immunoreactivity around senile plaques and 4) enhanced IDE activity in IDE and AβPP double transgenic mice associated with a decrease in Aβ and prevention of AD (Leissring et al., 2003).
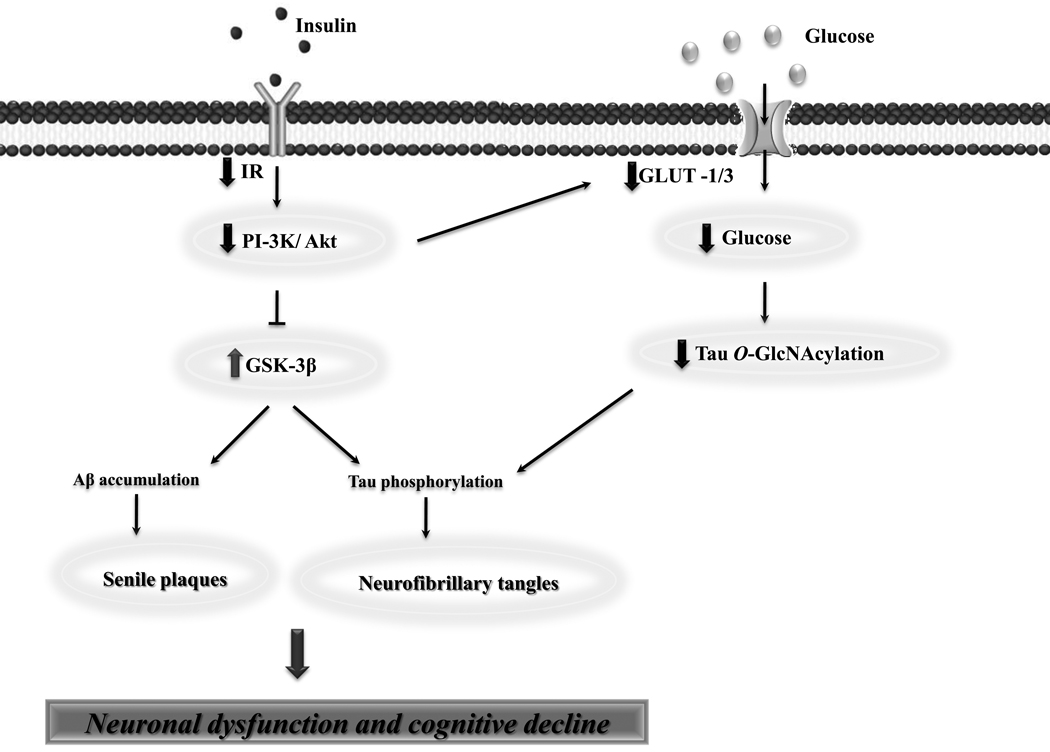
Figure 1.
The role of impaired brain insulin signaling in sporadic Alzheimer’s disease (sAD) pathology. Disturbance of brain insulin signaling has been suggested to be a key causative event underlying sAD etiopathogenesis, being linked to the presence of the two major pathological features of this neurodegenerative disorder, senile plaques and neurofibrillary tangles (NFTs). Indeed, impaired insulin/insulin receptor (IR) signaling leads to decreased insulin-mediated activation of phosphoinositide 3-kinase (PI3-K)/Akt signaling activity, resulting in overactivation of glycogen synthase kinase-3β (GSK-3β). Consequently, GSK-3β overactivation directly promotes tau hyperphosphorylation and formation of NFTs, as well as amyloid-β (Aβ) accumulation and senile plaques formation. Additionally, disruption of insulin signaling leads to a decreased glucose transporter-1 (GLUT-1) and -3 (GLUT-3) expression, culminating in impaired cerebral glucose uptake/metabolism. Moreover, it was recently proposed that decreased neuronal glucose metabolism results in decreased level of UDP-GlcNAc via the hexosamine biosynthetic pathway (HBP) and, consequently, decreased tau O-GlcNAcylation, thus potentiating tau hyperphosphorylation. Overall, impaired brain insulin signaling seems to be a plausible trigger of neuronal dysfunction and cognitive decline in sAD.
Figure 2.
Schematic illustration of insulin resistance-mediated amyloid-β (Aβ) peptide deposition via a mechanism involving insulin-degrading enzyme (IDE). Aβ is mainly degraded by IDE, in the extracellular space. Under hyperinsulinemic conditions associated with insulin resistance, IDE might be competitively inhibited by insulin, resulting in an increased Aβ deposition and senile plaques formation
The regulation of tau protein phosphorylation is another potential mechanism by which insulin seems to be implicated in AD pathology (Figure 1). Insulin has been shown to activate the major kinases involved in tau phosphorylation, including GSK-3β, ERK1/2 and cyclin-dependent kinase 5 (de la Monte and Wands, 2005; Li and Holscher, 2007; van der Heide et al., 2006). It has been previously shown that insulin and IGF-1 inhibit abnormal tau hyperphosphorylation by stimulating Akt-induced phosphorylation/inactivation of GSK-3β in both human and animal neurons (de la Monte and Wands, 2005; Hong and Lee, 1997; Li and Holscher, 2007; Moloney et al., 2010). Accordingly, it was previously shown that insulin and IGF-1 reduce tau phosphorylation promoting its binding to microtubules by inhibition of GSK-3β via the PI3-K pathway (Ho et al., 2004). Therefore, disturbance in insulin and/or IGF-1 signaling cascade leads to an increase in tau hyperphosphorylation potentiating the formation of NFT (Cheng et al., 2005). Furthermore, hyperphosphorylated tau fails to be transported into axons, accumulating and aggregating into NFTs in neuronal perikarya, contributing to neurodegeneration by enhancing oxidative stress, and triggering pathophysiological cascades that lead to increased apoptosis, mitochondrial dysfunction, and necrosis (de la Monte and Wands, 2005).
Collectively, these findings support the hypothesis that dysfunctional brain insulin metabolism is critically involved in the pathogenesis of AD.
Central administration of streptozotocin as an experimental approach for sporadic Alzheimer’s disease
Brain insulin system dysfunction has been suggested to be a possible event underlying the neurodegenerative events that occur in sAD. Considering the presence of both insulin and IRs in the brain, the administration of STZ intracerebroventricularly in rats to induce brain insulin system dysfunction emerged as a suitable experimental approach for sAD (Grunblatt et al., 2007; Salkovic-Petrisic and Hoyer, 2007). In the periphery, the administration of the diabetogenic drug STZ induces a selective destruction of the pancreatic β-cells. It has been postulated that this selective β-cell toxicity of STZ is related to the glucose moiety in its chemical structure, which enables STZ to enter into these cells via the GLUT-2 (Elsner et al., 2000). Moreover, STZ exerts β-cell toxicity effects mostly by causing alkylation of DNA, which triggers activation of poly ADP-ribosylation consequently leading to depletion of cellular NAD+ and ATP, and damaging the main β-cell function - insulin production and secretion (Szkudelski, 2001). Even though the mechanism of action of STZ is still unknown in the brain, it has been speculated to be similar to the peripheral one. Indeed, GLUT-2 is distributed throughout the rat brain, especially in the limbic areas and related nuclei, most concentrated in the ventral and medial regions close to the midline, and involved in glucose sensing and regulation of neurotransmitter release (Arluison et al., 2004a; Arluison et al., 2004b). Compelling evidence demonstrates that single or multiple injections of low doses of the diabetogenic drug STZ, either uni- or bilaterally into the lateral cerebral ventricles produce neurochemical and brain glucose metabolism changes, as well as long-term and progressive deficits in learning, memory and cognitive behavior, that resemble features of AD patients (Grunblatt et al., 2007; Salkovic-Petrisic and Hoyer, 2007).
Severe abnormalities in brain glucose/energy metabolism as well as reduced CBF have been documented after icvSTZ administration (Tota et al., 2010). For instance, Duelli and collaborators (1994) reported reduced glucose utilization in 17 of 35 brain areas, predominantly in the frontal, parietal, sensory motor, auditory and entorhinal cortex and in all hippocampal subfields, 6 weeks post-icvSTZ administration; 3 weeks after icvSTZ administration by others (Pathan et al., 2006). Nitsch and Hoyer (1991) also verified an increase in the concentrations of glucose and ADP in cerebral cortex, reinforcing that idea that icvSTZ administration promotes a disturbance of brain energy metabolism. Specifically, icv administration of STZ results in reduced activities of glycolytic key enzymes in the cerebral cortex and hippocampus (Plaschke and Hoyer, 1993), which in turn lead to a decline in the levels of energy-rich compounds, ATP and creatine phosphate (Lannert and Hoyer, 1998; Nitsch and Hoyer, 1991). Altogether, these effects are associated with significantly reduced brain oxidative metabolism and progressive deficit in learning and memory and cerebral energy balance (Duelli et al., 1994).
As a consequence of an impaired brain energy metabolism and reduced synthesis of acetyl coenzyme A, disturbances in the cholinergic transmission have also been implicated in the marked reduction in learning and memory capacities promoted by icvSTZ administration. In particular, decreased choline acetyltransferase (ChAT) activity in the hippocampus of icvSTZ treated rats has been consistently reported as early as both 1 and 3 weeks following the administration of the compound (Blokland and Jolles, 1993, 1994; Hellweg et al., 1992; Ishrat et al., 2006; Prickaerts et al., 1999; Terwel et al., 1995). Additionally, increased acetylcholinesterase (AChE) activity has been observed in the brain of icvSTZ rats which could result in increased degradation of acetylcholine, thereby enhancing the acetylcholine (ACh) deficits caused by reduced ChAT expression in these animals (Agrawal et al., 2009; de la Monte et al., 2006; Ishrat et al., 2006; Sonkusare et al., 2005). Furthermore, spatial discrimination performance in the Morris task is correlated with decreased hippocampal ChAT activity in icvSTZ-treated animals, which parallels the relationship between cognitive and biochemical changes observed in AD (Blokland and Jolles, 1993, 1994). However, in postmortem AD brain the cholinergic deficit is characterized by reductions in ACh levels and impaired ChAT and AChE activities (McGeer et al., 1984; Whitehouse et al., 1982).
Grunblatt and collaborators (2007) found that icvSTZ administration also promotes a significant decrease in IRs expression in cortex and hippocampus, insulin-1 mRNA in hippocampus, insulin-2 mRNA in cortex and a significant increase of tau phosphorylation in hippocampus, alterations associated with the impairment of memory and learning. Accordingly, time-dependent changes in the phosphorylated and total GSK-3α/β were found in rat brain after icvSTZ administration, which have been suggested to be related to the formation of Aβ peptide-like aggregates in brain capillaries in rats (Salkovic-Petrisic et al., 2006). Similarly, de la Monte and collaborators (2006) also reported an increase of GSK-3β, phospho-tau, ubiquitin, AβPP and Aβ and decreased levels of tau protein in icvSTZ-treated animals. More recently, impaired insulin signaling pathway, overactivation of GSK-3β, decreased GLUT-1 and GLUT-3, downregulation of protein O-GlcNAcylation, increased phosphorylation of tau and NFTs, and decreased microtubule-binding activity of tau were found in the brain of the icvSTZ-treated rat (Deng et al., 2009). These evidences lead the authors to propose that brain insulin resistance, which occurs in AD-affected brain and in the STZ-injected rat brain, could lead to neurofibrillary degeneration via two additive, or synergistic mechanisms (Deng et al., 2009). On the one hand, impaired insulin signaling contributes to GSK-3β overactivation, which leads to hyperphosphorylation of tau and causes cognitive impairments (Deng et al., 2009). On the other hand, insulin resistance decreases GLUT-1 and GLUT-3 expression and thus glucose uptake/metabolism in the brain, which in turn decreases tau O-GlcNAcylation, facilitating tau hyperphosphorylation and neurofibrillary degeneration (Deng et al., 2009). As previously mentioned, data from postmortem AD brains revealed a decreased expression of both GLUT-1 and -3 protein levels, which were associated with decreased O-GlcNAcylation, hyperphosphorylation of tau, and density of NFTs in human brains (Liu et al., 2008). Evidence from diet-induced insulin resistance and genetic models reinforces the crucial role of insulin signaling in amyloidogenesis and tau pathology. In fact, it was reported that induction of insulin resistance by high fat diet (Ho et al., 2004) or intake of sucrose-sweetened water (Cao et al., 2007) leads to an aggravation of amyloid pathology in mouse models of fAD. Additionally, a recent study designed to explore the consequences of disturbed cerebral insulin signaling on AβPP metabolism in a transgenic mouse model of fAD found that icv STZ-induced “insulin-resistant brain state” may aggravate AD-related cognitive impairment and increase the formation of pathomorphological AD hallmarks via GSK-3α/β pathway (Plaschke et al., 2010). On the other hand, loss of insulin-mediated activation of PI3-K and subsequent reduction of phosphorylation of Akt and GSK-3β are reported to result in a substantial increase in the levels of phosphorylated tau in the brains of neuron specific IR knockout mice (Schubert et al., 2004). However, these mice did not show any special learning deficits or long-term memory disturbance, which raises some doubts if the mere lack of the IR is sufficient to cause all AD-like symptoms.
Direct histopathological evidence of specific-neurotoxic damage caused by icvSTZ administration to axons and myelin in the fornix, anterior hippocampus and periventricular structures that are essential for learning and spatial memory have been reported (Shoham et al., 2003; Weinstock and Shoham, 2004). Glial fibrillary acidic protein (GFAP), an indicator of reactive astroglial changes, was also found to be increased in the septum, hippocampus and striatum of icvSTZ-treated rats (Prickaerts et al., 1999). Furthermore, the authors observed that ChAT activity was decreased only in the hippocampus, which was correlated with the hippocampal GFAP contents, suggesting that cholinergic deficit is a consequence of a direct damage to the hippocampus (Prickaerts et al., 1999). Central administration of STZ also promotes extensive cell loss, inferred from the increase in the volume of the ventricular system (Prickaerts et al., 2000; Shoham et al., 2003). Grieb and collaborators (2004) also verified a significant enlargement of the trans-Golgi segments 3 weeks after STZ administration. Despite the effects of STZ did not resemble Golgi atrophy and fragmentation described in neurons from disease-prone brain structures of patients with AD, the authors proposed that proamyloidogenic processing of AβPP may occur preferentially in the trans-Golgi segment, such that the observed early response of neuronal ultrastructure to desensitization of IR may predispose cells to form Aβ deposits (Grieb et al., 2004). Additionally, icvSTZ administration was also shown to induce brain atrophy, mainly due to neuronal and oligodendroglial cell loss mediated by apoptosis, mitochondrial dysfunction, neuroinflammation and oxidative stress (Lester-Coll et al., 2006). Indeed, impaired insulin signaling has been linked to increased oxidative stress (Droge and Kinscherf, 2008) and mitochondrial dysfunction in neuronal cells (Hoyer and Lannert, 1999). Accordingly, significant elevation in malondialdehyde levels and decreased levels of glutathione have been described after icvSTZ administration, which suggest that STZ-induced learning and memory impairment is associated with exacerbated oxidative stress in rats (Ishrat et al., 2006; Pathan et al., 2006; Sharma and Gupta, 2001, 2002). Interestingly, icvSTZ induce reactive gliosis and oxidative stress at 1 week post-treatment, preceding the induction of memory deficits at 3 weeks post-treatment (Sharma and Gupta, 2001; Shoham et al., 2007). Moreover, it was clearly demonstrated that icvSTZ upregulates genes such as glial derived neurotrophic factor, brain derived neurotrophic factor (BDNF) and integrin-alpha-M and downregulates early gene-transcription-factor nerve growth factor-IB and methallothionein-1/2 demonstrating that transcription and inflammatory factors, apoptosis inducers, cell survival and oxidative stress-involving proteins are altered (Grunblatt et al., 2006). In contrast, decreased levels of BDNF were found in postmortem AD temporal cortex (Lee et al., 2005). It was recently reported that BDNF serum values are increased in early stages of AD, probably representing a compensatory repair mechanism in early neurodegeneration (Laske et al., 2006), which could explain the upregulation of BDNF in icvSTZ rat model of sAD.
Overall, the similarities between human sAD and the icvSTZ experimental model are obvious. icvSTZ administration: 1) induces biochemical alterations, characterized by impaired cerebral glucose and energy metabolism, reduction in CBF, oxidative stress and cholinergic dysfunction; 2) causes morphological alterations resulting in neuronal and oligodendroglial cell damage and loss; and 3) results in a progressive deterioration of learning and memory. That said, icvSTZ rat model represent a feasible experimental approach to explore the underlying cellular and molecular mechanisms involved in the initial stages of sAD pathology that are not detectable in the human postmortem brain.
Conclusions
Insulin has been documented to play multifaceted roles in the brain and is intimately involved in brain glucose and energy metabolism as well as in cognitive function. Not surprisingly, impairments in brain insulin and disturbed insulin signal transduction have been suggested to be triggers and/or mediators of sAD. Indeed, sAD is characterized by early abnormalities on brain glucose and energy metabolism, proposing that these early metabolic changes caused by an “insulin resistant brain state” are responsible for the increased Aβ burden, tau hyperphosphorylation and impairment in cognitive function. Accumulating evidence shows that the icv injection of diabetogenic STZ desensitizes neuronal IRs, producing neurochemical and brain glucose metabolism changes, as well as long-term and progressive deficit in learning, memory and cognitive behavior that resemble changes found in the brain of patients with AD. In this way, icvSTZ could represent a promising experimental tool to target/modulate brain insulin signaling and bring new insights on the mechanistic events underlying sAD. Central STZ administration will be also helpful to investigate the feasibility of new therapeutic strategies aimed to prevent and/or mitigate sAD.
Acknowledgments
Work in the authors’ laboratories is supported by the National Institutes of Health (AG031852 to XWZ and AG028679 to MAS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamo M, Raizada MK, LeRoith D. Insulin and insulin-like growth factor receptors in the nervous system. Mol. Neurobiol. 1989;3:71–100. doi: 10.1007/BF02935589. [DOI] [PubMed] [Google Scholar]
- Agrawal R, Tyagi E, Shukla R, Nath C. A study of brain insulin receptors, AChE activity and oxidative stress in rat model of ICV STZ induced dementia. Neuropharmacology. 2009;56:779–787. doi: 10.1016/j.neuropharm.2009.01.005. [DOI] [PubMed] [Google Scholar]
- Arluison M, Quignon M, Nguyen P, Thorens B, Leloup C, Penicaud L. Distribution and anatomical localization of the glucose transporter 2 (GLUT2) in the adult rat brain--an immunohistochemical study. J. Chem. Neuroanat. 2004a;28:117–136. doi: 10.1016/j.jchemneu.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Arluison M, Quignon M, Thorens B, Leloup C, Penicaud L. Immunocytochemical localization of the glucose transporter 2 (GLUT2) in the adult rat brain. II. Electron microscopic study. J. Chem. Neuroanat. 2004b;28:137–146. doi: 10.1016/j.jchemneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Azari NP, Pettigrew KD, Schapiro MB, Haxby JV, Grady CL, Pietrini P, Salerno JA, Heston LL, Rapoport SI, Horwitz B. Early detection of Alzheimer's disease: a statistical approach using positron emission tomographic data. J. Cereb. Blood Flow Metab. 1993;13:438–447. doi: 10.1038/jcbfm.1993.58. [DOI] [PubMed] [Google Scholar]
- Banks WA. The source of cerebral insulin. Eur. J. Pharmacol. 2004;490:5–12. doi: 10.1016/j.ejphar.2004.02.040. [DOI] [PubMed] [Google Scholar]
- Baskin DS, Browning JL, Pirozzolo FJ, Korporaal S, Baskin JA, Appel SH. Brain choline acetyltransferase and mental function in Alzheimer disease. Arch. Neurol. 1999;56:1121–1123. doi: 10.1001/archneur.56.9.1121. [DOI] [PubMed] [Google Scholar]
- Bhat NR. Linking cardiometabolic disorders to sporadic Alzheimer's disease: a perspective on potential mechanisms and mediators. J. Neurochem. 2010;115:551–562. doi: 10.1111/j.1471-4159.2010.06978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blokland A, Jolles J. Spatial learning deficit and reduced hippocampal ChAT activity in rats after an ICV injection of streptozotocin. Pharmacol. Biochem. Behav. 1993;44:491–494. doi: 10.1016/0091-3057(93)90497-h. [DOI] [PubMed] [Google Scholar]
- Blokland A, Jolles J. Behavioral and biochemical effects of an ICV injection of streptozotocin in old Lewis rats. Pharmacol. Biochem. Behav. 1994;47:833–837. doi: 10.1016/0091-3057(94)90284-4. [DOI] [PubMed] [Google Scholar]
- Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA, Murri L, Rapoport SI, Solaini G. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer's disease. Neurobiol. Aging. 2002;23:371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- Bryant NJ, Govers R, James DE. Regulated transport of the glucose transporter GLUT4. Nat Rev Mol Cell Biol. 2002;3:267–277. doi: 10.1038/nrm782. [DOI] [PubMed] [Google Scholar]
- Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Ann. Neurol. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- Buee L, Hof PR, Bouras C, Delacourte A, Perl DP, Morrison JH, Fillit HM. Pathological alterations of the cerebral microvasculature in Alzheimer's disease and related dementing disorders. Acta Neuropathol. 1994;87:469–480. doi: 10.1007/BF00294173. [DOI] [PubMed] [Google Scholar]
- Burns JM, Donnelly JE, Anderson HS, Mayo MS, Spencer-Gardner L, Thomas G, Cronk BB, Haddad Z, Klima D, Hansen D, Brooks WM. Peripheral insulin and brain structure in early Alzheimer disease. Neurology. 2007;69:1094–1104. doi: 10.1212/01.wnl.0000276952.91704.af. [DOI] [PubMed] [Google Scholar]
- Cao D, Lu H, Lewis TL, Li L. Intake of sucrose-sweetened water induces insulin resistance and exacerbates memory deficits and amyloidosis in a transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2007;282:36275–36282. doi: 10.1074/jbc.M703561200. [DOI] [PubMed] [Google Scholar]
- Cardoso S, Correia S, Santos RX, Carvalho C, Santos MS, Oliveira CR, Perry G, Smith MA, Zhu X, Moreira PI. Insulin is a two-edged knife on the brain. Journal of Alzheimer's Disease. 2009;18:483–507. doi: 10.3233/JAD-2009-1155. [DOI] [PubMed] [Google Scholar]
- Castellani RJ, Harris PL, Sayre LM, Fujii J, Taniguchi N, Vitek MP, Founds H, Atwood CS, Perry G, Smith MA. Active glycation in neurofibrillary pathology of Alzheimer disease: N(epsilon)-(carboxymethyl) lysine and hexitol-lysine. Free Radic. Biol. Med. 2001;31:175–180. doi: 10.1016/s0891-5849(01)00570-6. [DOI] [PubMed] [Google Scholar]
- Cheng CM, Tseng V, Wang J, Wang D, Matyakhina L, Bondy CA. Tau is hyperphosphorylated in the insulin-like growth factor-I null brain. Endocrinology. 2005;146:5086–5091. doi: 10.1210/en.2005-0063. [DOI] [PubMed] [Google Scholar]
- Cole GM, Frautschy SA. The role of insulin and neurotrophic factor signaling in brain aging and Alzheimer's Disease. Exp. Gerontol. 2007;42:10–21. doi: 10.1016/j.exger.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch. Gen. Psychiatry. 1999;56:1135–1140. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Czech MP, Corvera S. Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 1999;274:1865–1868. doi: 10.1074/jbc.274.4.1865. [DOI] [PubMed] [Google Scholar]
- Davis S, Laroche S. Mitogen-activated protein kinase/extracellular regulated kinase signalling and memory stabilization: a review. Genes, brain, and behavior. 2006;5 Suppl 2:61–72. doi: 10.1111/j.1601-183X.2006.00230.x. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr, Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: relevance to Alzheimer's disease. J. Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J. Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer's disease. J. Alzheimers Dis. 2006;9:167–181. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- Deng Y, Li B, Liu Y, Iqbal K, Grundke-Iqbal I, Gong CX. Dysregulation of insulin signaling, glucose transporters, O-GlcNAcylation, and phosphorylation of tau and neurofilaments in the brain: Implication for Alzheimer's disease. Am. J. Pathol. 2009;175:2089–2098. doi: 10.2353/ajpath.2009.090157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaskar SU, Giddings SJ, Rajakumar PA, Carnaghi LR, Menon RK, Zahm DS. Insulin gene expression and insulin synthesis in mammalian neuronal cells. J. Biol. Chem. 1994;269:8445–8454. [PubMed] [Google Scholar]
- Doreulee N, Sergeeva OA, Yanovsky Y, Chepkova AN, Selbach O, Godecke A, Schrader J, Haas HL. Cortico-striatal synaptic plasticity in endothelial nitric oxide synthase deficient mice. Brain Res. 2003;964:159–163. doi: 10.1016/s0006-8993(02)04121-5. [DOI] [PubMed] [Google Scholar]
- Droge W, Kinscherf R. Aberrant insulin receptor signaling and amino acid homeostasis as a major cause of oxidative stress in aging. Antioxid. Redox Signal. 2008;10:661–678. doi: 10.1089/ars.2007.1953. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Duelli R, Schrock H, Kuschinsky W, Hoyer S. Intracerebroventricular injection of streptozotocin induces discrete local changes in cerebral glucose utilization in rats. Int. J. Dev. Neurosci. 1994;12:737–743. doi: 10.1016/0736-5748(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Phan T, Han S, Wang H, Chan GC, Scheiner ZS, Storm DR. Circadian oscillation of hippocampal MAPK activity and cAmp: implications for memory persistence. Nat. Neurosci. 2008;11:1074–1082. doi: 10.1038/nn.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsner M, Guldbakke B, Tiedge M, Munday R, Lenzen S. Relative importance of transport and alkylation for pancreatic beta-cell toxicity of streptozotocin. Diabetologia. 2000;43:1528–1533. doi: 10.1007/s001250051564. [DOI] [PubMed] [Google Scholar]
- English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J. Biol. Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- Erol A. An integrated and unifying hypothesis for the metabolic basis of sporadic Alzheimer's disease. J. Alzheimers Dis. 2008;13:241–253. doi: 10.3233/jad-2008-13302. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Bernstein HG, Engelsberger S, Humrich J, Laufer S, Muschner D, Thalheimer A, Turk A, Hoyer S, Zochling R, Boissl KW, Jellinger K, Riederer P. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J. Neural Transm. 1998;105:423–438. doi: 10.1007/s007020050068. [DOI] [PubMed] [Google Scholar]
- Frolich L, Blum-Degen D, Riederer P, Hoyer S. A disturbance in the neuronal insulin receptor signal transduction in sporadic Alzheimer's disease. Ann. N. Y. Acad. Sci. 1999;893:290–293. doi: 10.1111/j.1749-6632.1999.tb07839.x. [DOI] [PubMed] [Google Scholar]
- Games D, Buttini M, Kobayashi D, Schenk D, Seubert P. Mice as models: transgenic approaches and Alzheimer's disease. J. Alzheimers Dis. 2006;9:133–149. doi: 10.3233/jad-2006-9s316. [DOI] [PubMed] [Google Scholar]
- Gasparini L, Gouras GK, Wang R, Gross RS, Beal MF, Greengard P, Xu H. Stimulation of beta-amyloid precursor protein trafficking by insulin reduces intraneuronal beta-amyloid and requires mitogen-activated protein kinase signaling. J. Neurosci. 2001;21:2561–2570. doi: 10.1523/JNEUROSCI.21-08-02561.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini L, Xu H. Potential roles of insulin and IGF-1 in Alzheimer's disease. Trends Neurosci. 2003;26:404–406. doi: 10.1016/S0166-2236(03)00163-2. [DOI] [PubMed] [Google Scholar]
- Gimenez-Llort L, Schiffmann SN, Shmidt T, Canela L, Camon L, Wassholm M, Canals M, Terasmaa A, Fernandez-Teruel A, Tobena A, Popova E, Ferre S, Agnati L, Ciruela F, Martinez E, Scheel-Kruger J, Lluis C, Franco R, Fuxe K, Bader M. Working memory deficits in transgenic rats overexpressing human adenosine A2A receptors in the brain. Neurobiol. Learn. Mem. 2007;87:42–56. doi: 10.1016/j.nlm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Goedert M, Spillantini MG. A century of Alzheimer's disease. Science. 2006;314:777–781. doi: 10.1126/science.1132814. [DOI] [PubMed] [Google Scholar]
- Grieb P, Kryczka T, Fiedorowicz M, Frontczak-Baniewicz M, Walski M. Expansion of the Golgi apparatus in rat cerebral cortex following intracerebroventricular injections of streptozotocin. Acta Neurobiol Exp (Wars) 2004;64:481–489. doi: 10.55782/ane-2004-1531. [DOI] [PubMed] [Google Scholar]
- Grunblatt E, Koutsilieri E, Hoyer S, Riederer P. Gene expression alterations in brain areas of intracerebroventricular streptozotocin treated rat. J. Alzheimers Dis. 2006;9:261–271. doi: 10.3233/jad-2006-9305. [DOI] [PubMed] [Google Scholar]
- Grunblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J. Neurochem. 2007;101:757–770. doi: 10.1111/j.1471-4159.2006.04368.x. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Havrankova J, Roth J, Brownstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- Hellweg R, Nitsch R, Hock C, Jaksch M, Hoyer S. Nerve growth factor and choline acetyltransferase activity levels in the rat brain following experimental impairment of cerebral glucose and energy metabolism. J. Neurosci. Res. 1992;31:479–486. doi: 10.1002/jnr.490310310. [DOI] [PubMed] [Google Scholar]
- Henneberg N, Hoyer S. Desensitization of the neuronal insulin receptor: a new approach in the etiopathogenesis of late-onset sporadic dementia of the Alzheimer type (SDAT)? Arch. Gerontol. Geriatr. 1995;21:63–74. doi: 10.1016/0167-4943(95)00646-3. [DOI] [PubMed] [Google Scholar]
- Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA. Mitochondrial abnormalities in Alzheimer's disease. J. Neurosci. 2001;21:3017–3023. doi: 10.1523/JNEUROSCI.21-09-03017.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer's disease. FASEB Journal. 2004;18:902–904. doi: 10.1096/fj.03-0978fje. [DOI] [PubMed] [Google Scholar]
- Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J. Biol. Chem. 1997;272:19547–19553. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Risk factors for Alzheimer's disease during aging. Impacts of glucose/energy metabolism. J. Neural Transm. Suppl. 1998;54:187–194. doi: 10.1007/978-3-7091-7508-8_18. [DOI] [PubMed] [Google Scholar]
- Hoyer S. The brain insulin signal transduction system and sporadic (type II) Alzheimer disease: an update. J. Neural Transm. 2002;109:341–360. doi: 10.1007/s007020200028. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Causes and consequences of disturbances of cerebral glucose metabolism in sporadic Alzheimer disease: therapeutic implications. Adv. Exp. Med. Biol. 2004a;541:135–152. doi: 10.1007/978-1-4419-8969-7_8. [DOI] [PubMed] [Google Scholar]
- Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur. J. Pharmacol. 2004b;490:115–125. doi: 10.1016/j.ejphar.2004.02.049. [DOI] [PubMed] [Google Scholar]
- Hoyer S, Lannert H. Inhibition of the neuronal insulin receptor causes Alzheimer-like disturbances in oxidative/energy brain metabolism and in behavior in adult rats. Ann. N. Y. Acad. Sci. 1999;893:301–303. doi: 10.1111/j.1749-6632.1999.tb07842.x. [DOI] [PubMed] [Google Scholar]
- Hoyer S, Nitsch R. Cerebral excess release of neurotransmitter amino acids subsequent to reduced cerebral glucose metabolism in early-onset dementia of Alzheimer type. J. Neural Transm. 1989;75:227–232. doi: 10.1007/BF01258634. [DOI] [PubMed] [Google Scholar]
- Huang HM, Ou HC, Xu H, Chen HL, Fowler C, Gibson GE. Inhibition of alpha-ketoglutarate dehydrogenase complex promotes cytochrome c release from mitochondria, caspase-3 activation, and necrotic cell death. J. Neurosci. Res. 2003;74:309–317. doi: 10.1002/jnr.10756. [DOI] [PubMed] [Google Scholar]
- Ishiguro K, Shiratsuchi A, Sato S, Omori A, Arioka M, Kobayashi S, Uchida T, Imahori K. Glycogen synthase kinase 3 beta is identical to tau protein kinase I generating several epitopes of paired helical filaments. FEBS Lett. 1993;325:167–172. doi: 10.1016/0014-5793(93)81066-9. [DOI] [PubMed] [Google Scholar]
- Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, Ahmad AS, Islam F. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav. Brain Res. 2006;171:9–16. doi: 10.1016/j.bbr.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Johnston AM, Pirola L, Van Obberghen E. Molecular mechanisms of insulin receptor substrate protein-mediated modulation of insulin signalling. FEBS Lett. 2003;546:32–36. doi: 10.1016/s0014-5793(03)00438-1. [DOI] [PubMed] [Google Scholar]
- Joseph J, Shukitt-Hale B, Denisova NA, Martin A, Perry G, Smith MA. Copernicus revisited: amyloid beta in Alzheimer's disease. Neurobiol. Aging. 2001;22:131–146. doi: 10.1016/s0197-4580(00)00211-6. [DOI] [PubMed] [Google Scholar]
- Kahn CR, White MF. The insulin receptor and the molecular mechanism of insulin action. J. Clin. Invest. 1988;82:1151–1156. doi: 10.1172/JCI113711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PV, Domoki F, Lenti L, Gaspar T, Institoris A, Snipes JA, Busija DW. Cerebrovascular responses to insulin in rats. J. Cereb. Blood Flow Metab. 2009a;29:1955–1967. doi: 10.1038/jcbfm.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katakam PV, Domoki F, Snipes JA, Busija AR, Jarajapu YP, Busija DW. Impaired mitochondria-dependent vasodilation in cerebral arteries of Zucker obese rats with insulin resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009b;296:R289–R298. doi: 10.1152/ajpregu.90656.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd M. Alzheimer's Disease--an Electron Microscopical Study. Brain. 1964;87:307–320. doi: 10.1093/brain/87.2.307. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Bergeron C, Rajput A, Dozic S, Mastrogiacomo F, Chang LJ, Wilson JM, DiStefano LM, Nobrega JN. Brain cytochrome oxidase in Alzheimer's disease. J. Neurochem. 1992;59:776–779. doi: 10.1111/j.1471-4159.1992.tb09439.x. [DOI] [PubMed] [Google Scholar]
- Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav. Neurosci. 1998;112:1199–1208. doi: 10.1037//0735-7044.112.5.1199. [DOI] [PubMed] [Google Scholar]
- Laske C, Stransky E, Leyhe T, Eschweiler GW, Wittorf A, Richartz E, Bartels M, Buchkremer G, Schott K. Stage-dependent BDNF serum concentrations in Alzheimer's disease. J. Neural Transm. 2006;113:1217–1224. doi: 10.1007/s00702-005-0397-y. [DOI] [PubMed] [Google Scholar]
- Lee J, Fukumoto H, Orne J, Klucken J, Raju S, Vanderburg CR, Irizarry MC, Hyman BT, Ingelsson M. Decreased levels of BDNF protein in Alzheimer temporal cortex are independent of BDNF polymorphisms. Exp. Neurol. 2005;194:91–96. doi: 10.1016/j.expneurol.2005.01.026. [DOI] [PubMed] [Google Scholar]
- Leissring MA, Farris W, Chang AY, Walsh DM, Wu X, Sun X, Frosch MP, Selkoe DJ. Enhanced proteolysis of beta-amyloid in APP transgenic mice prevents plaque formation, secondary pathology, and premature death. Neuron. 2003;40:1087–1093. doi: 10.1016/s0896-6273(03)00787-6. [DOI] [PubMed] [Google Scholar]
- Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. J. Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- Li L, Holscher C. Common pathological processes in Alzheimer disease and type 2 diabetes: a review. Brain Res Rev. 2007;56:384–402. doi: 10.1016/j.brainresrev.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Letters. 2008;582:359–364. doi: 10.1016/j.febslet.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizcano JM, Alessi DR. The insulin signalling pathway. Curr. Biol. 2002;12:R236–R238. doi: 10.1016/s0960-9822(02)00777-7. [DOI] [PubMed] [Google Scholar]
- Lopez-Lopez C, Dietrich MO, Metzger F, Loetscher H, Torres-Aleman I. Disturbed cross talk between insulin-like growth factor I and AMP-activated protein kinase as a possible cause of vascular dysfunction in the amyloid precursor protein/presenilin 2 mouse model of Alzheimer's disease. J. Neurosci. 2007;27:824–831. doi: 10.1523/JNEUROSCI.4345-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luse SA, Smith KR., Jr The ultrastructure of senile plaques. Am. J. Pathol. 1964;44:553–563. [PMC free article] [PubMed] [Google Scholar]
- Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron. 2000;25:649–662. doi: 10.1016/s0896-6273(00)81067-3. [DOI] [PubMed] [Google Scholar]
- Margolis RU, Altszuler N. Insulin in the cerebrospinal fluid. Nature. 1967;215:1375–1376. doi: 10.1038/2151375a0. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Gary DS, Chan SL, Duan W. Perturbed endoplasmic reticulum function, synaptic apoptosis and the pathogenesis of Alzheimer's disease; Biochem. Soc. Symp; 2001. pp. 151–162. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur. J. Pharmacol. 2004;490:13–24. doi: 10.1016/j.ejphar.2004.02.041. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG, Suzuki J, Dolman CE, Nagai T. Aging, Alzheimer's disease, and the cholinergic system of the basal forebrain. Neurology. 1984;34:741–745. doi: 10.1212/wnl.34.6.741. [DOI] [PubMed] [Google Scholar]
- Moloney AM, Griffin RJ, Timmons S, O'Connor R, Ravid R, O'Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer's disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol. Aging. 2010;31:224–243. doi: 10.1016/j.neurobiolaging.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J. Biol. Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Duarte AI, Santos MS, Rego AC, Oliveira CR. An integrative view of the role of oxidative stress, mitochondria and insulin in Alzheimer's disease. J. Alzheimers Dis. 2009;16:741–761. doi: 10.3233/JAD-2009-0972. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Harris PL, Zhu X, Santos MS, Oliveira CR, Smith MA, Perry G. Lipoic acid and N-acetyl cysteine decrease mitochondrial-related oxidative stress in Alzheimer disease patient fibroblasts. J. Alzheimers Dis. 2007a;12:195–206. doi: 10.3233/jad-2007-12210. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Honda K, Zhu X, Nunomura A, Casadesus G, Smith MA, Perry G. Brain and brawn: parallels in oxidative strength. Neurology. 2006;66:S97–S101. doi: 10.1212/01.wnl.0000192307.15103.83. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Oliveira CR. Alzheimer's disease: a lesson from mitochondrial dysfunction. Antioxid. Redox Signal. 2007b;9:1621–1630. doi: 10.1089/ars.2007.1703. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Santos MS, Seica R, Oliveira CR. Brain mitochondrial dysfunction as a link between Alzheimer's disease and diabetes. J. Neurol. Sci. 2007c;257:206–214. doi: 10.1016/j.jns.2007.01.017. [DOI] [PubMed] [Google Scholar]
- Nitsch R, Hoyer S. Local action of the diabetogenic drug, streptozotocin, on glucose and energy metabolism in rat brain cortex. Neurosci. Lett. 1991;128:199–202. doi: 10.1016/0304-3940(91)90260-z. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Pappolla MA, Wade R, Hirai K, Chiba S, Smith MA. RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer's disease. J. Neurosci. 1999;19:1959–1964. doi: 10.1523/JNEUROSCI.19-06-01959.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CR. Cognitive effects of insulin in the central nervous system. Neurosci. Biobehav. Rev. 2001;25:311–323. doi: 10.1016/s0149-7634(01)00016-1. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Mahr NJ, Filley CM, Parks JK, Hughes D, Young DA, Cullum CM. Reduced platelet cytochrome c oxidase activity in Alzheimer's disease. Neurology. 1994;44:1086–1090. doi: 10.1212/wnl.44.6.1086. [DOI] [PubMed] [Google Scholar]
- Pathan AR, Viswanad B, Sonkusare SK, Ramarao P. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006;79:2209–2216. doi: 10.1016/j.lfs.2006.07.018. [DOI] [PubMed] [Google Scholar]
- Paz K, Voliovitch H, Hadari YR, Roberts CT, Jr, LeRoith D, Zick Y. Interaction between the insulin receptor and its downstream effectors. Use of individually expressed receptor domains for structure/function analysis. J. Biol. Chem. 1996;271:6998–7003. doi: 10.1074/jbc.271.12.6998. [DOI] [PubMed] [Google Scholar]
- Pei JJ, Khatoon S, An WL, Nordlinder M, Tanaka T, Braak H, Tsujio I, Takeda M, Alafuzoff I, Winblad B, Cowburn RF, Grundke-Iqbal I, Iqbal K. Role of protein kinase B in Alzheimer's neurofibrillary pathology. Acta Neuropathol. 2003;105:381–392. doi: 10.1007/s00401-002-0657-y. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Plaschke K, Hoyer S. Action of the diabetogenic drug streptozotocin on glycolytic and glycogenolytic metabolism in adult rat brain cortex and hippocampus. Int. J. Dev. Neurosci. 1993;11:477–483. doi: 10.1016/0736-5748(93)90021-5. [DOI] [PubMed] [Google Scholar]
- Plaschke K, Kopitz J, Siegelin M, Schliebs R, Salkovic-Petrisic M, Riederer P, Hoyer S. Insulin-resistant brain state after intracerebroventricular streptozotocin injection exacerbates Alzheimer-like changes in Tg2576 AbetaPP-overexpressing mice. J. Alzheimers Dis. 2010;19:691–704. doi: 10.3233/JAD-2010-1270. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, De Vente J, Honig W, Steinbusch H, Ittersum MMV, Blokland A, Steinbusch HW. Nitric oxide synthase does not mediate neurotoxicity after an i.c.v. injection of streptozotocin in the rat. J. Neural Transm. 2000;107:745–766. doi: 10.1007/s007020070056. [DOI] [PubMed] [Google Scholar]
- Prickaerts J, Fahrig T, Blokland A. Cognitive performance and biochemical markers in septum, hippocampus and striatum of rats after an i.c.v. injection of streptozotocin: a correlation analysis. Behav. Brain Res. 1999;102:73–88. doi: 10.1016/s0166-4328(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Qiu WQ, Walsh DM, Ye Z, Vekrellis K, Zhang J, Podlisny MB, Rosner MR, Safavi A, Hersh LB, Selkoe DJ. Insulin-degrading enzyme regulates extracellular levels of amyloid beta-protein by degradation. J. Biol. Chem. 1998;273:32730–32738. doi: 10.1074/jbc.273.49.32730. [DOI] [PubMed] [Google Scholar]
- Querfurth HW, LaFerla FM. Alzheimer's disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Coupled reductions in brain oxidative phosphorylation and synaptic function can be quantified and staged in the course of Alzheimer disease. Neurotox. Res. 2003;5:385–398. doi: 10.1007/BF03033167. [DOI] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer's disease: link to brain reductions in acetylcholine. J. Alzheimers Dis. 2005;8:247–268. doi: 10.3233/jad-2005-8304. [DOI] [PubMed] [Google Scholar]
- Rocchi A, Orsucci D, Tognoni G, Ceravolo R, Siciliano G. The role of vascular factors in late-onset sporadic Alzheimer's disease. Genetic and molecular aspects. Curr. Alzheimer Res. 2009;6:224–237. doi: 10.2174/156720509788486644. [DOI] [PubMed] [Google Scholar]
- Rocchi A, Pellegrini S, Siciliano G, Murri L. Causative and susceptibility genes for Alzheimer's disease: a review. Brain Res. Bull. 2003;61:1–24. doi: 10.1016/s0361-9230(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Salkovic-Petrisic M, Hoyer S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach. J. Neural Transm. Suppl. 2007:217–233. doi: 10.1007/978-3-211-73574-9_28. [DOI] [PubMed] [Google Scholar]
- Salkovic-Petrisic M, Tribl F, Schmidt M, Hoyer S, Riederer P. Alzheimer-like changes in protein kinase B and glycogen synthase kinase-3 in rat frontal cortex and hippocampus after damage to the insulin signalling pathway. J. Neurochem. 2006;96:1005–1015. doi: 10.1111/j.1471-4159.2005.03637.x. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol. 2002;12:65–71. doi: 10.1016/s0962-8924(01)02207-3. [DOI] [PubMed] [Google Scholar]
- Santos MS, Pereira EM, Carvaho AP. Stimulation of immunoreactive insulin release by glucose in rat brain synaptosomes. Neurochem. Res. 1999;24:33–36. doi: 10.1023/a:1020971812098. [DOI] [PubMed] [Google Scholar]
- Schechter R, Abboud M. Neuronal synthesized insulin roles on neural differentiation within fetal rat neuron cell cultures. Brain Res. Dev. Brain Res. 2001;127:41–49. doi: 10.1016/s0165-3806(01)00110-9. [DOI] [PubMed] [Google Scholar]
- Schechter R, Beju D, Gaffney T, Schaefer F, Whetsell L. Preproinsulin I and II mRNAs and insulin electron microscopic immunoreaction are present within the rat fetal nervous system. Brain Res. 1996;736:16–27. doi: 10.1016/0006-8993(96)00664-6. [DOI] [PubMed] [Google Scholar]
- Schechter R, Whitmire J, Holtzclaw L, George M, Harlow R, Devaskar SU. Developmental regulation of insulin in the mammalian central nervous system. Brain Res. 1992;582:27–37. doi: 10.1016/0006-8993(92)90313-x. [DOI] [PubMed] [Google Scholar]
- Scheepers A, Joost HG, Schurmann A. The glucose transporter families SGLT and GLUT: molecular basis of normal and aberrant function. JPEN. J. Parenter. Enteral Nutr. 2004;28:364–371. doi: 10.1177/0148607104028005364. [DOI] [PubMed] [Google Scholar]
- Schubert M, Gautam D, Surjo D, Ueki K, Baudler S, Schubert D, Kondo T, Alber J, Galldik N, Kustermann E, Arndt S, Jacobs AH, Krone W, Kahn CR, Bruning JC. Role for neuronal insulin resistance in neurodegenerative diseases. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3100–3105. doi: 10.1073/pnas.0308724101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J. Alzheimers Dis. 2001;3:75–80. doi: 10.3233/jad-2001-3111. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68:1021–1029. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002;71:2489–2498. doi: 10.1016/s0024-3205(02)02083-0. [DOI] [PubMed] [Google Scholar]
- Shoham S, Bejar C, Kovalev E, Schorer-Apelbaum D, Weinstock M. Ladostigil prevents gliosis, oxidative-nitrative stress and memory deficits induced by intracerebroventricular injection of streptozotocin in rats. Neuropharmacology. 2007;52:836–843. doi: 10.1016/j.neuropharm.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Shoham S, Bejar C, Kovalev E, Weinstock M. Intracerebroventricular injection of streptozotocin causes neurotoxicity to myelin that contributes to spatial memory deficits in rats. Exp. Neurol. 2003;184:1043–1052. doi: 10.1016/j.expneurol.2003.08.015. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Small GW, Chang CY, Lu CS, Kung De Aburto MA, Chen W, Czernin J, Rapoport SI, Pietrini P, Alexander GE, Schapiro MB, Jagust WJ, Hoffman JM, Welsh-Bohmer KA, Alavi A, Clark CM, Salmon E, de Leon MJ, Mielke R, Cummings JL, Kowell AP, Gambhir SS, Hoh CK, Phelps ME. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- Sims NR, Bowen DM, Allen SJ, Smith CC, Neary D, Thomas DJ, Davison AN. Presynaptic cholinergic dysfunction in patients with dementia. J. Neurochem. 1983;40:503–509. doi: 10.1111/j.1471-4159.1983.tb11311.x. [DOI] [PubMed] [Google Scholar]
- Skoog I. Vascular aspects in Alzheimer's disease. J. Neural Transm. Suppl. 2000;59:37–43. doi: 10.1007/978-3-7091-6781-6_6. [DOI] [PubMed] [Google Scholar]
- Small GW, Komo S, La Rue A, Saxena S, Phelps ME, Mazziotta JC, Saunders AM, Haines JL, Pericak-Vance MA, Roses AD. Early detection of Alzheimer's disease by combining apolipoprotein E and neuroimaging. Ann. N. Y. Acad. Sci. 1996;802:70–78. doi: 10.1111/j.1749-6632.1996.tb32600.x. [DOI] [PubMed] [Google Scholar]
- Smith MA, Richey Harris PL, Sayre LM, Beckman JS, Perry G. Widespread peroxynitrite-mediated damage in Alzheimer's disease. J. Neurosci. 1997;17:2653–2657. doi: 10.1523/JNEUROSCI.17-08-02653.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano DC, Sironi M, Bonfini C, Solerte SB, Govoni S, Racchi M. Insulin regulates soluble amyloid precursor protein release via phosphatidyl inositol 3 kinase-dependent pathway. FASEB Journal. 2000;14:1015–1022. doi: 10.1096/fasebj.14.7.1015. [DOI] [PubMed] [Google Scholar]
- Sonkusare S, Srinivasan K, Kaul C, Ramarao P. Effect of donepezil and lercanidipine on memory impairment induced by intracerebroventricular streptozotocin in rats. Life Sci. 2005;77:1–14. doi: 10.1016/j.lfs.2004.10.036. [DOI] [PubMed] [Google Scholar]
- Steen E, Terry BM, Rivera EJ, Cannon JL, Neely TR, Tavares R, Xu XJ, Wands JR, de la Monte SM. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease--is this type 3 diabetes? J. Alzheimers Dis. 2005;7:63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- Straface E, Matarrese P, Gambardella L, Vona R, Sgadari A, Silveri MC, Malorni W. Oxidative imbalance and cathepsin D changes as peripheral blood biomarkers of Alzheimer disease: a pilot study. FEBS Lett. 2005;579:2759–2766. doi: 10.1016/j.febslet.2005.03.094. [DOI] [PubMed] [Google Scholar]
- Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001;50:537–546. [PubMed] [Google Scholar]
- Takeda S, Sato N, Uchio-Yamada K, Sawada K, Kunieda T, Takeuchi D, Kurinami H, Shinohara M, Rakugi H, Morishita R. Diabetes-accelerated memory dysfunction via cerebrovascular inflammation and Abeta deposition in an Alzheimer mouse model with diabetes. Proc. Natl. Acad. Sci. U. S. A. 2010;107:7036–7041. doi: 10.1073/pnas.1000645107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarkowski E, Issa R, Sjogren M, Wallin A, Blennow K, Tarkowski A, Kumar P. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer's disease and vascular dementia. Neurobiol. Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- Terry RD, Gonatas NK, Weiss M. Ultrastructural Studies in Alzheimer's Presenile Dementia. Am. J. Pathol. 1964;44:269–297. [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Prickaerts J, Meng F, Jolles J. Brain enzyme activities after intracerebroventricular injection of streptozotocin in rats receiving acetyl-L-carnitine. Eur. J. Pharmacol. 1995;287:65–71. doi: 10.1016/0014-2999(95)00475-4. [DOI] [PubMed] [Google Scholar]
- Tota S, Awasthi H, Kamat PK, Nath C, Hanif K. Protective effect of quercetin against intracerebral streptozotocin induced reduction in cerebral blood flow and impairment of memory in mice. Behav. Brain Res. 2010;209:73–79. doi: 10.1016/j.bbr.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Inhibition of Krebs cycle enzymes by hydrogen peroxide: A key role of [alpha]-ketoglutarate dehydrogenase in limiting NADH production under oxidative stress. J. Neurosci. 2000;20:8972–8979. doi: 10.1523/JNEUROSCI.20-24-08972.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger J, McNeill TH, Moxley RT, 3rd, White M, Moss A, Livingston JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience. 1989;31:143–157. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- Valla J, Schneider L, Niedzielko T, Coon KD, Caselli R, Sabbagh MN, Ahern GL, Baxter L, Alexander G, Walker DG, Reiman EM. Impaired platelet mitochondrial activity in Alzheimer's disease and mild cognitive impairment. Mitochondrion. 2006;6:323–330. doi: 10.1016/j.mito.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog. Neurobiol. 2006;79:205–221. doi: 10.1016/j.pneurobio.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Maher F, Simpson IA. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia. 1997;21:2–21. doi: 10.1002/(sici)1098-1136(199709)21:1<2::aid-glia2>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Vekrellis K, Ye Z, Qiu WQ, Walsh D, Hartley D, Chesneau V, Rosner MR, Selkoe DJ. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 2000;20:1657–1665. doi: 10.1523/JNEUROSCI.20-05-01657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABA(A) receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- Wang B, Yang L, Wang Z, Zheng H. Amyolid precursor protein mediates presynaptic localization and activity of the high-affinity choline transporter. Proc. Natl. Acad. Sci. U. S. A. 2007;104:14140–14145. doi: 10.1073/pnas.0704070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson GS, Craft S. Modulation of memory by insulin and glucose: neuropsychological observations in Alzheimer's disease. Eur. J. Pharmacol. 2004;490:97–113. doi: 10.1016/j.ejphar.2004.02.048. [DOI] [PubMed] [Google Scholar]
- Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S. Insulin increases CSF Abeta42 levels in normal older adults. Neurology. 2003;60:1899–1903. doi: 10.1212/01.wnl.0000065916.25128.25. [DOI] [PubMed] [Google Scholar]
- Weinstock M, Shoham S. Rat models of dementia based on reductions in regional glucose metabolism, cerebral blood flow and cytochrome oxidase activity. J. Neural Transm. 2004;111:347–366. doi: 10.1007/s00702-003-0058-y. [DOI] [PubMed] [Google Scholar]
- White MF, Kahn CR. The insulin signaling system. J. Biol. Chem. 1994;269:1–54. [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237–1239. doi: 10.1126/science.7058341. [DOI] [PubMed] [Google Scholar]
- Wozniak M, Rydzewski B, Baker SP, Raizada MK. The cellular and physiological actions of insulin in the central nervous system. Neurochem. Int. 1993;22:1–10. doi: 10.1016/0197-0186(93)90062-a. [DOI] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Young WS., 3rd Periventricular hypothalamic cells in the rat brain contain insulin mRNA. Neuropeptides. 1986;8:93–97. doi: 10.1016/0143-4179(86)90035-1. [DOI] [PubMed] [Google Scholar]
- Yu S, Ding WG. The 45 kDa form of glucose transporter 1 (GLUT1) is localized in oligodendrocyte and astrocyte but not in microglia in the rat brain. Brain Res. 1998;797:65–72. doi: 10.1016/s0006-8993(98)00372-2. [DOI] [PubMed] [Google Scholar]
- Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur. J. Pharmacol. 2004;490:71–81. doi: 10.1016/j.ejphar.2004.02.045. [DOI] [PubMed] [Google Scholar]