Abstract
Recent theoretical advances describing consciousness from information and integration have highlighted the unique role of the thalamocortical system in leading to integrated information and thus, consciousness. Here, we examined the differential distributions of specific and nonspecific thalamocortical functional connections using resting-state fMRI in a group of healthy subjects and vegetative-state patients. We found that both thalamic systems were widely distributed, but they exhibited different patterns. Nonspecific connections were preferentially associated with brain regions involved in higher-order cognitive processing, self-awareness and introspective mentalizing (e.g., the dorsal prefrontal and anterior cingulate cortices). In contrast, specific connections were prevalent in the ventral and posterior part of the prefrontal and precuneus, known involved in representing externally-directed attentions. Significant reductions of functional connectivity in both systems, especially the nonspecific system, were observed in VS. These data suggest that brain networks sustaining information and integration may be differentiated by the nature of their thalamic functional connectivity.
Keywords: Consciousness, Specific and nonspecific thalamocortical functional connectivity, Information and integration, Resting-state functional magnetic resonance imaging (fMRI), Vegetative state (VS)
Introduction
Recent theoretical advances characterizing neural processes giving rise to consciousness have highlighted that information and integration may account for the essential properties of conscious experience (Tononi, 2004, 2008). According to the theory, the level of consciousness is related to the amount of integrated information, which is determined by the repertoire of causal states (information) and the causal interactions of its elements (integration). A graded reduction in either component (information or integration) would result in a graded reduction in the level of consciousness, as seen, for example, in general anesthesia (Alkire et al. 2008).
One neural system in the brain particularly central for carrying out integrative functionalities is the thalamocortical system, with its rich thalamocortical interconnectivity and the reciprocal nature that establishes oscillatory circuits with several cortical layers (Llinas, et al, 1998, 2001). In agreement with the theoretical implications, converging evidence from empirical lesion and stimulation studies has suggested that part of the distributed neural organizations within the thalamocortical system is most certainly essential for determining the content of conscious experience (Tononi and Edelman, 1998; Tononi and Laureys, 2009).
As a central node of brain networks, the thalamus plays an important role supporting consciousness in at least two major ways. First, the specific thalamic nuclei relay sensory and motor messages that may become the contents of consciousness, and second, the nonspecific nuclei are likely involved in the control of cortical arousal originating from the brainstem reticular formation. Accordingly, investigations into the mechanism of loss of consciousness have examined either the interruption of thalamocortical information transfer at the level of the relay nuclei (Angel, 1991; Detsch et al, 1999; Alkire et al., 2000), or alternatively, the failure of nonspecific thalamocortical functional connections to enable the conscious state (Bogen, 1997; Laureys et al, 2000). Of these two divisions of the thalamus, the role of the nonspecific thalamocortical connectivity, involving primarily the intralaminar nuclei, in supporting consciousness has been consistently reported (Llinas and Ribary, 1998, 2001; Van-der-Werf et al. 2002; Green, 2003). It has been known for some time that a selective lesion in the medial/intralaminar thalamus area invariably causes a loss of consciousness (Bogen, 1995; Schiff and Plum, 1999). Recently, pharmacological or electrical stimulation of certain intralaminar nuclei have been used to restore consciousness in anesthetized animals (Alkire et al. 2007) and in one instance, in a minimally conscious patient (Schiff et al., 2007). For patients in the vegetative state (VS), several neuroimaging studies also suggest that the incapacity of VS patients to generate consciousness is most likely linked to a disruption of thalamocortical functional and corticocortical connections (Laureys et al., 1999, 2000; Cauda et al., 2008; Boly et al, 2009). Particularly, in one such study, Laureys and colleagues found using PET imaging that loss and recovery of consciousness in a VS patient were paralleled respectively by impaired and restored thalamocortical functional connectivity between a seed placed in the area of the intralaminar nuclei and the prefrontal and anterior cingulate cortices (Laureys et al., 2000). Despite these advances made in understanding the role of the thalamocortical system to consciousness, the whole brain thalamocortical functional connections with respect to the specific and nonspecific components have not been systematically delineated.
A novel strategy to examine function connectivity in the brain is offered by an imaging technique that measures the spontaneous, low-frequency BOLD (blood oxygenation level dependent) response in the resting-state (Biswal et al., 1995; Fox and Raichle, 2007). Recent studies have established that the resting-state fMRI signal correlates particularly with the power coherence of neuronal activities in low-frequency EEG bands (δ, 1-4 Hz) (Lu et al., 2007), and such slow cortical potentials (SCP) may play an important role for large-scale information integration in the brain (He and Raichle, 2009). Here, we used resting-state BOLD imaging to examine for the first time the specific and nonspecific thalamocortical functional connectivity in healthy subjects and age-matched patients diagnosed with vegetative state.
Our work had two major goals: to determine (1) how cortical regions are functionally partitioned according to their functional connections with the specific and nonspecific thalami in healthy subjects, and (2) how these functional connectivities are altered in VS. To address these questions, we conducted voxelwise functional connectivity analysis using seed voxels manually defined within either the specific or the nonspecific (centromedian (CM) and parafascicular (Pf)) thalamic nuclei in healthy subjects and VS patients. MRI scans were performed in the resting-state and used to derive functional connectivity from the spontaneous low-frequency fluctuations in BOLD signal. We hypothesized that (1) the specific and nonspecific thalamocortical functional connections will demonstrate different spatial patterns, particularly in regions previously implicated in supporting consciousness, and (2) the two thalamocortical systems may be differentially affected in VS patients as compared to healthy individuals.
Methods
Participating Patients
A total of 14 subjects participated in this study including seven healthy volunteers and seven patients diagnosed with vegetative state. Experimental protocols were approved by the Ethics Committee of Capital Medical University (Beijing, China). Informed written consent was obtained from healthy controls and the families of all VS patients. The healthy control subjects are age matched with the VS patients, free of any drug administration, and have no history of neurological or psychiatric conditions or structural brain abnormalities. All seven patients were diagnosed with vegetative state (VS) after repeated clinical tests using both the standard Glasgow Coma Scale (GCS) and the Chinese Vegetative State Scale (CVSS). The clinical profiles of these patients were summarized in Table 1.
Table 1.
Summary of the clinical profiles of participating VS patients.
| Patient | Diagnosis | Age | G | Time (d) | GCS | CVSS |
|---|---|---|---|---|---|---|
| 1 | hydrocephalus, L frontal Contusion, SAH (subarachnoid hemorrhage) | 32 | M | 42 | 9 | 8 |
| 2 | DAI (diffuse axonal injury) | 19 | F | 188 | 11 | 9 |
| 3 | Bi frontal and temporal contusion | 24 | M | 63 | 8 | 6 |
| 4 | R temporal and frontal contusion, thalamus hemorrhage | 45 | M | 46 | 10 | 9 |
| 5 | Bi frontal and R occipital contusion | 48 | M | 59 | 10 | 9 |
| 6 | L temporal and parietal hemorrhage | 43 | F | 66 | 8 | 7 |
| 7 | R frontal, parietal and temporal hemorrhage, hydrocephalus | 61 | F | 82 | 9 | 8 |
L = left, R = right, Bi = bilateral.
MRI Acquisition
Imaging acquisition was performed using a Siemens Trio 3T scanner with a standard head coil. Foam padding and headphones were used to limit head motion and reduce scanner noise. An automated shimming protocol was used to improve B0 magnetic field homogeneity and reduce image distortions. During the scan, all healthy subjects were instructed to relax with eyes closed and avoid any structured imaginations. In healthy and VS subjects, functional axial images were obtained in a duration of 6 minutes using a single-shot gradient EPI pulse sequence (TE, 25 ms; TR, 2s; in-plane resolution 3.75 × 3.75 mm; flip angle, 900; number of slice, 25; slice thickness, 5mm; slice spacing, 1mm; matrix size, 64 × 64), followed by a scan of the high-resolution MPRAGE images for the anatomical reference (TE, 4ms; TR, 10ms; TI, 450ms; flip angle, 120; number of slices, 144; slice thickness, 1 mm; matrix size, 256×192).
Drawing the Regions of Interest
The regions of interest (ROIs) within the thalamus used as seed regions for connectivity analysis were defined in the coronal plane of each individual’s high-resolution MPRAGE images. Specifically, the intralaminar nuclei, CM and Pf, constituting the seed for nonspecific connections, are located at the ventro-medial corners of the left and right thalami (Fig. 1). Anatomical references that could be used to enhance the accuracy of defining these structures include the lateral maximum point of the third ventricle, red nucleus, and the inter-thalamic adhesion. These references are clearly identifiable in the high-resolution anatomical image. The remaining parts of the thalamus were used as an aggregate for seeding the specific connections.
Fig. 1.
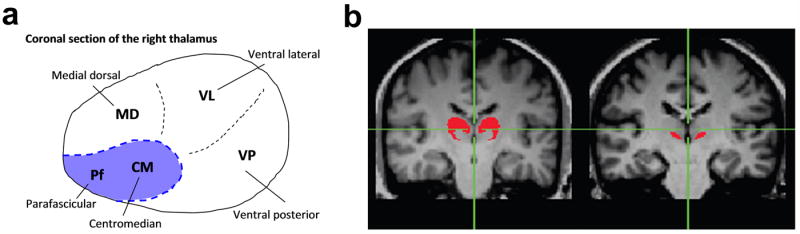
The locations of the thalamic nuclei and seed regions used in connectivity analysis. (a) Coronal section at the level of intralaminar nuclei of interest (i.e., the centromedian (CM) and parafascicular (Pf) nuclei, indicated by the shaded area) in the right thalamus. (b) Anatomical image across the same region of interest illustrating the delineation of the specific thalamic nuclei (left) and the nonspecific nuclei (right) used as seeds for the connectivity analysis.
Data Processing
Imaging data analysis, including drawing the ROIs as described above, was conducted using AFNI (http://afni.nimh.nih.gov/afni). Data preprocessing included despiking, detrending (3dDetrend in AFNI, using the Legendre polynomials with an order of 3), and motion correction (3dvolreg in AFNI using three translational and three rotational parameters obtained for each image). The first four points of the time series for each voxel were discarded to reduce the transient effects. The potential contaminating signals from the white matter (WM) and central spinal fluid (CSF) were extracted from each subject, using segments of WM and CSF manually drawn from the individual’s anatomical images. Then, we constructed eight regressors using signals corresponding to the six-motion parameters obtained from volume registration, WM, and CSF. In the next step, a general linear model (GLM) fitting (3dConvolve in AFNI) was performed, using these regressors to fit the imaging data. The residual signals, after passing through a band-pass filter to only preserve the low-frequency fluctuations within 0.015 to 0.1 Hz (Biswal et al, 1995; Fox and Raichle, 2007), were considered representative of the resting-state activity with potential contaminations minimized.
The averaged voxel time courses of ROIs (the specific and nonspecific thalamic nuclei) were engaged separately in performing voxelwise Pearson cross-correlation (3dfim+ in AFNI) across the whole brain, followed by a Fisher’s linear discriminant analysis applied to the obtained correlation coefficients (r), resulting m = 0.5*ln(1 + r)/(1 - r)). Then, m-values were registered to each individual’s high-resolution anatomic images, which were subsequently transformed into the Talairach space and resampled into 2-mm cubic voxels (adwarp in AFNI). Spatial smoothing was performed using a 6-mm full-width half maximum (FWHM) Gaussian kernel filter to compensate for the intersubject variability. At the final stage, group contrasts were constructed by applying multiple one-sample and two-sample t-tests, as well as an analysis of variance (ANOVA) for the hypothesis testing (p=0.025). Corrections for multiple comparisons were conducted by using the probability and cluster thresholding technique (AlphaSim in AFNI). Here, we applied a mask (in AlphaSim) that restricts consideration to only those voxels that showed significant thalamocortical functional connectivities in healthy subjects to relax the cluster size threshold. Such a procedure resulted in a minimum cluster thresholding of 105 voxels (2-mm cubic) in the Talairach space.
Results
The main results of our study can be summarized in four points. First, in the healthy subjects, both the specific (Fig. 2a) and nonspecific (Fig. 3a) thalamocortical functional connections were distributed in large clusters across the brain. The largest clusters connected with both thalami were in the frontoparietal region (63.4% of the voxels connected with specific nuclei, and 87.4% of the voxels connected with nonspecific nuclei). The specific functional connectivity alone had another significant cluster in the cerebellum (31.2% of voxels). Second, cortical functional connectivity of the nonspecific thalami was more extensive than that of the specific thalami (24,165 vs. 13,407 voxels connected with nonspecific and specific thalamus, respectively). Nonspecific thalamocortical functional connectivity was particularly evident in the dorsolateral and medial frontal cortices. Third, in the VS patients, functional connectivity was significantly reduced in the specific (Fig. 2b) and the nonspecific (Fig. 3b) systems (specific: 9,665 vs. 24,708, nonspecific: 1,324 vs. 30,218 voxels in VS and control, respectively). The nonspecific thalamic functional connectivity suffered a greater reduction, such that the ratio of connected voxels in VS to that in control subjects was 8.9 times larger in the specific system than in the nonspecific. Fourth, functional connections of the two types of the thalamic nuclei showed a limited overlap. In healthy subjects, this overlap occupied 23.7% of the total number of connected voxels (Fig. 4a); in VS patients, the overlap was 8.61% of the significant between-group differences (Fig. 4b). In the following summary, we will present the areal distribution of functional connectivity in greater detail.
Fig. 2.
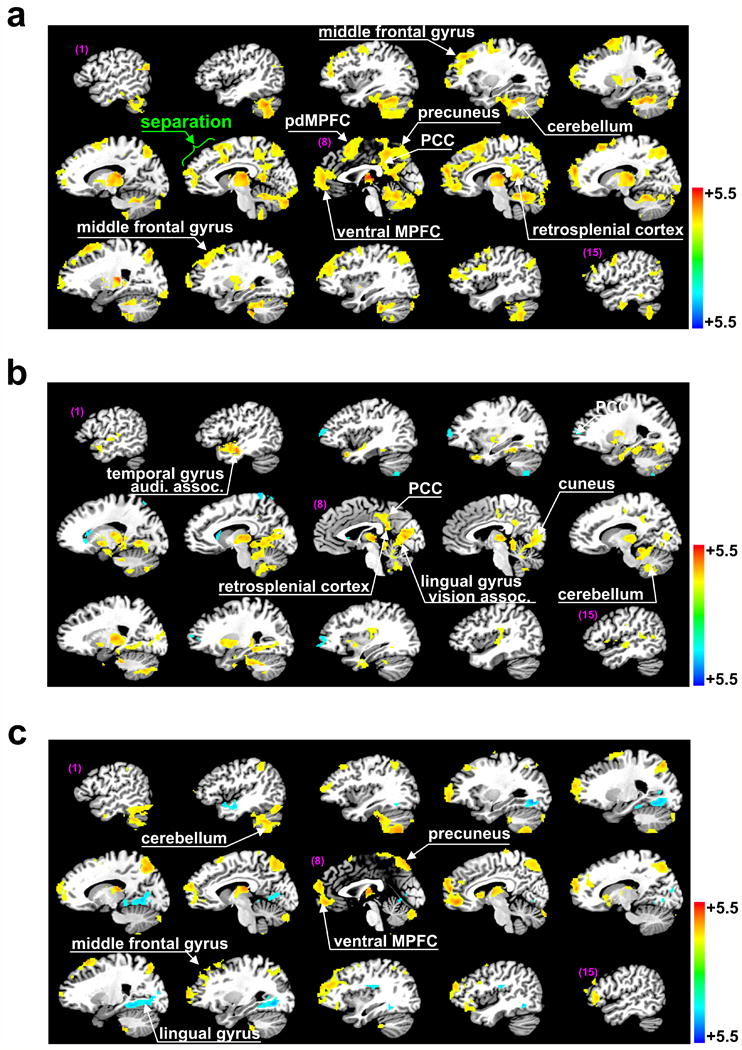
Brain regions demonstrating significant specific thalamic functional connections. Regions of particular interest are highlighted by white arrows. (a) Brain regions identified by one-sample t-tests with significant thalamic functional connectivity in healthy controls (Z-sore, p<0.025 after correction for multiple comparisons for here and elsewhere). (b) The same for VS patients. (c) Brain regions identified by two-sample t-tests (p<0.025) for a significant difference in specific thalamic functional connectivity between healthy subjects and VS patients.
Fig. 3.
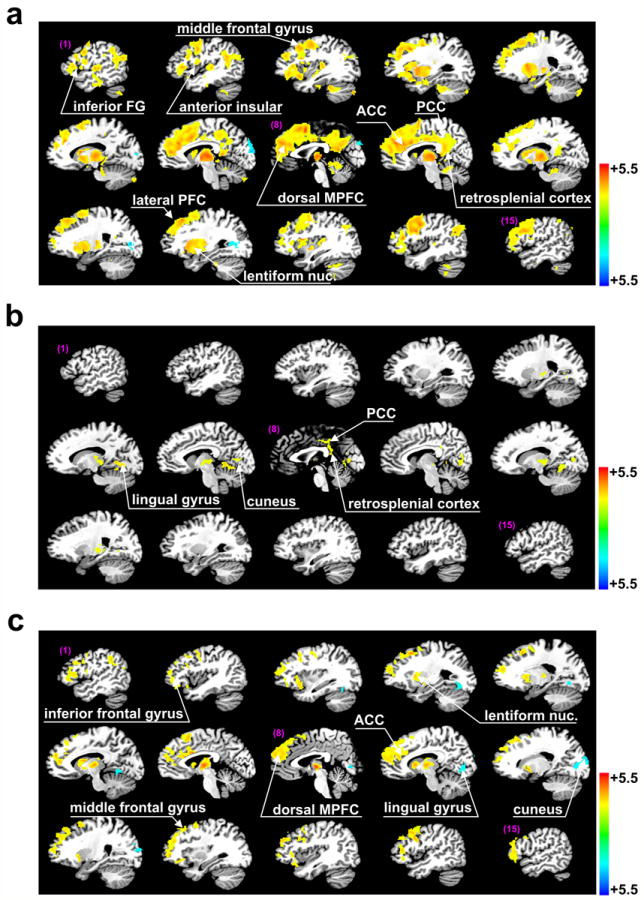
Brain regions demonstrating significant nonspecific thalamic functional connections. Regions of particular interest were highlighted by white arrows. (a) Brain regions identified by one-sample t-tests (p<0.025) with significant thalamic functional connectivity in healthy subjects. (b) The same for VS patients. (c) Brain regions identified by two-sample t-tests (p<0.025) for a significant difference in nonspecific thalamic functional connectivity between healthy subjects and VS patients.
Fig. 4.
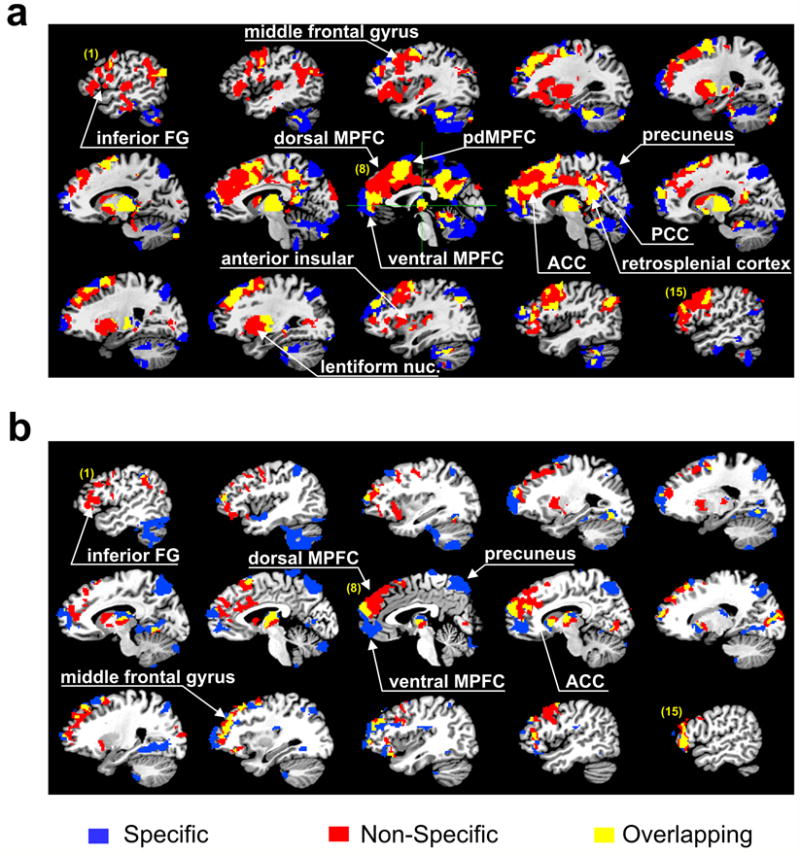
Comparison of brain regions with significant specific and nonspecific thalamic functional connections. Brain regions that demonstrated significant connectivity are identified by the same color regardless of the strength of connectivity. Regions of particular interest are marked by white arrows. (a) Distribution of specific (blue) and the nonspecific (red) and overlapping (yellow) thalamic functional connectivity in healthy subjects. This plot was obtained by collapsing Fig. 2a and Fig. 3a. (b) Distribution of regions with specific (blue), nonspecific (red), and overlapping (yellow) thalamic functional connections that showed a significant difference between VS patients and healthy subjects as obtained by two-sample t-tests. This plot was obtained by collapsing Fig. 2c and Fig. 3c.
Specific thalamocortical functional connectivity in healthy and VS subjects
Brain regions with significant specific thalamic functional connectivity in the healthy subjects (one-sample t-test) included scattered spots in the middle and superior frontal gyrus, part of the ventral medial prefrontal cortex (vMPFC), a posterior segment of the dorsal MPFC (pdMPFC, Fig. 2a7-9), precuneus, limited anterior and dorsal part of the cingulate cortex, part of the posterior cingulate cortex (PCC), retrosplenial cortex, reticular nucleus, and a large cluster of the cerebellum (Fig. 2a, Table 2). In contrast, VS patients demonstrated a complete loss of thalamic functional connections in the prefrontal and cingulate cortices, and the precuneus, whereas thalamic functional connections in the PCC and adjacent retrosplenial cortex (number of voxels, 436), and the cerebellum were partially preserved. VS patients also preserved specific functional connections in small areas of the middle and superior temporal gyrus (secondary auditory association regions, BA 20, 21, Fig. 2b1-2), lingual gyrus and adjacent cuneus (secondary visual association regions, Fig. 2b7-9). Two-sample t-tests indicated a significant difference between healthy subjects and VS patients in part of the vMPFC, precuneus, scattered spots of the middle and superior frontal gyrus, cerebellum, lingual gyrus, cuneus, and a small fraction of the middle temporal gyrus (Fig. 2c, Table 3).
Table 2.
Talairach coordinates of brain regions showing significant specific thalamic functional connections in healthy subjects.
| Brain regions | Side | BA | Talairach coordinates (LPI) |
Z-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle frontal gyrus | L | 9 | -29 | 38 | 26 | 3.31 |
| R | 10 | 37 | 42 | 22 | 2.87 | |
| Superior frontal gyrus | L | 6 | -15 | 6 | 58 | 2.93 |
| R | 6 | 15 | 20 | 54 | 3.46 | |
| Medial frontal gyrus | L | 10 | -2 | 52 | 8 | 3.83 |
| R | 10 | 2 | 52 | 8 | 3.31 | |
| Precuneus | L | 7 | -3 | -70 | 52 | 2.92 |
| R | 7 | 5 | -74 | 47 | 3.38 | |
| ACC | L | 32 | -3 | 39 | 12 | 2.58 |
| R | 32 | 4 | 37 | 15 | 2.50 | |
| Dorsal cingulate gyrus | L | 24 | -8 | 6 | 37 | 3.24 |
| R | 24 | 6 | 6 | 36 | 2.47 | |
| PCC | L | 31 | -3 | -41 | 35 | 2.99 |
| R | 31 | 4 | -35 | 37 | 2.76 | |
| Retrosplenial cortex | L | 29 | -3 | -45 | 14 | 3.29 |
| R | 29 | 4 | -45 | 14 | 3.06 | |
| Reticular nucleus | L | - | -17 | -22 | 10 | 3.52 |
| R | - | 22 | -22 | 10 | 4.71 | |
| Cerebellum | L | - | -24 | -40 | -30 | 3.56 |
| R | - | 11 | -57 | -22 | 4.13 | |
L = left, R = right, BA = Broadman’s Area.
Table 3.
Talairach coordinates of brain regions showing significant difference of specific thalamic functional connections between healthy subjects and VS patients.
| Brain regions | Side | BA | Talairach coordinates (LPI) |
Z-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Middle frontal gyrus | L | 8 | -34 | 22 | 44 | 2.67 |
| R | 10 | 36 | 40 | 21 | 2.86 | |
| Superior frontal gyrus | L | 10 | -13 | 63 | 18 | 2.36 |
| R | 10 | 27 | 62 | 11 | 2.34 | |
| Medial frontal gyrus | L | 10 | -3 | 50 | -1 | 3.15 |
| R | 10 | 3 | 50 | -6 | 3.13 | |
| Precuneus | L | 7 | -18 | -69 | 46 | 3.65 |
| R | 7 | 20 | -72 | 48 | 3.52 | |
| Cerebellum | L | - | -42 | -52 | -38 | 3.33 |
| R | - | 28 | -35 | -42 | 2.90 | |
| Middle temporal gyrus | L | 21 | -43 | -6 | -10 | 2.58 |
| R | - | - | - | - | - | |
| Lingual gyrus | L | 19 | -14 | -62 | -2 | -2.88 |
| R | 19 | 21 | -70 | -4 | -3.10 | |
| Cuneus | L | - | - | - | - | - |
| R | 17 | 18 | -83 | 10 | -3.19 | |
L = left, R = right, BA = Broadman’s Area.
Nonspecific thalamocortical functional connectivity in healthy and VS subjects
Brain regions with significant nonspecific thalamocortical functional connectivity in healthy subjects (one-sample t-test) included large areas of the inferior, middle, and superior frontal gyrus, anterior insular, most of the dorsal MPFC, anterior cingulate cortex (ACC), PCC, retrosplenial cortex, reticular nucleus, lentiform nucleus and a small fraction of the cerebellum (Fig. 3a, Table 4). In contrast, VS patients demonstrated a loss of nearly all nonspecific thalamic functional connections, except those in a small brain area of the PCC and adjacent retrosplenial cortex (108 voxels). Small scattered spots in the lingual gyrus and cuneus showed thalamic functional connectivity that was absent in the healthy subjects (Fig. 3b). Two-sample t-tests indicated significant differences between healthy subjects and VS patients for extensive areas in the inferior, middle and superior frontal gyrus, most of the dorsal MPFC, ACC, lentiform nucleus, and small areas in the lingual gyrus and cuneus (Fig. 3c, Table 5).
Table 4.
Talairach coordinates of brain regions showing significant nonspecific thalamic functional connections in healthy subjects.
| Brain regions | Side | BA | Talairach coordinates (LPI) |
Z-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Anterior insular | L | 13 | -43 | 1 | 14 | 3.10 |
| R | 13 | 35 | 11 | 12 | 3.36 | |
| Inferior frontal gyrus | L | 13 | -43 | 24 | 7 | 3.70 |
| R | 9 | 56 | 19 | 22 | 3.39 | |
| Middle frontal gyrus | L | 6 | -35 | -6 | 44 | 3.74 |
| R | 8 | 46 | 13 | 39 | 3.99 | |
| Superior frontal gyrus | L | 6 | -20 | 14 | 49 | 3.42 |
| R | 8 | 22 | 28 | 46 | 3.37 | |
| Medial frontal gyrus | L | 9 | -1 | 45 | 27 | 4.25 |
| R | 9 | 3 | 44 | 29 | 4.85 | |
| ACC | L | 32 | -4 | 32 | 23 | 3.86 |
| R | 32 | 5 | 30 | 26 | 3.98 | |
| Dorsal cingulate gyrus | L | 24 | -4 | 12 | 33 | 3.64 |
| R | 24 | 4 | -9 | 35 | 3.31 | |
| PCC | L | 31 | -4 | -44 | 33 | 2.35 |
| R | 31 | 4 | -33 | 35 | 3.89 | |
| Retrosplenial cortex | L | 30 | -3 | -47 | 15 | 3.39 |
| R | 30 | 4 | -43 | 20 | 3.48 | |
| Lentiform Mucleus | L | - | -23 | 5 | 5 | 4.02 |
| R | - | 23 | 14 | 0 | 3.63 | |
| Cerebellum | L | - | -32 | -40 | -28 | 3.96 |
| R | - | 3 | -52 | -15 | 3.13 | |
L = left, R = right, BA = Broadman’s Area.
Table 5.
Talairach coordinates of brain regions showing significant difference of nonspecific thalamic functional connections between healthy subjects and VS patients.
| Brain regions | Side | BA | Talairach coordinates (LPI) |
Z-score | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Inferior frontal gyrus | L | 45 | -47 | 21 | 9 | 3.03 |
| R | 45 | 52 | 27 | 8 | 3.23 | |
| middle frontal gyrus | L | 8 | -29 | 19 | 46 | 3.18 |
| R | 9 | 30 | 38 | 25 | 3.32 | |
| Superior frontal gyrus | L | 8 | -22 | 15 | 47 | 4.05 |
| R | 8 | 23 | 35 | 46 | 3.09 | |
| Medial frontal gyrus | L | 9 | -1 | 45 | 27 | 3.52 |
| R | 9 | 4 | 56 | 24 | 3.14 | |
| ACC | L | 32 | -8 | 42 | 12 | 3.59 |
| R | 32 | 9 | 37 | 22 | 3.35 | |
| Lentiform Nucleus | L | - | -22 | 7 | 5 | 3.16 |
| R | - | 20 | 5 | -3 | 3.42 | |
| Lingual gyrus | L | 19 | -21 | -65 | -6 | -2.48 |
| R | 18 | 14 | -73 | 3 | -2.77 | |
| Cuneus | L | - | - | - | - | - |
| R | - | 21 | -88 | 11 | -2.82 | |
L = left, R = right, BA = Broadman’s Area.
Specific vs. nonspecific thalamocortical functional connectivity in healthy and VS subjects
To aid in the visual comparison of the distributions of specific and nonspecific thalamocortical functional connections, we constructed color maps of all connected brain regions (Fig. 4). In the healthy controls (Fig. 4a), the overlapping areas that were significant from one-sample t-test include part of the anterior MPFC near the transition between the ventral and dorsal MFC, a small fraction of the posterior region of the dorsal MPFC, a significant part of brain areas in the PCC and retrosplenial cortex, scattered spots of the bilateral frontal cortices, and the entire reticular nucleus. A plot of the difference in functional connectivity between healthy controls and VS patients (Fig. 4b, two-sample t-test) reveals disparate distributions of the specific and nonspecific thalamocortical functional connectivities, with overlapping areas limited to a small fraction of the anterior MPFC at the transition between the ventral and dorsal MPFC, and scattered spots in the bilateral prefrontal cortices.
Finally, in order to facilitate the comparison of our results to other neuroimaging studies of VS patients, we integrated our results by collapsing main effects induced by the factor of connectivity (i.e. the specific vs. the nonspecific) to describe the overall differences of thalamocortical functional connectivity between the subject groups (ANOVA, repeated measure on subjects). The brain regions identified by the main effects of ANOVA are widely distributed, but mainly include the bilateral and medial prefrontal cortex, ACC, and precuneus (Fig. 5).
Fig. 5.
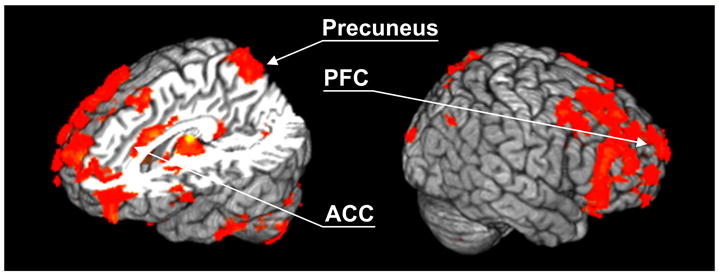
Brain regions showing the main effects of subject groups as identified by F-tests. The results were acquired by collapsing specific and nonspecific thalamic functional connections for each subject group (ANOVA, repeated measure, p<0.025 after the correction for multiple comparisons). Brain regions showing markedly different thalamic functional connections between VS patients and healthy controls include the prefrontal cortex, anterior cingulate cortex (ACC) and the precuneus, consistent with early studies on vegetative state (Laureys et al., 2000, 2004; Owen et al., 2008).
Discussion
The primary purpose of this study was to examine resting-state specific and nonspecific thalamocortical functional connections in the brain in healthy subjects and VS patients using fMRI. We hypothesized that while the specific thalamocortical system is concerned with the representation of various types of information, it is possible that the nonspecific system may play a primarily integrative role. Therefore, the neural components related to information and integration that are essential to consciousness (Tononi, 2004) may partition, according to the supporting specific and nonspecific thalamic systems. Consistent with our initial expectations, the specific and nonspecific thalamocortical functional connections in healthy subjects demonstrated highly organized neural structures, whose elements were widely distributed, yet organized in distinct patterns (Fig 2a, 3a). In contrast, VS patients demonstrated significant reductions in both specific and nonspecific thalamic functional connections, in which the nonspecific connections suffered a more severe loss than the specific connections (Fig 2b, 3b). The differential distributions of thalamic functional connectivities in the frontal and parietal lobes with respect to their functional significance in sustaining information integration will now be discussed.
Differential thalamocortical functional connectivity in the frontal lobe
One of the most noteworthy differences between the specific and nonspecific thalamocortical functional connectivity was observed in the frontal lobe, particularly in the medial frontal cortices (MFC, including the anterior cingulate cortices). In healthy subjects, the nonspecific connectivity was distributed in a nearly continuous manner, extending from the anterior to the dorsal region of the MFC (Fig. 3a7-9). In contrast, the specific connections demonstrated a more complex pattern: brain regions showing significant connectivity were spatially segmented, with one part mainly located in the ventral MFC and another part located in the more posterior region of the dorsal MFC (pdMFC Fig. 2a7-9). The overlapping areas included part of the pdMFC and a small anterior segment of the MFC near the transition between the dorsal and ventral sections (Fig. 4a7-9).
The prefrontal cortex, a major anterior node of the default mode network (DMN), is one of the most metabolically active brain regions in the resting-state (Gusnard and Raichle, 2001). A distinct feature of the prefrontal cortex is its prominent regional specialization for tasks involving a wide variety of cognitive and emotional processes (Raichle, 1998; Gusnard et al., 2001; Northoff et al., 2006; Amodio and Frith, 2006). Specifically, the dorsal MFC is activated and the ventral MFC is deactivated when tasks involve self-referential mental processing. The converse occurs during tasks requiring externally focused attention (Gusnard and Raichle, 2001). A more detailed functional division of the frontal cortex from a recent meta-analysis (Amodio and Frith, 2006) concludes the involvement of the ventral/orbital MFC and the more posterior part of the dorsal MFC in outcome monitoring and action monitoring, respectively, and a large area of the dorsal MFC in tasks involving self-knowledge, person perception and mentalizing. The distribution of the specific and nonspecific thalamocortical functional connections in the MFC matches well with the identified regional specializations, especially the segmentation of the specific component into two separate areas that surround the nonspecific component located in the dorsal MFC. Taken together, the distinct roles of the specific and nonspecific thalamocortical systems in the medial frontal regions in resting-state emerge as follows. The specific thalamocortical functional connectivity involves the ventral/orbital MFC and the more posterior part of the dorsal MFC; these regions appear to represent information about the external world. The dorsal MFC is functionally connected with the nonspecific thalamic nuclei and is activated in tasks involving self-referential or introspective mental activity that require high-order information integration. VS patients, however, demonstrated an entire loss of all thalamic functional connections in the frontal cortex.
Significant thalamocortical functional connections also were observed in the anterior insular cortex (AIC) and inferior frontal gyrus (IFG) (Fig. 4a1-3, 14-15). Specifically, both brain regions demonstrated almost exclusive nonspecific thalamic functional connections, with little specific connectivity (Fig. 4a1-3, 14-15). Recent functional-imaging studies showed that the AIC (often together with ACC) and the adjacent IFG play a fundamental role in various human awareness functions, including anger, fear, heart pain, happiness, sadness, disbelief, social exclusion, time perception, self-recognition, and so on (see Crag, 2009 for a comprehensive review). These subjective feelings and various state of emotional awareness are from the categories requiring the involvement of self-referential and/or higher-order processing that demand integrating information from multiple sensory modalities. We surmise that the dominance of the nonspecific thalamocortical functional connections in the AIC and IFG may be a result of higher-order information integration conducted by the nonspecific system in the resting-state condition. In contrast, VS patients exhibited an entire loss of such connections in the areas of AIC and IFG.
Differential thalamocortical functional connectivity in the parietal lobe
Three neural structures in the posterior part of the brain, including the precuneus, retrosplenial cortex, and PCC, which constitute part of the posterior DMN (Raichle, 1998; Fox and Raichle, 2007), showed significant thalamocortical functional connections. Specifically, the precuneus was solely in connection with the specific thalamus, whereas the PCC and retrosplenial cortex demonstrated connections with both the specific and the nonspecific thalami.
The importance of the precuneus as related to consciousness is highlighted by the fact that its deactivation has been reported in a number of unconscious states, such as sleep, vegetative state, and anesthesia (Cavanan and Trimble, 2006). Parallel with these findings, VS patients demonstrated a complete loss of the specific thalamic functional connections with the precuneus in both one-sample and two-sample t-tests (Fig. 4b). Neuroimaging studies have repeatedly reported that the precuneus, PCC, and adjacent areas are actively involved in various mnemonic functions, such as working memory, episodic-memory retrieval (Wagner et al., 2005; Cavanan and Trimble, 2006; Tulving et al., 1996; Nestor et al., 2003). However, the activation appears to be independent from the imagery content and presentation modalities (Krause et al., 1999; Schmidt, 2002). Similar results also were obtained in interpreting the incoming semantic information (Ferstl & von Cramon 2002, Xu et al. 2005, Whitney et al. 2009). Besides, the PCC and adjacent retrosplenial cortex frequently are activated by emotionally salient stimuli (Maddock, 1999). One major hypothesis about the brain regions of the posterior DMN is that these neural organizations are collectively involved in continuously evaluating information about the external world in the resting-state condition, providing a set of automatic and continuously available monitoring mechanisms before any intended voluntary actions can take place (Gusnard and Raichle, 2001). Our findings are consistent with this view, given the mixed pattern of the resting-state thalamic functional connectivities in these regions. In contrast to healthy subjects, the PCC and adjacent retrosplenial cortex are the only brain areas that remain partially connected with both the specific and nonspecific thalamic nuclei in VS (the only remaining connections of the nonspecific component). Such limited connectivity is, however, supposedly incapable of sustaining conscious perception.
Summary of the thalamocortical connections
Taken together, our results support three major conclusions. First, in healthy subjects, both the specific and nonspecific thalamic functional connectivity are widely distributed, but mostly segregated from each other with only a small degree of overlap. This wide distribution of thalamocortical functional connections makes sense from the point of view that consciousness facilitates widespread access in the brain among otherwise independent brain functions (the conscious access hypothesis, Baars, 2002, 2003). Second, we found a consistent division of brain regions such that all the neural correlates that have been identified in association with higher-order cognitive functions are either predominantly (e.g., dorsal MPFC, AIC, IFG, ACC) or at least partially (e.g., PCC, retrosplenial cortex) connected with the nonspecific thalamic nuclei. In contrast, brain regions presumably responsible for representing information about the external world were found connected with the specific thalamus (e.g., the ventral/orbital MFC and the pdMFC). Third, VS patients exhibited an almost complete loss of both the specific and nonspecific thalamic functional connectivity in the medial and bilateral frontal cortex, and the specific connectivity in the precuneus (Figs. 2, 3). Of the observed reductions, the loss of specific thalamic functional connectivity in VS was more incomplete, consistent with the residual sensory responses typically observed in VS patients (Laureys et al., 2004).
Interpretation in the context of information integration
Over the years, various brain regions have been suggested as candidates for the “seat” of consciousness. Despite the richness of experiments and evidence, it is still difficult to identify the minimal set of brain regions necessary and sufficient for supporting consciousness (Alkire et al, 2008, Tononi and Laureys, 2009). Nevertheless, it is quite certain that a part of neural organizations located within the thalamocortical system is essential for consciousness (Plum, 1991; Llinas and Ribary, 1998, 2001; Tononi, 1998, 2003, 2004). In this study, we intended to bridge the neuroanatomical findings about the thalamocortical system with the theoretical formulation of consciousness by hypothesizing that the two divisions of the thalamocortical system may be specifically concerned with the two criteria of consciousness. As discussed above, the obtained results provided supporting evidence to the hypothesized view. As the reduction of thalamic functional connectivities in VS is considered, whether the critical damage that led to the loss of consciousness in VS was primarily related to the failure of the specific or nonspecific systems may depend on the type and extent of brain injury. With respect to other forms of unconsciousness, Alkire and other investigators (Alkire et al., 2008; Hudetz, 2006, 2009) recently suggested that the degradation of conscious perception during general anesthesia may be best described as “information received but not perceived” within the context of information integration theory of consciousness. However, in VS patients who often suffer from complex traumatic brain injuries, the decrease on both specific and nonspecific functional connections can be attributed to the fact that the injury not only causes a breakdown of network integration (via the nonspecific thalamic network), but also damages, at least in part, the capability of gathering or accessing information in the brain. It will be of interest in future studies, using a similar neuroimaging methodology, to examine whether in a reversible model of unconsciousness, i.e. general anesthesia, the nonspecific functional connectivity (integration) will vary following the same trend of how the consciousness level changes, while the specific thalamic functional connectivity (information) remains relatively stable across different conscious states.
Study limitations
A potential limitation to the present study was that no cardiac or respiratory data were recorded during the scan. Thus, it is possible that some of the unwanted signals were not removed during regressions. Nevertheless, our concerns were mitigated, because the contrast of thalamic connectivity between the subject groups showed consistency with what many other neuroimaging studies reported about VS (Fig. 5; cf. Laureys et al., 2004; Owen and Coleman, 2008, and many others). Moreover, the differential thalamic functional connections provided consistent interpretations to neural activation patterns observed in prior cognition and consciousness studies involving tasks of different nature. We believe this was not a coincidence, but a reflection that our methodology captured the essential differences existing in these two thalamocortical divisions, as well as their functional meanings to information and integration (Tononi, 2004). A second limitation is the structural deformation of the thalamus in VS patients. This added extra difficulties to accurately define the ROIs. We circumvented this problem by using all available spatial references (see Methods) to locate the nonspecific nuclei in the coronal plane. Third, the current technique is useful in identifying brain regions presumably involved in integrating information, but is incapable in telling how, and in what magnitude, the integration occurs. Future studies will address how such processes within the neural networks can be computationally described. A fourth limitation is that in defining the seed voxels for connectivity analysis, only two nonspecific thalamic nuclei were considered. The specific nuclei were lumped together and no further differentiation of sensory motor and other nuclei was performed. A more refined differentiation of thalamocortical functional connectivity will be in order as a result of future technological improvements in functional brain imaging.
Acknowledgments
We would like to thank Douglas B. Ward, Wenjun Li, and Chunming Xie for help with data analysis. We thank Ms. Carrie O’Connor, MA, for editorial assistance. This work was supported in part by National Institute of Health Grant, NIH AG20279, and Chinese National High-tech R&D Program (863 Program), 2008AA02Z414.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alkire MT, Haier RJ, et al. Toward a unified theory of narcosis: brain imaging evidence for a thalamocortical switch as the neurophysiologic basis of anesthetic- induced unconsciousness. Conscious Cogn. 2000;9(3):370–386. doi: 10.1006/ccog.1999.0423. [DOI] [PubMed] [Google Scholar]
- 2.Alkire MT, Hudetz AG, Tononi G. Consciousness and Anesthesia. Science. 2008;322:876–880. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107(2):264–72. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 4.Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci. 2006;7(4):268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- 5.Angel A. The G. L. Brown lecture. Adventures in anaesthesia. Exp Physiol. 1991;76(1):1–38. doi: 10.1113/expphysiol.1991.sp003471. [DOI] [PubMed] [Google Scholar]
- 6.Baars BJ. The conscious access hypothesis: origins and recent evidence. Trends Cogn Sci. 2002;6(1):47–52. doi: 10.1016/s1364-6613(00)01819-2. [DOI] [PubMed] [Google Scholar]
- 7.Baars BJ, Ramsøy TZ, Laureys S. Brain, conscious experience and the observing self. Trends Neurosci. 2003;26(12):671–675. doi: 10.1016/j.tins.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 8.Biswal BB, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Mag Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 9.Bogen JE. On the neurophysiology of consciousness: I. An overview. Consciousness and Cognition. 1995;4:52–62. doi: 10.1006/ccog.1995.1003. [DOI] [PubMed] [Google Scholar]
- 10.Bogen JE. Some neurophysiologic aspects of consciousness. Semin Neurol. 1997;17:95–103. doi: 10.1055/s-2008-1040918. [DOI] [PubMed] [Google Scholar]
- 11.Boly M, Tshibanda L, et al. Functional connectivity in the default network during resting state is preserved in a vegetative but not in a brain dead patient. Human brain mapping. 2009;30(8):2393–400. doi: 10.1002/hbm.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cauda F, Micon BM, Sacco K, et al. Disrupted intrinsic functional connectivity in the vegetative state. J Neurol Neurosurg Psychiatry. 2009;80:429–431. doi: 10.1136/jnnp.2007.142349. [DOI] [PubMed] [Google Scholar]
- 13.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- 14.Craig AD. How do you feel - now? The anterior insula and human awareness. euroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 15.Detsch O, Vahle-Hinz C, et al. Isoflurane induces dose-dependent changes of thalamic somatosensory information transfer. Brain Res. 1999;829(1-2):77–89. doi: 10.1016/s0006-8993(99)01341-4. [DOI] [PubMed] [Google Scholar]
- 16.Green A. The Science and Philosophy of Consciousness. 2003 www.users.globalnet.co.uk/~lka/conz.htm.
- 17.Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 19.Ferstl EC, von Cramon DY. What does the frontomedian cortex contribute to language processing: coherence or theory of mind? Neuroimage. 2002;17:1599–1612. doi: 10.1006/nimg.2002.1247. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.He BJ, Raichle ME. The fMRI signal, slow cortical potential and consciousness. Trends Cognition Sci. 2009;13(7):302–309. doi: 10.1016/j.tics.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudetz AG. Suppressing consciousness: Mechanisms of general anesthesia. Seminars in Anesthesia, Perioperative Medicine and Pain. 2006;25:196–204. [Google Scholar]
- 23.Hudetz AG. Feedback suppression in anesthesia. Is it reversible? Consciousness and Cognition. 2009;18(4):1079–1081. doi: 10.1016/j.concog.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Krause BJ, Schmidt D, Mottaghy FM, Taylor J, Halsband U, Herzog H, et al. Episodic retrieval activates the precuneus irrespective of the imagery content of word pair associates: a PET study. Brain. 1999;122:255–263. doi: 10.1093/brain/122.2.255. [DOI] [PubMed] [Google Scholar]
- 25.Laureys S, et al. Restoration of thalamocortical connectivity after recovery from persistent vegetative state. Lancet. 2000;355:1790–1791. doi: 10.1016/s0140-6736(00)02271-6. [DOI] [PubMed] [Google Scholar]
- 26.Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol. 2004;3:537–546. doi: 10.1016/S1474-4422(04)00852-X. [DOI] [PubMed] [Google Scholar]
- 27.Llinás R, Ribary U, Contreras D, Pedroarena C. The neuronal basis for consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1841–1849. doi: 10.1098/rstb.1998.0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llinás R, Ribary U. Consciousness and the brain. The thalamocortical dialogue in health and disease. Ann N Y Acad Sci. 2001;929:166–175. [PubMed] [Google Scholar]
- 29.Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. PNAS. 2007;104:18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maddock RJ. The retrosplenial cortex and emotion: new insights from functional neuroimaging of the human brain. Trends Neurosci. 1999;22:310–316. doi: 10.1016/s0166-2236(98)01374-5. [DOI] [PubMed] [Google Scholar]
- 31.Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) Eur J Neurosci. 2003;18(9):2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- 32.Northoff G, Heinzel A, Greck M, et al. Self-referential processing in our brain - a meta-analysis of imaging studies on the self. NeuroImage. 2006;31(1):440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 33.Owen AM, Coleman MR. Functional neuroimaging of the vegetative state. Nature Reviews Neuroscience. 2008;9:235–243. doi: 10.1038/nrn2330. [DOI] [PubMed] [Google Scholar]
- 34.Raichle ME. The neural correlates of consciousness: an analysis of cognitive skill learning. Philos Trans R Soc Lond B Biol Sci. 1998;353:1889–901. doi: 10.1098/rstb.1998.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schiff ND, Giacino JT, Kalmar K, Victor JD, Baker K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448(7153):600–603. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 36.Schiff ND, Plum F. The Neurology of Impaired Consciousness: Global Disorders and Implied Models. Association for the Scientific Study of Consciousness. 1999 [Target article] http://www.phil.vt.edu/assc/niko.html.
- 37.Schmidt D. Brain systems engaged in encoding and retrieval of word-pair associates independent of their imagery content or presentation modalities. Neuropsychologia. 2002;40:457–470. doi: 10.1016/s0028-3932(01)00102-6. [DOI] [PubMed] [Google Scholar]
- 38.Tononi G. An information integration theory of consciousness. BMC Neuroscience. 2004;5(1):42. doi: 10.1186/1471-2202-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tononi G. Consciousness as integrated information: a provisional manifesto. Biological Bulletin. 2008;215:216–42. doi: 10.2307/25470707. [DOI] [PubMed] [Google Scholar]
- 40.Tononi G, Edelman GM. Consciousness and Complexity. Science. 1998;282:1846–1851. doi: 10.1126/science.282.5395.1846. [DOI] [PubMed] [Google Scholar]
- 41.Tononi G, Laureys S. The neurology of consciousness: an overview. In: Laureys S, Tononi G, editors. The Neurology of Consciousness: Cognitive Neuroscience and Neuropathology. Elsevier Ltd.; 2008. [Google Scholar]
- 42.Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6:71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 43.Van-der-Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Rev. 2002;39:107–140. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 44.Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9(9):445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Whitney C, Grossman M, Kircher TT. The influence of multiple primes on bottom-up and top-down regulation during meaning retrieval: evidence for 2 distinct neural networks. Cereb Cortex. 2009;19(11):2548–60. doi: 10.1093/cercor/bhp007. [DOI] [PubMed] [Google Scholar]
- 46.Xu J, Kemeny S, Park G, Frattali C, Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. Neuroimage. 2005;25:1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]


