Abstract
Plasma insulin enters the CNS where it interacts with insulin receptors in areas that are related to energy homeostasis and elicits a decrease of food intake and body weight. Here, we demonstrate that consumption of a high-fat (HF) diet impairs the central actions of insulin. Male Long-Evans rats were given chronic (70-day) or acute (3-day) ad libitum access to HF, low-fat (LF), or chow diets. Insulin administered into the 3rd-cerebral ventricle (i3vt) decreased food intake and body weight of LF and chow rats but had no effect on HF rats in either the chronic or the acute experiment. Rats chronically pair-fed the HF diet to match the caloric intake of LF rats, and with body weights and adiposity levels comparable to those of LF rats, were also unresponsive to i3vt insulin when returned to ad lib food whereas rats pair-fed the LF diet had reduced food intake and body weight when administered i3vt insulin. Insulin’s inability to reduce food intake in the presence of the high-fat diet was associated with a reduced ability of insulin to activate its signaling cascade, as measured by pAKT. Finally, i3vt administration of insulin increased hypothalamic expression of POMC mRNA in the LF-but not the HF-fed rats. We conclude that consumption of a HF diet leads to central insulin resistance following short exposure to the diet, and as demonstrated by reductions in insulin signaling and insulin-induced hypothalamic expression of POMC mRNA.
Keywords: obesity, insulin, insulin receptor, food intake, hypothalamus
Introduction
Insulin is important in the regulation of food intake and energy expenditure by the brain (1–6). Plasma insulin is positively correlated with body weight and adiposity (7, 8), and circulating insulin enters the brain by a receptor-mediated transport process (9–11). Obese humans have elevated CSF insulin compared to normal-weight controls, and the levels of both groups decrease following fasting (12). Collectively, these findings imply that insulin, once it gains access to the brain, provides a signal that is proportional to body fat (1, 13, 14). Consistent with this, chronic or acute intracerebroventricular administration of insulin reduces food intake and body weight of baboons, mice and rats (14–17) Reduction of insulin activity in the brain by local administration of antibodies (18, 19), by selective knockout of neuronal insulin receptors (20), by a selective decrease in hypothalamic insulin receptor protein following administration of insulin receptor antisense oligonucleotides (21), or by interference with the insulin signaling cascade (22), results in hyperphagia and weight gain. Hence, manipulations that increase or decrease central insulin signaling cause predictable changes of energy balance, and there is evidence that animals selectively bred to be susceptible to diet-induced obesity have decreased hypothalamic insulin signaling capacity prior to being exposed to a high-energy diet (23, 24). Finally, central insulin activates POMC neurons and reduces NPY mRNA in animals that are maintained on a low fat diet (25).
Consumption of a high-fat (HF) diet is associated with an increased incidence of obesity in animals and humans (26–30). This increase in body adiposity can impair peripheral insulin sensitivity (31) as demonstrated by reductions in insulin signaling (32); and this can be ameliorated by reducing dietary fat content (33–35). It is not known to what extent systemic insulin insensitivity is manifest in the brain. Centrally administered insulin is relatively ineffective in obese Zucker (fa/fa) rats (36, 37) and in rats fed a calorically dense diet that does not result in adiposity gain (38, 39). The present experiments were designed to determine whether chronic or acute consumption of a HF diet, independent of the development of obesity (determined by body weight and body adiposity), results in central insulin resistance, attenuated insulin signaling, and/or reductions in insulin-induced changes in hypothalamic gene expression.
Methods
Animals
Adult male Long-Evans rats (250–300 g; Harlan, IN) were housed in individual tub cages and maintained in a room illuminated from 0100 h to 1300 h in an AAALAC-accredited facility. They had ad lib access to water and pelleted chow unless otherwise noted. All protocols were approved by the University of Cincinnati Institutional Animal Care and Use Committee.
Diets
Three diets were used: A nonpurified low-fat pelleted chow diet (chow: Harlan Teklad Rat Chow), a nutritionally complete, semi-purified HF diet (20% fat by weight, 40% of the calories from fat), and a matched LF diet (4% fat by weight, or 20% of calories from fat AIN93 Dyets, Inc, Bethlehem, PA). Protein, essential minerals and vitamins were equalized per calorie for the HF and LF diets (see Table 1) (40).
Table 1.
Composition (g/kg of diet) of the low-fat (LF) and high-fat (HF) diets.
| Macronutrients | Low-Fat Diet | High-Fat Diet |
|---|---|---|
| Protein, kcal/g | 0.5 | 0.6 |
| Carbohydrate, kcal/g | 2.7 | 2.0 |
| Fat, kcal/g | 0.4 | 1.8 |
| Total, kcal/g | 3.6 | 4.4 |
| Protein, % of total kcal | 14 | 14 |
| Carbohydrate, % of total kcal | 75 | 45 |
| Fat, % of total kcal | 11 | 41 |
| Casein (high nitrogen) | 140 | 164 |
| Cornstarch | 455.7 | 303.1 |
| Dyetrose | 155 | 115 |
| Sucrose | 100 | 89.9 |
| Cellulose | 50 | 58.6 |
| Butter oil | 30 | 190 |
| Soybean oil | 10 | 10 |
| Mineral mix (AIN-93G-MX) | 35 | 41 |
| Vitamin mix (AIN-93-VX) | 10 | 11.7 |
| L-cystine | 1.8 | 2.1 |
| Choline bitartrate | 2.5 | 2.9 |
| Mineral mix (AIN-93M-MX) | 10 | 11.7 |
Chronic Experiment
Groups
Rats (n = 12/group) were maintained on HF, LF, or chow diets for 70 days prior to testing. Previously published data demonstrate that, rats maintained on the HF-diet are obese, hyperleptinemic, hyperinsulinemic and insulin resistant relative to chow and LF rats (30, 41). A fourth group (pair-fed HF or PHF group) consumed the HF diet, but the amount was yoked to the daily caloric intake of the ad lib LF rats and averaged 83% of the intake of the ad lib HF rats over the 70 days. A fifth group (pair-fed LF or PLF group) was fed the LF diet, but was restricted by the same percent of calories as the PHF rats.
I3vt cannulation
Surgery was performed after 9 weeks on the respective diets in the chronic experiment and 10 days prior to the start of the acute experiment. Rats were anesthetized with 1 ml/kg ip injections of a mixture of 70 mg/kg ketamine (Fort Dodge Animal Health, Fort Dodge, IA) and 2 mg/kg xylazine (Lloyd Laboratories, Shenandoah, IA). Rats were placed in a stereotaxic instrument with the skull held horizontally. The sagittal sinus was displaced laterally, and a 21-gauge stainless-steel guide cannula (Plastics One, Roanoke, VA) was lowered directly on the midline, 2.2 mm posterior to bregma, and 7.5 mm ventral to the dura and fixed to the skull with anchor screws and dental acrylic. Obturators that extended 0.5 mm beyond the cannula tips were inserted. When rats regained their pre-operative body weights following surgery, placement of i3vt cannulas was confirmed functionally by verifying that i3vt injection of 10 ng angiotensin II (American Peptides, Sunnyvale CA ) in 1 μl normal saline in water-replete rats elicited an intake of at least 5 ml water within 30 minutes, as has been reported many times (e.g., (39, 42, 43); and see (44)). Of the 40 animals cannulated, 10 animals did not meet this criterion, and therefore were not used.
I3vt Injection Protocol
One week following the angiotensin tests, the HF, LF and chow animals were adapted to a regimen on which food was removed for 4 hr at the same time at the end of the light period each day (0900 to 1300 h). The rats were individually handled for 5 min over 4 successive days to equilibrate their arousal levels before the experiment. Water was available at all times. The animals were maintained on the same diets (HF, LF or chow) throughout the experiment. On separate days, spaced 7 to 10 days apart, rats received insulin (4 mU, i3vt, Iletin II Regular pork insulin, Eli Lilly, Indianapolis, IN, 2 μl) or physiological saline (2 μl) in random order at 0900 h as the food was removed that day. Beginning a week later the protocol was repeated, but with 8 mU insulin or saline as the two injections. Food was returned at 1300 h and intake was assessed after 2, 4 and 20 h.
Insulin Signaling
Seven days after the final procedure, and after all animals had returned to baseline body weight, rats were fasted for 24-hrs. Rats were again injected using the same paradigm above, only this time rats were sacrificed 10 min following insulin (8mU) or saline injections following the protocol of Niswender et al. (45). Whole brains were rapidly removed from anesthetized animals, and the tissue rostral and caudal to the hypothalamus was dissected using a brain block guide. Medial-basal hypothalamus was then rapidly excised and divided in half. One portion was placed in homogenization buffer and stored on ice for Western blot analysis for analysis of insulin signaling.
Western Blots
Tissue was homogenized in ice-cold buffer; (20mM Tris-HCl (pH 7.5), (0.32mM sucrose, 2mM EGTA, 2mM ETA, 0.2mM Na orthovanadate, 50 mM Na+ floride, 0.3 mM PMSF, 5 ug/ml Aprotinin, 5 ug/ml Leupeptin). Samples were then centrifuged at 800g for 10 min at 4 °C and transferred to separate tubes. Supernatant was centrifuged at 20,000 g for 60 min. The supernatant was collected into a separate tube (Cytosolic fraction). The pellet was then resuspended in buffer containing 1% Triton X-100 and solubilized for 1 hr at 4 °C and spun at 20,000g for 30 min. The supernatant (membrane fraction) was collected into a separate tube. Samples were then added with 4X sample buffer (SDS/DTT) and boiled for 1 min. Proteins were resolved on 7% PAGE and transferred to a nitrocellulose membrane. Membranes were then blocked with 5% non-fat milk in TTBS adding appropriate antibody overnight. Membranes were washed and placed in secondary antibody (milk/TTBS) for 1 h. They were then washed 4 times in TTBS and incubated with chemiluminescence reagent for 1 min. and visualized using film. Antibodies used for pAKT (catalogue number (cat #): 7985-R) and AKT (cat # 8312) from Santa Cruz, CA. Data were expressed and analyzed relative to non-phosphorylated protein and/or GAPDH.
Pair Feeding Paradigm
The pair-fed animals(PHF and PLF groups ) also received i3vt cannulas after 9 weeks on the protocol. After 4 days of adaptation to handling, they were returned to ad lib food; i.e., the PHF rats consumed the HF diet ad lib and the PLF rats consumed the LF diet ad lib. On Days 1 through 4 of the return to ad lib food, half of each group was administered 4 mU insulin i3vt once each day and half was administered saline, and food intake and body weight were measured daily. Following this experiment, the animals were maintained on their respective diets ad libitum for one week. The animals were fasted overnight, injected with 8mU insulin or vehicle, and sacrificed 2hrs after the insulin injection, the basal medial hypothalamus was excised for determination of hypothalamic gene expression utilizing qPCR.
Hypothalamic RNA
The hypothalamus was placed in 15 ml of RNAlater (Ambion, Austin, TX) for hypothalamic gene expression. After equilibrating for 48 h at 4° C in RNAlater, hypothalami were dissected and placed in fresh RNAlater (1.5 ml) and held at −80° C until use. TRI-Reagent (MRC, Cincinnati, OH) was used to isolate RNA from individual hypothalami according to manufacturer’s recommendations. A ratio of 50 mg of tissue per ml TRI Reagent was maintained for each sample and bromochloropropane was used in place of chloroform. Real-time quantitative RT-PCR was performed using the SYBR Green (Biorad, Hercules, CA) intercalation method, according to manufacturer’s recommendations. Primer3 design software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) was used to identify a probe:primer set for NPY, AgRP, and POMC (see table 3) on the Bio-Rad iCycler iQ real-time PCR detection. Expression of the target gene was normalized to expression of the L32 housekeeping gene (which did not change as a function of dietary condition) and samples were run in triplicate. The standard curve samples used for RT-PCR were prepared by serial dilution of a RNA sample of known concentration to cover the range of 3000 ng to 3 ng of total RNA.
Body Composition Analysis
Separate weight-matched cohorts of rats were given chronic or acute access to the HF or the LF diets for the same duration as the previously described cohort. These animals were placed in the NMR (Nuclear Magnetic Resonance Machine) for determination of body adiposity on the same day as the other cohort was being sacrificed. Magnetic resonance imaging techniques provided an estimate of total adiposity, lean tissue, fat tissue and water using nuclear magnetic resonance (NMR, EchoMRI, EchoMedical Systems, Houston TX). Unanesthetized rats were placed in a restraint tube and inserted into the device. NMR results were validated by ether extraction at the end of an experiment (46).
Acute Experiment
I3vt insulin and food intake
Comparable groups of rats (ns = 5 to 8/group) were maintained on the chow diet for 9 weeks to make them comparable with the animals in the chronic experiment. They then received i3vt cannulas and upon recovery from surgery and confirmation of cannula placement, were placed on HF or LF diets for 72 h prior to receiving i3vt saline or 10 mU insulin (a higher dose of insulin was used based on preliminary results with lower doses) 8 h into the light phase in the non-fasted condition. Food intake was assessed after 24 h.
Statistical analysis
Data were analyzed by analysis of variance, followed by the Fisher Protected Least Squares Difference (PLSD) test. The bases of significant main effects and interactions were assessed by post-hoc LSD tests. Minimum significance was α < 0.05. Data are expressed as mean ± SEM throughout.
Results
Chronic experiment
Metabolic parameters
Table 2 describes plasma insulin, glucose, body weight and body fat measurements of separate groups of rats maintained on the same 5 diets for 70 days. One cohort of animals was maintained on chow for that duration, and a subset of animals had ad libitum access to the HF diet for 72 hrs prior to the experiment (rats in these experiments did not receive i3vt cannulas). The HF rats weighed significantly more (P < 0.05) than each of the other groups, and the LF and chow groups did not differ statistically (Table 2).
Table 2.
Plasma immunoreactive insulin (IRI), plasma glucose and carcass fat (g fat/100 g carcass) of identically treated rats maintained either on the HF, LF, Chow, PHF, and PLF conditions for 70 days or from animals exposed to HF diet for 72 hrs (from (30), n = 8/group). In each column, values with different superscripts are statistically different (P < 0.05).
| . | Carcass fat (g) | IRI (μU/ml) | Glucose (mM/L) | Body Weight (g) |
|---|---|---|---|---|
| HF | 148.74 ± 8.46a | 178 ± 14a | 5.8 ± 0.4a | 548.93 ± 21.35 a |
| LF | 111.0 ± 4.75b | 122 ± 11b | 5.3 ± 0.3a | 515.41 ± 22.1 |
| CHOW | 100.6 ± 4.0b | 109 ± 10b | 5.3 ± 0.2a | 499.79 ± 16.45 |
| PHF | 113.8 ± 3.0b | 111 ± 10b | 5.4 ± 0.4a | 500.39 ± 8.01 |
| PLF | 108.6 ± 5.2c | 97 ± 13b | 5.0 ± 0.5a | 518.7 ± 7.92 |
| 72 hr HF | 98.9 ± 10.2 | 112 ± 10.7 | 5.3 ± 0.4 | 510.76 ± 14.76 |
Response to i3vt insulin in free-feeding rats
In the LF and chow groups, both doses of i3vt insulin (4 and 8 mU) significantly decreased food intake at each time point relative to saline (p < 0.05 in each group at each point). Figure 1 depicts the data at 24 hr. In contrast, neither dose of insulin altered food intake at any time in the HF rats. Figure 2 depicts change in body weight over 24 h following the administration of saline and insulin. Body weight was significantly reduced following both doses of insulin in the LF and chow groups (p < 0.05 in each group) and not changed relative to the saline condition in the HF group. Hence, insulin reduced food intake and body weight in lean rats consuming either of two low-fat diets but had no effect in obese rats consuming a HF diet.
Figure 1.
Effect of i3vt saline or insulin [4 mU (a) or 8 mU (b)] on 24-hr caloric intake in rats maintained for 10 weeks on chow, the LF diet, or the HF diet. Values represent mean + SE. * = P < 0.05 compared with saline.
Figure 2.
Effect of i3vt saline or insulin [4 mU (a) or 8 mU (b)] on change of body weight in rats maintained for 10 weeks on chow, the LF diet, or the HF diet. Values represent mean + SE. * = P < 0.05 compared with saline.
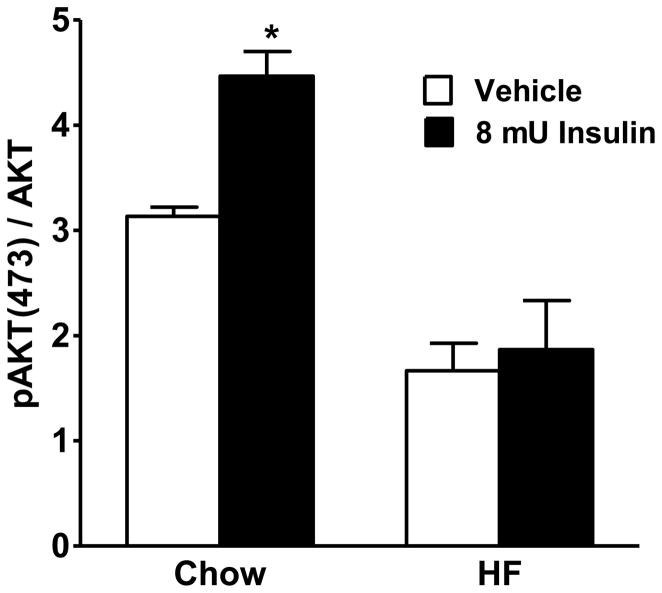
Hypothalamic Insulin Signaling
Consistent with previous reports (45), insulin induced increases in phosphorylation of AKT in the animals maintained on the LF diet, but not in the animals maintained on the HF diet (Figure 3). These observations suggest that, in the presence of a HF diet, insulin is less effective to activate its downstream signaling cascade.
Figure 3.
Insulin-induced increased pAKT relative to AKT in the basal medial hypothalamus of HF and LF rats following 8 mU of insulin or saline. Values represent mean percentage (± SE). * = P < 0.05 compared with 100%.
Response to i3vt insulin in pair-fed rats
Daily administration of insulin significantly reduced food intake relative to saline administration in the PLF group (Figure 4b). In contrast, there was no effect of daily insulin administration on food intake of PHF rats (Figure 4d). All animals in both groups gained weight on Day 1 of the return to ad lib food. Daily insulin elicited a continuous weight loss starting on Day 2 in the PLF group, and there was a significant weight loss by Day 4 (Figure 4a). In contrast, there was no significant difference in body weight between the insulin-treated rats and the saline-treated PHF rats over the four days (Figure 4b).
Figure 4.
Effect of saline or insulin (4 mU/day) by a single daily i3vt administration on body weight (a) and food intake (c) of PLF, and body weight (b) and food intake (d) in PHF groups. Values represent mean (± SE). * = P < 0.05 compared with saline.
Insulin-induced changes in hypothalamic gene expression
The sensitivity of the quantitative PCR assay was determined by amplifying target genes from serial dilutions of an RNA sample of known concentration. The standard curves for NPY, AgRP, and POMC mRNA were obtained by plotting the cycle number of each serial solution. The correlation coefficients were >0.99 for both assays, indicating that under the conditions used, there was a precise log-linear relation in the range between 3000 and 3 ng of total mRNA. Figure 5a depicts the percent change in AgRP mRNA expression in the hypothalamus of HF and LF rats relative to chow rats. Figure 5b depicts changes in NPY, and 5c depicts insulin-induced changes in POMC mRNA. There were no differences in insulin-induced changes in NPY or AgRP in either the LF or the HF groups. However, insulin increased POMC gene expression, consistent with previous data (25) in the LF group but not the HF group.
Figure 5.
Mean percent change of hypothalamic mRNA for AgRP (a), NPY (b) and POMC (c) following i3vt saline or insulin (4 mU). * = P < 0.05 compared with saline.
Acute experiment
The HF rats consumed more calories than the chow rats over the 72 hrs (379 ± 14 vs 240 ± 18 kcal, respectively, p < 0.05). Chow, but not HF rats, significantly reduced 24-hr food intake in response to i3vt insulin (Figure 6).
Figure 6.
Effect of i3vt saline or insulin (10 mU) on 24-hr caloric intake in rats maintained on LF or HF diets for 3 days. * = P < 0.05 compared with saline.
DISCUSSION
Insulin reduces food intake and body weight when it is chronically or acutely administered into the 3rd-cerebral ventricle (i3vt) of normal male rats consuming chow or a LF diet (16, 25, 38, 39, 46–54). The present set of experiments assessed whether i3vt insulin is equally effective in rats maintained chronically on a matched HF diet and, further, whether insulin administration prevents overeating and weight gain in underweight rats returned to free feeding following restricted access to a HF diet. The results are clear and indicate that i3vt insulin decreases food intake and body weight of LF and chow-fed rats but has no effect on HF-fed rats at the doses tested. These studies also assessed whether a short period of prior access to a HF diet is sufficient for central insulin resistance to develop; importantly, 72-hrs consumption of a HF diet was found to be sufficient to reduce central insulin sensitivity, independent of statistically significant changes in body weight or body adiposity (table 2). The magnitude of the decrease in food intake in LF and chow rats is comparable to reductions in food intake previously observed following i3vt insulin administration in male rats at comparable doses (16, 25, 39, 46–48, 51, 54). Additionally, insulin was ineffective in altering hypothalamic POMC gene expression and activating the insulin signaling cascade in animals maintained on the HF diet. These data support the conclusion that, in the presence of a HF diet, insulin is less effective.
The LF and chow diets employed have comparable low fat content, and previous reports have demonstrated that rats consuming such a low proportion of dietary fat are sensitive to i3vt insulin whereas rats consuming higher levels are not (38, 39). However, those experiments failed to address whether insulin insensitivity was a result of increased levels of dietary fat or a result of increased body adiposity. In an attempt to circumvent this ambiguity, rats were maintained with restricted access to the HF diet, matching their caloric intake precisely to that of the LF rats. Where these animals do have increased body adiposity relative to LF fed animals, their adiposity does not reach the increase seen in animals chronically ad libitum fed the HF diet. When these pair-fed rats were returned to ad lib food, those consuming the LF diet (PLF rats) were sensitive to central insulin’s catabolic action, were relatively hypophagic, and maintained a reduced body weight. In contrast, pair-fed rats consuming the HF diet (PHF rats) were hyperphagic when allowed free access to the food and regained lost body weight despite their increased body weights and body fat content, compared to PLF rats. Hence, 10-week consumption of a high proportion of dietary fat, independent of increased adiposity relative to ad libitum fed HF animals, renders the brain insensitive to exogenous insulin, at least with regard to its catabolic action.
Morgan et al. have recently reported that maintenance on a HF diet for 72 hours renders rats relatively insensitive to the catabolic effect of i3vt oleic acid (55). Since oleic acid and insulin, when administered centrally, each reduce food intake as well as hepatic glucose output (56–58), one group of rats was placed on HF diet for only 72 hrs and also exhibited resistance to i3vt insulin independent of changes in body adiposity or increases in body weight. Data from the chronic and the acute experiments suggest that consumption of a HF diet rapidly reduces central insulin sensitivity, and that may be independent of developing increases in body weight and adiposity. However, what was neither tested in the Morgan study nor here is the fact that during the first 72hrs of exposure to the HF diet, rats are hyperphagic presumably due to increased palatability of the HF diet. In rats, paradigms that result in insulin insensitivity also bring about resistance to the effects of leptin and the melanocortin agonist MTII.
Previous studies have demonstrated that a reduction of dietary fat is able to improve peripheral insulin sensitivity in humans (59, 60). In rats, reducing dietary fat from 40% to 30% of total energy rapidly restored peripheral insulin sensitivity (34). Hence, the present findings are consistent with the more general hypothesis that dietary fat induces both central and peripheral insulin resistance, and that this is independent of body adiposity.
To determine the mechanism underlying the decrease of central insulin sensitivity in the HF rats, we assessed activation of the insulin-signaling cascade. We observed that HF-fed rats have attenuated insulin signaling compared to LF animals. In the LF animals, insulin increased pAKT, consistent with insulin-induction of insulin signaling. Importantly, it was previously demonstrated that the anorexigenic effects of insulin are mediated by induction of pPI3K (45). Here, it was demonstrated that the HF diet attenuates the ability of insulin to activate the insulin signaling cascade as demonstrated by the lack of changes in pAKT. These data are consistent with findings that insulin has no effect to regulate food intake and body weight in HF-fed rats. Furthermore, rats that have been selectively bred to be susceptible to become obese when fed a high-energy diet have decreased hypothalamic insulin as well as leptin binding, and this defect is apparent before exposure to the obesity-inducing diet (23, 24). Collectively, these data support the hypothesis that reduced brain insulin signaling (i.e., increased central insulin resistance), whether genetically determined or secondary to consuming a high-fat diet, facilitates becoming obese.
Insulin receptors are expressed in several regions of the central nervous system, with a high density in the hypothalamus (61–64). In particular, neurons in the arcuate nucleus (ARC) that express proopiomelanocorticotropin (POMC), and others that express NPY, both express insulin receptors (1, 25, 65, 66). Because of this, we assessed insulin’s ability to regulate the expression of NPY, AgRP and POMC mRNA. The data here demonstrate that, in animals maintained on a LF diet, insulin induces the expression of POMC mRNA consistent with its anorexigenic action. In rats maintained on a HF diet however, there was no effect of insulin to augment POMC mRNA.
A hallmark characteristic of obesity as well as the metabolic syndrome is peripheral insulin resistance. While the cause(s) of the obesity, as well as of the insulin resistance, is undoubtedly multifactorial, consumption of a HF diet is considered by many to have a causal role. Using a well-controlled model of HF-diet-induced obesity, the present studies demonstrate that obese rats with peripheral insulin resistance also have central insulin resistance; i.e., unlike lean rats maintained on either of two low-fat diets, the HF-obese rats did not reduce their food intake or body weight when administered insulin into the 3rd ventricle, nor do they have changes in insulin-induced activation of the insulin signaling cascade. Further, the development of central insulin resistance occurs within days of exposure to a HF diet. These findings support a role for reduced brain insulin signaling in the development of diet-induced obesity, and suggest that this system is differentially regulated by high fat diet exposure.
Acknowledgments
This research was supported by NIH awards DK 17844, DK 54890, DK 54080, DK 54504, DK 56910, DK 54263, DK 56863 and DK 061857.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Niswender KD, Schwartz MW. Insulin and leptin revisited: adiposity signals with overlapping physiological and intracellular signaling capabilities. Frontiers in Neuroendocrinology. 2003;24:1–10. doi: 10.1016/s0091-3022(02)00105-x. [DOI] [PubMed] [Google Scholar]
- 2.Porte D, Jr, Baskin DG, Schwartz MW. Insulin signaling in the central nervous system: a critical role in metabolic homeostasis and disease from C. elegans to humans. Diabetes. 2005;54:1264–76. doi: 10.2337/diabetes.54.5.1264. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz MW, Porte D., Jr Diabetes, obesity, and the brain. Science. 2005;307:375–9. doi: 10.1126/science.1104344. [DOI] [PubMed] [Google Scholar]
- 5.Woods SC, Seeley RJ, Porte D, Jr, Schwartz MW. Signals that regulate food intake and energy homeostasis. Science. 1998;280:1378–83. doi: 10.1126/science.280.5368.1378. [DOI] [PubMed] [Google Scholar]
- 6.Plum L, Schubert M, Bruning JC. The role of insulin receptor signaling in the brain. Trends Endocrinol Metab. 2005;16:59–65. doi: 10.1016/j.tem.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Bagdade JD, Bierman EL, Porte D., Jr The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J Clin Invest. 1967;46:1549–1557. doi: 10.1172/JCI105646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polonsky KS, Given E, Carter V. Twenty-four-hour profiles and pulsatile patterns of insulin secretion in normal and obese subjects. Journal of Clinical Investigation. 1988;81:442–448. doi: 10.1172/JCI113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baura G, Foster D, Porte D, Jr, Kahn SE, Bergman RN, Cobelli C, Schwartz MW. Saturable transport of insulin from plasma into the central nervous system of dogs in vivo: a mechanism for regulated insulin delivery to the brain. Journal of Clinical Investigation. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz MW, Bergman RN, Kahn SE, Taborsky GJ, Jr, Fisher LD, Sipols AJ, Woods SC, Steil GM, Porte D., Jr Evidence for uptake of plasma insulin into cerebrospinal fluid through an intermediate compartment in dogs. J Clin Invest. 1991;88:1272–1281. doi: 10.1172/JCI115431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods SC, Seeley RJ, Baskin DG, Schwartz MW. Insulin and the blood-brain barrier. Current Pharmaceutical Design. 2003;9:795–800. doi: 10.2174/1381612033455323. [DOI] [PubMed] [Google Scholar]
- 12.Owen OE, Reichard GAJ, Boden G, Shuman C. Comparative measurements of glucose, beta-hydroxybutyrate, acetoacetate, and insulin in blood and cerebrospinal fluid during starvation. Metabolism. 1974;23:7–14. doi: 10.1016/0026-0495(74)90098-5. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MW, Figlewicz DP, Baskin DG, Woods SC, Porte D., Jr Insulin in the brain: a hormonal regulator of energy balance. Endocrine Reviews. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 14.Woods SC. Insulin and the brain: A mutual dependency. Progress in Psychobiology and Physiological Psychology. 1996;16:53–81. [Google Scholar]
- 15.Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–91. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Chavez M, Kaiyala K, Madden LJ, Schwartz MW, Woods SC. Intraventricular insulin and the level of maintained body weight in rats. Behavioral Neuroscience. 1995;109:528–531. doi: 10.1037//0735-7044.109.3.528. [DOI] [PubMed] [Google Scholar]
- 17.Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 18.McGowan MK, Andrews KM, Grossman SP. Chronic intrahyphothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physol-Behav. 1992;51:753–766. doi: 10.1016/0031-9384(92)90112-f. [DOI] [PubMed] [Google Scholar]
- 19.Strubbe JH, Mein CG. Increased feeding in response to bilateral injection of insulin antibodies in the VMH. Physiol Behav. 1977;19:309–313. doi: 10.1016/0031-9384(77)90343-2. [DOI] [PubMed] [Google Scholar]
- 20.Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289:2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- 21.Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci. 2002;5:566–72. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- 22.Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol. 2005;288:R981–6. doi: 10.1152/ajpregu.00675.2004. [DOI] [PubMed] [Google Scholar]
- 24.Irani BG, Dunn-Meynell AA, Levin BE. Altered hypothalamic leptin, insulin, and melanocortin binding associated with moderate-fat diet and predisposition to obesity. Endocrinology. 2007;148:310–6. doi: 10.1210/en.2006-1126. [DOI] [PubMed] [Google Scholar]
- 25.Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. Journal of Neuroscience. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astrup A, Buemann B, Western P, Toubro S, Raben A, Christensen NJ. Obesity as an adaptation to a high-fat diet: evidence from a cross-sectional study. Am J Clin Nutr. 1994;59:350–5. doi: 10.1093/ajcn/59.2.350. [DOI] [PubMed] [Google Scholar]
- 27.Lissner L, Heitmann BL. Dietary fat and obesity: evidence from epidemiology. European Journal of Clinical Nutrition. 1995;49:79–90. [PubMed] [Google Scholar]
- 28.Macdiarmid JI, Cade JE, Blundell JE. High and low fat consumers, their macronutrient intake and body mass index: further analysis from the National Diet and Nutrition Survey of British Adults. British Journal of Clinical Nutrition. 1996;50:505–512. [PubMed] [Google Scholar]
- 29.Roberts SB, Pi-Sunyer FX, Dreher M, Hahn R, Hill JO, Kleinman RE, Peters JC, Ravussin E, Rolls BJ, Yetley E, Booth SL. Physiology of fat replacement and fat reduction: Effects of dietary fat and fat substitutes on energy regulation. Nutrition Reviews. 1998;56:S29–S41. doi: 10.1111/j.1753-4887.1998.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 30.Woods SC, Seeley RJ, Rushing PA, D'Alessio DA, Tso P. A controlled high-fat diet induces an obese syndrome in rats. Journal of Nutrition. 2003;133:1081–1087. doi: 10.1093/jn/133.4.1081. [DOI] [PubMed] [Google Scholar]
- 31.Storlien LH, Baur LA, Kriketos AD, Pan DA, Cooney GJ, Jenkins AB, Calvert GD, Campbell LV. Dietary fats and insulin action. Diabetologia. 1996;39:621–631. doi: 10.1007/BF00418533. [DOI] [PubMed] [Google Scholar]
- 32.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cha MC, Johnson JA, Hsu CY, Boozer CN. High-fat hypocaloric diet modifies carbohydrate utilization of obese rats during weight loss. American Journal of Physiology. 2001;280:E797–E803. doi: 10.1152/ajpendo.2001.280.5.E797. [DOI] [PubMed] [Google Scholar]
- 34.Harris RB, Kor H. Insulin insensitivity is rapidly reversed in rats by reducing dietary fat from 40 to 30% of energy. Journal of Nutrition. 1992;122:1811–1822. doi: 10.1093/jn/122.9.1811. [DOI] [PubMed] [Google Scholar]
- 35.Muurling M, Jong MC, Mensink RP, Hornstra G, Dahlmans VE, Pijl H, Voshol PJ, Havekes LM. A low-fat diet has a higher potential than energy restriction to improve high-fat diet-induced insulin resistance in mice. Metabolism. 2002;51:695–701. doi: 10.1053/meta.2002.32725. [DOI] [PubMed] [Google Scholar]
- 36.Ikeda H, West DB, Pustek JJ, Figlewicz DP, Greenwood MRC, Porte D, Jr, Woods SC. Intraventricular insulin reduces food intake and body weight of lean but not obese Zucker rats. Appetite. 1986;7:381–386. doi: 10.1016/s0195-6663(86)80006-x. [DOI] [PubMed] [Google Scholar]
- 37.Carvalheira JB, Ribeiro EB, Araujo EP, Guimaraes RB, Telles MM, Torsoni M, Gontijo JA, Velloso LA, Saad MJ. Selective impairment of insulin signalling in the hypothalamus of obese Zucker rats. Diabetologia. 2003;46:1629–40. doi: 10.1007/s00125-003-1246-x. [DOI] [PubMed] [Google Scholar]
- 38.Arase K, Fisler JS, Shargill NS, York DA, Bray GA. Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol. 1988;255:R974–R981. doi: 10.1152/ajpregu.1988.255.6.R974. [DOI] [PubMed] [Google Scholar]
- 39.Chavez M, Riedy CA, van Dijk G, Woods SC. Central insulin and macronutrient intake in the rat. American Journal of Physiology. 1996;271:R727–R731. doi: 10.1152/ajpregu.1996.271.3.R727. [DOI] [PubMed] [Google Scholar]
- 40.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets of laboratory rodents: Final report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76 Rodent Diet. Journal of Nutrition. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 41.Woods SC, D'Alessio DA, Tso P, Rushing PA, Clegg DJ, Benoit SC, Gotoh K, Liu M, Seeley RJ. Consumption of a high-fat diet alters the homeostatic regulation of energy balance. Physiol Behav. 2004;83:573–8. doi: 10.1016/j.physbeh.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 42.Clegg DJ, Benoit SC, Barrera JG, Woods SC. Estrogen Mediates Body Fat Distribution and Brain Sensitivity to Adiposity Signals. Diabetes. 2003;52(supplement 1) [Google Scholar]
- 43.Clegg DJ, Brown LM, Kemp CJ, Strader AD, Benoit SC, Woods SC, Mangiaracina M, Geary N. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007 doi: 10.2337/db06-0015. [DOI] [PubMed] [Google Scholar]
- 44.Lotter EC, McKay LD, Mangiapane ML, Simpson JB, Vogel KW, Porte D, Jr, Woods SC. Intraventricular angiotensin elicits drinking in the baboon. Proc Soc Exp Biol Med. 1980;163:48–51. doi: 10.3181/00379727-163-40720. [DOI] [PubMed] [Google Scholar]
- 45.Niswender KD, Morrison CD, Clegg DJ, Olson R, Baskin DG, Myers MG, Jr, Seeley RJ, Schwartz MW. Insulin activation of phosphatidylinositol 3-kinase in the hypothalamic arcuate nucleus: a key mediator of insulin-induced anorexia. Diabetes. 2003;52:227–31. doi: 10.2337/diabetes.52.2.227. [DOI] [PubMed] [Google Scholar]
- 46.Clegg DJ, Brown LM, Woods SC, Benoit SC. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–87. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 47.Air EL, Benoit SC, Blake Smith KA, Clegg DJ, Woods SC. Acute third ventricular administration of insulin decreases food intake in two paradigms. Pharmacol Biochem Behav. 2002;72:423–9. doi: 10.1016/s0091-3057(01)00780-8. [DOI] [PubMed] [Google Scholar]
- 48.Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–52. doi: 10.1210/endo.143.6.8948. [DOI] [PubMed] [Google Scholar]
- 49.Carvalheira JB, Siloto RM, Ignacchitti I, Brenelli SL, Carvalho CR, Leite A, Velloso LA, Gontijo JA, Saad MJ. Insulin modulates insulin-induced STAT3 activation in rat hypothalamus. FEBS Letters. 2001;500:119–124. doi: 10.1016/s0014-5793(01)02591-1. [DOI] [PubMed] [Google Scholar]
- 50.Brief DJ, Davis JD. Reduction of food intake and body weight by chronic intraventricular insulin infusion. Brain Res Bull. 1984;12:571–575. doi: 10.1016/0361-9230(84)90174-6. [DOI] [PubMed] [Google Scholar]
- 51.Clegg DJ, Riedy CA, Smith KA, Benoit SC, Woods SC. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–7. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 52.Plata-Salaman CR, Oomura Y, Shimizu N. Dependence of food intake on acute and chronic ventricular administration of insulin. Physiol Behav. 1986;37:717–734. doi: 10.1016/0031-9384(86)90177-0. [DOI] [PubMed] [Google Scholar]
- 53.Plata-Salaman CR, Oomura Y. Effect of intra-third ventricular administration of insulin on food intake after food deprivation. Physiol Behav. 1986;37:735–739. doi: 10.1016/0031-9384(86)90178-2. [DOI] [PubMed] [Google Scholar]
- 54.Riedy CA, Chavez M, Figlewicz DP, Woods SC. Central insulin enhances sensitivity to cholecystokinin. Physiology & Behavior. 1995;58:755–760. doi: 10.1016/0031-9384(95)00108-u. [DOI] [PubMed] [Google Scholar]
- 55.Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. Journal of Biological Chemistry. 2004;279:31139–31148. doi: 10.1074/jbc.M400458200. [DOI] [PubMed] [Google Scholar]
- 56.Lam TK, Pocai A, Gutierrez-Juarez R, Obici S, Bryan J, Aguilar-Bryan L, Schwartz GJ, Rossetti L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–7. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 57.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–5. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]
- 58.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–82. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 59.Bisschop PH, de Metz J, Ackermans MT, Endert E, Pijl H, Kuipers F, Meijer AJ, Sauerwein HP, Romijn JA. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. American Journal of Clinical Nutrition. 2001;73:554–559. doi: 10.1093/ajcn/73.3.554. [DOI] [PubMed] [Google Scholar]
- 60.Lovejoy JC, Windhauser MM, Rood JC, de la Bretonne JA. Effect of a controlled high-fat versus low-fat diet on insulin sensitivity and leptin levels in African-American and Caucasian women. Metabolism. 1998;47:1520–1524. doi: 10.1016/s0026-0495(98)90080-4. [DOI] [PubMed] [Google Scholar]
- 61.Baskin DG, Sipols AJ, Schwartz MW, White MF. Insulin receptor substrate-1 (IRS-1) expression in rat brain. Endocrinology. 1994;134:1952–1955. doi: 10.1210/endo.134.4.7511094. [DOI] [PubMed] [Google Scholar]
- 62.Corp ES, Woods SC, Porte D, Jr, Dorsa DM, Figlewicz DP, Baskin DG. Localization of 125I-insulin binding sites in the rat hypothalamus by quantitative autoradiography. Neurosci Lett. 1986;70:17–22. doi: 10.1016/0304-3940(86)90430-1. [DOI] [PubMed] [Google Scholar]
- 63.Figlewicz DP, Ikeda H, Hunt TR, Stein LJ, Dorsa DM, Woods SC, Porte D., Jr Brain insulin binding is decreased in Wistar Kyoto rats carrying the “fa” gene. Peptides. 1986;7:61–65. doi: 10.1016/0196-9781(86)90062-8. [DOI] [PubMed] [Google Scholar]
- 64.Havrankova J, Roth J, Browstein M. Insulin receptors are widely distributed in the central nervous system of the rat. Nature. 1978;272:827–829. doi: 10.1038/272827a0. [DOI] [PubMed] [Google Scholar]
- 65.Baskin DG, Schwartz MW, Seeley RJ, Woods SC, Porte DJ, Breininger JF, Yonak Z, Schaefer J, Krouse M, Burghardt C, Campfield LA, Burn P, Kochan JP. Leptin receptor long form splice variant protein expression in neuron cell bodies of the brain and colocalization with neuropeptide Y mRNA in the arcuate nucleus. Journal of Histochemistry and Cytochemistry. 1999;47:353–362. doi: 10.1177/002215549904700309. [DOI] [PubMed] [Google Scholar]
- 66.Carvalheira JB, Torsoni MA, Ueno M, Amaral ME, Araujo EP, Velloso LA, Gontijo JA, Saad MJ. Cross-talk between the insulin and leptin signaling systems in rat hypothalamus. Obes Res. 2005;13:48–57. doi: 10.1038/oby.2005.7. [DOI] [PubMed] [Google Scholar]