Abstract
We synthesized disulfide-based cyclic RGD pentapeptides bearing a near-infrared fluorescent dye (cypate), represented by cypate-c(CRGDC) (1) for integrin-targeted optical imaging. These compounds were compared with the traditional lactam-based cyclic RGD counterpart, cypate-c(RGDfK) (2). Molecular modeling suggests that the binding affinity of 2 to integrin αvβ3 is an order of magnitude higher than that of 1. This was confirmed experimentally, which further showed that substitution of Gly with Pro, Val and Tyr in 1 remarkably hampered the αvβ3 binding. Interestingly, cell microscopy with A549 cells showed that 1 exhibited higher cellular staining than 2. These results indicate that factors other than receptor binding affinity to αvβ3 dimeric proteins mediate cellular uptake. Consequently, 1 and its analogs may serve as valuable molecular probes for investigating the selectivity and specificity of integrin targeting by optical imaging.
Keywords: Disulfide-based cyclization, RGD peptide, integrin αvβ3 binding, Near-infrared fluorescent probe, Optical imaging
The RGD (arginine-glycine-aspartic acid) tripeptide motif plays an essential role in the molecular recognition of integrin αvβ3 and some other integrin subtypes.1–6 The over-expression of integrin αvβ3 found in various types of tumors and neo-vasculatures and this dimeric protein complex is involved in regulating tumor growth, angiogenesis, and metastasis. Therefore, RGD-based integrin αvβ3 targeting has provided an effective approach for improving tumor imaging, and drug delivery. Cyclic RGD compounds such as the conventional lactam-based cyclic pentapeptide c(RGDfK) (where “f” represents D-phenylalanine) exhibit remarkable binding affinity and selectivity for integrin αvβ3.7–9 For example, [18F]galacto-RGD and cilengitide have been tested for cancer imaging and therapy in the clinic.7, 10–12
Despite some promising results, many aspects of integrin targeting, tumor imaging, and therapy that employ RGD peptides remain unclear.13 In particular, integrins have 24 subtypes through different combinations of α and β subunits.14, 15 Therefore, it is important to explore novel integrin-targeted ligands that are distinguishable from c(RGDfK) in structure as well as in receptor binding selectivity and specificity. We envision that such compounds with different structural and functional features may further enhance our understanding of integrin expressions, signal transduction, and their roles in cancer biology and pathology.
Over the past years, various types of disulfide-based cyclic RGD peptides have been reported.16–27 Recently, an internalizing disulfide bond-containing RGD peptide, called iRGD, has been developed to enhance both cancer detection and treatment by deep tissue penetration. 6, 28 Nevertheless, such cyclic RGD peptides have not been explored fully for integrin targeting and tumor imaging compared to the conventional lactam-based cyclic RGD peptide analogs. Optical imaging has emerged as a powerful modality for studying molecular recognition and molecular imaging in a noninvasive, sensitive, and real-time way. The advantages of optical imaging include cost-effectiveness, convenience, and safety. The method is also complementary to other imaging modalities such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), and magnetic resonance imaging (MRI). Considerable advances have been made in tumor optical imaging by using integrin-targeting compounds in both preclinical and clinical studies.11, 29–37
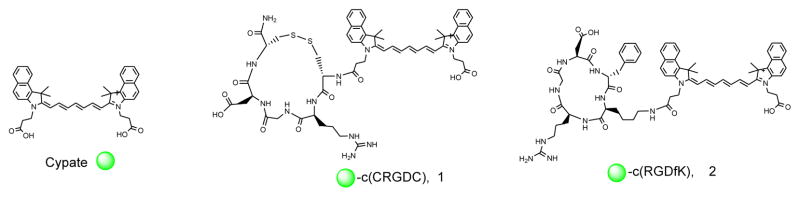
In this work, we synthesized a disulfide-based cyclic penta-RGD peptides conjugated with a near-infrared fluorescent carbocyanine dye (cypate) (1) and its analogs for potential integrin targeting and optical imaging. They were evaluated for their integrin αvβ3 binding and cellular staining in comparison with a lactam-based cypate-c(RGDfK) (2),38 where the cypate moiety was connected to the ε-amino group of lysine.
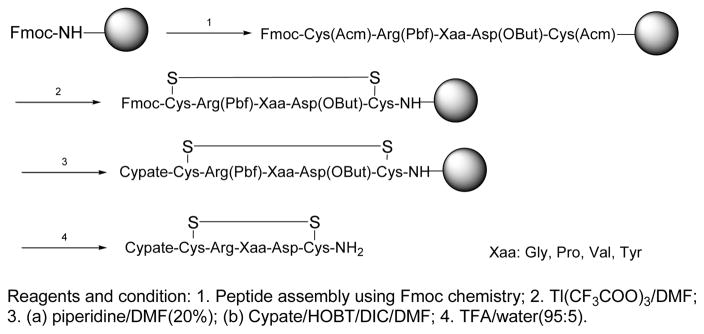
Based on the previous methods for synthesis of cypate-peptide conjugates on solid support,37 we used a similar strategy to prepare the disulfide cyclic RGD peptides and their conjugates. The protected RGD peptide Fmoc-C(Acm)-R(Pbf)-G-D(OBut)-C(Acm) was first assembled on Rink amide MBHA resin (1 equiv) using conventional Fmoc chemistry (Scheme 1). The disulfide-based cyclization was realized by swirling the resin-bound peptide with a solution of Tl(F3CCOO)3 in DMF for 2 h, yielding the disulfide-containing cyclic peptide on a resin, Fmoc-c[CR(Pbf)GD(OBut)C]-Resin.39, 40 After the Fmoc protecting group was removed with piperidine/DMF, the free amino group at the N-terminus was conjugated with cypate in the presence of DIC and HOBT. Finally, the desired product, cypate-c(CRGDC)-NH2 (1) was cleaved from the resin with aqueous TFA (95%) and purified by semi-preparative HPLC.
Scheme 1.
Synthesis of cypate-labeling cyclic disulfide RGD peptide.
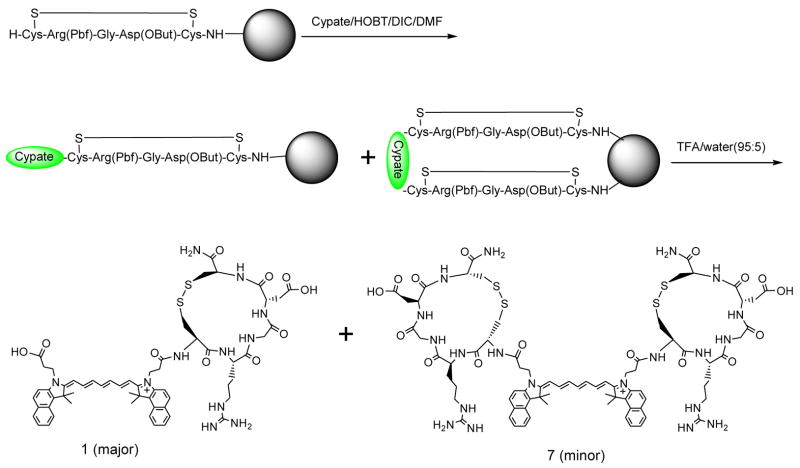
Similarly, several analogs were also successfully synthesized by varying the glycine residue with other amino acids including Pro, Val, and Tyr (3, 4, and 5), as shown in Table 1. In addition, we synthesized the lactam-based cyclic RGD peptides: cypate-c(RGDfK) (2) and c(RGDfV) (6) for comparison.38 As reported previously,37 we also identified the simultaneous formation of their dimeric analogs such as the dimeric analog of 1 cypate-[c(CRGDC)]2 (7) (ES-MS: [MH2]2+1000.05, [MH3]3+ 667.35, and [MH4]4+ 500.70) as shown in Scheme 2. All the dimeric analogs showed shorter retention time compared to their monomeric analogs, but its yield was low compared to the monomeric analogs.
Table 1.
The major ES-MS peaks and integrin αvβ3 binding affinities of 1, 2, and their analogs.
| Entry No. | Compounds | [MH]+/[MH2]2+ | IC50 (M) |
|---|---|---|---|
| 1 | Cypate-c(CRGDC) | 1157/579 | 4.6 × 10−6 |
| 2 | Cypate-c(RGDfK) | 1210/606 | 7.2 × 10−7 |
| 3 | Cypate-c(CRPDC) | 1196/598 | >10−5 |
| 4 | Cypate-c(CRVDC) | 1198/600 | >10−5 |
| 5 | Cypate-c(CRYDC) | 1262/632 | >10−5 |
| 6 | c(RGDfV) | 575 | 3.25 × 10−7 |
Scheme 2.
The simultaneous preparation of two compounds: the monomeric and dimeric cyclic disulfide RGD peptide analogs on solid support.
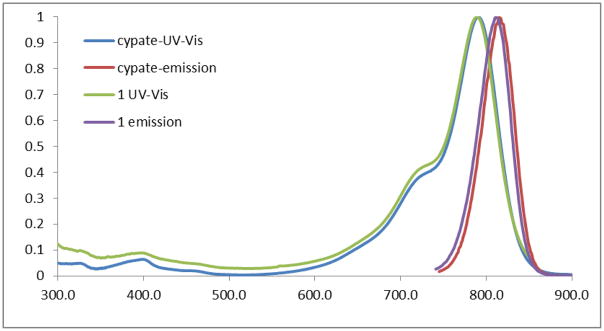
All the compounds were fully identified by both HPLC and ESI-MS after semi-preparative HPLC purification. As shown in Figure 2, all the compounds have similar UV-Vis absorption and fluorescence emission spectra ( and ) in the near-infrared region in 20% aqueous DMSO.
Figure 2.
Normalized UV-Vis and emission spectra of cypate and its conjugate cypate-c(CRGDC) (1).
Integrin αvβ3 binding affinities were determined based on the competitive binding between purified integrin αvβ3 and peptide ligands using radiolabeled echistatin as a tracer. Echistatin is a polypeptide that binds irreversibly with high affinity and specificity to the integrin αvβ3. In particular, 125I-echistatin has been widely widely used as a a tracer in integrin αvβ3 binding assays.41–43 The lactam-based cyclic RGD pentapeptide, c(RGDfV), was used as a reference standard because it is known to bind integrin αvβ3 with high affinity.8, 29 The obtained IC50 values are summarized in Table 1. The disulfide cyclic penta-peptide (1) showed lower receptor binding affinity compared to the two lactam-based cyclic RGD pentapeptide 2 and c(RGDfV), both of which display similar binding affinity. This might be ascribed to the more flexible structure of 1 compared to the lactam-based analog 2, as was further confirmed by molecular modeling.
Molecular modeling performed according to energy calculation protocol described elsewhere 44 found 24 low-energy conformations of the peptide backbone for compound 6, 18 for compound 2 and 68 for compound 1. Expectedly, RGD peptide analogues with more compatible conformations to the structure of c(R1-G2-D3-f4-NMe-V5) in complex with integrin αvβ3 (as revealed by the X-ray spectroscopy15) display tighter specific binding to integrin αvβ3. Upon overlapping of Cα-atoms in the “template” X-ray structure of c(R1-G2-D3-f4-NMe-V5) to the corresponding atoms of compound 6, 19 out of 24 low-energy conformations for compound 6 (ca. 79%) showed the root mean square deviation (rms) values ≤ 1 A. For compound 2, the same percentage was also ca. 78% (14 out of 18), but it was significantly lower at ca. 62% (42 out of 68) for compound 1. These results correlate well with experimental data on integrin binding (see Table 1) showing binding affinities of compounds 2 and 6 of about one order of magnitude higher than that of compound 1.
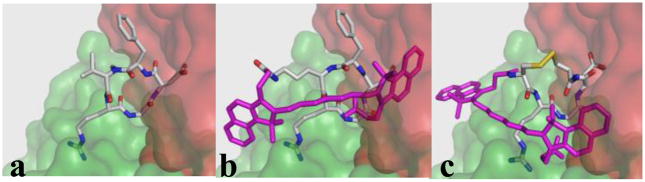
Some of the low-energy conformations for compounds 1 and 2 compatible with the template X-ray structure displayed plausible orientation of the cypate moiety in the cavity between the two subunits of integrin αvβ3. As a representative examples, Figure 3a–c show spatial orientations of selected conformations of compounds 1 and 2 in complex with integrin αvβ3 as compared with the orientation of the template compound.
Figure 3.
Representative conformations of c(RGDf-NMe-V) [a, template conformation], cypate-c(RGDfK) [b], and cypate-c(CRGDC) [c] in complex with integrin. All hydrogens are omitted. The cypate moiety is shown in magenta. αv and β3 subunits of integrin are shown as semi-transparent surfaces in green and red, respectively.
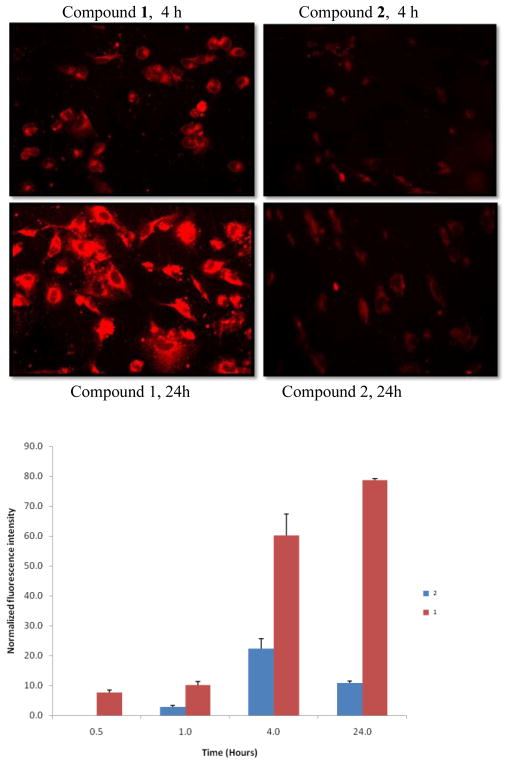
The A549 cells have been widely used for integrin-targeted tumor imaging due to their overexpression of integrin αvβ3. Compounds 1 and 2 were incubated with A549 cells for cellular staining and imaging by fluorescence microscopy (775/50 nm excitation and 845/55 nm emission filters). As shown in Figure 4, 1 stained the cells more strongly than 2.
Figure 4.
Representative fluorescence images of live A549 cells (top) and relative fluorescence intensity of cellular staining after incubation of A549 cells (bottom) with compounds 1 and 2 at 1 μM.
Disulfide bridges represent important evolutionarily conserved structural motifs in many biologically important peptides and proteins. Our results demonstrated that disulfide-based cyclization may provide an efficient approach for studying receptor binding affinity and selectivity of RGD peptides as well as enzymatic and metabolic stability of RGD peptides employed for integrin targeting.1, 45, 46 As described above, we have successfully synthesized the near-infrared fluorescent disulfide cyclic RGD peptide and evaluated their integrin αvβ3 binding. The results also confirmed the importance of Gly in maintaining the binding affinity of such RGD peptides to integrin αvβ3.
Based on our results from receptor binding assay and molecular modeling, 1 could not compete efficiently with 2 in binding affinity with integrin αvβ3. However, the remarkable cellular uptake of 1 suggests the potential use of this class of RGD for imaging and treating cancer cells. These preliminary data suggest that, besides the receptor binding affinities, receptor binding selectivity and other structural factors such as lipophilicity may also play important roles in the cellular internalization. Therefore, all the disulfide-RGD compounds synthesized for this study deserve further investigation in vitro and in vivo. Further work is underway to unravel the integrin selectivity, signal transduction, and related biological activities as well as its potential in integrin-targeted tumor imaging as we reported previously. 29, 37
This work further demonstrated that the dicarboxylic acid-containing cypate can serve as a scaffold for constructing diverse near-infrared fluorescent agents for optical imaging as we reported previously.37 Especially, the compound 1 can serve as an attractive template for further molecular design and structural modification to discover some novel innovative integrin-targeted agents for tumor optical imaging, therapy, and drug delivery. For example, a library of novel diverse disulfide RGD peptides can be synthesized by solid phase peptide synthesis. The acid group at the side chain of cypate motif also provides a site for structural modification to improve the physicochemical properties and integrin targeting ability. As described above, some dimeric analogs bearing two disulfide-RGD peptide motifs such as 7 can be obtained simultaneously. All these will provide some insights into molecular design and structural modifications to further improve the integrin-targeting activities.
In summary, we have prepared and evaluated some cypate-labeled near-infrared fluorescent disulfide-based cyclic RGD peptide analogs. Although 1 has relatively lower receptor binding affinity for integrin αvβ3 compared to 2, it exhibited higher cellular staining. This class of near-infrared fluorescent RGD compounds deserve further exploration for their integrin targeting in cancer biology, optical imaging, and targeted therapy.
Supplementary Material
Figure 1.
The two types of cyclic RGD pentapeptides bearing a near-infrared fluorescent dye, cypate.
Acknowledgments
This work was supported in part by the US National Institutes of Health grants NCI R33 CA123537, R01 CA109754 and NIBIB R01 EB00811, R01 EB007276.
Footnotes
Supplementary data associated with this article can be found in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Assa-Munt N, Jia X, Laakkonen P, Ruoslahti E. Biochemistry. 2001;40(8):2373. doi: 10.1021/bi002101f. [DOI] [PubMed] [Google Scholar]
- 2.Pasqualini R, Koivunen E, Ruoslahti E. Nat Biotechnol. 1997;15(6):542. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 3.Ruoslahti E. Annu Rev Cell Dev Biol. 1996;12:697. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 4.Ruoslahti E. Matrix Biol. 2003;22(6):459. doi: 10.1016/s0945-053x(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 5.Ruoslahti E, Pierschbacher MD. Science. 1987;238(4826):491. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 6.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Girard OM, Hanahan D, Mattrey RF, Ruoslahti E. Cancer Cell. 2009;16(6):510. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heckmann D, Kessler H. Methods Enzymol. 2007;426:463. doi: 10.1016/S0076-6879(07)26020-3. [DOI] [PubMed] [Google Scholar]
- 8.Dechantsreiter MA, Planker E, Mathä B, Lohof E, Hölzemann G, Jonczyk A, Goodman SL, Kessler H. J Med Chem. 1999;42(16):3033. doi: 10.1021/jm970832g. [DOI] [PubMed] [Google Scholar]
- 9.Pfaff M, Tangemann K, Müller B, Gurrath M, Müller G, Kessler H, Timpl R, Engel J. J Biol Chem. 1994;269(32):20233. [PubMed] [Google Scholar]
- 10.Haubner R, Kuhnast B, Mang C, Weber WA, Kessler H, Wester HJ, Schwaiger M. Bioconjug Chem. 2004;15(1):61. doi: 10.1021/bc034170n. [DOI] [PubMed] [Google Scholar]
- 11.Schottelius M, Laufer B, Kessler H, Wester HJ. Acc Chem Res. 2009;42(7):969. doi: 10.1021/ar800243b. [DOI] [PubMed] [Google Scholar]
- 12.Tucker GC. Curr Opin Investig Drugs. 2003;4(6):722. [PubMed] [Google Scholar]
- 13.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Nat Med. 2009;15(4):392. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 14.Kim M, Carman CV, Springer TA. Science. 2003;301(5640):1720. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 15.Xiong JP, Stehle T, Diefenbach B, Zhang R, Dunker R, Scott DL, Joachimiak A, Goodman SL, Arnaout MA. Science. 2001;294:5541–339. doi: 10.1126/science.1064535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duggan ME, Naylor-Olsen AM, Perkins JJ, Anderson PS, Chang CT-C, Cook JJ, Gould RJ, Ihle NC, Hartman GD. J Med Chem. 1995;38(17):3332. doi: 10.1021/jm00017a017. [DOI] [PubMed] [Google Scholar]
- 17.Mukhopadhyay S, Barnés CM, Haskel A, Short SM, Barnes KR, Lippard SJ. Bioconjug Chem. 2008;19(1):39. doi: 10.1021/bc070031k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamada T, Kidera A. FEBS Lett. 1996;387(1):11. doi: 10.1016/0014-5793(96)00409-7. [DOI] [PubMed] [Google Scholar]
- 19.Yamada T, Uyeda A, Kidera A, Kikuchi M. Biochemistry. 1994;33(39):11678. doi: 10.1021/bi00205a002. [DOI] [PubMed] [Google Scholar]
- 20.Kimura RH, Cheng Z, Gambhir SS, Cochran JR. Cancer Res. 2009;69(6):2435. doi: 10.1158/0008-5472.CAN-08-2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miao Z, Ren G, Liu H, Kimura RH, Jiang L, Cochran JR, Gambhir SS, Cheng Z. Bioconjug Chem. 2009;20(12):2342. doi: 10.1021/bc900361g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beer AJ, Schwaiger M. Cancer Metastasis Rev. 2008;27(4):631. doi: 10.1007/s10555-008-9158-3. [DOI] [PubMed] [Google Scholar]
- 23.Edwards D, Jones P, Haramis H, Battle M, Lear R, Barnett DJ, Edwards C, Crawford H, Black A, Godden V. Nucl Med Biol. 2008;35(3):365. doi: 10.1016/j.nucmedbio.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Yin R, Zheng H, Xi T, Xu HM. Bioconjug Chem. 2010;21(7):1142. doi: 10.1021/bc900292y. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, Lee HS. Int J Mol Med. 2004;14(4):529. [PubMed] [Google Scholar]
- 26.Nagel H, Maag S, Tassis A, Nestlé FO, Greber UF, Hemmi S. Gene Ther. 2003;10(19):1643. doi: 10.1038/sj.gt.3302058. [DOI] [PubMed] [Google Scholar]
- 27.Smolarczyk R, Cicho T, Graja K, Hucz J, Sochanik A, Szala S. Acta Biochim Pol. 2006;53(4):801. [PubMed] [Google Scholar]
- 28.Sugahara KN, Teesalu T, Karmali PP, Kotamraju VR, Agemy L, Greenwald DR, Ruoslahti E. Science. 2010;328(5981):1031. doi: 10.1126/science.1183057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Achilefu S, Bloch S, Markiewicz MA, Zhong T, Ye Y, Dorshow RB, Chance B, Liang K. Proc Natl Acad Sci U S A. 2005;102(22):7976. doi: 10.1073/pnas.0503500102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai W, Chen K, Li ZB, Gambhir SS, Chen X. J Nucl Med. 2007;48(11):1862. doi: 10.2967/jnumed.107.043216. [DOI] [PubMed] [Google Scholar]
- 31.Cai W, Shin DW, Chen K, Gheysens O, Cao Q, Wang SX, Gambhir SS, Chen X. Nano Lett. 2006;6(4):669. doi: 10.1021/nl052405t. [DOI] [PubMed] [Google Scholar]
- 32.Chen X, Conti PS, Moats RA. Cancer Res. 2004;64(21):8009. doi: 10.1158/0008-5472.CAN-04-1956. [DOI] [PubMed] [Google Scholar]
- 33.Cheng Z, Wu Y, Xiong Z, Gambhir SS, Chen X. Bioconjug Chem. 2005;16(6):1433. doi: 10.1021/bc0501698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin ZH, Josserand V, Razkin J, Garanger E, Boturyn D, Favrot MC, Dumy P, Coll JL. Mol Imaging. 2006;5(3):188. [PubMed] [Google Scholar]
- 35.Li C, Wang W, Wu Q, Ke S, Houston J, Sevick-Muraca E, Dong L, Chow D, Charnsangavej C, Gelovani JG. Nucl Med Biol. 2006;33(3):3498. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Liu S, Niu G, Wang F, Liu S, Chen X. Mol Imaging. 2010;9(1):21. [PMC free article] [PubMed] [Google Scholar]
- 37.Ye Y, Bloch S, Xu B, Achilefu S. J Med Chem. 2006;49(7):2268. doi: 10.1021/jm050947h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye Y, Bloch S, Xu B, Achilefu S. Bioconjug Chem. 2008;19(1):225. doi: 10.1021/bc7003022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Edwards WB, Fields CG, Anderson CJ, Pajeau TS, Welch MJ, Fields GB. J Med Chem. 1994;37(22):3749. doi: 10.1021/jm00048a011. [DOI] [PubMed] [Google Scholar]
- 40.Limal D, Briand JP, Dalbon P, Jolivet M. J Pept Res. 1998;52(2):121. doi: 10.1111/j.1399-3011.1998.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen X, Hou Y, Tohme M, Park R, Khankaldyyan V, Gonzales-Gomez I, Bading JR, Laug WE, Conti PS. J Nucl Med. 2004;45(10):1776. [PubMed] [Google Scholar]
- 42.Hantgan RR, Stahle MC, Connor JH, Horita DA, Rocco M, McLane MA, Yakovlev S, Medved L. Protein Sci. 2006;15(8):1893. doi: 10.1110/ps.052049506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar CC, Nie H, Rogers CP, Malkowski M, Maxwell E, Catino JJ, Armstrong L. J Pharmacol Exp Ther. 1997;283(2):843. [PubMed] [Google Scholar]
- 44.Ye Y, Li W, Anderson CJ, Kao J, Nikiforovich GV, Achilefu S. J Am Chem Soc. 2003;125:7766. doi: 10.1021/ja034186o. [DOI] [PubMed] [Google Scholar]
- 45.Nowlin DM, Gorcsan F, Moscinski M, Chiang SL, Lobl TJ, Cardarelli PM. J Biol Chem. 1993;268(27):20352. [PubMed] [Google Scholar]
- 46.Yang W, Meng L, Wang H, Chen R, Wang R, Ma X, Xu G, Zhou J, Wang S, Lu Y, Ma D. Oncol Rep. 2006;15(1):113. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.