Abstract
Targeted disruption of the transcription factor NKX2.3 gene in mice results in anatomical defects of intestine and secondary lymphoid organs. Here, we report that spleen and Peyer’s patches of NKX2.3-deficient mice are considerably reduced in size and lack the ordered tissue architecture. T and B cells are misplaced within the spleen and mesenteric lymph nodes and fail to segregate into the appropriate T and B cell areas. Furthermore, splenic marginal zones, characterized by specific B cells and various types of macrophage-derived cells around the marginal sinus, are absent in mutants. Homozygous NKX2.3 mutants lack the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) that is normally expressed in mucosa-associated lymphoid tissue (MALT) and spleen. We provide evidence that NKX2.3 can activate MAdCAM-1 transcription directly, suggesting that MAdCAM-1 is at least partly responsible for the migration and homing defects of lymphocytes and macrophages in mutants. Therefore, expression of MAdCAM-1 seems to be required for building functional structures in spleen and MALT, a prerequisite for unimpaired migration and segregation of B and T cells to and within these organs.
Keywords: lymphocyte homing/MAdCAM-1 expression/NKX2.3 knock-out/spleen anatomy/T and B lymphocytes
Introduction
NKX genes in vertebrates form a relatively large family of related genes that encode homeodomain-containing transcription factors implicated in many aspects of cell type specification and maintenance of differentiated tissue functions (for review, see Harvey, 1996). Six members of the NKX2 subfamily are known in mice, three of them are expressed predominantly in neuro-ectoderm and tissues derived thereof (NKX2.1, NKX2.2 and NKX2.9) and three in cells derived from mesendoderm and mesoderm (NKX2.3, NKX2.5 and NKX2.6) (Pabst et al., 2000). The NKX2.3 gene in mouse is expressed in the epithelium of branchial arches and tongue, in restricted areas of the developing jaws, in midgut and hindgut mesoderm, and in spleen parenchyme during embryonic development and postnatally (Pabst et al., 1997). Targeted disruption of the NKX2.3 gene in mice results in severe defects of gut development, primarily in the epithelium of the small intestine (Pabst et al., 1999). Perturbations of the gut tissue architecture lead to early postnatal death presumably due to digestive malfunctions. It was also observed that NKX2.3 mutant mice are sometimes asplenic or contain a spleen that is markedly smaller than normal with considerable morphological aberrations most obviously characterized by abundant filling with red blood cells. Secondary lymphoid organs including the spleen, lymph nodes (LN), and mucosa-associated lymphoid tissues (MALT), such as Peyer’s patches (PP) and less prominent clusters of lymphoid cells in the gastrointestinal, genitourinary and respiratory tracts are located at sites in the body where antigens are concentrated and presented to immune-competent cells in order to optimize cellular interactions for efficient removal of pathogens (reviewed by Fu and Chaplin, 1999). Lymphocytes present in these tissues are generally located in distinct regions of the organ with T and B cells segregated into different areas resulting in a unique anatomical architecture. The spleen, the largest single lymphoid organ in mammals, is separated into two major structures: the red pulp and the white pulp. While the red pulp, containing variable numbers of plasma cells as well as stroma cells and a large population of macrophages, mainly serves as a filter to remove aged or damaged erythrocytes from the circulation, the white pulp represents the organized lymphocyte compartment associated with regulated activation and maturation of antigen-dependent B and T cells. The T cell-rich compartment, designated the periarteriolar lymphoid sheath (PALS), surrounds the central arterioles that sprout into the white pulp nodules. After penetrating the PALS the central arteriole opens into a marginal sinus that is lined with endothelium and macrophages that allow immigration of lymphocytes into the spleen (Tanaka et al., 1996). The marginal zone is located adjacent to the marginal sinus and contains various specialized cell types including marginal macrophages, metallophilic macrophages, fiber-forming reticular cells and sessile B cells (Fu and Chaplin, 1999). In particular, the metallophilic macrophages and sinus-lining non-lymphoid cells that express the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) are believed to control entry of lymphocytes and antigens from the blood into the white pulp (Kraal et al., 1995; Tanaka et al., 1996). B cells in the white pulp are located in two compartments (Chaplin and Fu, 1998). Naive B cells and some memory B cells concentrate in an area adjacent to the marginal sinus as part of the marginal zone (Oldfield et al., 1988; van Krieken et al., 1989). A second population of B cells is organized in primary follicles surrounding follicular dendritic cells (FDC) similar to the primary follicles in LN. Proper regulation of immune responsiveness in the spleen is thought to be critically dependent on the highly ordered microarchitecture of the cellular components in the white pulp (MacLennan, 1994; Steinman et al., 1997). As observed in the spleen, separated T and B cell areas are also present in LN and PP, although their organization is distinct. In LN and PP, naive B and T lymphocytes enter from the blood by crossing specialized high endothelial venules (HEVs), while memory T cells and antigen-presenting cells are brought into the nodes from peripheral tissues via afferent lymphatics.
Although the anatomical structures of secondary lymphoid organs are well defined, the mechanisms that establish the various cellular compartments within these organs and regulate trafficking of cells through defined regions of the mature tissues are not well understood. Recent evidence suggests that the cytokine lymphotoxins and tumor necrosis factor (Fu and Chaplin, 1999) as well as homeostatic chemokines (Gunn et al., 1999) and chemokine receptors (Forster et al., 1996, 1999) essentially regulate lymphocyte trafficking and positioning. However, there is considerable lack of knowledge about factors bringing together hematopoietic and stroma cells to form a functional three-dimensional reticular network that is a prerequisite for filling and precise positioning of lymphoid cells.
We have reported previously that targeted mutation of the NKX2.3 gene in the mouse leads to gross alterations of spleen histology and tissue architecture (Pabst et al., 1999). Here, we describe a detailed analysis of this mutant phenotype and demonstrate that the transcription factor NKX2.3 is essential for the expression of the cell adhesion molecule MAdCAM-1. Lack of MAdCAM-1 in spleen is correlated with the failure to form a marginal zone that normally locates between follicles and the red pulp. As defined marginal sinus-lining macrophages and marginal zone B cells are missing in this organ, it seems that impaired migration of lymphoid and non-lymphoid cells at this port of cell entry essentially contributes to the disturbed microarchitecture. Our observations suggest that MAdCAM-1 is one of the crucial homing factors for the correct anatomical organization of the marginal zone. In addition, MAdCAM-1 also seems essential for establishing the correct tissue architecture of mucosal lymphoid tissues, in particular the PP.
Results
Preliminary analysis of the phenotype in homozygous NKX2.3 mutant mice indicated that spleens were generally smaller and sometimes entirely absent. On the mixed genetic background of 129Sv/C57BL6 mice the spleen phenotype appeared variable in degree, a phenomenon that was not observed in mutants of the 129Sv inbred mouse strain. In order to investigate defects in secondary lymphoid organs caused by the NKX2.3 mutation in more detail, 129Sv mouse mutants were used throughout the current study.
The correct anatomical localization of lymphocytes in spleen is disturbed in NKX2.3 mutant mice
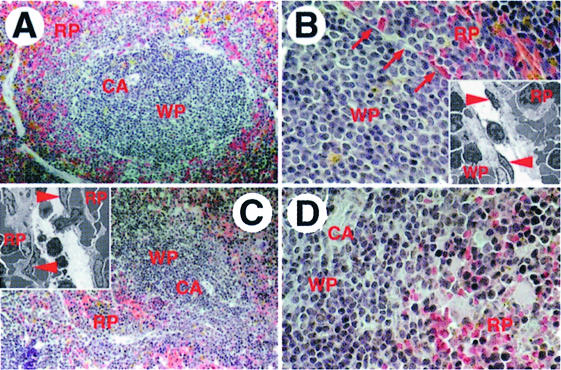
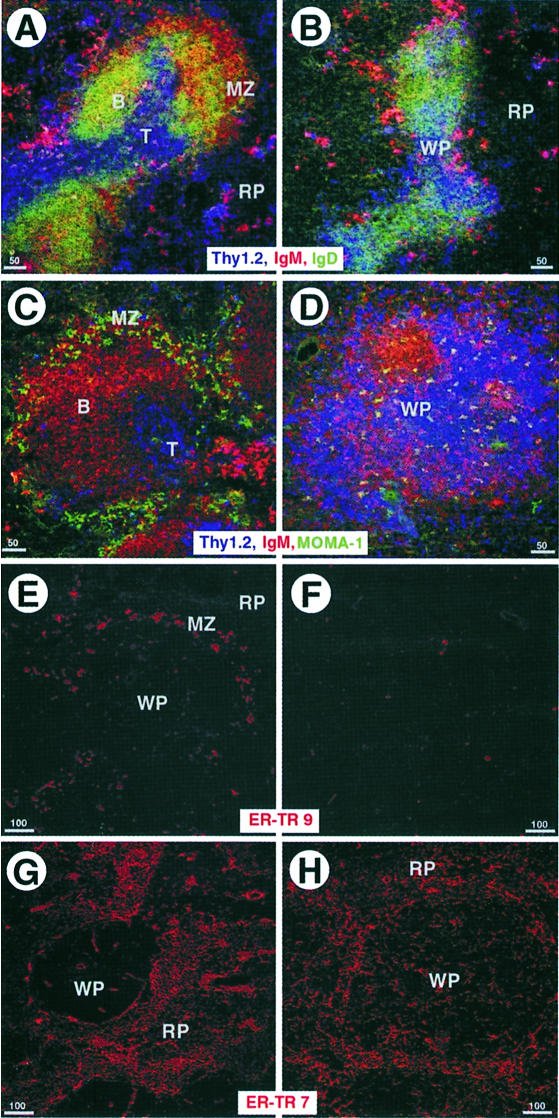
The majority of lymphocytes in the spleen are organized around central arterioles in a defined anatomical structure termed the white pulp. This region is sharply delimited and separated from the red pulp, which contains mainly erythrocytes, by the marginal zone that prevents substantial mixing of cells from both areas. Hematoxylin and eosin (H&E)-stained sections of spleens from wild-type animals showed that the white pulp was sharply demarcated by the marginal sinus (Figure 1A and B) but similar sections of spleens from NKX2.3 mutants revealed that lymphocytes were extensively intermingled with red blood cells at the periphery of the white pulp area and the clear separation of red and white pulp was not present (Figure 1C and D). Electron micrographs of the marginal sinus in the wild type illustrated the presence of endothelial cells and periluminal reticular cells associated with macrophages and B cells. This structure clearly segregated lymphocytes to one side and erythrocytes to the other side of the sinus (insets in Figure 1B). In contrast, the structure resembling the marginal sinus in mutant spleens appeared as large interstitial gaps with discontinuous endothelium that failed to separate the red from the white pulp (inset in Figure 1C). We also analyzed the distribution of various cell types in spleens of wild-type and mutant mice with antibodies that specifically recognize different cell populations. Triple immunostainings on spleen sections using anti-IgM and anti-IgD antibodies to detect B cells, and anti-Thy1 antibody to stain T cells indicated a pronounced reduction of IgM+ and IgDlow B cells in the marginal zone and probably fewer T cells in the PALS of mutants, while an apparently normal population of scattered IgM+ plasma cells was present in the red pulp (Figure 2A and B). The local disarrangement of cells in mutant spleen was also clearly documented in triple immunostainings including MOMA1 antibody to visualize metallophilic macrophages in the marginal zone. As expected, in wild-type animals the PALS contained primarily T cells around the central arteriole surrounded by IgM+ B cells, and both were lined by MOMA1+ macrophages (Figure 2C). In contrast, mutant spleens exhibited no defined PALS, and T and B cells appeared extensively mixed (Figure 2D). Moreover, substantially fewer MOMA1+ macrophages were present and these were not associated with the marginal zone. ER-TR-9 antibody was used to identify macrophages that normally locate close to the marginal sinus (Dijkstra et al., 1985; van Vliet et al., 1985). Figure 2E confirms the localization of ER-TR-9+ cells in a seam around the white pulp in wild type. In mutant spleen these cells appeared drastically reduced in numbers and the residual cells were randomly scattered throughout the tissue (Figure 2F). Antibody ER-TR-7, which stains mesenchymal spleen cells referred to as a reticular meshwork (van Vliet et al., 1986), detected these cells mainly in the red pulp surrounding but sparing the PALS areas in wild type (Figure 2G). Immunohistology of NKX2.3 mutant spleens showed a more even distribution of ER-TR-7+ cells including the white pulp, indicating that the microarchitecture of the spleen stroma has been disarranged (Figure 2H). Taken together these results confirm and extend our previous observations that the spleen anatomy is grossly disturbed in the absence of the transcription factor NKX2.3, suggesting that the mutation primarily affects the correct positioning of lymphoid and stroma cells.
Fig. 1. Abnormal histology of spleen in the NKX2.3 mutant mouse. Paraffin sections of spleens from wild-type (A and B) and NKX2.3 mutant (C and D) mice were stained with H&E. The white pulp (WP) around the central arteriole (CA) is sharply separated from the red pulp (RP) in wild-type (A) but exhibits a diffuse outer margin in mutant spleen (C). Higher magnification shows the marginal sinus [arrows in (B)] as a distinct demarcation of WP and RP in normal spleen and the lack of this border structure in mutant spleen (D), which leads to mixing of nucleated and red blood cells. Transmission electron microscopy illustrates the endothelial lining of the marginal sinus (endothelial cells indicated by arrowheads) and the different cell types associated with it on both sides in wild-type spleen [inset in (B)]. While this structure is not seen in mutant spleen, one finds large interstitial gaps that resemble dilated marginal sinus containing an apparently discontinous endothelium that fails to separate WP and RP [inset in (C)]. Magnifications: A and C, 100×; B and D, 200×; insets, 2000×.
Fig. 2. Spleen from NKX2.3 mutants lacks the typical marginal zone. Cryostat sections from adult wild-type (A, C, E and G) and mutant (B, D, F and H) spleens were immunostained with specific antibodies as indicated: Thy 1 (blue) for T cells, IgM (red) and IgD [green in (A and B)] for B cells, and MOMA1 [green in (C and D)] for metallophilic macrophages. Note the absence of marginal zone B cells (B) and metallophilic macrophages (D) in mutants compared with wild type (A and C). Also note the mixing of B and T cells in mutant spleen. ER-TR-9 antibody (red) detects a subpopulation of macrophages in the marginal zone of wild type (E), which is scarce and scattered in mutant spleen (F). ER-TR-7 (red) identifies the reticular meshwork in RP (G), which is disorganized and extends into the WP in mutant spleen (H).
NKX2.3 mutants exhibit anatomical defects in PP and mesenteric LN
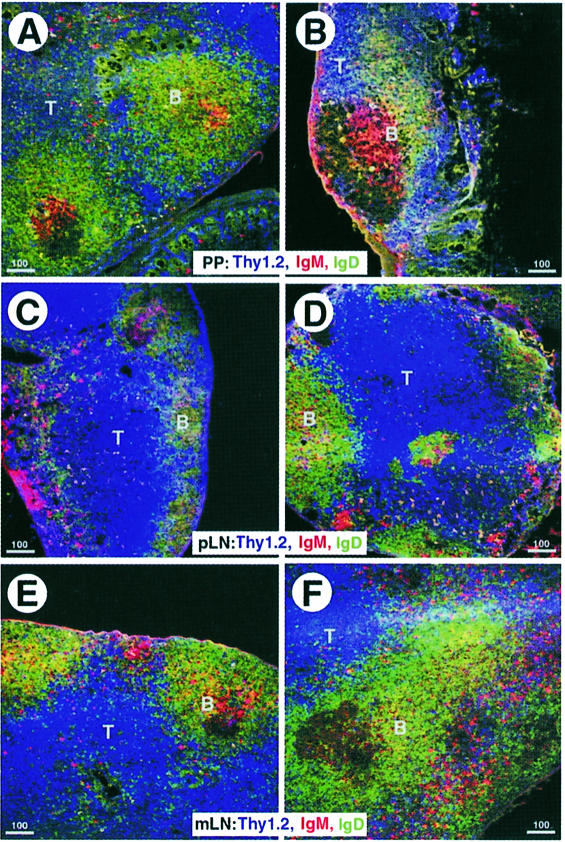
Considering the massive perturbations of the spleen anatomy, we wanted to know whether other secondary lymphoid organs were also affected by the NKX2.3 mutation. Macroscopic inspection of the gut wall in mutant animals failed to reveal PP, suggesting that they were either completely absent or greatly reduced in size. On sections of the gut from mutant animals very small PP with one or two follicles were occasionally found. Immunostainings for T and B cells showed that these cells were present in approximately the correct location in remnant PP of mutants but the proportion of IgD+ B cells appeared diminished (Figure 3A and B). In contrast, peripheral LN (pLN) from mutant mice appeared normal and essentially indistinguishable from their wild-type littermates (Figure 3C and D). However, mesenteric LN (mLN) in mutants occasionally showed an altered follicle structure with T cells partially dislocated into the cortical region (Figure 3E and F). Thus, lack of NKX2.3 seems to affect not only the histology of the spleen but also the formation of PP and to some degree the follicular structure in mLN.
Fig. 3. Tissue architecture of Peyer’s patches (PP) and mesenteric lymph nodes (mLN) but not peripheral lymph nodes (pLN) is affected by the NKX2.3 mutation. Cryostat sections of PP (A and B), pLN (C and D) and mLN (E and F) from wild-type (A, C and E) and mutant (B, D and F) mice were immunostained with anti-Thy 1 (blue), anti-IgM (red) and anti-IgD (green) to reveal the paracortical T cell region and B cell follicles. The few PP identified in mutants are considerably smaller (B). The follicle structure in mLN of mutants is ill-defined and T cells appear misplaced into the cortical region.
The leukocyte composition in spleen and mLN of NKX2.3 mutant mice is abnormal
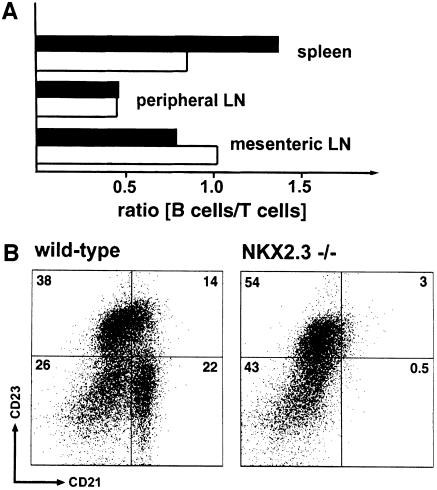
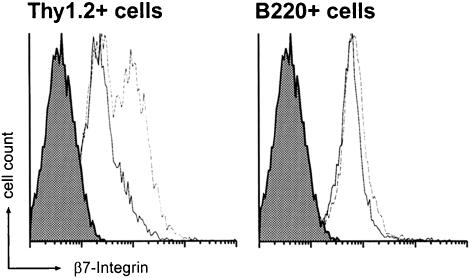
The absolute number of lymphocytes in spleen of NKX2.3-deficient mice was found to be reduced on average by 40% compared with wild type, which is also reflected by the markedly reduced spleen size (mean wet weight 85 mg in wild type versus 56 mg in mutant; data not shown). In order to quantitate the relative distribution of residual lymphocytes in the mutant we used flow cytometry. First, we wanted to know whether the overall ratio of B and T cells was affected in view of their extensive mixing within the white pulp of mutants. We observed no significant changes of cell composition in blood, bone marrow (data not shown) and pLN of mutant mice; however, a considerable shift in the relative ratio of B220+ B cells to CD4+ and CD8+ T cells in favor of T cells in mutant spleen and a moderate but significant relative reduction of T cells in mLN (Figure 4A) were observed. Interestingly, marginal zone phenotype B cells that were characterized as CD21high CD23low B220+ cells were largely absent in mutant spleens (Figure 4B), while the population of IgM+ IgD– cells was not changed significantly (data not shown). In addition, MOMA1+ metallophilic macrophages were found to be reduced in numbers in mutant spleen (data not shown; compare Figure 2C and D). These results confirmed that essential cellular constituents of the marginal zone were either completely lacking or partially misplaced in mutant spleen. We next looked for β7 integrin, which is known to be expressed on lymphocytes and thought to be involved in lymphocyte homing to MALT (Butcher and Picker, 1996; Salmi and Jalkanen, 1997) but not into pLN (Hu et al., 1992; Hamann et al., 1994). Interestingly, T cells but not B cells in blood, and mLN, as well as in spleen, showed increased expression of β7 integrin in mutants as compared with wild type (Figure 5). These findings suggested to us that altered leukocyte composition and incorrect positioning of lymphocytes and possibly macrophages in the NKX2.3 mutant mice were not caused by lack of β7 integrin and were unlikely to be the result of β7 integrin deregulation.
Fig. 4. Lymphocyte composition in spleen and mLN of NKX2.3 mutant mice is altered. (A) The ratio of B220+ B cells and T cells was determined by flow cytometry in spleens, pLN and mLN from wild-type (filled bar) and mutant (open bar) mice. The T cell population in mutant spleen but not in pLN is increased, while the relative number of T cells in mLN is decreased. Note that the overall size of mutant spleens is markedly reduced. (B) The frequency of marginal zone-phenotype B cells was determined by flow cytometry on B220+ cells using CD23 and CD21 antibodies. CD21high CD23low marginal zone B cells are largely absent from NKX2.3 mutant spleen.
Fig. 5. Integrin β7 expression is elevated on T cells but not B cells of NKX2.3 mutant mice. Lymphocytes from blood or spleen of wild type (solid line) and mutant (dotted line) were stained with anti-integrin β7 antibody and anti-Thy 1.2 or anti-B220 antibody for flow cytometry. Integrin β7 expression is increased on T cells of mutant mice. The shaded areas represent staining with isotype control antibodies.
NKX2.3 mutants lack MAdCAM-1 expression
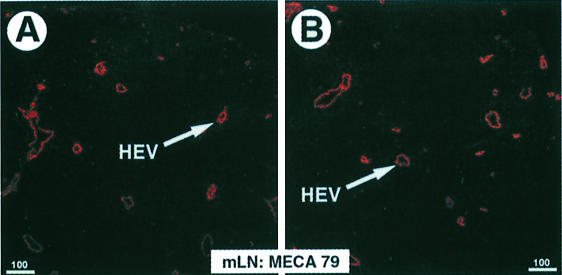
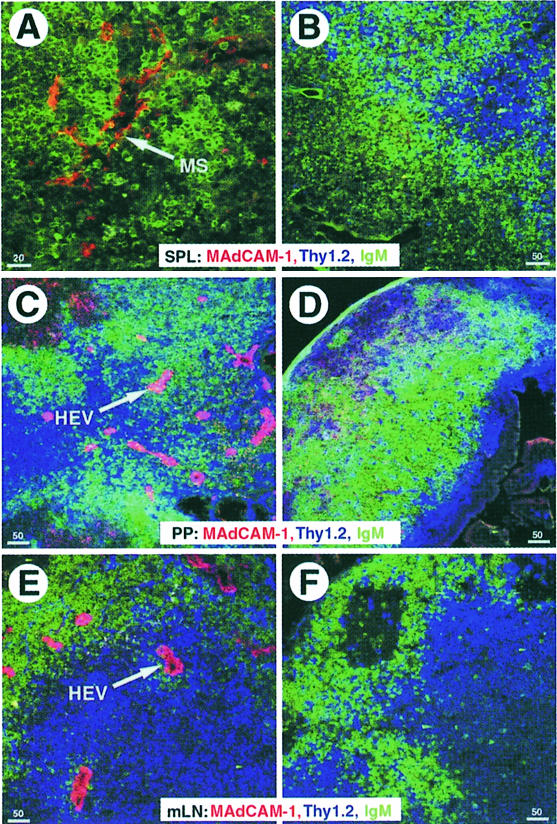
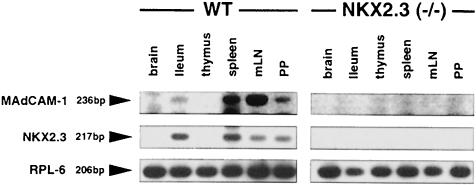
The upregulation of integrin β7 in NKX2.3 mutants prompted us to investigate the expression of potential ligands such as VCAM-1 and MAdCAM-1, which are both normally present on HEVs of PP and mLN and around the marginal sinus in spleen. Similarly, GlyCAM-1 and CD34 are also present on HEVs, whereas MAdCAM-1 is not expressed on HEVs of pLN (Briskin et al., 1993). Using the MECA 79 antibody, which reacts with GlyCAM-1, CD34 and MAdCAM-1, we detected positive cells on sections of wild-type and mutant spleens as well as on HEVs in LN, indicating that postcapillary endothelium was present and essentially unaltered in secondary lymphoid tissues of NKX2-3–/– mutants (Figure 6). FACS analysis with antibodies specific for VCAMs, ICAMs and CD18 (LFA-1) did not provide evidence for changed expression of these cell adhesion proteins in the NKX2.3 mutant (data not shown). In contrast, a MAdCAM-1-specific antibody used on sections of spleen, mLN and residual PP from mutant mice failed to detect any MAdCAM-1+ structures, although it readily decorated marginal sinus and HEVs on sections of wild-type organs (Figure 7). We also failed to identify MAdCAM-1 in the lamina propria of the small intestine from mutants where it is normally expressed together with NKX2.3 in wild-type mice (data not shown). These results indicate that MAdCAM-1 is not expressed in the NKX2.3 mutant mouse. This observation was confirmed by RT–PCR analysis of RNA preparations from spleen, mLN, PP and gut, as well as thymus and brain, the latter two serving as negative controls. While MAdCAM-1 transcripts were readily detected in the appropriate tissues of wild-type mice, no MAdCAM-1 transcripts were present in the mutants (Figure 8). The experiment also demonstrated that MAdCAM-1 and NKX2.3 were always co-expressed in the tissues examined, suggesting that NKX2.3 may be specifically required for MAdCAM-1 expression and in its absence homing of lymphocytes into spleen, PP and mLN is severely compromised.
Fig. 6. MECA 79 antibody detects normal HEVs in lymph nodes of NKX2.3 mutants. Cryostat sections from mLN of wild-type (A) and mutant (B) mice were stained for HEV-associated antigens with MECA 79 antibody.
Fig. 7. MAdCAM-1 is not expressed in secondary lymphoid organs of NKX2.3 mutant mice. Cryostat sections of spleen (A and B), PP (C and D) and mLN (E and F) of wild-type (A, C and E) and mutant (B, D and F) mice were immunostained for the distribution of T and B cells, and MAdCAM-1 expressing non-lymphoid cells using the following antibodies: anti-Thy 1 (blue), anti-IgM (green) and anti-MAdCAM-1 (red). Marginal sinus (MS) and HEVs that express MAdCAM-1 in the controls are indicated by arrows.
Fig. 8. RT–PCR analysis of MAdCAM-1 and NKX2.3 transcripts in various tissues of wild-type and NKX2.3 mutant mice. Note that both genes are co-expressed in small intestine, spleen, mLN and PP in wild type and absent from all tissues in the NKX2.3 mutant. The L6 ribosomal protein transcript was used as internal control.
NKX2.3 transactivates a reporter gene driven by the MAdCAM-1 promoter
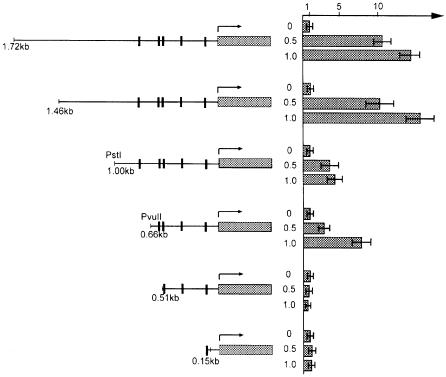
Inspection of the mouse MAdCAM-1 5′-upstream gene sequence including the promoter region revealed five potential binding sites for NKX2 transcription factors as judged by the sequence that has been determined for NKX2.5 binding (Chen and Schwartz, 1995). Gel mobility shift assays confirmed that NKX2.3 and NKX2.5 bind to these sites in vitro (data not shown). Significantly, two potential NKX2 binding sites are also present in the human MAdCAM-1 promoter, one of which is at approximately the same position in reference to the putative start site (sequence from DDBJ/EMBL/GenBank accession No. AC005775). To test whether a murine MAdCAM-1 promoter fragment containing the NKX2 consensus binding sites was sufficient to direct transactivation by NKX2.3, transient transfections in 10T1/2 fibroblasts were performed. We used a luciferase reporter gene linked to 1.8 kb of 5′-upstream sequence of the mouse MAdCAM-1 gene encompassing all potential NKX2 binding sites together with increasing concentrations of an expression vector for NKX2.3. We also tested 5′-truncated promoter fragments with successively deleted individual binding sites. As shown in Figure 9, cotransfections of reporter constructs and NKX2.3 expression plasmid resulted in transactivation of the 1.8 and 1.4 kb promoter fragments up to 15-fold over background in a concentration-dependent manner. A 0.9 kb promoter fragment still containing all potential NKX2 binding sites was activated 5-fold only, suggesting that a potential enhancer element might be present between –1.4 and –0.9 kb of the MAdCAM-1 promoter. Removal of the most upstream NKX2 binding site had no effect on transactivation, whereas deletion of the next successive site rendered the promoter completely inactive. This result indicates that NKX2.3 is able to activate MAdCAM-1 transcription and suggests that at least one of the consensus binding sites mediates activation. This finding is in agreement with the idea that MAdCAM-1 expression may be directly controlled by NKX2.3.
Fig. 9. NKX2.3 transactivates the MAdCAM-1 promoter. Promoter fragments of the MAdCAM-1 gene encompassing all or part of the potential NKX2.3 binding sites (indicated by vertical bars) were cloned in front of the firefly luciferase reporter and cotransfected with 0.5 or 1 µg of NKX2.3 expression vector into 10T1/2 fibroblasts. Relative reporter activities are shown by filled columns as fold activation over control. Basal activity in the absence of NKX2.3 was ∼150 light units per 0.5 s. Each column represents the mean of at least three independent transfection experiments.
Discussion
The organized structure of secondary lymphoid tissues is believed to enhance efficiency of antigen presentation required for effective activation and maturation of antigen-responsive lymphocytes. Little information is available on molecules that control development of secondary lymphoid organs and maintain the defined cellular composition and the typical microenvironment between the lymphoid and nonlymphoid components in spleen and LN.
Here we report evidence that the transcription factor NKX2.3 is necessary for proper structure and normal development of spleen, PP and mLN. In NKX2.3–/– mutant mice the overall size of the spleen is reduced and the white pulp is generally smaller and less branched. Only small remnants of PP exist in mutant intestine and the follicular structure in mLN is abnormal. This phenotype is variable depending on the genetic background, ranging from occasional asplenia in a mixed 129Sv/C57BL6 background and less severe size reduction of spleens in 129Sv inbred mice. Independent of the genetic background, however, cellular composition and tissue structure of mutant spleens are always disturbed. The observation that lymphocyte composition in NKX2.3 mutant mice appears normal in bone marrow, blood and pLN argues that the observed phenotype is most likely to be attributable to stromal defects rather than to alterations of the lymphocytes. Most significantly, the marginal zone that separates the white pulp from the red pulp is apparently absent in spleen of the NKX2.3–/– mutant as judged by the complete loss of all cell types characteristic for this structure. The marginal zone consists of a three-dimensional network of reticular tissue that is associated with various cell types. These include marginal zone phenotype B cells, ER-TR-9+ marginal zone macrophages, MOMA1+ metallophilic macrophages and MAdCAM-1+ fiber-forming reticular cells, which all seem to be missing in the mutant spleen by immunohistological criteria. Moreover, no correct PALS, the discrete T cell zone around a central arteriole, can be recognized in mutant spleens. FACS analysis, however, shows that, although reduced in numbers, at least some lymphocytes are present in mutant spleens, suggesting that loss of NKX2.3 leads to their displacement within the spleen rather than their complete elimination. In this context it is a crucial finding of this investigation that the mucosal homing receptor, MAdCAM-1, is not expressed in the NKX2.3 mutant. Together with the observation that NKX2.3 can directly activate the MAdCAM-1 promoter this implies that a primary defect in the mutant is loss of MAdCAM-1. All other adhesion molecules that we tested in secondary lymphoid organs were expressed normally in the mutant, which suggests that MAdCAM-1 plays a specific role that can not be substituted by other adhesins. However, as we have not demonstrated a direct link between the absence of MAdCAM-1 and the defects in secondary lymphoid organs, it is formally possible that the observed phenotype in NKX2.3 mutants is not solely due to the lack of MAdCAM-1, and other effectors may also be influenced directly or indirectly in NKX2.3-deficient mice.
MAdCAM-1 is a member of the superfamily of immunoglobulin-like proteins containing several structural motifs with homology to ICAM-1, VCAM-1 and IgA1 as well as a mucin-like domain (Briskin et al., 1993; Sampaio et al., 1995). It is expressed on mucosal venules and is involved in directing lymphocyte traffic into PP and intestinal lamina propria (Butcher and Picker, 1996). MAdCAM-1 is also expressed in sinus-lining cells in the spleen surrounding the PALS and follicle areas (Kraal et al., 1995; Koike et al., 1996, 1997; Tanaka et al., 1996). It has been demonstrated that MAdCAM-1 serves as the main binding partner for α4β7 integrin on lymphocytes and plays a role in l-selectin-mediated lymphocyte rolling (Berg et al., 1993; Berlin et al., 1993; Erle et al., 1994; Hamann et al., 1994; Viney et al., 1996). Short-term homing experiments clearly demonstrated that lymphocytes entering the spleen first accumulate in the marginal zone before they migrate into the white pulp and their appropriate compartments (Brelinska and Pilgrim, 1982; Willfuhr et al., 1990; Pabst and Westermann, 1991). The regulated process of lymphocyte retention in the marginal zone and transmigration into the PALS involves selective adhesion mechanisms. MAdCAM-1 may directly mediate adherence of β7 integrin-positive lymphocytes to the marginal zone, possibly initiated by secondary lymphoid organ chemokine (SLC; Pachynski et al., 1998). However, the role of the MAdCAM-1–β7 interaction has been questioned, because injections of MAdCAM-1 blocking antibody or β7 integrin antibody into mice failed to prevent immigration of lymphocytes into the white pulp, while it effectively blocked lymphocyte entry into PP (Kraal et al., 1995). In addition, mice lacking expression of SLC or its cognate chemokine receptor CCR7 have no defect in lymphocyte homing to the spleen per se, although lymphocytes are severely impaired in migrating from the red to the white pulp in these animals (Forster et al., 1999; Gunn et al., 1999). Marginal zone macrophages may also play a role in the homing process, as depletion of macrophages from the marginal zone impairs the ability of lymphocytes to localize in the PALS area (Kraal et al., 1989a,b, 1994). As we have shown in this study, the spleen marginal zone in NKX2.3 mutants lacks MOMA1+ metallophiles. Although we were unable to demonstrate convincing co-expression of β7 integrin and MOMA1 on metallophilic macrophages by flow cytometry or immunostaining, it seems quite possible that these cells may be retained in the marginal zone by this interaction with MAdCAM-1. The lack of MAdCAM-1 in spleen of the NKX2.3 mutant would then help to explain the deficiency of marginal metallophilic macrophages and underline the importance of MAdCAM-1 for the development of a proper marginal zone in recruiting immigrating blood cells. Marginal metallophilic macrophages express sialoadhesins that can serve as receptors for interactions with lymphocytes (van den Berg et al., 1992), allowing for the possibility that these macrophages participate in the recruitment of lymphocytes to the marginal zone. Whether sialoadhesins and other receptors or additional signals emanating from macrophages play a role in lymphocyte homing in vivo has yet to be determined. Given the well documented importance of the marginal zone for lymphocyte entrance into the white pulp, we would like to suggest that the observed displacement of B and T cells within the PALS is likely to be caused indirectly by the structurally impaired and functionally defective marginal zone in the NKX2.3 mutant.
Besides the defects in spleen, NKX2.3 mutants also exhibit phenotypic alterations in PP and mLN, while pLN appear to be normal. This observation is consistent with the expression of MAdCAM-1 only in the affected secondary lymphoid organs but not in pLN. Lympho cyte extravasation in LN occurs on HEVs involving organ-specific sets of cell adhesion receptors (Butcher and Picker, 1996; Salmi and Jalkanen, 1997). Integrin α4β1 recognizes VCAM-1 and fibronectin and is believed to have a major role in inflammation (Issekutz, 1991; Yednock et al., 1992; Weg et al., 1993), while integrin α4β7 also recognizes MAdCAM-1 on HEVs of PP and mLN where it mediates lymphocyte homing to mucosal sites (Streeter et al., 1988; Berlin et al., 1993). In line with this distinct receptor–function relationship are anti-MAdCAM-1 antibodies that inhibit lymphocyte homing to mLN and PP but not to pLN. A recent publication, however, challenges the view that the inflammatory response is mediated solely by VCAM-1 and homing to mLN and PP distinctly by MAdCAM-1 and suggests cooperative action of both receptors in mLN and PP (Berlin-Rufenach et al., 1999). Thus, lymphocytes may also be able to enter these organs by integrin α4β7 interaction with VCAM-1, which would then help to explain why lymphocytes, although reduced in numbers, are present in mucosal LN in the absence of MAdCAM-1 in the NKX2.3 mouse. Quite clearly, however, this pathway should be less effective, as mutant PP contain drastically fewer lymphocytes. Moreover, formation of correct primary follicles in mLN is also affected.
Similarly to the NKX2.3 mutant mouse, the recessive alymphoplasia (aly) mutation also causes a splenic phenotype that exhibits B and T cell mixing in the white pulp, lack of the marginal zone and absence of MAdCAM-1 (Koike et al., 1996, 1997). In contrast to NKX2.3–/– mice, however, aly/aly mice also have total aplasia of LN and severe immunodeficiency involving both cellular and humoral immune responses (Miyawaki et al., 1994). Preliminary analysis of the NKX2.3 mouse indicates a normal antibody response to a T cell-dependent antigen, which may be due to the intact pLN. More specific challenges, for example, with a mucosal pathogen or T cell-independent humoral response antigen, have not yet been investigated but may yield different results. The aly gene is located on mouse chromosome 11 (Kuramoto et al., 1994) and is clearly not allelic to MAdCAM-1, which maps to mouse chromosome 10 (Koike et al., 1997). aly is also distinct from NKX2.3, which is located on mouse chromosome 19 (Copeland et al., 1994). A recent publication reports the aly allele as a point mutation in the gene encoding NF-κB-inducing kinase (Shinkura et al., 1999). Interestingly, the MAdCAM-1 promoter contains NF-κB binding sites (Sampaio et al., 1995), which may be important for its expression in some tissues and therefore explain the overlapping phenotypes of aly/aly and NKX2.3–/– mutants. In summary, the genetic model of the NKX2.3 mouse mutant provides strong evidence that the transcription factor NKX2.3 is crucial for the expression of MAdCAM-1 and this cell adhesion molecule has a specific function in the correct formation of the marginal zone in spleen and lymphocyte homing in mucosal lymphoid tissues. It also demonstrates that the precise anatomical relationship around the marginal sinus is important for cellular functions pertaining to lymphocyte traffic within and between lymphatic tissues.
Materials and methods
Generation of NKX2.3–/– mice
A null mutation of the NKX2.3 gene was obtained by eliminating the entire coding region through homologous recombination in ES cells as described previously (Pabst et al., 1999). For this study, chimeric mice were bred to 129Sv mice and the mutation was maintained on the 129Sv inbred strain.
Antibodies and flow cytometry
The following antibodies and conjugates were used in this study: anti-Ly5 (B220; clone RA3-6B2; Caltech), anti-CD4 (L3-T4; clone YTS 191.1; Caltech), rat anti-mouse-IgM (clone R6-60.2; PharMingen), anti-Thy1.2 (Becton Dickinson), anti-mouse-IgM–FITC (clone LO-MM-9; Biosource), anti-mouse-IgD–FITC (clone SBA 1; Southern Biotechnology), anti-MAdCAM-1 (clone MECA-89; PharMingen), anti-PNAd carbohydrate epitope (clone MECA-79; PharMingen), anti-MZ-macrophages (clone ER-TR 9; Dianova), anti-metallophile macrophages (clone MOMA-1; Dianova), anti-β7 integrin and anti-ER-TR 7 (BMA Biomedicals AG, Switzerland). Differential counts were determined by flow cytometry with monoclonal antibodies and forward–side scatter properties as described previously (Forster et al., 1996).
Light and electron microscopy
For light microscopy, spleens were fixed in 4% paraformaldehyde overnight, dehydrated with increasing concentrations of ethanol, embedded in paraffin and sectioned at 6 µm. Staining with H&E was performed using standard protocols. For electron microscopy mice were anesthetized and fixed by perfusion through the tail vein with 5% formaldehyde/3% glutaraldehyde in cacodylate buffer (0.1 M cacodylate, 0.09 M sucrose, 0.01 M MgCl2, 0.01 M CaCl2 pH 6.9). Excised spleens were further fixed in the same fixation solution overnight at 4°C. After washing with cacodylate buffer, spleens were dehydrated with a graded series of acetone on ice and embedded as described (Spurr, 1969). Ultra-thin sections were cut with diamond knives and counterstained with uranyl acetate for 30 min and lead citrate for 3 min using the Leica Ultrostainer. Sections were examined in a Zeiss transmission electron microscope EM910 at an acceleration voltage of 80 kV and calibrated magnifications.
Immunohistology
Dissected organs were embedded in tissue tek (Miles, IN) and frozen on dry ice. Cryostat sections (8 µm thick) on gelatine-covered slides were dried overnight and fixed with acetone. Slides were rehydrated and pre-incubated for 1 h in staining buffer containing 0.1 M Tris pH 8.0, 0.1% Tween-20, supplemented with 5% heat-inactivated rat serum. Sections were incubated with antibodies for 1 h at room temperature or overnight at 4°C. After three washes in staining buffer, binding of biotinylated antibodies was revealed with streptavidin–TRITC (1:150; Jackson) or streptavidin-Cy5 (1:150; Amersham). After three final washes, slides were mounted in Moviol (Calbiochem) and analyzed by confocal microscopy (Leica, TCS 4D-I).
RT–PCR
Total RNA from brain, ileum, thymus, spleen, mLN and PP was isolated using RNA Clean (AGS) and subjected to RT–PCR. The reverse transcription was carried out with 1 µg of total RNA as template and 100 ng of randomized hexanucleotides as primers according to standard procedures. Specific PCR primers were used for MADCAM-1, NKX2.3 and ribosomal protein L6:
MAdCAM-1 upstream primer: CAGCTTGGGCAGTGTACAGAC;
MAdCAM-1 downstream primer: TCTGCAGGCCAGATGTTGTGG;
NKX2.3 upstream primer: CTCACTTGCAGGCGGAATTG;
NKX2.3 downstream primer: GGCTCCGTTCGCTCACAACC;
L6 upstream primer: GAAGCTCATCTATGAGAAGGC;
L6 downstream primer: AAGACGAAGGAGCTGCAGAAC.
Amplification was carried out in 34 cycles (MAdCAM-1 and NKX2.3) or 28 cycles (L6) each composed of 45 s at 94°C, 30 s at 55°C and 30 s at 72°C. Amplification products were analyzed on a 1% agarose gel.
Expression and reporter plasmids
Full-length NKX2.3 cDNA was subcloned into the pCS2 expression vector (Roth et al., 1991). MAdCAM-1 promotor fragments were amplified by PCR from genomic DNA using the following primers:
1.72 kb upstream primer: TTCCACCGGTGCCTTAGGAAAGC;
1.46 kb upstream primer: ACCCTCAGAACTCCAGCCTGAG;
0.51 kb upstream primer: GAGTCTGTCAGGCCTGCAAGTGGAA;
0.15 kb upstream primer: GAGCTCTGCCTCCTACAAAGTGGGG;
MAdCAM-1 dowstream primer: GTAAGCTTCTTGTGACCGCCAG GGGAAG.
PCR fragments were cloned into the pGL3-luciferase vector (Promega). The 1.0 and 0.66 kb MAdCAM-1 promotor luciferase construct was derived from the 1.72 kb construct by digestion of two single restriction sites with PstI and PvuII, respectively.
Cell culture and transient transfections
10T1/2 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum. For transient transfection assays 105 cells were plated in 24-well plates 18 h prior to transfection. Cells were transfected by standard calcium phosphate precipitation with 250 ng of the luciferase reporter plasmid together with 500 ng or 1 µg of the NKX2.3 expression construct, and 100 ng of CMV β-galactosidase vector used as internal control. Cells were harvested 36 h after transfection and assayed for luciferase and β-galactosidase activity. Results shown represent the mean of three independent transfections, normalized to β-galactosidase activity.
Acknowledgments
Acknowledgements
We thank D.Breitfeld and E.Müller for excellent technical assistance and M.Rohde for the EM images. This work was supported by the Deutsche Forschungsgemeinschaft SFB 271, TP A1 and by the Fonds der Chemischen Industrie.
References
- Berg E.L., McEvoy,L.M., Berlin,C., Bargatze,R.F. and Butcher,E.C. (1993) L-selectin-mediated lymphocyte rolling on MAdCAM-1. Nature, 366, 695–698. [DOI] [PubMed] [Google Scholar]
- Berlin C., Berg,E.L., Briskin,M.J., Andrew,D.P., Kilshaw,P.J., Holzmann,B., Weissman,I.L., Hamann,A. and Butcher,E.C. (1993) α4β7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell, 74, 185. [DOI] [PubMed] [Google Scholar]
- Berlin-Rufenach C., Otto,F., Mathies,M., Westermann,J., Owen,M.J., Hamann,A. and Hogg,N. (1999) Lymphocyte migration in lymphocyte function-associated antigen (LFA)-1-deficient mice. J. Exp. Med., 189, 1467–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brelinska R. and Pilgrim,C. (1982) The significance of the subcompartments of the marginal zone for directing lymphocyte traffic within the splenic pulp of the rat. Cell Tissue Res., 226, 155–165. [DOI] [PubMed] [Google Scholar]
- Briskin M.J., McEvoy,L.M. and Butcher,E.C. (1993) MAdCAM-1 has homology to immunoglobulin and mucin-like adhesion receptors and to IgA1. Nature, 363, 461–464. [DOI] [PubMed] [Google Scholar]
- Butcher E.C. and Picker,L.J. (1996) Lymphocyte homing and homeo stasis. Science, 272, 60–66. [DOI] [PubMed] [Google Scholar]
- Chaplin D.D. and Fu,Y. (1998) Cytokine regulation of secondary lymphoid organ development. Curr. Opin. Immunol., 10, 289–297. [DOI] [PubMed] [Google Scholar]
- Chen C.Y. and Schwartz,R.J. (1995) Identification of novel DNA binding targets and regulatory domains of a murine tinman homeodomain factor, nkx-2.5. J. Biol. Chem., 270, 15628–15633. [DOI] [PubMed] [Google Scholar]
- Copeland N.G., Jenkins,N.A. and Harvey,R.P. (1994) The murine homeobox genes Nkx2.3 and Nkx2.6 are located on chromosomes 19 and 14, respectively. Genomics, 22, 655–656. [DOI] [PubMed] [Google Scholar]
- Dijkstra C.D., van Vliet,E., Dopp,E.A., van der Lelij,A.A. and Kraal,G. (1985) Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology, 55, 23–30. [PMC free article] [PubMed] [Google Scholar]
- Erle D.J., Briskin,M.J., Butcher,E.C., Garcia-Pardo,A., Lazarovits,A.I. and Tidswell,M. (1994) Expression and function of the MAdCAM-1 receptor, integrin α4β7, on human leukocytes. J. Immunol., 153, 517–528. [PubMed] [Google Scholar]
- Forster R., Mattis,A.E., Kremmer,E., Wolf,E., Brem,G. and Lipp,M. (1996) A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell, 87, 1037–1047. [DOI] [PubMed] [Google Scholar]
- Forster R., Schubel,A., Breitfeld,D., Kremmer,E., Renner-Muller,I., Wolf,E. and Lipp,M. (1999) CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell, 99, 23–33. [DOI] [PubMed] [Google Scholar]
- Fu Y.X. and Chaplin,D.D. (1999) Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol., 17, 399–433. [DOI] [PubMed] [Google Scholar]
- Gunn M.D., Kyuwa,S., Tam,C., Kakiuchi,T., Matsuzawa,A., Williams,L.T. and Nakano,H. (1999) Mice lacking expression of secondary lymphoid organ chemokine have defects in lymphocyte homing and dendritic cell localization. J. Exp. Med., 189, 451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann A., Andrew,D.P., Jablonski-Westrich,D., Holzmann,B. and Butcher,E.C. (1994) Role of α4-integrins in lymphocyte homing to mucosal tissues in vivo.J. Immunol., 152, 3282–3293. [PubMed] [Google Scholar]
- Harvey R.P. (1996) NK-2 homeobox genes and heart development. Dev. Biol., 178, 203–216. [DOI] [PubMed] [Google Scholar]
- Hu M.C., Crowe,D.T., Weissman,I.L. and Holzmann,B. (1992) Cloning and expression of mouse integrin βp (β7): a functional role in Peyer’s patch-specific lymphocyte homing. Proc. Natl Acad. Sci. USA, 89, 8254–8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issekutz T.B. (1991) Inhibition of in vivo lymphocyte migration to inflammation and homing to lymphoid tissues by the TA-2 monoclonal antibody. A likely role for VLA-4 in vivo.J. Immunol., 147, 4178–4184. [PubMed] [Google Scholar]
- Koike R., Nishimura,T., Yasumizu,R., Tanaka,H., Hataba,Y., Watanabe,T., Miyawaki,S. and Miyasaka,M. (1996) The splenic marginal zone is absent in alymphoplastic aly mutant mice. Eur. J. Immunol., 26, 669–675. [DOI] [PubMed] [Google Scholar]
- Koike R., Watanabe,T., Satoh,H., Hee,C.S., Kitada,K., Kuramoto,T., Serikawa,T., Miyawaki,S. and Miyasaka,M. (1997) Analysis of expression of lymphocyte homing-related adhesion molecules in ALY mice deficient in lymph nodes and Peyer’s patches. Cell Immunol., 180, 62–69. [DOI] [PubMed] [Google Scholar]
- Kraal G., Rodrigues,H., Hoeben,K. and Van Rooijen,N. (1989a) Lymphocyte migration in the spleen: the effect of macrophage elimination. Immunology, 68, 227–232. [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Ter Hart,H., Meelhuizen,C., Venneker,G. and Claassen,E. (1989b) Marginal zone macrophages and their role in the immune response against T-independent type 2 antigens: modulation of the cells with specific antibody. Eur. J. Immunol., 19, 675–680. [DOI] [PubMed] [Google Scholar]
- Kraal G., Hoeben,K., Breve,J. and van den Berg,T.K. (1994) The role of sialic acid in the localization of lymphocytes in the spleen. Immunobiology, 190, 138–149. [DOI] [PubMed] [Google Scholar]
- Kraal G., Schornagel,K., Streeter,P.R., Holzmann,B. and Butcher,E.C. (1995) Expression of the mucosal vascular addressin, MAdCAM-1, on sinus-lining cells in the spleen. Am. J. Pathol., 147, 763–771. [PMC free article] [PubMed] [Google Scholar]
- Kuramoto T., Mashimo,T., Koike,R., Miyawaki,S., Yamada,J., Miyasaka,M. and Serikawa,T. (1994) The alymphoplasia (aly) mutation co-segregates with the intercellular adhesion molecule-2 (lcam-2) on mouse chromosome 11. Int. Immunol., 6, 991–994. [DOI] [PubMed] [Google Scholar]
- MacLennan I.C. (1994) Germinal centers. Annu. Rev. Immunol., 12, 117–139. [DOI] [PubMed] [Google Scholar]
- Miyawaki S., Nakamura,Y., Suzuka,H., Koba,M., Yasumizu,R., Ikehara,S. and Shibata,Y. (1994) A new mutation, aly, that induces a generalized lack of lymph nodes accompanied by immunodeficiency in mice. Eur. J. Immunol., 24, 429–434. [DOI] [PubMed] [Google Scholar]
- Oldfield S., Liu,Y.J., Beaman,M. and MacLennan,C.M. (1988) Memory B cells generated in T cell-dependent antibody responses colonise the splenic marginal zone. Adv. Exp. Med. Biol., 237, 93–98. [DOI] [PubMed] [Google Scholar]
- Pabst O., Schneider,A., Brand,T. and Arnold,H.H. (1997) The mouse Nkx2-3 homeodomain gene is expressed in gut mesenchyme during pre- and postnatal mouse development. Dev. Dyn., 209, 29–35. [DOI] [PubMed] [Google Scholar]
- Pabst O., Zweigerdt,R. and Arnold,H.H. (1999) Targeted disruption of the homeobox transcription factor Nkx2-3 in mice results in postnatal lethality and abnormal development of small intestine and spleen. Development, 126, 2215–2225. [DOI] [PubMed] [Google Scholar]
- Pabst O., Herbrand,H., Takuma,N. and Arnold,H.H. (2000) NKX2 gene expression in neuroectoderm but not in mesendodermally derived structures depends on sonic hedgehog in mouse embryos. Dev. Genes Evol., 210, 47–50. [DOI] [PubMed] [Google Scholar]
- Pabst R. and Westermann,J. (1991) The role of the spleen in lymphocyte migration. Scanning Microsc., 5, 1075–1080. [PubMed] [Google Scholar]
- Pachynski R.K., Wu,S.W., Gunn,M.D. and Erle,D.J. (1998) Secondary lymphoid-tissue chemokine (SLC) stimulates integrin α4β7-mediated adhesion of lymphocytes to mucosal addressin cell adhesion molecule-1 (MAdCAM-1) under flow. J. Immunol., 161, 952–956. [PubMed] [Google Scholar]
- Roth M.B., Zahler,A.M. and Stolk,J.A. (1991) A conserved family of nuclear phosphoproteins localized to sites of polymerase II transcription. J. Cell Biol., 115, 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M. and Jalkanen,S. (1997) How do lymphocytes know where to go: current concepts and enigmas of lymphocyte homing. Adv. Immunol., 64, 139–218. [DOI] [PubMed] [Google Scholar]
- Sampaio S.O., Li,X., Takeuchi,M., Mei,C., Francke,U., Butcher,E.C. and Briskin,M.J. (1995) Organization, regulatory sequences and alternatively spliced transcripts of the mucosal addressin cell adhesion molecule-1 (MAdCAM-1) gene. J. Immunol., 155, 2477–2486. [PubMed] [Google Scholar]
- Shinkura R., Kitada,K., Matsuda,F., Tashiro,K., Ikuta,K., Suzuki,M., Kogishi,K., Serikawa,T. and Honjo,T. (1999) Alymphoplasia is caused by a point mutation in the mouse gene encoding NF-κB-inducing kinase. Nature Genet., 22, 74–77. [DOI] [PubMed] [Google Scholar]
- Spurr A.R. (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res., 26, 31–43. [DOI] [PubMed] [Google Scholar]
- Steinman R.M., Pack,M. and Inaba,K. (1997) Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev., 156, 25–37. [DOI] [PubMed] [Google Scholar]
- Streeter P.R., Berg,E.L., Rouse,B.T., Bargatze,R.F. and Butcher,E.C. (1988) A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature, 331, 41–46. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Hataba,Y., Saito,S., Fukushima,O. and Miyasaka,M. (1996) Phenotypic characteristics and significance of reticular meshwork surrounding splenic white pulp of mice. J. Electron Microsc. (Tokyo), 45, 407–416. [DOI] [PubMed] [Google Scholar]
- van den Berg T.K., Breve,J.J., Damoiseaux,J.G., Dopp,E.A., Kelm,S., Crocker,P.R., Dijkstra,C.D. and Kraal,G. (1992) Sialoadhesin on macrophages: its identification as a lymphocyte adhesion molecule. J. Exp. Med., 176, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Krieken J.H., von Schilling,C., Kluin,P.M. and Lennert,K. (1989) Splenic marginal zone lymphocytes and related cells in the lymph node: a morphologic and immunohistochemical study. Hum. Pathol., 20, 320–325. [DOI] [PubMed] [Google Scholar]
- van Vliet E., Melis,M. and van Ewijk,W. (1985) Marginal zone macrophages in the mouse spleen identified by a monoclonal antibody. Anatomical correlation with a B cell subpopulation. J. Histochem. Cytochem., 33, 40–44. [DOI] [PubMed] [Google Scholar]
- van Vliet E., Melis,M., Foidart,J.M. and van Ewijk,W. (1986) Reticular fibroblasts in peripheral lymphoid organs identified by a monoclonal antibody. J. Histochem. Cytochem., 34, 883–890. [DOI] [PubMed] [Google Scholar]
- Viney J.L. et al. (1996) Mucosal addressin cell adhesion molecule-1: a structural and functional analysis demarcates the integrin binding motif. J. Immunol., 157, 2488–2497. [PubMed] [Google Scholar]
- Weg V.B., Williams,T.J., Lobb,R.R. and Nourshargh,S. (1993) A monoclonal antibody recognizing very late activation antigen-4 inhibits eosinophil accumulation in vivo.J. Exp. Med., 177, 561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willfuhr K.U., Westermann,J. and Pabst,R. (1990) Absolute numbers of lymphocytes subsets migrating through the compartments of the normal and transplanted rat spleen. Eur. J. Immunol., 20, 903–911. [DOI] [PubMed] [Google Scholar]
- Yednock T.A., Cannon,C., Fritz,L.C., Sanchez-Madrid,F., Steinman,L. and Karin,N. (1992) Prevention of experimental autoimmune encephalomyelitis by antibodies against α4β1 integrin. Nature, 356, 63–66. [DOI] [PubMed] [Google Scholar]