Abstract
We identified a temperature-sensitive mutant of the plant pathogenic fungus Ustilago maydis that is defective in the polar distribution of cell wall components and shows abnormal morphology. The affected gene, yup1, was cloned by complementation. It encodes a putative target soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (t-SNARE), suggesting a function in membrane fusion. A Yup1–GFP fusion protein localized to vesicles that showed rapid saltatory motion along microtubules. These vesicles are part of the endocytic pathway and accumulate at sites of active growth, thereby supporting the expansion of the hyphal tip. In yup1ts cells, endocytosis is impaired and accumulation of Yup1-carrying endosomes at cell poles is abolished, resulting in apolar distribution of wall components and morphological alterations. This suggests that a membrane recycling process via early endosomes supports polar growth of U.maydis.
Keywords: endocytosis/membrane recycling/microtubules/organelle transport/t-SNARE
Introduction
Cell motility enables cells to explore their environment and is of crucial importance for developmental processes (Bray, 1992). In contrast to most protozoan and vertebrate cells, plant and fungal cells are surrounded by a cell wall that counteracts internal hydrostatic pressure. Unlike most plant cells, fungal cells expand at defined regions, which results in directed growth. This allows a certain degree of motility, which appears to be important for substrate invasion (Wessels, 1986). Directed tip growth requires polar delivery of biosynthetic and hydrolytic enzymes as well as membrane and wall components to the hyphal apex or the growing bud. It is widely accepted that growth supplies such as chitin synthase are transported in microvesicles (reviewed in Gow, 1995). Therefore, polarized growth is dependent on localized vesicle exocytosis, in which the cytoskeleton (reviewed by Heath, 1995) as well as associated molecular motors (reviewed by Steinberg, 2000) have a central role.
Growth of hyphae is accompanied by an accumulation of vesicles in the expanding tip, the so-called Spitzenkörper (Grove and Bracker, 1970). Several lines of evidence suggest that this structure might serve as an intermediate storage compartment that supplies the growing tip with vesicles for regulated exocytosis (reviewed in Bartnicki-Garcia, 1996). It has been estimated that in fast-growing hyphae of Neurospora crassa up to 38 000 vesicles fuse with the apex each minute (Collinge and Trinci, 1974). This surprisingly high number might even be an underestimation because postulated membrane-recycling processes between endosomes and the plasma membrane are not taken into account (Wessels, 1986). In fact, electron microscopic (EM) studies on plant pollen tubes indicated that membrane recycling can have a considerable part in polar growth (Wessels, 1986). These specialized plant cells, similar to fungal hyphae, expand by tip growth, and this process is supported by recycling of membranes (Picton and Steer, 1983). Indications for membrane recycling in fungi exist only in Uromyces fabae (Hoffmann and Mendgen, 1998) and in Saccharomyces cerevisiae (Chuang and Schekman, 1996). Detailed knowledge about molecular components of the tip growth machinery is almost exclusively restricted to S.cerevisiae and Schizosaccharomyces pombe (reviewed by Mata and Nurse, 1998).
In this study we set out to elucidate aspects of polar growth and dimorphism in Ustilago maydis. This facultative plant pathogen can be propagated in a haploid yeast-like cell form that is accessible to both genetic and molecular methods (reviewed in Banuett, 1995) and is well suited for cytological studies (Lehmler et al., 1997; Steinberg et al., 1998). During its life cycle, U.maydis undergoes several distinct morphological transitions. These include a dimorphic switch when haploid cells of different mating types fuse and give rise to a filamentous dikaryon (reviewed in Banuett, 1995). On the plant surface, the dikaryotic hyphae expand by polar tip growth, leaving empty sections behind, a process that requires the microtubule-dependent motor kinesin (Lehmler et al., 1997). Inside the host, the fungus proliferates and tumor formation is induced, followed by karyogamy and sporogenesis (reviewed in Banuett, 1995).
Here we describe the isolation of a temperature-sensitive (ts) U.maydis mutant, yup1, which is defective in morphogenesis. By complementation we identified a gene encoding a putative endosomal target soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor (t-SNARE). Unexpectedly, Yup1 mediates both endocytic membrane fusion and polar growth. Our data suggest that endocytosis and exocytosis are tightly coupled via early endosomes and that membrane recycling processes play a central role in polar growth of U.maydis.
Results
Isolation of the yup1 gene
In a screen for ts mutants, the haploid U.maydis wild-type strain FB1 (a1 b1) was mutagenized by UV. ts survivors were identified by replica plating followed by incubation at 24 and 34°C, respectively. One of these ts mutants displayed a characteristic morphological phenotype (see below). By segregation analysis it was shown that the ts phenotype co-segregated with the observed morphological defects. One of these segregants, RWS1 (a2b1yup1ts), was complemented with a genomic library on an autonomously replicating plasmid. We identified a 5.6 kb DNA fragment that rescued the ts phenotype as well as the morphological defects of strain RWS1. By generating subclones, the complementing activity was confined to a 1.6 kb DNA fragment, which contained a single open reading frame of 903 bp, yup1.
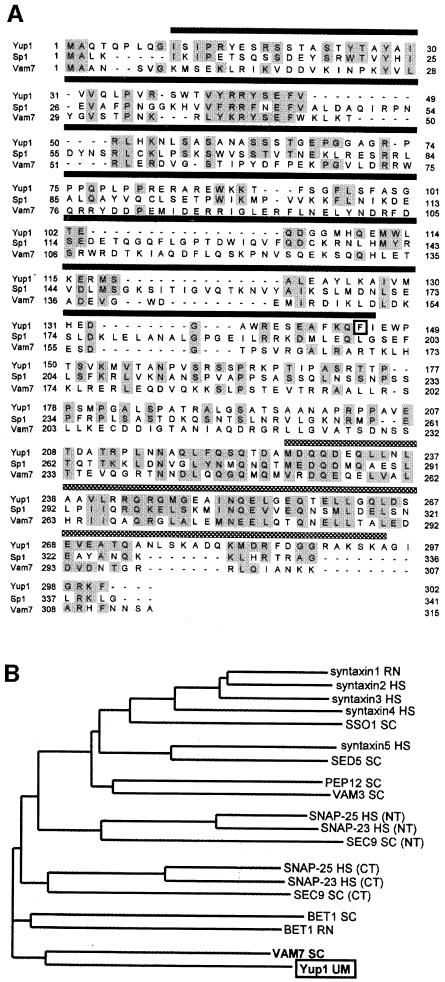
The yup1 gene is predicted to encode a protein of 33 kDa. Sequence analysis indicates that Yup1 contains a putative NADPH oxidase p40 (PX) domain, a region that might be important for protein–protein interaction (Ponting, 1996). Based on different calculations, the PX domain is predicted to span amino acids 4–149 (p: 1.9e-5, PFAM; Bateman et al., 1999), amino acids 4–149 (p: 1.7e-7; SMART; Schulz et al., 1998) or amino acids 10–146 (NScore 13.661, PROSITE profiles). According to these results we defined the PX domain in Yup1 as the region that appeared in all predictions (amino acids 10–146, Figure 1A). The C-terminal part of Yup1 (amino acids 227–295, Figure 1A) is predicted to adopt an α-helical coiled coil (COILS; Lupas et al., 1991).
Fig. 1. Sequence analysis of Yup1. (A) Alignment of the predicted amino acid sequence of Yup1 with related sequences. Yup1 shares homology (gray boxes) with the t-SNARE Vam7p from S.cerevisiae and the hypothetical protein AL031523 from S.pombe (Sp1). All three proteins are predicted to contain an N-terminal PX domain (amino acids 10–146 in Yup1; dark bar) and a C-terminal coiled-coil region (amino acids 227–295 in Yup1; gray bar) that is characteristic for t-SNAREs. In the ts allele, Phe145 in the PX domain of Yup1 is substituted by Ala (black frame). (B) Dendrogram of members of the t-SNARE superfamily. The tree is based on the C-terminal coiled-coil region and was kindly provided by Dr K.Hofmann. RN, Rattus norvegicus; HS, Homo sapiens; SC, Saccharomyces cerevisiae; UM, Ustilago maydis. NT, N-terminal; CT, C-terminal.
Sequence comparison using BLAST (Altschul et al., 1997) revealed homology to a hypothetical protein from S.pombe (AL031523; p: 5e-10), syntaxin 8 from human (p: 5e-7), rat (p: 2e-6) and mouse (p: 2e-6), followed by another hypothetical protein from S.pombe (Z98533, p: 3e-5) and Vam7p (p: 1e-4), a t-SNARE involved in vacuolar organization and membrane traffic in S.cerevisiae (Wada and Anraku, 1992; Sato et al., 1998; Ungermann and Wickner, 1998). A more sensitive general profile alignment using the C-terminal coiled-coil region typical for t-SNAREs (Weimbs et al., 1997) revealed that Yup1 is a new member of the t-SNARE superfamily (p: 1e-4; Figure 1B) and belongs to a subgroup containing Vam7p (p: 1e-8; p-values for the general profile search were kindly provided by K.Hofmann). In addition, the SMART server identified a highly significant t-SNARE domain in Yup1 (p: 1.7e-7).
The ts protein contains a single amino acid substitution at position 145 (Phe to Ala; square in Figure 1A). This exchange near the C-terminal border of the putative PX domain of Yup1 appears to be responsible for the observed morphological phenotype.
yup1ts mutant cells show altered morphology and abnormal distribution of cell wall components
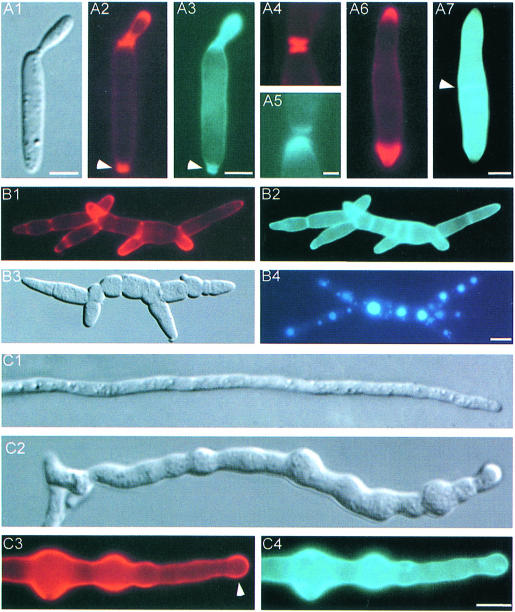
Haploid U.maydis cells grow by budding. The newly formed bud emerges at the pole of the elongated mother cell (Figure 2A1). To visualize zones of active growth, the cell wall was stained with rhodamine-labeled wheat germ agglutinin (WGA), a lectin that binds to oligomeric chitin (Nagata and Burger, 1974). In addition, calcofluor was used to stain newly synthesized fungal wall polymers (Mitchison and Nurse, 1985). In wild-type FB6a (a2b1) cells, both dyes stained similar regions of bud growth (Figure 2A2 and A3), the bud scar (arrowhead in Figure 2A2 and A3) as well as newly formed septa at the stage of cell separation (Figure 2A4 and A5). The dyes did not exactly co-localize, however (Figure 2A4 and A5), suggesting that their molecular targets are not identical.
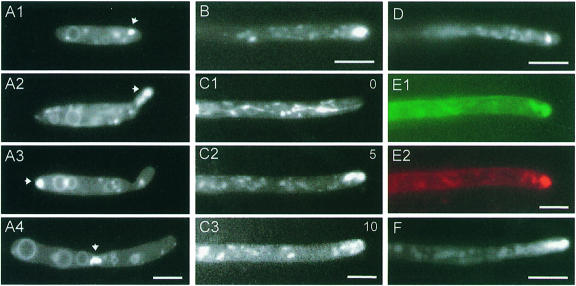
Fig. 2. Phenotype of RWS1 at 34°C. (A) RWS1 cells at permissive temperature (A1–A5) and after 2 h at 34°C (A6 and A7). Cell wall was stained with WGA (red) and calcofluor (blue). Both dyes localize to the growing bud (A2 and A3), the bud scar (arrowhead in A2 and A3), as well as newly formed septa (A4 and A5). Note that the dyes do not exactly co-localize (A4 and A5). After 2 h at 34°C, WGA staining is restricted to the growing tips (A6), whereas calcofluor is absent from the tips and concentrated in lateral walls and in abnormal septa (arrowhead in A7). Bar in A1–A3 and A6–A7: 3 µm; bar in A4–A5: 1 µm. (B) Phenotype of RWS1 12 h after temperature shift. Cells showed altered morphology (B3). The elongated multicellular structures contain multiple growth sites that are marked by WGA (B1). Calcofluor concentrates in old cell walls and septa (B2). Vacuole morphology is unaltered and each cell contains 1–2 large vacuoles visualized with the vital dye CellTracker™ blue (B4). Bar: 5 µm. (C) Phenotype of dikaryotic hyphae resulting from fusion of RWS1 and RWS2. At permissive temperature, mutant hyphae show normal morphology (C1). At 34°C, these hyphae remain shorter than the respective hyphae at permissive temperature and their morphology is altered (C2). WGA concentrates at the tip region (arrowhead in C3) and in the swollen parts of the hyphae (C3). Calcofluor is absent from the tips (C4) and co-localizes with WGA in the lateral walls. Bar: 5 µm.
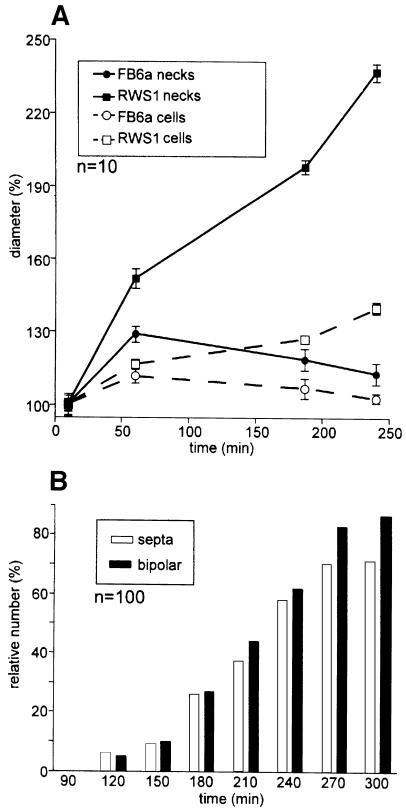
Upon shift to 34°C, FB6a cells continued normal budding and only minor changes in bud neck and cell diameter were seen (Figure 3A). This probably resulted from a transiently disturbed actin cytoskeleton after temperature shift (not shown). After 1–2 h at 34°C, wild-type cells recovered fully. In contrast, at 34°C strain RWS1 lost its typical budding pattern and dramatically changed morphology. Two hours after temperature shift, RWS1 cells had increased their bud neck and cell diameter (Figure 3A) and divided by septation (Figure 3B). These defects were accompanied by the disappearance of calcofluor staining from the tips (Figure 2A7), although WGA staining suggested that chitin was still concentrated at the cell poles (Figure 2A6). Instead calcofluor, but not WGA, reacted strongly with the lateral cell wall and septa (arrowhead in Figure 2A7). At 34°C, the chitin concentration at both cell poles became more prominent with time, suggesting that the cells grew in a bipolar fashion (Figure 3B). After 12 h, this resulted in long chains of cells, which were branched (Figure 2B3) and still bound WGA at their tips (Figure 2B1), but showed an apolar distribution of calcofluor (Figure 2B2). Each cell in these chains contained a single nucleus (not shown) and often only a single large vacuole (Figure 2B4).
Fig. 3. Morphology and growth of haploid RWS1 cells after temperature shift. (A) Quantification of morphological changes of the RWS1 mutant and corresponding FB6a wild-type cells at 34°C. After the temperature shift, FB6a cells enlarge in cell diameter (open circles) and bud necks (closed circles), but resume normal growth within 4 h. In contrast, RWS1 cells continuously expand their cell diameter (open squares) and lose their bud constrictions (closed squares). (B) Quantification of bipolar growth and septation after temperature shift. In RWS1, the first bipolar cells and septa appear after 2 h at 34°C.
To analyze the role of yup1 during hyphal growth, compatible haploid strains were co-spotted on agar plates containing charcoal. At room temperature as well as at 34°C, the tip cells of wild-type hyphae resulting from fusion of FB6a with FB6b (a1 b2) had a length of 120.7 ± 12.3 µm (n = 21) and a diameter of 1–2 µm. They contained two nuclei and showed tip growth, as suggested by a polar chitin distribution in growing tips (not shown). At permissive temperature, dikaryotic mutant hyphae resulting from fusion of RWS1 and RWS2 (a1 b2 yup1ts) showed no significant differences to wild-type hyphae (Figure 2C1). Six hours after mating, these hyphae were shifted to 34°C and analyzed after incubation for an additional 12–16 h. All mutant hyphae were significantly shorter than wild-type hyphae (78.0 ± 9.1 µm, n = 7) and showed drastic morphological aberrations (Figure 2C2). In contrast to wild-type hyphae (not shown), WGA and calcofluor staining was not restricted to the growing apex, but was found along the length of the hypha with strong accumulations at swollen areas (Figure 2C3 and C4). Comparable to haploid yup1ts cells, apical WGA staining was observed (arrowhead in Figure 2C3), whereas calcofluor was absent from the tip (Figure 2C4), again suggesting a defect in polar secretion of the wall component stained by calcofluor.
A Yup1–GFP fusion protein localizes to rapidly moving organelles
To gain further insight into the function of Yup1, C-terminal fusions of Yup1 and green fluorescent protein (GFP) (Chalfie et al., 1994) were generated. In plasmid pYup1SG1, the fusion protein is under the control of the yup1 promoter, while transcription in plasmid pYup1SG2 is driven by the strong otef promoter (Spellig et al., 1996). The ectopic integration of either plasmid in RWS1 could rescue the yup1ts phenotype, indicating that both Yup1–GFP fusion proteins were biologically active (not shown). Expression of yup1–GFP under either the endogenous yup1 promoter (strain RWS3; Figure 4A) or the otef promoter (strain RWS4; Figure 4B1–B4) led to identical subcellular localization (see below), but the latter fusion protein gave a stronger signal. Therefore, subsequent analysis of vesicle motility and cellular localization of Yup1–GFP was performed with RWS4. Western blot analysis showed that expression of Yup1–GFP in this strain was 7-fold higher than in RWS3. However, the overexpression of Yup1–GFP did not influence either doubling time or cell morphology (not shown).
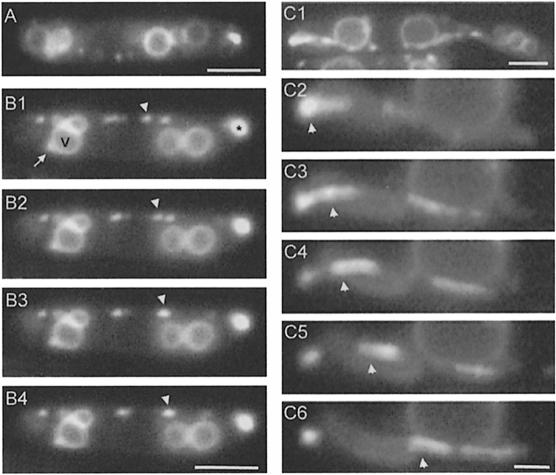
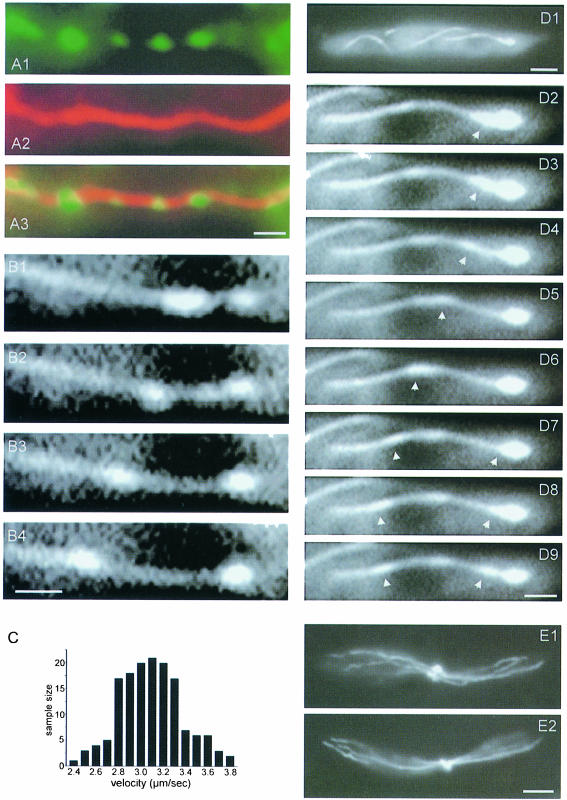
Fig. 4. Cellular localization of a Yup1–GFP fusion protein. (A) Epifluorescence microscopy of Yup1–GFP-carrying vesicles in a cell expressing the fusion protein under the control of the yup1 promoter (RWS3). Bar: 3 µm. (B) Epifluorescence microscopy of Yup1–GFP-carrying vesicles in a strain that overexpressed Yup1–GFP (RWS4). Photographs of a single cell were taken at 0.4 s intervals (B1–B4). Yup1–GFP localized predominantly to BSDs (asterisk in B1) and rapidly moving organelles (arrowhead in B1–B4). In addition, Yup1–GFP is found in the vacuolar membranes (V; B1) and vacuole-associated dots (arrow, B1). Note that RWS3 and RWS4 show identical subcellular localization of Yup1–GFP. Bar: 3 µm. (C) Yup1–GFP vesicle motion in RWS4. A BSD was located at one pole of the cell (C1) and disintegrated within several seconds into rapidly moving vesicles (arrowhead in C2–C6). Time interval between frames: ∼1 s; bar: 3 µm (C1) and 1 µm (C2–C6).
Yup1–GFP localized to four distinct structures (Figure 4B1): (i) rapidly moving vesicles of <0.5 µm in diameter (arrowhead); (ii) brightly stained dots (BSDs) of ∼1 µm diameter (0.94 ± 0.14 µm, n = 13; asterisk); (iii) one or two dots that were in close contact with vacuoles (arrow); and (iv) weakly stained vacuolar membranes (V).
Yup1–GFP vesicles moved along the periphery of the cell (Figure 4B1–B4). This motion was bidirectional, often saltatory and occurred along defined tracks. The vesicles were undergoing numerous fusion and fission processes and had dynamic contact with the BSDs, which appeared to be a sink as well as a source for Yup1–GFP vesicles as they entered and left these structures frequently. Occasionally, the BSDs disintegrated into numerous small vesicles (Figure 4C1–C6). To analyze this process in more detail, we compared the number of vesicles that entered small growing buds with that of vesicles leaving the growth region. We found significantly more vesicles entering small buds that contained BSDs than vesicles leaving (in: 53.9 ± 3.7%; out: 46.1 ± 3.7%, n = 429 vesicles in 10 cells, α = 0.01; p = 0.0002), suggesting an involvement of these vesicle accumulations in secretion.
The BSDs were almost stationary and showed only slow to-and-fro motion. There was a clear cell-cycle-dependent localization of BSDs (Figure 5A1–A4). In unbudded cells, polar BSDs were observed in 56.0 ± 8.3% (n = 3 experiments, >100 cells). Cells with small buds carried BSDs in their growing bud (81.2 ± 9.7%, n = 3 experiments with >100 cells; Figure 5A2, arrow). In cells with larger buds, BSDs appeared at the opposing cell pole (96.2 ± 0.1%, n = 3 experiments with >200 cells; Figure 5A3; see also Figure 4C1), which is the region where the next bud will arise (Jacobs et al., 1994). Finally, during cell division, the newly formed septum was flanked by two BSDs (80.7 ± 2.3%, n = 3 experiments, n >100 cells; Figure 5A4), suggesting that BSDs support polar secretion of wall components. To exclude the possibility that the appearance of BSDs at the cell poles was due to overexpression of Yup1–GFP, the quantification was repeated in three experiments, each time analyzing >100 cells of strain RWS3. Again >80% of all budded cells contained polar BSDs. A functional relationship between BSDs and the growth process is also inferred from the observation that similar structures were present in the apices of growing hyphae (Figure 5B–F). Comparable to haploid sporidia, these vesicle accumulations could be observed in hyphae from crosses of FB2 with RWS3 (Figure 5B) and of FB2 with RWS4 (Figure 5C–E).
Fig. 5. Localization of BSDs in haploid sporidia and dikaryotic hyphae. (A) Localization of Yup1–GFP vesicle accumulations during growth stages of U.maydis cells (RWS4). BSDs localize to poles of unbudded cells (arrow in A1) and to small buds (arrow in A2). In large budded cells, BSDs localize to the opposing cell pole (arrow in A3) and localize to both sides of the new septum prior to cell separation (arrow in A4). Bar: 3 µm. (B) A BSD in the apex of a hypha that expresses Yup1–GFP under the control of the yup1 promoter (RWS3 × FB2). Bar: 5 µm. (C) Dynamics of the Yup1–GFP cluster in the tip of dikaryotic hyphae obtained from a cross of RWS4 and FB2. Upon mechanical disturbance, Yup1–GFP vesicles dispersed within the hypha (C1). After 5 min, the apical vesicle cluster reappeared (C2) and remained there for an additional 5 min (C3). Tip expansion was only detected while Yup1–GFP vesicles were located in the apex. Time in minutes given in upper right corner. Bar: 3 µm. (D) A hypha obtained from a cross of RWS4 and FB2 was fixed with 1% formaldehyde. The apical BSD is still visible, suggesting its existence in the unfixed hypha. Bar: 5 µm. (E) Co-localization of apical Yup1–GFP vesicle clusters with the endocytic marker FM4-64. Dikaryotic hyphae were obtained from a cross of RWS4 and FB2, stained with FM4-64 for 2 min and chased for 7 min. The Yup1–GFP accumulation (green, E1) co-localizes with endosomal vesicles (red, E2). Bar: 3 µm. (F) Accumulation of Yup1–GFP in the tip of SG200Δkin2 hyphae. The kinesin-deficient hyphae contain apical Yup1–GFP clusters, but lack apical vesicles at the ultrastructural level (Lehmler et al., 1997). Bar: 5 µm.
Mechanical disturbance during the preparation of dikaryotic hyphae from agar plates led to disintegration of the BSD, giving rise to several Yup1–GFP-carrying vesicles and tubes that moved away from the apex (Figure 5C1). No expansion of the hyphal apex could be detected as long as the BSD was absent from the tip, although Yup1–GFP vesicles were rapidly moving within these hyphae. After 5 min, the apical vesicle cluster reappeared (Figure 5C2). Within the next 5 min, this cluster remained in the apex (Figure 5C3), and tip expansion was observed at rates around 0.4–0.8 µm/min. After >10 min of observation, the BSD disintegrated and the GFP-carrying vesicles were found scattered throughout the hyphae (not shown). The absence of vesicle clusters at the tip again correlated with loss of cell expansion. To confirm the existence of these apical vesicle clusters in normally growing hyphae, we fixed Yup1–GFP-containing hyphae with 1% formaldehyde prior to microscopic preparation. In several hyphae, vesicle accumulations could be detected (Figure 5D), suggesting that they were naturally occurring in the growing apex of U.maydis hyphae. Interestingly, BSDs could be stained with the endocytic dye FM4-64 (Figure 5E1 and E2). This was an indication that Yup1–GFP-carrying vesicles might be part of the endosomal compartment (see below).
Yup1–GFP vesicles move along microtubules
Yup1–GFP vesicles moved bidirectionally with an average velocity of 3.1 ± 0.3 µm/s (n = 150; Figure 6C). To investigate the underlying cytoskeletal elements, strain RWS4 was treated with various drugs that inhibit the microtubule (MT) and actin cytoskeleton, respectively. Benomyl (10 µM) was found to be an efficient inhibitor for the MT cytoskeleton in U.maydis wild-type cells (A.Brill and G.Steinberg, unpublished). Using immunofluorescence techniques, we confirmed the absence of MTs from RWS4 30 min after benomyl addition (not shown). This treatment did not alter the morphology of Yup1–GFP-carrying vesicles, but abolished their motion. Motility could be rescued within several minutes by washing out the drug with phosphate-buffered saline (PBS). In contrast, 10 µM cytochalasin D, an inhibitor of F-actin (Selden et al., 1980), as well as 10 mM 2,3-;butanedione monoxime, a drug that inhibits myosin-dependent transport in fungi (Steinberg and McIntosh, 1998), had no significant effect on Yup1–GFP vesicle motility (not shown), suggesting that Yup1–GFP vesicle motion is an MT-dependent process. In agreement with such a role of the tubulin cytoskeleton, Yup1–GFP vesicles (Figure 6A1) co-localized with MTs, which were stained by immunofluorescence (Figure 6A2; overlay in A3).
Fig. 6. Analysis of Yup1–GFP vesicle movement. (A) Co-localization of Yup1–GFP vesicles (A1) with MTs (A2) stained by immunofluorescence. The overlay is given in A3. Bar: 1 µm. (B) Movement of a Yup1–GFP vesicle along a single MT containing a GFP–α-tubulin fusion protein in strain RWS5 (B1–B4). Frames were taken at 0.2 s intervals. Bar: 1 µm. (C) Movement velocities in RWS4 cells. GFP-stained vesicles show rapid bidirectional motion at 3.1 ± 0.3 µm/s (n = 150). (D) Brightly stained dot at the end of GFP-microtubules in strain RWS5. Note the little vesicles that leave the BSD (arrowheads in D2–D9), which is located at the end of a MT close to one pole of the cell (overview in D1). Bar: 3 µm (D1) and 1 µm (D2–D9). (E) Microtubule distribution in FB6a (E1) and RWS1 cells (E2) 2 h after the shift to 34°C. Staining was peformed using antibodies against α-tubulin. Bar: 3 µm.
In a U.maydis strain that expressed both Yup1–GFP and GFP–tubulin (RWS5), and thereby allowed the in vivo observation of MT-dependent dynamics (G.Steinberg and A.Brill, unpublished), Yup1–GFP vesicles moved exclusively along MTs (Figure 6B1–B4) and were in contact with BSDs that accumulated at the ends of MTs (Figure 6D1–D9). The BSDs occasionally kept in contact with the end of MTs during shrinking (not shown). To exclude the possibility that alterations of the MT cytoskeleton were responsible for the observed defects in morphogenesis, we compared tubulin distribution in FB6a and RWS1 strains at permissive temperature as well as at 34°C. No difference in MT organization could be detected between wild-type and yup1ts cells either at 24°C (not shown) or at 34°C (Figure 6E1 and E2). Surprisingly, Yup1–GFP vesicle clusters were also found in kinesin-deficient hyphae that were described to lack a Spitzenkörper-like apical vesicle accumulation (Lehmler et al., 1997; Figure 5F), suggesting that conventional kinesin is not responsible for Yup1–GFP vesicle motion.
The PX domain is involved in localization of Yup1–GFP to moving endosomes
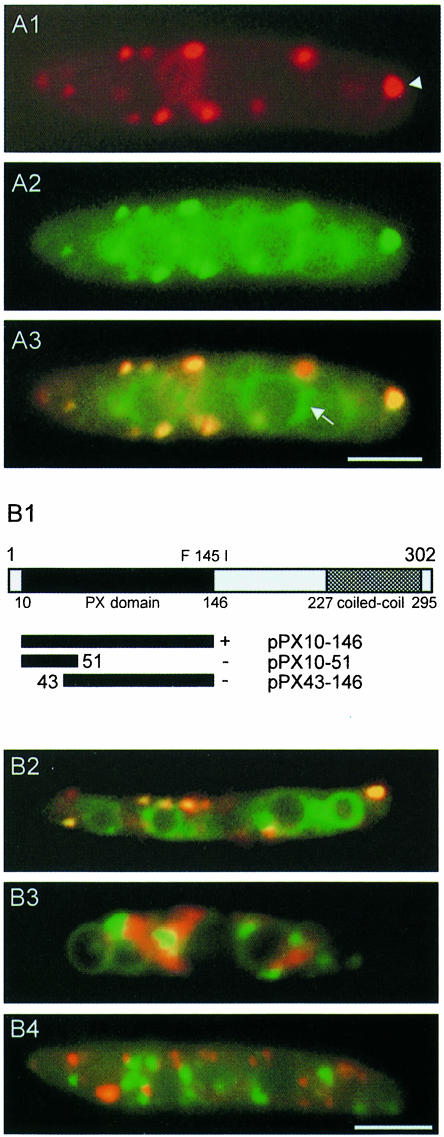
The endocytic pathway in fungi can be visualized using lipophilic dyes like FM4-64 (Vida and Emr, 1995). Staining the endosomal compartment of haploid U.maydis cells (RWS4) with FM4-64 in pulse–chase experiments as well as staining with another endocytic tracer, RH414 (not shown), revealed co-localization of motile endocytic vesicles at the periphery (Figure 7A1) with Yup1–GFP vesicles (Figure 7A2; overlay in A3). BSDs were also stained with FM4-64 (arrowhead in Figure 7A1). To assay the role of the PX domain in the localization of Yup1 to its target membranes, we fused the complete as well as truncated versions of the putative PX domain to GFP and tested localization of the respective fusion proteins in wild-type FB1 cells (Figure 7B1). The complete PX domain (amino acids 10–146; pPX10–146) was found to mediate localization identical to that of the full-length fusion protein. Truncations on both the N- and C-termini of the PX domain, however, led to complete loss of localization. Next we introduced the pPX10–146 construct into RWS1. At permissive temperature, the GFP fusion protein co-localized with FM4-64 in early endosomes and BSDs (overlay in Figure 7B2). However, within 10 min after the shift to 34°C (see Materials and methods), the PX10-146–GFP fusion protein no longer co-localized with FM4-64 and appeared in immobile aggregates within the cell (green in Figure 7B3). The endocytic dye FM4-64 was also mislocalized in cloudy aggregates, indicating a defect in endocytosis (red in Figure 7B3; see below). In addition, we expressed a Yup1ts–GFP fusion protein in wild-type cells. At permissive temperature, this fusion protein completely co-localized with FM4-64 (not shown), while at 34°C the Yup1ts–GFP fusion protein appeared in immobile aggregates (green in Figure 7B4) and did not co-localize with FM4-64 that normally entered the fast-moving early endosomes (red in Figure 7B4). These results argue for a critical role of the PX domain in the proper localization of Yup1 to endosomal membranes.
Fig. 7. Localization of Yup1–GFP on endosomal vesicles. (A) Co-localization of Yup1–GFP vesicles with endosomes in strain RWS4. The Yup1–GFP fusion protein (green) localizes to small peripheral vesicles (green, A1) that co-localize with the endocytic dye FM4-64 (red, A2). An overlay of both (A3) reveals additional staining of vacuoles by Yup1–GFP (arrow in A3). Bar: 3 µm. (B) Functional analysis of the N-terminal PX domain of Yup1. Truncations of yup1 were fused to GFP and the cellular localization of the respective fusion protein in wild-type cells was monitored. The complete PX domain (PX10-146–GFP) is sufficient for localization (indicated by +, B1). Deletions removing either the N- or C-terminal portions of the PX domain abolish localization (indicated by –, B1). In RWS1 at permissive temperature, PX10-146–GFP co-localizes with FM4-64-stained endosomes (overlay in yellow, B2), but is localized within immobile aggregates at 34°C (green, B3), where FM4-64 appears in diffuse structures throughout the cytoplasm (red, B3). A Yup1ts–GFP fusion protein (green, B4) expressed in wild-type cells also localizes to aggregates distinct from endosomes stained by FM4-64 (red dots, B4) at 34°C. Bar: 3 µm.
yup1ts mutants are defective in the endocytic pathway
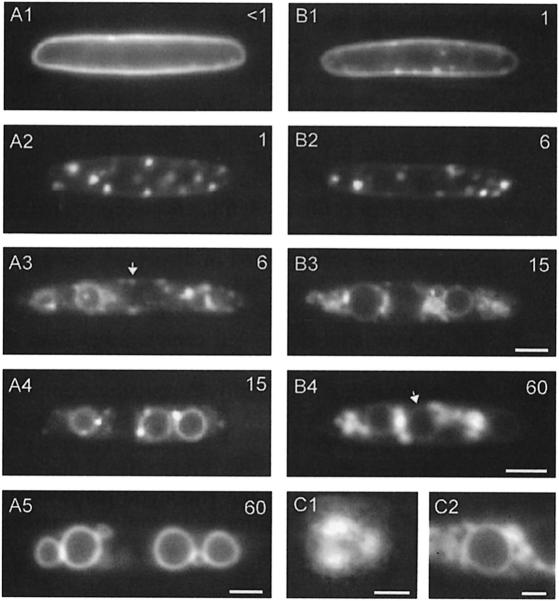
In typical pulse–chase experiments in wild-type cells (FB6a) using FM4-64 or RH414 (not shown), endocytosis began with the uptake of the dye into the plasma membrane within <1 min (Figure 8A1). This was followed by a rapid accumulation of FM4-64 in defined areas of the membrane, which were immobile and distributed regularly (Figure 8A2). These regions had a diameter of 0.76 ± 0.04 µm (n = 9) and were often trilobed (Figure 8C1). To exclude the possibility that these structures are regions of the cell wall, we confirmed their existence in protoplasts (not shown). After a 4–6 min chase period, the dye appeared in rapidly moving tubules and vesicles (Figure 8A3) corresponding to the endosomes that co-localized with Yup1–GFP vesicles (see Figure 7A). After 12–15 min, the dye appeared in vacuole-associated dots (Figure 8A4) and in the vacuolar membrane. After 30–60 min, the dye exclusively stained vacuolar membranes (Figure 8A5). To test whether uptake of endocytic tracers is ATP dependent, cells were poisoned with 5 mM NaN3 for 5–10 min. This treatment did not abolish uptake of the dye into the plasma membrane and into the trilobed regions, suggesting that the initial steps of endocytosis are ATP independent (not shown). However, neither FM4-64 nor RH414 appeared in the subsequent endosomes and vacuoles.
Fig. 8. Analysis of the endocytic pathway in haploid U.maydis cells. (A) The endocytic pathway in wild-type cells was traced in pulse–chase experiments using the vital marker FM4-64. Chase periods in minutes are given in the upper right corner. The dye entered the plasma membrane (A1), subsequently concentrated in stationary trilobed regions (A2) and appeared in rapidly moving early endosomes (arrow in A3). Finally, FM4-64 appeared in vacuole-associated dots (A4) and reached the vacuolar membrane (A5). Bar: 3 µm. (B) The endocytic pathway in RWS1 cells after 2 h at 34°C. The dye is taken up into the plasma membrane (B1) and the trilobed regions (B2), but neither reaches the early endosomes nor the subsequent compartments. Instead, vesicular accumulations appear within the cell (B3) and even after 60 min only small amounts of marker dye were found in the vacuolar membrane (arrow in B4). Bar: 3 µm. (C) Detailed view of a trilobed region in wild-type cells (C1) and of vesicular accumulations in RWS1 grown at 34°C after a 60 min chase period (C2). Bar: 0.5 µm in C1 and 1 µm in C2.
In RWS1, first defects of FM4-64 uptake into early endosomes were observed after 30 min at 34°C and the endocytic pathway was interrupted in all cells after 2 h. After a 1 min chase period, the plasma membrane still carried FM4-64 (Figure 8B1) and the marker appeared in the trilobed regions only after 5–6 min chase (Figure 8B2). More strikingly, the dye did not reach rapidly moving early endosomes, but accumulated in disperse aggregates arranged in patches (Figure 8B3). In contrast to early endosomes, these aggregates were stationary, often smaller and accumulated in the cell center rather than at the periphery. Although in most cells these aggregations appeared unstructured, occasionally a vesicular composition was observed (Figure 8C2). Even after a 1 h chase period, only small amounts of the dye had reached the vacuolar membrane (arrow, Figure 8B4). These findings are consistent with a defect in the endocytic pathway in yup1ts cells resulting in aggregation of incoming transport vesicles.
Discussion
In this study we have identified a gene, yup1, which is essential for morphogenesis in U.maydis. ts mutants showed drastic morphological changes and defects in cell separation. Yup1 is a putative t-SNARE and Yup1–GFP fusion protein localized to vesicles that moved along MTs. Surprisingly, these vesicles co-localized with the endocytic dye FM4-64, suggesting that they are early endosomes. Clusters of these vesicles accumulated at sites of active growth. In yupts cells, fusion of FM4-64-carrying transport vesicles with early endosomes was impaired at 34°C, suggestive of defects in endocytosis.
Yup1 is a putative t-SNARE
The yup1 gene encoding a predicted polypeptide of 302 amino acids complemented the temperature sensitivity as well as the morphological defects of the yup1ts mutant at 34°C. The predicted sequence of Yup1 contains a C-terminal coiled-coil region of ∼60 amino acids, which was shown to be characteristic for the recently defined t-SNARE superfamily (Weimbs et al., 1997). Within this region, Yup1 exhibits highest similarity to a subgroup of t-SNAREs including Vam7p from S.cerevisiae. Vam7p was identified in a screen for mutants defective in vacuolar organization (Wada and Anraku, 1992) and recent studies describe a role of Vam7p in homotypic fusion of vacuoles (Ungermann and Wickner, 1998) as well as fusion of transport vesicles originating from late endosomes with the vacuole (Sato et al., 1998). Interestingly, Yup1 and Vam7p are similar in length and both contain an N-terminal PX domain, which is supposed to be involved in protein–protein interaction and is required for Vam7p function (Sato et al., 1998). Based on this sequence similarity and domain structure, we predict Yup1 from U.maydis to be a putative t-SNARE that might function in membrane trafficking.
Membrane trafficking and organelle organization require rapid fusion and fission of membranous organelles. According to the SNARE hypothesis (Söllner et al., 1993), specific recognition protein complexes, consisting of vesicle (v) SNAREs on the transport vesicle and interacting counterparts on the target membrane (the t-SNAREs), play a crucial role in these processes. Therefore, we expected the putative t-SNARE Yup1 to be located on membranes. In agreement with this, we found Yup1–GFP on rapidly moving vesicles, and our data indicate that the PX domain of Yup1 mediates this subcellular localization. Remarkably, the amino acid substitution, which is responsible for the observed ts phenotype in RWS1, resides within the predicted PX domain of Yup1 and results in a mislocalization of Yup1 at 34°C. This suggests that the observed defects in polar growth and morphogenesis are a consequence of Yup1 mislocalization. The exact mechanism by which Yup1 is attached to endosomal membranes remains to be elucidated.
Yup1 is necessary for delivery of cell wall components and polar growth
The most striking effect of the ts allele in RWS1 cells at 34°C was an altered morphology. The shape of fungal cells depends on the cell wall, which counteracts osmotic pressure (Wessels, 1986). Polysaccharides like chitin have a central role in strengthening the fungal wall and apolar distribution of such components might impair their mechanical function. Using calcofluor, a probe with a broad target spectrum (Wood, 1980), which is commonly used to stain newly formed plant (Meadows, 1984) and fungal cell walls (Mitchison and Nurse, 1985), we could show that morphological changes in yup1ts cells are accompanied by alterations in cell wall composition. In particular, calcofluor staining disappeared from the poles of yup1ts cells, but was found in the lateral wall and septa upon temperature shift. These findings suggest a defect in polar delivery of either structural components or wall-modifying enzymes. It should be noted that polar growth in yup1ts mutants was not completely abolished and chitin (stained by WGA) still appeared at growing tips. This argues for a more complex tip growth process in which Yup1 plays an essential but non-exclusive role.
Surprisingly, after 2 h at 34°C, yup1ts mutant cells started to form septa, which in contrast to wild-type septa seen in dikaryotic hyphae (not shown) did not react with WGA, but could instead be stained with calcofluor. At present, we cannot provide a molecular explanation for these results. However, the generation of multicellular structures where each compartment contains a single nucleus suggests a primary defect in cell separation. A simple explanation for these findings could be that delivery of vesicles containing wall-degrading enzymes is disturbed in yup1ts cells at 34°C, resulting in incomplete cell separation. In mutant hyphae at permissive temperature, WGA and calcofluor stain components at the hyphal tip, suggesting that hyphae expand by apical exocytosis. In contrast, at 34°C in yup1ts strains, both WGA and calcofluor were found primarily in swellings along the hypha. This, again, indicates a severe defect in polar delivery of wall components.
A role for Yup1 in exocytosis is also obvious from localization data. Vesicles that carried a biologically active Yup1–GFP fusion protein accumulated at sites of active growth, namely the bud, regions of septum formation and the growing tip of hyphae. Moreover, tip growth could only be detected in hyphae that contained apical Yup1–GFP clusters, and growth was abolished in the absence of these vesicle accumulations, although rapid saltatory movement of Yup1–GFP vesicles could still be observed. Together with the observed morphological phenotype, these data strongly support a role of Yup1 in polar exocytosis and growth in haploid cells as well as in dikaryotic hyphae of U.maydis.
Yup1 functions in endocytosis
A role of the putative t-SNARE Yup1 in exocytosis would predict its localization in the plasma membrane where it should mediate fusion of Golgi-derived exocytic vesicles at sites of active growth. However, Yup1–GFP fusion protein localizes to endosomal vesicles, suggesting a role in endocytosis rather than exocytosis. To solve this puzzle, we analyzed the endocytic pathway of U.maydis using the styryl dyes FM4-64 and RH414. These markers are well established endocytic tracers that were used to monitor endocytosis in vertebrate cells (Betz and Bewick, 1992) as well as fungal cells (e.g. Vida and Emr, 1995; Hoffmann and Mendgen, 1998). After uptake through the plasma membrane, the tracer dyes appeared in two distinct endosomal compartments and ended up in the vacuole, which is the fungal equivalent of the lysosomal compartment of higher eukaryotes (Klionsky et al., 1990). Based on these studies, the endocytic pathway of U.maydis appears very similar to that of S.cerevisiae (Vida and Emr, 1995). Even the trilobed regions of the plasma membrane, which so far have not been reported in yeast, could be detected upon careful examination using FM4-64 or RH414 and 5 mM azide (not shown). Usually, yeast cells contained only a single trilobed region, which had the same size as trilobed regions in U.maydis (t-test, not significantly different, α = 0.05, p = 0.338). These regions appear to concentrate FM4-64 in an ATP-independent manner before internalization, indicating that diffusion processes might account for the concentration within these regions. Subsequent internalization of the dye, however, requires active transport. It remains to be seen which biological role these trilobed regions have in fungal endocytosis.
A role of Yup1 in endocytosis is strongly supported by the observed phenotype after a shift to 34°C. Although early steps of endocytosis took place, the dye did not reach the motile early endosomes and, instead, appeared in irregular accumulations in the cytoplasm. Consequently, transport to the vacuole was interrupted and FM4-64 remained trapped within these vesicular accumulations. Unfortunately, all attempts to visualize these accumulations in yup1ts cells by EM were unsuccessful, as were attempts to demonstrate clusters of early endosomes in growth regions by various fixation techniques (not shown).
In its presumed function as a t-SNARE, Yup1 should be specific for a defined membrane. Surprisingly, we found Yup1–GFP in the vacuolar membrane in addition to its localization on endosomes, irrespective of whether Yup1–GFP was expressed from its own promoter or from the stronger otef promoter. The sequence similarity of Yup1 with the vacuolar t-SNARE Vam7p from S.cerevisiae and recent reports that a single SNARE could function in different membranous compartments (Fischer von Mollard and Stevens, 1999) could argue for an additional function of Yup1 on vacuoles. However, in sharp contrast to vam7 mutants, yup1ts mutants showed no defect in vacuole organization. Therefore, we consider it more likely that the vacuolar localization of Yup1–GFP results from a GFP fusion protein that is on its way for degradation in the vacuole.
Endosomal movements along microtubules
In contrast to yeast where early endosomes are not motile (data not shown), Yup1–GFP-carrying early endosomes in U.maydis showed rapid saltatory motion along MTs. Several studies describe similar endosomal movements along MTs in vertebrate cells (reviewed in Lane and Allan, 1998), although in these systems velocities are 50–100 times lower than in U.maydis (Herman and Albertini, 1984). Endosomal traffic in vertebrates is likely to involve molecular motors like kinesin and dynein (e.g. Pol et al., 1997). It remains an attractive possibility that fungal kinesins, which show unusual fast in vitro transport velocities (e.g. Steinberg and Schliwa, 1996), are responsible for the rapid movement of early endosomes in U.maydis. However, Kin2, a conventional kinesin from U.maydis (Lehmler et al., 1997), is not responsible for the endosomal movements observed. Interestingly, in S.cerevisiae the MT cytoskeleton appears to have no function in membrane trafficking (Madden and Snyder, 1998) and motors like Kin2 are absent. Owing to their small size, yeast cells might not require long-distance transport, a process that is MT based in vertebrate cells (Lane and Allan, 1998). The elongated cell shape of U.maydis, particularly during its hyphal stage, and the existence of a prominent MT cytoskeleton, suggest a central role of MT-based, long-distance transport processes in this organism. Such an MT-based transport system could facilitate contact between early endosomes and endocytic transport vesicles, as suggested for vertebrates (Murphy et al., 1996). Moreover, MTs in U.maydis are required for polar organization of endosomal vesicle clusters at sites of active growth. Parallels exist to the fission yeast S.pombe, where MTs are thought to target the growth machinery to the poles of the cell (reviewed by Mata and Nurse, 1998). This raises the intriguing possibility of a more general role for MTs in polar growth of fungal cells. In this respect, it would be interesting to analyze the function of the S.pombe protein that shows similarity to Yup1.
The endosomal compartment and the fungal Spitzenkörper
Fungal tip growth is accompanied by an apical vesicle accumulation, the so-called Spitzenkörper. This structure is part of the apical hyphal growth region and participates in morphogenesis and polar growth of hyphae (reviewed in Bartnicki-Garcia, 1996). Only little is known about the nature of Spitzenkörper vesicles, but exoenzymes and wall components were found in these organelles, suggesting that the Spitzenkörper consists of secretory vesicles and is part of the exocytic system (Wessels, 1986).
Our data show that endosomal vesicle accumulations are prominent in growing tips of U.maydis hyphae. This is in agreement with studies on FM4-64 localization in U.fabae (Hoffmann and Mendgen, 1998), which demonstrate an endocytic vesicle cluster in growing hyphae of this fungus. The apical localization of these endocytic vesicles suggests that they might participate in the Spitzenkörper. Moreover, like the Spitzenkörper, the Yup1–GFP vesicle clusters are sensitive to mechanical disturbance and are required for cell expansion. In agreement with this, U.maydis hyphae contain an apical Spitzenkörper-like vesicle cluster (Lehmler et al., 1997), suggesting that these vesicles coincide with the Yup1–GFP vesicle accumulations. However, no vesicle accumulation was seen in EM preparations of U.maydis Δkin2 hyphae (Lehmler et al., 1997), although such mutant hyphae still contain the apical Yup1–GFP vesicle cluster. Therefore, we find it most likely that the Yup1–GFP accumulations were not preserved during EM preparations. Unfortunately, we were not able to detect early endosomes using several EM preparation techniques. This raises the possibility that the use of GFP allows in vivo observation of a class of vesicles that have not yet been visualized by EM studies.
Are endocytosis and exocytosis connected via membrane recycling?
Our data suggest that the putative t-SNARE Yup1 functions in endocytosis by mediating membrane recognition and fusion of incoming endocytic transport vesicles with early endosomes. On the other hand, loss of Yup1 function results in a defective polar delivery of wall components and thus in abnormal wall composition and altered morphology. These divergent functions could be reconciled if one assumes that, through its location on endosomes, Yup1 could couple exocytosis and endocytosis by assisting in membrane recycling processes.
Membrane recycling between endosomes and the plasma membrane is a prominent process in vertebrate cells, and is best understood in axons where it is involved in the dynamics of synaptic vesicles (reviewed in Pearse and Bretscher, 1981) and supports apical cell expansion (Dai and Sheetz, 1995). Moreover, endosome-dependent recycling processes appear to support the development of cell polarity in mouse embryos (Fleming et al., 1986) and might be important for the formation of certain membrane protrusions in vertebrate cells (Bretscher and Aguado-Velasco, 1998). In contrast, indications for fungal membrane recycling so far only come from U.fabae (Hoffmann and Mendgen, 1998) and S.cerevisiae. In the latter, chitin synthase appears to cycle between endosomes and the plasma membrane during growth (Chuang and Schekman, 1996).
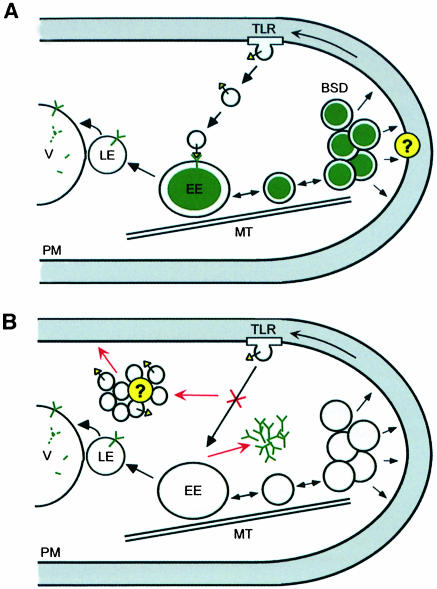
To accommodate our findings on Yup1, we suggest a model (Figure 9A) where Yup1 is located on early endosomes. Its primary function is to mediate fusion of incoming endocytic vesicles with early endosomes. Rapid long-distance transport along MTs might facilitate these fusion processes and could be involved in organizing endosomal clusters at sites of active growth. These clusters might support cell expansion by delivering cell-wall-modifying enzymes to the plasma membrane. The enzymes can then be recycled back to early endosomes via trilobed regions in the plasma membrane (TLR, Figure 9A). In RWS1 cells (Figure 9B), Yup1 aggregates in the cytoplasm because the mutation in the PX domain prevents proper localization on endosomes. As a result, the putative v-SNAREs on incoming transport vesicles cannot find their fusion partner and the vesicles accumulate within the cytoplasm. Consequently, recycling and polar delivery of wall components are abolished and this leads to altered cell wall structure and abnormal morphology. The role of endosomes in fungal growth uncovered here suggests parallels to vertebrate cell expansion. Moreover, the existence of apical endocytic vesicles in U.fabae hyphae (Hoffmann and Mendgen, 1998) indicates that membrane recycling plays a fundamental role in fungal morphogenesis in general.
Fig. 9. Model for Yup1 function. (A) Yup1–GFP (green Y) is located on early endosomes (EE), which are moving rapidly along MTs. These endosomes cluster in regions of growth, where they form the BSDs and might deliver components (marked by ?) that are needed for proper wall synthesis to the plasma membrane (PM). Such components could be wall-modifying enzymes that are recycled back to the early endosomes via the trilobed regions (TLR). Fusion of endocytic transport vesicles with the early endosomes requires the putative t-SNARE Yup1 and a corresponding v-SNARE on the transport vesicle (yellow triangle). In addition, part of Yup1–GFP travels to the vacuole (V) via late endosomes (LE) for degradation. (B) In RWS1 cells at 34°C the mutation in the PX domain of yup1 results in a mislocalization of the t-SNARE Yup1, which aggregates in the cytoplasm. As a result, incoming transport vesicles are unable to fuse with early endosomes and accumulate in the cell. Thus, transport of recycled wall components to growth regions via early endosomes is interrupted, resulting in altered cell wall composition and abnormal morphology.
Materials and methods
Strains and growth conditions
Ustilago maydis strains FB1 (a1 b1), FB2 (a2 b2), FB6a (a2 b1) and FB6b (a1 b2) have been described previously (Banuett and Herskowitz, 1989). A kinesin-deficient strain was generated by gene replacement of kin2 in SG200 (a1 mfa2 bW2 bE; S.Genin and R.Kahmann, unpublished) using previously described constructs (Lehmler et al., 1997). RWS1 (a2 b1 yup1ts) and RWS2 (a1 b2 yup1ts) are strains that contain the yup1ts allele. RWS3 contains the pYup1SG1 plasmid integrated ectopically into strain FB1. RWS4 contains the pYup1SG2 plasmid integrated ectopically into strain FB1. In RWS5, the pYup1SG2 plasmid is integrated ectopically into FB2Tub1EG#10. This latter strain contains an α-tubulin gene (tub1) fused to eGFP and inserted in the cbx locus of FB2 (A.Brill and G.Steinberg, unpublished). If not stated otherwise, strains were grown in 2.5% potato dextrose (PD) or 0.4% (w/v) bacto-peptone and 0.4% (w/v) sucrose (YEPS; modified from Tsukuda et al., 1988) at 28°C. Solid media contained 2% (w/v) bacto-agar. Mating assays and plant infections were carried out as described (Gillissen et al., 1992). For temperature shift experiments, liquid cultures were grown at room temperature (21–24°C, permissive temperature) overnight, diluted and shifted to 34°C. To generate dikaryotic hyphae, compatible strains were co-spotted on charcoal-containing agar plates and incubated at room temperature. To visualize yup1ts phenotypes, plates were shifted to 34°C 6 h after co-spotting and hyphae were grown for an additional 12–16 h at 34°C.
Cloning procedures
For cloning purposes, the Escherichia coli K12 strain DH5α (Bethesda Research Laboratories) was used and molecular methods followed described protocols (Sambrook et al., 1989). DNA isolation from U.maydis and transformation procedures were carried out as described (Schulz et al., 1990). Sequencing and cloning was performed in plasmids pUC19, pTZ18R, pTZ19R, pSL1180 (Pharmacia) and pBluescript II KS+ (Stratagene).
Sequence analysis
Analysis of the predicted amino acid sequence was carried out using BLAST (Altschul et al., 1997; http://www.ncbi.nlm.nih.gov/blast/blast.cgi?Jform=0), PFAM (Bateman et al., 1999; http://www.sanger.ac.uk/Software/Pfam/index.shtml), SMART (Schulz et al., 1998; http://smart.embl-heidelberg.de), PROSITE profiles (Swiss Institute of Bioinformatics, Geneva, Switzerland; http://www.isrec.isb-sib.ch/software/PFSCAN_form.html) and COILS (Lupas et al., 1991; http://www.ch.embnet.org/software/COILS_form.html).
Generation of mutants and isolation of the yup1 gene
To generate ts mutants of U.maydis, the wild-type strain FB1 was treated with UV at 254 nm to 1% survival rate. After mutagenesis, cells were grown for 48 h at room temperature, followed by replica plating and incubation at room temperature and 34°C, respectively. Colonies that grew at permissive temperature but not at 34°C were collected and retested for temperature sensitivity. About 0.1% of the survivors were temperature sensitive. One ts mutant that showed an abnormal morphology at 34°C was chosen for further studies. Segregation analysis was performed after crossing the mutant strain with FB2. In two segregants, RWS1 and RWS2, the morphological phenotype co-segregated with temperature sensitivity. For complementation studies, RWS1 was transformed with a pCM54-derived genomic library (Tsukuda et al., 1988), in which U.maydis DNA fragments of 6–11 kb are cloned in a self-replicating plasmid. Two overlapping plasmids, pCM54-yup1 (5.6 kb) and pCM54-yup2 (9.6 kb), complemented the growth defect as well as the morphological defects of RWS1. By subcloning, an EcoRI–XbaI fragment of 1.6 kb was shown to rescue the ts phenotype of RWS1.
GFP fusion constructs
pYup1SG2. The C-terminal Yup1–sGFP fusion under the control of the otef promoter was generated in three steps. The open reading frame of yup1 was amplified with two primers, RS1 (GTACCCATGGCACAA ACACAGCCA) and RS2 (GTACTCATGAATCCTGCTCCTGCGC CGGCAAACTTTCTTCCTATCCC), generating a NcoI site at the start codon and adding a linker (AGAGAGF) with a BspHI site at the 3′-end of yup1. This fragment was cloned into pTZ19R, sequenced, and the yup1 gene was excised as a 900 bp NcoI–BspHI fragment and cloned into the NcoI site of pOTEF-SG (Spellig et al., 1996).
pYup1SG1. The Yup1–GFP fusion construct was placed under the control of the native yup1 promoter, by excision of a 1.4 kb HindIII–SphI fragment containing the otef promoter from pYup1SG2 and replacing it with a 1.1 kb HindIII–SphI fragment from pCM54-yup2 carrying the yup1 promoter.
pPX10–146, pPX10–51, pPX43–146. Subfragments of yup1 were amplified by PCR, creating NcoI sites on both ends, sequenced and inserted into the NcoI site of p123, which contains eGFP under the control of the otef promoter and a carboxin resistance gene (C.Aichinger and R.Kahmann, unpublished), creating C-terminal fusions with eGFP (Clontech).
Light microscopy and image processing
Microscopic analysis was performed using a Zeiss Axiophot microscope. Frames were taken with a cooled CCD camera (Hamamatsu, C4742-95) or a SIT camera (Hamamatsu, C2400-08), respectively. Epifluorescence was observed using standard fluorescein isothiocyanate, 4′,6-diamidino-2-phenylindole (DAPI) and rhodamine filter sets. For co-localization studies, eGFP fluorescence was observed by a specific filter set (BP 470/20, FT 493, BP 505–530). Quantifications and processing of images were performed with Image-Pro Plus (Media Cybernetics) and Photoshop (Adobe). Statistical analysis was carried out using PRISM (GraphPad).
Staining procedures and inhibitor studies
For cytological studies on yeast-like cells, logarithmically growing liquid cultures were used. Hyphae were taken from plates between 12 and 20 h after co-spotting, and observed in water. For nuclear staining, cells were fixed with 1% formaldehyde for 15 min followed by incubation with 1 µg/ml DAPI (Sigma) in PBS (pH 7.2) for 15 min at 65°C and subsequent washing with PBS. To stain the endocytic pathway, cells were pulsed for 2 min with 16 µM FM4-64 (Molecular Probes; Vida and Emr, 1995) or 16 µM RH414 (Molecular Probes; Betz and Bewick, 1992) and chased for 1–60 min with water or PBS essentially as described previously (Steinberg et al., 1998). To monitor endocytosis in the ts mutant, cells were shifted to 34°C for 2 h and subsequently stained with FM4-64 in pulse–chase experiments as described above. Cells were kept below 34°C during the staining procedure and analyzed immediately to minimize alterations from the ts phenotype. For co-localization studies with GFP, cells were briefly fixed with 1% formaldehyde and washed once in 1× PBS before microscopic analysis. For in vivo labeling of vacuoles, CellTracker™ blue (Molecular Probes) was used as described previously (Steinberg et al., 1998). Cell wall components were stained with 25 µg/ml tetramethyl rhodamine isothiocyanate-labeled WGA (Sigma) or 2 µg/ml Calcofluor-white (Sigma) in PBS, respectively. Indirect immunofluorescence of MTs was carried out using monoclonal antibodies against α-tubulin (N356; Amersham) according to Steinberg et al. (1998). For visualizing GFP after fixation, cells were treated with 1% formaldehyde (EM grade, Polyscience) for 30 min at room temperature.
Accession numbers
DDBJ/EMBL/GenBank accession Nos are: BankIt326921, AF247648.
Acknowledgments
Acknowledgements
We are grateful to Dr K.Hofmann for providing the dendrogram of t-SNAREs and for his invaluable help with sequence analysis. We thank M.Artmeier for technical assistance, and express our gratitude to Dr K.Mendgen and Dr T.Jezirowski for numerous attempts to visualize endosomes by EM. Our work was supported by a grant of the Deutsche Forschungsgemeinschaft through SFB 413.
References
- Altschul S.F., Madden,T.L., Schäffer,A.A., Zhang,J., Zhang,Z., Miller,W. and Lipman,D.J. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res., 25, 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuett F. (1995) Genetics of Ustilago maydis, a fungal pathogen that induces tumors in maize. Annu. Rev. Genet., 29, 179–208. [DOI] [PubMed] [Google Scholar]
- Banuett F. and Herskowitz,I. (1989) Different a alleles of Ustilago maydis are necessary for maintenance of filamentous growth but not for meiosis. Proc. Natl Acad. Sci. USA, 86, 5878–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. (1996) The hypha: unifying thread of the fungal kingdom. In Sutton,B.C. (ed.), A Century of Mycology. Cambridge University Press, Cambridge, UK, pp. 105–133. [Google Scholar]
- Bateman A., Birney,E., Durbin,R., Eddy,S.R., Finn,R.D. and Sonnhammer,E.L.L. (1999) Pfam3.1: 1313 multiple alignments and profile HMMs match the majority of proteins. Nucleic Acids Res., 27, 260–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz W.J. and Bewick,G.S. (1992) Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science, 255, 200–203. [DOI] [PubMed] [Google Scholar]
- Bray D. (1992) Cell Movements. Garland Publishing, New York, NY. [Google Scholar]
- Bretscher M.S. and Aguado-Velasco,C. (1998) EGF induces recycling membrane to form ruffles. Curr. Biol., 8, 721–724. [DOI] [PubMed] [Google Scholar]
- Chalfie M., Tu,Y., Euskirchen,G., Ward,W.W. and Prasher,D.C. (1994) Green fluorescent protein as a marker for gene expression. Science, 263, 802–805. [DOI] [PubMed] [Google Scholar]
- Chuang J.S. and Schekman,R.W. (1996) Differential trafficking and timed localization of two chitin synthase proteins, Chs2p and Chs3p. J. Cell Biol., 135, 597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collinge A.J. and Trinci,A.P.J. (1974) Hyphal tips of wild type and spreading colonial mutants of Neurospora crassa.Arch. Microbiol., 99, 353–368. [DOI] [PubMed] [Google Scholar]
- Dai J. and Sheetz,M.P. (1995) Axon membrane flows from the growth cone to the cell body. Cell, 83, 693–701. [DOI] [PubMed] [Google Scholar]
- Fischer von Mollard G. and Stevens,T.H. (1999) The Saccharomyces cerevisiae v-SNARE Vti1p is required for multiple membrane transport pathways to the vacuole. Mol. Biol. Cell, 10, 1719–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming T.P., Cannon,P.M. and Pickering,S.J. (1986) The cytoskeleton, endocytosis and cell polarity in the mouse preimplantation embryo. Dev. Biol., 113, 406–419. [DOI] [PubMed] [Google Scholar]
- Gillissen B., Bergemann,J., Sandmann,C., Schroeer,B., Bolker,M. and Kahmann,R. (1992) A two-component regulatory system for self/non-self recognition in Ustilago maydis.Cell, 68, 647–657. [DOI] [PubMed] [Google Scholar]
- Gow N.A.R. (1995) Tip growth and polarity. In Gow,N.A.R. and Gadd,G.M. (eds), The Growing Fungus. Chapman & Hall, London, pp. 277–299. [Google Scholar]
- Grove S.N. and Bracker,C.E. (1970) Protoplasmic organization of hyphal tips among fungi: vesicles and Spitzenkörper. J. Bacteriol., 104, 989–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath I.B. (1995) The cytoskeleton. In Gow,N.A.R. and Gadd,G.M. (eds), The Growing Fungus. Chapman & Hall, London, UK, pp. 99–134. [Google Scholar]
- Herman B. and Albertini,D.F. (1984) A time-lapse video image intensification analysis of cytoplasmic organelle movements during endosome translocation. J. Cell Biol., 98, 565–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. and Mendgen,K. (1998) Endocytosis and membrane turnover in the germ tube of Uromyces fabae.Fungal Genet. Biol., 24, 77–85. [DOI] [PubMed] [Google Scholar]
- Jacobs C.W., Mattichak,S.J. and Knowles,J.F. (1994) Budding patterns during the cell cycle of the maize smut pathogen Ustilago maydis.Can. J. Bot., 72, 1675–1680. [Google Scholar]
- Klionsky D.J., Herman,P.K. and Emr,S.D. (1990) The fungal vacuole: composition, function and biogenesis. Microbiol. Rev., 54, 266–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J. and Allan,V. (1998) Microtubule-based membrane movements. Biochim. Biophys. Acta, 1376, 27–55. [DOI] [PubMed] [Google Scholar]
- Lehmler C., Steinberg,G., Snetselaar,K.M., Schliwa,M., Kahmann,R. and Boelker,M. (1997) Identification of a motor protein required for filamentous growth in Ustilago maydis.EMBO J., 16, 3464–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke,M. and Stock,J. (1991) Predicting coiled coils from protein sequences. Science, 252, 1162–1164. [DOI] [PubMed] [Google Scholar]
- Madden K. and Snyder,M. (1998) Cell polarity and morphogenesis in budding yeast. Annu. Rev. Microbiol., 52, 687–744. [DOI] [PubMed] [Google Scholar]
- Mata J. and Nurse,P. (1998) Discovering the poles in yeast. Trends Cell Biol., 8, 163–167. [DOI] [PubMed] [Google Scholar]
- Meadows M.G. (1984) A batch assay using calcofluor fluorescence to characterize cell wall regeneration in plant protoplasts. Anal. Biochem., 141, 38–42. [DOI] [PubMed] [Google Scholar]
- Mitchison J.M. and Nurse,P. (1985) Growth in cell length in the fission yeast Schizosaccharomyces pombe.J. Cell Sci., 75, 357–376. [DOI] [PubMed] [Google Scholar]
- Murphy C. et al. (1996) Endosome dynamics regulated by a Rho protein. Nature, 384, 427–432. [DOI] [PubMed] [Google Scholar]
- Nagata Y. and Burger,M.M. (1974) Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J. Biol. Chem., 249, 3116–3122. [PubMed] [Google Scholar]
- Pearse B.M. and Bretscher,M.S. (1981) Membrane recycling by coated vesicles. Annu. Rev. Biochem., 50, 85–101. [DOI] [PubMed] [Google Scholar]
- Picton J.M. and Steer,M.W. (1983) Membrane recycling and the control of secretory activity in pollen tubes. J. Cell Sci., 63, 303–310. [DOI] [PubMed] [Google Scholar]
- Pol A., Ortega,D. and Enrich,C. (1997) Identification of cytoskeleton-associated proteins in isolated rat liver endosomes. Biochem. J., 327, 741–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponting C.P. (1996) Novel domains in NADPH oxidase subunits, sorting nexins and PtdIns 3-kinases: binding partners of SH3 domains? Protein Sci., 5, 2353–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. [Google Scholar]
- Sato T.K., Darsow,T. and Emr,S.D. (1998) Vam7p, a SNAP-25-like molecule and Vam3p, a syntaxin homolog, function together in yeast vacuolar protein trafficking. Mol. Cell. Biol., 18, 5308–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B., Banuett,F., Dahl,M., Schlesinger,R., Schaefer,W., Martin,T., Herskowitz,I. and Kahmann,R. (1990) The b alleles of Ustilago maydis whose combinations program pathogenic development code for polypeptides containing a homeodomain-related motif. Cell, 60, 295–306. [DOI] [PubMed] [Google Scholar]
- Schulz J., Milpetz,F., Bork,P. and Ponting,C.P. (1998) SMART, a simple modular architecture research tool: Identification of signalling domains. Proc. Natl Acad. Sci. USA, 95, 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selden L.A., Gershman,L.C. and Estes,J.E. (1980) A proposed mechanism of action of cytochalasin D on muscle actin. Biochem. Biophys Res. Commun., 95, 1854–1860. [DOI] [PubMed] [Google Scholar]
- Söllner T., Whiteheart,S.W., Brunner,M., Erdjument-Bromage,H., Geromanos,S., Tempst,P. and Rothman,J.E. (1993) SNAP receptors implicated in vesicle targeting and fusion. Nature, 362, 318–324. [DOI] [PubMed] [Google Scholar]
- Spellig T., Bottin,A. and Kahmann,R. (1996) Green fluorescent protein (GFP) as a new vital marker in the phytopathogenic fungus Ustilago maydis.Mol. Gen. Genet., 252, 503–509. [DOI] [PubMed] [Google Scholar]
- Steinberg G. (2000) The cellular role of molecular motors in fungi. Trends Microbiol., 4, 162–168. [DOI] [PubMed] [Google Scholar]
- Steinberg G. and McIntosh,J.R. (1998) Effects of the myosin inhibitor 2,3-butanedione on the physiology of fission yeast. Eur. J. Cell Biol., 77, 284–293. [DOI] [PubMed] [Google Scholar]
- Steinberg G. and Schliwa,M. (1996) Characterization of the biophysical and motility properties of kinesin from the fungus Neurospora crassa.J. Biol. Chem., 271, 7516–7521. [DOI] [PubMed] [Google Scholar]
- Steinberg G., Schliwa,M., Lehmler,C., Bölker,M., Kahmann,R. and McIntosh,J.R. (1998) Kinesin from the plant pathogen Ustilago maydis is involved in vacuole formation and cytoplasmic migration. J. Cell Sci., 111, 2235–2246. [DOI] [PubMed] [Google Scholar]
- Tsukuda T., Carleton,S., Fotheringham,S. and Holloman,W.K. (1988) Isolation and characterization of an autonomously replicating sequence from Ustilago maydis.Mol. Cell. Biol., 8, 3703–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C. and Wickner,W. (1998) Vam7p, a vacuolar SNAP-25 homolog, is required for SNARE complex integrity and vacuole docking and fusion. EMBO J., 17, 3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida T. and Emr,S.D. (1995) A new vital stain for visualizing vacuolar membrane dynamics and endocytosis in yeast. J. Cell Biol., 128, 779–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada Y. and Anraku,Y. (1992) Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. II. Vam7, a gene for regulating morphogenic assembly of vacuoles. J. Biol. Chem., 267, 18671–18674. [PubMed] [Google Scholar]
- Weimbs T., Low,S.H., Chapin,S.J., Mostov,K.E., Bucher,P. and Hofmann,K. (1997) A conserved domain is present in different families of vesicular fusion proteins: a new superfamily. Proc. Natl Acad. Sci. USA, 94, 3046–3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels J.G.H. (1986) Cell wall synthesis in apical hyphal growth. Int. Rev. Cytol., 104, 387–413. [Google Scholar]
- Wood P.J. (1980) Specificity in the interaction of direct dyes with polysaccharides. Carbohydr. Res., 85, 271–288. [Google Scholar]