Abstract
The lateral habenula (LHb) plays a role in prediction of negative reinforcement, punishment and aversive responses. In the current study, we examined the role that the LHb plays in the regulation of negative reward responses and aversion. First, we tested the effect of intervention in LHb activity on sucrose reinforcing behavior. An electrode was implanted into the LHb and rats were trained to self-administer sucrose (20%; 16 days) until at least three days of stable performance were achieved (as represented by the number of active lever presses in self-administration cages). Rats subsequently received deep brain stimulation (DBS) of the LHb, which significantly reduced sucrose self-administration levels. In contrast, lesion of the LHb increased sucrose-seeking behavior, as demonstrated by a delayed extinction response to substitution of sucrose with water. Furthermore, in a modified non-rewarding conditioned-place-preference paradigm, DBS of the LHb led to aversion to the context associated with stimulation of this brain region.
We postulate that electrical stimulation of the LHb attenuates positive reward-associated reinforcement by natural substances.
Keywords: deep brain stimulation, depression, sucrose self-administration, lateral habenula, reward
Introduction
The lateral habenula (LHb) innervates multiple brain regions, including sites that contain high concentrations of dopaminergic neurons such as the ventral tegmental area (VTA) (Herkenham and Nauta, 1979; Ji and Shepard, 2007). A previous study (Lisoprawski et al., 1980) showed that lesions of the LHb led to increases in dopamine utilization in the prefrontal cortex. In the same direction, further studies revealed that electrical stimulation of the LHb inhibits firing of up to 97% of the dopaminergic neurons in the substantia nigra compacta and VTA (Christoph et al., 1986; Ji and Shepard, 2007). These findings led Hikosaka and colleagues to suggest the potential ability of the LHb to influence dopaminergic functions such as incentive motivation (Hikosaka et al., 2008).
In a recent study carried out by (Matsumoto and Hikosaka, 2007), recordings of LHb and dopaminergic neurons were taken in the brain of a rhesus monkey during performance of a visual task with positionally biased reward outcomes. While LHb neurons were excited in response to no-reward-predicting stimuli, they were inhibited by reward-predicting stimuli. In contrast, mesolimbic dopamine neurons exhibited a reverse response. This, taken together with the finding that stimulation of the LHb results in inhibition of dopamine neurons, led the researchers to propose that the LHb is involved in negative reward processes, i.e. processes which encode situations where no reward is available, as opposed to dopaminergic neurons that engage in positive reward processes (Hikosaka).
These findings imply the LHb as a potential location for intervention in reward processes. We recently showed that electrical stimulation of the LHb attenuates cocaine seeking behavior during both self-administration and extinction training of rats. In contrast, lesion of the LHb increases cocaine-seeking behavior in rats, as demonstrated by a delayed extinction response (Friedman et al., 2010). Drug-seeking behavior involves processes of reward, as well as other processes such as compulsive behavior and memory (Everitt et al., 2001; Kauer and Malenka, 2007). In order to shed light on the effect of DBS on reward in general, the current study aimed to test how alterations in the normal functioning of the LHb affect natural reward processes. We hypothesized that stimulation of the LHb would attenuate sucrose intake in the sucrose self-administration paradigm, whereas lesions of the LHb would result in delayed extinction of sucrose-seeking behavior after substitution of sucrose with water. Furthermore, we evaluated the effect of LHb stimulation on the naïve brain via measurement of the effect of LHB stimulation in a non-rewarding environment, using a modified conditioned place preference paradigm. Taken together, our findings indicate that LHb DBS induces reward devaluation.
Materials and Methods
Animals
Male (250-300 g) Sprague Dawley rats were maintained under conditions of unvarying temperature (25°C) and humidity (50%), in a 12:12h light/dark cycle, with free access to food and water. All animal procedures were approved by the Bar-Ilan University Animal Care Committee and were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals
Sucrose self-administration
Rats were transferred into operant conditioning chambers (Med-Associates, Inc.; St Albans Vermont) for daily 30 min sessions, during their dark cycle. Each self-administration chamber (30×25×22 cm) had two levers, active and inactive, located 5 cm above the floor of the chamber. An active lever press delivered sucrose (20% sucrose solution; 0.13 ml/infusion; duration of 5 sec) through an infusion pump and into a liquid drop receptacle for oral consumption. A light located above the active lever was lit during the 5 second-long sucrose infusion periods, and remained lit for another 15 sec. Throughout these 15-sec intervals, active lever presses were recorded, but no additional sucrose reinforcement was provided. Stable maintenance levels were defined as at least 3 days of 35-42 active lever presses. Presses on the inactive lever were recorded, but they did not activate the infusion pump and light. Rats were returned to their home cages at the end of the daily session.
Guide-cannula implantation for LHb lesion
Prior to stereotaxic surgery, rats were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.). A guide-cannula (Plastic One, 30 gauge) was implanted into the LHb (anterior -3.8, lateral -0.9, ventral -3.8 mm from Bregma) (Paxinos and Watson, 1998) and sealed by a cannula-dummy (Plastic one). The implantation was secured to the skull with screws and dental acrylic cement. Rimadyl (Carprofen) (2 mg/kg, s.c.) was injected post-surgery to relieve pain. All lesions were performed unilaterally, in the right hemisphere.
Lesion procedure
After reaching post-operational maintenance of sucrose, a cannula (Plastic One) with a 1 mm projection was inserted into the guide-cannula. Quinolinic acid (Sigma Chemicals) was dissolved in 1 N NaOH and diluted with phosphate-buffered saline (PBS) to a final pH of 7.4 and a concentration of 120 nmol/μl. This solution was infused through the cannula using an electronic-driven pump (CMA 400, CMA/ Microdialysis) for 6 min, to a total amount of 1.2μl. Following infusion of quinolinic acid or vehicle, the guide-cannula was sealed by a cannula-dummy (Plastic One) in order to reduce upward diffusion of the solution.
DBS electrode construction and implantation
Animals were anesthetized with ketamine hydrochloride (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) prior to stereotaxic surgery, A bipolar self-designed stimulating electrode (2 stainless steel electrodes, 0.01 mm diameter, 1 mm between cathode and anode, non-isolated electrode tip 1 mm) was inserted into the brain in such a way that the LHb was situated between the cathode and anode electrodes (cathode/anode anterior -3.8/-3.8, lateral -1.4/-2.4, ventral 4.74/4.74 mm from Bregma, with 14° angle). We tested site-specificity by stimulation of the medial rostral part of the lateral posterior thalamic nucleus (LPMR), which is within close proximity to the LHb (cathode/anode anterior -3.8/-3.8, lateral -2.4/-3.4, ventral 4.74/4.74 mm from Bregma, with a 14° angle). The electrode was secured to the skull with screws and dental acrylic cement. Rimadyl (2 mg/kg, i.p.) was injected post-surgery. All stimulations were performed unilaterally, in the right hemisphere.
Electrical Deep Brain Stimulation procedure
Animals received DBS for 15 min, after being transferred to the self-administration chambers. The parameters for the DBS were 200 μ-amperes, with a spike-duration of 0.5 msec. In a preliminary study we continually adjusted the stimulation pattern, in order to achieve a reduction in the number of active lever presses within the sucrose self-administration task. We found that low frequency stimulation (10 Hz) for 15 min elevated the number of presses (120% over baseline), while high frequency stimulation (100 Hz) for 15 min had no effect at all. We then found that a specific combination of alternating low (10 Hz) and high (100 Hz) frequency stimulations achieved an optimal reductive effect on lever presses. Therefore we decided to employ a combined stimulation pattern which consisted of four different sets of patterns: 1) low frequency stimulation of 10 Hz, 2) high frequency stimulation of 100 Hz, 3) low-frequency-burst stimulation (180 msec intervals between bursts; within each burst there were 5 spikes with 80 msec intervals between spikes. Total stimulation was 10 Hz), and 4) high-frequency-burst stimulation (300 msec intervals between bursts; within each burst there were 30 spikes with 3 msec intervals between spikes. Total stimulation was 60 Hz). Spike duration was 1msec. Alternations between high and low frequency patterns were performed constantly. The duration of high frequency stimulation (pattern 2 and 4) was 4 sec per stimulation, with 20 second-long pauses between stimulations (in order to avoid tissue damage). The duration of low frequency stimulation (patterns 1 and 3) was 60 sec (Fig. 1 C-E)
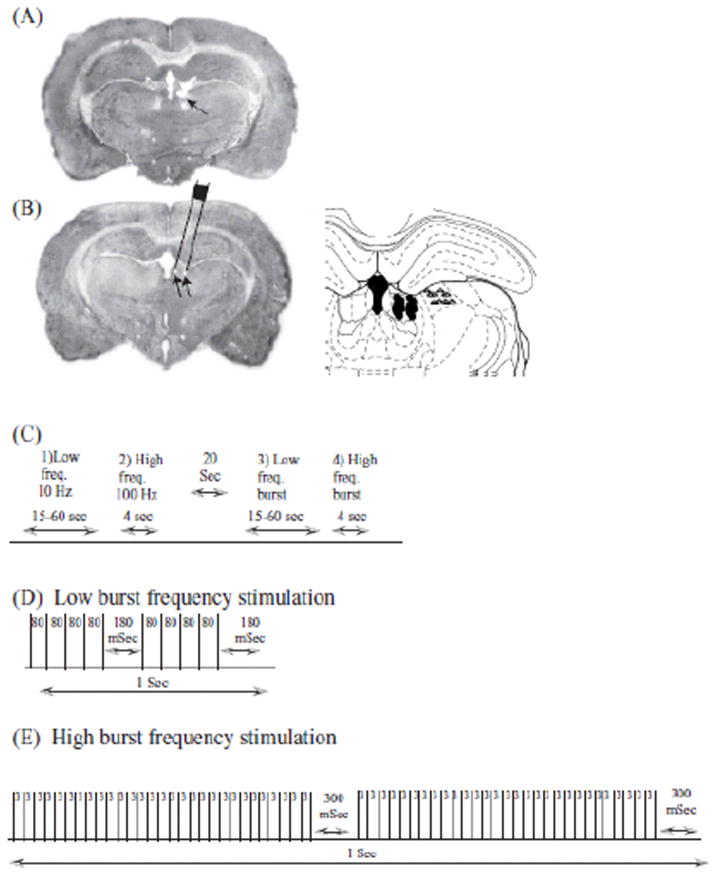
Fig. 1. Histology and stimulation parameters.
Panel A: A representative brain section stained with Cresyl violet indicating of the LHb lesion site (arrow) Panel B: Left hand- A representative brain section stained with Cresyl violet indicating the electrode implantation site (arrow indicates electrode tip). Illustration of the electrode was superimposed to demonstrate its position. Magnification ×5 (insert ×10) in both panels. Right hand- Illustration of the electrodes-tips placements within the LHb (circles) and LPMR (triangles) is depicted. Panel C: Stimulation scheme. Panel D: Low frequency burst stimulation. Panel E: High frequency burst stimulation.
Conditioned Place Preference (CPP)
The CPP apparatus consisted of a white plastic box (22 cm w×90 cm l×30 cm h), which was divided into two compartments (22 cm w×40 cm l×30 cm h) with a separation section between them (22 cm w×10 cm l×30 cm h). The two compartments were different from each other both visually and in their texture. One featured walls with black and white vertical stripes, and the other featured gray walls of an intermediate hue and equally spaced perforations on its floor. The separation section was neutral in color, with white walls. On training days, the dividers between the compartments were closed (Buccafusco, 2009). On test days (measurement of baseline and response to DBS), each rat was placed in the separation section in the center of the apparatus. A 10-cm gate was opened between each compartment and the rat was allowed free access to all compartments. The amount of time spent in each of the zones (the 2 compartments and separation section) was recorded. Training and testing session durations were 30 min. Between each recording (in both training and testing sessions) the apparatus was turned around in order to control for any possible confounding effects. Time spent in each zone was detected using EthoVision 3.1 (Noldus, Holland), an automated video tracking, motion analysis and behavior recognition system, designed to sample and process moving video images.
Histology
At the conclusion of the experiments, animals were anesthetized and transcardially perfused with PBS×1 followed by 4% paraformaldehyde. The brains were removed and immersed in 4% paraformaldehyde for 24 hrs, and then in a phosphate buffer with 30% sucrose for 48 hrs. The brains were then frozen on dry ice and sliced (40 μm sections) using a cryostat microtome. Sections were mounted on glass slides (coated with 2% gelatin), stained with Cresyl violet and subsequently examined under a microscope to verify the placement of the electrode (Fig. 1 A-B).
Statistics
All data are expressed as mean ± standard error of the mean (SEM). Significance was determined by analyses of variance (ANOVA) with repeated measures (days) to examine the effect of the experimental procedures on active lever presses in the sucrose self-administration task. ANOVAs were followed by Student–Newman–Keuls post hoc tests to determine which treatment groups were altered, and p<0.05 was considered significant. Significance between the low and high DBS groups and their controls was determined by Student’s t-tests. Significance of CPP test was determined by one way ANOVA for time spent in the DBS compartment.
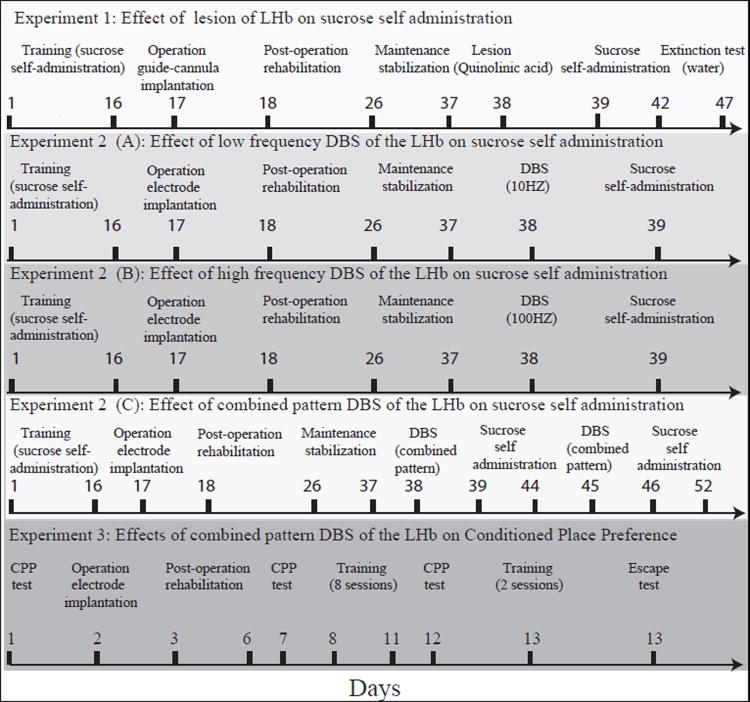
Experimental Design (Fig. 2)
Fig. 2. Flow chart of behavioral procedures.
Experiment 1: Effect of LHb lesioning on extinction of sucrose seeking behavior
Rats were trained to self-administer sucrose, and when stable maintenance levels were reached, a guide cannula was implanted into the LHb. Rats were then monitored for two weeks until it was established that post-operational performance during self-administration sessions reached that of the pre-operational naive state. On day 3 of recurred stable maintenance levels, one group of rats underwent chemical lesioning of the LHb. A parallel control group of rats was sham-operated. The next day, 10 rats from each group were transferred to the self-administration cages and sucrose self-administration was measured daily for 3 consecutive days. Concurrently, 10 rats from each group (lesioned and sham-operated) were denied sucrose by substitution with water, and the level of extinction response was measured daily, for 6 consecutive days after the lesion. An additional group of 10 sham-operated rats continued to receive sucrose throughout the extinction testing, to control for a possible effect of the operational procedure on general consumption behavior.
Experiment 2: Effect of DBS of the LHb on sucrose-seeking behavior
Rats were trained to self-administer sucrose, and when stable maintenance levels were reached, an electrode was implanted into the LHb. Rats were then monitored for two weeks until it was established that post-operational performance in self-administration sessions reached that of the pre-operational naive state. On day 3 of recurred stable maintenance levels, rats received DBS (15 min) in the self-administration chambers. One group of rats (n=10) received low frequency DBS (10 Hz), a second group (n=10) received high frequency DBS (100 Hz), and the third group (n=16) received the combined DBS set. The level of sucrose self-administration in rats receiving low and high DBS was measured for 30 min (Fig. 3B), and compared to the rats’ own previous sham-operated state. The level of sucrose self-administration in rats which received combined pattern DBS was measured daily, for 5 days. On day 6 the rats received a second combined pattern DBS session and the level of sucrose self-administration was measured daily for an additional 7 days (a total of 12 days in whole; see Fig. 3C). Results were compared to two control groups: sham-operated rats (n=10) which received sucrose throughout the experiment, and sham-operated rats (n=10) that during maintenance received sucrose, which was then substituted for water (i.e., an extinction paradigm) during the testing period (on the same day that treatment rats received their first DBS session).
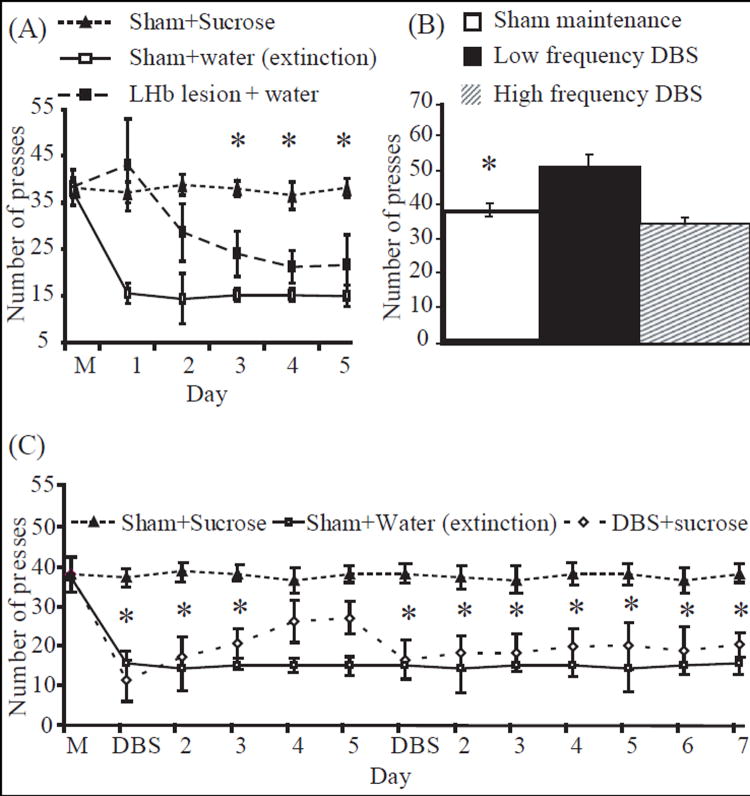
Fig. 3.
Panel A: Effect of LHb lesioning on extinction of sucrose-seeking behavior Rats were trained to self-administer sucrose until they reached stable maintenance levels. The LHb was then lesioned and the mean number of active lever presses was recorded in daily 30 min sessions. The lesioned group, in contrast to sham-operated control rats, responded in a delayed extinction response (*p<0.01, ANOVA repeated measures). Active lever presses of the sham-operated control group which continued to receive sucrose were not altered. M represents mean of last 3 days of maintenance. Panel B: Effect of low frequency DBS of the LHb on sucrose-seeking behavior. Rats were trained to self-administer sucrose until they reached stable maintenance levels. The LHb was then stimulated and the mean number of active lever presses was recorded in daily 30 min sessions. The stimulated group showed a significantly higher mean number of active lever presses in comparison to their previous sham-operated state (i.e., mean of last 3 days of maintenance prior to stimulation). *p <0.01, Student’s t-test. Panel C: Effect of combined pattern DBS of the LHb on sucrose-seeking behavior. Rats were trained to self-administer sucrose until reaching stable maintenance levels. They were then submitted to two sessions of combined pattern DBS treatment (15 min) during a period of 12 days (once on the first day and again on the sixth). The stimulated group demonstrated a decreased degree of sucrose consumption compared to controls (*p<0.001; ANOVA repeated measures), reaching levels similar to those of sham-operated rats receiving water in the extinction paradigm. Sucrose consumption of sham-operated control rats remained at normal levels, thus negating possible confounding effects of the implantation procedure on consumption behavior. Mean number of active lever presses is shown for 30 min sessions. M represents the mean of the last 3 days of maintenance.
Experiment 3: Effects of combined pattern DBS of the LHb on Conditioned Place Preference (CPP)
A separate group of rats (n=10) were placed in a CPP apparatus and baseline place preference was measured. On the next day, DBS electrodes were implanted into the LHb of the rats. The rats were allowed 4 days of rehabilitation following surgery and on day 7 their place preference was once again recorded. Throughout the next four days (days 8-11) the rats underwent one daily combined pattern DBS training session and one sham training session, 30 min each, separated by a 4-hour interval. During the DBS training session rats were placed in their preferred compartment (based on the results of the baseline tests). In the sham training session, rats were placed in their non-preferred compartments and connected to the stimulating device, but no stimulation was administered. On day 12 of the experiment, rats from each group were placed in the central separation section and time spent in each zone was then recorded (Fig. 4A). DBS session results were compared to those of sham-operated rats (n=10) and to results of control (naive) rats that underwent two daily sham sessions (n=10). On day 13 the rats once again underwent a short combined pattern DBS and sham training session (3 min each) in the CPP apparatus, with a one-hour interval between sessions. They were then placed again in the compartment in which they had earlier received DBS. The latency of the first exit from the DBS zone (i.e., the amount of time spent in the DBS compartment prior to escape) and the time spent in each zone were recorded (Fig. 4B). The DBS session result of each rat was compared to sham operated group.
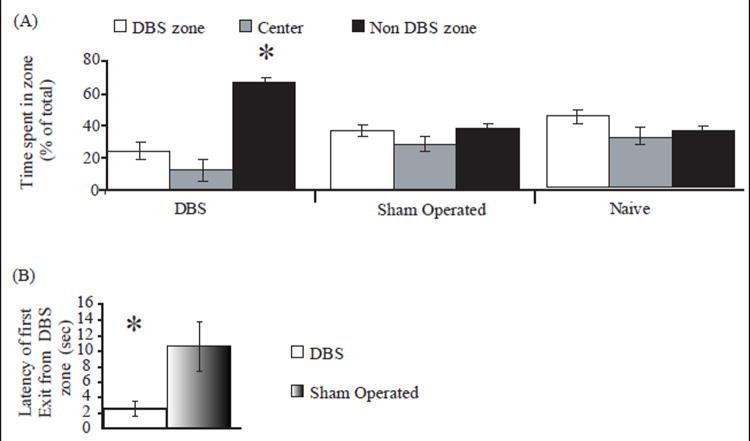
Fig. 4. Effects of combined pattern DBS of the LHb on Conditioned Place Preference.
Panel A After 4 days of daily DBS and sham sessions, rats were again placed in the separation section of the CPP apparatus. Time spent in each of the three zones was recorded. DBS-treated rats showed a significant preference for the non-DBS zone, as compared to sham operated rats and to results of control (naive) rats that underwent two daily sham sessions (*p<0.01, one-way ANOVA for time spent in the DBS zone). Panel B: Rats underwent short DBS and sham sessions. They were then placed again in the compartment in which they had received DBS, and showed significantly reduced latency (time spent in the DBS compartment prior to first exit) as compared to sham- operated group (F[1, 9]=2.3, *p<0.01, Student’s t-test).
Results
Experiment 1: Effect of lesioning of the LHb on extinction of sucrose self-administration
Lesion of the LHb (for histology see Fig. 1A) did not affect maintenance levels of sucrose self-administration as measured by the number of active lever presses (sham-operated: 39±4.0 active lever presses vs. lesioning of the LHb: 40±3.7 active lever presses; mean of 3 days). These findings indicate that implantation and lesion procedures did not affect general consumption behavior.
The second group of rats that underwent lesioning of the LHb after reaching maintenance levels was placed in the self-administration cages, in which sucrose was substituted with water. The extinction response was measured daily for 6 days, by recording the number of active lever presses. The lesioned group, in contrast to sham-operated rats which also underwent sucrose extinction, exhibited a delayed and attenuated extinction response. The amount of lever pressing of lesioned rats was similar to that of sham-operated rats which continued to self-administer sucrose. A repeated measures ANOVA for the number of active lever presses revealed a main effect of group [F(2,27)=56.31; p<0.0001], of days [F(5,135)=23; p<0.0001], and an interaction between group and days [F(10, 135)=19.3; p<0.03]. The Student-Newman-Keuls post-hoc test showed significant differences between the three groups: sham-operated sucrose extinction rats vs. LHb lesioned rats (p=0.01, for days 1-4), sham-operated rats which self-administered sucrose vs. sham-operated sucrose extinction rats (p<0.01, for days 1-5), and sham-operated rats which self-administered sucrose vs. LHb lesioned rats (p<0.01, for days 3-5) (Fig. 3A). No significant differences in inactive lever presses were noted between experimental groups (sham-operated sucrose extinction rats: 4.0±2.0 inactive lever presses, sham-operated rats which self-administered sucrose: 3.8 ±2.1 inactive lever presses, and LHb lesioned rats: 4.1±1.9 inactive lever presses; mean of 5 days of experiment).
Experiment 2: Effect of DBS of the LHb on sucrose-seeking behavior
Rats implanted with an electrode were allowed to self-administer sucrose until they reached maintenance levels. The first group then received low frequency DBS treatment (for detailed histology see Fig. 1B) and exhibited a significant increase in the number of active lever presses compared to their previous sham-operated, maintenance state (Fig. 3B, F[1,18]; p<0.05, Student’s t-test). This indicates that low frequency stimulation of the LHb increases the rewarding effects of sucrose. However, the group that received high frequency DBS treatment showed a similar amount of active lever presses compared to their previous sham-operated, maintenance state (Fig. 3B).
In contrast, the group that received combined pattern DBS treatment showed a significantly lower number of lever presses compared to those of the sham-operated, sucrose self-administering rats (Fig. 3C). This indicates the ability of LHb DBS to devalue the rewarding effects of sucrose. A repeated measures ANOVA for the number of active lever presses revealed a main effect of group [F(2, 27)=71.41; p<0.0001], a main effect of days [F(12, 324)=39.51; p<0.001], and an interaction between group and days [F(24, 324)=18.47; p<0.02]. The Student-Newman-Keuls post-hoc test showed significant differences between groups: sham-operated, sucrose self-administering rats vs. DBS-treated rats (p<0.01 for day 1 of the first DBS session to day 3, and p<0.01 for day 1 of the second DBS session until day 7), and sham-operated, sucrose self-administering rats vs. sham-operated sucrose extinction group (p<0.01, for the complete testing period (days 2-12). No significant differences were found between active lever presses of the DBS-treated group and sham-operated sucrose extinction group (positive control), except for days 4-5 following the first DBS treatment (p<0.01). At this point, the effect of DBS treatment began to wear off, and therefore DBS was again applied. This second session prolonged the attenuation effect, and rats again demonstrated a mean amount of active lever presses similar to the sham-operated sucrose extinction group, up to the end of experiment 2(C).
No significant differences in inactive lever presses were noted between experimental groups (sham-operated, sucrose self-administrating rats: 3.9±2.4 inactive lever presses; sham-operated sucrose extinction group: 4.1±3.1 inactive lever presses; and DBS-treated rats: 4±3 inactive lever presses; mean of 12 days). During the 15 minutes of stimulation, the mean number of active lever presses was approximately 4±1 presses.
Stimulation of rat brain in an adjacent region, the medial rostral part of the lateral posterior thalamic nucleus (LPMR), resulted in no effect on sucrose seeking behavior, measured by a sucrose self-administration test conducted immediately after the stimulation session (sham-operated rats: 38.1±4.8 active lever presses, vs. rats stimulated in LPMR: 39.2±6.2 active lever presses).
Experiment 3: Effects of combined pattern DBS of the LHb on Conditioned Place Preference
Following baseline measurements, rats received one daily combined pattern DBS session (in their preferred compartment) and one daily sham session (in their non-preferred compartment), with a 4-hr interval between sessions, for 4 days. On the next day, DBS-treated rats demonstrated a significant decrease in the amount of time spent in the initially preferred compartment. These results were in contrast to sham-operated rats’ results and to the results of control (naïve) rats that underwent two daily sham sessions; these groups showed no distinctive preference for any of the three zones (see Fig. 4A; one way ANOVA for time spent in the DBS zone: F[2, 27]=19.31, p<0.001).
Next, rats received another short DBS- and sham-training session (3 min each, with a 1-hr interval between sessions). They were then placed again in the compartment in which they had received DBS. The rats showed significantly reduced latency (time spent in the DBS compartment prior to first exit) as compared to sham-operated rats (see Fig. 4B; F[1, 18]=10.3, p<0.001, Student’s t-test).
Discussion
The findings presented in this study demonstrate that alterations in the normal functioning of the LHb, induced through lesioning and electrical stimulation, affect natural reward processes.
The LHb is composed of an intricate circuitry of neuronal extensions that convey its influence to diverse regions of the brain (Morissette and Boye, 2008), including dopaminergic areas such as the VTA. A possible explanation for the delayed extinction response following LHb lesions when sucrose was substituted with water, could be that the lesions eliminated the LHb inhibitory effect on response of dopaminergic cells to no-reward stimuli (Christoph et al., 1986). This, in turn, prevented the rats from distinguishing between reward stimuli (i.e., sucrose) and no-reward stimuli (i.e., substitution of sucrose with water). These results further support earlier findings that indicate a role for the LHb in negative reward processing (Matsumoto and Hikosaka, 2007), probably via a strong inhibiting effect of the LHb on mesolimbic dopaminergic cells (Christoph et al., 1986; Ji and Shepard, 2007).
The LHb receives inputs mainly from the basal ganglia and limbic regions, including the VTA and median and dorsal raphe (Gruber et al., 2007; Lecourtier and Kelly, 2007). Neurons from the globus pallidus internus (Gpi) that project to the LHb respond to a no-reward prediction cue by firing at a frequency of approximately 110-150 Hz (Hong and Hikosaka, 2008), which strongly activates the LHb. Intense LHb activation occurs especially when reward is expected and not received, which eventually leads to reward devaluation. Dopaminergic and serotonergic neurons that project to the LHb respond to reward-predicting cues by firing at a frequency of approximately 8-12 Hz (Fiorillo et al., 2003; Nakamura et al., 2008), which inhibits LHb activity. Therefore, it is possible that by use of low frequency stimulation (10 Hz), we were able to mimic dopaminergic signaling of reward expectation, which inhibited the LHb and consequently increased sucrose craving. On the other hand, high frequency stimulation (100 Hz) which resembles GPi firing was expected to signal no-reward and thus lower sucrose craving, yet it had no effect on rats’ response. We suggest that in order to significantly decrease craving, negative signaling must be immediately available on every occurrence of positive, reward-predictive signaling. This requires a timed stimulation schedule for each expected reward-related neuronal response. Therefore, we used an exogenous combined stimulation pattern to time the negative prediction trigger immediately after the positive predictor was provided. We presented the brain with low stimulation of 10 Hz for 60 sec, which resembles an elongated reward-prediction time. This caused the brain to wait for a positive event to occur, but then receive negative stimuli – by switching to a quick, 4-sec stimulation of 80/100 Hz (burst/non-burst). It is possible that by using this combined pattern of stimulation, we were able to mimic the electrical inputs received by the LHb from both the GPi and dopaminergic areas. We suggest that this treatment led to reward devaluation, which effectively attenuated reward seeking behavior.
A recent paper pointed to the connectivity that exists between the habenulae i.e left and right hemispheres by the habenular commissure (Kim, 2009). Indeed, in our study, unilateral stimulation proved to be sufficient in order to attain the desired effect and therefore bilateral stimulation was unnecessary. Similar findings have been reported in treatment of Parkinson’s disease, chronic depression and cocaine self-administration, where unilateral stimulation is successful in producing the desired effects (Friedman et al., 2009; Friedman et al., 2010; Germano et al., 2004).
It is possible that the decrease in sucrose self-administration demonstrated herein stems from the strengthening of LHb functioning as a result of DBS treatment. This strengthening could lead to reward devaluation, but could also result in a general depressive-like state, since anhedonia is one of the two main core symptoms of depression (Friedman et al., 2009; Yadid and Friedman, 2008). Animal models of depression demonstrate hyperactivity of the LHb as a common denominator (Shumake and Gonzalez-Lima, 2003). In addition, habenular lesions alleviate depressive behavior in several models of depression, including learned helplessness (Amat et al., 2001) and forced-swim immobility (Yang et al., 2008). Moreover, electrical stimulation of the LHb induces avoidance deficits resembling learned helplessness (Shumake et al.) in gerbils. Following LHb electrical stimulation, these rats also demonstrate reduced sucrose consumption (Enkel et al.; Shumake et al., 2004) and accelerated extinction of sucrose self-administration (Vollmayr et al., 2004). In addition, similar to the data demonstrated in the current study, Shumake et al. (2010) also found prolonged suppression of operant responding up to 5 sessions after LHb stimulation was discontinued. The relevance of the LHb to depressive states is further strengthened by a recent study in a patient suffering from therapy-resistant, severe major depressive disorder. By continual bilateral stimulation of the LHb, the patient achieved sustained full remission of depressive symptoms (Sartorius et al., 2010).
The findings of this study, combined with those of previous studies (Amat et al., 2001; Pobbe and Zangrossi, 2008; Shumake and Gonzalez-Lima, 2003; Yang et al., 2008), point to the central role that the LHb plays in the regulation of negative reward responses. It is noteworthy that only one or two sessions of LHb stimulation were required to induce prolonged effects on reward-seeking behavior. This finding in particular implicates the function of the LHb not merely as a constant regulator of dopaminergic firing, but also as a “negative reward tutor”. We use this term to refer to the ability of single external stimulation occurrences to generate enduring inhibitive effects on reward-seeking behavior.
LHb neurons respond similarly to conditioned stimulus that is paired with a displeasing context, whether it is the absence of reward or presence of an aversive stimulus (Matsumoto and Hikosaka, 2009). This is in accordance with the results of our CPP test which showed that rats responded to LHb DBS by escaping and avoiding the site of stimulation, exhibiting a clear place aversion response. We therefore suggest that attenuation of lever pressing observed following LHb DBS is a consequence of reward devaluation or, possibly, signaling of an unpleasant event. The brain thus receives indication that previously gratifying circumstances are no longer rewarding or, alternatively, are competing with aversion.
In conclusion, the findings presented herein strongly support the hypothesis that electrical stimulation of the LHb attenuates processes of positive reward-associated reinforcement.
Acknowledgments
This study was supported by NIH (grant #R21DA027776) to GY. AF and EL were supported by a President’s Fellowship, Bar-Ilan University. The research reported in this article was completed as part of AF’s Ph.D. dissertation.
Footnotes
Financial Disclosures All the authors state that they have no conflict of interest to declare.
GY (corresponding author) certifies that all authors have agreed to all the content in the manuscript, including the data as presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Sparks PD, Matus-Amat P, Griggs J, Watkins LR, Maier SF. The role of the habenular complex in the elevation of dorsal raphe nucleus serotonin and the changes in the behavioral responses produced by uncontrollable stress. Brain Res. 2001;917:118–126. doi: 10.1016/s0006-8993(01)02934-1. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ. Conditioned Place Preference. In: Prus AJ, James JR, Rosecrans JA, editors. Methods of Behavior Analysis in Neuroscience 2nd Frontiers in Neuroscience. Medical College of Georgia. Augusta CRC Press; Boca Raton (FL): 2009. [Google Scholar]
- Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986;6:613–619. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enkel T, Gholizadeh D, von Bohlen Und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, Gass P, Vollmayr B. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology. 35:1008–1015. doi: 10.1038/npp.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299:1898–1902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Friedman A, Frankel M, Flaumenhaft Y, Merenlender A, Pinhasov A, Feder Y, Taler M, Gil-Ad I, Abeles M, Yadid G. Programmed acute electrical stimulation of ventral tegmental area alleviates depressive-like behavior. Neuropsychopharmacology. 2009;34:1057–1066. doi: 10.1038/npp.2008.177. [DOI] [PubMed] [Google Scholar]
- Friedman A, Lax E, Dikshtein Y, Abraham L, Flaumenhaft Y, Sudai E, Ben-Tzion M, Lavi A, Yaka R, Yadid G. Electrical stimulation of the lateral habenula produces enduring inhibitory effect on cocaine seeking behavior. Neuropharmacology. 2010 doi: 10.1016/j.neuropharm.2010.06.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germano IM, Gracies JM, Weisz DJ, Tse W, Koller WC, Olanow CW. Unilateral stimulation of the subthalamic nucleus in Parkinson disease: a double-blind 12-month evaluation study. J Neurosurg. 2004;101:36–42. doi: 10.3171/jns.2004.101.1.0036. [DOI] [PubMed] [Google Scholar]
- Gruber C, Kahl A, Lebenheim L, Kowski A, Dittgen A, Veh RW. Dopaminergic projections from the VTA substantially contribute to the mesohabenular pathway in the rat. Neurosci Lett. 2007;427:165–170. doi: 10.1016/j.neulet.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Nauta WJ. Efferent connections of the habenular nuclei in the rat. J Comp Neurol. 1979;187:19–47. doi: 10.1002/cne.901870103. [DOI] [PubMed] [Google Scholar]
- Hikosaka O. The habenula: from stress evasion to value-based decision-making. Nat Rev Neurosci. 11:503–513. doi: 10.1038/nrn2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008;28:11825–11829. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007;27:6923–6930. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kim U. Topographic commissural and descending projections of the habenula in the rat. J Comp Neurol. 2009;513:173–187. doi: 10.1002/cne.21951. [DOI] [PubMed] [Google Scholar]
- Lecourtier L, Kelly PH. A conductor hidden in the orchestra? Role of the habenular complex in monoamine transmission and cognition. Neurosci Biobehav Rev. 2007;31:658–672. doi: 10.1016/j.neubiorev.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Lisoprawski A, Herve D, Blanc G, Glowinski J, Tassin JP. Selective activation of the mesocortico-frontal dopaminergic neurons induced by lesion of the habenula in the rat. Brain Res. 1980;183:229–234. doi: 10.1016/0006-8993(80)90135-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Representation of negative motivational value in the primate lateral habenula. Nat Neurosci. 2009;12:77–84. doi: 10.1038/nn.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette MC, Boye SM. Electrolytic lesions of the habenula attenuate brain stimulation reward. Behav Brain Res. 2008;187:17–26. doi: 10.1016/j.bbr.2007.08.021. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Matsumoto M, Hikosaka O. Reward-dependent modulation of neuronal activity in the primate dorsal raphe nucleus. J Neurosci. 2008;28:5331–5343. doi: 10.1523/JNEUROSCI.0021-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Pobbe RL, Zangrossi H., Jr Involvement of the lateral habenula in the regulation of generalized anxiety- and panic-related defensive responses in rats. Life Sci. 2008;82:1256–1261. doi: 10.1016/j.lfs.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Sartorius A, Kiening KL, Kirsch P, von Gall CC, Haberkorn U, Unterberg AW, Henn FA, Meyer-Lindenberg A. Remission of major depression under deep brain stimulation of the lateral habenula in a therapy-refractory patient. Biol Psychiatry. 2010;67:e9–e11. doi: 10.1016/j.biopsych.2009.08.027. [DOI] [PubMed] [Google Scholar]
- Shumake J, Conejo-Jimenez N, Gonzalez-Pardo H, Gonzalez-Lima F. Brain differences in newborn rats predisposed to helpless and depressive behavior. Brain Res. 2004;1030:267–276. doi: 10.1016/j.brainres.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Shumake J, Gonzalez-Lima F. Brain systems underlying susceptibility to helplessness and depression. Behav Cogn Neurosci Rev. 2003;2:198–221. doi: 10.1177/1534582303259057. [DOI] [PubMed] [Google Scholar]
- Shumake J, Ilango A, Scheich H, Wetzel W, Ohl FW. Differential neuromodulation of acquisition and retrieval of avoidance learning by the lateral habenula and ventral tegmental area. J Neurosci. 2010;30:5876–5883. doi: 10.1523/JNEUROSCI.3604-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmayr B, Bachteler D, Vengeliene V, Gass P, Spanagel R, Henn F. Rats with congenital learned helplessness respond less to sucrose but show no deficits in activity or learning. Behav Brain Res. 2004;150:217–221. doi: 10.1016/S0166-4328(03)00259-6. [DOI] [PubMed] [Google Scholar]
- Yadid G, Friedman A. Dynamics of the dopaminergic system as a key component to the understanding of depression. Prog Brain Res. 2008;172:265–286. doi: 10.1016/S0079-6123(08)00913-8. [DOI] [PubMed] [Google Scholar]
- Yang LM, Hu B, Xia YH, Zhang BL, Zhao H. Lateral habenula lesions improve the behavioral response in depressed rats via increasing the serotonin level in dorsal raphe nucleus. Behav Brain Res. 2008;188:84–90. doi: 10.1016/j.bbr.2007.10.022. [DOI] [PubMed] [Google Scholar]