Abstract
Background
Irinotecan hydrochloride and S-1, an oral fluoropyrimidine, have shown antitumor activity against advanced gastric cancer as single agents in phase I/II studies. The combination of irinotecan and S-1 (IRI-S) is also active against advanced gastric cancer. This study was conducted to compare the efficacy and safety of IRI-S versus S-1 monotherapy in patients with advanced or recurrent gastric cancer.
Methods
Patients were randomly assigned to oral S-1 (80 mg/m2 daily for 28 days every 6 weeks) or oral S-1 (80 mg/m2 daily for 21 days every 5 weeks) plus irinotecan (80 mg/m2 by intravenous infusion on days 1 and 15 every 5 weeks) (IRI-S). The primary endpoint was overall survival. Secondary endpoints included the time to treatment failure, 1- and 2-year survival rates, response rate, and safety.
Results
The median survival time with IRI-S versus S-1 monotherapy was 12.8 versus 10.5 months (P = 0.233), time to treatment failure was 4.5 versus 3.6 months (P = 0.157), and the 1-year survival rate was 52.0 versus 44.9%, respectively. The response rate was significantly higher for IRI-S than for S-1 monotherapy (41.5 vs. 26.9%, P = 0.035). Neutropenia and diarrhea occurred more frequently with IRI-S, but were manageable. Patients treated with IRI-S received more courses of therapy at a relative dose intensity similar to that of S-1 monotherapy.
Conclusions
Although IRI-S achieved longer median survival than S-1 monotherapy and was well tolerated, it did not show significant superiority in this study.
Keywords: Irinotecan S-1, Gastric cancer, Phase III, Randomized controlled trial
Introduction
Gastric cancer is the second leading cause of cancer-related deaths after lung cancer in Japan, and it was responsible for approximately 50,000 deaths in 2005 [1]. While surgery and appropriate adjuvant chemotherapy have resulted in superior stage-by-stage survival when compared with that in other parts of the world [2], the prognosis of unresectable or recurrent gastric cancer remains dismal. The development of more effective chemotherapeutic regimens is therefore warranted.
In Western countries where a combination of 5-fluorouracil (5-FU) and cisplatin (CDDP) [3] has served as a reference arm in several phase III studies [4–6], triplets employing epirubicin [7] or docetaxel [5] in addition to this combination are the current standards, with modifications such as the replacement of CDDP with oxaliplatin and the replacement of infusional 5-FU with oral agents such as capecitabine [8]. Failure with the first-line treatment usually denotes the termination of chemotherapy, and second-line treatments are rarely considered outside of clinical trials. In Japan, where a phase III study (JCOG9205) failed to show superiority of a 5-FU/CDDP combination over 5-FU alone [9], the 5-FU monotherapy remained a standard of care, and other cytotoxic agents were usually delivered sequentially as second-line and third-line therapies rather than concurrently as combination therapy. With this strategy, the median survival time (MST) of patients with advanced gastric cancer whose treatment started with infusional 5-FU alone actually reached 10.8 months [9].
In the 1990s, S-1 (TS-1; Taiho Pharmaceutical, Tokyo, Japan), an oral derivative of 5-FU, was developed for the treatment of gastric cancer [10–12]. With an exceptionally high response rate of 46% as a single agent, this drug rapidly established itself as a community standard in Japan and was used widely in clinical practice. Phase III trials eventually proved the non-inferiority of S-1 when compared with infusional 5-FU in the advanced/metastatic setting [13], along with the superiority of S-1 monotherapy over observation alone in the postoperative adjuvant setting [14]. In addition, S-1 was found to be a unique cytotoxic drug, in that Japanese patients tolerated higher doses than Western patients, due to differences in the gene polymorphism of relevant enzymes [15]. Thus, the development of novel chemotherapeutic regimens in Japan during the 2000s has inevitably centered around this drug.
The establishment of doublets to enhance response rates and improve on survival was the next important step, and several phase I/II studies were performed to explore combinations of S-1 with other cytotoxic drugs such as CDDP [16], docetaxel [17], paclitaxel [18], and irinotecan (Yakult Honsha, Tokyo, Japan; Daiichi Sankyo, Tokyo, Japan) [19]. All these combinations were found to be promising, with response rates of around 50% and relatively favorable safety profiles. A series of phase III trials comparing these doublets with S-1 monotherapy were subsequently planned and conducted to seek optimal first-line treatments. Of these, a phase III trial to explore S-1/CDDP was the first to complete accrual, and a significant improvement in MST of this combination over S-1 monotherapy was proven [20]. The present study, entitled GC0301/TOP-002, represents another of these attempts, exploring the efficacy of a combination of S-1 and irinotecan (IRI-S). The dose and schedule for this combination had been established by a phase I trial [21], and treatment at the recommended dose has shown a response rate of 47.8% [95% confidence interval (CI) 27.4–68.2%] with an MST of 394 days in a phase II study [19]. Given these earlier results and the synergistic effect of irinotecan and 5-FU observed in preclinical studies, the results of this present trial have been eagerly awaited.
Patients and methods
Eligibility
The eligibility criteria were histologically and cytologically confirmed unresectable or recurrent gastric adenocarcinoma; oral food intake possible; age between 20 and 75 years; no prior radiotherapy or chemotherapy; expected survival for ≥12 weeks; Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; and adequate major organ function before chemotherapy (leukocyte count of 4,000–12,000/mm3, hemoglobin ≥ 8.0 g/dl, platelet count ≥ 100,000/mm3, total bilirubin ≤ 1.5 mg/dl, aspartate aminotransferase ≤ 100 IU/l, alanine aminotransferase ≤ 100 IU/l, creatinine ≤ 1.2 mg/dl). The main exclusion criteria were massive ascites, active concomitant malignancy, uncontrolled diabetes mellitus, and pregnancy or breast-feeding. Written informed consent was obtained from each patient. Institutional review board approval was obtained at each participating institution. An independent data monitoring committee evaluated safety throughout this study. The study was performed in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. This trial was registered with the Japan Pharmaceutical Information Center (JapicCTI-050083).
Treatment schedule
In the S-1 monotherapy group, patients received oral S-1 twice daily for 28 days every 6 weeks. In the IRI-S group, S-1 (80 mg/m2) was given orally for 21 days and irinotecan (80 mg/m2) was infused intravenously on days 1 and 15 every 5 weeks. In both groups, the dose of S-1 was based on body surface area: 40 mg if the area was <1.25 m2; 50 mg for 1.25–1.5 m2, and 60 mg for ≥1.5 m2. Dose modification criteria were defined in the protocol. Treatment was discontinued if there was documented disease progression, unacceptable toxicity, or withdrawal of consent.
Assessment of response and toxicity
All patients who had at least one measurable lesion were evaluated for tumor response according to the Response Evaluation Criteria in Solid Tumors (RECIST) [22]. All radiologic assessments were confirmed by extramural review. Toxicity was evaluated according to the National Cancer Institute Common Toxicity Criteria (version 2.0).
Statistical analysis
Eligible patients were registered with the data center and randomized by centralized dynamic allocation with stratification for advanced/recurrent disease (with or without adjuvant chemotherapy), performance status (0/1/2), and institution. The full analysis set was defined as all patients who received treatment at least once and met all inclusion criteria. The per-protocol set was defined as all patients who received treatment at least once and had no major protocol violations.
The primary endpoint was overall survival, which was compared between groups using the stratified log-rank test. Secondary endpoints were the time to treatment failure (TTF), the 1- and 2-year survival rates, the response rate, and safety. Overall survival time was defined as the interval from the date of registration to the date of death (patients who remained alive at the final follow-up were censored at that time). Survival curves were estimated by the Kaplan–Meier method, and differences were analyzed with the stratified log-rank test. Hazard ratios (HRs) for various prognostic factors were calculated using a stratified Cox proportional hazards model. TTF was defined as the time from the date of registration to the date of detection of progressive disease, death, or treatment discontinuation.
In addition, subset analyses were conducted, using the Cox proportional hazards model, to identify factors that influenced overall survival in each group. As well as the predetermined variables such as gender, age, performance status, and disease status (whether the disease was unresectable or recurrent), subset analyses were conducted for 6 additional variables; the presence or absence of a measurable lesion by the RECIST, hepatic metastasis, peritoneal metastasis, existent of primary focus, metastasis the number of metastatic foci, and tumor histology. All analyses were performed using SAS system version 8.2 (SAS Institute, Cary, NC, USA).
This study was designed to detect a 40% improvement in MST at a two-tailed significance level of P ≤ 0.05 with 80% power. The MST for S-1 monotherapy was assumed to be 8.5 months, based on the results of previous phase I/II studies [12, 23]. A total of 142 patients per group were required according to calculations made with nQuery Advisor version 4.0 (Statistical Solutions, Boston, MA, USA), and the sample size was set as 300 (150 patients per group).
We initially planned to continue follow-up for ≥1.5 years after the registration of all patients, with a cut-off date of April 2007. However, an unexpectedly high survival rate of 22% (68 of 315 patients) at the cut-off date prompted the Coordinating Committee, the medical expert, and the biostatistician to advise the sponsor to continue follow-up for a further year before performing the final analysis. Thus, the MST was also calculated using 2.5-year follow-up data.
Results
Patient characteristics
Between June 2004 and November 2005, a total of 326 patients (S-1 monotherapy, n = 162; IRI-S, n = 164) were enrolled from 54 institutions and randomized (Fig. 1). Seven patients were subsequently found to be ineligible or withdrew before receiving any treatment. Another 4 patients were found to be ineligible after starting treatment and were not included in the analysis. Therefore, 315 patients (S-1 monotherapy, n = 160; IRI-S, n = 155) were evaluable and were included in the full analysis set to assess overall survival and TTF. In addition, 187 patients were evaluable for tumor response. Baseline patient characteristics are shown in Table 1.
Fig. 1.
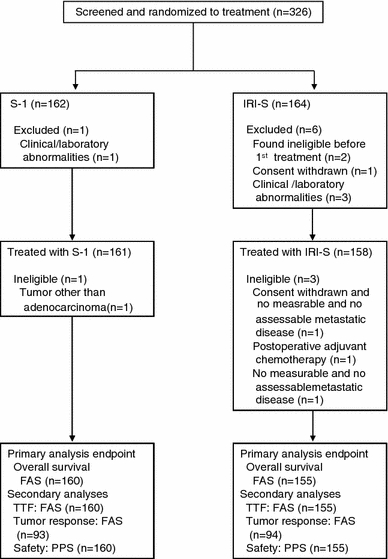
Patient disposition. FAS Full analysis set, IRI-S S-1 plus irinotecan, PPS per-protocol set, TTF time to treatment failure
Table 1.
Baseline characteristics and prior therapy
| Characteristic | Treatment | |||||
|---|---|---|---|---|---|---|
| S-1 | IRI-S | Total | ||||
| n | % | n | % | n | % | |
| Patients randomized | 162 | 164 | 326 | |||
| Patients receiving at least one dose of study medication (full analysis set) | 160 | 155 | 315 | |||
| Sex | ||||||
| Male | 127 | 79 | 110 | 71 | 237 | 75 |
| Female | 33 | 21 | 45 | 29 | 78 | 25 |
| Age (years) | ||||||
| Median | 63 | 63 | 63 | |||
| Range | 27–75 | 33–75 | 27–75 | |||
| ECOG performance status | ||||||
| 0 | 109 | 68 | 102 | 66 | 211 | 67 |
| 1 | 46 | 29 | 48 | 31 | 94 | 30 |
| 2 | 5 | 3 | 5 | 3 | 10 | 3 |
| Tumor histology | ||||||
| Intestinal | 71 | 44 | 61 | 39 | 132 | 42 |
| Diffuse | 88 | 55 | 93 | 60 | 181 | 57 |
| Other | 1 | 1 | 1 | 1 | 2 | 1 |
| Resection of primary tumor | ||||||
| + | 93 | 58 | 93 | 60 | 186 | 59 |
| − | 67 | 42 | 62 | 40 | 129 | 41 |
| Advanced | 133 | 83 | 129 | 83 | 262 | 83 |
| Recurrent | ||||||
| Adjuvant chemotherapy (+) | 5 | 3 | 5 | 3 | 10 | 3 |
| Adjuvant chemotherapy (−) | 22 | 14 | 21 | 14 | 43 | 14 |
IRI-S S-1 plus irinotecan, ECOG Eastern Cooperative Oncology Group
Treatments given
The median number of treatment courses was three (range 1–19) for S-1 monotherapy whose duration was 6 weeks, and four (range 1–25) for IRI-S whose duration was 5 weeks. The main reasons for treatment discontinuation were disease progression [S-1 monotherapy vs. IRI-S, 116/160 (72.5%) vs. 89/155 (57.4%)], adverse events [12/160 (7.5%) vs. 23/155 (14.8%)], attending physician’s decision [18/160 (11.3%) vs. 18/155 (11.6%)], and consent withdrawal [11/160 (6.9%) vs. 17/155 (11.0%)]. The median TTF was 3.6 months (95% CI 2.9–4.1) and 4.5 months (95% CI 3.7–5.3), respectively (P = 0.157). The relative dose intensity was 88.9% for S-1 monotherapy, versus 90.0% for S-1 and 86.2% for irinotecan among those treated with IRI-S. Most patients in both groups received the scheduled dose of chemotherapy.
Second-line chemotherapy was administered to 240 patients (76%; S-1 monotherapy, n = 112; IRI-S, n = 128) (Table 2). The most common second-line therapy in both groups was a taxane alone (S-1 monotherapy, 26.9%; IRI-S, 40.6%). Among patients initially treated with S-1, 13 received crossover treatment with IRI-S, while 31 patients originally treated with IRI-S received second-line S-1 monotherapy.
Table 2.
Second-line chemotherapy
| Regimen | S-1 (n = 160) | IRI-S (n = 155) | ||
|---|---|---|---|---|
| n | % | n | % | |
| IRI-S | 13 | 8.1 | – | – |
| Irinotecan-based regimena | 27 | 16.9 | 4 | 2.6 |
| S-1 alone | – | – | 31 | 20.0 |
| S-1-based regimenb | 9 | 5.6 | 11 | 7.1 |
| Taxane alone | 43 | 26.9 | 63 | 40.6 |
| Others | 20 | 12.5 | 19 | 12.3 |
| None | 48 | 30.0 | 27 | 17.4 |
IRI-S S-1 plus irinotecan
aIrinotecan/cisplatin, irinotecan/taxane
bS-1/cisplatin, S-1/taxane
Response and survival
The overall response rate was determined in 187 patients evaluable by the RECIST, and was significantly higher with IRI-S than with S-1 monotherapy (39/94, 41.5% vs. 25/93, 26.9%; P = 0.035) (Table 3).
Table 3.
Response to treatment
| S-1 (n = 93) | IRI-S (n = 94) | |||
|---|---|---|---|---|
| n | % | n | % | |
| Complete response | 0 | 0 | 0 | 0 |
| Partial response | 25 | 27 | 39 | 41 |
| Stable disease | 35 | 38 | 40 | 43 |
| Progressive disease | 30 | 32 | 12 | 13 |
| Not assessable | 3 | 3 | 3 | 3 |
| Overall response rate | 26.9 | 41.5* | ||
| 95% CI | 18.2–37.1 | 31.4–52.1 | ||
CI confidence interval
* P = 0.035 (χ2 test)
The MST at the predetermined cut-off date was 12.8 months with IRI-S compared with 10.5 months with S-1 monotherapy (HR 0.856, P = 0.233) (Fig. 2), but the difference was not statistically significant. The 1-year survival rates were 44.9% [95% CI 37.2–52.6%] with S-1 monotherapy and 52.0% (95% CI 44.1–59.9%) with IRI-S, while the 2-year survival rates were 19.5% (95% CI 12.6–26.4%) and 18.0% (95% CI 11.2–24.8%), respectively.
Fig. 2.
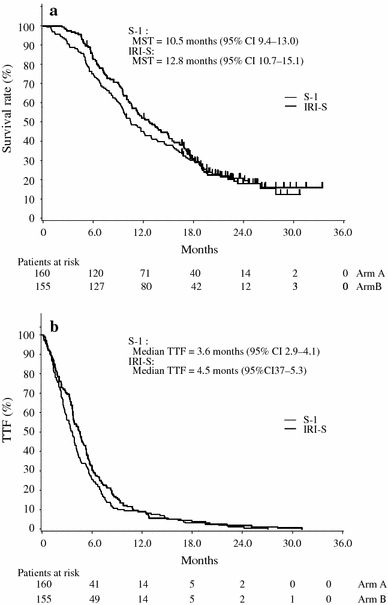
Kaplan–Meier estimates of overall survival (a) and time to treatment failure (b) for 315 evaluable patients treated with S-1 monotherapy or S-1 plus irinotecan (IRI-S). MST Median survival time, TTF time to treatment failure, CI confidence interval
MST was additionally calculated as an exploratory analysis after 2.5 years of follow-up, but the result was identical to the initial analysis at 12.8 months for IRI-S and at 10.5 months for S-1 monotherapy (HR 0.927; log-rank test P = 0.536). Again, the difference was not statistically significant.
Prognostic factors of all patients and factors that favored treatment with IRI-S
Baseline risk factors with a significant influence on the overall survival of all patients accrued (P < 0.05) were performance status (HR 1.348, 95% CI 1.079–1.686, Wald test P = 0.009), tumor histology (HR 1.720, 95% CI 1.161–2.548, P = 0.007), target lesion (HR 1.525, 95% CI 1.164–1.999, P = 0.002), and surgery for the primary tumor (HR 0.698, 95% CI 0.538–0.906, P = 0.007).
Stratified analysis according to baseline patient characteristics (Fig. 3) showed that IRI-S was significantly more effective than S-1 monotherapy for patients with diffuse-type histology (HR 0.632, 95% CI 0.454–0.880) and for those with an ECOG performance status of 1 or 2 (HR 0.614, 95% CI 0.401–0.940). No differences were observed for the other factors assessed.
Fig. 3.
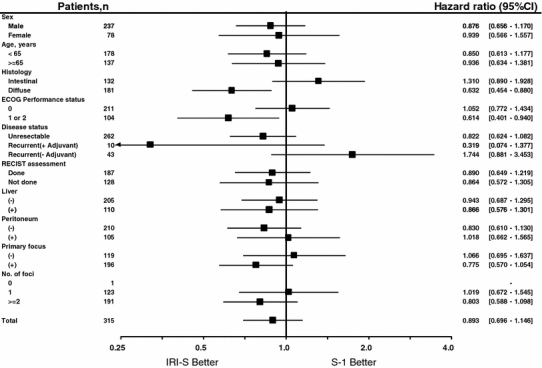
Subset analysis of overall survival stratified by baseline patient characteristics. CI Confidence interval, ECOG Eastern Cooperative Oncology Group, RECIST Response Evaluation Criteria in Solid Tumors
Safety
Adverse events that occurred in each group are listed in Table 4. The incidence of major hematological toxicities was higher with IRI-S than with S-1 monotherapy. Grade 3 or 4 neutropenia was observed in 10.6% of patients treated with S-1 monotherapy versus 27.1% of patients treated with IRI-S, while the corresponding incidences of infection/febrile neutropenia were 3.8 versus 1.9%. The most common grade 3 or 4 non-hematological toxicities were diarrhea (S-1 monotherapy vs. IRI-S, 5.6 vs. 16.1%), anorexia (18.8 vs. 17.4%), nausea (5.6 vs. 7.1%), and vomiting (1.9 vs. 3.2%). Hand-foot skin reaction, a characteristic adverse event associated with some oral fluoropyrimidines, was confined to grade 2 or less and was observed in only 4.4 and 5.2% of patients treated with S-1 monotherapy and IRI-S, respectively. There were no treatment-related deaths among patients treated with S-1 monotherapy, whereas two patients in the IRI-S died of potentially treatment-related conditions (severe bone marrow dysfunction, multiple organ failure that was probably associated with multiple duodenal ulcers).
Table 4.
Summary of adverse events
| S-1 (n = 160) | IRI-S (n = 155) | |||||||
|---|---|---|---|---|---|---|---|---|
| All events | Grade 3/4 | All events | Grade 3/4 | |||||
| n | % | n | % | n | % | n | % | |
| Anemia | 83 | 51.9 | 19 | 11.5 | 113 | 72.9 | 24 | 15.5 |
| Leukopenia | 83 | 51.9 | 5 | 3.1 | 115 | 74.2 | 18 | 11.6 |
| Neutropenia | 86 | 53.8 | 17 | 10.6 | 113 | 72.9 | 42 | 27.1 |
| Infection/febrile neutropenia | 28 | 17.5 | 6 | 3.8 | 40 | 25.8 | 3 | 1.9 |
| Thrombocytopenia | 18 | 11.3 | 6 | 3.8 | 17 | 11.0 | 2 | 1.3 |
| Increased AST | 75 | 46.9 | 8 | 5.0 | 69 | 44.5 | 5 | 3.2 |
| Increased ALT | 58 | 36.3 | 3 | 1.9 | 69 | 44.5 | 3 | 1.9 |
| Increased bilirubin | 74 | 46.3 | 9 | 5.6 | 56 | 36.1 | 5 | 3.2 |
| Increased creatinine | 17 | 10.6 | 2 | 1.3 | 19 | 12.3 | 3 | 1.9 |
| Fatigue | 101 | 63.1 | 12 | 7.5 | 123 | 79.4 | 10 | 6.5 |
| Alopecia | 13 | 8.1 | 0 | 0.0 | 87 | 56.1 | 0 | 0.0 |
| Anorexia | 104 | 65.0 | 30 | 18.8 | 125 | 80.6 | 27 | 17.4 |
| Diarrhea | 63 | 39.4 | 9 | 5.6 | 103 | 66.5 | 25 | 16.1 |
| Nausea | 84 | 52.5 | 9 | 5.6 | 115 | 74.2 | 11 | 7.1 |
| Vomiting | 60 | 37.5 | 3 | 1.9 | 68 | 43.9 | 5 | 3.2 |
| Stomatitis/pharyngitis | 27 | 16.9 | 2 | 1.3 | 34 | 21.9 | 4 | 2.6 |
| Hand-foot skin reaction | 7 | 4.4 | 0 | 0.0 | 8 | 5.2 | 0 | 0.0 |
| Pigmentation changes | 74 | 46.3 | 0 | 0.0 | 77 | 49.7 | 0 | 0.0 |
Adverse events were graded according to National Cancer Institute Common Toxicity Criteria, version 2.0
ALT alanine aminotransferase, AST aspartate aminotransferase, IRI-S S-1 plus irinotecan
Discussion
This study was conducted to determine whether IRI-S could prolong MST compared with S-1 monotherapy. Basic studies have indicated that irinotecan has a multifactorial synergistic effect with the anti-tumor activity of 5-FU [24, 25]. In addition, several trials exploring combinations of S-1 and irinotecan have reported promising response rates [19, 23, 26, 27]; the dose and schedule in the present study was selected based on the lower incidence of grade 3 neutropenia and gastrointestinal toxicity evidenced from phase II studies among these trials.
Although the combination therapy in the present study achieved a significantly higher response rate, the initial expectation that the addition of irinotecan would improve the MST by 40% was not met. Thus, the combination of S-1 and CDDP remains the first-line chemotherapy that can be recommended for Japanese patients, while patients who are frail or those who wish to refrain from the short stay in the hospital required for hydration could turn to S-1 monotherapy. Another standard treatment could be available pending the results of a phase III trial comparing S-1 with an S-1/docetaxel combination [17]. A combination of CDDP with 5-FU or its derivative capecitabine has been used as a platform for molecularly targeting agents in recent international trials [28]; however, the place of platinum agents in the first-line treatment of gastric cancer would seem indispensible at present.
Irinotecan has often been delivered in combination with CDDP for gastric cancer in the West [29]. This combination was also explored in Japan in a phase II trial [30] and subsequently in a phase III trial [13], but failed to show statistically significant superiority over infusional 5-FU alone. Irinotecan was more recently found to be similarly effective to CDDP when delivered with 5-FU [31], with benefit in terms of a more favorable toxicity profile. The combination then went on to be compared with a 5-FU/CDDP combination [4], but, again, failed to show a survival advantage. With similar results obtained from the present study, irinotecan-based chemotherapy would no longer be expected to surpass 5-FU or its derivatives with or without CDDP in the first-line setting.
Our stratified analysis revealed that IRI-S had a significant effect on overall survival in patients with diffuse-type histology and an ECOG performance status of 1 or 2 (Fig. 3). IRI-S was more effective in symptomatic patients. This finding may be related to its higher response rate, resulting from tumor shrinkage, with subsequent attenuation of clinical symptoms, possibly leading to enhanced survival time. The effect of IRI-S in cancer with diffuse-type histology was in line with the finding of the subset analysis of another phase III study that an irinotecan/CDDP combination improved the survival of patients with undifferentiated gastric cancer [13]. However, these data are contradictory to data from a phase II study of the combination of S-1 and irinotecan [19], where a higher response rate was observed for intestinal-type histology. It would not seem feasible at this time, therefore, to attempt to identify patients who may benefit from the IRI-S, using clinicopathologic factors that are easily accessible.
As mentioned previously, cytotoxic drugs tend to be used sequentially as second-line and third-line therapies in some countries, including Japan. Recently, Thuss-Patience et al. [32] reported on second-line treatment for metastatic gastric cancer, and stated that irinotecan monotherapy significantly extended survival compared with best supportive care. A retrospective study exploring a combination of irinotecan and CDDP for patients who failed first-line therapy with S-1 has shown a promising response rate of 28.6% and a MST of 9.4 months from the first day of the second-line treatment [33]. Another retrospective study, also in the second-line setting, has shown promising MSTs, ranging from 9.5 to 10.1 months [34]. These studies suggest a role for irinotecan after the failure of a 5-FU-based first-line treatment, provided that the patients retain sufficient performance status to tolerate this drug. Because definite evidence remains unavailable, further prospective studies in the second-line and third-line settings are warranted to confirm the place of irinotecan in the treatment of gastric cancer. IRI-S uses up one of promising drug combination for the second line treatment without sufficient prolongation of TTF when compared with S-1 monotherapy. It could partially explain why the combination failed to attain significant gain in MST in the present study.
IRI-S was generally well tolerated in the present study. The dose intensity of S-1 in patients treated with IRI-S was equivalent to that in patients receiving S-1 monotherapy, demonstrating the good tolerability of the IRI-S. The most common grade 3 or 4 adverse events associated with this regimen included neutropenia (27.1%) and diarrhea (16.1%), both of these being more frequent than in patients receiving S-1 monotherapy. IRI-S appears to be better tolerated than either the S-1/CDDP or irinotecan/CDDP regimens explored in other phase III studies [13, 20]. Grade 3 or 4 neutropenia was less common with IRI-S than with the S-1/CDDP and irinotecan/CDDP regimens (27 vs. 40% and 65%, respectively), as was anorexia (17 vs. 30% and 33%) and nausea (7 vs. 12% and 21%). Only diarrhea was more common with IRI-S than with the S-1/CDDP and irinotecan/CDDP regimens (16 vs. 4% and 9%, respectively) [13, 20]. However, it is of note that, in the present study, two patients who received IRI-S died of potentially treatment-related conditions. The evaluation of uridine 5′-diphospho-glucuronosyl-transferase gene polymorphism, which had not been approved at the time the trial was conducted, could now identify a small number of patients who may suffer from overt adverse reactions to IRI-S [35].
Although manageable in most cases, the IRI-S was found to be more toxic than S-1 monotherapy. To conclude, the improvement in the response rate observed with the IRI-S did not translate into the predicted prolongation of MST.
Acknowledgments
This study was supported by Yakult Honsha Co., Ltd. and Daiichi Sankyo Co., Ltd. (GC0301/TOP002). The funding source had no role in the study design, data collection, data analysis, or interpretation. We thank the patients, clinicians, and support staff who participated in this study. We also thank Y. Takada, K. Miyakawa, and K. Tamura for performing extramural review to assess objective responses, as well as T. Taguchi, S. Yoshida, and H. Origasa for their helpful advice.
Conflict of interest
Chikuma Hamada has received advisory fees from Yakult Honsha Co., Ltd. (Tokyo, Japan) and Daiichi Sankyo Co., Ltd. (Tokyo, Japan). Yuh Sakata has received advisory fees and honoraria from Yakult Honsha Co., Ltd. (Tokyo, Japan), Daiichi Sankyo Co., Ltd. (Tokyo, Japan), and Taiho Pharmaceutical Co., Ltd. (Tokyo, Japan). The other authors have no conflicts of interest to declare.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Foundation for Promotion of Cancer Research. Cancer Statistics in Japan 2007. Available from URL http://ganjoho.ncc.go.jp/public/statistics/backnumber/2007_en.html. Accessed June 21, 2010.
- 2.Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, et al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 3.Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E, et al. Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol. 2000;18:2648–2657. doi: 10.1200/JCO.2000.18.14.2648. [DOI] [PubMed] [Google Scholar]
- 4.Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C, et al. Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol. 2008;19:1450–1457. doi: 10.1093/annonc/mdn166. [DOI] [PubMed] [Google Scholar]
- 5.Ajani JA, Moiseyenko VM, Tjulandin S, Majilis A, Constenla M, Boni C, et al. Clinical benefit with docetaxel plus fluorouracil and cisplatin compared with cisplatin and fluorouracil in a phase III trial of advanced gastric or gastroesophageal adenocarcinoma: the V-325 Study Group. J Clin Oncol. 2007;25:3205–3209. doi: 10.1200/JCO.2006.10.4968. [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Rodriguez W, Bodoky G, Moiseyenko CM, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010;28:1547–1553. doi: 10.1200/JCO.2009.25.4706. [DOI] [PubMed] [Google Scholar]
- 7.Waters JS, Norman A, Cunningham D, Scarffe JH, Webb A, Harper P, et al. Long-term survival after epirubicin, cisplatin and fluorouracil for gastric cancer: results of a randomized trial. Br J Cancer. 1999;80:269–272. doi: 10.1038/sj.bjc.6690350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 9.Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H, et al. Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: the Japan Clinical Oncology Group Study (JCOG9205) J Clin Oncol. 2003;21:54–59. doi: 10.1200/JCO.2003.04.130. [DOI] [PubMed] [Google Scholar]
- 10.Fukushima M, Satake H, Uchida J, Shimamoto Y, Kato T, Takechi T, et al. Preclinical antitumor efficacy of S-1: a new oral formulation of 5-fluorouracil on human tumor xenografts. Int J Oncol. 1998;13:693–698. doi: 10.3892/ijo.13.4.693. [DOI] [PubMed] [Google Scholar]
- 11.Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191–197. doi: 10.1159/000012099. [DOI] [PubMed] [Google Scholar]
- 12.Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–1720. doi: 10.1016/S0959-8049(98)00211-1. [DOI] [PubMed] [Google Scholar]
- 13.Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A, et al. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009;10(11):1027–1028. doi: 10.1016/S1470-2045(09)70259-1. [DOI] [PubMed] [Google Scholar]
- 14.Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–1820. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 15.Ajani JA, Faust J, Ikeda K, Yao JC, Anbe H, Carr KL, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol. 2005;23:6957–6965. doi: 10.1200/JCO.2005.01.917. [DOI] [PubMed] [Google Scholar]
- 16.Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207–2212. doi: 10.1038/sj.bjc.6601413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshida K, Ninomiya M, Takakura N, Hirabayashi N, Takiyama W, Sato Y, et al. Phase II study of docetaxel and S-1 combination therapy for advanced or recurrent gastric cancer. Clin Cancer Res. 2006;12:3402–3407. doi: 10.1158/1078-0432.CCR-05-2425. [DOI] [PubMed] [Google Scholar]
- 18.Narahara H, Fujitani K, Takiuchi H, Sugimoto N, Inoue K, Uedo N, et al. Phase II study of a combination of S-1 and paclitaxel in patients with unresectable or metastatic gastric cancer. Oncology. 2008;74:37–41. doi: 10.1159/000138978. [DOI] [PubMed] [Google Scholar]
- 19.Uedo N, Narahara H, Ishihara R, Takiuchi H, Goto M, Fujitani K, et al. Phase II study of a combination of irinotecan and S-1 in patients with advanced gastric cancer (OGSG0002) Oncology. 2007;73:65–71. doi: 10.1159/000120630. [DOI] [PubMed] [Google Scholar]
- 20.Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:2. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 21.Takiuchi H, Narahara H, Tsujinaka T, Gotoh M, Kawabe S, Katsu K, et al. Phase I study of S-1 combined with irinotecan (CPT-11) in patients with advanced gastric cancer (OGSG 0002) Jpn J Clin Oncol. 2005;35:520–525. doi: 10.1093/jjco/hyi148. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Inokuchi M, Yamashita T, Yamada H, Kojima K, Ichikawa W, Nihei Z, et al. Phase I/II study of S-1 combined with irinotecan for metastatic advanced gastric cancer. Br J Cancer. 2006;94:1130–1135. doi: 10.1038/sj.bjc.6603072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters GJ, van der Wilt CL, van Moorsel CJ, Kroep JR, Bergman AM, Ackland SP. Basis for effective combination cancer chemotherapy with antimetabolites. Pharmacol Ther. 2000;87:227–253. doi: 10.1016/S0163-7258(00)00086-3. [DOI] [PubMed] [Google Scholar]
- 25.Guichard S, Hennebelle I, Bugat R, Canal P. Cellular interactions of 5-fluorouracil and the camptothecin analogue CPT-11 (irinotecan) in a human colorectal carcinoma cell line. Biochem Pharmacol. 1998;55:667–676. doi: 10.1016/S0006-2952(97)00541-8. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Yasui H, Goto A, Arai T, Ura T, Hamaguchi T, et al. Phase I study of irinotecan and S-1 combination therapy in patients with metastatic gastric cancer. Int J Clin Oncol. 2003;8:374–380. doi: 10.1007/s10147-003-0359-z. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu Y, Yuki S, Miyagishima T, Asaka M. Irinotecan plus oral S-1 in patients with advanced gastric cancer biweekly IRIS regimen. Gan To Kagaku Ryoho (Cancer and Chemotherapy) 2006;33(Suppl 1):131–134. [PubMed] [Google Scholar]
- 28.Van Cutsem E, Kang Y, Chung H, Shen L, Sawaki A, Lordick F, et al. Efficacy results from the ToGA trial: a phase III study of trastuzumab added to standard chemotherapy (CT) in first-line human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer (GC) [abstract]. J Clin Oncol. 2009;27(Suppl 18S):LBA4509.
- 29.Enzinger PC, Ilson DH, Saltz LB, O’Reilly EM, Kelsen DP. Irinotecan and cisplatin in upper gastrointestinal malignancies. Oncology. 1998;12:110–113. [PubMed] [Google Scholar]
- 30.Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, et al. Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol. 1999;17:319–323. doi: 10.1200/JCO.1999.17.1.319. [DOI] [PubMed] [Google Scholar]
- 31.Pozzo C, Barone C, Szanto J, Padi E, Peschel I, Bukki J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal–gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol. 2004;15:1773–1781. doi: 10.1093/annonc/mdh473. [DOI] [PubMed] [Google Scholar]
- 32.Thuss-Patience PC, Kretzschmar A, Deist T, Hinke A, Bichev D, Lebedinzew B, et al. Irinotecan versus best supportive care (BSC) as second-line therapy in gastric cancer: a randomized phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) [abstract]. J Clin Oncol. 2009; 27 (Suppl 15S):4540.
- 33.Takahari D, Shimada Y, Takeshita S, Nishitani H, Takashima A, Okita N, et al. Second-line chemotherapy with irinotecan plus cisplatin after the failure of S-1 monotherapy for advanced gastric cancer. Gastric Cancer. 2010;13:186–190. doi: 10.1007/s10120-010-0557-0. [DOI] [PubMed] [Google Scholar]
- 34.Sakamoto T, Yasui H, Boku N, Onozawa Y, Hironaka S, Fukutomi A, et al. Comparison of combination chemotherapy with irinotecan and cisplatin regimen administered every 2 or 4 weeks in pretreated patients with unresectable or recurrent gastric cancer: retrospective analysis. Int J Clin Oncol. 2010;15:287–293. doi: 10.1007/s10147-010-0054-9. [DOI] [PubMed] [Google Scholar]
- 35.Ando Y, Fujita K, Sasaki Y, Hasegawa Y. UGT1A1*6 and UGT1A1*27 for individualized irinotecan chemotherapy. Curr Opin Mol Ther. 2007;9:258–262. [PubMed] [Google Scholar]


