Abstract
Impairment in the executive control of attention has been found in youth with chromosome 22q11.2 deletion syndrome (22q11.2DS). However, how this impairment is modified by other factors, particularly age, is unknown. Forty-six typically developing and 53 children with 22q11.2DS were tested with the attention networks task (ANT) in this cross-sectional study. We used logarithmic transform and linear modeling to assess age effects on the executive index of the ANT. Mixed modeling accounted for between subject variability, age, handedness, catecholamine-O-transferase (COMT; codon 158) genotype, and gender on performance for all experimental conditions (cue × flanker) and their two-level interactions. Children with 22q11.2DS showed a relative, age-dependent executive index impairment but not orienting or alerting network index impairments. In factorial analysis, age was a major predictor of overall performance. There was a significant effect of the 22q11.2DS on overall performance. Of note, children with 22q11.2DS are specifically vulnerable to incongruent flanker interference, especially at younger ages. We did not find an overall effect of COMT genotype or handedness. Children with 22q11.2DS demonstrated age-related impairment in the executive control of attention. Future investigation will likely reveal that there are different developmental trajectories of executive attentional function likely related to the development of schizophrenia in 22q11.2DS.
Keywords: Chromosome 22q11.2 deletion syndrome, Cognitive control, Attention networks task
Introduction
Chromosome 22q11.2 deletion syndrome (22q11.2DS; OMIM accession nos. 188400, 192430, and 145410) is caused by a commonly occurring (∼1:4,000 live births (Oskarsdottir et al. 2004; Tezenas Du Montcel et al. 1996)) microdeletion on the long arm of chromosome 22. The deletion confers on children a variety of physical and psychological phenotypes (Gothelf et al. 2008; Robin and Shprintzen 2005). In addition, they have mild, general intellectual impairment in both verbal and nonverbal domains, though most are more affected in the latter (De Smedt et al. 2007). Various aspects of attention might underlie the nonverbal cognitive impairments. For example, selective/spatial attention has been related to spatial and numerical processing impairment (Simon et al. 2005; Bish et al. 2005) and more recently to temporal processing (Simon 2010) while executive aspects of attention (such as inhibition) have been related to impairments in cognitive control, such as working memory (Gothelf et al. 2007; Kates et al. 2007).
It is possible to test several attentional systems with a single task, the attention networks task (ANT). Fan et al. (2002) created the ANT to efficiently assay the function of three proposed attention networks with distinct functional–anatomic correlations (Posner and Petersen 1990). Named for their function, these networks are the executive, orienting, and alerting networks. The alerting network is responsible for overall vigilance, the orienting network directs attention to salient stimuli or locations, and the executive network maintains control, by processes such as inhibiting response to irrelevant stimuli. The ANT uses flanker interference (Eriksen and Eriksen 1974) to test the executive network, spatial cueing (Posner 1980) to test the orienting network, and unpredictably occurring nonspatial cues to test the alerting network. For flanker interference, nontarget arrows are presented alongside the target arrow, either pointing in the same direction (congruent) or in the opposite direction (incongruent). Fan et al. (2002) proposed that specific contrasts between the different flanking, spatial cueing, and alerting conditions defined performance indices that represent each network. For example, the executive index is calculated by subtracting a performance measure (e.g., reaction time) on congruent flanking conditions from performance on incongruent flanking conditions. It has been reported that these performance indices did not correlate and have appeared statistically independent in typically developing adults (Fan et al. 2002) and in children (Konrad et al. 2005; Rueda et al. 2004).
Several studies have reported a specific impairment in the executive network when testing children with 22q11.2DS on the ANT. Sobin et al. (2004) detected impairments in executive network performance in 32 children with 22q11.2DS compared with their typically developing siblings. In addition to the original ANT indices, they compared the flanker and cueing conditions’ independent effects on performance measures (reaction time, accuracy, and missed trials). On post hoc testing, they found that increased errors and missed trials occurred significantly more often in the incongruent flanking condition. Their results were consistent with prior reports based on neuropsychological tests that suggested impaired mental flexibility and poor visual attentional focus in these children (Woodin et al. 2001). Bish et al. (2005) also found impaired performance specific to the executive index of the ANT. Hypothesizing impaired flanker interference processing, they analyzed sequences of flanking trial conditions to assess adaptation to incongruent flanker information. Generally, when participants are confronted with an incongruent flanking trial, they tend to adapt by becoming more efficient if on the next trial they are again confronted by incongruent flankers. Referred to as the Gratton effect (Gratton et al. 1992), this pattern is assumed to indicate efficient control of cognitive resources. However, Bish et al. (2005) found that, as a group, children with 22q11.2DS demonstrated an inability to benefit from a prior incongruent flanker trial. Using a modification of the same task, Takarae et al. (2009) reported that, while most children with 22q11.2DS in her study did adapt when an extended series of three incongruent flankers was presented, there was a small group that did not. The notable thing about this nonadapting group was that all shared a particular variant of the catecholamine-O-transferase (COMT) gene, located in the chromosome 22q11.2 deleted region. A codominant COMT polymorphism results in a substitution at polypeptide position 158 yielding a low (Met158) or high (Val158) activity form of the enzyme COMT. COMT is involved in dopamine catabolism, and its synaptic degradation, particularly in the prefrontal cortex (Chen et al. 2004). While many factors affect its impact (Tunbridge et al. 2006), this COMT Val158Met polymorphism affects prefrontal cortex-dependent cognitive function (e.g., (Bilder et al. 2002; Egan et al. 2001; Joober et al. 2002; Malhotra et al. 2002)), which includes attentional control measured by flanker task performance (Blasi et al. 2005). It was a subgroup of those with COMT Met158 who failed to adapt in the study by Takarae et al. (2009). In summary, many children with 22q11.2DS demonstrate ANT performance impairment specific to the executive network and altered conflict adaptation and this difference may be modulated by the Val158Met polymorphism of COMT.
Impairment in the executive control of attention is evident in schizophrenia and is measurable by the ANT (Wang et al. 2005). It has been proposed that schizophrenia-related cognitive impairments may be a risk factor for schizophrenia in 22q11.2DS (e.g., (Lewandowski et al. 2007)). Already, there is some limited evidence that standardized measures dependent on executive functioning might be associated with psychotic symptoms in youth with 22q11.2DS (Antshel et al. 2010; Gothelf et al. 2005; Rockers et al. 2009). As 22q11.2DS is a genetic model for the development of schizophrenia (relative risk of ∼25) (Bassett and Chow 1999; Murphy and Owen 2001), specific schizophrenia-related impairments serve as endophenotypes for further investigation. Despite detection of schizophrenia-related impairments in small samples of youth with 22q11.2DS, little is known about how they develop. An understanding of the development and mechanism of schizophrenia-related executive dysfunction in people with 22q11.2DS will provide clues to its biology in schizophrenia.
We do not assess conversion to schizophrenia longitudinally in the current study. However, unlike prior studies of executive attention in 22q11.2DS, we have accrued data from a large-enough sample to allow us to assess age as a factor in the executive control of attention. This allows us to assess whether or not development is typical with respect to the executive control of attention for the group. We hypothesized that our study group of children with 22q11.2DS, some of whose performance has been reported elsewhere (Takarae et al. 2009), would show a specific impairment on the executive control of attention. Moreover, reflecting atypical development, we hypothesized that the executive control of attention in 22q11.2DS will differ relative to typically developing children as a function of age. Also, distinct from prior investigations, the current sample size permits us to simultaneously evaluate candidate effect modifiers such as the COMT polymorphism, sex (Antshel et al. 2005) and handedness on performance.
Methods
Participants
A total of 106 children between 7 and 14 years of age participated in this study. The protocol was approved by the Institutional Review Board at the University of California, Davis, and all participants and their parents consented to take part. None had participated in our initial study (Bish et al. 2005) but some (27 with 22q11.2DS and 13 typically developing (TD) control participants) had participated in another (Takarae et al. 2009). This report (Takarae et al. 2009) was of a study of trial adaptation and COMT genotype. We do not report or use any ANT outcome variables or analyses reported elsewhere. 46 of the participants were TD and 60 had 22q11.2DS as determined by fluorescence in situ hybridization (FISH) testing. Participant characteristics are given in Table 1.
Table 1.
Participant characteristics
| Diagnostic group | Comparison | ||||
|---|---|---|---|---|---|
| 22q | TD | t test | Pearson’s chi-square | Unequal variances (p) | |
| Age (months) (mean (SD), N) | 128.8 (24.2), 53 | 120.5 (28.6), 46 | −1.548 | 0.125 | |
| Male sex (N (valid %a)) | 25 (47.2%) | 25 (54.3%) | 0.580 | 0.476 | |
| Right handed (N (valid %)) | 38 (76.0%) | 39 (88.6%) | 2.523 | 0.112 | |
| COMT Val158Met (N (valid %)) | 26 (55.3%) | N/A | |||
aPercentage of the number of participants without a missing value
Attention networks task
We used a version of the ANT that was modified from the original children’s ANT (Rueda et al. 2004) as in our prior study (Takarae et al. 2009). We replaced each fishlike stimulus with a friendly alien flying an arrow-shaped spaceship with an arrow on its side. We also used a central (not at the target location), neutral cue in place of the original double cue. We also arranged the trials in such a way as to provide for several runs of three incongruent flanker trials in a row (see Takarae et al. (2009) for details). Flanker interference (executive) had the following conditions: (1) congruent (flanking arrows in the same direction as the stimulus arrow), and (2) incongruent (flanking arrows in the opposite direction). The spatial cueing (orienting) conditions were the following: (1) valid (cue at the target location), (2) invalid (cue opposite the target location), and (3) neutral (cue appears at the central fixation point). We tested the Alerting system by comparing response times on trials in which neutral cues were presented to those where there was no cue. To manipulate predictability, primarily for this condition, intertrial intervals were pseudorandomly distributed at 200 ms intervals between 400 and 1,600 ms between the last trial and the onset of the cue. An interstimulus interval of 400 ms elapsed between the appearance of the cue and the target.
Genetic analysis
Determination of COMT polymorphism using serum or saliva samples was completed by PCR and restriction enzyme digest as previously described (Takarae et al. 2009).
Data analysis
Data were processed using MatLab (version 7.4) on an Apple OS X platform to generate outcome variables from raw data. Trials with response times less than 150 ms were excluded as anticipatory trials. Such exclusions were rare for participants included in analysis; no more than four trials of 144 were excluded for any individual due to anticipation. Trials 2.5 standard deviations from the mean of the condition were excluded as outlying trials. Participants with error rates >50% for any condition were excluded. Because of this, seven participants were excluded from the 22q11.2DS group leaving 53 for analysis. None of the TD group was excluded. These participants differed significantly from the other children with 22q11.2DS in that they were younger (mean (SD) age in months 97.1 (12.9) versus 128.8 (24.2); Mann–Whitney U = 327.5; p < 0.001). Six of the seven children who were excluded provided genetic material available for analysis. All of these six had the COMT Met158 allele, a characteristic that trends towards significance when compared with the other participants with 22q11.2DS of whom 55.3% have the Met158 allele (p = 0.070; Fisher’s exact test, two-tailed). Gender and handedness did not differ.
The main outcome variable was the adjusted reaction time. Median reaction time (RT) of combined correct and incorrect responses for each experimental condition was calculated. RTs, three for flanking and four for cueing, were adjusted for performance by dividing by one minus the error rate for their respective cueing or flanking condition (AdjRT), that is AdjRT = RT/(1% error). Such a reaction time adjustment is a well established way to account for speed accuracy trade-off (Townsend and Ashby 1983). Given the notable positive skew of the unimodal distributions of AdjRT for each condition, we used median rather than mean as a measure of central tendency. Then, for each individual, we calculated three indices. The alerting index was calculated for an individual by subtracting the median AdjRT of all trials with a neutral (central) cue from the median AdjRT of all trials with no cue. The orienting index was calculated by subtracting the median AdjRT of valid trials from the median AdjRT of invalid trials. The executive index was calculated by subtracting the median AdjRT for all congruent trials from the median AdjRT of all incongruent trials.
The effect of age on the executive index was modeled by simple linear regression. The executive index was natural logarithm transformed before analysis to remedy non-normality of residuals and unequal variances between the diagnostic groups. Several participants had executive indices below zero after processing. To avoid taking a logarithm of a negative number, 150 adjusted milliseconds were added to the executive index. However, one typically developing participant had an executive index that was −420.24 adjusted milliseconds and was therefore excluded as an outlier. Age was centered at 80 months, the age of the youngest participant, to provide a baseline for estimating age-specific differences. Letting del22q represent a zero–one indicator variable coding the presence of 22q11.2DS, the regression model was:
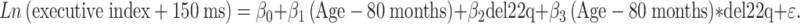 |
To address the effects of the three flanking conditions and four cueing conditions, a mixed-model 3 × 4 factorial analysis was carried out, adjusted for the effects of age, handedness, and gender on performance. To preserve power, we limited ourselves to studying the two-way interactions. The outcome was reaction time under each of the  cue × flanker conditions. The median reaction time across all trials for each condition was divided by the error rate to generate AdjRT. AdjRT was transformed by adding 1 to their value and taking the natural logarithm, LnAdjRT, to reduce variance spread at extreme values and correct the positively skewed distribution of raw scores. The mixed model allowed for a random intercept to account for within-child correlation in performance. Fixed factors were: cue type, flanker type, presence or absence of chromosome 22q11.2 microdeletion, age (in months), sex, and handedness, as well as two-way interactions. In the group with 22q11.2DS, we also investigated the COMT Val158Met polymorphism (rs4680). Models were built using PASW version 18.0 in a forward, stepwise fashion. A factor was included if a type III sum of squares demonstrated a significant contribution to the model, p ≤ 0.05. Once included, a factor was not excluded until a type III sum of squares analysis demonstrated no trend, p ≥ 0.10. All factors, first main effects and then interactions, were entered in an arbitrary sequence and then, as a secondary analysis, in a reverse sequence to assess reliability of the stepwise algorithm. The results were the same in both directions. For significant group or two-way interactions, pairwise comparisons were subsequently carried out. p Values were Bonferroni corrected to adjust for multiple comparisons.
cue × flanker conditions. The median reaction time across all trials for each condition was divided by the error rate to generate AdjRT. AdjRT was transformed by adding 1 to their value and taking the natural logarithm, LnAdjRT, to reduce variance spread at extreme values and correct the positively skewed distribution of raw scores. The mixed model allowed for a random intercept to account for within-child correlation in performance. Fixed factors were: cue type, flanker type, presence or absence of chromosome 22q11.2 microdeletion, age (in months), sex, and handedness, as well as two-way interactions. In the group with 22q11.2DS, we also investigated the COMT Val158Met polymorphism (rs4680). Models were built using PASW version 18.0 in a forward, stepwise fashion. A factor was included if a type III sum of squares demonstrated a significant contribution to the model, p ≤ 0.05. Once included, a factor was not excluded until a type III sum of squares analysis demonstrated no trend, p ≥ 0.10. All factors, first main effects and then interactions, were entered in an arbitrary sequence and then, as a secondary analysis, in a reverse sequence to assess reliability of the stepwise algorithm. The results were the same in both directions. For significant group or two-way interactions, pairwise comparisons were subsequently carried out. p Values were Bonferroni corrected to adjust for multiple comparisons.
Results
ANT indices
ANT index performance is presented in Table 2. As in prior reports, performance on the executive index differed between those with 22q11.2DS and TD control participants. We found no correlations between these indices (data not shown). Further investigation of the relationships between these networks is not the focus of this study, but is currently being investigated by our lab.
Table 2.
Efficiency of the attention networks
| Group | Number | Mean (SD) | Comparisona | ||
|---|---|---|---|---|---|
| t (df) | p | ||||
| Alerting Index | TD | 46 | −9.9 (66.9) | −1.287 (81.8) | 0.202 |
| 22q11.2DS | 53 | 15.5 (124.8) | |||
| Orienting Index | TD | 46 | 124.8 (110.0) | −0.473 (96.4) | 0.637 |
| 22q11.2DS | 53 | 135.6 (117.0) | |||
| Executive Index | TD | 46 | 80.0 (137.6) | −2.053 (84.4) | 0.043 |
| 22q11.2DS | 53 | 158.6 (236.5) | |||
aIndependent samples, two-tailed, t test with equal variances not assumed
Executive function levels are plotted by age and diagnostic group in Fig. 1. Age trends, group differences, and a difference in age trends between groups accounted overall for 11% of variation in executive performance in a linear regression model (r = 0.375; p = 0.002). The model estimates that at 6 2/3 years (80 months), TD children have a mean executive index of 88 (95% confidence interval (CI), 31 to 163) adjusted ms while those with 22q11.2DS have an executive index of 276 (95% CI, 61 to 707) adjusted milliseconds. For TD children, age did not significantly predict performance (β = −0.002; p = 0.441). However, for those with 22q11.2DS, the executive index reduced by 0.9% per month of age (β = −0.009; 95% CI = −0.017 to −0.001; p = 0.035). Since data are modeled on a logarithmic scale, the variation, a SE of ±0.4% per month age, has a higher impact on performance at a younger age as observed in the raw data (Fig. 1). Because of this variation and its distribution by age, we modeled other candidate effect modifiers: COMT genotype, gender and handedness. Furthermore, because the ANT tests several attention networks simultaneously, we estimated the effects of flanking and cueing using mixed models.
Fig. 1.
The effect of age on the executive index. For each participant, the median reaction time, adjusted for error, of trials with congruent flankers is subtracted from the median-adjusted reaction time of trials with incongruent flankers. This executive index differs in an age-dependent fashion between those with 22q11.2DS and typically developing control participants. The variation within groups appears most prominent at the younger ages (80 months) for both groups. Group performance converges at adolescence. A fit line with 95% CI is shown for the typically developing group, but is omitted for children with 22q11.2DS where a line does not describe the distribution of their raw performance by age
ANT factorial analysis
Table 3 shows group means of median reaction time and error rate for each condition. Overall, it appears that those with 22q11.2DS were globally impaired on the ANT with both slower reaction times and more errors in all conditions. We assessed the effect of presence of 22q11.2DS on the natural logarithm of error-adjusted raw reaction time (LnAdjRT). The highly variable ANT performance in the group with 22q11.2DS prompted our use of a mixed linear model.
Table 3.
Median reaction time and error rate means for each condition by diagnostic group
| Cue condition | Flanker type | |||||
|---|---|---|---|---|---|---|
| 22q11.2 DS (N = 53) | Typically developing (N = 46) | |||||
| None | Congruent | Incongruent | None | Congruent | Incongruent | |
| Median response times (ms) | ||||||
| None | 832 | 908 | 980 | 749 | 846 | 874 |
| Neutral | 806 | 886 | 975 | 724 | 803 | 886 |
| Valid | 731 | 740 | 861 | 653 | 712 | 756 |
| Invalid | 814 | 915 | 1,001 | 786 | 804 | 921 |
| Error rates (%) | ||||||
| None | 3.8 | 5.6 | 8.9 | 2.9 | 3.3 | 2.1 |
| Neutral | 4.2 | 3.5 | 10.1 | 2.1 | 3.9 | 6.6 |
| Valid | 4.3 | 5.5 | 5.6 | 1.8 | 2.4 | 3.4 |
| Invalid | 5.2 | 4.4 | 7.3 | 3.6 | 1.4 | 3.3 |
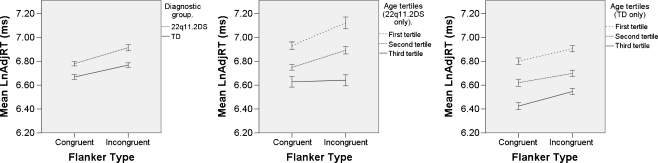
Cue type, flanker type, presence or absence of chromosome 22q11.2 microdeletion, age (in months), sex, handedness, and their two-way interactions were entered in a stepwise forward fashion. Surviving factors and model parameters are presented in Table 4. Age was the dominant predictor of performance, interacting with both cue and flanker effects. Figure 2 aids in interpretation of the model parameters. It demonstrates that as they become older, children perform better when confronted with flanker interference in general. It shows an interaction between age and flanker effects within the 22q11.2DS group. The effect of the 22q11.2DS itself is large, a 14.3% cost. Males received a substantial benefit, though not statistically significant, which is diminished relative to females on the incongruent condition.
Table 4.
Surviving factors and interactions
| Selected factorsa | Mixed model estimates of fixed effects on LnAdjRT | |||
|---|---|---|---|---|
| Estimate (β) (×102)b | Standard error (×102) | Significance (p)c | Description (e β followed by specified significant condition) | |
| Age | −0.651 | 0.0868 | <0.001 | 0.65% benefit per month of age. |
| Age × flanker | −0.145 | 0.0410 | 0.009 | 0.15% benefit per month on incongruent flankers. |
| Age × cue | No significant specific interactions. | |||
| Flanker | 26.9 | 5.44 | <0.001 | 30.8% cost on incongruent flankers. |
| Diagnosis | 13.4 | 4.15 | 0.039 | 14.3% cost to those with 22q11.2DS. |
| Diagnosis × flanker | No significant specific interactions. | |||
| Gender | No significant specific interactions. | |||
| Gender × flanker | 7.2 | 3.51 | 0.011 | 7.5% cost to males on incongruent flankers |
| Cue × flanker | No significant specific interactions. | |||
| Variance | ||||
| Residual | 1.95 | 0.09 | 2.0% of the total variation | |
| Participants | 3.43 | 0.54 | 3.5% of the total variation | |
aDetermined by type III sum of squares (see “Methods” for criteria)
bSome parameters were set to 0 because they are used as the reference parameter for modeling. They are diagnosis = typically developing, gender = female, flanker = single, and cue = valid. For interactions, if any condition tested contains a reference, that estimate is set to 0
cFor clarity, parameter estimates are reported only if the factor it modifies significantly contributed to the model. For example, age × flanker survived entry criteria, but of the age × flanker interactions, only the age by incongruent condition significantly contributed to the model and is presented here. Despite inclusion of the cue × flanker interaction in the model due to a type III sum of squares significance of p = 0.042, none of the 12 specific cue × flanker possibilities significantly contributed to the mixed linear model. This may be due to the large number of paired comparisons to be considered or their colinearity. p Values are Bonferroni adjusted for 23 comparisons
Fig. 2.
Effect of diagnosis and age on flanking conditions. These profile plots demonstrate the overall effect of diagnosis and age on performance. Units are the natural logarithm of the error-adjusted reaction time (LnAdjRT; see “Methods”). The interaction between the diagnosis of 22q11.2DS and age is apparent for the incongruent flanking condition (middle profile plot) where the typically developing children display a similar flanker interference profile across all age groups. Error bars represent standard error of the mean
Within the 22q11.2DS group analysis
We excluded TD children to focus on 22q11.2 specific factor effects including the effect of the COMT Val158Met polymorphism. As above, all factors including the COMT polymorphism and their two-way interactions were entered in a stepwise forward fashion. Surviving factors and parameter estimates are presented in Table 5. The incongruent flanker effect is large, resulting in a 67% reaction time cost at 80 months of age. Age significantly improves performance on incongruent flanking conditions above all other conditions, cueing or flanking. Of note, the effect of any type of cue is not seen to be mediated by age as it was when both study groups were entered into model. A deeper analysis of the sources of variance within the 22q11.2DS group for the Orienting and Alerting networks is being prepared for separate manuscript (Simon, Harvey, and Stoddard, in preparation). In addition, gender is not a significant factor that determines performance. COMT genotype did not significantly contribute to the model nor interact with either cue or flanking condition.
Table 5.
22q11.2DS only: surviving factors and interactions
| Selected factorsa | Mixed model estimates of fixed effects on LnAdjRT | |||
|---|---|---|---|---|
| Estimate (β) (×102)b | Standard error (×102) | Significance (p)c | Description (e β followed by specified condition) | |
| Age | −0.684 | 0.158 | <0.001 | 0.69% benefit per month of age |
| Age × flanker | −0.231 | 0.078 | 0.025 | 0.23% additional benefit per month of age on incongruent flankers |
| Flanker | 51.4 | 9.9 | <0.001 | 67% cost on incongruent flankers |
| Cue | 17.1 | 1.9 | <0.001 | 19% cost on invalid cues |
| 14.8 | 1.9 | <0.001 | 16% cost on no cue | |
| 17.2 | 1.9 | <0.001 | 19% cost on neutral cue | |
| Variance | ||||
| Residual | 2.6 | 1.6 | 2.6% of the total variation | |
| Participants | 4.6 | 1.0 | 4.7% of the total variation | |
aDetermined by type III sum of squares (see “Methods” for criteria)
bSome parameters were set to 0 because they are used as the reference parameter for modeling. They are diagnosis = typically developing, gender = female, flanker = single, and cue = valid. For interactions, if any condition tested contains a reference, that estimate is set to 0
cFor clarity, parameter estimates are reported only if the factor it modifies significantly contributed to the model. For example, age × flanker survived entry criteria, but of the age × flanker interactions, only the age by incongruent condition significantly contributed to the model and is presented here. p Values are Bonferroni adjusted for eight comparisons
Discussion
We demonstrate that executive control of attention is substantially affected by age in a large group of children with 22q11.2DS. While impairment exists in the whole group of children with 22q11.2DS, it is more pronounced and variable for children who are younger. When testing typically developing children, Rueda et al. (2004) found that they approached adult performance by about age 7 years on the executive network subtask of the ANT. We found the same result in our typically developing group. However, performance for those with 22q11.2DS was distributed across age in a funnel shape, suggesting that a subgroup of children with 22q11.2DS may have a delayed development in the processing of flanker interference. In our second analysis, we demonstrate the relative effect of several factors that determine ANT performance in children with 22q11.DS. Of note, children with 22q11.2DS are specifically vulnerable to incongruent flanker interference, especially at younger ages. As a group, they have a 67% adjusted reaction time cost relative to their performance on trials with no flankers at 80 months of age. However, this impairment lessens rapidly, by ∼0.92% per month age afterwards. Thus, this study replicates smaller studies on two independent samples showing specific executive index impairment (Bish et al. 2005; Sobin et al. 2004), provides new information about the magnitude and specificity of the impairment on the incongruent flanking condition, demonstrates the effect of age and factors associated with cognitive impairment in 22q11.2DS, and suggests a statistical approach to account for variability in the 22q11.2DS group (Sobin et al. 2004).
As before (Takarae et al. 2009), we did not find an overall effect of the COMT Val158Met polymorphism on flanker interference. However, Takarae et al. (2009) did find that all children who failed to adapt to a series of three incongruent flanking trials (n = 6 of 27) had the COMT Met158 polymorphism. All 27 of these children were included in this study. However, unlike this earlier analysis, we did not analyze the relationship of the COMT Val158Met polymorphism to trial to trial adaptation to flanker interference. Trial to trial adjustment on conflict tasks is a more dynamic and engaging way to measure cognitive systems managing conflict than simple flanker interference tasks (e.g. (Carter and van Veen 2007)). Of the seven excluded from the study for random-appearing performance, six were genotyped all of whom had the Met158 polymorphism. The exclusion of these individuals biased the study against finding an association between Met158 and impaired performance on flanking conditions. The most significant characteristic of these excluded children was their young age. Interestingly, the effect of age has been suggested (Takarae et al. 2009; Tunbridge et al. 2006; Gothelf et al. 2005) as an explanation of inconsistent reports with regards to the COMT Val158Met polymorphism in 22q11.2DS that have tested a variety of aspects of cognition (Baker et al. 2005; Bearden et al. 2004; Kates et al. 2006; Shashi et al. 2006). None of these studies, including this one, accounted for the effects of the common synonymous SNPs in COMT which are known to determine its protein expression (Nackley et al. 2006).
The flanker task embedded in the ANT tests conflict processing and its associated anatomical areas (Fan et al. 2005), specifically the ability to monitor and inhibit the processing of irrelevant stimuli that would generate an incorrect response. Though these adolescents with 22q11.2DS have a typically appearing executive index, the examination of flanker effect shown in Fig. 2 reveals otherwise. It appears that they do not demonstrate the typical profile of either facilitation by response-congruent distracters or interference by response-incompatible distracters. Post hoc analysis (Fig. 3) reveals that they were impaired in resolving incongruent flanker interference relative to typically developing adolescents. These observations are consistent with the theory of anterior cingulate gyrus dependent conflict processing that has been frequently shown to be impaired in individuals with schizophrenia and attention deficit/hyperactivity disorder (ADHD) (e.g., (Carter and van Veen 2007)). Both syndromes are prevalent in people with 22q11.2DS. However, we did not find a correlation between executive index and ADHD symptom severity in any category as measured by the Swanson, Nolan, and Pelham Questionnaire, 4th revision (Swanson 1992). Of note, when testing the executive control of attention with the ANT, others have also unexpectedly found (Nestor et al. 2007) and replicated (Urbanek et al. 2009) reduced incongruent flanker interference in patients with chronic schizophrenia that is similar to the pattern in our results.
Fig. 3.
Effect of diagnosis on flanker effect. This bar graph represents the effect of incongruent flanker interference and congruent flanker facilitation in the third age tertile (12.3 to 14.9 years; n = 9 each group). Contrasts between the conditions with and without flankers represent flanker facilitation (congruent flanker condition—no flanking condition) and interference (incongruent flanker condition—no flanking condition). Compared to typically developing adolescents (n = 9), those with 22q11.2DS (n = 9) show a reduced reaction time cost to interference (Mann–Whitney U = 13; p = 0.015) but not to facilitation (Mann–Whitney U = 40; p = 0.965). Error bars represent the 95% CI of the median
While schizophrenia-related cognitive impairments in children with 22q11.2DS is of particular interest, direct associations between cognitive impairment and the development of psychotic disorders in 22q11.2DS have yet to be established (Lewandowski et al. 2007; Antshel et al. 2010; Gothelf et al. 2005; Rockers et al. 2009). Like all but one of these former studies, we do not examine the presence of clinical psychosis and link it to schizophrenia-associated impairment in the executive control of attention. However, our analysis does provide further information about more specified schizophrenia-related cognitive impairment in 22q11.2DS with some caveats. Firstly, while flanker interference demands the cognitive operations of conflict monitoring, it also depends on other cognitive operations, specifically priming (Mayr et al. 2003). These effects are not a sufficient alternative explanation for the involvement of impaired conflict processing, and its previously observed effects might be accounted for by study design (Botvinick et al. 2004). Secondly, little is known about its neural substrate and functioning in children which differs from adults (Konrad et al. 2005) in typical populations and might be even more different and more variable in the case of congenital disorders. This is particularly important in 22q11.2DS in which schizophrenia-related functional neural connectivity (Sigurdsson et al. 2010) and anatomic neural connectivity are atypical, e.g., (Karayiorgou et al. 2010). Together with our prior reports (Bish et al. 2005; Takarae et al. 2009), these results strongly motivate theoretically guided investigations of specific impairments in cognition and their relationship to the development of psychotic disorders in 22q11.2DS.
This study has several limitations. The ANT was designed as a rapid test of several attention networks rather than a complete survey of the executive control of attention. However, in a population with known spatiotemporal processing impairments, the ANT is a well-validated and effective method of testing the executive control of attention as it simultaneously probes other attentional systems for interactions. A limitation in the factorial analysis is the possible over fit of the model to the data because a stepwise selection includes only significant factors. However, the inclusion of factors already known to affect executive control of attention (age, gender, and COMT polymorphism) and spatial processing (handedness) protects against spurious associations. The purpose of the second analysis was to assess their effect of covariates on flanker interference rather than discover new associations. Despite multiple pairwise comparisons in the second analysis, there is limited risk of type I error as mixed models make a conservative inference of significance for fixed factors and the Bonferonni familywise correction is severe. There are many factors not assessed in this study which might be related to our results including other genetic effects, prescription drug effects, other somatic disease effects, and prior interventions, such as cardiac surgery, with cognitive sequelae. This pattern of impairment we observed may not be specific to 22q11.2DS with respect to other neurodevelopmental disorders. However, because a genetic lesion is identified in 22q11.2DS, how this pattern changes over time and its functional consequences will prepare for studies of their genetic determinants.
This study demonstrates how age affects the executive control of attention in a group of children with 22q11.2DS. It demonstrates that cross-sectional cognitive research in 22q11.2DS with summary measures of performance for groups with a wide age range may not be valid, risking type II error. It also suggests that there may be a critical developmental window for detection of some types of cognitive impairment. This is especially important for studies seeking associations between cognitive impairment a risk factor for the later development of schizophrenia or studies of the pathogenesis of schizophrenia in 22q11.2DS.
Funding
This work was supported by the University of California, Davis, Health System Department of Psychiatry and Behavioral Sciences as well as National Institutes of Health (NIH) grants R01HD42974 to TJS and UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the NIH, and NIH Roadmap for Medical Research. The NIH had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Acknowledgments
We would like to thank the youth and their families who made this work possible, Yukari Takarae, Ph.D., and Joel Bish, Ph.D., for their contribution to the design of the study and initial work on this experimental paradigm, Heather Shapiro, B.A., for critical review and programming support, and Flora Tassone, Ph.D., for genetic analysis.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Antshel KM, AbdulSabur N, Roizen N, Fremont W, Kates WR. Sex differences in cognitive functioning in velocardiofacial syndrome (VCFS) Dev Neuropsychol. 2005;28(3):849–69. doi: 10.1207/s15326942dn2803_6. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Shprintzen R, Fremont W, Higgins AM, Faraone SV, Kates WR. Cognitive and psychiatric predictors to psychosis in velocardiofacial syndrome: a 3-year follow-up study. J Am Acad Child Adolesc Psychiatry. 2010;49(4):333–44. [PMC free article] [PubMed] [Google Scholar]
- Baker K, Baldeweg T, Sivagnanasundaram S, Scambler P, Skuse D. COMT Val108/158 Met modifies mismatch negativity and cognitive function in 22q11 deletion syndrome. Biol Psychiatry. 2005;58(1):23–31. doi: 10.1016/j.biopsych.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EW. 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry. 1999;46(7):882–91. doi: 10.1016/S0006-3223(99)00114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Jawad AF, Lynch DR, Sokol S, Kanes SJ, McDonald-McGinn DM, et al. Effects of a functional COMT polymorphism on prefrontal cognitive function in patients with 22q11.2 deletion syndrome. Am J Psychiatry. 2004;161(9):1700–2. doi: 10.1176/appi.ajp.161.9.1700. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Volavka J, Czobor P, Malhotra AK, Kennedy JL, Ni X, et al. Neurocognitive correlates of the COMT Val(158)Met polymorphism in chronic schizophrenia. Biol Psychiatry. 2002;52(7):701–7. doi: 10.1016/S0006-3223(02)01416-6. [DOI] [PubMed] [Google Scholar]
- Bish JP, Ferrante SM, McDonald-McGinn D, Zackai E, Simon TJ. Maladaptive conflict monitoring as evidence for executive dysfunction in children with chromosome 22q11.2 deletion syndrome. Dev Sci. 2005;8(1):36–43. doi: 10.1111/j.1467-7687.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevag B, Callicott JH, Das S, et al. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25(20):5038–45. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Yeung N, Ullsperger M, Carter C, Cohen JD. Conflict monitoring: computational and empirical studies. In: Posner MI, editor. Cognitive neuroscience of attention. New York: The Guilford Press; 2004. pp. 91–102. [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7(4):367–79. doi: 10.3758/CABN.7.4.367. [DOI] [PubMed] [Google Scholar]
- Chen J, Lipska BK, Halim N, Ma QD, Matsumoto M, Melhem S, et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am J Hum Genet. 2004;75(5):807–21. doi: 10.1086/425589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smedt B, Devriendt K, Fryns JP, Vogels A, Gewillig M, Swillen A. Intellectual abilities in a large sample of children with velo-cardio-facial syndrome: an update. J Intellect Disabil Res. 2007;51(Pt 9):666–70. doi: 10.1111/j.1365-2788.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- Egan MF, Goldberg TE, Kolachana BS, Callicott JH, Mazzanti CM, Straub RE, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci. 2001;98(12):6917–22. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B, Eriksen C. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept Psychophys. 1974;16(1):143–9. doi: 10.3758/BF03203267. [DOI] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. J Cogn Neurosci. 2002;14(3):340–7. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss B, Fossella J, Flombaum J, Posner M. The activation of attentional networks. Neuroimage. 2005;26(2):471–9. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Eliez S, Thompson T, Hinard C, Penniman L, Feinstein C, et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nat Neurosci. 2005;8(11):1500–2. doi: 10.1038/nn1572. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Hoeft F, Hinard C, Hallmayer JF, Stoecker JV, Antonarakis SE, et al. Abnormal cortical activation during response inhibition in 22q11.2 deletion syndrome. Hum Brain Mapp. 2007;28(6):533–42. doi: 10.1002/hbm.20405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Schaer M, Eliez S. Genes, brain development and psychiatric phenotypes in velo-cardio-facial syndrome. Dev Disabil Res Rev. 2008;14(1):59–68. doi: 10.1002/ddrr.9. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. Optimizing the use of information: strategic control of activation of responses. J Exp Psychol Gen. 1992;121(4):480–506. doi: 10.1037/0096-3445.121.4.480. [DOI] [PubMed] [Google Scholar]
- Joober R, Gauthier J, Lal S, Bloom D, Lalonde P, Rouleau G, et al. Catechol-O-methyltransferase Val-108/158-Met gene variants associated with performance on the Wisconsin Card Sorting Test. Arch Gen Psychiatry. 2002;59(7):662–3. doi: 10.1001/archpsyc.59.7.662. [DOI] [PubMed] [Google Scholar]
- Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11(6):402–16. doi: 10.1038/nrn2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Antshel KM, Abdulsabur N, Colgan D, Funke B, Fremont W, et al. A gender-moderated effect of a functional COMT polymorphism on prefrontal brain morphology and function in velo-cardio-facial syndrome (22q11.2 deletion syndrome) Am J Med Genet B Neuropsychiatr Genet. 2006;141B(3):274–80. doi: 10.1002/ajmg.b.30284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Krauss BR, Abdulsabur N, Colgan D, Antshel KM, Higgins AM, et al. The neural correlates of non-spatial working memory in velocardiofacial syndrome (22q11.2 deletion syndrome) Neuropsychologia. 2007;45(12):2863–73. doi: 10.1016/j.neuropsychologia.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konrad K, Neufang S, Thiel CM, Specht K, Hanisch C, Fan J, et al. Development of attentional networks: an fMRI study with children and adults. Neuroimage. 2005;28(2):429–39. doi: 10.1016/j.neuroimage.2005.06.065. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Shashi V, Berry PM, Kwapil TR. Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(1):27–36. doi: 10.1002/ajmg.b.30379. [DOI] [PubMed] [Google Scholar]
- Malhotra AK, Kestler LJ, Mazzanti C, Bates JA, Goldberg T, Goldman D. A functional polymorphism in the COMT gene and performance on a test of prefrontal cognition. Am J Psychiatry. 2002;159(4):652–4. doi: 10.1176/appi.ajp.159.4.652. [DOI] [PubMed] [Google Scholar]
- Mayr U, Awh E, Laurey P. Conflict adaptation effects in the absence of executive control. Nat Neurosci. 2003;6(5):450–2. doi: 10.1038/nn1051. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Owen MJ. Velo-cardio-facial syndrome: a model for understanding the genetics and pathogenesis of schizophrenia. Br J Psychiatry. 2001;179:397–402. doi: 10.1192/bjp.179.5.397. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, et al. Human catechol-o-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 2006;314:1930–3. doi: 10.1126/science.1131262. [DOI] [PubMed] [Google Scholar]
- Nestor PG, Kubicki M, Spencer KM, Niznikiewicz M, McCarley RW, Shenton ME. Attentional networks and cingulum bundle in chronic schizophrenia. Schizophr Res. 2007;90(1–3):308–15. doi: 10.1016/j.schres.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsdottir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch Dis Child. 2004;89(2):148–51. doi: 10.1136/adc.2003.026880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Q J Exp Psychol. 1980;32(1):3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Robin NH, Shprintzen RJ. Defining the clinical spectrum of deletion 22q11.2. J Pediatr. 2005;147(1):90–6. doi: 10.1016/j.jpeds.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Rockers K, Ousley O, Sutton T, Schoenberg E, Coleman K, Walker E, et al. Performance on the Modified Card Sorting Test and its relation to psychopathology in adolescents and young adults with 22q11.2 deletion syndrome. J Intellect Disabil Res. 2009;53(7):665–76. doi: 10.1111/j.1365-2788.2009.01178.x. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Fan J, McCandliss BD, Halparin JD, Gruber DB, Lercari LP, et al. Development of attentional networks in childhood. Neuropsychologia. 2004;42(8):1029–40. doi: 10.1016/j.neuropsychologia.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Shashi V, Keshavan MS, Howard TD, Berry MN, Basehore MJ, Lewandowski E, et al. Cognitive correlates of a functional COMT polymorphism in children with 22q11.2 deletion syndrome. Clin Genet. 2006;69(3):234–8. doi: 10.1111/j.1399-0004.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464(7289):763–7. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ. A new account of the neurocognitive foundations of impairments in space, time and number processing in children with chromosome 22q11.2 deletion syndrome. Dev Disabil Res Rev. 2010;14(1):52–8. doi: 10.1002/ddrr.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ, Bish JP, Bearden CE, Ding L, Ferrante S, Nguyen V, et al. A multilevel analysis of cognitive dysfunction and psychopathology associated with chromosome 22q11.2 deletion syndrome in children. Dev Psychopathol. 2005;17(3):753–84. doi: 10.1017/S0954579405050364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Blundell M, Anyane-Yeboa K, Karayiorgou M. Networks of attention in children with the 22q11 deletion syndrome. Dev Neuropsychol. 2004;26(2):611–26. doi: 10.1207/s15326942dn2602_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JM. School-based assessments and interventions for ADD students. Irvine: KC Publishing; 1992. [Google Scholar]
- Takarae Y, Schmidt L, Tassone F, Simon TJ. Catechol-O-methyltransferase polymorphism modulates cognitive control in children with chromosome 22q11.2 deletion syndrome. Cogn Affect Behav Neurosci. 2009;9(1):83–90. doi: 10.3758/CABN.9.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezenas Du Montcel S, Mendizabai H, Ayme S, Levy A, Philip N. Prevalence of 22q11 microdeletion. J Med Genet. 1996;33(8):719. doi: 10.1136/jmg.33.8.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend J, Ashby F. The stochastic modeling of elementary psychological processes. Cambridge: CUP Archive; 1983. [Google Scholar]
- Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60(2):141–51. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Urbanek C, Neuhaus A, Opgen-Rhein C, Strathmann S, Wieseke N, Schaub R, et al. Attention network test (ANT) reveals gender-specific alterations of executive function in schizophrenia. Psychiatry Res. 2009;168(2):102–9. doi: 10.1016/j.psychres.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Wang K, Fan J, Dong Y, Wang C, Lee T, Posner M. Selective impairment of attentional networks of orienting and executive control in schizophrenia. Schizophr Res. 2005;78(2–3):235–41. doi: 10.1016/j.schres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Woodin MF, Wang PP, Aleman D, McDonald-McGinn DM, Zackai EH, Moss EM. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genet Med. 2001;3(1):34–9. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]