Abstract
The α4 laminin subunit is a component of endothelial cell basement membranes. An antibody (2A3) against the α4 laminin G domain stains focal contact-like structures in transformed and primary microvascular endothelial cells (TrHBMECs and HMVECs, respectively), provided the latter cells are activated with growth factors. The 2A3 antibody staining colocalizes with that generated by αv and β3 integrin antibodies and, consistent with this localization, TrHBMECs and HMVECs adhere to the α4 laminin subunit G domain in an αvβ3-integrin–dependent manner. The αvβ3 integrin/2A3 antibody positively stained focal contacts are recognized by vinculin antibodies as well as by antibodies against plectin. Unusually, vimentin intermediate filaments, in addition to microfilament bundles, interact with many of the αvβ3 integrin-positive focal contacts. We have investigated the function of α4-laminin and αvβ3-integrin, which are at the core of these focal contacts, in cultured endothelial cells. Antibodies against these proteins inhibit branching morphogenesis of TrHBMECs and HMVECs in vitro, as well as their ability to repopulate in vitro wounds. Thus, we have characterized an endothelial cell matrix adhesion, which shows complex cytoskeletal interactions and whose assembly is regulated by growth factors. Our data indicate that this adhesion structure may play a role in angiogenesis.
INTRODUCTION
In cultured cells, the focal contact or focal adhesion is a region of close interaction between cells and the matrix on their substrate (Dogic et al., 1999). Focal contacts are dynamic structures that show motility even in stationary cells and are the conduits of signals from the matrix to the cytoplasm of the cell and vice versa (Howe et al., 1998; Smilenov et al., 1999; Zamir et al., 2000). Microfilament bundles associate indirectly with the cytoplasmic domains of matrix receptors of the integrin family that are enriched in focal contacts (Simon and Burridge, 1994; Dogic et al., 1999). The extracellular domains of integrins bind matrix elements such as fibronectin, vitronectin, and laminin in the connective tissue, thereby providing a structural link between the extracellular matrix and the actin-based cytoskeleton system of a cell (Hynes, 1992).
In fibroblasts at least two types of matrix adhesions have been identified (Zamir et al., 1999, 2000; Katz et al., 2000). These can be distinguished by their molecular composition and distribution. The classic focal contact tends to be located at the periphery of the cell and is enriched in such proteins as vinculin and paxillin. On the other hand, so-called fibrillar adhesions tend to be concentrated toward the center of the cells and contain little if any paxillin and vinculin, while being rich in tensin. Both the focal contact and fibrillar adhesion are associated with the microfilament cytoskeleton.
In addition to focal contacts and, possibly, fibrillar adhesions, certain epithelial cells utilize a structurally distinct matrix adhesive device called a hemidesmosome to interact with the connective tissue (Jones et al., 1998). Like a focal contact, each hemidesmosome contains an integrin, namely, the α6β4 heterodimer, and is involved in both adhesion and signal transduction (Mainiero et al., 1995; Jones et al., 1998). However, the hemidesmosome is quite different from the focal contact in that it interacts with the keratin cytoskeleton network and fails to show any obvious association with microfilament bundles (Simon and Burridge, 1994; Jones et al., 1998).
The hemidesmosome links epithelial cells to laminin in the basement membrane. Specifically, the α6β4 integrin of the hemidesmosome binds to the G-domain of the α3-subunit of the laminin-5 heterotrimer (Spinardi et al., 1993; Baker et al., 1996; Jones et al., 1998). Of all the laminin α-chains so far identified, the α3-subunit is structurally closest to the α4 laminin subunit in that they both contain a “truncated” N-terminal “short arm” domain, containing a single rod-like segment consisting of epidermal growth factor-like repeats. By comparison, the short arms of the α1, α2, and α5 laminin subunits contain three globular subdomains that are separated by rod-like segments (Ryan et al., 1994; Iivanainen et al., 1995; Frieser et al., 1997; Miner et al., 1997; Niimi et al., 1997; Aumailley and Smyth, 1998).
Whereas the α3 laminin subunit is synthesized primarily by epithelial cells, the α4 laminin subunit is expressed in endothelium, bone marrow, adipocytes, lung fibroblasts, heart, lung, and skeletal muscle, smooth muscle, and dermis (Iivanainen et al., 1995; Liu and Mayne, 1996; Richards et al., 1996; Frieser et al., 1997; Miner et al., 1997; Niimi et al., 1997; Pierce et al., 1998; Gu et al., 1999). Furthermore, the α4 laminin subunit is a component of laminin-8 and 9, heterotrimeric matrix molecules composed of α4-, β1-, and γ1-chains and α4-, β2-, and γ1-chains, respectively (Aumailley and Smyth, 1998; Kortesmaa et al., 2000). The goal of this study was to analyze the organization of the α4 laminin subunit in endothelial extracellular matrix using a new monoclonal antibody we developed. During the course of our studies, we have identified a matrix adhesion site that, like the hemidesmosome, associates with the intermediate filament cytoskeleton. However, this site of adhesion also possesses many of the characteristics of a focal contact in that it is enriched in vinculin, associates with the microfilament system, and is found predominantly at the periphery of endothelial cells. We provide evidence for the role of this matrix adhesion in dynamic processes, including those involved in angiogenesis.
MATERIALS AND METHODS
Cell Lines
Human microvascular endothelial cells (HMVEC) were purchased from Cascade Biologics (Portland, OR) and were maintained in MED131 supplemented with microvascular growth supplement. In some experiments, HMVECs were maintained in MED131 in the absence of supplements for at least 24 h before use. To stimulate the latter cells, basic fibroblast growth factor (bFGF, obtained from Life Technologies-BRL, Gaithersburg, MD), was added directly to the MED131 culture medium at a concentration of 5 ng/ml for at least 24 h. Immortalized human bone marrow endothelial cells (TrHBMEC) (Schweitzer et al., 1997) were maintained in DMEM containing a final concentration of 2 mM l-glutamine, 10% fetal bovine serum, and 1× RPMI vitamins. These were obtained from Dr. Denise Paulin (Universite Paris VII and Institut Pasteur, Paris, France). SCC12 cells were maintained in culture as detailed elsewhere (Goldfinger et al., 1998).
Antibodies and Actin Probe
The rabbit antisera against αv-integrin (AB1930), β3-integrin (AB1932), and the LM609 mouse monoclonal antibody against the αvβ3-heterodimer (MAB1976Z) were purchased from Chemicon International, Inc. (Temecula, CA). The monoclonal β1-blocking antibody P4C10 was obtained from Life Technologies (Gaithersburg, MD) (Carter et al., 1990). RG13 antibody against the α3 laminin subunit was described previously (Gonzales et al., 1999). Mouse monoclonal antibodies specific for plectin (clone 7A8), vimentin (clone V9), and vinculin (clone hVIN-1) were purchased from Sigma Chemical Co. (St. Louis, MO). The α4 laminin subunit rabbit antiserum was the kind gift of Dr. Jeffrey Miner (Washington University, St. Louis, MO).
Rhodamine-conjugated phalloidin was obtained from Molecular Probes (Eugene, OR). Secondary antibodies conjugated to fluorescein, rhodamine, indodicarbocyanine (Cy5), and various sized gold particles were purchased from Jackson ImmunoResearch Labs Inc. (West Grove, PA).
Extracellular Matrix Proteins and Production of Recombinant α4-Protein
Human fibronectin and mouse laminin-1 were purchased from Collaborative Research (Bedford, MA) and Life Technologies-BRL, respectively. An 833-base pair cDNA fragment encoding amino acid residues 918–1213 of the G1/2 domains of the α4 laminin subunit was generated from TrHBMEC cDNA and subcloned into the pBAD TOPO TA expression vector (Invitrogen, Inc., San Diego, CA). This vector was then transfected into the Escherichia coli strain LMG194 (Guzman et al., 1995). The His-tagged α4 laminin protein fragment protein was induced in the cells by the addition of arabinose, and the fragment was purified using column chromatography (Novagen, Inc., Madison, WI). The purity of the recombinant polypeptide was assessed by visualizing protein samples by SDS-PAGE and, after transfer to nitrocellulose, using a His probe (Pierce, Rockford, IL).
Production of Monoclonal Antibody 2A3
To prepare an α4 laminin subunit antibody, the recombinant α4 laminin fragment was used to immunize BALB/C mice. Spleen cells of immunized mice were fused to SP2 hybridoma cells according to standard procedures (Harlow and Lane, 1988). Populations of fused cells producing antibody against the α4 laminin fragment were identified by Western blotting and then cloned three times by limiting cell dilution. One of the cloned cell lines, termed 2A3, produced an immunoglobulin (Ig) M class antibody. Desmos, Inc. (San Diego, CA) prepared a 2A3 ascites fluid.
Endothelial Cell Adhesion Assays
Approximately 2 × 105 TrHBMECs or 5 × 104 HMVECs were plated onto uncoated or specific protein-coated wells of a 96-well plate (Sarstedt, Newton, NC). After 90 or 120 min at 37°C, the cells were washed extensively with phosphate-buffered saline (PBS) to remove nonadhering cells, and then adherent cells were fixed in 3.7% formaldehyde in PBS for 15 min at room temperature. The fixed cells were incubated at room temperature with 0.5% crystal violet for 15 min and then solubilized with 1% SDS. A570 was measured with a Vmax plate reader (Molecular Devices, Menlo Park, CA).
Immunofluorescence
Endothelial cells were grown on glass coverslips and were fixed in 3.7% formaldehyde in PBS for 5 min and extracted in 0.5% Triton X-100 in PBS for 10 min at 4°C to allow subsequent antibody penetration. After extensive washing in PBS, the fixed and extracted cells were incubated with primary antibodies, diluted in PBS, at 37°C in a humid chamber for at least 1 h, washed three times in PBS, and then incubated with the appropriate mixture of fluorochrome-conjugated secondary antibodies for an additional 1 h at 37°C. Rhodamine-conjugated phalloidin was diluted in PBS and was incubated with the fixed and extracted cells at 37°C for 1 h. Stained specimens were viewed using an LSM510 laser scanning confocal microscope (Zeiss Inc., Thornwood, NY).
Immunoelectron Microscopy
Endothelial cells, maintained on glass coverslips, were fixed for 15 min in 0.5% glutaraldehyde in PBS, extracted in 0.5% Triton X-100 in PBS for 30 min at 4°C, washed in PBS, and then incubated for 15 min in 1 μg/ml NaBH4 in PBS. A mixture of primary antibody was overlaid on the cells, which were then incubated overnight at 4°C. After thorough washing, the cells on coverslips were incubated for 6 h at room temperature in a mixture of gold-conjugated secondary antibodies. After washing, the cells were prepared for electron microscopy as described elsewhere (Riddelle et al., 1992). Ultrathin sections were viewed in a 100CX or 1220 JEOL electron microscope at 60 kV (JEOL USA, Peabody, MA).
Angiogenesis Assay
Matrigel was purchased from Collaborative Biomedical Products (Bedford, MA) and coated as a thin gel onto the surface of the wells of a 24-well tissue culture plate (Corning, Corning, NY) according to the instructions of the supplier. Coated dishes were incubated at 37°C for 30 min before use. Approximately 6.25 × 104 cells were plated on top of the Matrigel in each well. The cells were incubated at 37°C for 18 h, fixed in 2% glutaraldehyde in PBS, and then photographed.
In Vitro Scrape Wound/Migration Assay
Endothelial cells were grown to confluence in tissue culture-treated six-well plates (Corning) and then wounded by scraping with a pipette tip in a single stripe. The culture medium was then removed and replaced with fresh medium. The wounded cultures were incubated at 37°C for 18 h, fixed in 2% glutaraldehyde in PBS, and then photographed.
Protein Preparations, SDS-PAGE, and Western Immunoblotting
Confluent cell cultures were solubilized in sample buffer consisting of 8 M urea, 1% SDS in 10 mM Tris-HCL, pH 6.8, and 15% β-mercaptoethanol. In the case of whole cell extract preparations, DNA was sheared by sonication using a 50-W Ultrasonic Processor (Vibracell Sonics and Materials, Inc., Danbury, CT) before SDS-PAGE. Endothelial cell matrix was prepared according to the method of Gospodarowicz (1984) with modifications detailed by Langhofer et al. (1993). The matrix proteins were collected from the culture dish by solubilization in the urea-SDS sample buffer. Proteins were separated by SDS-PAGE, transferred to nitrocellulose, and processed for immunoblotting as previously described (Laemmli 1970; Zackroff et al., 1984; Harlow and Lane, 1988; Klatte et al., 1989).
RESULTS
Preparation of an α4 Laminin Subunit Antibody (2A3)
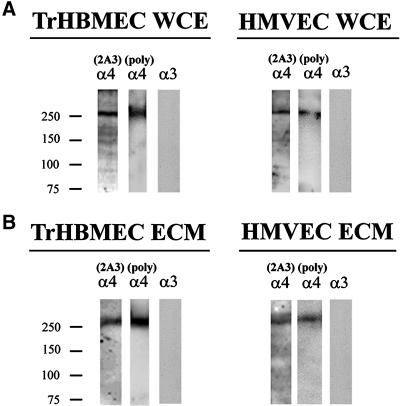
In this study we first determined the localization of the α4 laminin subunit in cultured endothelial cells. To do so, we prepared a monoclonal antibody probe (2A3) against a recombinant G domain fragment (amino acid residues 918–1213) of the α4 laminin subunit. This fragment includes both the G1 and G2 subdomains of the α4-laminin. Antibody 2A3 recognizes a 250-kDa protein in whole cell extracts and extracellular matrix derived from both transformed (TrHBMEC) and primary (HMVEC) endothelial cells (Figure 1). A similar 250-kDa protein in these same preparations is recognized by a rabbit antiserum against the α4 laminin subunit (Figure 1) (Miner et al., 1997; Pierce et al., 1998). In addition, the molecular weight of the 2A3 reactive protein is consistent with the size of the α4 laminin subunit reported by other groups (Frieser et al., 1997; Gu et al., 1999). In contrast, antibodies against the α3 laminin subunit show no reactivity with these protein preparations (Figure 1).
Figure 1.
An antibody termed 2A3, against the G-domain of the α4 laminin subunit, was characterized by Western immunoblotting using SDS-PAGE preparations of whole cell extracts (WCE) (A) and matrix (ECM) (B) of TrHBMECs and HMVECs. In the Western blots of the whole cell extract and the matrix preparations, 2A3 antibody primarily reacts with a protein of an approximate molecular mass of 250 kDa. A polyclonal serum against the α4 laminin subunit recognizes a similar sized polypeptide in these preparations. A monoclonal antibody (RG13) against the α3 laminin subunit fails to recognize any polypeptides in either the extract or matrix of either cell type.
Immunofluorescence Analyses of Endothelial Cells
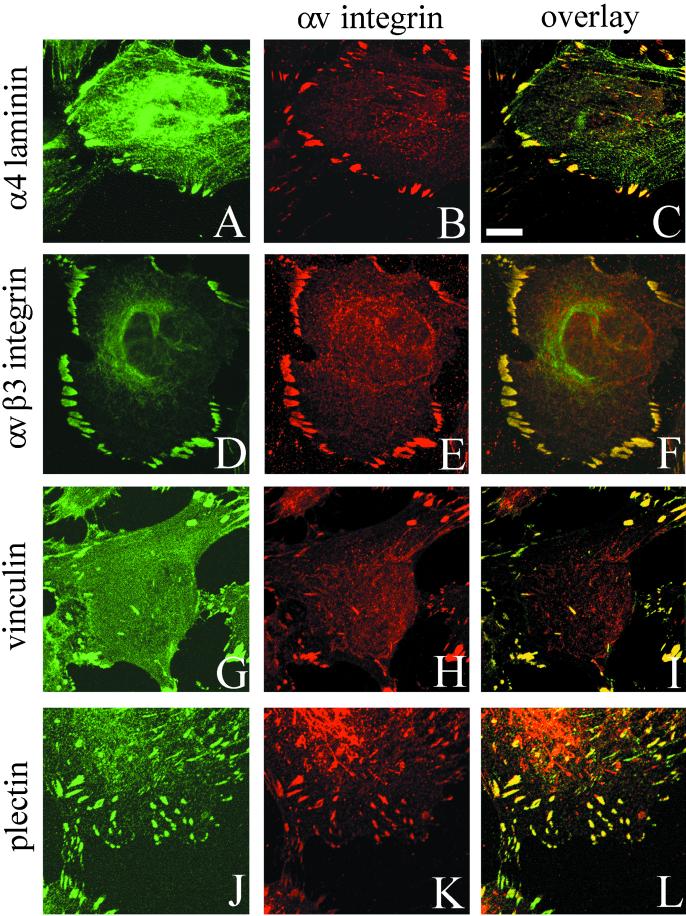
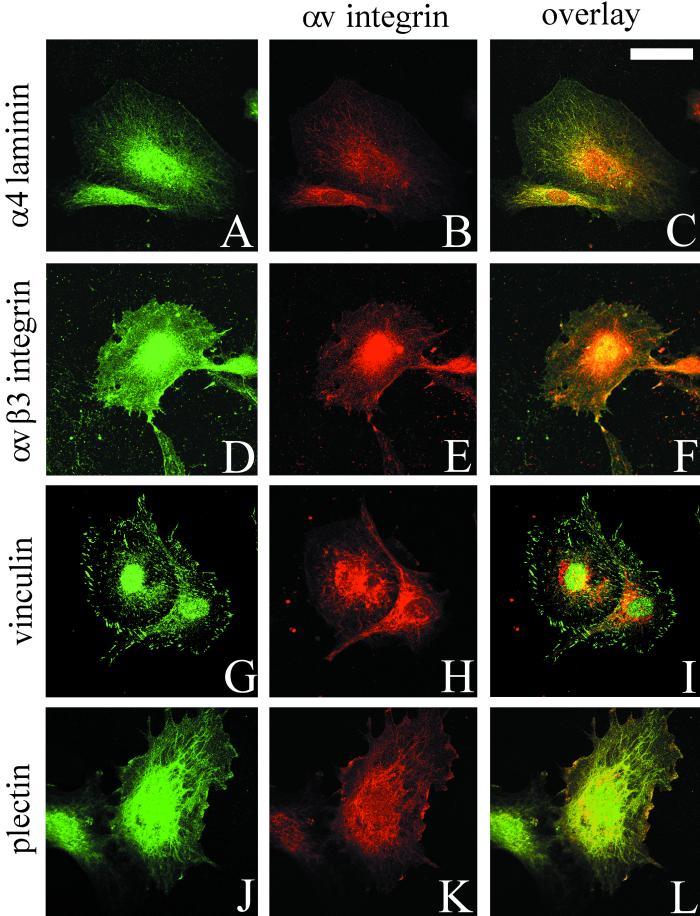
The distribution of the α4 laminin subunit in subconfluent TrHBMEC cultures was determined by confocal immunofluorescence microscopy. Antibody 2A3 stains in a focal contact-like pattern along the substratum-attached surface of the cells and codistributes with staining generated by a polyclonal antiserum against αv-integrin, a major cell surface matrix receptor expressed by endothelial cells in vitro and in vivo (Figure 2, A–C) (Brooks et al., 1994–1995; Varner, 1997). In TrHBMECs, αv integrin antibody colocalizes with staining generated by probes against the αvβ3 integrin complex, the focal contact protein vinculin and plectin, and a cytoskeleton cross-linking protein (Wiche et al., 1982; Wiche 1998) (Figure 2, D–F, G–I, and J–L, respectively). Thus, the vinculin-positive, plectin-positive focal contacts in TrHBMECs are enriched in αvβ3 integrin heterodimers.
Figure 2.
TrHBMECs were processed for double-label immunofluorescence using a combination of antibodies against α4 laminin (2A3) and αv integrin subunit (A and B), the αvβ3 integrin complex and the αv-subunit (D and E), vinculin and the αv integrin subunit (G and H), and plectin and the αv integrin subunit (J and K). Cells were viewed by confocal microscopy. The focal plane is close to the substratum-attached surface of the cells. C, F, I, and L are overlays of the fluorescence images. Bar in C, 10 μm.
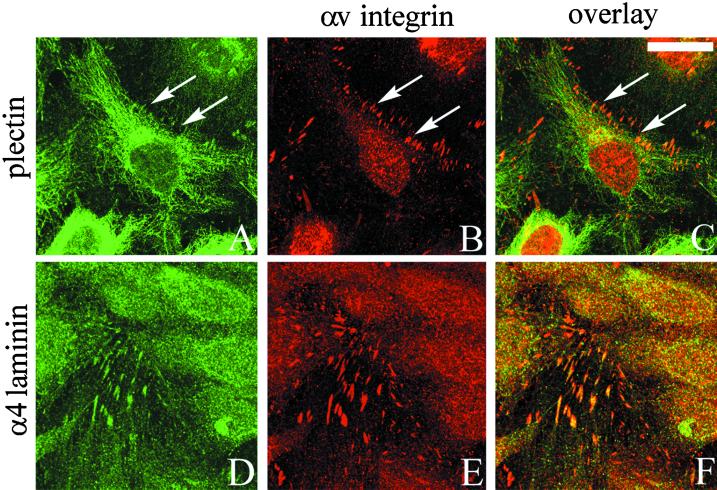
If the TrHBMECs are grown to confluence, such that they become contact inhibited, then little plectin is found along sites of cell-substrate interaction (Figure 3A). Indeed, plectin shows primarily a filamentous staining pattern throughout the cytoplasm of the cells and does not localize extensively with αv-integrin (Figure 3, A–C). In contrast, antibodies against αv-integrin and the α4 laminin subunit, as well as vinculin and the αvβ3 integrin complex (LM609) (Gonzales and Jones, unpublished observations), codistribute in a focal contact-staining pattern in populations of confluent TrHBMECs (Figure 3, D–F).
Figure 3.
TrHBMECs were maintained on glass coverslips until the cells reached confluence. Approximately 24 h later the cells were processed for double-label immunofluorescence microscopy using an antibody against plectin (A) or 2A3 antibody against the α4 laminin subunit (D) in combination with an antiserum against αv-integrin (B and E). Cells were viewed by confocal microscopy. The focal plane is close to the substratum-attached surface of the cells. C and F show the overlays of the fluorescence images. Arrows in A–C indicate areas of focal contacts stained by αv-antibodies in B that are not recognized by plectin antibodies in A. Rather, the plectin antibodies in A generate a predominantly filamentous staining pattern. Bar in C, 20 μm.
Little, if any, basal surface staining was detected in serum-starved HMVECs processed for immunocytochemistry using antibody 2A3, an antiserum against αv-integrin, antibodies against the αvβ3 integrin heterodimer, or antibodies against plectin (Figure 4, A, B, D, E, H, J, and K). The plectin antibody shows filamentous staining in the perinuclear cytoplasm of these cells (Figure 4J). In contrast, antibodies to vinculin generate staining of small, but clearly defined, focal contacts (Figure 4G). However, when serum-starved HMVECs were stimulated with 5 ng/ml bFGF for 24 h before processing, 2A3, αvβ3-integrin, and vinculin antibodies show obvious colocalization in focal contacts that are also stained by αv-antibodies (Figure 5, A–C, D–F, and G-I, respectively). Furthermore, plectin codistributes with αv-integrin in focal contacts in the growth factor-stimulated HMVECs (Figure 5, J–L).
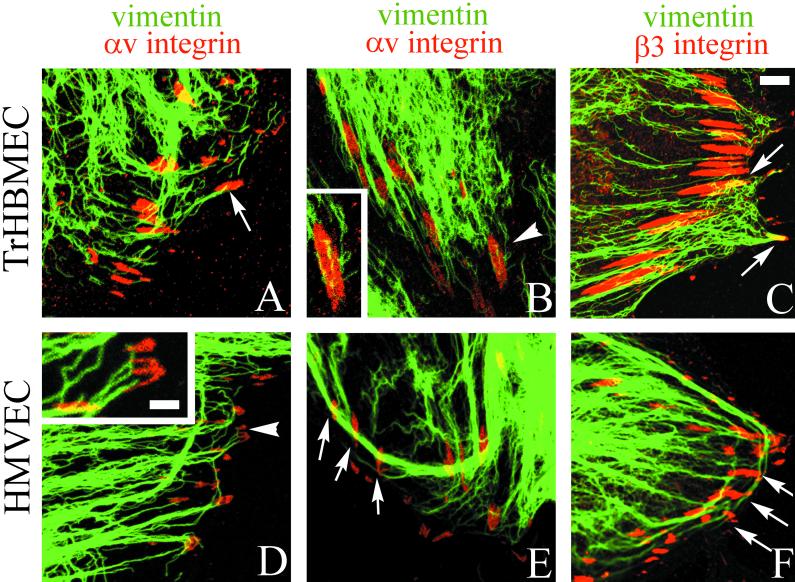
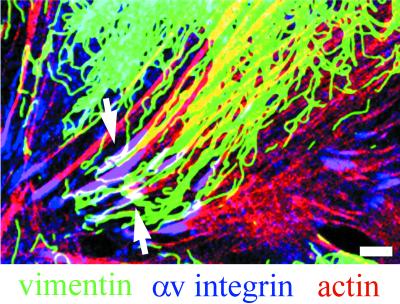
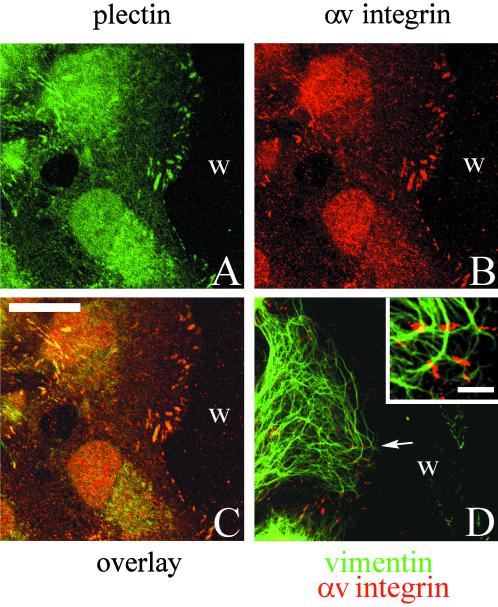
Because plectin is known to link the intermediate filament cytoskeleton to the cell surface, we next looked at the organization of the vimentin cytoskeleton systems in subconfluent TrHBMECs and in HMVECs that had been stimulated with bFGF. Vimentin bundles associate with a large number of αv-integrin and β3 integrin antibody-stained focal contact structures in both cell types (Figure 6). Microfilament bundles also terminate on these vimentin-associated focal contacts as shown in the triple-label image where colocalized proteins appear white (Figure 7, only an image of TrHBMECs is shown).
Figure 6.
TrHBMECs (A–C) and HMVECs (D–F) were processed for double-label immunofluorescence using a monoclonal antibody against vimentin (green) in combination either with an antiserum against the αv integrin subunit (red) (A, B, D, and E) or an antiserum against β3 integrin (red) (C and F). Overlays of the staining patterns are shown. In TrHBMECs some vimentin filament bundles can be seen to terminate at αv-integrin–containing focal contacts (arrow in A). The focal contact indicated by the arrowhead in B is shown at higher magnification in the inset. Vimentin filaments appear to be wrapped around the focal contact. In HMVECs vimentin bundles are also seen in association with αv-integrin–containing focal contacts (arrowhead and arrows in D and E). The inset in D shows a higher power view of one focal contact (arrowhead) in the HMVEC cell shown in D. Note that three individual vimentin filament bundles appear to terminate at the site of the focal contact. Vimentin filaments and bundles also associate with focal contacts stained by β3 integrin antibodies in both TrHBMECs and HMVECs (arrows in C and F). Bar in C, 5 μm; bar in D (insets), 1 μm.
Figure 7.
TrHBMECs were processed for triple-label fluorescence microscopy using antibody probes against vimentin (green) and αv-integrin (blue), together with rhodamine-conjugated phalloidin (red). The overlay of the three labels is shown. White color indicates an overlap in the staining patterns (arrows). Bar, 5 μm.
There seem to be three distinct modes of interaction of vimentin intermediate filaments with the αv integrin subunit antibody-stained focal contacts. In Figure 6A and the inset in Figure 6D, vimentin bundles appear to terminate at the site of focal contacts. In Figure 6B, vimentin bundles often appear to wrap around or loop close to the αv antibody-stained focal contacts. Finally, in Figure 6E a relatively thick vimentin bundle appears to loop past three different focal contacts. Vimentin bundles and filaments associate with focal contacts stained by a β3 integrin antiserum in a similar manner (Figure 6, C and F). Table 1 shows quantification of the number of αv and β3 integrin antibody-stained focal contacts that show vimentin association in both TrHBMECs and HMVECs as determined by double-labeling studies. In both cell types, >60% of the αv and β3 integrin-positive plaques show interaction with the intermediate filament cytoskeleton.
Table 1.
Quantification of vimentin/focal contact interactions
| Cell line | TrHBMECsa | HMVECsa |
|---|---|---|
| % of β3-integrin–positive focal contacts associated with vimentin | 74.4% | 67.6% |
| Number of focal contacts counted in 10 cells | 558 | 421 |
| % of αv-integrin–positive focal contacts associated with vimentin | 73.5% | 63.3% |
| Number of focal contacts counted in 10 cells | 245 | 308 |
Cells were costained with vimentin and integrin antibody probes.
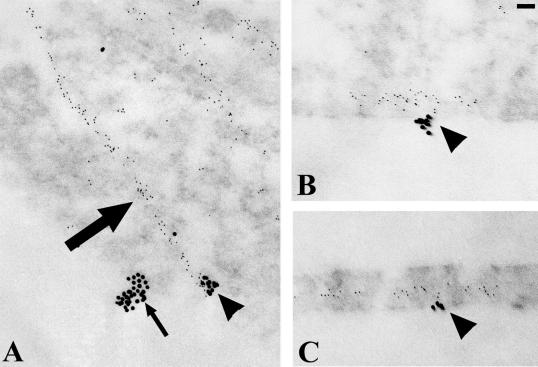
To provide further evidence that the vimentin cytoskeleton associates with focal contact proteins in endothelial cells, TrHBMECs were processed for double-label immunogold localization using antibody preparations against β3-integrin and vimentin. Sections were prepared both en face and perpendicular to the growth substratum (Figure 8A). In en face sections the β3 integrin subunit, visualized with 18-nm gold particles, occurs in clusters along the substratum-attached surface of the cells (Figure 8A). A filament bundle, which is stained by vimentin antibodies and visualized with 6-nm gold particles, shows association with one of two β3 integrin aggregates in the cytoplasm (Figure 8A). This is consistent with the fluorescence observations shown in Figure 6 (see also Table 1). In the cross-sections of the cells shown in Figure 8, B and C, 6-nm gold particles are associated with clusters of 18-nm gold particles concentrated along the substratum-attached surface of the cells.
Figure 8.
TrHBMECs were processed for double-label immunogold localization using a mixture of mouse monoclonal antibodies against vimentin and a rabbit antiserum against β3-integrin. The binding of these probes was visualized with 6-nm gold-conjugated goat anti-mouse IgG and 18-nm gold-conjugated goat anti-rabbit IgG. Sections of the cells were prepared either parallel (A) or perpendicular (B and C) to their substratum-attached surface. In A, linear arrays of 6-nm gold particles arise from a cluster of 18-nm gold particles. A second cluster of 18-nm gold particles shows no association with the smaller sized gold particles in A. In B and C, strings of 6-nm particles interact with 18-nm gold clusters along the basal surface of the cells. Bar, 60 nm.
Endothelial Cell Adhesion Assays
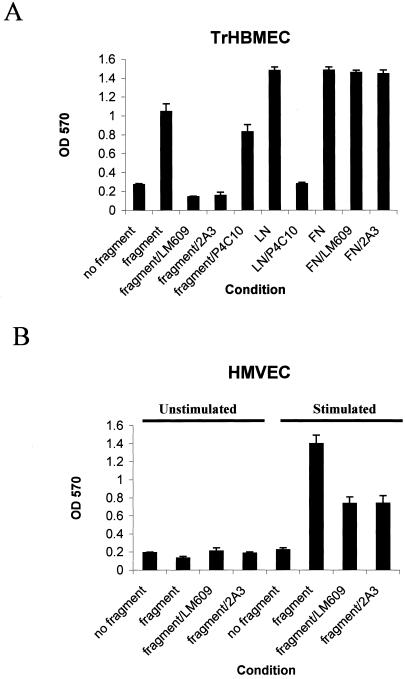
Because αvβ3-integrin colocalizes with α4-laminin in our endothelial cell populations, we next assessed whether endothelial cells bind the α4 laminin subunit in an αvβ3-integrin–dependent manner. To do so, TrHBMECs and HMVECs were plated in uncoated wells, in wells coated with the recombinant α4 laminin G1/G2 fragment, in fibronectin-coated wells, or in laminin-1–coated wells of culture plates. The proportion of attached cells was determined after either 90 or 120 min of incubation (Figure 9). In the case of HMVECs, the cells were plated in the presence or absence of growth factors. TrHBMECs adhere much better to a surface coated with the α4 laminin fragment (Figure 9A). Attachment of TrHBMECs to the α4 laminin subunit was significantly inhibited by the αvβ3 integrin-blocking antibody LM609 (25 μg/ml) and by antibody 2A3 (1:40 dilution) (Figure 9A). To ensure that the LM609 and 2A3 antibodies were specifically inhibiting cell adhesion to the α4 laminin fragment, we determined their ability to perturb TrHBMEC attachment to fibronectin. TrHBMEC adhesion to fibronectin is not inhibited by either LM609 (Babic et al., 1999) or 2A3 antibodies (Figure 9A). TrHBMEC adhesion to the α4-laminin–coated surface is decreased to a small degree by the β1-integrin-inhibitory antibody P4C10 (at 25 μg/ml) (Figure 9A). To confirm that P4C10 at this concentration inhibits β1-integrin in TrHBMECs, we plated TrHBMECs onto laminin-1–coated surfaces in the presence of P4C10 (Figure 9A). P4C10 antibody efficiently inhibits TrHBMEC adhesion to laminin-1.
Figure 9.
The TrHBMECs and HMVECs attach to a laminin α4-fragment in an αvβ3-integrin–dependent manner. (A) TrHBMECs were plated in a 96-well plate in uncoated wells, wells coated with the α4 laminin subunit fragment, laminin-1 (LN), or fibronectin (FN) in the presence or absence of antibody LM609 against αvβ3, antibody 2A3 against the α4 laminin subunit, P4C10 against the β1-integrin or control IgG. TrHBMECs attach efficiently to the fragment, laminin-1, and to fibronectin within 90 min after plating. Antibodies 2A3 and LM609 inhibit cell attachment to the α4 laminin fragment, whereas the same antibodies do not inhibit cell attachment to fibronectin. P4C10 has a minor inhibitory effect on attachment of TrHBMECs to the α4 chain fragment, whereas the same antibody significantly inhibits attachment to laminin-1. (B) HMVECs were plated onto uncoated wells or wells coated with the recombinant fragment in the presence or absence of growth factors. The attachment of cells was assayed after 120 min. Attachment of HMVECs to the α4 laminin fragment is stimulated by growth factors but can be inhibited by antibody 2A3 and LM609.
HMVECs show poor adhesion to uncoated and the α4 laminin fragment-coated surfaces after being maintained in culture in the absence of growth factors for 24 h before introduction into the adhesion assay (Figure 9B). To test the impact of growth factors on attachment of HMVECs to the α4 laminin fragment, HMVECs were growth factor stimulated for 24 h before the adhesion assay. The growth factor-stimulated cells show a significant increase in their capacity to attach to the α4 laminin fragment compared with unstimulated HMVECs. Furthermore, antibodies LM609 and 2A3 inhibit the attachment of stimulated HMVECs to the α4 laminin fragment (Figure 9B).
As a control for these studies, we also assessed epithelial cell attachment to the α4 laminin fragment. SCC12 keratinocytes show no specific adherence to the α4 laminin fragment because they adhere similarly to wells coated with the α4 laminin fragment as to uncoated tissue culture plastic within 90 min after plating (Gonzales and Jones, unpublished observations). Moreover, TrHBMECs show the same adherence to wells coated with control His-tagged recombinant proteins as they do to uncoated wells (Gonzales and Jones, unpublished observations).
Angiogenesis Assays
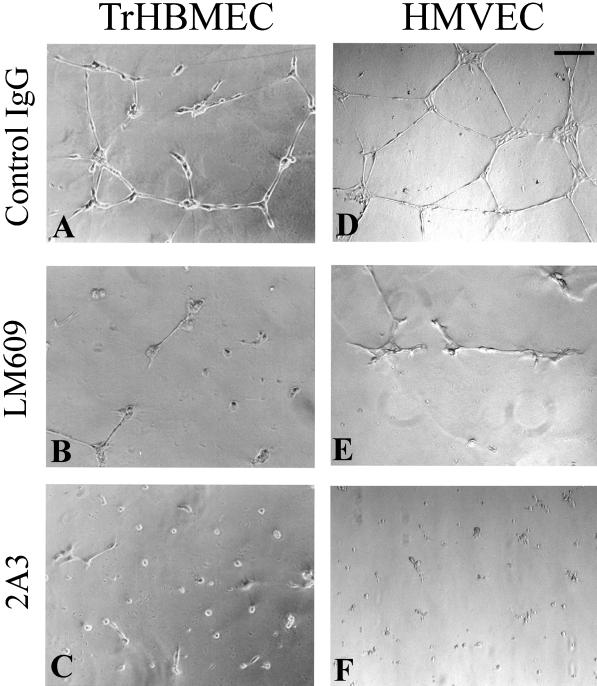
Because αvβ3-integrin is known to play an important role in angiogenesis (Brooks et al., 1994a, 1995; Ruoslahti and Engvall, 1997) and because we have observed a colocalization of the α4 laminin subunit and the αv-integrin in our cultured cell populations, we analyzed the possible function of the α4 laminin subunit in certain aspects of angiogenesis such as branching morphogenesis and cell migration of endothelial cells (Stromblad and Cheresh, 1996). We first used the Matrigel morphogenesis assay (Grant et al., 1989). TrHBMECs and HMVECs, the latter cells being stimulated with growth factors for 24 h, were plated onto Matrigel. After 18 h of incubation, even when cultured in the presence of control IgG, both cell types organize into extensive tubular arrays (Figure 10, A and D). However, when the endothelial cells are plated onto Matrigel in the presence of either antibody LM609 against the αvβ3 integrin complex or 2A3 antibody against the α4 laminin subunit, the formation of tubular arrays is inhibited and, in the case of cells treated with 2A3 antibody, cells appear as spheroid aggregates (Figure 10, B and E and C and F, respectively).
Figure 10.
TrHBMECs (A–C) and HMVECs (D–F) were plated onto Matrigel, and after 18 h were photographed. In A and D the cells were maintained in the presence of control IgG, and both cell types show assembly into tube-like structures. This morphogenesis is inhibited by LM609 antibodies against αvβ3 (B and E) and antibody 2A3 against the α4 laminin chain (C and F). Bar in D, 100 μm.
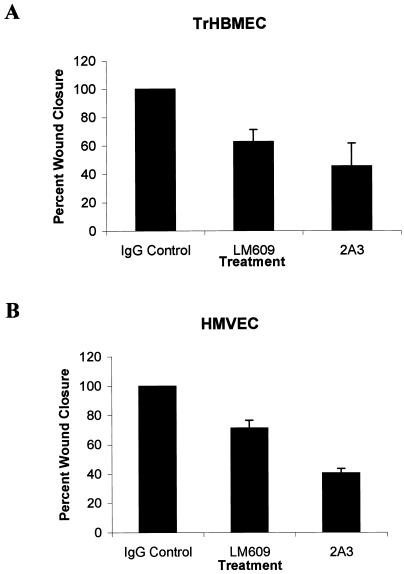
To test the role of the α4 laminin subunit in endothelial cell motility, TrHBMECs and HMVECs were first grown to confluence. After in vitro “wounding” of the cell monolayers, the cell cultures were allowed to “heal” in the presence of various antibodies or control IgG (Figure 11). The cells incubated in control IgG migrate to cover the wound site within 18 h (Figure 11, A and D). In contrast, in wounded cultures, treated with either antibody 2A3 or antibody LM609 against αvβ3-integrin, wound closure is incomplete after the same time interval (Figures 11, B, C, E, and F, and 12).
Figure 11.
Confluent monolayers of TrHBMECs (A–C) and HMVECs (D–F) were wounded by scraping with a pipette-man tip. The cultures were allowed to recover for 18 h in the presence of control IgG (A and D), antibody LM609 against αvβ3-integrin (B and E), and antibody 2A3 against the α4 laminin subunit (C and F). The cells in A and D have completely covered the wound site, whereas in B, C, E, and F the wound sites (w) are incompletely closed (as indicated by the line). Bar in D, 100 μm.
These wound-healing studies suggest that the matrix adhesion structure composed of a plectin/αvβ3-integrin/α4-laminin complex may be involved in migration. To assess this possibility we prepared wounded TrHBMEC cultures for double-label immunofluorescence at a time when cells are actively migrating into the wound site (at ∼4 h postwounding). The αv integrin subunit and plectin, as well as α4-laminin (Gonzales and Jones, unpublished observations), appear to be concentrated in focal contacts at the leading edge of cells as they migrate over the wound site (Figure 13, A–C). In addition, vimentin intermediate filaments are associated with these focal contact-like structures (Figure 13, D and inset).
Figure 13.
A confluent monolayer of TrHBMECs was wounded by scraping with a pipette-man tip. The culture was allowed to recover for 4 h, at which time cells had begun to migrate into the wound area. The preparation was then processed for double-label immunofluorescence microscopy using antibodies against plectin (A) together with an antiserum against αv-integrin (B). Cells were viewed by confocal microscopy. The focal plane is close to the substratum-attached surface of the cells. C is the overlay of the two fluorescence images. Preparations were also made of wounded cultures for double-label immunofluorescence using the antiserum against the αv-integrin (red) combined with a monoclonal vimentin antibody (green). The overlay of the two images is shown in D. Inset in D shows a higher power view of several αv-integrin positively stained focal contacts with which vimentin interacts in a cell that has migrated into the wound site (w). Bar in C, 20 μm; bar in D, inset, 3 μm.
DISCUSSION
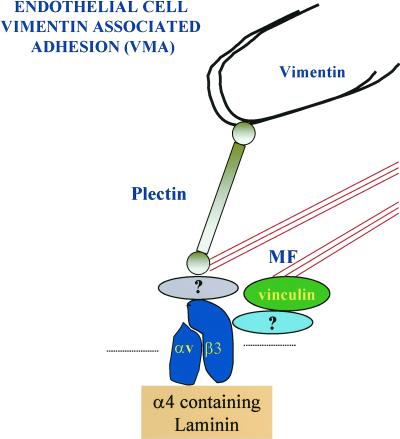
An antibody that we have prepared against the α4 laminin subunit antibody binds focal contact-like structures in transformed and nontransformed endothelial cells in vitro. These focal contacts are recognized also by antibodies against the αvβ3 integrin heterodimer and vinculin. In addition, we detected the cytoskeleton cross-linking protein plectin as a cytoplasmic constituent of the structures, and we have demonstrated that they constitute sites at the basal side of the cells at which both microfilaments and, in many instances, vimentin intermediate filaments appear to interact (Figure 14). For the latter reason we suggest the term vimentin-associated matrix adhesions (VMAs) for these adhesion sites. These may be comparable to the vimentin-associated focal contacts demonstrated by Bershadsky et al. (1987) in quail embryo fibroblasts. We assume that plectin mediates the interaction of vimentin with these focal contact-like devices, based on its known vimentin-binding properties, its localization, and its function in linking other types of intermediate filaments to the cell surface (Wiche et al., 1982; Steinböck and Wiche, 1999). Plectin, via its actin-binding domain, may also facilitate microfilament-cell surface binding at the basal surface of endothelial cells (Elliot et al., 1997).
Figure 14.
This diagram shows the molecular components of the VMA we have begun to characterize. The αvβ3-integrin associates with a laminin subunit at the core of the structure. We speculate that vimentin-type intermediate filaments interact with the VMA via an association with plectin (Wiche et al., 1982; Steinböck et al., 2000). Because plectin possesses an actin-binding motif, plectin, together with focal contact proteins such as vinculin, may be involved in mediating microfilament (MF) bundle-cell surface association (Elliot et al., 1997). The protein that links vinculin to the αvβ3-integrin may be α-actinin (Simon and Burridge, 1994), whereas the identity of the protein that links plectin to αvβ3 is yet to be determined.
The VMA bears some similarities in its molecular makeup to classic or type I hemidesmosomes of stratified squamous epithelial cells and type II hemidesmosomes present in some simple epithelial cell types (Hieda et al., 1992; Jones et al., 1998). Both type I and type II hemidesmosomes and the VMA contain an integrin (the α6β4 integrin heterodimer in epithelial cells and the αvβ3-integrin in endothelial cells), a truncated laminin isoform (laminin-5 and an α4-containing laminin isoform), and a plectin or plectin-related molecule (HD1) through which they appear to associate with intermediate filaments (Hieda et al., 1992; Jones et al., 1998). In this regard, Homan et al. (1998) have also described a hemidesmosome-like structure in cultured endothelial cells in which plectin associates with the α6β4-integrin along sites of cell-substrate interaction. However, the assembly of this structure was observed only in endothelial cells in which β4 integrin expression was induced artificially by molecular means (Homan et al., 1998). Our results reveal that plectin is found in association with matrix connectors in both primary and transformed endothelial cells in tissue culture, without any genetic modification of the cells and under conditions where we have been unable to detect any expression of the β4 integrin subunit (Gonzales and Jones, unpublished observations).
Despite the similarities, there are also structural and functional differences between hemidesmosomes and the VMAs of endothelial cells. Whereas hemidesmosomes in epithelial cells associate exclusively with the keratin bundle networks of epithelial cells, the VMAs interact with a different type of intermediate filament protein as well as the microfilament cytoskeleton network (Figure 14). Moreover, whereas the hemidesmosome is considered to provide a stable anchorage point, equivalent to a spot weld for nonmigrating epithelial cells, the VMA in endothelial cells is assembled by actively migrating cells and may be necessary to support motility. Functionally, therefore, the VMA may be more closely related to the focal contact than the hemidesmosome. Indeed, the VMA may be one of a growing number of members of a cell-matrix adhesion site family that includes the classic focal contact, enriched in vinculin, α-actinin, paxillin, and focal adhesion kinase, and fibrillar adhesions that contain tensin but little or no vinculin and paxillin (Katz et al., 2000).
In the case of the primary endothelial cells, our data indicate that the assembly of the VMA is induced by growth factors. It has already been shown that surface expression of αvβ3-integrin in endothelial cells can be regulated by growth factors, and we have confirmed this here (Sepp et al., 1994). Moreover, it is now widely accepted that, when an integrin heterodimer couples to a growth factor receptor, cytoplasmic effectors such as focal adhesion kinase and integrin-linked kinase initiate a signal cascade (Schaepfer and Hunter, 1998; Dedhar et al., 1999). We speculate that in endothelial cells one consequence of signaling mediated by such cytoplasmic effectors is nucleation of assembly of the VMA.
TrHBMECs and HMVECs adhere to a recombinant fragment of the G-domain of the α4 laminin subunit in an αvβ3-integrin–dependent manner. This suggests the possibility that the α4 laminin subunit is a ligand for the αvβ3-integrin. This is somewhat surprising because the αvβ3-integrin binds to RGD tripeptide sites in its other known ligands, including fibronectin, vitronectin, Cyr61 and Fisp12, two immediate early gene products, and DEL1, a protein encoded by a unique developmentally regulated endothelial cell locus (Hidai et al., 1998; Kireeva et al., 1998; Babic et al., 1999). In contrast, our G domain fragment of α4-laminin does not contain an RGD motif, and thus any αvβ3-mediated cell adhesion to the laminin G domain α4-fragment we have used in our assays must occur in a non-RGD–dependent manner. Of course, we cannot entirely rule out the possibility that the αvβ3-integrin plays a role in trans-activating another integrin in endothelial cells. In such a scenario, the other integrin, rather than the αvβ3-heterodimer, would mediate direct cell interaction with the α4 laminin fragment. Because an antibody that inhibits the β1 integrin subunit has only a marginal impact on endothelial cell attachment to the α4 laminin subunit fragment, this other integrin heterodimer does not contain a β1-subunit.
Matrix components and integrins are believed to play an important role in formation of blood vessels (angiogenesis), a process that occurs during normal development, during wound repair, during the female reproductive cycle, and in various diseases such as cancer (Brooks et al., 1994b; Ruoslahti and Engvall, 1997). These factors act by modulating endothelial cell motility as well as enabling cells to aggregate to form capillary structures. Our data indicate that the VMA may be involved in these phenomena because 2A3 antibody, against the α4 laminin subunit, and LM609 antibody, against the αvβ3-integrin, both inhibit branching morphogenesis of endothelial cells and delay healing of wounded endothelial cell cultures in vitro. The idea that a matrix adhesion device that binds to the intermediate filament network of endothelial cells is involved in dynamic processes such as migration and tissue morphogenesis at first would appear to be counterintuitive because intermediate filament-binding sites at the cell surface are considered to play an essential role in stabilizing tissues. For example, although certain hemidesmosome components, such as BP230 (BPAG1) and the α6β4 integrin heterodimer, may play roles during migration, leading to wound healing and metastasis, it is generally accepted that hemidesmosomes in epithelial cells are stable substrate anchor points that are present in contact-inhibited cells (Guo et al., 1995; Rabinovitz and Mercurio, 1997). In contrast, the VMA is partially disassembled in contact-inhibited, presumably quiescent cells. Furthermore, whereas hemidesmosomes are disassembled when cells undergo wound healing (migrate) or are activated by growth factors, the VMA in endothelial cells is assembled under the same conditions (Mainiero et al., 1995; Goldfinger et al., 1998; Jones et al., 1998). Indeed, we see an array of VMAs at the leading front of actively moving cells repopulating a wound site. Because these are associated with plectin and vimentin intermediate filaments, we suggest that vimentin, through binding to a matrix adhesion site via plectin, may play an active role in migration, a possibility proposed recently by others (Eckes et al., 2000). This idea is consistent with recent reports that both vimentin-deficient and plectin-deficient cells show impaired motility (Eckes et al., 1998; Wiche, 1998).
One other aspect of our studies is quite intriguing. Plectin shows a dramatic change in its localization in growth factor-depleted endothelial cells and cells that are contact inhibited. In such cells plectin shows association primarily with the cytoplasmic network of vimentin. In sharp contrast, when endothelial cell cultures are stimulated by growth factor or wounding, the organization of plectin rapidly changes. It becomes polarized to the substratum-attached surface of the cell where it associates with the αvβ3-integrin. The dynamics of plectin localization in endothelial cells may be regulated by the same sort of phosphorylation mechanism that has been shown to control plectin binding to different types of intermediate filament proteins (Foisner et al., 1991; Wiche, 1998).
In summary, we have identified an endothelial cell-matrix adhesion complex that shows heterogeneous cytoskeleton association. This structure has at its core the αvβ3-integrin and the α4 laminin subunit. In addition, we have provided in vitro data that support the possibility that the structure is involved in regulating processes leading to angiogenesis, including cell migration.
Figure.
s 4 and 5. HMVECs maintained in medium lacking growth factors (Figure 4) or medium supplemented with bFGF (Figure 5) were processed for double labeling using a combination of antibodies against α4-laminin (2A3) and αv integrin subunit (A and B), the αvβ3 integrin complex and the αv integrin subunit (D and E), vinculin and the αv integrin subunit (G and H), and plectin and the αv integrin subunit (J and K). Cells were viewed by confocal microscopy. The focal plane is close to the substratum-attached surface of the cells. C, F, I, and L are overlays of the fluorescence images. Bar in Figure 4C, 20 μm. Bar in Figure 5C, 10 μm.
Figure 12.
The extent of wound closure in the experiments in Figure 11 was quantitated. Wounds were photographed 0 and 18 h after wounding. For each trial 40 measurements across the wound at distances at least 100 μm apart were made 18 h after wounding. These measurements were used to determine the average wound closure (average width of the wound at 0 h minus average width of the wound at 18 h). This is presented as a percentage of the average width of the wound at 0 h.
ACKNOWLEDGMENTS
We thank Drs. Jeffrey Miner and Denise Paulin for generous gifts of antibody and cells. We are grateful for the technical assistance of Xiang He and Shaunak Rana. This work was supported by grants to J.C.R.J. (GM38470 and DE12328) from the National Institutes of Health and by a Wellcome Trust International Biomedical Collaboration Grant (047898/Z/96/Z) to F.W.F. and R.D.G.
Abbreviations used:
- bFGF
basic fibroblast growth factor
- G-domain
globular domain
- HMVEC
human microvascular endothelial cell
- Ig
immunoglobulin
- PBS
phosphate-buffered saline
- TrHBMEC
transformed human bone marrow endothelial cell
- VMA
vimentin-associated matrix adhesion
REFERENCES
- Aumailley M, Smyth N. The role of laminins in basement membrane function. J Anat. 1998;193:1–21. doi: 10.1046/j.1469-7580.1998.19310001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babic AM, Chen C-C, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SE, Hopkinson SB, Fitchmun M, Andreason GL, Frasier F, Plopper G, Quaranta V, Jones JCR. Laminin-5 and hemidesmosomes: role of the alpha 3 chain subunit in hemidesmosome stability and assembly. J Cell Sci. 1996;109:2509–2520. doi: 10.1242/jcs.109.10.2509. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Tint IS, Svitkina TM. Association of intermediate filaments with vinculin-containing adhesion plaques of fibroblasts. Cell Motil Cytoskeleton. 1987;8:274–283. doi: 10.1002/cm.970080308. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Clark RAF, Cheresh DA. Requirement of vascular integrin αvβ3 for angiogenesis. Science. 1994a;264:569–571. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Montgomery AMP, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin αvβ3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994b;79:1157–1164. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Strömblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Anti-integrin αvβ3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–1822. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter WG, Wayner EA, Bouchard TS, Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedhar S, Williams B, Hannigan G. Integrin-linked kinase (ILK): a regulator of integrin and growth factor signaling. Trends Cell Biol. 1999;9:319–323. doi: 10.1016/s0962-8924(99)01612-8. [DOI] [PubMed] [Google Scholar]
- Dogic D, Eckes B, Aumailley M. Extracellular matrix, integrins and focal adhesions. Curr Top Pathol. 1999;93:75–85. doi: 10.1007/978-3-642-58456-5_8. [DOI] [PubMed] [Google Scholar]
- Eckes B, Colucci-Guyon E, Smola H, Nodder S, Babinet C, Krieg T, Martin P. Impaired wound healing in embryonic and adult mice lacking vimentin. J Cell Sci. 2000;113:2455–2462. doi: 10.1242/jcs.113.13.2455. [DOI] [PubMed] [Google Scholar]
- Eckes B, Dogic D, Colucci-Guyon E, Wang N, Maniotis A, Ingber I, Merckling A, Langa F, Aumailley M, Delouvee A, Koteliansky V, Babinet C, Krieg T. Impaired mechanical stability, migration, and contractile capacity in vimentin-deficient fibroblasts. J Cell Sci. 1998;111:1897–1907. doi: 10.1242/jcs.111.13.1897. [DOI] [PubMed] [Google Scholar]
- Elliot CE, Becker B, Oehler S, Castoñón MJ, Hauptmann R, Wiche G. Plectin transcript diversity: identification and tissue distribution of variants with distinct first coding exons and rodless isoforms. Genomics. 1997;42:115–125. doi: 10.1006/geno.1997.4724. [DOI] [PubMed] [Google Scholar]
- Foisner R, Traub P, Wiche G. Protein kinase A- and protein kinase C-regulated interaction of plectin with lamin B and vimentin. Proc Natl Acad Sci USA. 1991;106:723–733. doi: 10.1073/pnas.88.9.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frieser M, Nöckel H, Pausch F, Röder C, Hahn A, Deutzmann R, Sorokin LM. Cloning of the mouse laminin α4 cDNA: expression in a subset of endothelium. Eur J Biochem. 1997;246:727–735. doi: 10.1111/j.1432-1033.1997.t01-1-00727.x. [DOI] [PubMed] [Google Scholar]
- Goldfinger LE, Stack MS, Jones JCR. Processing of laminin-5 and its functional consequences: role of plasmin and tissue-type plasminogen activator. J Cell Biol. 1998;141:255–265. doi: 10.1083/jcb.141.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Haan K, Baker SE, Fitchmun MI, Todorov I, Weitzman S, Jones JCR. A cell signal pathway involving laminin-5, α3β1 integrin, and mitogen-activated protein kinase can regulate epithelial cell proliferation. Mol Biol Cell. 1999;10:259–270. doi: 10.1091/mbc.10.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Preparation of extracellular matrices produced by cultured bovine corneal endothelial cells and PF-HR-9 endodermal cells: their use in cell culture. In: Barnes DW, Sirbasku DA, Stao GH, editors. Methods for Preparation of Media. Supplements and Substrata. Vol. 1. New York: Alan R. Liss; 1984. pp. 275–293. [Google Scholar]
- Grant DS, Tashiro KI, Segui-Real B, Yamada Y, Martin GR, Kleinman HK. Two different laminin domains mediate the differentiation of human endothelial cells into capillary-like sturctures in vitro. Cell. 1989;58:933–943. doi: 10.1016/0092-8674(89)90945-8. [DOI] [PubMed] [Google Scholar]
- Gu Y, Sorokin L, Durbeej M, Hjalt T, Jönsson J-I, Ekblom M. Characterization of bone marrow laminins and identification of α5-containing laminins as adhesive proteins for multipotent hematopoietic FDCP-mix cells. Blood. 1999;93:2533–2542. [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Guzman L-M, Barondess JJ, Carson MJ, Beckwith J. Tight regulation, modulation, and high level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E, Lane D. Monoclonal Antibodies. In: Laboratory C S H, editor. Antibodies: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1988. pp. 92–121. [Google Scholar]
- Hidai C, Zupanic T, Penta K, Mikhail A, Kawana M, Quertermous EE, Aoka Y, Fukagawa M, Matsui Y, Platika D, Auerback R, Hogan BLM, Snodgrass R, Quertermous T. Cloning and characterization of developmental endothelial locus-1: An embryonic endothelial cell protein that binds the αvβ3 integrin receptor. Genes Dev. 1998;12:21–33. doi: 10.1101/gad.12.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieda Y, Nishizawa Y, Uematsu J, Owaribe K. Identification of a new hemidesmosomal protein, HD1: a major, high molecular mass component of isolated hemidesmosomes. J Cell Biol. 1992;116:1497–1506. doi: 10.1083/jcb.116.6.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homan SM, Mercurio AM, LaFlamme SE. Endothelial cells assemble two distinct α6β4 containing vimentin-associated structures: roles for ligand binding and the β4 cytoplasmic tail. J Cell Sci. 1998;111:2717–2728. doi: 10.1242/jcs.111.18.2717. [DOI] [PubMed] [Google Scholar]
- Howe A, Aplin AE, Alahari SK, Juliano RL. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Iivanainen A, Sainio K, Sariola H, Tryggvason K. Primary structure and expression of a novel human laminin α4 chain. FEBS Lett. 1995;365:183–188. doi: 10.1016/0014-5793(95)00462-i. [DOI] [PubMed] [Google Scholar]
- Jones JCR, Hopkinson SB, Goldfinger LE. Structure and assembly of hemidesmosomes. BioEssays. 1998;20:488–494. doi: 10.1002/(SICI)1521-1878(199806)20:6<488::AID-BIES7>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Katz B-Z, Zamir E, Bershadsky A, Kam Z, Yamada KM, Geiger B. Physical state of the extracellular matrix regulates the structure and molecular composition of the cell-matrix adhesions. Mol Biol Cell. 2000;11:1047–1060. doi: 10.1091/mbc.11.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Lam SC-T, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through the integrin αvβ3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- Klatte DH, Kurpakus MA, Grelling KA, Jones JCR. Immunochemical characterization of three components of the hemidesmosome and their expression in cultured epithelial cells. J Cell Biol. 1989;109:3377–3390. doi: 10.1083/jcb.109.6.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortesmaa J, Yurchenco P, Tryggvason K. Recombinant laminin-8 (α4β1γ1) J Biol Chem. 2000;275:14853–14859. doi: 10.1074/jbc.275.20.14853. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;277:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langhofer M, Hopkinson SB, Jones JCR. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. J Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Liu J, Mayne R. The complete cDNA coding sequence and tissue specific expression of the mouse laminin α4 chain. Matrix Biol. 1996;15:433–437. doi: 10.1016/s0945-053x(96)90162-6. [DOI] [PubMed] [Google Scholar]
- Mainiero F, Pepe A, Wary KK, Spinardi L, Mohammadi M, Schlessinger J, Giancotti FG. Signal transduction by the α6β4 integrin: distinct β4 subunit sites mediate recruitment of Shc/Grb2 and association with the cytoskeleton of hemidesmosomes. EMBO J. 1995;14:4470–4481. doi: 10.1002/j.1460-2075.1995.tb00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: expression, development transitions, and chromosomal locations of α1–5, identification of heterotrimeric laminins 8–11, and cloning of a novel α3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niimi T, Kumagai C, Okano M, Kitagawa Y. Differentiation-dependent expression of laminin-8 (α4β1γ1) mRNAs in mouse 3T3–L1 adipocytes. Matrix Biol. 1997;16:223–230. doi: 10.1016/s0945-053x(97)90011-1. [DOI] [PubMed] [Google Scholar]
- Pierce RA, Griffin GL, Mudd MS, Moxley MA, Longmore WJ, Sanes JR, Miner JH, Senior RM. Expression of laminin α3, α4, and α5 chains by alveolar epithelial cells and fibroblasts. Am J Respir Cell Mol Biol. 1998;19:237–244. doi: 10.1165/ajrcmb.19.2.3087. [DOI] [PubMed] [Google Scholar]
- Rabinovitz I, Mercurio AM. The integrin alpha 6 beta 4 functions in carcinoma cell migration on laminin-1 by mediating the formation and stabilization of actin-containing motility structures. J Cell Biol. 1997;139:1873–1884. doi: 10.1083/jcb.139.7.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A, Al-Imara L, Pope FM. The complete cDNA sequence of laminin α4 and its relationship to the other human laminin α chains. Eur J Biochem. 1996;238:813–821. doi: 10.1111/j.1432-1033.1996.0813w.x. [DOI] [PubMed] [Google Scholar]
- Riddelle KS, Hopkinson SB, Jones JCR. Hemidesmosomes in the epithelial cell line 804G: their fate during wound closure, mitosis and drug induced reorganization of the cytoskeleton. J Cell Sci. 1992;103:475–490. doi: 10.1242/jcs.103.2.475. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Engvall E. Integrins and vascular extracellular matrix assembly. J Clin Invest. 1997;100(11 Suppl):S53–S56. [PubMed] [Google Scholar]
- Ryan M, Tizard R, VanDevanter DR, Carter WG. Cloning of the LamA3 gene encoding the α3 chain of the adhesive ligand epiligrin. J Biol Chem. 1994;269:22779–22787. [PubMed] [Google Scholar]
- Schaepfer DD, Hunter T. Integrin signaling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Schweitzer KM, Vicart P, Delouis C, Paulin D, Dräger AM, Langenhuijsen MMAC, Weksler BB. Characterization of a newly established human bone marrow endothelial cell line: distinct adhesive properties for hematopoietic progenitors compared with human umbilical vein endothelial cells. Lab Invest. 1997;76:25–36. [PubMed] [Google Scholar]
- Sepp NT, Li L-J, Lee KH, Brown EJ, Caughman SW, Lawley TJ, Swerlick RA. Basic fibroblast growth factor increases expression of the αvβ3 integrin complex on the human microvascular endothelial cells. J Invest Dermatol. 1994;103:295–299. doi: 10.1111/1523-1747.ep12394617. [DOI] [PubMed] [Google Scholar]
- Simon KO, Burridge K. Interactions between integrins and the cytoskeleton: structure and regulation. In: Cheresh DA, Mecham RP, editors. Integrins: Molecular and Biological Responses to the Extracellular Matrix. San Diego, CA: Academic Press; 1994. pp. 49–78. [Google Scholar]
- Smilenov LB, Mikhailov A, Pelham RJ, Marcantonio EE, Gunderson GG. Focal adhesion motility revealed in stationary fibroblasts. Science. 1999;286:1172–1174. doi: 10.1126/science.286.5442.1172. [DOI] [PubMed] [Google Scholar]
- Spinardi L, Ren Y-L, Sanders R, Giancotti FG. The β4 subunit cytoplasmic domain mediates the interaction of α6β4 integrin with the cytoskeleton of hemidesmosomes. Mol Biol Cell. 1993;4:871–884. doi: 10.1091/mbc.4.9.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinböck FA, Branislav N, Coulombe PA, Fuchs E, Traub P, Wiche G. Dose dependent linkage, assembly inhibition and disassembly of vimentin and cytokeratin 5/14 filaments through plectin's intermediate filament-binding domain. J Cell Sci. 2000;113:483–491. doi: 10.1242/jcs.113.3.483. [DOI] [PubMed] [Google Scholar]
- Steinböck FA, Wiche G. Plectin: a cytolinker by design. Biol Chem. 1999;380:151–158. doi: 10.1515/BC.1999.023. [DOI] [PubMed] [Google Scholar]
- Stromblad S, Cheresh DA. Cell adhesion and angiogenesis. Trends Cell Biol. 1996;6:462–468. doi: 10.1016/0962-8924(96)84942-7. [DOI] [PubMed] [Google Scholar]
- Varner JA. The role of vascular cell integrins αvβ3 and αvβ5 in angiogenesis. In: Goldberg ID, Rosen EM, editors. Regulation of Angiogenesis. Basel, Switzerland: Birhäuser Verlag; 1997. pp. 361–390. [Google Scholar]
- Wiche G. Role of plectin in cytoskeleton organization and dynamics. J Cell Sci. 1998;111:2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- Wiche G, Hermann H, Leichtfried F, Pytela R. Plectin: a high-molecular-weight cytoskeletal polypeptide component that copurifies with intermediate filaments of the vimentin type. Cold Spring Harbor Symp Quant Biol. 1982;46:475–482. doi: 10.1101/sqb.1982.046.01.044. [DOI] [PubMed] [Google Scholar]
- Zackroff RV, Goldman AE, Jones JCR, Steinert PM, Goldman RD. Isolation and characterization of keratin-like proteins from cultured cells with fibroblastic morphology. J Cell Biol. 1984;98:1231–1237. doi: 10.1083/jcb.98.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamir E, Katz B-Z, Aota S, Yamada KM, Geiger B, Kam Z. Molecular diversity of cell-matrix adhesions. J Cell Sci. 1999;112:1655–1669. doi: 10.1242/jcs.112.11.1655. [DOI] [PubMed] [Google Scholar]
- Zamir E, Katz M, Posen Y, Erez N, Yamada KM, Batz B-Z, Lin S, Lin DC, Bershadsky A, Kam Z, Geiger B. Dynamics and segregation of cell-matrix adhesions in cultured fibroblasts. Nature Cell Biol. 2000;2:191–197. doi: 10.1038/35008607. [DOI] [PubMed] [Google Scholar]