Abstract
Among more than 2050 brains in our Alzheimer disease brain banks we came across three brains with well demarcated indurated white-yellow nodules in the amygdalas. Microscopically, these nodules were composed of numerous lipid-laden macrophages located in the central region surrounded by an eosinophilic hyaline-like material with minimal reactive gliosis in the periphery. Neurons within these lesions had a normal appearance but were moderately decreased in number. Beta-amyloid, tau and alpha-synuclein immunostaining revealed no abnormal deposits within the nodules. The three patients had long histories of dementia (one linked to a presenilin-1 mutation). The neuropathological diagnoses were Alzheimer disease in two of them and an unclassified tauopathy with argyrophilic grains in the third.
Conclusion
The pathogenesis of these lesions is uncertain. We favor that the degeneration has some relationship to the underlying dementing disease, either secondary to deafferentation or an alteration in metabolic demand, perhaps related to the bi-directional anatomical and functional connections that exist between the amygdala and the hippocampus or less likely as a primary event.
Keywords: Amygdala, Alzheimer Disease, Neurodegeneration
Introduction
The amygdala is a component of the limbic system that has a modulatory effect on the acquisition and consolidation of memories that evoke an emotional response [3,4]. In humans, selective amygdala lesions have only rarely been reported. Bilateral amygdaloid lesions are a typical feature of Lipoid Proteinosis or Urbach-Wiethe disease, a rare autosomal recessive genodermatosis due to mutations in the extracellular matrix protein 1 (ECM1) gene [7]. Among more than 2050 brains in our Alzheimer Disease Research Center brain banks we came across three brains with well demarcated unusual nodular lesions in the amygdalas from two patients with Alzheimer disease and one with an unclassifiable tauopathy and no evidence of other underlying disease. In two of the brains the nodules were recorded as bilateral. Curiously, all three patients came to autopsy in the two Centers within an 18 month period.
Patients
Patient 1 was a woman, with no family history of dementia or other neurological diseases, who died aged 50 years after a ten-year period of intellectual decline progressing to profound dementia. She had a history of depression beginning in her twenties. Approximately 10 years prior to death she was fired as a data analyst because of mistakes due to memory problems and possible paranoid delusions. She became a grocery store bagger but frequently forgot to pick up her paycheck. Five years prior to death she moved into her mother's house because she could no longer manage her affairs. The same year she made several suicide attempts and was hospitalized. On examination, she was alert and oriented with 0/3 memory at zero and five minutes and was unable to subtract serial sevens. All laboratory tests were within normal range. Magnetic resonance imaging (MRI) of the brain revealed mild to moderate atrophy. She was started on Prozac. A year later she began to lose her way driving familiar routes. Other mental status evaluations over the next two years revealed severe recent memory deficits but intact long term memory and 'normal higher function'. She was started on Aricept and Zoloft but her memory continued to deteriorate and she increasingly accused others of stealing from her. She was eventually placed in a nursing home 18 months prior to her death. Her mother was alive and well aged 78. Her father died of esophageal carcinoma aged 71. She had no family history of neurological disease. Over the last months of her life she lost the ability to use a knife and fork, her speech output diminished to just a few words and she needed a feeding tube. There was no history of seizures.
Patient 2 was a woman who died aged 87 years who presented to the Memory Disorders Clinic 13 years prior to her death with declining memory and personality changes for the previous two years and a remote right cerebellar infarct. She had a Blessed Dementia Scale score of 9 and a Hachinski Ischemia Scale score of 8. She had no family history of dementia. She progressively lost her ability to perform the activities of daily living and was placed in a nursing home 9 years prior to her death. She was last evaluated 4 years prior to death at which time her Blessed Dementia Scale score was 25 and she had mild dystonia, shuffling gait and '4+ en bloc' turning.
Patient 3 was a man who died aged 46 years following an eleven-year period of progressive cognitive decline beginning with memory difficulties and depression at the age of 35 years. His only evaluation in the Cognitive Disorders Program at the University of Michigan took place about one month before his death. At this time he was severely demented, wheelchair bound and living in a nursing home. He was unable to cooperate with testing but was awake and alert. He used few words but tended to curse. He had gait ataxia, 3+ rigidity, without cogwheel rigidity, occasional myoclonic jerks and diffuse symmetric hyperreflexia. MRI revealed diffuse atrophy. His mother died aged 42 with a post-mortem diagnosis of Alzheimer disease. A half-brother died at the age of 42 with severe dementia. Another two full siblings and three full siblings were alive and well. A presenilin 1 mutation was found. He died a month later.
Pathological examination
All autopsies were limited to examination of the brain and were performed according to the protocols of our Alzheimer Disease Research Centers [22]. Briefly, the cerebral hemispheres were divided in the sagittal plane and one hemisphere was coronally sectioned in the fresh state and the slices flash frozen and stored in a −80° C freezer. The other half was fixed in formalin for one to two weeks and then sectioned in the coronal plane on standardized landmarks. Paraffin sections from standardized areas were stained according to the protocol outlined in Vonsattel et al [22] including Luxol fast blue/ hematoxylin and eosin (LHE), modified Bielschowsky, beta amyloid, tau and alpha synuclein. Additional stains performed on the amygdala nodules included trichrome, phosphotungstic acid hematoxylin (PTAH), Congo red, PAS, reticulin, von Kossa (for calcium), Grocott’s methenamine silver, and hematoxylin and eosin and immunohistochemical stains for CD68, GFAP, CD45, Neu N and neurofilament. Samples of the formalin fixed amygdala nodules were dissected from the 8 and 9 year formalin fixed tissue (cases 1 and 2), post fixed in glutaraldehyde and examined with the electron microscope. Photographs of the fresh and fixed slices were available for patients one and two. CERAD scores [13] were estimated as outlined by Vonsattel et al. [22] and NIA Reagan scores of neurofibrillary degeneration were estimated as described in Newell et al. [14].
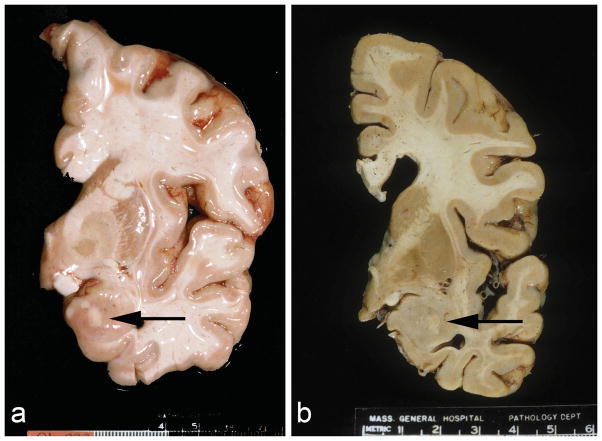
Patient 1: The brain weighed 992 grams and had marked frontal and temporal lobe atrophy. Both amygdalas were moderately atrophic. The anterior aspects of both amygdalas contained multiple yellow nodular indurated lesions ranging from 0.1 to 1.5 cm in greatest diameter (Figure 1a). X-rays of the slices showed no calcification. The hippocampi were severely atrophic with numerous plaques and neurofibrillary tangles (NFT) but no ischemic type changes. Numerous NFT and plaques were present in all areas of the cortex. The pathological diagnosis was severe Alzheimer disease, Braak and Braak stage VI and definite AD by CERAD criteria.
Fig. 1.
Coronal sections of the brains at the level of the infundibulum. Note the well demarcated white-yellow nodules mainly located in the basolateral nuclear group of the amygdalas. (a: unfixed brain of patient 1. b: formalin fixed brain of patient 2)
Patient 2: The brain weighed 1010 grams and had mild frontal, parietal and temporal lobe atrophy and enlarged lateral ventricles (19 ml on left, normal 5–7 ml). The substantia nigra was pale and the hippocampus was mildly atrophic. Both amygdalas had central 0.7 cm areas of white discoloration (Figure 1b). Microscopic exam showed rare NFT in frontal, temporal and cingulate gyri and large numbers in the entorhinal cortex and amygdala. Tau immunostain revealed that most of these tangles were perinuclear. There were no diffuse or neuritic plaques in the cortex or hippocampus. The hippocampus was well preserved with scattered more conventional NFT as well as pretangles, and silver and ubiquitin positive granules in the neuropil. Several hippocampal neurons contained ubiquitin positive nucleolar sized intranuclear inclusions and occasional faint tau positive cytoplasmic inclusions, but no diffuse or neuritic amyloid plaques. The pathological diagnosis was an unclassified tauopathy, not fitting into most of the defined tauopathies, with argyrophilic grains. There were two small old infarcts in the basal ganglia. The larger was 1.0 cm in greatest dimension.
Patient 3: The brain weighed 1120 grams and had moderate diffuse atrophy of all lobes with moderate to marked dilatation of the lateral ventricles, particularly the temporal horns. Microscopically numerous NFT were present in the hippocampus, parahippocampal gyrus and amygdala as well as moderate numbers in the occipital and insular cortex (Braak and Braak Stage VI), thus fulfilling the NIA-Reagan criteria for a ‘High Likelihood’ that the dementia was due to Alzheimer disease and the CERAD criteria for Definite Alzheimer Disease. The gross appearance of the amygdalas was not commented upon but more than one section was taken from it implying that an abnormality was recognized at the time of the gross examination. The method of freezing precluded obtaining useful information about the other amygdala in this case.
Amygdala Lesions
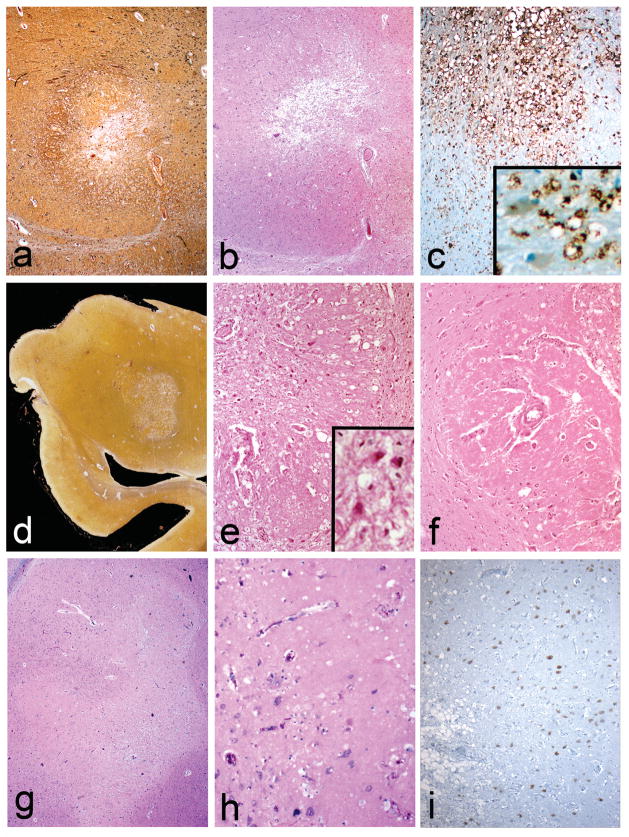
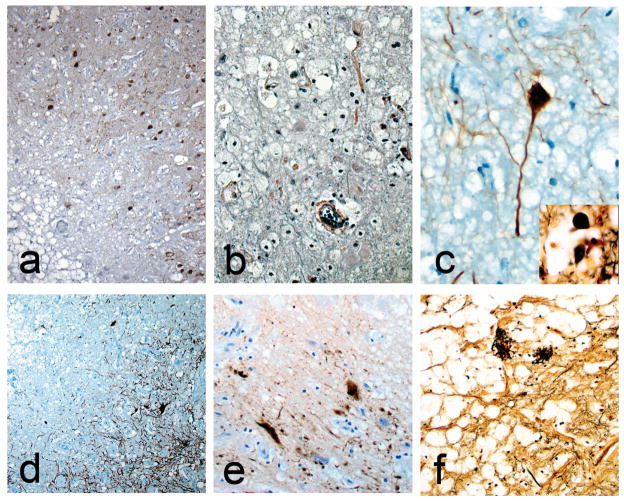
The nodules in all three brains were mainly located in the basolateral nuclear group of the amygdala while the remaining amygdala nuclei were preserved. The microscopic characteristics of all three cases were similar. They consisted of sharply defined paucicelllular areas within the amygdala with numerous foamy lipid-laden cells in their centers (Figure 2a–i), surrounded by eosinophilic hyaline-like material with minimal reactive gliosis at the periphery (Figure 2b,e,f,h). Some of the foamy cells contained luxol fast blue positive material and most of them were positive for CD68 (Figure 2c,e,i). The few remaining neurons within these lesions appeared normal (Figure 2e,f,i and Figure 3). No calcium accumulation was demonstrated. Trichrome and PTAH stains confirmed the lack of collagen (Figure 3b). The hyaline material did not stain for Congo Red or react with beta amyloid. CD-68 confirmed that most of the foamy cells were macrophages (Figure 2c). CD45 revealed scattered mononuclear cells and microglia both in and around the nodules. NeuN illustrated the presence of viable neurons (Figure 2i). GFAP and PTAH confirmed minimal reactive astrocytosis at the periphery (Figure 3b and d). Bielschowsky silver and neurofilament antibody revealed relatively spared axons and occasional axonal spheroids (Figure 3c and inset) at the periphery as well as neurofibrillary tangles in the surrounding amygdala (Figure 3a). No Congo red, beta-amyloid, tau or alpha-synuclein deposits were found within the lesions. Electron microscopy of tissue retrieved after 8 years in formalin from case 1 revealed vascular basal lamina thickening but no other specific alterations. Case 2 had markedly thickened small blood vessel walls in the amygdalas as well as in other areas of the brain consistent with hypertensive arteriosclerotic changes (Figure 2f).
Fig. 2.
The nodules are sharply defined and composed of numerous lipid-laden macrophages located in a central region (a–i) surrounded by an eosinophilic hyaline-like material (b,e,h). CD68 stain reveals numerous positive macrophages (c). Neurons within these lesions appeared normal but were moderately decreased in number. (e,f,i and e inset). In i, the normal amygdala is on the right and the macrophage filled center is on the left of the figure. Thick walled blood vessels can be seen in e and f. Case 1 (a–c), Case 2 (d–f) and case 3 (g–i). a and d: Bielschowsky silver; b,e–h LHE stain; c: CD68 immunostain; i: NeuN immunostain. Original magnifications: a,b,c,e,f,g,i 100x; c inset 200x; d, whole mount; h:200x)
Fig. 3.
Neurofibrillary tangles are present around the margin of the nodule in case 1 (a). Glial fibrils are present but no collagen in case 3 (b). Intact neurons can be found within the lesions (c, case 2). Inset c shows an axonal spheroid at the edge of the nodule in case 1. Gliosis forms part of the rims of the nodules (d). Tau stained dystrophic neurites at the edge of edge of the nodule (e, case 1) Note the foamy cells in the upper right. Some neuritic plaques remain within the nodules (f, case 1). a and e: immunostain for tau; b: PTAH stain; c: immunostain for neurofilament; d: immunostain for GFAP; f and inset c: Bielschowsky silver. Original magnifications: a and d 100x; b 400x; c,e,f 200x
Discussion
We present three cases with striking and selective amygdala damage found among more than 2050 brains in our Alzheimer disease brain banks. The pathogenesis of these lesions is uncertain.
One possible etiology considered was ischemia. Among the amygdaloid nuclei, the basolateral nuclear group (lateral, laterobasal, accessory basal and mediobasal nuclei) is the most likely to suffer from ischemic changes but these lesions are usually associated with damage in the hippocampus [20]. In our cases the hippocampus and entorhinal cortex had no evidence of ischemic changes and there were no other histological signs of ischemia within the amygdala lesions. The possibility that these lesions are remnants of bilateral infarcts in the territory of the anterior choroidal arteries or in the proximal branches of the middle cerebral arteries seems highly unlikely since in at least two of the cases the lesions are bilateral and no damage in other areas of these vascular territories was present. In addition, neurons within them are intact although there are a large number of macrophages in the center of the lesions.
Bilateral amygdaloid lesions are a typical feature of Lipoid Proteinosis or Urbach-Wiethe disease, a rare autosomal recessive genodermatosis due to mutations in the extracellular matrix protein 1 (ECM1) gene [7]. Lipoid proteinosis is characterized by multisystem involvement with intracellular deposition of amorphous hyaline material. Our patients had no skin abnormalities described clinically or recognized at the time of autopsy. None of them had the bilateral amygdala calcifications that are characteristic of this syndrome. Microscopic findings of Lipoid Proteinosis in brain [11] include gross amorphous calcification encompassed by gliotic tissue and calcified thickened vessels walls.
The possibility of parenchymal amyloidomas was ruled out by lack of Congo red and beta-amyloid staining and by electron microscopy. Histologically, the lesions have a resemblance to some of the abnormalities found in Wernicke encephalopathy, but although the neurons were mostly spared, the vasculature was not prominent and the typical locations affected in this disease were normal.
Epilepsy, especially status epilepticus, can also affect the amygdala resulting in histological similarities to those found in diffuse ischemia [1,12] but in most of those patients the changes have been accompanied by profound damage to the hippocampus and other areas of the cortex [12]. The patients that we report do not have a history of epilepsy, the hippocampus is well preserved and the amygdalas did not show a significant neuronal loss or major reactive gliosis. Other entities that could affect selectively the amygdala and the rest of the limbic system bilaterally are herpes simplex [3] and paraneoplastic encephalitis [5] but the very well defined lesions, the absence of inflammation, neuronophagia or significant neuronal loss make these etiologies very unlikely.
Can the amygdala lesions be related to the underlying dementing diseases? Could the damage be a result of alterations in the bi-directional anatomical or functional connections between the amygdala and the hippocampus [6,15,16,19], either as a result of deafferentation or metabolic demand? This possibility is supported by previous studies that demonstrated significant anomalies in the amygdala in patients with AD [9,10]. Amygdaloid atrophy and neuronal loss are more prominent in the basolateral nuclear group, especially in patients with long lasting dementia [17,20]. In our patients, the basolateral nuclear group was also almost selectively affected, but analogous macroscopic or microscopic findings to these three cases have not been previously reported in any neurodegenerative disease. The major hippocampal connections reciprocally link the basolateral group with the entorhinal cortex, subiculum and the ammonic hippocampal regions [15,16]. Theories of anterograde and retrograde degenerative processes support the suggestion of a “spreading” mechanism via well-defined sets of connecting fibers [8]. Another possible explanation for this distribution pattern might be neurochemical differences in the subdivisions of the amygdala, with the basolateral group having a richer cholinergic innervation [2,10].
Unfortunately our patients were too demented for testing of amygdala function but there is no evidence in the medical records of any of the typically described symptoms associated with amygdala damage, such as impairment of emotions linked to facial expression, alteration in memories with an emotional component [18], loss of precautionary behavior or monetary loss aversion [4]. Patients with bilateral amygdala damage show cognitively little deviation from normal subjects [18] therefore it is very unlikely that these amygdala lesions played a role in the dementia symptoms in our patients
In summary, we report unusual degeneration of the amygdalas of unknown etiology in three patients with long-standing dementia due to different pathophysiologies. The connection between these lesions and these patients’ illnesses is obscure.
Supplementary Material
Acknowledgments
This study was supported by Grant Nos. P50 AG005134 and P50 AG08671 from the National Institutes of Health and the Contrato Rio Hortega del Instituo Salud Carlos III.
Footnotes
Case 2 was discussed at the Annual Diagnostic Slide Session of the Annual Meeting of the American Association of Neuropathologists in 2003.
The authors declare that they have no conflicts of interest.
References
- 1.Auer RN, Siesjö BK. Biological differences between ischemia, hypoglycemia, and epilepsy. Ann Neurol. 1988;24:699–707. doi: 10.1002/ana.410240602. [DOI] [PubMed] [Google Scholar]
- 2.Brashear HR, Godec MS, Carlsen J. The distribution of neuritic plaques and acetylcholinesterase staining in the amygdala in Alzheimer's disease. Neurology. 1988;38:1694–1699. doi: 10.1212/wnl.38.11.1694. [DOI] [PubMed] [Google Scholar]
- 3.Broks P, Young AW, Maratos EJ, et al. Face processing impairments after encephalitis: amygdala damage and recognition of fear. Neuropsychologia. 1998;36:59–70. doi: 10.1016/s0028-3932(97)00105-x. [DOI] [PubMed] [Google Scholar]
- 4.De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proc Natl Acad Sci U S A. 2010;107:3788–3792. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duyckaerts C, Derouesne C, Signoret JL, Gray F, Escourolle R, Castaigne P. Bilateral and limited amygdalohippocampal lesions causing a pure amnesic syndrome. Ann Neurol. 1985;18:314–9. doi: 10.1002/ana.410180307. [DOI] [PubMed] [Google Scholar]
- 6.French SJ, Hailstone JC, Totterdell S. Basolateral amygdala efferents to the ventral subiculum preferentially innervate pyramidal cell dendritic spines. Brain Res. 2003;981:160–167. doi: 10.1016/s0006-8993(03)03017-8. [DOI] [PubMed] [Google Scholar]
- 7.Hamada T, McLean WH, Ramsay M, et al. Lipoid proteinosis maps to 1q21 and is caused by mutations in the extracellular matrix protein 1 gene (ECM1) Hum Mol Genet. 2002;11:833–840. doi: 10.1093/hmg/11.7.833. [DOI] [PubMed] [Google Scholar]
- 8.Hardy JA, Mann DM, Wester P, Winblad B. An integrative hypothesis concerning the pathogenesis and progression of Alzheimer's disease. Neurobiol Aging. 1986;7:489–502. doi: 10.1016/0197-4580(86)90086-2. [DOI] [PubMed] [Google Scholar]
- 9.Herzog AG, Kemper TL. Amygdaloid changes in aging and dementia. Arch Neurol. 1980;37:625–629. doi: 10.1001/archneur.1980.00500590049006. [DOI] [PubMed] [Google Scholar]
- 10.Kromer Vogt LJ, Hyman BT, Van Hoesen GW, Damasio AR. Pathological alterations in the amygdala in Alzheimer's disease. Neuroscience. 1990;37:377–385. doi: 10.1016/0306-4522(90)90408-v. [DOI] [PubMed] [Google Scholar]
- 11.Meenan FO, Bowe SD, Dinn JJ, et al. Lipoid proteinosis; a clinical, pathological and genetic study. Q J Med. 1978;47:549–561. [PubMed] [Google Scholar]
- 12.Meyer A, Beck E, Shepherd M. Unusually severe lesions in the brain following status epilepticus. J Neurol Neurosurg Psychiat. 1955;18:24–33. doi: 10.1136/jnnp.18.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mirra SS, Heyman A, McKeel D, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 14.Newell KL, Hyman BT, Growdon JH, Hedley-Whyte ET. Application of the National Institute on Aging (NIA)-Reagan Institute criteria for the neuropathological diagnosis of Alzheimer Disease. J Neuropathol Exper Neurol. 1999;58:1147–55. doi: 10.1097/00005072-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Saunders RC, Rosene DL. A comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: I. Convergence in the entorhinal, prorhinal, and perirhinal cortices. J Comp Neurol. 1988;271:153–184. doi: 10.1002/cne.902710202. [DOI] [PubMed] [Google Scholar]
- 16.Saunders RC, Rosene DL, Van Hoesen GW. Comparison of the efferents of the amygdala and the hippocampal formation in the rhesus monkey: II. Reciprocal and non-reciprocal connections. J Comp Neurol. 1988;271:185–207. doi: 10.1002/cne.902710203. [DOI] [PubMed] [Google Scholar]
- 17.Scott SA, DeKosky ST, Scheff SW. Volumetric atrophy of the amygdala in Alzheimer's disease: quantitative serial reconstruction. Neurology. 1991;41:351–356. doi: 10.1212/wnl.41.3.351. [DOI] [PubMed] [Google Scholar]
- 18.Siebert M, Markowitsch HJ, Bartel P. Amygdala, affect and cognition: evidence from 10 patients with Urbach-Wiethe disease. Brain. 2003;126:2627–2637. doi: 10.1093/brain/awg271. [DOI] [PubMed] [Google Scholar]
- 19.Smith AP, Stephan KE, Rugg MD, Dolan RJ. Task and content modulate amygdala-hippocampal connectivity in emotional retrieval. Neuron. 2006;49:631–638. doi: 10.1016/j.neuron.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Vereecken TH, Vogels OJ, Nieuwenhuys R. Neuron loss and shrinkage in the amygdala in Alzheimer's disease. Neurobiol Aging. 1994;15:45–54. doi: 10.1016/0197-4580(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 21.Volpe BT, Petito CK. Dementia with bilateral medial temporal lobe ischemia. Neurology. 1985;35:1793–1797. doi: 10.1212/wnl.35.12.1793. [DOI] [PubMed] [Google Scholar]
- 22.Vonsattel JPG, Aizawa H, Ge P, et al. An improved approach to prepare human brains for research. J Neuropathol Exper Neurol. 1995;54:42–56. doi: 10.1097/00005072-199501000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.