SUMMARY
Purpose
Pediatric lymphoblastic lymphoma (LL) has utilized treatment strategies similar to childhood acute lymphoblastic leukemia (ALL) with prolonged maintenance chemotherapy. We report the results of a pilot study to estimate feasibility, toxicity and efficacy of a 12 month aggressive multiagent chemotherapy regimen in children and adolescents with advanced LL.
Patients and Methods
Between July 1994 and June 1997, 85 eligible children and adolescents with advanced LL (Stage III/IV) were enrolled on this pilot study. Patients achieving a complete response following induction and consolidation received 6 cycles of maintenance chemotherapy for a total duration of 12 months.
Results
Grade III/IV toxicities included: hematologic (80%), infections (20%), stomatitis and elevated transaminases,(29%). There were a total of 19 events, 13 relapses, two secondary AML and four toxic deaths (5%). The five-year event-free survival (EFS) and overall survival (OS) was 78% ± 4.5% and 85% ± 3.9%, respectively. Relapsed patients had a 5 yr OS of only 33±14%. Multivariate analysis failed to demonstrate age, gender, LDH level, presence of marrow and/or CNS disease to have independent prognostic value.
Conclusions
These results suggest that this experimental approach is safe and results in similar outcomes as more prolonged childhood ALL regimens.
INTRODUCTION
The Children’s Cancer Group (CCG) began its studies in the treatment of children and adolescents with advanced lymphoblastic lymphoma (LL) in 1977 (CCG-551), which compared COMP (a cyclic lymphoma regimen) to a modification of the LSA2-L2 (leukemia) regimen developed at Memorial Sloan-Kettering Cancer Center, both regimens were given for an 18 month duration and areas of bulk disease received local irradiation (Anderson, et al 1993, Anderson, et al 1983, Mora, et al 2003). This study demonstrated that LSA2-L2 therapy was superior to COMP with a five-year event-free survival (EFS) for LSA2L2 of 64% compared to 35% for COMP (p<0.001) (Anderson, et al 1993, Anderson, et al 1983). The successor study CCG-502 compared LSA2-L2 to a COMP-based regimen plus L-asparaginase and daunomycin (ADCOMP) with again local irradiation to areas of bulky disease.(Tubergen, et al 1995) Although EFS was superior for LSA2-L2 (74%) versus ADCOMP (64%), the difference was not statistically significant. In the subsequent CCG-123 study, patients with "lymphomatous" ALL, i.e. bulky disease, were treated with either a modified BFM (Berlin-Frankfurt-Munster) 76/79 regimen, which included a re-induction/re-consolidation phase and a total of 36 months of therapy but no radiotherapy to bulky sites or modified LSA2-L2 with or without prophylactic cranial irradiation or modified intensified LSA2-L2 (New York) regimen with 5-drug induction, intensified consolidation but still total of 18 months of therapy (Steinherz, et al 1986). The modified BFM and New York regimens without prophylactic cranial irradiation provided better EFS than the LSA2-L2 regimen. Based on the results from CCG-123 that suggested that the outcome for patients with disseminated LL might benefit from an intensification of therapy, we designed a pilot study to assess the toxicity and feasibility of a compressed (a total of 12 months of therapy) aggressive multi-agent induction and consolidation chemotherapy regimen using non cross resistant maintenance therapy for a total duration of 12 months in children and adolescents with newly diagnosed stage III and IV LL.
PATIENTS AND METHODS
Eligibility Criteria
Newly diagnosed and previously untreated patients aged 21 years and under with disseminated lymphoblastic lymphoma (St. Jude stage III and IV) were eligible. All patients underwent physical exam, computed tomography (CT) scans, skeletal scintigraphy, complete blood count, bone marrow exam, cerebrospinal fluid exam, and LDH level to determine extent of disease. Gallium scans were not required but were recommended. Patients with central nervous system (CNS) disease defined as more than five white blood cells per high power field with lymphoblasts on cytospin examination, cranial nerve palsy, and/or radiographic evidence of a mass lesion in the CNS were eligible except those requiring emergency radiation therapy. Emergency therapy for management of airway obstruction and/or superior vena cava syndrome was allowed providing that diagnostic bone marrow and CSF examinations were obtained prior to steroid therapy and provided that protocol therapy was begun within 72 hours of initiation of the emergency therapy. The protocol was reviewed and approved by local institutional review boards and written informed consent was obtained on every patient enrolled.
Central Pathology Review
All cases with available tissue underwent central pathology review by two independent pathologists (SLP and DZ) to confirm the diagnosis and immunohenotype (precursor T or precursor B) with over 85% of cases entered onto the protocol undergoing central pathology review. Institutional histology and immunophenotying (flow cytometry or immunohistochemical staining of paraffin-fixed tissue) were reviewed. If sufficient phenotypic data were not available from the submitting institution, an attempt to determine phenotype by immunoperoxidase staining was made when unstained slides from paraffin-embedded diagnostic tissue samples were available. The battery of immunohistochemical stains used to determine phenotype and diagnosis included CD79a and CD20 (Dako, Carpinteria, CA) for B-cell disease, terminal deoxytidyl transferase (TdT) (Supertechs, Bethesda, MD), as well as CD1a (Immunotech/Coulter, Hialeah, FL), CD3, CD5, CD45RO (Dako, Carpinteria, CA), and/or CD43 (Ventana, Tucson, AZ) for T-cell disease. Dependent on the numbers of unstained slides available and the commercial availability of each specific antibody at the time of testing, the antibodies used to characterize each case were chosen to allow for the most definitive immunophenotyping possible. Central immunophenotyping was not required for study eligibility.
Treatment Protocol
The treatment protocol is shown in Table 1. A three week induction therapy was followed by a three-week consolidation period. Days 0 and 16 of consolidation were dependent on neutrophil and platelet recovery with granulocyte-colony stimulating factor (G-CSF) utilized during these phases. Those patients in complete remission after consolidation received six courses of maintenance non cross resistant chemotherapy at seven week intervals. Day 0 and day 35 of each maintenance course began when the absolute neutrophil count and platelet count recovered greater then 1000/mm3 and 100k/mm3, respectively. During maintenance, G-CSF was given for delays in therapy secondary to myelosuppression. Patients not in complete remission after one course of maintenance chemotherapy were considered early failures and discontinued protocol therapy. Only patients that were CNS positive at diagnosis received 1800 cGy cranial irradiation therapy at the end of therapy.
Table 1.
Treatment
| Induction 3 weeks |
Consolidation 3 weeks |
Maintenance 42 weeks (6 Courses × 7 weeks) |
||||||
|---|---|---|---|---|---|---|---|---|
| DIAGNOSIS | Vincristine | 1.5 mg/m2 IV d0, 7, 14 | ASSESSMENT | Vincristine | D0, d7 | ASSESSMENT | Pulse A | |
| Prednisone | 60 mg/m2 po d0−d20, taper over 7 days | Cytarabine | 2000 mg/m2 IV d0 + d1 | IT Methotrexate | By age | |||
| Daunamycin | 60 mg/m2 IV over 48 hrs d1−d2 | Etoposide | 200 mg/m2 IV d0 + d1 | Cyclophosphamide | 1200 mg/m2 IV | |||
| Cyclophosphamide | 1200 mg/m2 IV d0 | Thioguanine | 300 mg/m2 po d0−d3 | Thioguanine | 300 mg/m2 po/d × 4 day | |||
| L-Asparaginase | Either PEG* L’ASP or E. Coli asp starting d3 | IT Methotrexate | By age d14, d21 | Pulse B | ||||
| IT Cyctocine Arabinoside / Methotrexate | By age d0, only/by age d7+, d14, d21+ | High Dose Methotrexate/Leukovorin | 1000 mg/m2 IV over 24 hrs | Vincristine | 15 mg/m2 IV d14, d21 | |||
| G-CSF | 5 mcg/Kg until ANC>10,000 | Prednisone | 180 mg/m2/d po for 7 days | |||||
| +CNS positive only | Doxorubicin | 30 mg/m2 IV d 14 | ||||||
| Pulse C | ||||||||
| Vincristine | 1.5 mg/m2 IV d28 | |||||||
| High Dose Methotrexate/Leukovorin | 1000 mg/m2 IV over 24 hrs | |||||||
| L-Asparaginase | PEG* L’asp or E. Coli L’asp, day 29 | |||||||
| Pulse D | ||||||||
| Cytarabine | 2000 mg/m2 IV d35 + d36 | |||||||
| Etoposide | 200 mg/m2 IV d35 + d36 | |||||||
| RT for CNS positive only | 1800 cGy cranial & 600 cGy spine at completion of chemotherapy | |||||||
| G-CSF all pulses | ||||||||
dependent on availability of PEG 1’asp 25,000 u/m2 IM/E. Coli l’asp 20,000 u/m2 IM weekly × 3
Response Criteria
Complete response (CR) was defined as the resolution of all measurable mass lesions by radiography, bone marrow <5% blasts and CSF white blood cells (WBC) <5/mm3 with no blasts on two consecutive taps. Patients were considered in partial response (PR) if there was a decrease of 50% or more in all measurable mass lesions. Patients were removed from protocol therapy if not in CR after one maintenance cycle.
Statistical Methods
The primary endpoint for statistical survival analysis was event free survival (EFS), defined as the minimum time from study entry to failure to induce remission, disease progression, disease relapse, the occurrence of a second malignant neoplasm, or death from any cause. Secondary endpoints included overall survival (OS) defined as time from study entry to death from any cause, and post-recurrence/progression survival (PRS) defined as time to death among patients who experienced disease progression or relapse. This computation excluded patients who died of treatment complications, who experience second malignant neoplasms, or who had no follow-up after recurrence. All analyses are based on “intent-to-treat,” whereby patients were not censored for reasons of deviations from protocol therapy.
Univariate analyses of differences in EFS, OS, and PRS among patient subgroups were based on the logrank test. Estimates and standard errors of EFS, OS, and PRS percent were obtained from the product limit estimate with Greenwood standard errors (Kalbfleisch and Prentice 1980) Standard errors of estimates are indicated with “±”. The cumulative incidence of grade 3–4 toxicity through 8 cycles of treatment were computed using life table methods (Kalbfleisch and Prentice 1980) using the first occurrence of a toxicity as the endpoint. Patients are censored from this analysis at the time they stopped receiving protocol therapy for any reason.
Toxicity
Toxicity was graded according to the NCI CTCAE 2.0 Criteria
RESULTS
Study patients
Between July 1994 and June 1997, 107 patients were enrolled on CCG-5941. Twenty-two patients were excluded for the following reasons: large cell lymphoma patients (7), mature B-cell lymphoma (4), natural killer cell lymphoma (1), one lymphoma specific phenotype indeterminable (1), acute lymphoblastic leukemia (3), neuroblastoma (1), rhabdomyosarcoma (1), ineligible due to prestudy treatment (2), brain dead at study entry (1) , and excluded in accordance with regulatory requirements (1). Demographic and disease characteristics of the 85 remaining patients are summarized in Table 2.
Table 2.
Patient characteristics and EFS outcome
| Variable | Subgroup | # Patients (%) | EFS ±S.E. @ 5 years |
P-value |
|---|---|---|---|---|
| Race | White | 65 (76) | 78 ± 5 | 0.65 |
| Non-White | 20 (24) | 79 ± 9 | ||
| Sex | Male | 54 (64) | 83 ± 5 | 0.2 |
| Female | 31 (36) | 70 ± 8 | ||
| Age at study entry | 0 to 4 | 14 (17) | 77 ± 12 | 0.17 |
| 5 to 9 | 23 (27) | 91 ± 6 | 0.63 (trend) | |
| 10+ | 48 (56) | 72 ± 7 | ||
| LDH | < 2X Normal | 58 (68) | 78 ± 6 | 0.72 |
| > 2X Normal | 27 (32) | 78 ± 8 | ||
| Marrow disease | No | 73 (86) | 79 ± 5 | 0.86 |
| Yes | 12 (14) | 74 ± 13 | ||
| CNS disease | No | 82 (94) | 79 ± 5 | <0.05(2) |
| Yes | 3 (5) | 2/3 fail | ||
| Ant. MM > 1/3(1) | Yes | 57 (67) | 77 ± 6 | 0.63 |
| No | 27 (32) | 80 ± 8 | ||
| Unknown | 1 (1) | |||
| Induction Response | CR1 | 36 (42) | 83 ± 6(3) | 0.73 |
| CR2 | 31 (37) | 76 ± 8(3) | ||
| Unknown | 18 (21) | - |
Ant MM: Anterior mediastinal mass;
Permutaton logrank test;
post-induction EFS
Seventy-seven cases (91%) showed a precursor T-cell phenotype by either institutional immunophenotypic data or central immunophenotyping, six cases (7.0%) showed a precursor B-cell phenotype and two cases (2.3%) showed expression of both precursor B and early T-cell antigens (CD79a and CD45RO staining). One case was notable for extensive tissue eosinophilia accompanying a T-lymphoblastic lymphoma. The diagnosis in 76 of the 85 eligible patients was confirmed by central histopathology review.
Toxicity and Complications of Treatment
The toxicity profile of the treatment regimen is shown in Table 3. Four patients (5%) died for reasons not related to disease progression (toxic deaths). Three deaths were due to infectious complications. The other death was due to venoclusive disease of the liver. Eighty percent of patients experienced at least one episode of grade III/IV neutropenia during treatment, 68% experienced grade III/IV thrombocytomia, and 20% experienced grade III/IV infection. Furthermore, there was a ≥ 21% incidence of grade III/IV stomatitis and 29% incidence of elevated liver transaminases.
Table 3.
Grade 3 or 4 toxicity through 8 cycles: Induction, Consolidation, Maintenance 1–6 (Frequency and percent per course, and cumulative incidence)
| Toxicity | Ind. (N=85) |
Cons. (N=82) |
Mnt. 1 (N=76) |
Mnt. 2 (N=73) |
Mnt. 3 (N=70) |
Mnt. 4 (N=68) |
Mnt. 5 (N=65) |
Mnt. 6 (N=65) |
Cum. % |
|---|---|---|---|---|---|---|---|---|---|
| ANC | 20 | 43 | 32 | 34 | 40 | 38 | 35 | 32 | |
| 23.5% | 52.4% | 42.1% | 46.6% | 57.1% | 55.9% | 53.8% | 49.2% | 80% | |
| PLT | 2 | 40 | 24 | 20 | 25 | 31 | 28 | 27 | |
| 2.4% | 48.8% | 31.6% | 27.4% | 35.7% | 45.6% | 43.1% | 41.5% | 68% | |
| HGB | 0 | 35 | 28 | 20 | 21 | 21 | 19 | 16 | |
| 0.0% | 42.7% | 36.8% | 27.4% | 30.0% | 30.9% | 29.2% | 24.6% | 65% | |
| LIVER: SGOT/SGPT/Alk. Phos. | 5 | 7 | 7 | 5 | 4 | 8 | 6 | 8 | |
| 5.9% | 8.5% | 9.2% | 6.8% | 5.7% | 11.8% | 9.2% | 12.3% | 29% | |
| LIVER: Bilirubin | 4 | 8 | 1 | 0 | 0 | 1 | 1 | 0 | |
| 4.7% | 9.8% | 1.3% | 0.0% | 0.0% | 1.5% | 1.5% | 0.0% | 15% | |
| PANCREAS: Amylase, Ultrasound | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 0.0% | 4.9% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 1.5% | 6% | |
| PANCREAS: Glucose | 4 | 2 | 2 | 2 | 1 | 2 | 3 | 1 | |
| 4.7% | 2.4% | 2.6% | 2.7% | 1.4% | 2.9% | 4.6% | 1.5% | 14% | |
| RENAL: BUN/Creat/CrClr | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | |
| 0.0% | 1.2% | 0.0% | 0.0% | 0.0% | 0.0% | 0.0% | 1.5% | 3% | |
| RENAL: SBP/DBP | 2 | 2 | 0 | 0 | 1 | 2 | 2 | 1 | |
| 2.4% | 2.4% | 0.0% | 0.0% | 1.4% | 2.9% | 3.1% | 1.5% | 8% | |
| GI: Stomatitis | 0 | 5 | 3 | 6 | 2 | 3 | 1 | 4 | |
| 0.0% | 6.1% | 3.9% | 8.2% | 2.9% | 4.4% | 1.5% | 6.2% | 21% | |
| GI: Pain/Constipation/Diarrhea | 1 | 7 | 1 | 1 | 1 | 2 | 1 | 0 | |
| 1.2% | 8.5% | 1.3% | 1.4% | 1.4% | 2.9% | 1.5% | 0.0% | 14% | |
| GI: Nausea/Vomiting | 3 | 4 | 0 | 1 | 2 | 0 | 1 | 0 | |
| 3.5% | 4.9% | 0.0% | 1.4% | 2.9% | 0.0% | 1.5% | 0.0% | 12% | |
| PULMONARY | 1 | 4 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 1.2% | 4.9% | 0.0% | 1.4% | 0.0% | 0.0% | 0.0% | 0.0% | 7% | |
| NERVOUS SYSTEM: Peripheral | 0 | 1 | 2 | 4 | 0 | 0 | 2 | 1 | |
| 0.0% | 1.2% | 2.6% | 5.5% | 0.0% | 0.0% | 3.1% | 1.5% | 8% | |
| NERVOUS SYSTEM: Central | 3 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | |
| 3.5% | 2.4% | 0.0% | 0.0% | 0.0% | 0.0% | 1.5% | 0.0% | 7% | |
| INFECTION | 1 | 5 | 4 | 3 | 6 | 1 | 4 | 5 | |
| 1.2% | 6.1% | 5.3% | 4.1% | 8.6% | 1.5% | 6.2% | 7.7% | 20% |
100% of induction courses and 72% of consolidation courses were completed within 35 days (i.e., within two weeks the nominal course length of 21 days). On the other hand, only 17% of maintenance courses were completed within 56 days (nominal length 42 days). The average duration of maintenance courses was 70 days.
Treatment outcome
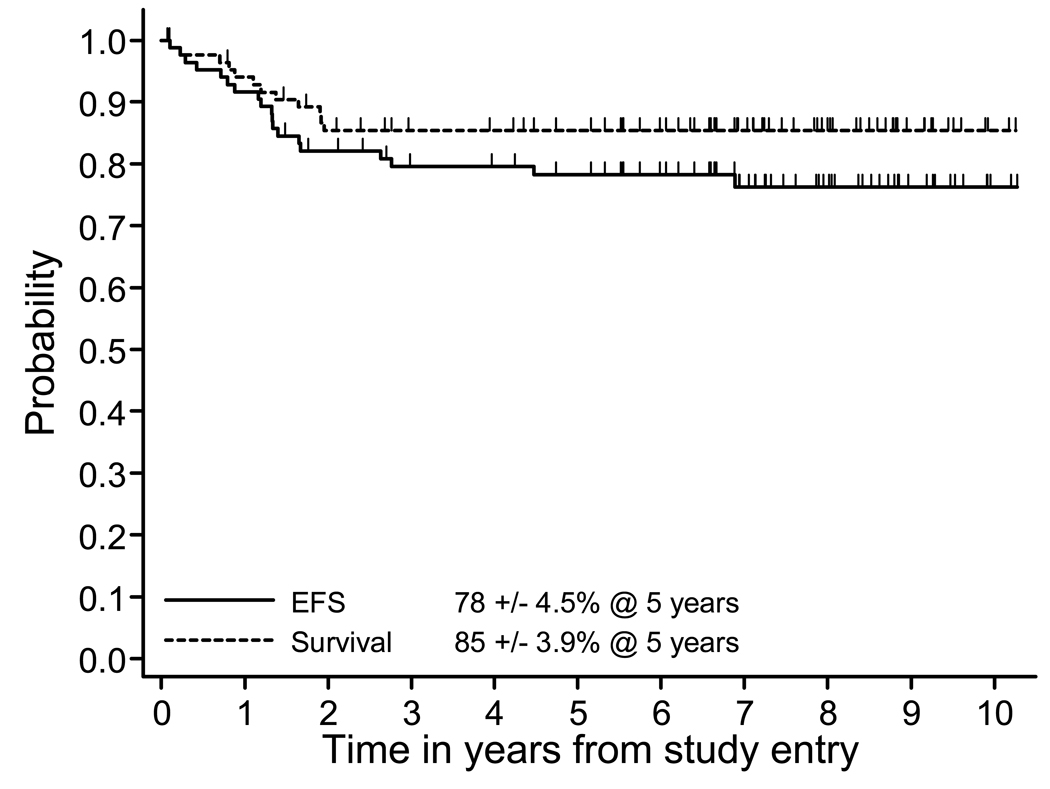
The five-year EFS and OS was 78 % ± 4.5 % and 85 % ± 3.9 %, respectively (Figure 1). Median follow-up among patients who had not experienced an event was 7.2 years, with 85% of patients followed at least five years from study entry. A total of 19 events occurred with only two events more than three years from study entry. Of the 19 events, 13 were relapses, two were secondary AML, and four were toxic deaths unrelated to disease progression. Five-year post recurrence survival (PRS) was 33±14% in 13 patients with post-event follow-up. There were 9 observed deaths, 8/9 occurring within 7 months and 1 at 2.5 years. Four patients were alive at last reported contact -- 1.9, 2.7, 5, and 7.5 years after the event. Analysis of selected prognostic factors including age, race, sex, presence of a mediastinal mass, LDH, induction response, marrow disease at diagnosis showed no impact on EFS. Only three patients presented with CNS disease and two failed (Table 2). More patients with CNS disease need to be treated with this regimen to determine if CNS involvement is a true prognostic factor.
Figure One. Event-Free Survival and Survival from Study Entry.
Probability of event free survival (EFS) and overall survival (OS) of all patients from diagnosis.
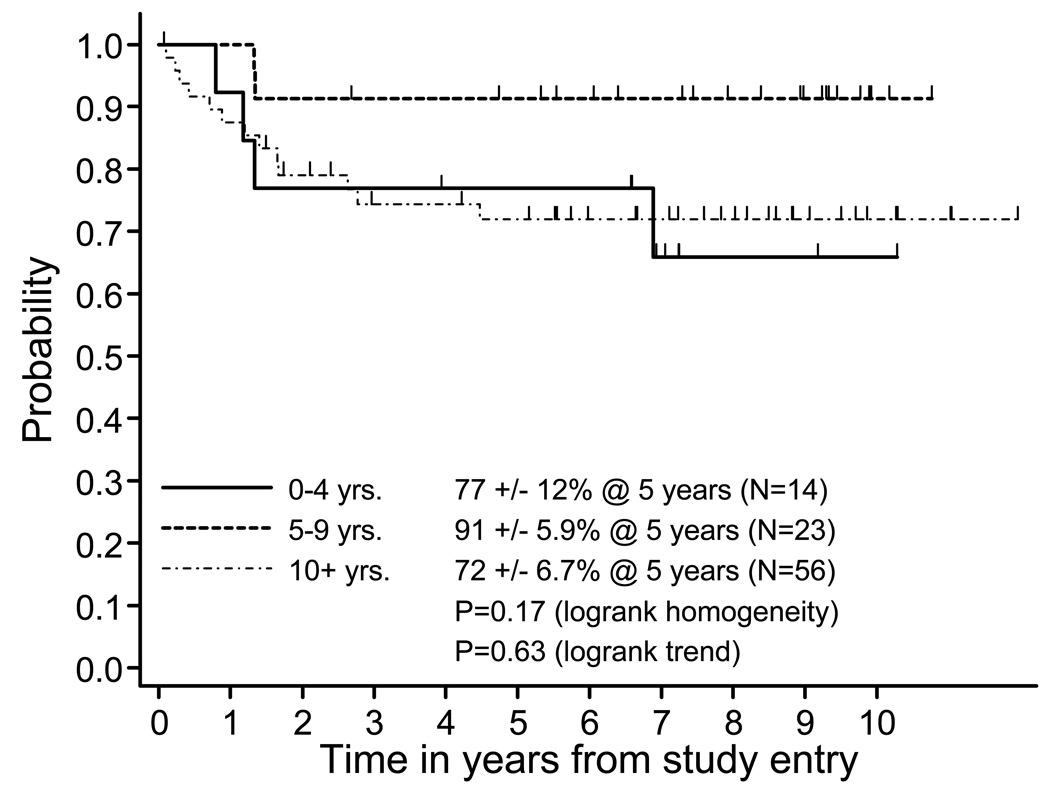
Although children between 5 and 9 yrs of age appeared to do slightly better, there was no significant difference in the 5 yr EFS by age (Figure 2).
Figure Two. Event-free survival by age at diagnosis.
Comparison of event free survival (EFS) stratified by age groups 0–4 years ( ———— ), 5–9 years ( --------- ), 10+ years (-.-.-.- -.- )of all patients from diagnosis. (p=0.17, logrank test for homogeneity, p=0.63, logrank test for trend)
DISCUSSION
The optimal treatment and duration of therapy for advanced childhood and adolescent LL has yet to be determined. Although there are many similarities between T-ALL and T-LL, recent microarray studies have suggested a significant biological heterogeniety between the two histological groups (Cairo, et al 2005, Raetz, et al 2006). Patients with greater than 25% lymphoblasts in the bone marrow are considered to have ALL and are usually treated on childhood acute lymphoblastic leukemia protocols. It remains to be determined whether advanced LL should be treated similar to T-ALL therapy with prolonged maintenance therapy (2 years) or because of biological differences treated differently and more like a B-NHL and/or like anaplastic large cell lymphoma with more aggressive induction and consolidation therapy and less maintenance therapy.
Outcome of disseminated LL from institutional and other pediatric cooperative group studies outside of the Children’s Oncology Group (COG) have reported disease-free survivals of 35–88% (Table 4). The Dana Farber consortium has utilized the APO backbone [adriamycin, prednisone, vincristine, 6-mercaptopurine (6-MP), L-asparaginase] (Weinstein, et al 1983) This regimen also utilized both cranial irradiation and intrathecal (IT) methotrexate for CNS prophylaxis(Weinstein, et al 1983). A United Kingdom trial addressed the question regarding the need for adjuvant low dose radiation therapy (RT) to areas of bulk disease. The four year EFS was only 46% and they concluded that RT therapy to sites of bulk disease provided no added benefit (Mott, et al 1984). A more recent study, UK-CCSG-8503, included an early intensification with etoposide and cytosine arabinoside in lower dosages and demonstrated a four year EFS of 65% (Eden, et al 1992). Investigators at St. Jude Children's Research Hospital added the Teniposide (VM-26) and cytosine arabinoside to a conventional three drug induction [prednisone, VCR and L-asparaginase (Total Therapy X)]. The maintenance backbone consisted of standard oral 6-MP and MTX with five pulses of VM-26 and cytosine arabinoside during the first year of therapy, for a total duration of therapy for 120 weeks (Dahl, et al 1985). They reported an estimated four year EFS in 22 patients of 73%. Patte et al report similar results in 82 children with stage III and IV lymphoblastic lymphoma treated on SFOP LMT 81 (Patte, et al 1992). The treatment was a modified LSA2-L2 therapy with the addition of 10 courses of systemic high dose methotrexate. The results demonstrated a EFS of 79%, 72% for stage III and IV patients, respectively (Patte, et al 1992) A more recent study SFOP LMT 96 incorporated HD MTX (3gm/m2) on day 8 of induction and additional maintenance therapy and reported 5 year EFS of 87% but included some stage I and II patients (Bergeron, et al 2006). The BFM protocols for lymphoblastic lymphoma, which utilized a ALL like backbone, have reported the best results to date with a 5 year EFS of 82± 3% with a toxic death rate of about 2–3% (Burkhardt, et al 2006b, Reiter, et al 2000). This protocol utilized systemic high dose methotrexate at 5 gm/m2 followed by 1800 cGy cranial irradiation for CNS positive patients only. Similary, the EORTC (Uyttebroeck, et al 2008), utilizing a BFM like backbone, reported a 79% and 81% 6 year EFS for Stage III and IV LL, respectively (Uyttebroeck, et al 2008).
Table 4.
Comparative Outcome in the Treatment of Advanced Childhood Lymphoblastic Lymphoma
| Author/Year | Treatment Protocol | # of Patients | Outcome (3–5 YR EFS) |
|---|---|---|---|
| Weinstein et al, 1983 | Dana Farber (APO) | 21 | 58±23% |
| Dahl et al, 1985 | St. Jude (Total Therapy X) | 22 | 72% |
| Eden et al, 1992 | UKCCSG 8503 | 95 | 65% |
| Patte et al, 1992 | SFOP LMT81 | 82 | *75% |
| Anderson et al, 1993 | CCG 551 (COMP) | 40 | 35% |
| Anderson et al, 1993 | CCG 551 (LSA2-L2) | 124 | 64% |
| Tubergen et al, 1995 | CCG 502 (LSA2-L2) | 143 | 74% |
| Tubergen et al, 1995 | CCG 502 (ADCOMP) | 138 | 64% |
| Reiter et al, 2000 | BFM NHL 90 | 105 | *90% |
| Mora et al, 2003 | MSKCC (LSA2-L2) | 95 | *75% |
| Burkkardt et al, 2006 | BFM-NHL 95 | 156 | 88% |
| Bergeron et al, 2006 | SFOP LMT96 | 87 | *87% |
| Uyttebroeck et al, 2008 | EORTC (CLG 58881) | 119 | 79% |
| Abromowitch et al, (Current Study) | CCG 5941 | 85 | 78±4.5% |
includes Stage I & II
This pilot study of a compressed (12 month) aggressive multi-agent chemotherapy regimen for newly diagnosed pediatric patients with disseminated LL demonstrated a 5 year EFS and OS of 78 ± 4.5% and 85±3.9%, respectively. (Figure 1) These results are similar as previously published for children with advanced T-LL regimens. However, there were four toxic deaths (5%) due to complications of grade III/IV hematopoietic toxicity. This compares to five early/toxic deaths (5/186 pts, 3.2%) in the BFM-NHL 95 advanced LL study (Burkhardt, et al 2006b). Delays of chemotherapy during the maintenance pulses were prevalent in this study. Additionally, this study demonstrates the difficulty in salvaging relapsed patients, i.e. 33% survival rate after relapse or progression .
Understanding the biological heterogeneity of T-LL and the prognostic importance will help tailor future therapy with more aggressive therapy for those patients with poor biological prognostic factors. Burkhardt et al reported that loss of heterozygosity (LOH) of 6q14-24 was associated with a significant increase risk of relapse in advanced childhood LL (Burkhardt, et al 2006a). Notch 1 mutations have been identified in 50–60% of T-ALL and it remains to be determined if Notch mutations are associated with T-LL and whether other mutations in childhood T-LL have any prognostic importance (Ferrando and Look 2003). Furthermore, Coustan-Smith et al has recently demonstrated minimal disease (MD) in the peripheral blood of children with Stage III LL at diagnosis by flow cytometry of double positive CD3 and TDT cells in 67% at diagnosis (Coustan-Smith, et al 2007). Persistent MD during and/or at the end of induction therapy may also identify a poor risk group of patients (Coustan-Smith, et al 2007).
In summary, with modern intensive chemotherapy, the outcome for most children and adolescents with advanced lymphoblastic lymphoma is excellent; however 15 to 20% of patients still fail and post recurrence survival is poor. It remains to be determined whether shorter aggressive treatment strategies like this study will provide similar results compared to more prolonged childhood ALL type regimens in children with advanced LL. This would require a randomized prospective comparison. However, as demonstrated in this study, clinical factors predictive of failure to therapy have been difficult to identify for children with advanced lymphoblastic lymphoma. Therefore, to improve outcome for these patients, future studies should focus on identifying biological factors at initial diagnosis or early into therapy, so that alternative therapies may be investigated. Current studies in the Children’s Oncology Group (COG) (COG A5971) are evaluating methods to identify patients at high risk for early failure by assessing rapidity of response by imaging techniques, measuring minimal disease in blood and bone marrow and molecular and genetic analysis of initial diagnostic tumor tissue. COG A5971 utilizes ALL therapy similar to BFM NHL-95 . It is a randomized trial to test the role of high dose methotrexate and early intensification. The results of this study will determine whether we pursue ALL or shortened intensified lymphoma therapy (CCG 5941) in the future.
ACKNOWLEDGMENTS
This work was supported in part by COG Grant CA 98543. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm.
We thank all the institutional investigators and staff for the enrollment and care of the patients. We would also like to thank Lauren Harrison, RN and Jennifer Gaecke for their assistance in the preparation of this manuscript.
Footnotes
Presented in part at the American Society of Hematology (ASH) Meeting, December 2006, Orlando, Florida
References
- Anderson JR, Jenkin RD, Wilson JF, Kjeldsberg CR, Sposto R, Chilcote RR, Coccia PF, Exelby PR, Siegel S, Meadows AT, et al. Long-term follow-up of patients treated with COMP or LSA2L2 therapy for childhood non-Hodgkin's lymphoma: a report of CCG-551 from the Childrens Cancer Group. J Clin Oncol. 1993;11:1024–1032. doi: 10.1200/JCO.1993.11.6.1024. [DOI] [PubMed] [Google Scholar]
- Anderson JR, Wilson JF, Jenkin DT, Meadows AT, Kersey J, Chilcote RR, Coccia P, Exelby P, Kushner J, Siegel S, Hammond D. Childhood non-Hodgkin's lymphoma. The results of a randomized therapeutic trial comparing a 4-drug regimen (COMP) with a 10-drug regimen (LSA2-L2) N Engl J Med. 1983;308:559–565. doi: 10.1056/NEJM198303103081003. [DOI] [PubMed] [Google Scholar]
- Bergeron C, Celine S, Pacquement H, Perel Y, Coze C, Gandemer V, Vannier JP, Mechinaud F, Schmitt C, Leverger G, Baruchel A, Perol D, Patte C. Childhood T-cell lymphoblastic lymphoma (TLL) results of the SFOP LMT96 strategy. Pediatr Blood Cancer. 2006;46:867a. [Google Scholar]
- Burkhardt B, Bruch J, Salzburg J, Zimmermann M, Reiter A. Clinical and biological significance of loss of heterozygosity at chromosome 6q in children and adolescents with T-cell lymphoblastic lymphoma. Pediatr Blood Cancer. 2006a;46:840a. [Google Scholar]
- Burkhardt B, Woessmann W, Zimmermann M, Kontny U, Vormoor J, Doerffel W, Mann G, Henze G, Niggli F, Ludwig WD, Janssen D, Riehm H, Schrappe M, Reiter A. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. 2006b;24:491–499. doi: 10.1200/JCO.2005.02.2707. [DOI] [PubMed] [Google Scholar]
- Cairo MS, Raetz E, Lim MS, Davenport V, Perkins SL. Childhood and adolescent non-Hodgkin lymphoma: new insights in biology and critical challenges for the future. Pediatr Blood Cancer. 2005;45:753–769. doi: 10.1002/pbc.20342. [DOI] [PubMed] [Google Scholar]
- Coustan-Smith E, Abromowitch M, Sandlund J, Campana D. A novel approach for minimal residual disease detection in childhood T-cell lymphoblastic lymphoma (T-LL): A Children's Oncology Group Report. American Society of Hematology. 2007 Submitted Abstract. [Google Scholar]
- Dahl GV, Rivera G, Pui CH, Mirro J, Jr, Ochs J, Kalwinsky DK, Abromowitch M, Look AT, Murphy SB. A novel treatment of childhood lymphoblastic non-Hodgkin's lymphoma: early and intermittent use of teniposide plus cytarabine. Blood. 1985;66:1110–1114. [PubMed] [Google Scholar]
- Eden OB, Hann I, Imeson J, Cotterill S, Gerrard M, Pinkerton CR. Treatment of advanced stage T cell lymphoblastic lymphoma: results of the United Kingdom Children's Cancer Study Group (UKCCSG) protocol 8503. Br J Haematol. 1992;82:310–316. doi: 10.1111/j.1365-2141.1992.tb06423.x. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Look AT. Gene expression profiling in T-cell acute lymphoblastic leukemia. Semin Hematol. 2003;40:274–280. doi: 10.1016/s0037-1963(03)00195-1. [DOI] [PubMed] [Google Scholar]
- Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley and Sons; 1980. [Google Scholar]
- Mora J, Filippa DA, Qin J, Wollner N. Lymphoblastic lymphoma of childhood and the LSA2-L2 protocol: the 30-year experience at Memorial-Sloan-Kettering Cancer Center. Cancer. 2003;98:1283–1291. doi: 10.1002/cncr.11615. [DOI] [PubMed] [Google Scholar]
- Mott MG, Chessells JM, Willoughby ML, Mann JR, Morris-Jones PH, Malpas JS, Palmer MK. Adjuvant low dose radiation in childhood T cell leukaemia/lymphoma (report from the United Kingdom Childrens' Cancer Study Group--UKCCSG) Br J Cancer. 1984;50:457–462. doi: 10.1038/bjc.1984.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patte C, Kalifa C, Flamant F, Hartmann O, Brugieres L, Valteau-Couanet D, Bayle C, Caillaud JM, Lemerle J. Results of the LMT81 protocol, a modified LSA2L2 protocol with high dose methotrexate, on 84 children with non-B-cell (lymphoblastic) lymphoma. Med Pediatr Oncol. 1992;20:105–113. doi: 10.1002/mpo.2950200204. [DOI] [PubMed] [Google Scholar]
- Raetz EA, Perkins SL, Bhojwani D, Smock K, Philip M, Carroll WL, Min DJ. Gene expression profiling reveals intrinsic differences between T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Pediatr Blood Cancer. 2006;47:130–140. doi: 10.1002/pbc.20550. [DOI] [PubMed] [Google Scholar]
- Reiter A, Schrappe M, Ludwig WD, Tiemann M, Parwaresch R, Zimmermann M, Schirg E, Henze G, Schellong G, Gadner H, Riehm H. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: a BFM group report. Blood. 2000;95:416–421. [PubMed] [Google Scholar]
- Steinherz PG, Gaynon P, Miller DR, Reaman G, Bleyer A, Finklestein J, Evans RG, Meyers P, Steinherz LJ, Sather H, et al. Improved disease-free survival of children with acute lymphoblastic leukemia at high risk for early relapse with the New York regimen--a new intensive therapy protocol: a report from the Childrens Cancer Study Group. J Clin Oncol. 1986;4:744–752. doi: 10.1200/JCO.1986.4.5.744. [DOI] [PubMed] [Google Scholar]
- Tubergen DG, Krailo MD, Meadows AT, Rosenstock J, Kadin M, Morse M, King D, Steinherz PG, Kersey JH. Comparison of treatment regimens for pediatric lymphoblastic non-Hodgkin's lymphoma: a Childrens Cancer Group study. J Clin Oncol. 1995;13:1368–1376. doi: 10.1200/JCO.1995.13.6.1368. [DOI] [PubMed] [Google Scholar]
- Uyttebroeck A, Suciu S, Laureys G, Robert A, Pacquement H, Ferster A, Marguerite G, Mazingue F, Renard M, Lutz P, Rialland X, Mechinaud F, Cave H, Baila L, Bertrand Y. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: Long term results of the EORTC CLG 58881 trial. Eur J Cancer. 2008;44:840–846. doi: 10.1016/j.ejca.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Weinstein HJ, Cassady JR, Levey R. Long-term results of the APO protocol (vincristine, doxorubicin [adriamycin], and prednisone) for treatment of mediastinal lymphoblastic lymphoma. J Clin Oncol. 1983;1:537–541. doi: 10.1200/JCO.1983.1.9.537. [DOI] [PubMed] [Google Scholar]