Abstract
A disintegrin and metalloproteinases (ADAMs) are members of a new gene family of transmembrane and secreted proteins, which belong to the zinc proteinase superfamily. These molecules are involved in various biological events such as cell adhesion, cell fusion, cell migration, membrane protein shedding, and proteolysis. Growing evidence now attests to the potential involvement of ADAMs proteinases in diverse processes such as skin wound healing, inflammation, pigmentation, tumor development, cell proliferation, and metastasis. This paper focuses on the roles of ADAMs proteinases in a wide variety of skin diseases.
1. Introduction
A disintegrin and metalloproteinases (ADAMs) are members of a new gene family of transmembrane and secreted proteins, which belong to the zinc protease superfamily. These molecules are involved in various biological processes such as cell adhesion, cell fusion, cell migration, membrane protein shedding, and proteolysis. It has become clear in recent years that ADAMs proteinases have important roles in skin homeostasis and in skin diseases, and the signaling cascades involved are gradually being identified [1, 2].
Many transmembrane proteins are processed by one or several proteolytic steps to their biologically active forms [1, 2]. Examples include growth factors such as epidermal growth factor (EGF), heparin-binding EGF-like growth factor (HB-EGF) and transforming growth factor-α (TGF-α), and cytokines such as tumor necrosis factor-α (TNF-α), all of which are synthesized as precursor proteins that need to be cleaved to gain their functional activity. In addition, there are a number of cell surface receptors that undergo cleavage near the transmembrane domain, a process called ectodomain shedding [1, 2]. These include the TNF-α receptor, CD44, L-selectin and KIT. The soluble, released ectodomains of those receptors may be part of their downmodulation in response to ligand activation and/or those fragments may have functions of their own.
In this paper, we discuss the implications of ADAMs proteinases in several physiological and pathological functions in skin tissue and their roles in various skin diseases.
2. Structure and Function of ADAMs Proteinases
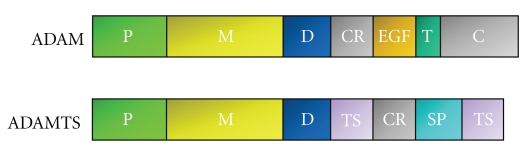
There are two distinct groups in the ADAMs family: the membrane-anchored ADAMs and the secreted-type ADAMTS (ADAM with thrombospondin motifs), which are not discussed in this paper. Each member of the ADAMs family contains common domains, including the propeptide, metalloproteinase, disintegrin, cysteine-rich, EGF-like (though this is absent in ADAM10 and ADAM17), transmembrane and cytoplasmic domains (Figure 1). Although the structure of ADAMs and ADAMTS proteinases is closely related, ADAMTS molecules are characterized by various numbers of thrombospondin type one (TSP-1) motifs at their C-terminal ends and the absence of transmembrane and cytoplasmic domains.
Figure 1.
Schematic of domain structure of ADAM proteinases. P: propeptide domain, M: metalloproteinase domain, D: disintegrin domain, CR: cysteine-rich domain, EGF: EGF-like domain, T: transmembrane domain, C: cytoplasmic domain, SP: spacer domain, TS: thrombospondin type I motif.
The ADAMs group is comprised of more than 30 members (reviewed in [1, 2]). Proteinase activities have been demonstrated for ADAM8, 9, 10, 12, 15, 17, 19, 20, 21, 28, and 33, and these molecules contain a common HEXGHXXGXXHD sequence. About 60% of the members of this group are nonproteolytic ADAMs molecules.
Several ADAMs are expressed in multiple spliced forms. For example, ADAM22, ADAM29, and ADAM30 have two to three forms that vary in the lengths of their cytoplasmic tails, although no functional differences in those isoforms have been reported. In contrast, ADAM12 has two splice forms, a membrane-bound form and a secreted form, which have markedly different activities.
ADAM17 is the most extensively investigated ADAM proteinase and is known to release soluble TNF-α from its membrane precursor, pro-TNF-α, which permits TNF-α paracrine signaling; thus, ADAM17 is also called TACE (TNF-α-converting enzyme) [3]. ADAM17 plays a critical role in the ectodomain shedding of many soluble proteins, including TNF-α, TNF receptors I and II, TGF-α, HB-EGF, amphiregulin (AR), and interleukin-6 receptor (IL-6R). The development of pharmaceutical inhibitors for ADAM17 has focused on its role as a modulator of TNF-α in rheumatoid arthritis [4]. As TNF-α is a potent proinflammatory cytokine, ADAM17 inhibitors may be of particular utility in respiratory disease, ulcerative colitis, and other diseases. ADAM9 cleaves and releases EGF, HB-EGF, and fibroblast growth factor receptor 2 IIIb. ADAM10 is involved in the ectodomain shedding of various substrates, including adhesion molecules such as L1 cell adhesion molecule (L1-CAM) and CD44, E-cadherin, and N-cadherin, IL-6R, CD30. ADAM12 is known to participate in the ectodomain shedding of several potential substrates, including HB-EGF and EGF [5]. Moreover, ADAM12 regulates transforming growth factor-β (TGF-β) receptor trafficking. ADAM12 interacts with TGF-β receptor and enhances TGF-β signaling by controlling the localization of TGF-β receptors to early endosomes [6].
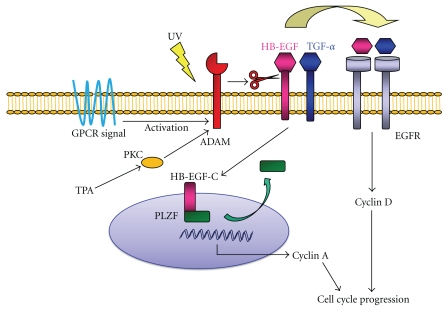
The ectodomain shedding of proHB-EGF actually produces two fragments, an extracellular fragment (HB-EGF), and a remnant fragment (HB-EGF-C). Recently, promyelocytic leukemia zinc finger (PLZF) was identified as a binding protein of the cytoplasmic tail of proHB-EGF [7]. PLZF is a transcriptional repressor of cyclin A and suppresses cell growth by inhibiting entry or progression into the S-phase of the cell cycle. Subsequent to proteolytic cleavage of proHB-EGF, HB-EGF-C translocates from the plasma membrane into the nucleus, which triggers nuclear export of the transcriptional repressor PLZF. Suppression of cyclin A and delayed entry into S-phase of cells expressing PLZF can be reversed by the production of HB-EGF-C. These results indicate that released HB-EGF-C functions as an intracellular signal and coordinates cell cycle progression with HB-EGF.
3. Involvement of ADAMs Proteinases in Skin Diseases
3.1. Skin Cancer
Since ADAMs can mediate the shedding of growth factors and regulate the adhesion and motility of cells, ADAMs family proteinases are involved in signaling events that are dysregulated in cancers and during tumor progression [8]. In many types of cancers, ADAMs are upregulated and several recent studies have highlighted the potential of targeting ADAMs family members as a new approach for antitumor therapy [9]. A schematic summarizing the functions of ADAMs proteins is shown in Figure 2.
Figure 2.
Physiological functions of ADAM proteinases in cancer.
3.1.1. Squamous Cell Carcinoma (SCC)
G protein-coupled receptors (GPCRs) have been shown to activate some ADAMs proteinases and to transactivate epidermal growth factor receptor (EGFR) [10]. Ultraviolet (UV) radiation of skin cancer cells activates ADAMs and induces EGFR ligand shedding and EGFR transactivation [11]. It is likely that UV irradiation induces reactive oxygen species (ROS) generation, and that those ROS activate ADAM9 and ADAM17, which then cleave EGFR ligands, particularly AR. The soluble form of AR subsequently binds to EGFR and can induce skin cancer proliferation.
Overexpression of protein kinase Cε (PKCε) in mouse epidermis results in the rapid development of papilloma-independent metastatic SCCs via the two-stage model of carcinogenesis [12]. PKCε transgenic mice have elevated serum TNF-α levels during skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate (TPA). Since TNF-α is linked to skin tumor promotion by TPA, this increase may be linked to the development of metastatic SCC. TPA-stimulated shedding of TNF-α could be completely prevented in PKCε transgenic mice and isolated keratinocytes by an ADAM17 inhibitor, TAPI-1. These results indicate that PKCε signal transduction pathways to TPA-stimulated TNF-α ectodomain shedding are mediated by ADAM17. Injection of a TNF-α synthesis inhibitor during skin tumor promotion completely prevented the development of metastatic SCC in PKCε transgenic mice. The sum of these results indicates that ADAM17 is involved in the development of SCC in PKCε transgenic mice.
3.1.2. Basal Cell Carcinoma (BCC)
BCC is the most common type of skin tumor. BCC rarely metastasizes but is locally invasive and highly destructive. ADAM10, ADAM12, and ADAM17 are increased at the peripheral tumor margin compared with central areas of BCC tumor cell nests. Expression of ADAM10 and ADAM12 is increased in the deep margin of invading tumor cell nests. In contrast, ADAM17 is increased in superficial BCC. ADAM10, ADAM12, and ADAM17 show different expression patterns in BCC histologic subtypes, indicating their different roles in the pathogenesis of BCC [13].
3.1.3. Malignant Melanoma (MM)
ADAM9, ADAM10, ADAM12, ADAM15, and ADAM17 are overexpressed in more than 10 MM cell lines, and HB-EGF and TGF-α are overexpressed in more than 10 MM cell lines [11].
ADAM10 expression was significantly elevated in melanoma metastases compared with primary melanomas [14]. Furthermore, expression of several components of the Notch pathway, which can be cleaved by ADAM10, were upregulated in MM compared with common melanocytic nevi [15]. Down-regulation of ADAM10 with specific siRNA resulted in the suppression of cell growth and the reduced migration of MM cells. In addition, overexpression of ADAM10 induced the migration of MM cells. Soluble L1-CAM can induce cell migration through binding to integrins. Elevated levels of L1-CAM in a metastatic variant of an MM cell line suggest a role for L1-CAM in tumor progression. Knockdown of L1-CAM reduced the migration of MM cells and abrogated their chemoresistance against cisplatin [14]. On the other hand, ADAM10 is the major protease responsible for constitutive CD44 cleavage from MM cells, and its expression can impair tumor cell proliferation [16]. CD44 proteins are cell surface receptors for hyaluronic acid (HA), a component of the extracellular matrix that has multiple effects on cell behavior. ADAM10, ADAM17, and matrix metalloproteinase (MMP) 14 have previously been implicated in the shedding of CD44 from various tumor cells. In MM cells, ADAM10 and ADAM17 but not MMP14 are significantly expressed in histological sections. However, only ADAM10 but not ADAM17 is involved in the constitutive shedding of native CD44 from MM cells. HA promotes the proliferation of MM cells [17], and soluble CD44 inhibits HA-stimulated proliferation of melanoma cells in vitro and in vivo [18].
ADAM9 is detected in MM cells and in peritumoral stromal fibroblasts, while it is absent in fibroblasts distal to the tumor site. In contrast, in nevi, ADAM9 expression is absent both in nevus cells and in stromal cells close to nevus cell nests [19].
3.2. Wound Healing
Wound healing is a complex process involving multiple cellular events, including cell proliferation, migration, and tissue remodeling. Members of the EGF family, such as EGF, TGF-α, HB-EGF, AR, and their receptor EGFR, are the most important factors in skin wound healing [20–22]. TGF-α, AR, and HB-EGF are autocrine growth factors in normal keratinocytes [23, 24], and both TGF-α and HB-EGF accelerate keratinocyte migration [25–28]. TNF-α regulates keratinocyte migration in vivo and in vitro via a MMP9 dependent pathway [29]. Blocking TNF-α function inhibits keratinocyte migration. HB-EGF mRNA is rapidly induced after scrape wounding of keratinocytes, with a slight increase in TGF-α, AR, and blocking HB-EGF inhibits keratinocyte migration [27, 28]. Therefore HB-EGF is the predominant growth factor involved in the epithelialization of skin wound healing in vivo and it functions by accelerating keratinocyte migration. ADAM9, ADAM10, ADAM12, and ADAM17 are considered candidate HB-EGF sheddases [30–33], which are also expressed in keratinocytes [34–37]. However it has been reported that ADAM9, ADAM10, and ADAM17 reduce cell motility in cDNA-transfected HaCaT cells [38].
Gene array data and immunohistochemistry from human venous reflux ulcers demonstrated that ADAM12 is upregulated in the nonhealing edge of chronic skin ulcers [39]. In addition, skin explants from ADAM12-deficient mice revealed a significant increase in keratinocyte migration and proliferation compared to skin explants from wild-type mice. Based on these findings, expression of ADAM12 in chronic wounds impairs wound healing through the inhibition of keratinocyte migration and proliferation.
ADAM10 represents the major E-cadherin sheddase [36]. ADAM10-mediated shedding of E-cadherin affects epithelial cell-cell adhesion as well as cell migration. The shedding of E-cadherin by ADAM10 modulates the β-catenin subcellular localization and downstream signaling. ADAM10 overexpression in epithelial cells increases the expression of the β-catenin downstream gene cyclin D1 in a dose-dependent manner and enhances cell proliferation. ADAM10 is also involved in the cleavage of CXC-chemokine ligand 16 (CXCL16), which is expressed as a transmembrane adhesion molecule and can be released as a chemoattractant [40]. CXCL16 is expressed in epidermal keratinocytes and is released into the wound exudate upon injury.
ADAM9 expression is increased during the first 7 days after-wounding in a mouse wound model [41]. ADAM9 knockout mice show accelerated wound repair compared with control littermates. No changes in the infiltration of inflammatory cells into the wound areas were observed. Since no differences in proliferation are observed in vivo or in vitro, the increased migration of keratinocytes is responsible for this effect.
3.3. Inflammatory Skin Diseases
3.3.1. Psoriasis
Psoriasis is a common hyperproliferative and chronic inflammatory skin disease, and it has been proposed that TNF-α is involved in its pathogenesis [42]. Increased concentrations of TNF-α have been detected in psoriatic skin lesions [43, 44]. Previous reports have shown that TGF-α, AR, and HB-EGF are overexpressed in psoriatic epidermis [45–47]. Further, soluble p55 and p75 TNF receptors are upregulated in synovial fluid from patients with psoriatic arthritis [48]. EGFR and its ligands are considered to be the most important mechanism for the proliferation of keratinocytes. So far, overexpression of ADAM10, ADAM12, and ADAM17 has been demonstrated in psoriatic skin [35, 49]. ADAM17 cleaves TNF-α and TNF receptors. ADAM10, ADAM12, and ADAM17 are critically involved in EGFR-ligand shedding including HB-EGF. By this mechanism, these sheddases may play a role in the regulation of keratinocyte proliferation and inflammation. Levels of ADAM17 are elevated in peripheral blood mononuclear cells of patients with active psoriasis (compared with healthy subjects), are correlated with the plasma concentration of soluble TNF receptor, with the severity of the disease, and are decreased after treatment [50].
ADAM33 has been identified as a novel psoriasis susceptibility gene [51]. This gene has been previously reported to be linked to asthma [52], which indicates that ADAM33 controls general effects on dermal inflammation and immunity.
3.3.2. Eczema
Acute eczema is an inflammatory skin disease characterized by scaling, redness, and itching. In acute eczema, small intraepidermal blisters may occur, which is characterized by reduced cohesion of keratinocytes. E-cadherin is a prime mediator of epithelial cell-to-cell interactions [53]. In several inflammatory skin diseases, such as psoriasis and pemphigus vulgaris, increased serum levels of soluble E-cadherin have been described [54]. Many cytokines, growth factors, and chemokines contribute to the pathogenesis of inflammatory epithelial skin diseases. Proinflammatory cytokines significantly increased levels of soluble E-cadherin in keratinocytes, which was abrogated in the presence of an ADAM10 inhibitor. ADAM10 levels increase in areas of blisters [55]. These findings indicate that inflammatory responses are able to activate ADAM10-mediated proteolysis of E-cadherin in keratinocytes.
3.4. Pigmentary Disorders
The signaling activated by the Kit ligand (Kitl), also referred to as stem cell factor (SCF), and its receptor KIT (membrane-bound KIT; m-KIT) plays an important role in melanocyte development, survival, proliferation, and melanogenesis [56]. Kitl is synthesized as a transmembrane protein and is processed to produce the soluble secreted form Kitl. Alternative splicing generates two Kitl RNA transcripts that encode two cell-associated Kitl proteins, Kitl1, and Kitl2. Membrane-bound KIT protein (m-KIT) is expressed as a transmembrane protein and has a tyrosine kinase domain [57]. A cleaved, soluble product of KIT, the soluble form of KIT (s-KIT), exists and has an ability to bind SCF, and functions as a decoy receptor to inhibit SCF/m-KIT signaling [58]. Mutations in the human gene encoding KIT results in piebaldism, a congenic disorder that is characterized by amelanotic patches on acral and/or ventral skin surfaces [59]. Intradermal injection of SCF enhances the number, size, and dendricity of melanocytes in normal human skin xenografts, whereas interruption of SCF binding to its receptor m-KIT decreases these parameters [60]. UVB radiation augments the expression of membrane-bound SCF in epidermal keratinocytes, and injection of a KIT-inhibitory antibody abolishes the UVB-induced increase in pigmentation in guinea pig skin [61]. SCF expression is increased in epidermal lentigo seniles [62]. SCF secreted by dermal fibroblasts stimulates melanocytes located in the epidermis overlying dermatofibroma [63]. s-KIT has been detected in vivo in human serum and plasma, and levels of s-KIT are increased in the sera of patients with mastocytosis and correlates with disease severity [64]. Additionally, the amount of s-KIT in the serum correlates with graft-versus-host disease following bone marrow transplantation [65]. Both ADAM17 and ADAM19 affect Kitl1 shedding in different ways: ADAM17 is the major sheddase for Kitl1 and Kitl2, while ADAM19 reduces ADAM17-dependent phorbol-ester-stimulated Kitl1 ectodomain shedding [66]. The production of s-KIT is enhanced by an ADAM17 activator in melanocytes, and the ADAM17 activator-induced production of s-KIT abolishes SCF-induced melanogenesis [67]. Moreover, melanogenesis is significantly suppressed by the addition of an ADAM17 activator, whereas TAPI-1, an inhibitor of ADAM17, is found to significantly stimulate melanin synthesis in a 3D skin model [67].
The melanocortin-1 receptor (MC1R) is a key regulator of pigmentation in mammals and is tightly linked to an increased risk of skin cancers, including melanoma, in humans [68, 69]. Agouti signal protein (ASP) antagonizes MC1R function, and is also associated with increased risk of skin cancer [70]. Many genes that are upregulated by ASP are involved in morphogenesis, cell adhesion, and extracellular matrix-receptor interactions. Microarray analysis indicates that ASP up-regulates several ADAM family genes such as ADAM11, ADAM12, ADAM19, and ADAM23 in melanocytes [71]. However the precise roles of these ADAM proteinases on melanocytes remain unclear.
Color loci in mammals are genetic loci in which mutations can affect pigmentation of the hair, skin, and/or eyes. In mice, over 800 phenotypic alleles are now known, at more than 200 identified color loci [72]. In the ADAMs family, ADAM17 and ADAMTS20 are known to be involved in regulating pigmentation. ADAM17 knockout mice reveal a disorganized distribution and structure of hair follicles, which contain hairs with irregular pigment deposition [73]. A defect of ADAMTS20 is the cause of the belted (bt) white-spotting mutation. It presents as a mostly pigmented mouse except for a region proximal to the hindlimbs that appears as a white belt [74]. ADAMTS20 is a secreted metalloprotease which shows a highly dynamic pattern of expression in the developing embryo that generally precedes the appearance of melanoblasts, and is not expressed in the migrating cells. It has been suggested that the ADAMTS20 proteinase plays a role in the regulation of cell migration [74]. In humans, ADAM17 and ADAMTS20 are also known to be candidate genes that regulate pigmentation in East Asians [75].
3.5. Others
3.5.1. UV Radiation
UV radiation is clearly an important environmental factor in human skin carcinogenesis. ADAMs family proteinases are involved in regulating these signaling pathways. UVA and UVB up-regulate ADAM17 mRNA expression in immortalized keratinocyte HaCaT cells [76]. A low, nonlethal dose of UVA (1–4 J/cm2) induces a dose-dependent EGFR activation, cyclin D1 accumulation, and cell cycle progression in HaCaT cells [77]. Knockdown of ADAM17 blocks UVA-induced EGFR activation and cell cycle progression, which demonstrates that ADAM17 mediates the EGFR/cyclin D1 pathway and cell cycle progression to the S phase induced by UVA radiation [77]. UVC also induces EGFR phosphorylation in melanocytes and keratinocytes, which is inhibited by a broad spectrum metalloproteinase inhibitor, BB94 [11]. AR is required for UV-induced EGFR activation in SCC-9 cells, which depends on ADAM9 and ADAM17.
3.5.2. Systemic Sclerosis
Systemic sclerosis (SSc) is a disease characterized by progressive fibrosis of multiple systems including the skin. Elevated serum concentrations of the soluble TNF receptor p55 and TNF-α are known to correlate with the severity of SSc [78–80]. Up-regulation of ADAM17 was observed in peripheral monocytes of patients with early SSc [81]. Furthermore, the ADAM17 inhibitor, TAPI-1, significantly suppressed skin sclerosis induced by bleomycin and reduced fibrogenic cytokines [82]. These findings indicate that ADAM17 could be a new target for the therapy of SSc.
3.5.3. Collagen XVII
Collagen XVII (also called BP180 or BPAG2) is a hemidesmosomal adhesion component in the skin and mucosa [83]. Mutations in the COL17A1 gene are associated with junctional epidermolysis bullosa, a genetic skin blistering disease [84]. Patients with bullous pemphigoid and related autoimmune bullous dermatoses have tissue-bound and circulating autoantibodies targeting collagen XVII. Collagen XVII is an epithelial adhesion molecule and is proteolytically released from the membrane via the action of several ADAM proteases. ADAM9, ADAM10, and ADAM17 are known to be collagen XVII sheddases [38, 85].
4. Concluding Remarks
The current status and future potential of ADAMs proteinase activities in the fields of cutaneous biology and skin disorders were discussed in this paper, and the functions of relevant ADAMs proteinases are summarized in Table 1. A better understanding of the regulatory mechanisms and physiological functions of ADAMs proteinases in human skin may generate novel targets for the diagnosis and/or therapeutics of skin diseases.
Table 1.
Principal ADAMs proteinases involved in skin diseases.
| ADAM | substrate | Pathology association |
|---|---|---|
| ADAM9 | HB-EGF, EGF, Collagen XVII | Cancer, Wound healing |
| ADAM10 | E-cadherin, N-cadherin, CD44, EGF, HB-EGF, L1-CAM, CXCL16 Collagen XVII | Cancer, Wound healing, Psoriasis, Eczema |
| ADAM12 | EGF, HB-EGF | Cancer, Wound healing, Psoriasis |
| ADAM17 | TNF-α, TNFR, TGF-α, HB-EGF, AR, Collagen XVII, KIT, SCF | Cancer, Migration, Psoriasis, Melanogenesis, SSc |
| ADAM33 | Psoriasis |
Conflict of Interests
The authors has no conflict of interests to declare.
Acknowledgment
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute.
Abbreviations
- ADAMs:
A disintegrin and metalloproteinases
- ADAMTS:
ADAM with thrombospondin motifs
- AR:
Amphiregulin
- ASP:
Agouti signal protein
- BCC:
Basal cell carcinoma
- CXCL16:
CXC-chemokine ligand 16
- EGF:
Epidermal growth factor
- EGFR:
EGF receptor
- GPCR:
G protein-coupled receptor
- HA:
Hyaluronic acid
- HB-EGF:
Heparin-binding EGF-like growth factor
- IL-6R:
Interleukin-6 receptor
- Kitl:
Kit ligand
- L1-CAM:
L1 cell adhesion molecule
- m-KIT:
Membrane-bound KIT
- s-KIT:
Soluble form of KIT
- MC1R:
Melanocortin-1 receptor
- MM:
Malignant melanoma
- MMP:
Matrix metalloproteinase
- PKC:
Protein kinase C
- PLZF:
Promyelocytic leukemia zinc finger
- ROS:
Reactive oxygen species
- SCC:
Squamous cell carcinoma
- SCF:
Stem cell factor
- SSc:
Systemic sclerosis
- TACE:
TNF-α-converting enzyme
- TGF-α:
Transforming growth factor-α
- TGF-β:
Transforming growth factor-β
- TNF-α:
Tumor necrosis factor-α
- TPA:
12-O-tetradecanoylphorbol-13-acetate
- TSP-1:
Thrombospondin type one
- UV:
Ultraviolet.
References
- 1.Edwards DR, Handsley MM, Pennington CJ. The ADAM metalloproteinases. Molecular Aspects of Medicine. 2009;29(5):258–289. doi: 10.1016/j.mam.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seals DF, Courtneidge SA. The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes and Development. 2003;17(1):7–30. doi: 10.1101/gad.1039703. [DOI] [PubMed] [Google Scholar]
- 3.Black RA. Tumor necrosis factor-α converting enzyme. International Journal of Biochemistry and Cell Biology. 2002;34(1):1–5. doi: 10.1016/s1357-2725(01)00097-8. [DOI] [PubMed] [Google Scholar]
- 4.DasGupta S, Murumkar PR, Giridhar R, Yadav MR. Current perspective of TACE inhibitors: a review. Bioorganic and Medicinal Chemistry. 2009;17(2):444–459. doi: 10.1016/j.bmc.2008.11.067. [DOI] [PubMed] [Google Scholar]
- 5.Kveiborg M, Albrechtsen R, Couchman JR, Wewer UM. Cellular roles of ADAM12 in health and disease. International Journal of Biochemistry and Cell Biology. 2008;40(9):1685–1702. doi: 10.1016/j.biocel.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 6.Atfi A, Dumont E, Colland F, et al. The disintegrin and metalloproteinase ADAM12 contributes to TGF-β signaling through interaction with the type II receptor. Journal of Cell Biology. 2007;178(2):201–208. doi: 10.1083/jcb.200612046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nanba D, Mammoto A, Hashimoto K, Higashiyama S. Proteolytic release of the carboxy-terminal fragment of proHB-EGF causes nuclear export of PLZF. Journal of Cell Biology. 2003;163(3):489–502. doi: 10.1083/jcb.200303017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Science. 2007;98(5):621–628. doi: 10.1111/j.1349-7006.2007.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kataoka H. EGFR ligands and their signaling scissors, ADAMs, as new molecular targets for anticancer treatments. Journal of Dermatological Science. 2009;56(3):148–153. doi: 10.1016/j.jdermsci.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 10.Ohtsu H, Dempsey PJ, Eguchi S. ADAMs as mediators of EGF receptor transactivation by G protein-coupled receptors. American Journal of Physiology. 2006;291(1):C1–C10. doi: 10.1152/ajpcell.00620.2005. [DOI] [PubMed] [Google Scholar]
- 11.Singh B, Schneider M, Knyazev P, Ullrich A. UV-induced EGFR signal transactivation is dependent on proligand shedding by activated metalloproteases in skin cancer cell lines. International Journal of Cancer. 2009;124(3):531–539. doi: 10.1002/ijc.23974. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler DL, Ness KJ, Oberley TD, Verma AK. Protein kinase Cε is linked to 12-O-tetradecanoylphorbol-13-acetate-induced tumor necrosis factor-α ectodomain shedding and the development of metastatic squamous cell carcinoma in protein kinase Cε transgenic mice. Cancer Research. 2003;63(19):6547–6555. [PubMed] [Google Scholar]
- 13.Oh ST, Schramme A, Stark A, Tilgen W, Gutwein P, Reichrath J. The disintegrin-metalloproteinases ADAM 10, 12 and 17 are upregulated in invading peripheral tumor cells of basal cell carcinomas. Journal of Cutaneous Pathology. 2009;36(4):395–401. doi: 10.1111/j.1600-0560.2008.01082.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee SB, Schramme A, Doberstein K, et al. ADAM10 is upregulated in melanoma metastasis compared with primary melanoma. Journal of Investigative Dermatology. 2010;130(3):763–773. doi: 10.1038/jid.2009.335. [DOI] [PubMed] [Google Scholar]
- 15.Massi D, Tarantini F, Franchi A, et al. Evidence for differential expression of Notch receptors and their ligands in melanocytic nevi and cutaneous malignant melanoma. Modern Pathology. 2006;19(2):246–254. doi: 10.1038/modpathol.3800526. [DOI] [PubMed] [Google Scholar]
- 16.Anderegg U, Eichenberg T, Parthaune T, et al. ADAM10 is the constitutive functional sheddase of CD44 in human melanoma cells. Journal of Investigative Dermatology. 2009;129(6):1471–1482. doi: 10.1038/jid.2008.323. [DOI] [PubMed] [Google Scholar]
- 17.Ahrens T, Assmann V, Fieber C, et al. CD44 is the principal mediator of hyaluronic-acid-induced melanoma cell proliferation. Journal of Investigative Dermatology. 2001;116(1):93–101. doi: 10.1046/j.1523-1747.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- 18.Ahrens T, Sleeman JP, Schempp CM, et al. Soluble CD44 inhibits melanoma tumor growth by blocking cell surface CD44 binding to hyaluronic acid. Oncogene. 2001;20(26):3399–3408. doi: 10.1038/sj.onc.1204435. [DOI] [PubMed] [Google Scholar]
- 19.Zigrino P, Mauch C, Fox JW, Nischt R. ADAM-9 expression and regulation in human skin melanoma and melanoma cell lines. International Journal of Cancer. 2005;116(6):853–859. doi: 10.1002/ijc.21087. [DOI] [PubMed] [Google Scholar]
- 20.Stoll S, Garner W, Elder J. Heparin-binding ligands mediate autocrine epidermal growth factor receptor activation in skin organ culture. Journal of Clinical Investigation. 1997;100(5):1271–1281. doi: 10.1172/JCI119641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastore S, Mascia F, Mariani V, Girolomoni G. The epidermal growth factor receptor system in skin repair and inflammation. Journal of Investigative Dermatology. 2008;128(6):1365–1374. doi: 10.1038/sj.jid.5701184. [DOI] [PubMed] [Google Scholar]
- 22.Repertinger SK, Campagnaro E, Fuhrman J, El-Abaseri T, Yuspa SH, Hansen LA. EGFR enhances early healing after cutaneous incisional wounding. Journal of Investigative Dermatology. 2004;123(5):982–989. doi: 10.1111/j.0022-202X.2004.23478.x. [DOI] [PubMed] [Google Scholar]
- 23.Coffey RJ, Derynck R, Wilcox JN. Production and auto-induction of transforming growth factor-α in human keratinocytes. Nature. 1987;328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- 24.Hashimoto K, Higashiyama S, Asada H, et al. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. Journal of Biological Chemistry. 1994;269(31):20060–20066. [PubMed] [Google Scholar]
- 25.Cha D, O’Brien P, O’Toole EA, Woodley DT, Hudson LG. Enhanced modulation of keratinocyte motility by transforming growth factor-α (TGF-α) relative to epidermal growth factor (EGF) Journal of Investigative Dermatology. 1996;106(4):590–597. doi: 10.1111/1523-1747.ep12345083. [DOI] [PubMed] [Google Scholar]
- 26.Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. Journal of Investigative Dermatology. 2006;126(9):2096–2105. doi: 10.1038/sj.jid.5700350. [DOI] [PubMed] [Google Scholar]
- 27.Shirakata Y, Kimura R, Nanba D, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. Journal of Cell Science. 2005;118(11):2363–2370. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- 28.Tokumaru S, Higashiyama S, Endo T, et al. Ectodomain shedding of epidermal growth factor receptor ligands is required for keratinocyte migration in cutaneous wound healing. Journal of Cell Biology. 2000;151(2):209–219. doi: 10.1083/jcb.151.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scott KA, Arnott CH, Robinson SC, et al. TNF-α regulates epithelial expression of MMP-9 and integrin αvβ6 during tumour promotion. A role for TNF-α in keratinocyte migration? Oncogene. 2004;23(41):6954–6966. doi: 10.1038/sj.onc.1207915. [DOI] [PubMed] [Google Scholar]
- 30.Izumi Y, Hirata M, Hasuwa H, et al. A metalloprotease-disintegrin, MDC9/meltrin-γ/ADAM9 and PKCδ are involved in TPA-induced ectodomain shedding of membrane-anchored heparin-binding EGF-like growth factor. The EMBO Journal. 1998;17(24):7260–7272. doi: 10.1093/emboj/17.24.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan Y, Shirakabe K, Werb Z. The metalloprotease Kuzbanian (ADAM10) mediates the transactivation of EGF receptor by G protein-coupled receptors. Journal of Cell Biology. 2002;158(2):221–226. doi: 10.1083/jcb.200112026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asakura M, Kitakaze M, Takashima S, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: metalloproteinase inhibitors as a new therapy. Nature Medicine. 2002;8(1):35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 33.Sahin U, Weskamp G, Kelly K, et al. Distinct roles for ADAM10 and ADAM17 in ectodomain shedding of six EGFR ligands. Journal of Cell Biology. 2004;164(5):769–779. doi: 10.1083/jcb.200307137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zigrino P, Steiger J, Fox JW, et al. Role of ADAM-9 disintegrin-cysteine-rich domains in human keratinocyte migration. Journal of Biological Chemistry. 2007;282(42):30785–30793. doi: 10.1074/jbc.M701658200. [DOI] [PubMed] [Google Scholar]
- 35.Oh ST, Schramme A, Stark A, Tilgen W, Gutwein P, Reichrath J. Overexpression of ADAM 10 and ADAM 12 in lesional psoriatic skin. British Journal of Dermatology. 2008;158(6):1371–1373. doi: 10.1111/j.1365-2133.2008.08513.x. [DOI] [PubMed] [Google Scholar]
- 36.Maretzky T, Reiss K, Ludwig A, et al. ADAM10 mediates E-cadherin shedding and regulates epithelial cell-cell adhesion, migration, and β-catenin translocation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(26):9182–9187. doi: 10.1073/pnas.0500918102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawaguchi M, Mitsuhashi Y, Kondo S. Localization of tumour necrosis factor-α converting enzyme in normal human skin. Clinical and Experimental Dermatology. 2004;29(2):185–187. doi: 10.1111/j.1365-2230.2004.01455.x. [DOI] [PubMed] [Google Scholar]
- 38.Franzke CW, Tasanen K, Schäcke H, et al. Transmembrane collagen XVII, an epithelial adhesion protein, is shed from the cell surface by ADAMS. The EMBO Journal. 2002;21(19):5026–5035. doi: 10.1093/emboj/cdf532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harsha A, Stojadinovic O, Brem H, et al. ADAM12: a potential target for the treatment of chronic wounds. Journal of Molecular Medicine. 2008;86(8):961–969. doi: 10.1007/s00109-008-0353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scholz F, Schulte A, Adamski F, et al. Constitutive expression and regulated release of the transmembrane chemokine CXCL16 in human and murine skin. Journal of Investigative Dermatology. 2007;127(6):1444–1455. doi: 10.1038/sj.jid.5700751. [DOI] [PubMed] [Google Scholar]
- 41.Mauch C, Zamek J, Abety AN, Grimberg G, Fox JW, Zigrino P. Accelerated wound repair in ADAM-9 knockout animals. Journal of Investigative Dermatology. 2010;130(8):2120–2130. doi: 10.1038/jid.2010.60. [DOI] [PubMed] [Google Scholar]
- 42.Gottlieb AB. Psoriasis: immunopathology and immunomodulation. Dermatologic Clinics. 2001;19(4):649–657. doi: 10.1016/s0733-8635(05)70306-5. [DOI] [PubMed] [Google Scholar]
- 43.Kristensen M, Chu CQ, Eedy DJ, Feldmann M, Brennan FM, Breathnach SM. Localization of tumour necrosis factor-alpha (TNF-α) and its receptors in normal and psoriatic skin: epidermal cells express the 55-kD but not the 75-kD TNF receptor. Clinical and Experimental Immunology. 1993;94(2):354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ettehadi P, Greaves MW, Wallach D, Aderka D, Camp RDR. Elevated tumour necrosis factor-alpha (TNF-α) biological activity in psoriatic skin lesions. Clinical and Experimental Immunology. 1994;96(1):146–151. doi: 10.1111/j.1365-2249.1994.tb06244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elder JT, Fisher GJ, Lindquist PB, et al. Overexpression of transforming growth factor α in psoriatic epidermis. Science. 1989;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- 46.Cook PW, Pittelkow MR, Keeble WW, Graves-Deal R, Coffey RJ, Shipley GD. Amphiregulin messenger RNA is elevated in psoriatic epidermis and gastrointestinal carcinomas. Cancer Research. 1992;52(11):3224–3227. [PubMed] [Google Scholar]
- 47.Stoll SW, Elder JT. Retinoid regulation of heparin-binding EGF-like growth factor gene expression in human keratinocytes and skin. Experimental Dermatology. 1998;7(6):391–397. doi: 10.1111/j.1600-0625.1998.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 48.Partsch G, Wagner E, Leeb BF, Dunky A, Steiner G, Smolen JS. Upregulation of cytokine receptors sTNF-R55, STNF-R75, and sIL-2r in psoriatic arthritis synovial fluid. Journal of Rheumatology. 1998;25(1):105–110. [PubMed] [Google Scholar]
- 49.Kawaguchi M, Mitsuhashi Y, Kondo S. Overexpression of tumour necrosis factor-α-converting enzyme in psoriasis. British Journal of Dermatology. 2005;152(5):915–919. doi: 10.1111/j.1365-2133.2005.06440.x. [DOI] [PubMed] [Google Scholar]
- 50.Serwin AB, Sokolowska M, Chodynicka B. Tumour necrosis factor α (TNF-α)-converting enzyme (TACE) and soluble TNF-α receptor type 1 in psoriasis patients treated with narrowband ultraviolet B. Photodermatology Photoimmunology and Photomedicine. 2007;23(4):130–134. doi: 10.1111/j.1600-0781.2007.00296.x. [DOI] [PubMed] [Google Scholar]
- 51.Lesueur F, Oudot T, Heath S, et al. ADAM33, a new candidate for psoriasis susceptibility. PLoS ONE. 2007;2(9, article e906) doi: 10.1371/journal.pone.0000906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van Eerdewegh P, Little RD, Dupuis J, et al. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002;418(6896):426–430. doi: 10.1038/nature00878. [DOI] [PubMed] [Google Scholar]
- 53.Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 54.Matsuyoshi N, Tanaka T, Toda K, Okamoto H, Furukawa F, Imamura S. Soluble E-cadherin: a novel cutaneous disease marker. British Journal of Dermatology. 1995;132(5):745–749. doi: 10.1111/j.1365-2133.1995.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 55.Maretzky T, Scholz F, Köten B, Proksch E, Saftig P, Reiss K. ADAM10-mediated E-cadherin release is regulated by proinflammatory cytokines and modulates keratinocyte cohesion in eczematous dermatitis. Journal of Investigative Dermatology. 2008;128(7):1737–1746. doi: 10.1038/sj.jid.5701242. [DOI] [PubMed] [Google Scholar]
- 56.Wehrle-Haller B. The role of Kit-ligand in melanocyte development and epidermal homeostasis. Pigment Cell Research. 2003;16(3):287–296. doi: 10.1034/j.1600-0749.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- 57.Lennartsson J, Jelacic T, Linnekin D, Shivakrupa R. Normal and oncogenic forms of the receptor tyrosine kinase kit. Stem Cells. 2005;23(1):16–43. doi: 10.1634/stemcells.2004-0117. [DOI] [PubMed] [Google Scholar]
- 58.Dahlen DD, Lin NL, Liu YC, Broudy VC. Soluble c-kit receptor blocks stem cell factor bioactivity in vitro. Leukemia Research. 2001;25(5):413–421. doi: 10.1016/s0145-2126(00)00122-3. [DOI] [PubMed] [Google Scholar]
- 59.Giebel LB, Spritz RA. Mutation of the KIT (mast/stem cell growth factor receptor) protooncogene in human piebaldism. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(19):8696–8699. doi: 10.1073/pnas.88.19.8696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grichnik JM, Burch JA, Burchette J, Shea CR. The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. Journal of Investigative Dermatology. 1998;111(2):233–238. doi: 10.1046/j.1523-1747.1998.00272.x. [DOI] [PubMed] [Google Scholar]
- 61.Hachiya A, Kobayashi A, Ohuchi A, Takema Y, Imokawa G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. Journal of Investigative Dermatology. 2001;116(4):578–586. doi: 10.1046/j.1523-1747.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- 62.Hattori H, Kawashima M, Ichikawa Y, Imokawa G. The epidermal stem cell factor is over-expressed in lentigo senilis: implication for the mechanism of hyperpigmentation. Journal of Investigative Dermatology. 2004;122(5):1256–1265. doi: 10.1111/j.0022-202X.2004.22503.x. [DOI] [PubMed] [Google Scholar]
- 63.Shishido E, Kadono S, Manaka I, Kawashima M, Imokawa G. The mechanism of epidermal hyperpigmentation in dermatofibroma is associated with stem cell factor and hepatocyte growth factor expression. Journal of Investigative Dermatology. 2001;117(3):627–633. doi: 10.1046/j.0022-202x.2001.01440.x. [DOI] [PubMed] [Google Scholar]
- 64.Akin C, Schwartz LB, Kitoh T, et al. Soluble stem cell factor receptor (CD 117) and IL-2 receptor alpha chain (CD25) levels in the plasma of patients with mastocytosis: relationships to disease severity and bone marrow pathology. Blood. 2000;96(4):1267–1273. [PubMed] [Google Scholar]
- 65.Hashino S, Imamura M, Kobayashi S, et al. Soluble c-kit levels in acute GVHD after allogeneic bone marrow transplantation. British Journal of Haematology. 1995;89(4):897–899. doi: 10.1111/j.1365-2141.1995.tb08431.x. [DOI] [PubMed] [Google Scholar]
- 66.Kawaguchi N, Horiuchi K, Becherer JD, Toyama Y, Besmer P, Blobel CP. Different ADAMs have distinct influences on Kit ligand processing: phorbol-ester-stimulated ectodomain shedding of Kitl1 by ADAM17 is reduced by ADAM19. Journal of Cell Science. 2007;120(6):943–952. doi: 10.1242/jcs.03403. [DOI] [PubMed] [Google Scholar]
- 67.Kasamatsu S, Hachiya A, Higuchi K, Ohuchi A, Kitahara T, Boissy RE. Production of the soluble form of KIT, s-KIT, abolishes stem cell factor-induced melanogenesis in human melanocytes. Journal of Investigative Dermatology. 2008;128(7):1763–1772. doi: 10.1038/jid.2008.9. [DOI] [PubMed] [Google Scholar]
- 68.Rouzaud F, Kadekaro AL, Abdel-Malek ZA, Hearing VJ. MC1R and the response of melanocytes to ultraviolet radiation. Mutation Research. 2005;571(1-2):133–152. doi: 10.1016/j.mrfmmm.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 69.Kadekaro AL, Kanto H, Kavanagh R, Abdel-Malek ZA. Significance of the melanocortin 1 receptor in regulating human melanocyte pigmentation, proliferation, and survival. Annals of the New York Academy of Sciences. 2003;994:359–365. doi: 10.1111/j.1749-6632.2003.tb03200.x. [DOI] [PubMed] [Google Scholar]
- 70.Scherer D, Kumar R. Genetics of pigmentation in skin cancer—a review. Mutation Research. 2010;705(2):141–153. doi: 10.1016/j.mrrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 71.Le Pape E, Passeron T, Giubellino A, Valencia JC, Wolber R, Hearing VJ. Microarray analysis sheds light on the dedifferentiating role of agouti signal protein in murine melanocytes via the Mc1r. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(6):1802–1807. doi: 10.1073/pnas.0806753106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bennett DC, Lamoreux ML. The color loci of mice—a genetic century. Pigment Cell Research. 2003;16(4):333–344. doi: 10.1034/j.1600-0749.2003.00067.x. [DOI] [PubMed] [Google Scholar]
- 73.Peschon JJ, Slack JL, Reddy P, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282(5392):1281–1284. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 74.Rao C, Foernzler D, Loftus SK, et al. A defect in a novel ADAMTS family member is the cause of the belted white-spotting mutation. Development. 2003;130(19):4665–4672. doi: 10.1242/dev.00668. [DOI] [PubMed] [Google Scholar]
- 75.McEvoy B, Beleza S, Shriver MD. The genetic architecture of normal variation in human pigmentation: an evolutionary perspective and model. Human Molecular Genetics. 2006;15(2):R176–R181. doi: 10.1093/hmg/ddl217. [DOI] [PubMed] [Google Scholar]
- 76.Skiba B, Neill B, Piva TJ. Gene expression profiles of TNF-α,TACE, furin, IL-1β and matrilysin in UVA- and UVB-irradiated HaCat cells. Photodermatology Photoimmunology and Photomedicine. 2005;21(4):173–182. doi: 10.1111/j.1600-0781.2005.00162.x. [DOI] [PubMed] [Google Scholar]
- 77.He YY, Council SE, Feng LI, Chignell CF. UVA-induced cell cycle progression is mediated by a disintegrin and metalloprotease/epidermal growth factor receptor/AKT/cyclin D1 pathways in keratinocytes. Cancer Research. 2008;68(10):3752–3758. doi: 10.1158/0008-5472.CAN-07-6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heilig B, Fiehn C, Brockhaus M, Gallati H, Pezzutto A, Hunstein W. Evaluation of soluble tumor necrosis factor (TNF) receptors and TNF receptor antibodies in patients with systemic lupus erythematodes, progressive systemic sclerosis, and mixed connective tissue disease. Journal of Clinical Immunology. 1993;13(5):321–328. doi: 10.1007/BF00920240. [DOI] [PubMed] [Google Scholar]
- 79.Gruschwitz MS, Albrecht M, Vieth G, Haustein UF. In situ expression and serum levels of tumor necrosis factor-α receptors in patients with early stages of systemic sclerosis. Journal of Rheumatology. 1997;24(10):1936–1943. [PubMed] [Google Scholar]
- 80.Scala E, Pallotta S, Frezzolini A, et al. Cytokine and chemokine levels in systemic sclerosis: relationship with cutaneous and internal organ involvement. Clinical and Experimental Immunology. 2004;138(3):540–546. doi: 10.1111/j.1365-2249.2004.02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bohgaki T, Amasaki Y, Nishimura N, et al. Up regulated expression of tumour necrosis factor α converting enzyme in peripheral monocytes of patients with early systemic sclerosis. Annals of the Rheumatic Diseases. 2005;64(8):1165–1173. doi: 10.1136/ard.2004.030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Terao M, Murota H, Kitaba S, Katayama I. Tumor necrosis factor-α processing inhibitor-1 inhibits skin fibrosis in a bleomycin-induced murine model of scleroderma. Experimental Dermatology. 2010;19(1):38–43. doi: 10.1111/j.1600-0625.2009.00973.x. [DOI] [PubMed] [Google Scholar]
- 83.Yancey KB. The pathophysiology of autoimmune blistering diseases. Journal of Clinical Investigation. 2005;115(4):825–828. doi: 10.1172/JCI24855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGrath JA, Gatalica B, Christiano AM, et al. Mutations in the 180-kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nature Genetics. 1995;11(1):83–86. doi: 10.1038/ng0995-83. [DOI] [PubMed] [Google Scholar]
- 85.Franzke CW, Bruckner-Tuderman L, Blobel CP. Shedding of collagen XVII/BP180 in skin depends on both ADAM10 and ADAM9. Journal of Biological Chemistry. 2009;284(35):23386–23396. doi: 10.1074/jbc.M109.034090. [DOI] [PMC free article] [PubMed] [Google Scholar]