Abstract
Objective
To describe the natural history, clinical, neurophysiological and histological features and outcomes of diabetic patients presenting with acute painful neuropathy associated with glycemic control, also referred to as ‘insulin neuritis’.
Methods
Sixteen subjects, presenting with acute painful neuropathy had neurological and retinal examinations, laboratory studies, autonomic testing and pain assessments over 18 months. Eight subjects had skin biopsies for evaluation of intra-epidermal nerve fiber density.
Results
All subjects developed severe pain within 8 weeks of intensive glucose control. There was a high prevalence of autonomic cardiovascular, gastrointestinal, genitourinary, and sudomotor symptoms in all subjects. Orthostatic hypotension and parasympathetic dysfunction were seen in 69% of subjects. Retinopathy worsened in all subjects. Reduced intra-epidermal nerve fiber density (IENFD) was seen in all tested subjects. After 18 months of glycemic control, there were substantial improvements in pain, autonomic symptoms, autonomic test results and IENFD. Greater improvements were seen after 18 months in type 1 vs. type 2 diabetic subjects in autonomic symptoms (cardiovascular p<0.01; gastrointestinal p<0.01; genitourinary p<0.01) and autonomic function tests (p<0.01, sympathetic and parasympathetic function tests).
Interpretation
Treatment induced neuropathy is characterized by acute, severe pain, peripheral nerve degeneration and autonomic dysfunction after intensive glycemic control. The neuropathy occurred in parallel with worsening diabetic retinopathy suggesting a common underlying pathophysiological mechanism. Clinical features and objective measures of small myelinated and unmyelinated nerve fibers can improve in these diabetic patients despite a prolonged history of poor glucose control, with greater improvement seen in patients with type 1 diabetes.
Keywords: diabetic neuropathy, painful neuropathy, autonomic neuropathy
While chronic neuropathic pain occurs frequently in diabetic patients, acute severe neuropathic pain is rarely encountered. Several different acute painful syndromes occur: 1) Pain may appear shortly after the initiation of intensive glycemic control, sometimes referred to as ‘insulin neuritis’ or treatment induced neuropathy.1-3 Characteristically, the painful neuropathy is preceded by rapid glycemic control. 2) Additional cases present in association with severe weight loss with or without a change in glycemic control, a disorder referred to as ‘diabetic neuropathic cachexia’.4-6 The weight loss in these patients is unintentional. 3) Pain may also present with intentional weight loss unrelated to changes in glucose control, a condition called “diabetic anorexia”.7 4) Rarely, an acute painful neuropathy may present with no apparent underlying cause.8-11
Treatment induced neuropathy was first described by Caravati in 1933. He reported a diabetic woman with numbness, tingling, and shooting pains in the lower extremities that appeared four weeks after the initiation of insulin.1 The pain increased despite the use of analgesics and sedatives, but resolved within 3 days of stopping insulin concurrent with severe hyperglycemia. Further attempts at insulin use resulted in similar levels of pain. He called the condition “insulin neuritis”.1
Recent reports have described treatment induced neuropathy in individuals with type 1 and type 2 diabetes treated with oral hypoglycemic agents or with insulin. Pre-treatment glycosylated hemoglobin (A1C) is typically high and glycemic control rapid. Pain, the most prominent symptom of treatment induced neuropathy, may occur distally in a length-dependent fashion or be more generalized and involve proximal sites including the trunk.2-4
Reports of treatment induced neuropathy have been predominantly small observational case studies with variable follow-up.2-5, 12, 13 In this manuscript, we report a cohort of 16 patients with the sudden onset of pain, autonomic dysfunction and microvascular complications after rapid glycemic control without associated weight loss. We provide the largest case-series, first detailed report of autonomic testing, autonomic symptoms, intra-epidermal nerve fiber density, concomitant microvascular complications and longitudinal follow-up of patients with treatment induced neuropathy.
Patients and Methods
This study was approved by the institutional review board of Beth Israel Deaconess Medical Center. Sixteen cases of acute painful neuropathy were referred to our diabetic neuropathy clinic over 4 years after rapid and sustained glycemic control and were followed prospectively for 18 months or more. All patients underwent a standard battery of autonomic tests within 3 months of the onset of pain with repeated autonomic testing 18 months later (±4 weeks). Subjects underwent detailed neurologic examinations approximately every 3 months, with pain scores measured at each visit. Selected patients underwent skin biopsy evaluation of epidermal nerve fiber density (IENFD). All subjects were evaluated for routine screening tests including complete blood count, erythrocyte sedimentation rate, thyroid function tests, serum B12, comprehensive metabolic panel (including renal and hepatic function) and serum and urine protein electrophoresis. Subjects had yearly (or more frequent) retinal examinations and spot urine tests for microalbuminuria as part of their routine care. All subjects had glycosylated hemoglobin (A1C) scores monitored every 3 months.
Physical Examination
The physical examination findings were quantified with the neuropathy impairment score in the lower limb (NIS-LL) [see Supplementary Table 1 for details].14
Pain Scores
Subjects (n=16) rated their pain on an 11 point Likert scale at every neurologic examination (every 3 months); a score of 0 denoted no pain and a score of 10 denoted the worst pain imaginable. Subjects were treated with medications to reduce neuropathic pain, including anti-convulsants, anti-depressants and opioids, alone or in combination. Pain scores were measured while on medication.
Autonomic Testing
Subjects (n=16) had tests of cardiovascular parasympathetic function (the heart rate response to Valsalva maneuver and deep respiration) and cardiovascular sympathetic function (the blood pressure response to tilt-table testing at 60 degrees and a Valsalva maneuver). Patients had continuous ECG monitoring, continuous beat-to-beat blood pressure recordings, and manual blood pressure measurements every minute during tilt-table testing. .
Autonomic Questionnaires
Subjects (n=16) completed a detailed questionnaire addressing autonomic symptoms related to cardiovascular, gastrointestinal, genito-urinary, vasomotor and sudomotor dysfunction at the time of autonomic testing. Autonomic symptoms were rated on an 11 point scale (where 0 = no symptoms and 10= severe symptoms). For determination of symptom prevalence, a symptom was considered clinically significant if score was >2.
Skin biopsy evaluation of intra-epidermal nerve fiber density (IENFD)
Subjects (n=10) underwent 3-mm punch skin biopsies at the lateral aspect of the distal leg, distal thigh, and proximal thigh within 5 months of the onset of pain and in 3 subjects 1 year later at the distal leg adjacent to the original biopsy sites using standard techniques.15 Specimens were fixed and stained with PGP 9.5 (ubiquitin hydrolase, Chemicon). All patients underwent IENFD counting by a blinded technician and results were expressed as a linear density (number of fibers per millimeter).16
Statistics
The group data are presented as mean±standard deviation. Autonomic test results were analyzed using analysis of variance and Student’s t test where applicable. A p value <0.05 was considered significant. All analysis performed using Systat 11 (Systat software, SPSS, Richmond CA).
Results
The complete demographic data for all 16 subjects is shown in Supplementary Table 1. Nine subjects had type 1 diabetes (7 female; mean age 25) and 7 had type 2 diabetes (2 female; mean age 47). None of the subjects had other abnormal laboratory values suggestive of other causes of neuropathy (mild anemia was seen in 4/7 of the female type 1 diabetic subjects). Vitamin B12 levels ranged from 632 to >2000 pg/ml. Compared with subjects with type 2 diabetes, subjects with type 1 diabetes had higher baseline A1C scores (15.5±1.3% vs 13.0±0.8%, p<0.01) and lower A1C scores after treatment (6.4±0.6% vs. 7.5±0.7%, p<0.01). Subjects with type 1 diabetes also had lower blood pressures (116/74±5/3 mmHg vs. 139/86±7/5 mmHg, p<0.01) and lower cholesterol levels (168±11 mg/dl vs 213±24 mg/dl, p<0.01) at baseline.
All 7 female subjects with type 1 diabetes had a remote history of diabetic anorexia (intentionally withholding insulin for weight loss). The average duration of diabetic anorexia prior to the development of painful neuropathy was 5.6 years (range 3-9 years) but there was no change in weight for an average of 1.8 years (range 6 months to 4 years) prior to the onset of pain. One male subject, with type 2 diabetes and an A1C of 12.1%, intentionally lost 21 lbs in 2 months by restricting himself to a 500 calorie per day diet in order to avoid taking oral hypoglycemic medications. His average daily blood glucose dropped from approximately 402 mg/dl to 126 mg/dl within a week of this dietary restriction. He noted tingling 4 weeks after starting his diet, and pain 2 weeks later. The male subjects with type 1 diabetes and all subjects with type 2 diabetes had historically poor glucose control due to treatment non-compliance. All patients reported a specific life event that caused them to rapidly improve glycemic control. Examples include death of a close friend or family member from diabetes, or personal hospitalization from a diabetes related complication.
Physical examinations, quantified by NIS-LL scores, are reported in supplementary Table 1. All subjects had normal motor strength examinations. The initial NIS-LL scores for individuals with type 2 diabetes were higher than those with type 1 diabetes (NIS-LL 10.5±2.2 type 2, 5.1±1.4 type 1, P<0.001). One year later, there were no significant changes in NIS-LL scores in either group (NIS-LL 10.8±1.9 type 2, 5.3±1.3 type 1, P<0.001). Reduced pain and thermal sensation was seen in 100% of subjects in the distal legs. All subjects reported severe 10/10 pain within 6-8 weeks of the onset of glucose control, independent of diabetes type. Most (81% of subjects) report pain in a stocking and glove distribution (67% with type 1 and 100% with type 2). Three subjects (19%), all with type 1 diabetes, reported more diffuse pain all over their body.
The A1C values and corresponding pain scores from each visit are shown in Figure 1. Despite maximal medical therapy, typically involving 2-3 drugs in combination, subjects still reported average pain scores of 7-9/10 (therapeutic regimen reported in Supplementary Table 1). The average duration of time required for a 50% reduction in pain (on maximal therapy) was 15 months, with a range of 12-28 months. In all cases, pain medications had been unchanged for several months prior to pain reduction. Hyperalgesia and allodynia was present in 57% of subjects (78% with type 1 and 29% with type 2). Hyperalgesia alone was seen in 1 patient with type 1 diabetes and 1 patient with type 2 diabetes. Allodynia was not seen without hyperalgesia in these subjects.
Figure 1.
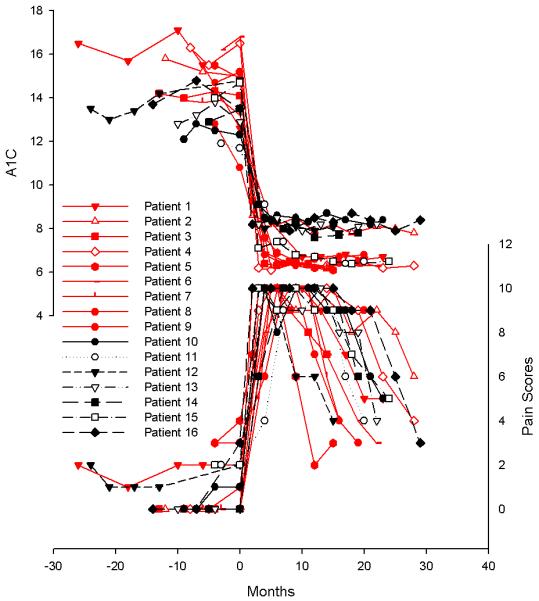
The upper portion of the graph reveals the glycosylated hemoglobin (A1C) scores over time and the lower portion of the graph reveals neuropathic pain scores at the same visits. Patient numbers correspond to the data shown in Supplementary Tables 1-3. Red lines represent those with type 1 diabetes, black lines represent those with type 2 diabetes. The pain relief that occurred after 1 year was not associated with any pharmaceutical intervention. All pain medications had been stable for more than 6 months at the time of pain improvement (detailed in supplementary Table 1).
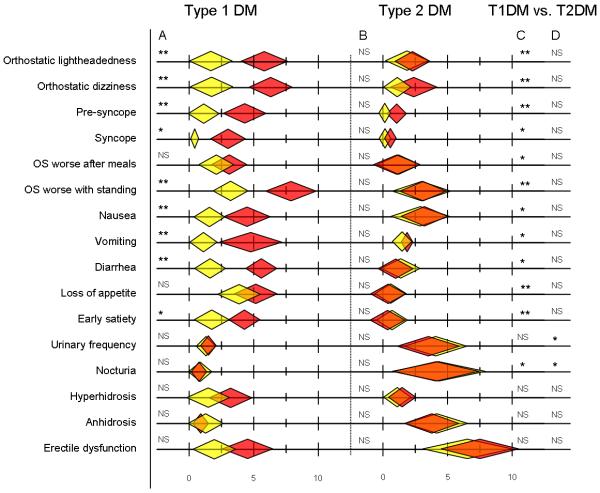
There was a high prevalence of cardiovascular, gastrointestinal, genitourinary, and sudomotor symptoms in all subjects (clinically meaningful responses defined as >2 on questionnaire; summarized in Figure 2). Orthostatic symptoms were the most common with lightheadedness seen in 69%, dizziness in 75%, presyncope in 52% and syncope in 31%. Symptoms of gastrointestinal dysfunction were also common; nausea in 69%, vomiting in 56%, diarrhea in 50%, loss of appetite in 43% and early satiety in 43%. Erectile dysfunction was seen in 86% of male subjects. All autonomic symptoms were worse in type 1 diabetes patients. Most symptoms improved after 18 months in subjects with type 1 diabetes, but little change was noted in those with type 2 diabetes. [Figure 2]
Figure 2.
Autonomic symptoms were rated using a modified Likert scale where 0=no symptoms, 10 = worst possible symptoms. The graph reveals the mean and standard deviations of the questionnaire scores at baseline (shown in red) and 18 months later (shown in yellow). The questionnaire was administered at the time of autonomic testing; clinically meaningful scores are > 2. Results of baseline vs. 18 months tests for type 1 diabetes (Column A) and type 2 diabetes (Column B); baseline comparisons of type1 vs. type 2 diabetes (Column C) and 18 month comparisons of type1 vs. type 2 diabetes (Column D) are shown. NS=not significant; *= p<0.005; **= p<0.001. OS = Orthostatic symptoms. All results include 9 subjects with type 1 diabetes and 7 subjects with type 2 diabetes, except erectile dysfunction which was limited to male subjects (2 individuals with type 1 diabetes and 5 with type 2).
There was a high prevalence of sympathetic and parasympathetic autonomic dysfunction (defined by age- and sex-derived normative values).
Parasympathetic function: abnormal heart rate response to deep breathing was present in 69%; abnormal expiratory to inspiratory ratio in 62% and abnormal Valsalva ratio in 56%. Sympathetic function: orthostatic hypotension (defined as systolic blood pressure fall of >20 mmHg within 3 minutes of standing or upright tilt),17 was present in 69% of individuals. All autonomic abnormalities were more frequent and more severe in patients with type 1 diabetes. Autonomic test results are summarized in Supplementary Table 2.
Eighteen months later there was a substantial reduction in the prevalence of autonomic dysfunction. Parasympathetic function: abnormal heart rate response to deep breathing was present in 48%, abnormal expiratory to inspiratory ratio in 19% and an abnormal Valsalva ratio in 43%. Sympathetic function: orthostatic hypotension was present in 31%. Results are shown in Supplementary Figure 1 and Supplementary Table 2.
Skin biopsies revealed abnormal or borderline nerve fiber densities at the distal leg in all 8 tested subjects. Large nerve-fiber swellings were noted at the distal leg in all tested subjects, with some small and medium size nerve fibers swellings noted at the distal thigh in 5/8 subjects. More diffuse patterns of large nerve fiber swellings, at all biopsy sites, were noted in 2 patients (1 and 4) that reported diffuse non-length dependent pain. Intra-epidermal nerve fiber density data is shown in Supplementary Table 3 and Supplementary Figure 2. The three individuals with skin biopsies repeated 1 year later had increased nerve fiber densities at the distal leg.
Retinal examination worsened in all patients within 1 year after the onset of glycemic control. At baseline 9/16 subjects had no retinopathy and 7/16 had non-proliferative retinopathy (3 mild, 2 moderate and 2 severe). After 6 months of sustained glycemic control 0/16 subject had no retinopathy, 8/16 subjects had non-proliferative retinopathy (2 moderate and 6 severe) and 8/16 had severe proliferative retinopathy. The presence of microalbuminuria was detected in 8/16 individuals at baseline and 13/16 patients one year later.
Discussion
We report the largest case series and first detailed analysis of autonomic symptoms, autonomic testing, cutaneous innervation, accompanying microvascular complications and longitudinal follow-up of patients with the acute onset of neuropathy associated with glycemic control. Our data show that treatment induced neuropathy is a reversible disorder characterized by severe pain, autonomic dysfunction and unmyelinated nerve fiber damage after rapid and sustained glucose regulation in individuals with historically poor glycemic control. All subjects reported an improvement in pain after many months of continued glucose control, and those with type 1 diabetes in particular had improved autonomic symptoms, autonomic testing and nerve fiber density. The data suggest diffuse damage to the unmyelinated and lightly myelinated nerve fibers that is temporally related to the rapid improvement in glucose control. Furthermore, there was parallel worsening of diabetic retinopathy, also a microvascular complication of diabetes, suggesting a possible common underlying pathophysiology.
The pain in our cohort differed from that observed in subjects with the generalized painful polyneuropathy associated with diabetes. First, pain was more severe and more refractory to therapeutic interventions; the pain was rated 10 on a scale of 10 by all subjects despite treatment with multiple medications. Second, all subjects had the onset of pain within 6 weeks of rapid glucose control. Third, although the pain was symmetric and length dependent in the majority of patients, one third of the type 1 diabetic patients reported generalized pain; proximal and/or generalized pain has been previously reported in this disorder.3, 5, 18, 19 Fourth, evoked pain - hyperalgesia and allodynia - was present in 60% of subjects (80% of type 1 subjects and 40% of type 2 patients); a greater prevalence than in distal symmetric polyneuropathy.20 Finally, pain was self-limited in all subjects.
Unlike many reports of acute painful neuropathy,2, 3, 13 none of the individuals in our cohort had diabetic neuropathic cachexia. Only one of our subjects, a male patient with type 2 diabetes, intentionally dieting to improve glycemic control, reported substantial weight loss. All 7 of the women with type 1 diabetes had a remote history of diabetic anorexia. These women intentionally withheld insulin (in most cases in adolescence) to induce weight loss. The weight loss preceded the onset of acute painful neuropathy by at least 6 months and an average of almost 6 years. Prior reports of diabetic anorexia have noted pain onset with weight loss but in our subjects there were no reports of weight loss with pain. In all cases weight remained stable until resumption of insulin use and consequent weight gain.7 Furthermore, in contrast to reports of diabetic neuropathic cachexia5 and other reports of treatment induced neuropathy4 where symptoms peaked at the nadir of weight loss and resolved with weight gain, pain developed in these women after the weight gain. While it is not likely that the diabetic anorexia was a direct precipitant of the acute painful neuropathy, it is possible that it created a predisposition to later nerve injury.
All individuals with treatment induced neuropathy had evidence of autonomic dysfunction on testing and exhibited symptoms of autonomic impairment that was more prevalent and more severe than in patients with generalized diabetic peripheral neuropathy.21 For example, 69% of our cohort had systolic blood pressure falls > 20mmHg (78% of type 1 and 43% of type 2). In comparison, in the Rochester population based study of generalized neuropathy blood pressure falls of > 20 mmHg were present in 22.9% of type 1 and 16.2% of type 2 patients. In our cohort, even after 18 months, 31% of patients had blood pressure falls of >20 mmHg (22% with type 1 and 43% with type 2).
Symptoms of autonomic dysfunction were more prevalent and more severe in subjects with type 1 diabetes, particularly with respect to symptoms of orthostatic intolerance and gastrointestinal function. Urinary frequency, nocturia and anhidrosis were reported more frequently in individuals with type 2 diabetes, although it is unclear if this increase is due to differences in age and gender.
All subjects also had worsening of their retinopathy within 1 year of rigorous control. This observation is consistent with prior reports of unanticipated worsening of retinopathy in individuals with type 1 and type 2 diabetes that occurred shortly after the initiation of intensive treatment with insulin. The risk of early worsening retinopathy increases with each percentage point decrease in A1C.22 The cause of the early worsening of retinopathy is not known. Cytokines and trophic factors including the mitogenic cytokine, vascular endothelial growth factor (VEGF), insulin growth factor (IGF), IL-6, IL-8 and TNF-α have been implicated in the pathogenesis of diabetic retinopathy. It is hypothesized that upregulation of these cytokines and trophic factors associated with intensive glycemic control is responsible for the early worsening of retinopathy.23-25
Similarly, the underlying pathophysiology of this acute treatment induced neuropathy is not known. Proposed mechanisms include the development of epineurial arterio-venous shunting causing endoneurial ischemia,2 apoptosis due to sudden glucose deprivation,26 recurrent hypoglycemia resulting in microvascular neuronal damage,27, 28 ectopic pain from regenerating nerve fibers,19 ectopic firing of regenerating axon sprouts19 (most likely due to channel or receptor upregulation) and insulin induced reduction in endoneurial oxygen tension due to opening of arteriovenous shunts.2 Nerves of streptozotocin-induced diabetic rats appear resistant to this hypoxic effect of insulin, but with control of hyperglycemia this susceptibility re-appears.29 A direct relationship to hypoglycemia seems unlikely; hypoglycemic neuropathy preferentially involves the motor nerves.30 While the possibility of a nutritional deficiency has been raised when treatment induced neuropathy occurs in association with weight loss, the absence of weight loss in our subjects makes that etiology unlikely.
We and others recently have observed an increase in proinflammatory cytokines in association with experimental hypoglycemia.31, 32 Elevated cytokine levels, including interleukin-1β, interleukin-6 and tumor necrosis factor-α have been associated with painful neuropathy.33-35 We have also observed impaired autonomic function after experimental hypoglycemia.36 Thus, acute treatment induced neuropathy and worsening of retinopathy after intensive glycemic control may have a common underlying pathophysiological mechanism that involves upregulation of proinflammatory cytokines. Recent data suggest that activation of microglia with subsequent cytokine production may underlie both the pathogenesis of worsening retinopathy after intensive glycemic therapy and treatment induced neuropathy.37, 38 Microglial activation is present in human and preclinical models of diabetic retinopathy, and in preclinical models of neuropathic pain in which microglial activation with subsequent upregulation of cytokines and chemokines contributes to the development and maintenance of neuropathic pain.39, 40 Taken together these data suggest an additional hypoglycemia related pathophysiological mechanism and providing potential targets for therapeutic intervention.
Sural nerve biopsies have been reported in 8 patients in 4 different studies, with results revealing variable loss of myelinated fibers, acute axonal degeneration and some clusters of regenerating, myelinated fibers,4-6, 13 findings similar to other published data on sural nerves pathology in diabetes.41, 42 There are no reports of a follow-up biopsy in these acute painful neuropathy subjects.
Of the 8 subjects in our study with skin biopsies, all had borderline or abnormal nerve fiber densities at the distal leg. Morphologic abnormalities, including large swellings on small nerve fibers, were seen in several individuals (Supplementary Figure 2). The decreased IENFD at proximal sites tended to be seen in those with more widespread distribution of pain, but not in all cases. Those individuals with proximal pain that had normal IENFD at the distal and proximal thighs did have more prominent nerve fiber swellings. We, and others, have reported large nerve fiber swellings are associated with a decline in intra-epidermal nerve fiber density.15, 43 However, those subjects with large swellings that had biopsies repeated 1 year later did not have a reduction in IENFD and did not have morphologic abnormalities. These data suggest that if the stimulus for nerve damage is removed, nerve fiber swellings need not necessarily portend a decline in intra-epidermal nerve fiber density.44, 45
The cases in this report highlight that symptoms, signs and objective measures of small myelinated and unmyelinated nerve fibers can improve in patients with a prolonged history of very poor glucose control. After 18 months of improved glucose control, there were improvements in pain, symptoms and tests of autonomic function, and IENFD. The improvements in individuals with type 2 diabetes were not as marked as in those with type 1 diabetes. There are several factors that may explain the differences between these groups. Specifically, individuals with type 1 diabetes were younger, had less co-morbid medical conditions such as hyperlipidemia and hypertension, known risk factors for diabetic polyneuropathy,46 and ultimately had better glucose control (A1C average of 6.3) compared to those with type 2 diabetes (A1C average of 8.1). Nevertheless, even in the patients with type 2 diabetes pain improved substantially. We suggest that ‘treatment induced neuropathy’ more accurately encompasses the disorder than the historic term ‘insulin neuritis’.
Supplementary Material
Acknowledgement
This research was supported by the Juvenile Diabetes Research Foundation 11-2007-143 and NIH K23NS050209.
Footnotes
Disclosure: The authors report no conflicts of interest.
References
- 1.Caravati CM. Insulin neuritis: a case report. Va.Med.Monthly. 1933;59:745–746. [Google Scholar]
- 2.Tesfaye S, Malik R, Harris N, et al. Arterio-venous shunting and proliferating new vessels in acute painful neuropathy of rapid glycaemic control (insulin neuritis) Diabetologia. 1996;39:329–335. doi: 10.1007/BF00418349. [DOI] [PubMed] [Google Scholar]
- 3.Dabby R, Sadeh M, Lampl Y, et al. Acute painful neuropathy induced by rapid correction of serum glucose levels in diabetic patients. Biomed Pharmacother. 2008 doi: 10.1016/j.biopha.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Archer AG, Watkins PJ, Thomas PK, et al. The natural history of acute painful neuropathy in diabetes mellitus. J.Neurol.Neurosurg.Psychiatry. 1983;46:491–499. doi: 10.1136/jnnp.46.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ellenberg M. Diabetic neuropathic cachexia. Diabetes. 1974;23:418–423. doi: 10.2337/diab.23.5.418. [DOI] [PubMed] [Google Scholar]
- 6.Castellanos F, Mascias J, Zabala JA, et al. Acute painful diabetic neuropathy following severe weight loss. Muscle Nerve. 1996;19:463–467. doi: 10.1002/(SICI)1097-4598(199604)19:4<463::AID-MUS6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 7.Steel JM, Young RJ, Lloyd GG, Clarke BF. Clinically apparent eating disorders in young diabetic women: associations with painful neuropathy and other complications. British Medical Journal - Clinical Research. 1987;294:859–862. doi: 10.1136/bmj.294.6576.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Said G, Goulon-Goeau C, Slama G, Tchobroutsky G. Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus. A clinical and pathological study. New England Journal of Medicine. 1992;326:1257–1263. doi: 10.1056/NEJM199205073261905. [DOI] [PubMed] [Google Scholar]
- 9.Young RJ, Zhou YQ, Rodriguez E, et al. Variable relationship between peripheral somatic and autonomic neuropathy in patients with different syndromes of diabetic polyneuropathy. Diabetes. 1986;35:192–197. doi: 10.2337/diab.35.2.192. [DOI] [PubMed] [Google Scholar]
- 10.Young RJ, Ewing DJ, Clarke BF. Chronic and remitting painful diabetic polyneuropathy. Correlations with clinical features and subsequent changes in neurophysiology. Diabetes Care. 1988;11:34–40. doi: 10.2337/diacare.11.1.34. [DOI] [PubMed] [Google Scholar]
- 11.Said G, Slama G, Selva J. Progressive centripetal degeneration of axons in small fibre diabetic polyneuropathy. Brain. 1983;106:791–807. doi: 10.1093/brain/106.4.791. [DOI] [PubMed] [Google Scholar]
- 12.Ellenberg M. Diabetic neuropathy precipitating after institution of diabetic control. American Journal of Medical Science. 1958;236:466. [PubMed] [Google Scholar]
- 13.Vital C, Vital A, Dupon M, et al. Acute painful diabetic neuropathy: two patients with recent insulin- dependent diabetes mellitus. J.Peripher.Nerv.Syst. 1997;2:151–154. [PubMed] [Google Scholar]
- 14.Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999;41(Suppl 1):8–13. doi: 10.1159/000052074. [DOI] [PubMed] [Google Scholar]
- 15.Gibbons CH, Griffin JW, Polydefkis M, et al. The utility of skin biopsy for prediction of progression in suspected small fiber neuropathy. Neurology. 2006;66:256–258. doi: 10.1212/01.wnl.0000194314.86486.a2. [DOI] [PubMed] [Google Scholar]
- 16.McCarthy BG, Hsieh ST, Stocks A, et al. Cutaneous innervation in sensory neuropathies: evaluation by skin biopsy. Neurology. 1995;45:1848–1855. doi: 10.1212/wnl.45.10.1848. [DOI] [PubMed] [Google Scholar]
- 17.Position paper: Orthostatic hypotension, multiple system atrophy (the Shy Drager syndrome) and pure autonomic failure. Journal of the Autonomic Nervous System. 1996;58:123–124. doi: 10.1016/0165-1838(96)90001-6. [DOI] [PubMed] [Google Scholar]
- 18.Archer AG, Roberts VC, Watkins PJ. Blood flow patterns in painful diabetic neuropathy. Diabetologia. 1984;27:563–567. doi: 10.1007/BF00276968. [DOI] [PubMed] [Google Scholar]
- 19.Llewelyn JG, Thomas PK, Fonseca V, et al. Acute painful diabetic neuropathy precipitated by strict glycemic control. Acta Neuropathol.(Berl) 1986;72:157–163. doi: 10.1007/BF00685978. [DOI] [PubMed] [Google Scholar]
- 20.Otto M, Bak S, Bach FW, et al. Pain phenomena and possible mechanisms in patients with painful polyneuropathy. Pain. 2003;101:187–192. doi: 10.1016/s0304-3959(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 21.Low PA, Benrud-Larson LM, Sletten DM, et al. Autonomic Symptoms and Diabetic Neuropathy: A population-based study. Diabetes Care. 2004;27:2942–2947. doi: 10.2337/diacare.27.12.2942. [DOI] [PubMed] [Google Scholar]
- 22.Anonymous Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Archives of Ophthalmology. 1998;116:874–886. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 23.Davis MD. Worsening of diabetic retinopathy after improvement of glycemic control. Arch Ophthalmol. 1998;116:931–932. doi: 10.1001/archopht.116.7.931. [DOI] [PubMed] [Google Scholar]
- 24.Chantelau E. Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Br J Ophthalmol. 1998;82:725–730. doi: 10.1136/bjo.82.7.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2009;127:1175–1182. doi: 10.1001/archophthalmol.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Honma H, Podratz JL, Windebank AJ. Acute glucose deprivation leads to apoptosis in a cell model of acute diabetic neuropathy. J Peripher Nerv Syst. 2003;8:65–74. doi: 10.1046/j.1529-8027.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- 27.Yasaki S, Dyck PJ. Duration and severity of hypoglycemia needed to induce neuropathy. Brain Res. 1990;531:8–15. doi: 10.1016/0006-8993(90)90752-w. [DOI] [PubMed] [Google Scholar]
- 28.Ohshima J, Nukada H. Hypoglycaemic neuropathy: microvascular changes due to recurrent hypoglycaemic episodes in rat sciatic nerve. Brain Res. 2002;947:84–89. doi: 10.1016/s0006-8993(02)02910-4. [DOI] [PubMed] [Google Scholar]
- 29.Kihara M, Zollman PJ, Smithson IL, et al. Hypoxic effect of exogenous insulin on normal and diabetic peripheral nerve. Am J Physiol. 1994;266:E980–985. doi: 10.1152/ajpendo.1994.266.6.E980. [DOI] [PubMed] [Google Scholar]
- 30.Mohseni S. Hypoglycemic neuropathy. Acta Neuropathol. 2001;102:413–421. doi: 10.1007/s004010100459. [DOI] [PubMed] [Google Scholar]
- 31.Dotson S, Freeman R, Failing HJ, Adler GK. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care. 2008;31:1222–1223. doi: 10.2337/dc07-2243. [DOI] [PubMed] [Google Scholar]
- 32.Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism. 2009;58:443–448. doi: 10.1016/j.metabol.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 33.Backonja MM, Coe CL, Muller DA, Schell K. Altered cytokine levels in the blood and cerebrospinal fluid of chronic pain patients. J Neuroimmunol. 2008;195:157–163. doi: 10.1016/j.jneuroim.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Uceyler N, Sommer C. Cytokine regulation in animal models of neuropathic pain and in human diseases. Neurosci Lett. 2008;437:194–198. doi: 10.1016/j.neulet.2008.03.050. [DOI] [PubMed] [Google Scholar]
- 35.Sommer C, Kress M. Recent findings on how proinflammatory cytokines cause pain: peripheral mechanisms in inflammatory and neuropathic hyperalgesia. Neurosci.Lett. 2004;361:184–187. doi: 10.1016/j.neulet.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 36.Adler GK, Bonyhay I, Failing H, et al. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes. 2009;58:360–366. doi: 10.2337/db08-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishikiori N, Osanai M, Chiba H, et al. Glial cell-derived cytokines attenuate the breakdown of vascular integrity in diabetic retinopathy. Diabetes. 2007;56:1333–1340. doi: 10.2337/db06-1431. [DOI] [PubMed] [Google Scholar]
- 38.Zeng HY, Green WR, Tso MO. Microglial activation in human diabetic retinopathy. Arch Ophthalmol. 2008;126:227–232. doi: 10.1001/archophthalmol.2007.65. [DOI] [PubMed] [Google Scholar]
- 39.Romero-Sandoval EA, Horvath RJ, DeLeo JA. Neuroimmune interactions and pain: focus on glial-modulating targets. Curr Opin Investig Drugs. 2008;9:726–734. [PMC free article] [PubMed] [Google Scholar]
- 40.Abbadie C, Bhangoo S, De Koninck Y, et al. Chemokines and pain mechanisms. Brain Res Rev. 2009;60:125–134. doi: 10.1016/j.brainresrev.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Llewelyn JG, Gilbey SG, Thomas PK, et al. Sural nerve morphometry in diabetic autonomic and painful sensory neuropathy. A clinicopathological study. Brain. 1991;114:867–892. doi: 10.1093/brain/114.2.867. [DOI] [PubMed] [Google Scholar]
- 42.Malik RA, Tesfaye S, Newrick PG, et al. Sural nerve pathology in diabetic patients with minimal but progressive neuropathy. Diabetologia. 2005;48:578–585. doi: 10.1007/s00125-004-1663-5. [DOI] [PubMed] [Google Scholar]
- 43.Lauria G, Morbin M, Lombardi R, et al. Axonal swellings predict the degeneration of epidermal nerve fibers in painful neuropathies. Neurology. 2003;61:631–636. doi: 10.1212/01.wnl.0000070781.92512.a4. [DOI] [PubMed] [Google Scholar]
- 44.Lauria G, McArthur JC, Hauer PE, et al. Neuropathological alterations in diabetic truncal neuropathy: evaluation by skin biopsy. J.Neurol.Neurosurg.Psychiatry. 1998;65:762–766. doi: 10.1136/jnnp.65.5.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nodera H, Barbano RL, Henderson D, Herrmann DN. Epidermal reinnervation concomitant with symptomatic improvement in a sensory neuropathy. Muscle Nerve. 2003;27:507–509. doi: 10.1002/mus.10336. [DOI] [PubMed] [Google Scholar]
- 46.Tesfaye S, Chaturvedi N, Eaton SE, et al. Vascular risk factors and diabetic neuropathy. New England Journal of Medicine. 2005;352:341–350. doi: 10.1056/NEJMoa032782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.