Abstract
Introduction
Total body dual-energy x-ray absorptiometry (DXA) data offer the opportunity to compare bone density of demographic groups across the entire skeleton.
Methods
The present study uses total body DXA data (Hologic QDR 4500A, Hologic Inc, Bedford MA) from the National Health and Nutrition Examination Survey (NHANES) 1999–2004 to examine bone mineral density (BMD) of the total body and selected skeletal subregions in a wide age range of adult men and women from three race/ethnic groups. Total body, lumbar spine, pelvis, right leg, and left arm BMD and lean mass from 13,091 adults age 20 years and older were used. The subregions were chosen to represent sites with different degrees of weight bearing.
Results
Mean BMD varied in expected ways for some demographic characteristics (men>women and non-Hispanic blacks>non-Hispanic whites) but not others (non-Hispanic whites>Mexican Americans). Differences in age patterns in BMD also emerged for some characteristics (sex) but not others (race/ethnicity). Differences in cross-sectional age patterns in BMD and lean mass by degree of weight-bearing in older adults were observed for the pelvis, leg and arm.
Conclusion
This information may be useful for generating hypotheses about age, race, and sex differences in fracture risk in the population.
Keywords: Total body bone mineral density, race/ethnic differences, gender differences, cross-sectional age patterns
Introduction
Total bone mineral density (BMD) from dual-energy x-ray absorptiometry (DXA) total body scans is routinely collected in body composition studies but rarely analyzed in detail [1]. These data provide a unique opportunity to assess differences in skeletal status between groups across the entire skeleton. Studies examining demographic patterns in total body BMD to date, however, have generally not included data for both sexes in multiple race/ethnic groups across a wide age range within the same study [2–9]. Total body DXA scans were conducted for the first time in the National Health and Nutrition Examination Survey (NHANES) starting in 1999. The present study uses total body DXA data from NHANES 1999–2004 to examine bone density patterns in men and women age 20 years and older from three race/ethnic groups: non-Hispanic whites, non-Hispanic blacks, and Mexican Americans. In addition, bone is responsive to mechanical loads [10], and Frost [11] has proposed that osteoporosis is a disuse phenomenon, in which bones adapt to decreased skeletal loading with age by remodeling to reduce their mass. In light of this possibility, demographic patterns in BMD and lean mass are also examined in the present study for selected skeletal subregions which vary in the degree of weight-bearing. Total BMD is not an appropriate surrogate for diagnosing osteoporosis or assessing fracture risk [12], because the relationship between total body BMD and fracture risk has not been adequately defined. Nonetheless, this information may be useful for generating hypotheses about age-, race- and sex-specific differences in fracture risk in the population.
Methods
Sample
Total body skeletal status was assessed using DXA data from the NHANES, which is conducted by the National Center for Health Statistics (NCHS), Centers for Disease Control and Prevention, to assess the health and nutritional status of a large representative cross-sectional sample of the non-institutionalized, civilian US population. All procedures in NHANES 1999–2004 were approved by the NCHS Institutional Review Board, and written informed consent was obtained from all subjects [13].
Data were collected in NHANES 1999–2004 via household interviews and direct standardized physical examinations that were conducted in specially equipped mobile examination centers [13]. NHANES 1999–2004 was designed to provide reliable estimates for three race/ethnic groups: non-Hispanic whites (NHW), non-Hispanic blacks (NHB), and Mexican Americans (MA). Race and ethnicity were self-reported by the participants.
Respondents with missing total body DXA data were not a random subset of the eligible sample1. Specifically, the percentage of eligible participants with valid data decreased with increasing age (primarily due to an increase in implants such as pacemakers, stents, and hip replacements) and with increasing body mass index (primarily due to truncal adiposity which interfered with the ability to obtain valid measurements). To address this potential bias, multiple imputation of missing data for the total body and subregions was performed using a sequential regression multivariate imputation procedure, the details of which have been published elsewhere [14]. The regression models used to impute the data employed a large number of predictor variables, including DXA data for any nonmissing total body subregions, as well as demographic, socioeconomic and geographic variables, anthropometric measurements, health indicators, dietary intake and medication use, blood test results, and variables related to the design of the NHANES sample. Data were multiply-imputed for all sample persons in the eligible age range for this examination except pregnant women and those with limb amputations other than fingers or toes. The imputed values of a small number of respondents were found to vary greatly among the different imputed datasets. Review of the data for these participants showed that their values for all DXA measurements were missing, as well as measured height and weight (important predictor variables in the imputation models). Because of the extreme variability of their imputed DXA data values, the data for these respondents were not included in the present study.
The present study uses the total body DXA measurements for 13,091 adults age 20 years and older with complete total body bone density data, including 3,049 with imputed values that were not highly variable (e.g., did not lack measurements of height, weight, and all DXA data, as described above). This represents 85% of the participants in this age range who were interviewed (n=15,332) and 92% of those who were interviewed and examined (n=14,213). Eight percent (n=1122) of the examinee sample in this age range were not included in the present study because no DXA values were imputed for them due to their physiological state (pregnant women, amputees) or because they had highly variable imputed values. Of these, 772 were pregnant women, 46 were amputees and 304 had highly variable imputed DXA values.
Variables
Total body scans were performed with Hologic QDR 4500A fan-beam densitometers (Hologic, Inc., Bedford, Massachusetts) using DOS software version 8.26:a3*. Scanning was done in the fast mode. Details of the DXA examination protocol have been published elsewhere [15]. Each scan was reviewed and analyzed by the Department of Radiology of the University of California, San Francisco, using standard radiologic techniques and study-specific protocols developed for the NHANES. Hologic Discovery software version 12.1 was used to analyze all scans for total body bone density.
BMD of the total body and four selected skeletal subregions (i.e., lumbar spine, pelvis, right leg, and left arm) were included in the present study: These subregions were chosen because they vary in the extent to which they are weight bearing, from the pelvis to the leg to the lumbar spine to the arm, which is considered non-weight bearing in this analysis. Since the right side is the dominant side in the majority of individuals [16], the right leg and left arm were chosen to enhance the contrast between weight-bearing and nonweight-bearing sites.
Skeletal load is determined by muscle forces as well as by gravity from bearing weight [10], so data on lean mass excluding fat mass and bone mineral content were also examined for the total body and for body subregions similar to those used to examine BMD. Specifically, lean mass data were available for the right leg and left arm. Lean mass for the pelvis was not available, however, so trunk lean mass was examined instead. The lean mass data were obtained using the same DXA instruments and procedures as described above.
An analysis of data from 608 respondents in NHANES who received two DXA scans was conducted to assess precision of the total body measurements. The root mean square coefficient of variation ranged from 1.1–3.9% for BMD and 1.5–3.5% for lean mass for the skeletal regions used in the present study.
Upper leg length measurements were included in the analyses of age patterns in BMD and lean mass, in order to control for differences in body size. Upper leg length was measured as the distance between the inguinal crease (just below the anterior superior iliac spine) to the top of the patella [17].
Statistical analysis
Analyses were conducted with PC-SAS (Version 9.1, SAS Institute, Cary NC) and SUDAAN (Version 9.03, Research Triangle Institute, NC). All analyses used sample weights and took into account the complex design of the survey and the multiple imputations in calculating statistical tests.
Means were age-standardized to the 2000 US Census. Linear regression was used to assess differences in means by age, sex, and race/ethnicity, as well as to assess interactions between BMD and these demographic characteristics. Linear regression was also used to compare the strength of the relationship between BMD and age at the different skeletal sites. Because the regression coefficients are based on variables that are measured on the same scale, a comparison of relative strength between the skeletal sites was made. For this analysis, the regression coefficients for age were calculated while adjusting for race/ethnicity or for race/ethnicity and upper leg length for each skeletal subregion separately by sex. These coefficients were compared between subregions after first performing an analysis similar to a multiple analysis of variance (MANOVA) in which the association between age and bone density was simultaneously assessed at the four different skeletal sites in order to account for within and between subjects correlations. This was done by restructuring the dataset to create a “skeletal site” variable (pelvis, left arm, right leg, lumbar spine) for each subject, and then testing whether an interaction term for “skeletal site” * age was significant using linear regression while also adjusting for race/ethnicity and leg length. This model was also used to determine significant differences between the individual skeletal sites when the interaction term indicated that the relationship between age and BMD differed significantly between sites.
Secondary analyses were performed to examine differences between race/ethnic groups within sex after excluding imputed data in order to assess whether including the imputed data (e.g., including individuals with more extreme values for body weight or body fat) influenced the observed patterns in BMD by race/ethnicity. Since inclusion of the imputed data did not influence these race/ethnic patterns, the patterns shown below include the imputed data.
Results
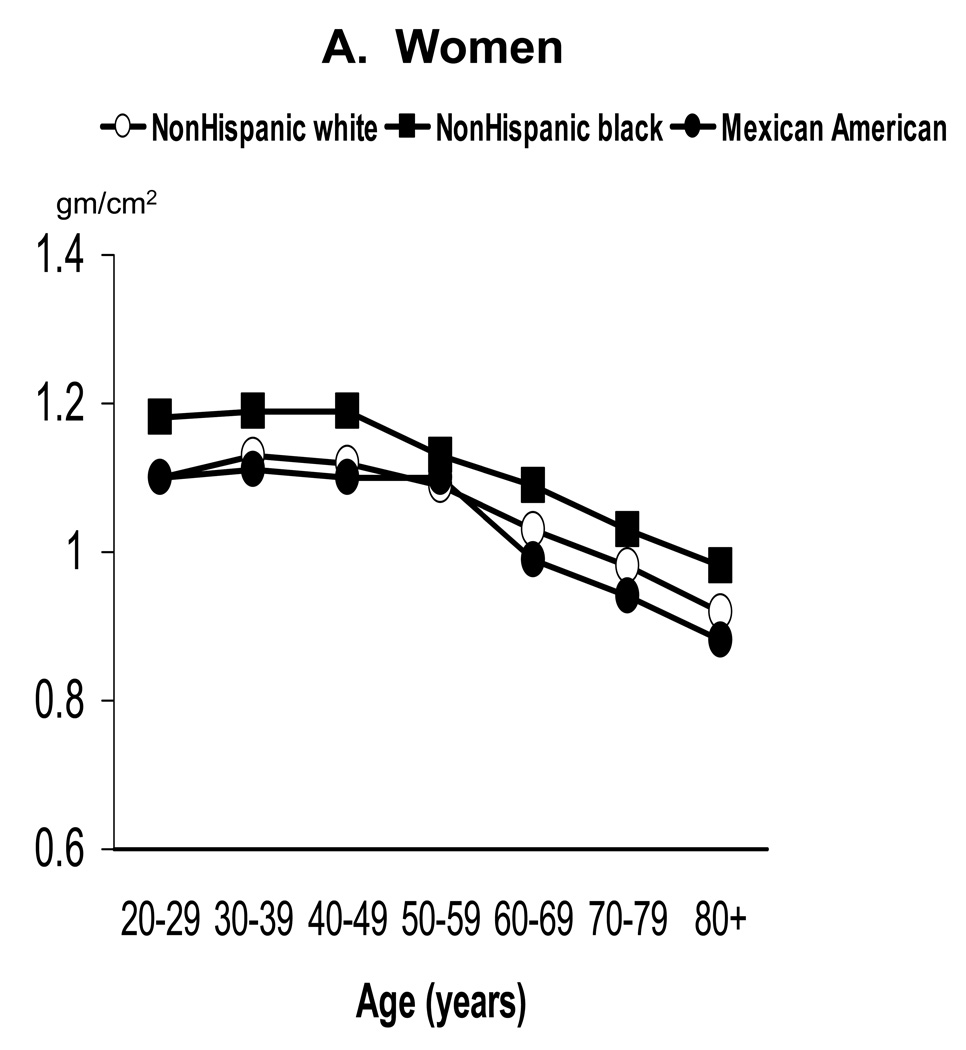
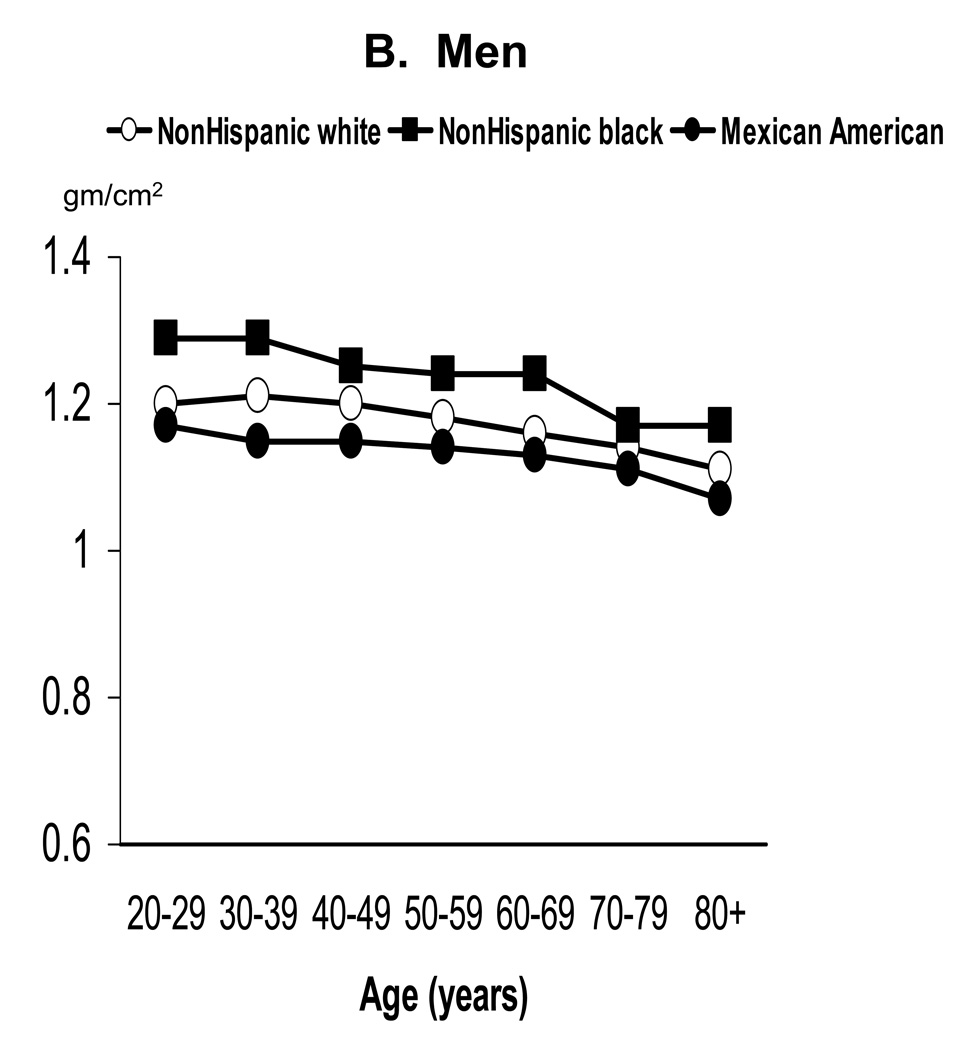
Age-standardized mean BMD by sex and race/ethnicity for adults age 20 years and older is shown in Table 1 for the total body and the four selected skeletal subregions. Within race/ethnic group, age-adjusted mean BMD was significantly higher in men than women at all skeletal sites examined (p<0.05). Within sex groups, age-adjusted mean BMD also differed significantly between each of the three race/ethnic groups at all skeletal sites examined (p<0.0001 for each comparison), with values being highest in NHB, intermediate in NHW, and lowest in MA. As illustrated with total body BMD in Figure 1, this race/ethnic pattern generally held in each decade of age. Specifically, NHB had significantly higher mean total body BMD than NHW or MA in every decade (p<0.02) in both sexes. NHW also had significantly higher mean total body BMD than MA in each decade (p<0.02), except for women under 50 years of age.
Table 1.
Age-standardized mean bone mineral density (BMD)* of the total body and selected subregions by sex and race/ethnicity among adults age 20 years and older, NHANES 1999–2004
| Skeletal site | Women | Men | ||||
|---|---|---|---|---|---|---|
| Race/ethnicity | N | Mean | SE* | n | Mean | SE* |
| Total body | ||||||
| All races*** | 6532 | 1.087 | 0.002 | 6559 | 1.184 | 0.002 |
| NonHispanic white | 3276 | 1.083 | 0.002 | 3347 | 1.184 | 0.003 |
| NonHispanic black | 1329 | 1.148 | 0.004 | 1262 | 1.252 | 0.005 |
| Mexican Americans | 1438 | 1.061 | 0.005 | 1488 | 1.142 | 0.006 |
| Pelvis | ||||||
| All races*** | 6532 | 1.233 | 0.003 | 6559 | 1.325 | 0.004 |
| NonHispanic white | 3276 | 1.226 | 0.003 | 3347 | 1.326 | 0.005 |
| NonHispanic black | 1329 | 1.326 | 0.007 | 1262 | 1.420 | 0.009 |
| Mexican Americans | 1438 | 1.202 | 0.007 | 1488 | 1.272 | 0.008 |
| Right leg | ||||||
| All races*** | 6532 | 1.110 | 0.002 | 6559 | 1.292 | 0.003 |
| NonHispanic white | 3276 | 1.111 | 0.003 | 3347 | 1.296 | 0.004 |
| NonHispanic black | 1329 | 1.158 | 0.005 | 1262 | 1.354 | 0.007 |
| Mexican Americans | 1438 | 1.074 | 0.006 | 1488 | 1.242 | 0.007 |
| Lumbar spine | ||||||
| All races*** | 6532 | 1.037 | 0.002 | 6559 | 1.054 | 0.003 |
| NonHispanic white | 3276 | 1.034 | 0.003 | 3347 | 1.052 | 0.004 |
| NonHispanic black | 1329 | 1.112 | 0.005 | 1262 | 1.144 | 0.008 |
| Mexican Americans | 1438 | 0.986 | 0.005 | 1488 | 1.001 | 0.006 |
| Left arm | ||||||
| All races*** | 6532 | 0.711 | 0.001 | 6559 | 0.859 | 0.002 |
| NonHispanic white | 3276 | 0.711 | 0.001 | 3347 | 0.862 | 0.002 |
| NonHispanic black | 1329 | 0.746 | 0.002 | 1262 | 0.893 | 0.003 |
| Mexican Americans | 1438 | 0.692 | 0.002 | 1488 | 0.837 | 0.004 |
Standardized to the 2000 Census population
Standard error
includes persons of other races not shown separately
Figure 1.
Mean total body BMD of adults ages 20 years and older by age, sex, and race/ethnicity: NHANES 1999–2004
Regression analyses for each skeletal site revealed that the pattern of mean BMD by age differed in those <50 years of age versus those 50 years and older. Specifically, the relationship of age and BMD was weak and variable in direction at the different sites in those < age 50 years (data not shown). As a result, subsequent analyses of age patterns were focused on age 50+ years only. Analyses for these older adults were done separately by sex because regression results indicated that the interaction of sex*age was significant at all skeletal sites (p<0.02). However, analyses were not done separately by race/ethnicity because the race*age interaction was not significant for any of the skeletal sites examined. Results were adjusted for effects of race/ethnicity by including it as an independent variable in the regression equation.
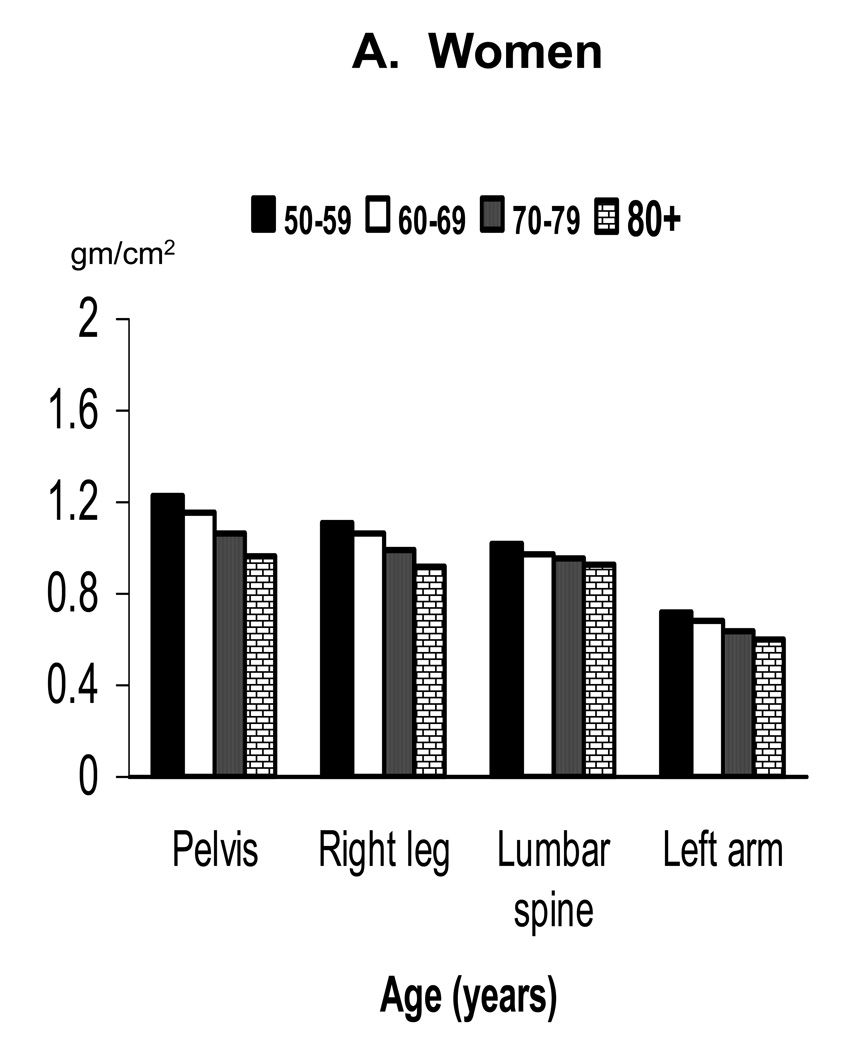
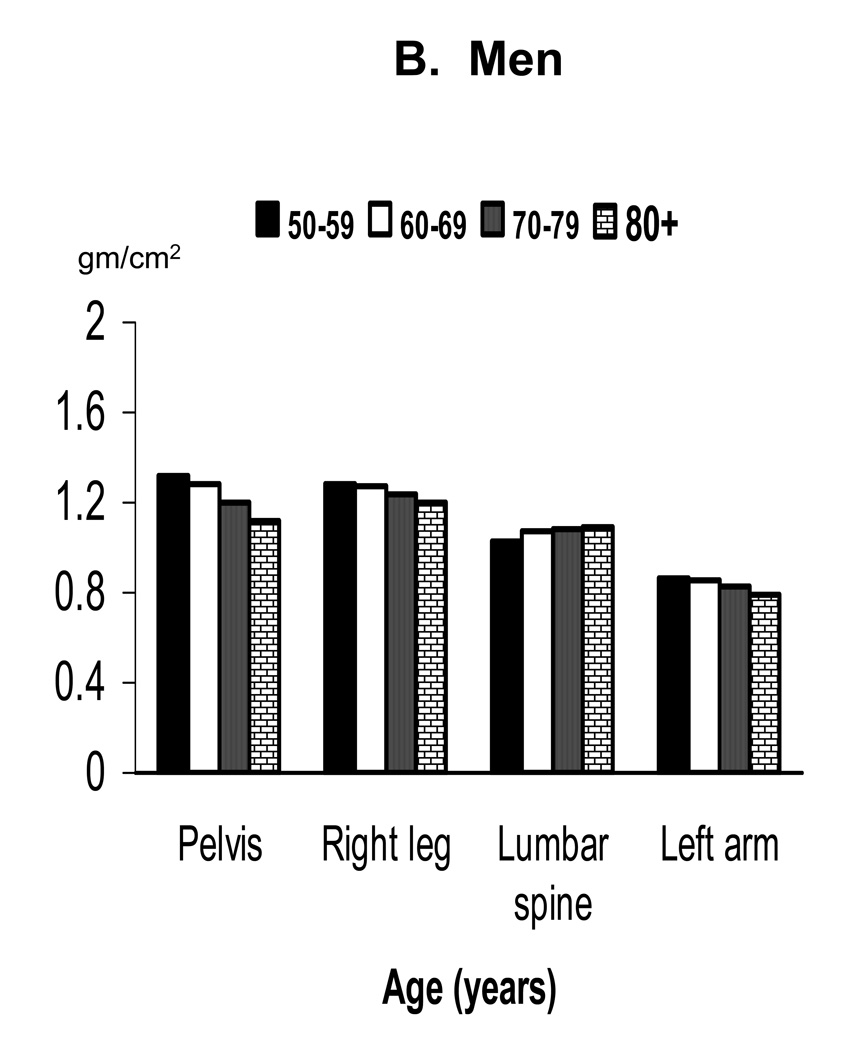
Mean BMD by decade in older adults at the different skeletal sites is shown separately by sex in Figure 2. Mean BMD was lower in each succeeding decade of age at all skeletal sites in both sexes except the lumbar spine in men. Table 2 provides a comparison of results for the regression of BMD on age in older adults after adjusting for race/ethnicity or for race/ethnicity and leg length. The regression coefficients indicate that age was significantly negatively related to BMD of the total body, pelvis, right leg and left arm in both sexes. Age was also significantly negatively related to lumbar spine BMD in women, but was significantly positively related to lumbar spine BMD in men.
Figure 2.
Mean BMD of selected skeletal subregions in older adults by age and sex: NHANES 1999–2004
Table 2.
Relationship of age with BMD or lean mass*at selected skeletal sites in adults age 50 years and older, NHANES 1999–2004
| Women (n=3326) | Men (n=3227) | |||||
|---|---|---|---|---|---|---|
| Regression Coefficient** |
SE*** | p value | Regression Coefficient** |
SE*** | p value | |
| BMD (gm/cm2) | ||||||
| Adjusted for race/ethnicity | ||||||
| Total body | −0.0056 | 0.0002 | 0.00001 | −0.0022 | 0.0002 | 0.00001 |
| Pelvis | −0.0088a,b,c | 0.0004 | 0.00001 | −0.0064a,b,c | 0.0004 | 0.00001 |
| Right leg | −0.0064 a,d,e | 0.0002 | 0.00001 | −0.0026a,d | 0.0002 | 0.00001 |
| Lumbar spine | −0.0033b,d | 0.0004 | 0.00001 | 0.0022b,d,e | 0.0005 | 0.0002 |
| Left arm | −0.0040c,e | 0.0001 | 0.00001 | −0.0022c,e | 0.0002 | 0.00001 |
| Adjusted for race/ethnicity and leg length | ||||||
| Total body | −0.0056 | 0.0002 | 0.00001 | −0.0019 | 0.0002 | 0.00001 |
| Pelvis | −0.0086 a,b,c | 0.0003 | 0.00001 | −0.0100a,b,c, | 0.0004 | 0.00001 |
| Right leg | −0.0063a,d,e | 0.0002 | 0.00001 | −0.0023 a,d | 0.0003 | 0.00001 |
| Lumbar spine | −0.0034b,d,f | 0.0004 | 0.00001 | 0.0022b,d,e | 0.0005 | 0.00001 |
| Left arm | −0.0039 c,e,f | 0.0001 | 0.00001 | −0.0019c,e | 0.0002 | 0.00001 |
| Lean mass (gms)* | ||||||
| Adjusted for race/ethnicity | ||||||
| Total body | −193.2 | 17.57 | 0.00001 | −320.7 | 16 | 0.00001 |
| Trunk | −93.2a,b,c | 8.97 | 0.00001 | −134.6a,b,c | 8.7 | 0.0001 |
| Right leg | −35.6b,d | 3.36 | 0.00001 | −59.2b,d | 3.28 | 0.00001 |
| Left arm | −12.0c,d | 1.04 | 0.00001 | −30.1c,d | 1.35 | 0.00001 |
| Adjusted for race/ethnicity and leg length | ||||||
| Total body | −170.3 | 16.96 | 0.00001 | −274 | 17.34 | 0.00001 |
| Trunk | −84.6a,b,c | 8.87 | 0.00001 | −114.4a,b,c | 8.67 | 0.0001 |
| Right leg | −29.6b,d | 3.08 | 0.00001 | −49.5b,d | 3.56 | 0.00001 |
| Left arm | −10.9c,d | 1.06 | 0.00001 | −26.9c,d | 1.34 | 0.00001 |
excluding fat mass and bone mineral content
Coefficients for skeletal subregions within sex sharing common superscripts differ significantly, p< 0.01
Standard error
The relative strength of the relationship between age and BMD, as reflected by the size of the regression coefficients in Table 2, varied at the four skeletal subregions. The strongest relationship emerged for the pelvis, where the regression coefficient was significantly more negative than those for the other sites examined in both sexes. The relationship between age and BMD at the right leg was also significantly more negative than that seen for the left arm in women, but not in men. The age-BMD relationship at the lumbar spine differed from that seen at the other subregions, being significantly weaker than that for the pelvis or leg in women and, as already noted, positive in direction in men. Adjusting for upper leg length tended to alter the magnitude of the regression coefficients for the 4 skeletal subregions, but did not affect conclusions (Table 2).
Differences in the strength of the relationship between lean mass and age were also seen for the trunk, leg and arm in both sexes (Table 2). The relationship with age was negative at all three body subregions, but was significantly strongest for the trunk, followed by the leg, and weakest for the arm. Adjusting for upper leg length did not affect conclusions.
Discussion
Our findings regarding differences in mean BMD for the total body and selected subregions in men versus women or in NHB versus NHW agree with previous studies [2,4,5, 18–20], and are also consistent with fracture differences observed for these groups [21]. However, BMD values among MA in NHANES 1999–2004 were lower than in NHW of the same age and sex at the different skeletal sites, which contrasts with findings for the proximal femur from NHANES III [19]. A comparison of selected relevant demographic, acculturation, and body size variables of Mexican Americans in NHANES III versus NHANES 1999–2004 did not identify a clear explanation for this discrepancy. BMD differences between Hispanics and whites (when not adjusted for body size) have also varied in direction in community-based studies [2,4,5,18] and, in some cases, have varied by skeletal site within studies. For example, Araujo et al [2] reported lower femur BMD in older white men compared to Hispanic men, but found no difference in BMD between these groups at the total body, lumbar spine, or forearm. In contrast, Morton et al [4] reported similar unadjusted BMD values in Hispanic and white women at the hip but lower values in Hispanic women at the lumbar spine and total body. Finally, Taaffe et al [5] reported similar BMD in whites and Hispanics at the hip, spine, or total body prior to adjusting for height.
The differences in BMD between age decades in older adults at the different skeletal sites were greater in women than men, which is consistent with longitudinal studies that have documented greater BMD loss with age in women than in men [22, 23]. Within sex, we did not find strong evidence for different age patterns in BMD between race/ethnic groups. This agrees with findings reported previously for the proximal femur from NHANES III and from other cross-sectional studies [2,19, 20]. There are few prospective data on ethnic differences in BMD change with age, and results have varied somewhat. For example, two longitudinal studies in elderly men and women showed lower rates of BMD loss from the hip in blacks when compared to Caucasians [24,25]. A third longitudinal study suggested that ethnic differences in the rate of loss varied by age and skeletal site, with white women having faster loss at the forearm than blacks at menopause but not in older age groups, and no difference in spine BMD loss between races regardless of age [26]. To date, longitudinal data by race for the skeletal subregions examined in this study have not been published, so it is not known whether these sites show differential BMD loss by age between whites and blacks. We are also unaware of comparable longitudinal data comparing rates of BMD loss in Hispanics with other groups for any skeletal site. Each of the three longitudinal studies comparing BMD loss rates of blacks versus whites had some limitations. Depending on the study, the limitations included a relatively short time period (1.5–4 years) between measurements, a small sample size, use of different densitometers for measurements at different time points, or use of different time intervals between measures in the different race/ethnic groups. On the other hand, the cross-sectional age patterns presented here can suffer from cohort effects, and they have been shown to overestimate loss from some skeletal sites and underestimate loss from other sites when compared to longitudinal rates of BMD loss at those sites [22, 23]. Thus, the cross-sectional age patterns by race/ethnicity seen in the present study should be interpreted with caution and need to be confirmed with longitudinal data.
The strength of the cross-sectional relationship between age and BMD in older adults differed among the skeletal subregions examined in the present study. Of particular interest is that the relationship strength tended to follow degree of weight-bearing. Specifically, the strongest relationships were observed for the pelvis, which differed from that seen at the leg as well as the arm in both sexes. The relationship at the right leg also differed from that seen at the arm in women, although not in men. Longitudinal data describing BMD loss at the pelvis and leg could not be located for comparison, but other cross-sectional studies have also reported greater decreases in BMD with age at the pelvis relative to other skeletal subregions in both sexes [3,9].
Finding a stronger negative relationship at these weight-bearing sites is consistent with Frost’s suggestion that osteoporotic fragility is a disuse phenomenon [11]. Melton et al [10] found declines in habitual skeletal loading were generally accompanied by reductions in bone strength indices at several skeletal sites. Loss of loading from gravitational, weight-bearing forces could contribute to lower skeletal loads with age. Both body weight and body mass index tend to decline with age in older adults [27], as do physical activity levels [28]. The importance of weight-bearing in maintaining BMD at the pelvis and leg is also consistent with results from a study of BMD loss during spaceflight, as most of the total loss that occurred during the space missions examined came from these two sites [29].
Changes in lean mass with age could also play a role in differences in the age-BMD relationships, since muscle forces also contribute to skeletal loads. Of particular interest is the consistency in the relative ranking of age patterns in lean mass (trunk>legs>arms) with the relative ranking of age patterns in BMD (pelvis>legs>arms) observed in the present study. These findings suggest that the loss of muscle forces on bone may be greatest in the pelvis, followed by the legs and then the arms. The stronger negative relationship between lean mass and age in the leg than arm observed in our study is consistent with results from several longitudinal studies that have reported greater loss of lean mass from the leg than arm [30–32]. One of these longitudinal studies also found larger changes in trunk lean soft tissue than in the legs (−1.2% vs. −0.8% in men and −0.6% vs −0.3% in women after two years) [30]. The differences between the trunk and legs in this longitudinal study were not tested statistically, but they appear consistent with the cross-sectional age patterns in lean mass observed in the present study.
Some caveats apply, however. For example, the trunk includes more skeletal regions than just the pelvis. In addition, trunk lean mass is not solely skeletal muscle. Magnetic resonance imaging data from a small sample of young adults suggest skeletal muscle makes up a greater proportion of trunk tissue volume than visceral organs [33], but it is not known whether this applies to older adults. It is also important to note that the age patterns in lean mass and BMD were not completely consistent in men: the age pattern in lean mass differed significantly between the arm and leg in men, whereas the age pattern in BMD did not. Age patterns in the present study are cross-sectional, and may not accurately reflect longitudinal lean mass loss. Finally, although Melton et al [10] found some correspondence in age-related declines in bone strength and habitual loading, none of the loading variables tested explained >41% of the bone variation in bone strength in their cross-sectional study. Thus, skeletal loading may only partially explain the loss of bone with age.
The relationship between age and BMD at the lumbar spine also differed significantly from that seen at the other three subregions examined. In men, the association was positive in direction. In women, the association was negative but weaker than at the other skeletal sites. Other cross-sectional studies have also reported either no BMD loss or apparent BMD gain in men when based on anterior-posterior spine scans [3, 6]. Interpretation of age patterns in lumbar spine BMD is likely confounded by changes in the spine with age that mask BMD loss [34]. Indeed, Melton et al [22] found substantial BMD loss at the lumbar spine with age when lateral spine scans were used to measure BMD and the same observation has been made in studies based on quantitative computed tomography (QCT) of the lumbar spine [35].
Limitations of the present study include use of cross-sectional data to describe changes with age and possible confounding in the observed age patterns by bone size. BMD is only partially corrected for bone size, so we also adjusted for leg length to reduce bone size confounding. However, it is not clear that this adjustment can effectively remove bone size effects for the different skeletal subregions considered. Other limitations include the potential for nonresponse bias in the sample, and the exclusion of institutionalized people, who may have lower BMD [38], from the survey sampling frame. Nonresponse bias due to refusal to participate in the physical examinations in NHANES is reduced by a nonresponse adjustment factor included in the calculation of the sample weights for use with examinee data. However, about 8% of the respondents age 20 years and older who came to the exam centers lacked usable total body DXA data, and this nonresponse is not addressed by the sample weight adjustments. These were mostly pregnant women; a smaller number either had highly variable imputed DXA values or were amputees. Imputed values for those with missing DXA data were used in the present study (unless highly variable) to help reduce nonresponse bias by other factors, such as increasing age or body mass index.
In summary, total body DXA data from NHANES 1999–2004 offer the opportunity to examine bone density of the total body and skeletal subregions for a wide age range of adult men and women from several race/ethnic groups. Mean BMD of the total body and selected subregions varied in expected ways for some demographic characteristics (men>women and NHB>NHW) but not in others (NHW>MA). Differences in age-related patterns in BMD also emerged for some characteristics (sex) but not others (race/ethnicity). Differences in age patterns in BMD by degree of weight-bearing in older adults were detected for the pelvis, leg and arm. In women, the relationship differed between all three sites, (e.g., pelvis > right leg > left arm). In men, the relationship differed for the pelvis when compared to the leg or arm, but not between the arm and leg. Similar patterns seen for lean mass in these three skeletal subregions suggest muscle forces may play a role in BMD differences by age. Age patterns for the lumbar spine seen in the present study are likely confounded by degenerative changes in the spine with age that mask BMD loss when standard anterior-posterior DXA scans are used. Longitudinal data are needed to confirm the age patterns seen in this study.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention, the National Institutes of Health, or the Department of Health and Human Services.
Participants were excluded from the DXA examination if they had a history of radiographic contrast material (barium) use in past 72 hours, nuclear medicine studies in the past 3 days, weighed over 300 pounds (self-reported) or were taller than 6’5”; the latter two characteristics were related to physical limitations of the DXA table. Females were excluded if they had a positive pregnancy test or said they were pregnant at the time of the examination.
References
- 1.Melton LJ, Looker AC, Shepherd JA, O’Connor MK, Achenbach SJ, Riggs BL, Khosla S. Osteoporosis assessment by whole body region vs. site-specific DXA. Osteoporos Int. 2005;16:1558–1564. doi: 10.1007/s00198-005-1871-y. [DOI] [PubMed] [Google Scholar]
- 2.Araujo AB, Travison TG, Harris SS, Holick MF, Turner AK, McKinlay JB. Race/ethnic differences in bone mineral density in men. Osteoporos Int. 2007;18:943–953. doi: 10.1007/s00198-006-0321-9. [DOI] [PubMed] [Google Scholar]
- 3.Fatayerji D, Cooper AM, Eastell R. Total body and regional bone mineral density in men: effect of age. Osteoporos Int. 1999;10:59–65. doi: 10.1007/s001980050195. [DOI] [PubMed] [Google Scholar]
- 4.Morton DJ, Barrett-Connor E, Kritz-Silverstein D, Wingard DL, Schneider DL. Bone mineral density in postmenopausal Caucasian, Filipina, and Hispanic women. Int J Epidemiol. 2003;32:150–156. doi: 10.1093/ije/dyg024. [DOI] [PubMed] [Google Scholar]
- 5.Taaffe DR, Villa ML, Holloway L, Marcus R. Bone mineral density in older non-Hispanic Caucasian and Mexican American women: relationship to lean and fat mass. Annals Human Biol. 2000;27:331–344. doi: 10.1080/03014460050044829. [DOI] [PubMed] [Google Scholar]
- 6.Melton LJ, Khosla S, Achenbach SJ, O’Connor MK, O’Fallon WM, Riggs BL. Effects of body size and skeletal site on the estimated prevalence of osteoporosis in women and men. Osteoporos Int. 2000;11:977–983. doi: 10.1007/s001980070037. [DOI] [PubMed] [Google Scholar]
- 7.Nuti R, Martini G, Gennari C. Age-related changes of whole skeleton and body composition in healthy men. Calcif Tissue Int. 1995;57:336–339. doi: 10.1007/BF00302068. [DOI] [PubMed] [Google Scholar]
- 8.Nuti R, Martini G. Effects of age and menopause on bone density of entire skeleton in healthy and osteoporotic women. Osteoporos Int. 1993;3:59–65. doi: 10.1007/BF01623374. [DOI] [PubMed] [Google Scholar]
- 9.Rico H, Revilla M, Villa LF, Alvarez de Buergo M. Age-related differences in total and regional bone mass: a cross-sectional study with DXA in 429 normal women. Osteoporos Int. 1993;3:154–159. doi: 10.1007/BF01623277. [DOI] [PubMed] [Google Scholar]
- 10.Melton LJ, Riggs BL, Achenbach SJ, Amin S, Camp JJ, Rouleau PA, Robb RA, Oberg AL, Khosla S. Does reduced skeletal loading account for age-related bone loss? J Bone Miner Res. 2006;21:1847–1855. doi: 10.1359/jbmr.060908. [DOI] [PubMed] [Google Scholar]
- 11.Frost HM. On our age-related bone loss: insights from a new paradigm. J Bone Miner Res. 1997;12:1539–1546. doi: 10.1359/jbmr.1997.12.10.1539. [DOI] [PubMed] [Google Scholar]
- 12.Kanis JA, McCloskey EV, Johansson H, Oden A, Melton LJ, Khaltaev N. A reference standard for the description of osteoporosis. Bone. 2008;42:467–475. doi: 10.1016/j.bone.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention, National Center for Health Statistics. Current National Health and Nutrition Examination Survey (NHANES) [accessed July 23, 2007];1999 Available at: http://www.cdc.gov/nchs/about/major/nhanes/currentnhanes.htm.
- 14.Centers for Disease Control and Prevention, National Center for Health Statistics. Technical documentation for the 1999–2004 dual-energy x-ray absorptiometry (DXA) multiple imputation data files. [Accessed March 10, 2008];2008 February; Available at: http://www.cdc.gov/nchs/data/nhanes/dxa/dxa_techdoc.pdf.
- 15.Centers for Disease Control and Prevention, National Center for Health Statistics. Body composition procedure manual. [accessed March 10, 2008];2004 January; Available at: http://www.cdc.gov/nchs/data/nhanes03_04/bc.pdf.
- 16.National Center for Health Statistics. Najjar MF, Rowland M. Anthropometric reference data and prevalence of overweight, United States 1976–80. [accessed Feb 9 2008];Washington: U.S. Government Printing Office; Vital and health statistics. 1987 Oct; Series 11, No. 238. DHHS Pub. No. (PHS) 87-1688. Public Health service http://www.cdc.gov/nchs/data/series/sr_11/sr11_238.pdf. [PubMed]
- 17.Centers for Disease Control and Prevention, National Center for Health Statistics. Anthropometry procedure manual. [Accessed March 10, 2008];2004 January; Available at: http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/BM.pdf.
- 18.Siris ES, Miller PD, Barrett-Connor E, Faulkner KG, Wehren LE, Abbott TA, Berger ML, Santora AC, Sherwood LM. Identification and fracture outcomes of undiagnosed low bone mineral density in postmenopausal women. Results from the National Osteoporosis Risk Assessment. JAMA. 2001;286:2815–2822. doi: 10.1001/jama.286.22.2815. [DOI] [PubMed] [Google Scholar]
- 19.Looker AC, Wahner JW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC, Lindsay R. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–469. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 20.Nelson DA, Jacobsen G, Barondess DA, et al. Ethnic differences in regional bone density, hip axis length, and lifestyle variables among healthy black and white men. J Bone Miner Res. 1995;10:782–787. doi: 10.1002/jbmr.5650100515. [DOI] [PubMed] [Google Scholar]
- 21.Baron J, Barret J, Berger M Centers for Disease Control. Incidence and costs to Medicare of fractures among Medicare beneficiaries aged greater than 65 years–United States, July 1991—June 1992. MMWR. 1996;45:877–883. [PubMed] [Google Scholar]
- 22.Melton LJ, Khosla S, Atkinson EJ, O’Connor MK, O’Fallon WM, Riggs BL. Cross-sectional versus longitudinal evaluation of bone loss in men and women. Osteoporos Int. 2000;11:592–599. doi: 10.1007/s001980070080. [DOI] [PubMed] [Google Scholar]
- 23.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 24.Cauley JA, Lui LY, Stone KL, Hillier TA, Zmuda JM, Hochberg M, Beck TJ, Ensrud KE. Longitudinal study of changes in hip bone mineral density in Caucasian and African-American women. J Am Geriatr Soc. 2005;53:183–189. doi: 10.1111/j.1532-5415.2005.53101.x. [DOI] [PubMed] [Google Scholar]
- 25.Tracy JK, Meyer WA, Flores RH, Wilson PD, Hochberg MC. Racial differences in rate of decline in bone mass in older men: the Baltimore men’s osteoporosis study. J Bone Miner Res. 2005;20:1228–1234. doi: 10.1359/JBMR.050310. [DOI] [PubMed] [Google Scholar]
- 26.Luckey MM, Wallenstein S, Lapinski R, Meier DE. A prospective study of bone loss in African-American and white women–a clinical research center study. J Clin Endocrinol Metab. 1996;81:2948–2956. doi: 10.1210/jcem.81.8.8768857. [DOI] [PubMed] [Google Scholar]
- 27.McDowell MA, Fryar CD, Hirsch R, Ogden C. Anthropometric data for children and adults: US population 1999–2002. Advance data from vital and health statistics no. 361. [accessed February 8, 2008];National Center for Health Statistics. 2005 Available at http://www.cdc.gov/nchs/data/ad/ad361.pdf.
- 28.Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 29.LeBlanc A, Lin C, Shackelford L, Sinitsyn V, Evans H, Belichenko O, Schenkman B, Kozlovskaya I, Oganov V, Bakulin A, Hedrick T, Feeback D. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J Appl Physiol. 2000;89:2158–2164. doi: 10.1152/jappl.2000.89.6.2158. [DOI] [PubMed] [Google Scholar]
- 30.Visser M, Pahor M, Tylavsky F, Kritchevsky SB, Cauley JA, Newman AB, Blunt BA, Harris TB. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol. 2003;94:2368–2374. doi: 10.1152/japplphysiol.00124.2002. [DOI] [PubMed] [Google Scholar]
- 31.Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R. Aging of skeletal muscle: a 12-yr longitudinal study. J Appl Physiol. 88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 32.Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer FX, Heymsfield SB. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab. 2000;279:E366–E375. doi: 10.1152/ajpendo.2000.279.2.E366. [DOI] [PubMed] [Google Scholar]
- 33.Ishiguro N, Kanchisa H, Miyatani M, Masuo Y, Fukunaga T. Applicability of segmental bioelectrical impedance analysis for predicting trunk skeletal muscle volume. J Appl Physiol. 2006;100:572–578. doi: 10.1152/japplphysiol.00094.2005. [DOI] [PubMed] [Google Scholar]
- 34.Orwoll ES, Oviatt SK, Mann T. The impact of osteophytic and vascular calcifications on vertebral mineral density measurements in men. J Clin Endocrinol Metab. 1990;70:1202. doi: 10.1210/jcem-70-4-1202. [DOI] [PubMed] [Google Scholar]
- 35.Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, Peterson JM, Rouleau PA, McCollough CH, Bouxsein ML, Khosla S. Population-based study of age and sex differences in bone volumetric density, size, geometry, and structure at different skeletal sites. J Bone Miner Res. 2004;19:1945–1954. doi: 10.1359/JBMR.040916. [DOI] [PubMed] [Google Scholar]
- 36.Looker AC, Orwoll ES, Johnston CC, Lindsay R, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP. Prevlance of low femoral bone density in older US adults from NHANES III. J Bone Mineral Res. 1997;12:1761–1768. doi: 10.1359/jbmr.1997.12.11.1761. [DOI] [PubMed] [Google Scholar]
- 37.Kanis JA on behalf of the World Health Organization Scientific Group. Assessment of osteoporosis at the primary health-care level. [accessed February 8, 2008];University of Sheffield, UK: World Health Organization; Technical Report. WHO Collaborating Centre. 2007 Available at http://www.who.int/chp/topics/Osteoporosis.pdf.
- 38.Zimmerman SI, Girman CJ, Buie VC, Chander J, Hawkes W, Martin A, Holder L, Hebel JR, Sloane PD, Magaziner J. The prevalence of osteoporosis in nursing home residents. Osteoporos Int. 1999;9:151–157. doi: 10.1007/s001980050129. [DOI] [PubMed] [Google Scholar]