PKCs are a group of serine/threonine kinases belonging to the AGC (protein kinase A, G, and C) super family, which can be classified in to 3 groups based on their lipid and cofactor requirements and 7 isoforms are expressed in platelets [1, 2]. Activation of protein kinase C (PKC) has been suggested to mediate several physiological responses including αIIbβ3 activation and dense granule secretion [3–7].
We have previously reported that classical PKC isoforms regulate GPVI-mediated, but not PAR-mediated, dense granule release [8]. As both PARs and GPVI are known to cause dense granule secretion in platelets through calcium and PKC pathways [3, 4], and both pathways activate phospholipase C, we questioned why the classical PKC isoforms selectively play a role in GPVI-mediated dense granule release. In this study, we employed a strategy of using the classical PKC inhibitor Go6976 to evaluate the role of α and β isoforms in human platelets downstream of GPVI pathways.
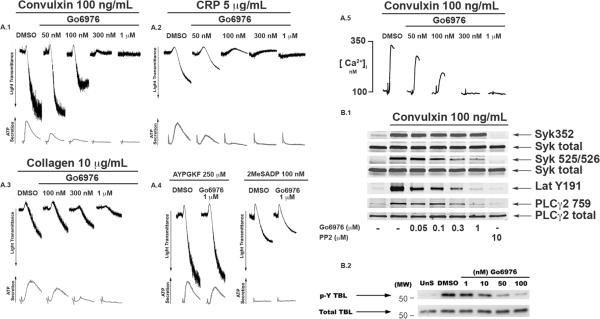
We used convulxin, collagen related peptide (CRP), or collagen as well as AYPGKF and 2MeSADP in order to evaluate the functional role of the classical PKC isoforms in platelet functional responses. As shown in Fig1A.1–A.3, vehicle (DMSO)-treated platelets underwent shape change, aggregation, and ATP secretion. Platelets pre-treated with increasing concentrations of the classical PKC isoform inhibitor Go6976, resulted in a concentration-dependent inhibition of both aggregation and secretion with all GPVI agonists. In contrast only a minimal effect in platelet functional responses was seen when platelets were activated with GPCR agonists AYPGKF or 2MeSADP in the presence of 1 μM Go6976 (Fig. 1 A.4). Platelets activated with either convulxin (Fig 1A.1) or CRP (Fig 1A.2) in the presence of 1 μM Go6976 also failed to aggregate, suggesting Go6976 may possibly cause blockade of intracellular calcium mobilization.
Figure 1.
Effects of Go6976 on platelet functional responses and phosphorylation. In Fig. A.1 platelet aggregation and secretion were measured in 0.5 mL of washed aspirin-treated platelets (2 × 108). Samples were pretreated with varying concentrations of Go6976 for 10 minutes at 37°C and activated with (A.1) convulxin, (A.2) collagen related peptide (CRP), (A.3) collagen, and (A.4) AYPGKF and 2MeSADP at 37° C while stirring for 3 minutes. In Fig. A.5 intracellular calcium was measured in 0.5 mL of (2 × 108) platelets preloaded with Fura-2AM (5 μM), following the addition of 100 ng/mL convulxin in the presence of Go6976 or vehicle control. In Fig B.1 platelets were activated for 30 seconds with convulxin in the presence of Go6976 or PP2 (10 μM). Phosphorylation of tyrosine residues 352 and 525/526 on Syk, 191 on Lat, and 759 on PLCγ2 were analyzed by western blot analysis and probed with phospho-specific antibodies. In Fig. B.2 recombinant active Syk was pre-treated with Go6976 or vehicle DMSO for 10 minutes at 37°C and the in vitro kinase assay was initiated by the addition of 2.5 μM ATP in the presence of the substrate tubulin.
Both calcium and PKCs have been shown to be important for platelet aggregation and secretion [3, 4]. Heemskerk and co-workers have shown that PKC isoforms might regulate increases in intracellular calcium levels [9]. Hence we investigated whether increasing concentrations of Go6976 had an effect on intracellular calcium mobilization following activation with convulxin. As shown in Fig. 1 A.5. a concentration-dependent inhibition of intracellular calcium mobilization was seen in platelets treated with Go6976. However, Go6976 did not affect AYPGKF- or 2MeSADP-induced intracellular calcium mobilization (not shown). Since the conventional class of PKCs require calcium for their activation, these results suggest Go6976 could possibly exert its effects on an off target molecule upstream of calcium, following the activation of GPVI pathways.
Agonist-induced platelet shape change is mediated through calcium-dependent and Rho kinase-dependent pathways [10], and is not regulated by protein kinase C isoforms [4]. The fact that in the presence of 1 μM Go6976 a classical PKC selective inhibitor, but not by a pan-PKC inhibitor RO 31–8220 [3], the convulxin-mediated platelet shape change was completely ablated suggesting that Go6976 exerts inhibition on a signaling molecule upstream of calcium and Rho A.
Upon examination of PLCγ2 Tyr 759 phosphorylation in the presence of Go6976, we observed a concentration- dependent inhibition of the convulxin-induced Tyr 759 phosphorylation levels in platelets (Fig. 1 B.2). We next evaluated whether the phosphorylation levels of upstream molecules Lat and Syk were affected by Go6976 treatment following activation of GPVI pathways. As shown in Fig. 1 B.1, platelets activated with convulxin in the presence of Go6976 elicited a concentration-dependent inhibition of Lat Tyr 191 and Syk Tyr 525/526 phosphorylation, while Syk Tyr 352 phosphorylation was unaffected. In contrast, the Src-family kinase inhibitor PP2, not only abrogated tyrosine phosphorylation of Lat 191 and Syk 525/526, but also Syk 352. These results suggest Go6976 exerts its inhibitory effects at the level of Syk as indicated by the dose-dependent inhibition of one of its immediate downstream targets Lat. Inhibition of Syk Tyr 525/526 was less pronounced, consistent with the possibility the multiple kinases including Syk autophosphorylation may be responsible for this event [11–14]. We ruled out the inhibitory effect of Go6976 on SFKs as Syk Tyr 352 was abrogated by PP2 but not by Go6976 (Fig. 1 B.1). The reduced tyrosine phosphorylation levels of Syk at 525/526 was not seen in platelets treated with a novel Syk kinase inhibitor R406 [14]. Our results with 1 μM Go6976, however, are in agreement with those seen in platelets treated with R406, wherein platelet aggregation and tyrosine phosphorylation levels of Lat and PLCγ2 were inhibited downstream of GPVI pathways [14]. Despite the different levels of Syk Tyr 525/526 phosphorylation seen with Go6976 and R406, these findings suggest Go6976 blocks signaling at the level of Syk and may utilize a slightly different mechanism for this inhibition than R406.
While the auto-phosphorylation of Syk Tyr 525/526 has been suggested to be a marker for its kinase activity there are some reports that phosphorylation of Syk may not reflect its kinase activity [14] or the phosphorylation at Tyr 352 alone is enough to cause downstream signaling [13]. To further investigate the effects of Go6976 on Syk kinase activity, we performed an in vitro kinase assay using tubulin as the substrate. As shown in Fig. 1 B.2, Go6976 inhibited Syk kinase activity, as measured by tubulin phosphorylation, in a concentration-dependent manner. These results suggest Go6976 non-selectively inhibits Syk kinase activity in platelets. It is important to note that Go6976 is a derivative of the broad spectrum PKC inhibitor staurosporine [15]. Several protein-tyrosine kinases including TPK-IIB, a variant of p72syk, have been reported to be inhibited by staurosporine [16, 17]. Considering Go6976 is a staurosporine derivative, our results suggest this compound retains the tyrosine kinase inhibiting properties of staurosporine.
In summary, we have used the staurosporine derivative Go6976 to block classical PKC isoforms and have identified its ability to inhibit Syk kinase activity in human platelets downstream of GPVI pathways. We demonstrate that Go6976 blocks downstream signaling events of Syk in platelets and the kinase activity of Syk in vitro. We suggest the use of Go6976 is not an appropriate strategy for investigating classical PKC isoforms downstream of GPVI pathways in human platelets.
Acknowledgements
This work was supported by Research Grants HL93231, HL60683, and HL81322 from the National Institute of Health (S.P.K).
Footnotes
Disclosure of conflict of interests: The authors declare that they have no conflict of interest.
References
- 1.Bynagari-Settipalli YS, Chari R, Kilpatrick L, Kunapuli SP. Protein Kinase C -Possible Therapeutic Target to Treat Cardiovascular Diseases. Cardiovasc Hematol Disord Drug Targets. 2010;10:292–308. doi: 10.2174/187152910793743869. [DOI] [PubMed] [Google Scholar]
- 2.Harper MT, Poole AW. Isoform-specific functions of protein kinase C: the platelet paradigm. Biochem Soc Trans. 2007;35:1005–8. doi: 10.1042/BST0351005. [DOI] [PubMed] [Google Scholar]
- 3.Quinton TM, Kim S, Dangelmaier C, Dorsam RT, Jin J, Daniel JL, Kunapuli SP. Protein kinase C- and calcium-regulated pathways independently synergize with Gi pathways in agonist-induced fibrinogen receptor activation. Biochem J. 2002;368:535–43. doi: 10.1042/BJ20020226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinton TM, Ozdener F, Dangelmaier C, Daniel JL, Kunapuli SP. Glycoprotein VI-mediated platelet fibrinogen receptor activation occurs through calcium-sensitive and PKC-sensitive pathways without a requirement for secreted ADP. Blood. 2002;99:3228–34. doi: 10.1182/blood.v99.9.3228. [DOI] [PubMed] [Google Scholar]
- 5.Chari R, Getz T, Nagy B, Jr., Bhavaraju K, Mao Y, Bynagari YS, Murugappan S, Nakayama K, Kunapuli SP. Protein kinase C[delta] differentially regulates platelet functional responses. Arterioscler Thromb Vasc Biol. 2009;29:699–705. doi: 10.1161/ATVBAHA.109.184010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagy B, Jr., Bhavaraju K, Getz T, Bynagari YS, Kim S, Kunapuli SP. Impaired activation of platelets lacking protein kinase C-theta isoform. Blood. 2009;113:2557–67. doi: 10.1182/blood-2008-07-169268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bynagari YS, Nagy B, Jr., Tuluc F, Bhavaraju K, Kim S, Vijayan KV, Kunapuli SP. Mechanism of activation and functional role of protein kinase Ceta in human platelets. J Biol Chem. 2009;284:13413–21. doi: 10.1074/jbc.M808970200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murugappan S, Tuluc F, Dorsam RT, Shankar H, Kunapuli SP. Differential role of protein kinase C delta isoform in agonist-induced dense granule secretion in human platelets. J Biol Chem. 2004;279:2360–7. doi: 10.1074/jbc.M306960200. [DOI] [PubMed] [Google Scholar]
- 9.Gilio K, Harper MT, Cosemans JM, Konopatskaya O, Munnix IC, Prinzen L, Leitges M, Liu Q, Molkentin JD, Heemskerk JW, Poole AW. Functional divergence of platelet protein kinase C (PKC) isoforms in thrombus formation on collagen. J Biol Chem. 285:23410–9. doi: 10.1074/jbc.M110.136176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Riondino S, Gazzaniga PP, Pulcinelli FM. Convulxin induces platelet shape change through myosin light chain kinase and Rho kinase. Eur J Biochem. 2002;269:5878–84. doi: 10.1046/j.1432-1033.2002.03305.x. [DOI] [PubMed] [Google Scholar]
- 11.Couture C, Williams S, Gauthier N, Tailor P, Mustelin T. Role of Tyr518 and Tyr519 in the regulation of catalytic activity and substrate phosphorylation by Syk protein-tyrosine kinase. Eur J Biochem. 1997;246:447–51. doi: 10.1111/j.1432-1033.1997.00447.x. [DOI] [PubMed] [Google Scholar]
- 12.El-Hillal O, Kurosaki T, Yamamura H, Kinet JP, Scharenberg AM. syk kinase activation by a src kinase-initiated activation loop phosphorylation chain reaction. Proc Natl Acad Sci U S A. 1997;94:1919–24. doi: 10.1073/pnas.94.5.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carsetti L, Laurenti L, Gobessi S, Longo PG, Leone G, Efremov DG. Phosphorylation of the activation loop tyrosines is required for sustained Syk signaling and growth factor-independent B-cell proliferation. Cell Signal. 2009;21:1187–94. doi: 10.1016/j.cellsig.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Spalton JC, Mori J, Pollitt AY, Hughes CE, Eble JA, Watson SP. The novel Syk inhibitor R406 reveals mechanistic differences in the initiation of GPVI and CLEC-2 signaling in platelets. J Thromb Haemost. 2009;7:1192–9. doi: 10.1111/j.1538-7836.2009.03451.x. [DOI] [PubMed] [Google Scholar]
- 15.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–7. [PubMed] [Google Scholar]
- 16.Meggio F, Donella Deana A, Ruzzene M, Brunati AM, Cesaro L, Guerra B, Meyer T, Mett H, Fabbro D, Furet P, et al. Different susceptibility of protein kinases to staurosporine inhibition. Kinetic studies and molecular bases for the resistance of protein kinase CK2. Eur J Biochem. 1995;234:317–22. doi: 10.1111/j.1432-1033.1995.317_c.x. [DOI] [PubMed] [Google Scholar]
- 17.Brunati AM, James P, Guerra B, Ruzzene M, Donella-Deana A, Pinna LA. The spleen protein-tyrosine kinase TPK-IIB is highly similar to the catalytic domain of p72syk. Eur J Biochem. 1996;240:400–7. doi: 10.1111/j.1432-1033.1996.0400h.x. [DOI] [PubMed] [Google Scholar]