Abstract
The anti-HER2 antibody Trastuzumab (Herceptin) has been proven to be effective in the treatment of HER2 overexpressing breast cancer; resistance, however invariably emerges in metastatic tumors. The expression of p95-HER2, a form of HER2 with a truncated extracellular domain that lacks the Trastuzumab binding epitope, has been implicated as a mechanism of resistance to the antibody. We utilized an in vivo tumor model that overexpresses p95-HER2 and demonstrate it to be resistant to the signaling and antitumor effects of Trastuzumab. We find that both full length and p95-HER2 interact with the HSP90 chaperone protein and are degraded in tumor cells exposed to HSP90 inhibitors in tissue culture and in vivo. Loss of expression of p95-HER2 is accompanied by downregulation of the PI3K/AKT and ERK signaling pathways and inhibition of cell proliferation. Chronic administration of HSP90 inhibitors in vivo results in sustained loss of HER2 and p95-HER2 expression and inhibition of AKT activation together with induction of apoptosis and complete inhibition of tumor growth in Trastuzumab-resistant, p95-HER2-overexpressing models. Thus, p95-HER2 is an HSP90 client protein, the expression and function of which can be effectively suppressed in vivo by HSP90 inhibitors. HSP90 inhibition is therefore a potentially effective therapeutic strategy for p95-HER2-mediated Trastuzumab-resistant breast cancer.
Keywords: p95-HER2, Trastuzumab, breast cancer, HER2
Introduction
The HER2/ERBB2 receptor tyrosine kinase is amplified in 20–30% of cases of breast cancer (Slamon et al., 1987). Amplification of HER2 is associated with activation of receptor tyrosine kinase-dependent signaling pathways, especially HER2/HER3-dimer dependent activation of PI3K/AKT signaling, with attendant increases in D-cyclin expression, deregulation of proliferation and desensitization of the tumor to apoptotic stimuli (Basso et al., 2002; Holbro et al., 2003; Yakes et al., 2002; Yu et al., 2001). HER2 amplification or mutational activation is oncogenic in many model systems and it is likely that, in these tumors, it is necessary for tumor initiation, progression or maintenance of the transformed phenotype (Finkle et al., 2004; Guy et al., 1992; Moody et al., 2002; Muller et al., 1988).
Trastuzumab (Herceptin), a humanized, monoclonal antibody that binds to HER2, has marked clinical benefit for patients with early or late stage breast cancers in which HER2 is overexpressed (Hudis, 2007). Trastuzumab is thought to exert multiple antitumor effects including inhibition of HER2 signaling, which leads to downregulation of the PI3K-AKT and RAS-ERK signaling pathways, and, in addition, activation of antibody dependent cell mediated cytotoxicity (Clynes et al., 2000; Cuello et al., 2001; Lane et al., 2000; Yakes et al., 2002). The antibody has antitumor activity when given alone and also enhances the effectiveness of certain chemotherapeutic agents, most notably taxanes, possibly by inhibiting antiapoptotic signaling pathways (Baselga et al., 1998; Pegram et al., 2000). Despite these pleiotropic activities, intrinsic or acquired resistance to Trastuzumab-based therapy is a common clinical phenomenon, especially in patients with metastatic disease, in whom tumor progression is almost invariable.
Several potential resistance mechanisms have been described in model systems, although none of these has been completely validated in patients. These include hyperactivation of the PI3K-AKT pathway due to mutation or diminished expression of PTEN (phosphatase and tensin homologue deleted on chromosome 10) or mutational activation of the p110 subunit of PI3K, upregulation of other receptor tyrosine kinases such as EGFR, c-MET, or IGF-1R, and accumulation of truncated forms of HER2 (Berns et al., 2007; Lu et al., 2001; Nagata et al., 2004; Ritter et al., 2007; Scaltriti et al., 2007; Shattuck et al., 2008). Expression of amino-terminal truncated forms of HER2 that have lost the Trastuzumab binding epitope has been demonstrated to occur in up to 30% of human breast tumors with HER2 overexpression (Molina et al., 2001). Amino-terminal truncated HER2 has been referred to as “p95-HER2” because the predominant form has an apparent molecular weight of 95kD. Expression of p95-HER2 in a transgenic mouse model is sufficient for tumorigenesis (Pederson et al., 2009). The expression of p95-HER2 is clinically associated with aggressive disease, poor prognosis, and lack of response to Trastuzumab (Molina et al., 2001; Molina et al., 2002; Saez et al., 2006; Scaltriti et al., 2007).
Tumors in which Trastuzumab resistance is mediated by p95-HER2 would still be expected to respond to effective inhibitors of the function or expression of the relevant species of HER2. Inhibition of the chaperone protein HSP90 is one way to achieve these ends. HSP90 is an abundant molecular chaperone that plays a role in the refolding of proteins in cells exposed to stress and is required for the conformational maturation of a subset of proteins that regulate signal transduction. Several natural products, including the ansamycin antibiotic geldanamycin, bind to the ATP/ADP binding pocket of HSP90 and inhibit its function (Workman et al., 2007). This results in the ubiquitination and proteasomal degradation of HSP90 client proteins, of which HER2 is among the most sensitive. Exposure of HER2 dependent breast tumors to HSP90 inhibitors in tissue culture and in vivo causes rapid and potent HER2 degradation, concomitant inhibition of PI3K/AKT signaling, and suppression of the growth in vivo of both xenograft and transgenic models (Benezra et al., 2005; Munster et al., 2002).
Trastuzumab-resistant tumors that remain dependent on HER2 activity or expression might be predicted to be sensitive to HSP90 inhibition. These would include those tumors in which Trastuzumab does not effectively inhibit HER2 activity, including those that overexpress p95-HER2. However, this supposes that (1) the activity of Trastuzumab is not primarily due to induction of ADCC, (2) p95-HER2 still requires HSP90 for function, and (3) p95-HER2 is potently degraded by HSP90 inhibitors in vivo.
We now report that p95-HER2 binds to HSP90 and that pharmacologic inhibitors of HSP90 cause a rapid degradation of p95-HER2 in tumor cells in tissue culture and in xenografted tumors. In a tumor model that is dependent upon p95-HER2 but not full length HER2 for its survival, HSP90 inhibition completely suppresses tumor growth. Similarly, in a Trastuzumab-resistant xenograft model that expresses high levels of both full length HER2 and p95-HER2, HSP90 inhibitors effectively induce the degradation of both proteins, inhibit PI3K/AKT signaling and suppress tumor growth in vivo. These studies support the utility of HSP90 inhibition as a rational strategy for the treatment of breast tumors in which Trastuzumab resistance is due to expression of p95-HER2.
Materials and Methods
Reagents
SNX-2112 and SNX-5422 were provided by Paul Steed at Serenex, Inc. (Durham, NC, USA) (Rice et al., 2008). SNX-2112 was dissolved in DMSO for in vitro studies, whereas SNX-5422 was formulated in 1% Carboxymethylcellulose/0.5%Tween-80 for in vivo studies. Lapatinib (Tykerb) was provided by Tona Gilmer at GlaxoSmithKline (RTP, NC, USA) and dissolved 0.5% hydroxypropylmethylcellulose/0.1% Tween-80 for in vivo studies. Trastuzumab (Herceptin) was purchased from the MSKCC Pharmacy and dissolved in sterile water at 21mg/ml. 17-AAG was obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program, NCI, (Bethesda, MD, USA) and was dissolved in DMSO to yield 50 mg/mL and 10 mmol/L stock solutions.
Cell Culture
T47D cells were transfected with full length HER2 and p95-HER2 cDNAs cloned into pIRES-Hyg under the CMV promoter as described in Scaltriti et al. 2007. Cells were maintained in DMEM-F12 medium supplemented with 100u/ml penicillin, 100mg/ml streptomycin, 4mM L-glutamine, 50µg/ml Hygromycin, and 10% heat-inactivated fetal bovine serum and incubated at 37°C in 5% CO2. Cell viability was determined by seeding 3000 cells/well in 96-well plates and treating with drug 24hr after plating in complete medium (200ul). Each drug concentration was tested in eight wells. Cells were exposed to drug for 96 hours and cell number was assayed with Alamar Blue reagent (TREK Diagnostics, Cleveland, OH) using a Molecular Devices Spectrophotometer.
Inducible p95-HER2
MEF-3T3 tet-off and MCF-7 tet-off cell lines, engineered to express the tetracycline-controlled transactivator (tTA) (Gossen et al., 1992), were obtained from Clontech Laboratories (Clontech, Oxford, UK) and maintained in Dulbecco’s modified Eagle medium/Ham F12 1:1 (DMEM/F12) supplemented with 10% fetal bovine serum (FBS), 2 mM L-glutamine (Life Technologies, Inc. Ltd., Paisley, UK) and 100 µg/ml G418 (Gibco), at 37°C in 5% CO2. Cells were stably transfected with the pUHD10-3h vector encoding the cDNAs of p95HER2 starting at methionine 611 (p95HER2-M611; (Pederson et al., 2009)) by using FuGENE6 (Roche) according to the manufacturer’s protocol. Independent clones were selected with 0.1mg/ml hygromycin B (Invitrogen). The expression of p95HER2-M611 was induced by doxycycline removal detaching the cells with 0.5% Trypsin-EDTA (GIBCO) and washing three times by centrifugation.
Animal Studies
4–6 week old nu/nu athymic BALB/c female mice were obtained from the NCI-Frederick Cancer Center and maintained in pressurized ventilated caging. All studies were performed in compliance with IACUC guidelines. F2#1282 tumors were kindly provided by Gail Lewis Phillips and Mark Sliwkowski and established in nude mice by subcutaneously implanting 2×2×2mm-sized tumor pieces. For efficacy studies, mice with well-established tumors were selected and randomized approximately fourteen days post-implantation (Size > 200mm3); BT-474 xenograft tumors were established in nude mice by subcutaneously implanting 0.72 mg sustained release 17β-estradiol pellets with a 10g trocar into one flank followed by injecting 1 × 107 cells suspended 1:1 (volume) with reconstituted basement membrane (Matrigel, Collaborative Research, Bedford, MA, USA) on the opposite side 3 days afterwards. Mice were treated with SNX-5422, 17-AAG, Trastuzumab, or Lapatinib with the indicated doses. Tumor dimensions were measured with vernier calipers and tumor volumes calculated (π/6 × larger diameter × (smaller diameter)2). For pharmacodynamic studies, mice with well-established tumors were treated and sacrificed pre-treatment and at indicated times post-treatment (two or three mice/timepoint).
For xenografted MEFs, six- to eight-week-old female athymic nude-Foxn1nu mice were purchased from Harlan Laboratories (Italy). Soon after Doxycycline removal, the cells were harvested and counted using the Guava ViaCount Assay on a Guava PCA Platform (Guava Technologies, Hayward, CA). 1 × 106 MEFs tet-off cells conditionally expressing p95HER2-M611 were injected into the right flanks of all animals. p95HER2-M611-dependent tumorigenicity of the MEF xenografts was confirmed by complete tumor shrinkage in a separate group of mice where 0.1% of Doxycycline was added to the drinking water. For the pharmacodynamics study, three groups of animals (four mice per group) were treated with a single dose of 75mg/kg of SNX5422 for 0, 6 or 24 hours respectively.
Immunoblotting/Immunoprecipitation
Tumor lysates were prepared by homogenization in SDS-lysis buffer (~1ml/mg tissue) (50mM Tris-HCl, (pH7.4) 2% SDS), boiling for 10 minutes, followed by brief sonication. Lysates were cleared by centrifugation at 14,000xg (10min) and the supernatant was collected. Lysates from cells in culture were prepared by washing twice in cold PBS followed by lysis with RIPA-lysis buffer (Pierce Chemical, Rockford, IL, USA) or NP40-lysis buffer ([50 mmol/L Tris (pH 7.4), 1% NP40, 150 mmol/L NaCl, 40 mmol/L NaF) for immunoprecipitations, supplemented with protease and phosphatase inhibitors (10µM/ml Na3VO4/phenylmethylsulfonyl fluoride/DTT and 1mg/ml leupeptin, aprotinin, and trypsin inhibitor). Protein concentration of each sample was determined using the BCA kit (Pierce) per manufacturer’s instructions. 25 or 50µg protein was loaded onto 7 or 10% SDS-PAGE minigels for immunoblotting. Transfer onto nitrocellulose membranes was followed by incubation with primary antibodies (Cell Signaling, Beverly, MA, USA except: HER2 – LabVision, Fremont, CA, USA for IP, Upstate Biotechnology, Lake Placid, NY, USA for Westerns; PI3K-p85 – Upstate Biotechnology; Cyclin D1 – Santa Cruz, Santa Cruz, CA, USA; HA- Santa Cruz, HER3 – LabVision). For immunoprecipitation, 1mg of protein lysate was immunoabsorbed with 20µg of indicated antibody or IgG control followed by protein G sepharose (or protein A-sepharose for Lane-4 of Figure-4). These conjugates were pelleted and washed 3 times with NP40 lysis buffer and resuspended in 2% SDS sample buffer.
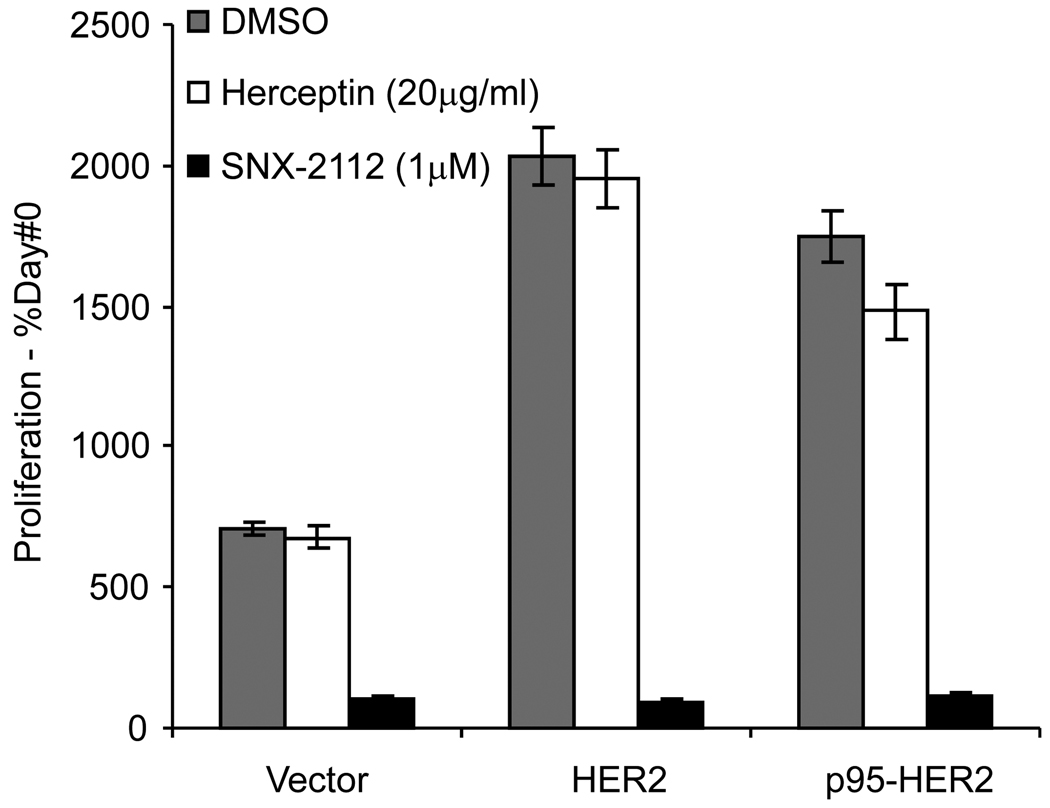
Figure 4. HSP90 inhibition but not Trastuzumab mediates downregulation of p95-HER2 and HER2 activated proliferation.
T47D cells stably transfected with HER2, p95-HER2, or vector were treated with 1 µM SNX-2112, 20µg/ml Trastuzumab, or DMSO and mean viable cells reported after 4 days. Proliferation is reported as percentage of viable cells compared to Day#0 with SD.
Results
Multiple laboratory models of Trastuzumab resistance have been derived from HER2 dependent breast cancer cell lines and murine tumors and have been associated with a variety of mechanisms of resistance. We surveyed models HER2 and p95-HER2 expression levels in tumor models and found that the F2#1282 expresses high levels of p95-HER2 [Figure-1A and data not shown]. The Trastuzumab-resistant, F2#1282 tumor was developed from a transgenic mouse model engineered to expresses human HER2 under the control of the MMTV promoter (Finkle et al., 2004; Lewis Phillips et al., 2008). Antibody therapy directed against the extracellular domain of HER2 in this model prevents tumor emergence, however, one tumor did grow despite treatment and was isolated and shown to express high levels of p95-HER2 (Lewis Phillips et al., 2008).
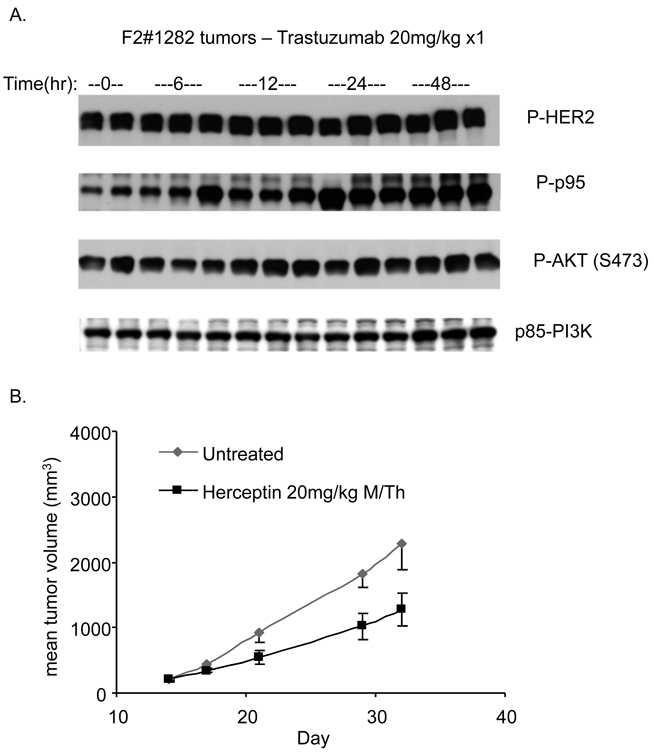
Figure 1. F2#1282 tumors are resistant to the signaling and antiproliferative effects of Trastuzumab.
A) Mice with established F2#1282 tumors were treated with a single dose of Trastuzumab (20mg/kg IP) and tumors collected at indicated times after dosing. Immunoblotting was performed on lysates using antibodies against Phosphorylated-HER2 (Y1221), Phosphorylated-AKT (S473), and p85-PI3K. B) Mice with established F2#1282 tumors (5 mice/group) were randomized to no treatment or treatment 2x/week with Trastuzumab (20mg/kg) and tumors measured at indicated times.
In multiple HER2-breast cancer models, Trastuzumab effectively inhibits PI3K/AKT signaling and tumor growth (Supplemental Figure-1, (Yakes et al., 2002)). The effects of Trastuzumab treatment on AKT activation and in vivo tumor growth in the resistant F2#1282 model (grown as subcutaneously implanted tumors in nude mice) were assessed in Figure-1. Mice bearing tumors were treated with a single dose of Trastuzumab (20mg/kg) and sacrificed at the indicated times after dose (3 mice/timepoint). Trastuzumab treatment caused no appreciable decline in HER2 or p95-HER2 phosphorylation up to 48 hours after administration (Figure-1A, Rows-1+2). Phosphorylated forms of AKT (S473 and T308) and ERK are not inhibited and appear to be slightly induced (Row-3 and data not shown) by Trastuzumab treatment. Expression of total and phosphorylated p95-HER2 was upregulated in response to Trastuzumab treatment, particularly at 24 and 48 hours (Row-2 and data not shown). The effect of chronic treatment of Trastuzumab upon tumor growth was determined in mice treated with twice weekly Trastuzumab (Figure-1B). Trastuzumab caused only a modest slowing of tumor growth compared to untreated controls. In contrast, treatment of the HER2 dependent BT474 breast tumor xenograft with Trastuzumab resulted in inhibition of AKT phosphorylation (~58% untreated control) and concomitant complete suppression of tumor growth (Supplemental Fig-1).
The resistance of F2#1282 to inhibition of AKT phosphorylation by Trastuzumab suggests that either the tumor has become HER2 independent by activating PI3K/AKT signaling by another mechanism or that the tumor remains dependent on HER2 signaling but is refractory to its inhibition by Trastuzumab. In support of the latter, treatment of these tumors with a kinase inhibitor of the catalytic activity of HER2 and HER1, Lapatinib, results in downregulation of phosphorylation of p95-HER2, HER2, AKT, and ERK and marked inhibition of tumor growth (data not shown, manuscript in preparation). We have also previously demonstrated that this model is dependent upon AKT activation as its growth in vivo is suppressed by a selective, allosteric inhibitor of AKT [She et al 2008]. Thus, both AKT signaling and tumor growth remain dependent on HER kinases in the F2#1282 model.
p95-HER2 is an HSP90 client protein
HER2 binds to HSP90 and is degraded is response to HSP90 inhibitors. This results in inhibition of HER2/PI3K/AKT signaling and tumor growth (Basso et al., 2002). As F2#1282 remains HER2 dependent, its sensitivity to HSP90 inhibitors will depend in part on whether Trastuzumab-resistant, active forms of HER2 such as p95-HER2 retain their dependence on HSP90. In the resistant F2#1282 model, loss of expression of p95-HER2 in response to HSP90 inhibitors may either be due to loss of full length HER2 or to a direct dependence of p95-HER2 upon HSP90 chaperone function for its own stability. In order to separate these effects, we utilized a cell line in which HA-tagged p95-HER2 was stably transfected into the T47D cell line (Anido et al., 2006; Scaltriti et al., 2007). The T47D line is an estrogen dependent model in which the HER2 gene is not amplified. In the parental T47D, HER2 is expressed at only moderate levels and expression of p95-HER2 is not detectable.
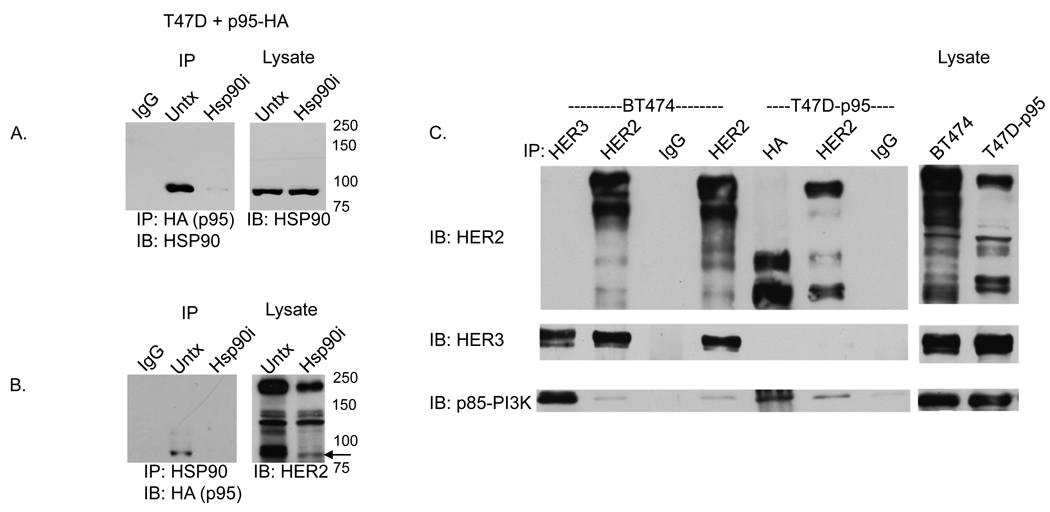
We investigated whether cellular p95-HER2 is present in a complex with HSP90. In Figure-2, HA-tagged p95-HER2 was expressed in T47D cells. Exposure of these cells to the selective HSP90 inhibitor SNX-2112 (Chandarlapaty et al., 2008) caused a marked reduction in the expression of full length and lower molecular weight forms of HER2, including p95-HER2 (Fig-2B-Lysate). Furthermore, (Fig-2A), HSP90 coimmunoprecipitates with p95-HER2-HA in anti-HA pull-downs (lane-2), but not in anti-IgG controls (lane-1) or in lysates of cells pretreated with SNX-2112 for 4 hours in which p95-HER2 has been degraded (lane-3). The complementary result can be demonstrated as well: p95-HER2-HA is immunoprecipitated with anti-HSP90 antisera (Figure-2B, lane-2) but not in IgG immunoprecipitates (Figure-2B, lane-1) or HSP90 inhibitor pre-treated immunoprecipitates (Figure-2B, lane-3). As HER2 and p95-HER2 are degraded in cells exposed to SNX-2112 for 4 hours, the absence of detectable complex in these lysates supports the specificity of the interaction.
Figure 2. p95-HER2 interacts with HSP90 and p85-PI3K.
A) Lysates from T47D cells stably transfected with HA-tagged-p95-HER2 were immunoprecipitated with an anti-HA antibody (or IgG) and immunoblotted for HSP90. In Lane-3, cells were treated with 1µM HSP90 inhibitor (SNX-2112) for 4 hours prior to collection. The right hand panel shows immunoblots of lysates without immunoprecipitation. B) Lysates of T47D transfectants immunoprecipitated with an anti-HSP90 antibody and Westerns immunoblotted for HA. Arrow points to p95-HER2 band. C) Lysates of BT474 cells were immunoprecipitated with antibodies against HER3, HER2 or IgG and Westerns immunoblotted with anti bodies to HER2, HER3, or p85-PI3K. These demonstrate coimmunoprecipitation of full length HER2 with HER3 and HER3 with p85. Lanes 5–7 contain lysates of T47D-p95 immunoprecipitated with antibodies against HA, HER2, or IgG immunoblotted with HER2, HER3, or p85-PI3K antibodies. These demonstrate p95-HER2 coimmunoprecipitation with p85. Corresponding whole cell lysates of BT474 and T47D-p95 are shown immunoblotted for HER2.
p95-HER2 is found in a complex with PI3K
Previous work demonstrated that both full length HER2 and p95-HER2 are found in a complex with HER3 which mediates activation of the PI3K-AKT survival pathway (Xia et al., 2004). This is supported by the data in Fig-2C. In the HER2-dependent, Trastuzumab-sensitive breast cancer cell line, BT474, HER2 coimmunoprecipates with HER3, a protein which, when phosphorylated, has a high affinity for the p85 regulatory subunit of PI3K. In these cells, HER3 is phosphorylated (data not shown), and coprecipitates with p85 (Fig-2C, lane-2+4) and with activated PI3K (Hermanto et al., 2001). In the T47D-p95 transfected cells, selective immunoprecipitation of p95-HER2 with anti-HA antisera coimmunoprecipitates PI3K-p85, suggesting that p95-HER2 can specifically activate the PI3K-AKT signaling pathway (lane-5). In the T47D model, p95-HER2 and HER3 do not coimmunoprecipitate raising the possibility that PI3K-p85 may bind directly to tyrosine phosphorylated p95-HER2 or to another docking protein in this model. Taken together, the data suggest that p95-HER2 is similar to full length HER2 in that it forms a complex with PI3K and thereby activates PI3K signaling.
Degradation of p95-HER2 in tumors exposed to HSP90 inhibitors
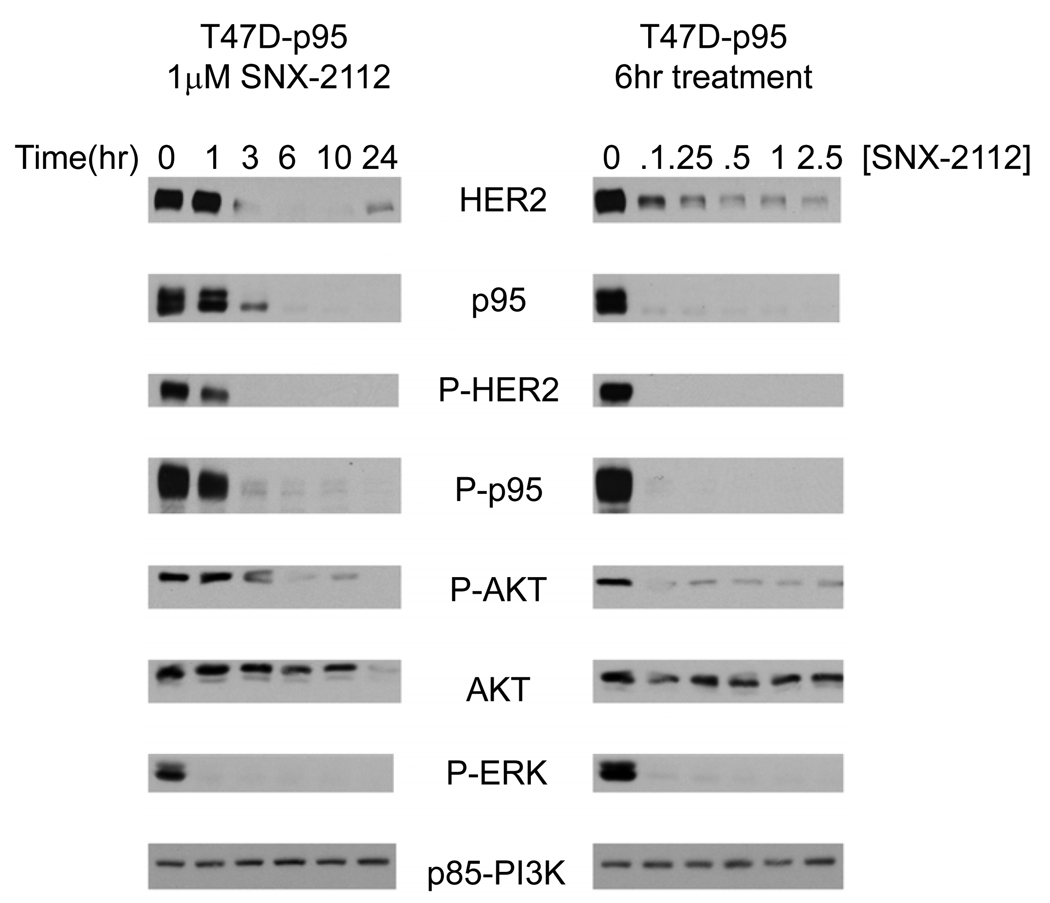
The dose of SNX-2112 required to cause degradation of p95-HER2 and the kinetics of loss of expression were determined in the HA-p95-HER2 expressing T47D cell line. HSP90 inhibition results in loss of both full length HER2 (Figure-3A, row-1) and p95-HER2 (row-2) with 3 hours of exposure to drug and loss of expression persisted for at least 24 hours (lanes 3–6) after treatment. Loss of p95-HER2 is observed on immunoblot with antibodies against either HER2 or HA, suggesting that the transfected version of p95-HER2 is specifically degraded. Treatment of these cells with concentrations of drug as low as 0.1 µM (Figure-3B, lane-2) causes both HER2 (row-1) and p95-HER2 (row-2) degradation but not degradation of non-HSP90 client proteins such as p85-PI3K (row-8). The degradation of p95-HER2 is not confined to the T47D model; it is also downregulated in response to HSP90 inhibition in mouse embryonic fibroblasts and MCF-7 cells into which it has been overexpressed [Supplemental Figure 2]. These data strongly suggest that, similar to full length HER2, the extracellular truncated p95-HER2 interacts with HSP90 and is degraded is cells exposed to HSP90 inhibitors.
Figure 3. HSP90 inhibition induces the degradation of p95-HER2 and HER2.
T47D-p95 cells were treated with an HSP90 inhibitor (1 µM SNX-2112) and collected at indicated timepoints afterwards or cells were treated with indicated doses and collected after 6 hours. Immunoblots of Westerns using indicated antibodies demonstrate degradation of both full-length HER2 and p95-HER2 with downregulation of the HER2, AKT, and ERK signaling.
HSP90 inhibitors suppress p95-HER2 activated signaling
HER2 heterodimerizes with other HER kinases and potently activates ERK and PI3K/AKT signaling. The latter event plays an important role in maintaining the growth of HER2-dependent breast cancer and is sensitive to induction of HER2 degradation (Basso et al., 2002). In T47D p95-HER2 transfectants exposed to SNX-2112, degradation of p95-HER2 and HER2 is temporally associated with downregulation of PI3K-AKT (Fig 3, row 5) and ERK signaling (row 7) as assessed by loss of activated AKT (S473) and ERK (T202/Y204). Although AKT is a client protein of HSP90, its degradation occurs much later (12 hours), than loss of P-AKT (rows 5 and 6, lanes 3–5), suggesting that downregulation of the pathway is a consequence of HER2 inhibition rather than of AKT degradation or direct inhibition. The loss of activated AKT prior to total AKT is also seen in MEFs and MCF-7 cells expressing p95-HER2 [Supplemental Figure 2]. Although the degradation of other HSP90 client proteins could contribute to PI3K/AKT inhibition, we have previously shown in breast and lung cancer models that HSP90 inhibitors rapidly inhibit PI3K/AKT signaling preferentially in tumors in which the upstream activator of the pathway is an HSP90 client protein that is sensitive to HSP90 inhibition (Basso et al., 2002; Munster et al., 2002; Sawai et al., 2008).
HSP90 inhibition antagonizes HER2 and p95-HER2 stimulated proliferation
T47D is a breast cancer cell line that expresses estrogen receptor and moderate levels of HER2 and harbors a PIK3CA mutation as well. Introduction of HER2 or p95-HER2 into T47D cells confers a growth advantage and renders them partially sensitive to HER kinase inhibition (Scaltriti et al., 2007). We compared the effect of Trastuzumab treatment and HSP90 inhibition upon proliferation of these cells. Cellular proliferation of the T47D cells is stimulated by transfection of either p95-HER2 (~2.5 fold) or full length HER2 (~2.9 fold) compared to proliferation of vector transfected cells (Figure-4). Trastuzumab treatment has little effect on the proliferation of either p95-HER2 (~2.2 fold above vector control) or HER2 (~2.9 fold) transfected cells. In contrast, HSP90 inhibition results in complete inhibition of cellular proliferation of the p95-HER2, HER2, or vector transfected cells. While the inhibition of vector transfected cells implies a role for other HSP90 clients in mediating survival, the inhibition of growth of the p95-HER2 transfected cells suggests that the drug may prevent rescue from growth by degrading the p95-HER2.
A p95-HER2 dependent in vivo tumor model is sensitive to HSP90 inhibitors
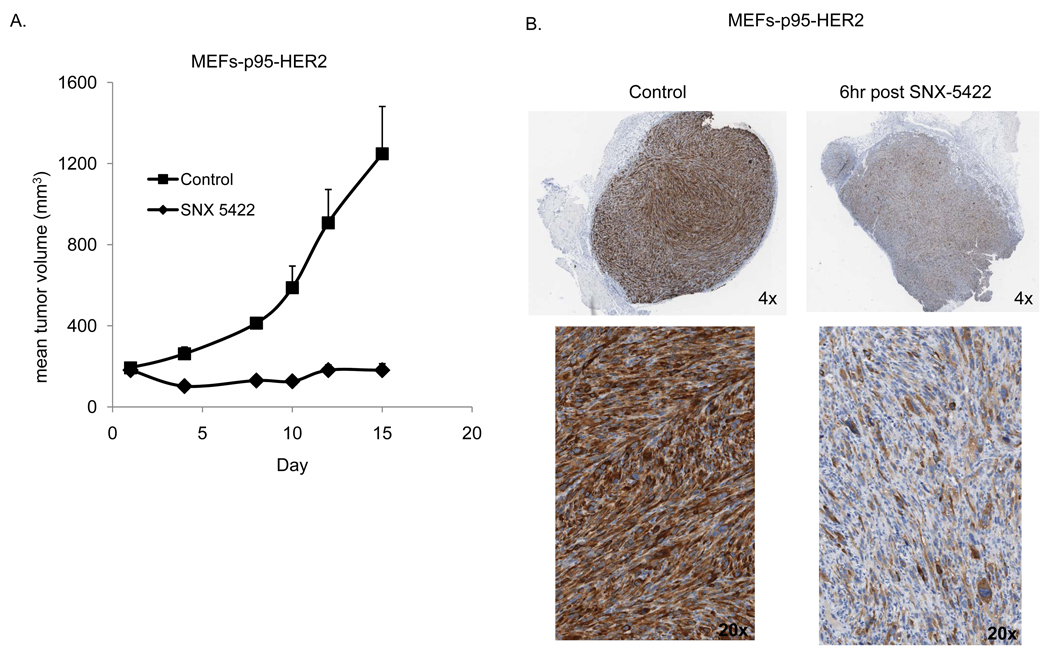
To assess the efficacy of HSP90 inhibition in targeting p95-HER2 in vivo we utilized MEFs expressing p95-HER2 under tet-off tetracycline-controlled transactivator. The cells lack expression of full length human HER2 and expression of p95-HER2 transforms these cells and enables them to grow as tumors in nude mice. Moreover, the addition of doxycycline to the drinking of water of tumor bearing mice represses p95-HER2 expression and results in complete tumor shrinkage (data not shown) confirming the dependence of these cells upon p95-HER2 for their tumorigenicity. In Figure-5A, MEFs expressing p95-HER2 were xenografted onto nude mice. These MEFs conditionally express a p95-HER2 cDNA (M611) in the absence of doxycycline (Pederson et al., 2009). In order to assess the HSP90 dependence of these tumors we utilized SNX5422 which is an oral prodrug of SNX-2112 that is rapidly converted to SNX-2112 and functions as an in vivo HSP90 inhibitor. In Figure 5A, tumor bearing mice were treated three times/week with SNX5422 (50mg/kg PO) resulting in complete inhibition of tumor growth over the two weeks of treatment. Tumors treated with a single dose of SNX-5422 (75 mg/kg) had a marked reduction in p95-HER2 expression as seen by both immunohistochemistry and immunoblotting (Figure 5B and Supplemental Figure 3). Whereas the MEF-p95-HER2 tumor model lacks expression of full length human HER2 and is insensitive to Trastuzumab (data not shown), it is dependent upon p95-HER2 expression for growth and sensitive to HSP90 inhibitors that induce the degradation of p95-HER2.
Figure 5. HSP90 inhibition downregulates HER2 and p95-HER2-mediated signaling and Trastuzumab-resistant tumor growth.
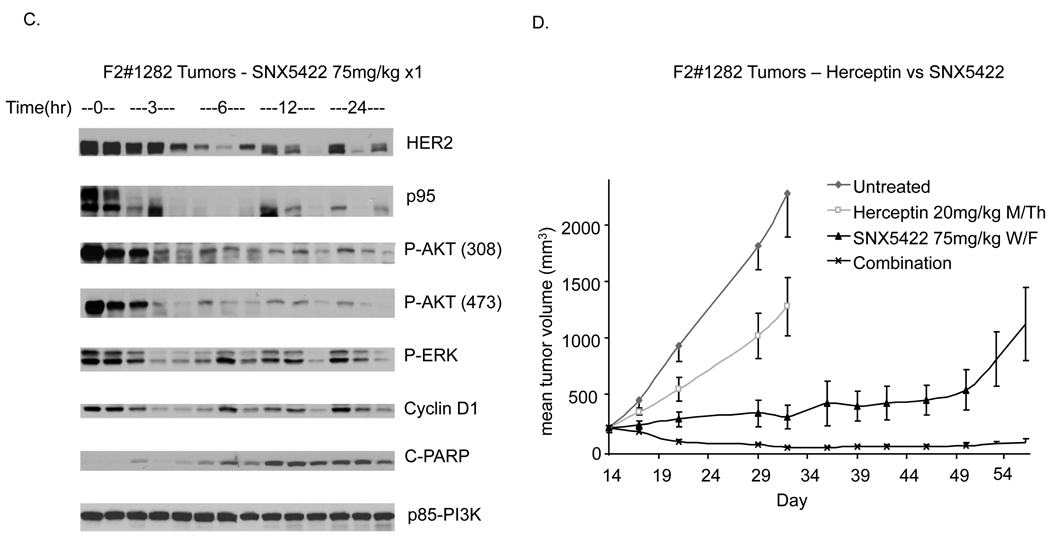
A. Mice bearing established tumors of p95-HER2 expressing MEFs were randomized to treatment with vehicle or SNX5422 (50mg/kg PO 3x/week). B. Mice bearing p95-HER2-MEF tumors were administered a single dose of SNX5422 (75mg/kg PO) and tumor removed at indicated times after dose. Immunohistochemistry with the DAKO antibody against HER2 was performed with representative sections displayed. C. Mice bearing F2#1282 tumors were administered a single dose of SNX5422 (75mg/kg PO) and tumors removed at indicated times after dose. Immunoblotting was performed on lysates using antibodies against the indicated proteins. D. Mice with established F2#1282 tumors (5 mice/group) were randomized to treatment with vehicle, SNX5422 (2x/week), Trastuzumab (2x/week), or the combination. Treatment was discontinued after Day#17 and measurements continued as indicated.
The F2#1282 Trastuzumab-resistant breast tumor model is sensitive to HSP90 inhibitors in vivo
In human breast tumors with p95-HER2 expression, full length HER2 is also typically overexpressed. To assess the HSP90 dependence of model in which p95-HER2 and full length HER2 are overexpressed, we again utilized the F2#1282 model. As shown in Figure-1, in the F2#1282 model, AKT activation and tumor growth are insensitive to Trastuzumab but the tumors retain a dependence upon HER kinase and AKT kinase function. In Figure-5C, mice bearing xenografted F2#1282 tumors were treated with a single dose of SNX5422 (75mg/kg) and sacrificed at the indicated times after dose (3 mice/timepoint). SNX5422 is an oral prodrug of SNX-2112 that is rapidly converted to SNX-2112 and functions as an in vivo HSP90 inhibitor. A single 75mg/kg dose of the oral HSP90 inhibitor is well tolerated and causes loss of expression of total and activated full length HER2 and p95-HER2 in the tumor (Figure-5C and data not shown). Decreased HER2 expression is associated with a greater than 75% decrease in phospho-AKT intensity evident three hours after drug administration and persisting at least 24 hours later. Inhibition of signaling is accompanied by loss of cyclin D1 expression and induction of apoptosis as measured by increased levels of cleaved PARP in the xenografts.
In contrast to the inactivity of Trastuzumab treatment in this model, twice weekly SNX5422 (75mg/kg PO) resulted in near complete tumor growth inhibition that was sustained two weeks beyond cessation of treatment (day#31). Furthermore, we find that combining HSP90 inhibition with Trastuzumab has more potent activity than either alone and results in tumor regressions that are also appreciable well beyond the time of cessation of treatment.
Discussion
The use of Trastuzumab as an agent to specifically target breast cancers with amplification of the HER2 oncogene was one of the first and most successful applications of targeted therapy for metastatic carcinomas. The broad use of Trastuzumab has resulted in an increasing prevalence of patients whose tumors have developed resistance to the therapy over time and the identification of a significant number who are resistant at the outset. However, the mechanisms underlying resistance to Trastuzumab remain obscure, in part because there is still considerable debate as to the mechanisms that underlie its antitumor activity. There are essentially two schools of thought as to its mechanism of action, one based on inhibition of HER2 functional signaling, the other focused on induction of antibody dependent cytotoxicity (Clynes et al., 2000; Yakes et al., 2002).
Although there is a significant amount of data supporting the latter, most of the hypotheses and experimental data on resistance have been directed at mechanisms that prevent or bypass inhibition of signaling by Trastuzumab. Despite many putative mechanisms described in experimental models, the actual mechanisms of resistance have not been defined in patients, in large part because of the lack of biopsy studies. The experimental models that have been developed have largely focused on mechanisms that cause activation of PI3K/AKT signaling to be less dependent or independent of HER2. These include decreased PTEN function, activation of other receptor tyrosine kinases (e.g. EGFR), or mutational activation of PI3K. Another potential mechanism of resistance, discussed in this paper, is the expression of forms of HER2 that are functionally active, but lack the Trastuzumab epitope. Such tumors would be predicted to remain HER2 dependent for activation of PI3K/AKT signaling but would be resistant to inhibition of the pathway by Trastuzumab. The recent finding that the HER2 kinase inhibitor Lapatinib has antitumor activity in a significant proportion of Trastuzumab-resistant, HER2 overexpressing breast cancer patients suggests that many of these tumors indeed retain a dependence on HER2.
p95-HER2 retains tyrosine kinase activity and has been shown to activate ERK and AKT signaling, but lacks the Trastuzumab binding site (Anido et al., 2006; Molina et al., 2002; Xia et al., 2004). Its expression in human tumors has been correlated with Trastuzumab resistance (Scaltriti et al., 2007). If this relationship is causal, such tumors would be expected to respond to modalities that inhibit p95-HER2 function or reduce its expression. HER2 is an HSP90 dependent protein that is ubiquitinated and degraded in the proteosome in response to HSP90 inhibitors. We demonstrate that p95-HER2 retains its dependence on HSP90. It is present in the cell in an HSP90 complex and is rapidly and potently degraded in cells exposed to the HSP90 inhibitor SNX-2112. This occurs at comparable concentrations of inhibitor required for degradation of full length HER2. This is compatible with work showing that HSP90 binds to a region in the catalytic domain of HER2 (Tikhomirov and Carpenter, 2003; Xu et al., 2001). HSP90 inhibitors rapidly degrade HER2 and inhibit PI3K/AKT and ERK signaling in tumor models with HER2 overexpression, whether or not they express high levels of endogenous or transfected p95-HER2 ((Basso et al., 2002; Munster et al., 2002), Figure-3–Figure-5). Moreover, we find that HSP90 inhibition potently induces degradation of HER2 and p95-HER2 in vivo and causes prolonged inhibition of AKT signaling in murine tumor models, at doses that are not toxic to the host.
These data suggest that HSP90 inhibition will be useful in Trastuzumab-resistant tumors that retain HER2 dependence, especially those in which resistance is due to p95-HER2 expression. We have previously demonstrated in tissue culture models that cells transfected with p95-HER2 do not respond to Trastuzumab therapy but are sensitized to the antiproliferative effects of the HER2 kinase inhibitor Lapatinib (Scaltriti et al., 2007). In this report we demonstrate that the F2#1282 Trastuzumab-resistant xenograft model expresses high levels of p95-HER2. In this model, Trastuzumab treatment appears to further increase p95-HER2, perhaps contributing to resistance. In contrast, Trastuzumab has been shown to decrease p95-HER2 expression in the sensitive BT474 model and this has been adduced as a putative mechanism of Trastuzumab activity (Molina et al., 2001). Whether upregulation of p95-HER2 expression is necessary for resistance in F2#1282 is not certain, however, it is clear that p95-HER2 expression and mitogenic signaling are not downregulated by Trastuzumab treatment in this model.
In contrast, the growth of F2#1282 tumors is quite sensitive to HSP90 inhibition. A single dose of HSP90 inhibitor is sufficient to induce rapid degradation of both p95-HER2 and full length HER2 and cause prolonged inhibition of AKT and ERK signaling, PARP cleavage, and complete cessation of tumor growth. Similarly, the HER1/2 kinase inhibitor Lapatinib also causes downregulation of HER2 signaling and significantly slows tumor growth. Taken together, these data establish that this Trastuzumab-resistant tumor model remains dependent upon HER2. In further support, we find that a genetically engineered model of p95-HER2 mediated tumorigenesis, the MEF-p95-HER2 model, is also resistant to Trastuzumab, completely dependent upon p95-HER2 expression for survival and highly sensitive to HSP90 inhibition. These data are consistent with the findings of clinical trials of alternative HER2 targeted therapies for patients with HER2 amplified breast cancer that have become resistant to Trastuzumab. Recent trials show that the HER kinase inhibitors, Lapatinib and HKI-272, and the HSP90 inhibitor, 17-AAG, have significant activity in HER2-overexpressing breast tumors that have progressed on Trastuzumab treatment (Geyer et al., 2006; Modi et al., 2007). The activity of both of these classes of agents is most likely due to their more potent or different mechanism of inhibition of HER2. This follows the pattern of resistance to other targeted therapies such as BCR-ABL inhibitors in CML or mutant EGFR inhibitors in NSCLC in which resistant tumors often retain their dependence on the targeted oncoprotein. Whether the tumors that are resistant to salvage therapy with a HER kinase or HSP90 inhibitor are still HER2 dependent, but refractory to these inhibitors, or whether they have progressed to a HER2-independent state is unknown.
The current data suggests that either an HSP90 inhibitor or an effective HER kinase inhibitor can inhibit tumors in which resistance is mediated by p95-HER2 and perhaps other HER2-dependent mechanisms. Both modalities effectively inhibit AKT activation, although in F2#1282 the effects of the HSP90 inhibitor on the pathway are much more prolonged. It is not at all clear which modality is superior and, since they inhibit HER2 by different mechanisms, coadministration could conceivably inhibit HER2 function more effectively than either drug alone and with enhanced clinical benefit.
Despite the resistance of F2#1282 tumors to Trastuzumab therapy alone, we have noted that it significantly enhances the antitumor activity of the HSP90 inhibitor. The combination is associated with significant tumor regression compared to the HSP90 inhibitor alone. The mechanisms through which Trastuzumab contributes to antitumor activity are unknown but could include more effective inhibition of the function of full-length HER2 or other effects of Trastuzumab on angiogenesis or tumor immunity. Recently, patients whose tumors had progressed during multiple Trastuzumab-based therapies were treated in a Phase I/II clinical trial, of the HSP90 inhibitor, 17-AAG, in the setting of continued Trastuzumab administration. The results of this trial were quite promising with a 26% objective response rate and 63% evidence of biologic response rate (Modi et al., 2008). There is no way to know whether the Trastuzumab had any effect on these results, but these and other data suggest that Trastuzumab/HSP90 inhibitor combinations are rational in patients who have not previously been treated as well as those with acquired Trastuzumab resistance.
Supplementary Material
Acknowledgments
We thank Gail Lewis Phillips and Mark Sliwkowski for providing the F2#1282 tumors for this study. We also thank Elisa DeStanchina and Wai Wong for assistance with animal studies.
Financial Support: This work is supported by the National Institute of Health Program Grant: P01-CA094060, the Breast Cancer Research Foundation, and the generous support of Arlene Taub. S. Chandarlapaty is supported by an ASCO Foundation Young Investigator Award.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
References
- Anido J, Scaltriti M, Bech Serra JJ, Santiago Josefat B, Todo FR, Baselga J, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. Embo J. 2006;25:3234–3244. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baselga J, Norton L, Albanell J, Kim YM, Mendelsohn J. Recombinant humanized anti-HER2 antibody (Herceptin) enhances the antitumor activity of paclitaxel and doxorubicin against HER2/neu overexpressing human breast cancer xenografts. Cancer Res. 1998;58:2825–2831. [PubMed] [Google Scholar]
- Basso AD, Solit DB, Munster PN, Rosen N. Ansamycin antibiotics inhibit Akt activation and cyclin D expression in breast cancer cells that overexpress HER2. Oncogene. 2002;21:1159–1166. doi: 10.1038/sj.onc.1205184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R, Henke E, Ciarrocchi A, Ruzinova M, Solit D, Rosen N, et al. Induction of complete regressions of oncogene-induced breast tumors in mice. Cold Spring Harb Symp Quant Biol. 2005;70:375–381. doi: 10.1101/sqb.2005.70.006. [DOI] [PubMed] [Google Scholar]
- Berns K, Horlings HM, Hennessy BT, Madiredjo M, Hijmans EM, Beelen K, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402. doi: 10.1016/j.ccr.2007.08.030. [DOI] [PubMed] [Google Scholar]
- Chandarlapaty S, Sawai A, Ye Q, Scott A, Silinski M, Huang K, et al. SNX2112, a synthetic heat shock protein 90 inhibitor, has potent antitumor activity against HER kinase-dependent cancers. Clin Cancer Res. 2008;14:240–248. doi: 10.1158/1078-0432.CCR-07-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- Cuello M, Ettenberg SA, Clark AS, Keane MM, Posner RH, Nau MM, et al. Down-regulation of the erbB-2 receptor by trastuzumab (herceptin) enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in breast and ovarian cancer cell lines that overexpress erbB-2. Cancer Res. 2001;61:4892–4900. [PubMed] [Google Scholar]
- Finkle D, Quan ZR, Asghari V, Kloss J, Ghaboosi N, Mai E, et al. HER2-targeted therapy reduces incidence and progression of midlife mammary tumors in female murine mammary tumor virus huHER2-transgenic mice. Clin Cancer Res. 2004;10:2499–2511. doi: 10.1158/1078-0432.ccr-03-0448. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- Guy CT, Webster MA, Schaller M, Parsons TJ, Cardiff RD, Muller WJ. Expression of the neu protooncogene in the mammary epithelium of transgenic mice induces metastatic disease. Proc Natl Acad Sci U S A. 1992;89:10578–10582. doi: 10.1073/pnas.89.22.10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanto U, Zong CS, Wang LH. ErbB2-overexpressing human mammary carcinoma cells display an increased requirement for the phosphatidylinositol 3-kinase signaling pathway in anchorage-independent growth. Oncogene. 2001;20:7551–7562. doi: 10.1038/sj.onc.1204964. [DOI] [PubMed] [Google Scholar]
- Holbro T, Beerli RR, Maurer F, Koziczak M, Barbas CF, 3rd, Hynes NE. The ErbB2/ErbB3 heterodimer functions as an oncogenic unit: ErbB2 requires ErbB3 to drive breast tumor cell proliferation. Proc Natl Acad Sci U S A. 2003;100:8933–8938. doi: 10.1073/pnas.1537685100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med. 2007;357:39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- Lane HA, Beuvink I, Motoyama AB, Daly JM, Neve RM, Hynes NE. ErbB2 potentiates breast tumor proliferation through modulation of p27(Kip1)-Cdk2 complex formation: receptor overexpression does not determine growth dependency. Mol Cell Biol. 2000;20:3210–3223. doi: 10.1128/mcb.20.9.3210-3223.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis Phillips GD, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res. 2008;68:9280–9290. doi: 10.1158/0008-5472.CAN-08-1776. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zi X, Zhao Y, Mascarenhas D, Pollak M. Insulin-like growth factor-I receptor signaling and resistance to trastuzumab (Herceptin) J Natl Cancer Inst. 2001;93:1852–1857. doi: 10.1093/jnci/93.24.1852. [DOI] [PubMed] [Google Scholar]
- Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. [DOI] [PubMed] [Google Scholar]
- Modi S, Sugarman S, Stopeck AT, Linden H, Ma W, Kersey K, et al. ASCO 2008 Annual Meeting; Chicago, IL. 2008. [Google Scholar]
- Molina MA, Codony-Servat J, Albanell J, Rojo F, Arribas J, Baselga J. Trastuzumab (herceptin), a humanized anti-Her2 receptor monoclonal antibody, inhibits basal and activated Her2 ectodomain cleavage in breast cancer cells. Cancer Res. 2001;61:4744–4749. [PubMed] [Google Scholar]
- Molina MA, Saez R, Ramsey EE, Garcia-Barchino MJ, Rojo F, Evans AJ, et al. NH(2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancer. Clin Cancer Res. 2002;8:347–353. [PubMed] [Google Scholar]
- Moody SE, Sarkisian CJ, Hahn KT, Gunther EJ, Pickup S, Dugan KD, et al. Conditional activation of Neu in the mammary epithelium of transgenic mice results in reversible pulmonary metastasis. Cancer Cell. 2002;2:451–461. doi: 10.1016/s1535-6108(02)00212-x. [DOI] [PubMed] [Google Scholar]
- Muller WJ, Sinn E, Pattengale PK, Wallace R, Leder P. Single-step induction of mammary adenocarcinoma in transgenic mice bearing the activated c-neu oncogene. Cell. 1988;54:105–115. doi: 10.1016/0092-8674(88)90184-5. [DOI] [PubMed] [Google Scholar]
- Munster PN, Marchion DC, Basso AD, Rosen N. Degradation of HER2 by ansamycins induces growth arrest and apoptosis in cells with HER2 overexpression via a HER3, phosphatidylinositol 3'-kinase-AKT-dependent pathway. Cancer Res. 2002;62:3132–3137. [PubMed] [Google Scholar]
- Nagata Y, Lan KH, Zhou X, Tan M, Esteva FJ, Sahin AA, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–127. doi: 10.1016/j.ccr.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Pederson K, Angelini P, Lao S, Bach-Faig A, Cunninngham MP, Ferrer-Ramon C, et al. A Naturally Occurring HER2 Carboxy-Terminal Fragment Promotes Mammary Tumor Growth and Metastasis. Mol Cell Biol. 2009;29:3319–3331. doi: 10.1128/MCB.01803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram MD, Lopez A, Konecny G, Slamon DJ. Trastuzumab and chemotherapeutics: drug interactions and synergies. Semin Oncol. 2000;27:21–25. discussion 92–100. [PubMed] [Google Scholar]
- Rice J, Fadden P, Huang K, Barabasz A, Foley B, Veal J, et al. AACR Annual Meeting; San Diego, CA. 2008. [Google Scholar]
- Ritter CA, Perez-Torres M, Rinehart C, Guix M, Dugger T, Engelman JA, et al. Human breast cancer cells selected for resistance to trastuzumab in vivo overexpress epidermal growth factor receptor and ErbB ligands and remain dependent on the ErbB receptor network. Clin Cancer Res. 2007;13:4909–4919. doi: 10.1158/1078-0432.CCR-07-0701. [DOI] [PubMed] [Google Scholar]
- Saez R, Molina MA, Ramsey EE, Rojo F, Keenan EJ, Albanell J, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006;12:424–431. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- Sawai A, Chandarlapaty S, Greulich H, Gonen M, Ye Q, Arteaga CL, et al. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res. 2008;68:589–596. doi: 10.1158/0008-5472.CAN-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M, Rojo F, Ocana A, Anido J, Guzman M, Cortes J, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007;99:628–638. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- Shattuck DL, Miller JK, Carraway KL, 3rd, Sweeney C. Met receptor contributes to trastuzumab resistance of Her2-overexpressing breast cancer cells. Cancer Res. 2008;68:1471–1477. doi: 10.1158/0008-5472.CAN-07-5962. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Tikhomirov O, Carpenter G. Identification of ErbB-2 kinase domain motifs required for geldanamycin-induced degradation. Cancer Res. 2003;63:39–43. [PubMed] [Google Scholar]
- Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann N Y Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. [DOI] [PubMed] [Google Scholar]
- Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004;23:646–653. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- Xu W, Mimnaugh E, Rosser MF, Nicchitta C, Marcu M, Yarden Y, et al. Sensitivity of mature Erbb2 to geldanamycin is conferred by its kinase domain and is mediated by the chaperone protein Hsp90. J Biol Chem. 2001;276:3702–3708. doi: 10.1074/jbc.M006864200. [DOI] [PubMed] [Google Scholar]
- Yakes FM, Chinratanalab W, Ritter CA, King W, Seelig S, Arteaga CL. Herceptin-induced inhibition of phosphatidylinositol-3 kinase and Akt Is required for antibody-mediated effects on p27, cyclin D1, and antitumor action. Cancer Res. 2002;62:4132–4141. [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P. Specific protection against breast cancers by cyclin D1 ablation. Nature. 2001;411:1017–1021. doi: 10.1038/35082500. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.