Abstract
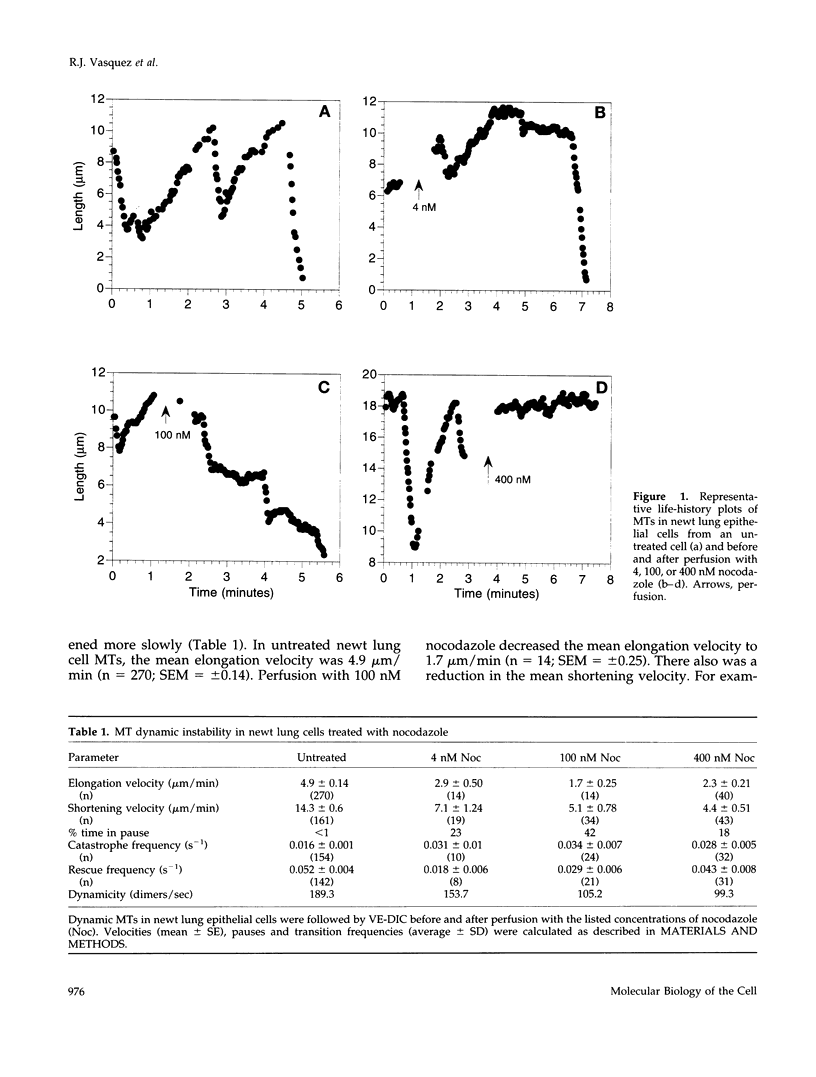
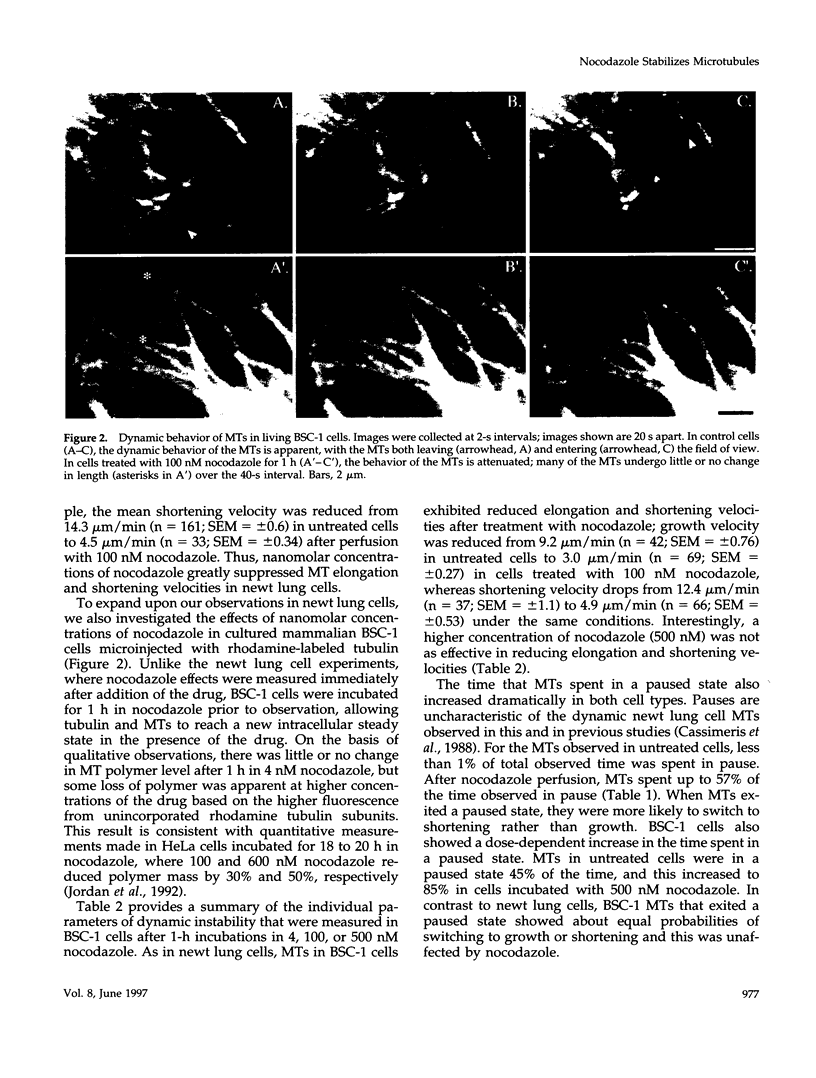
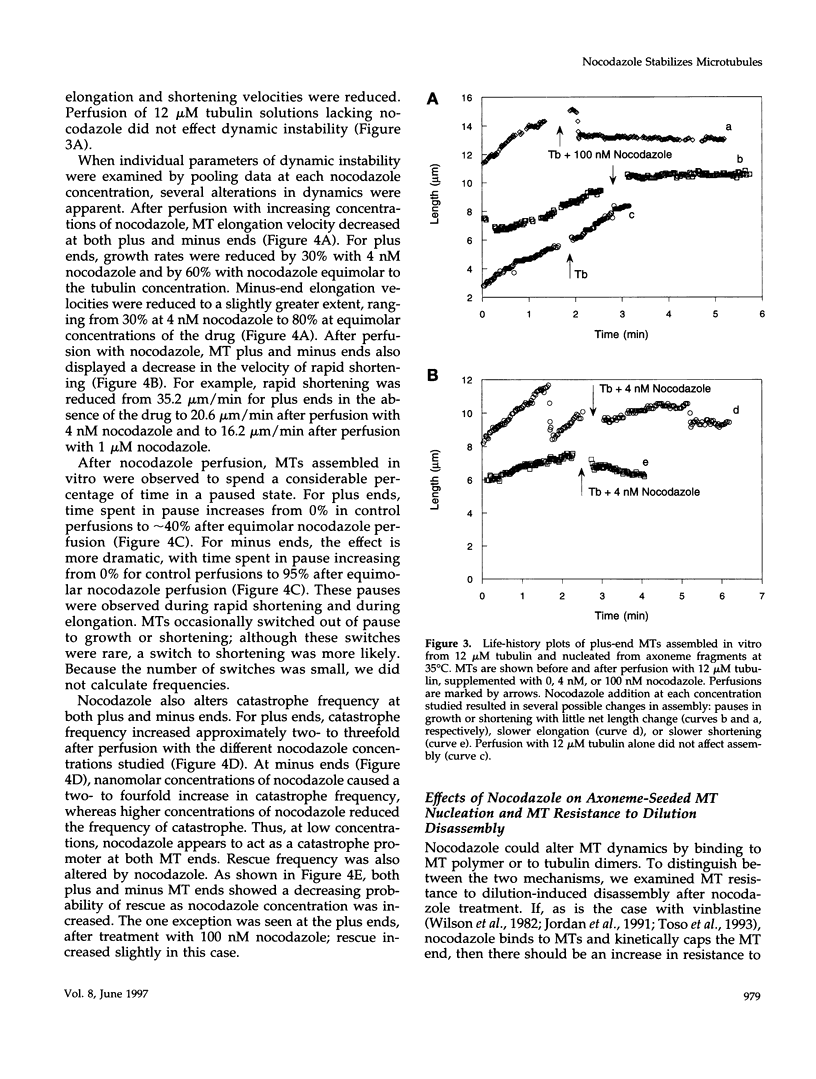
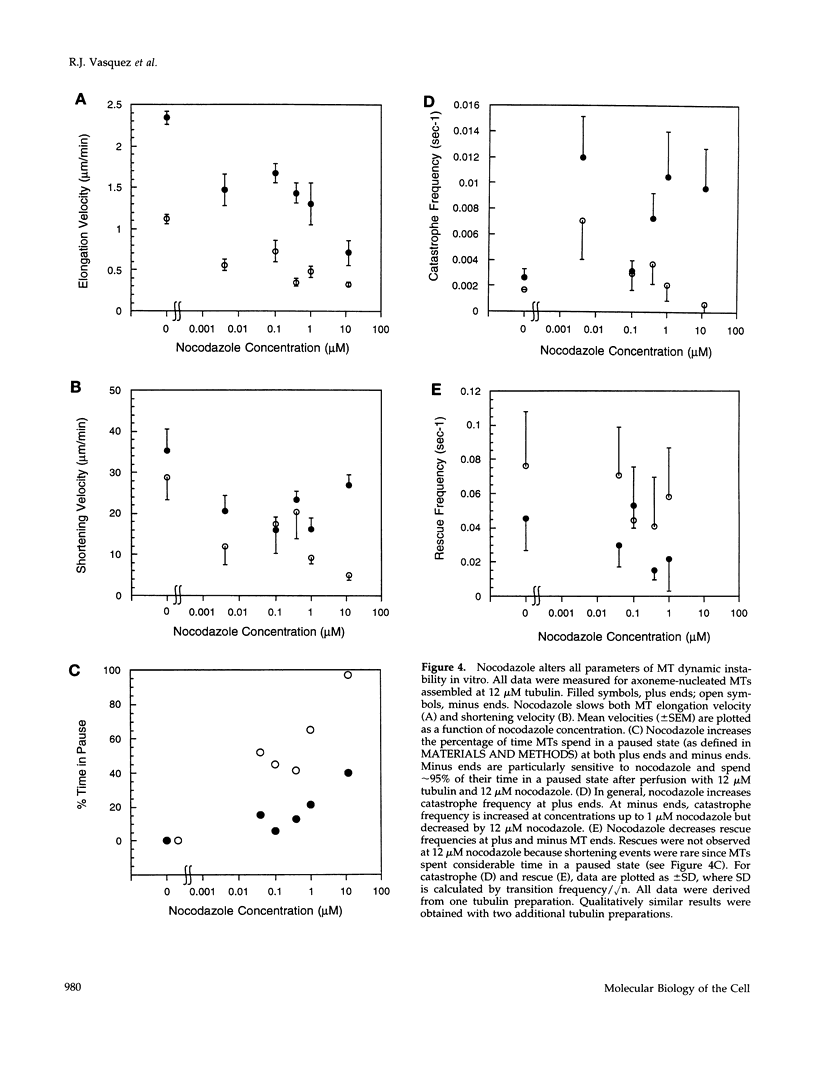
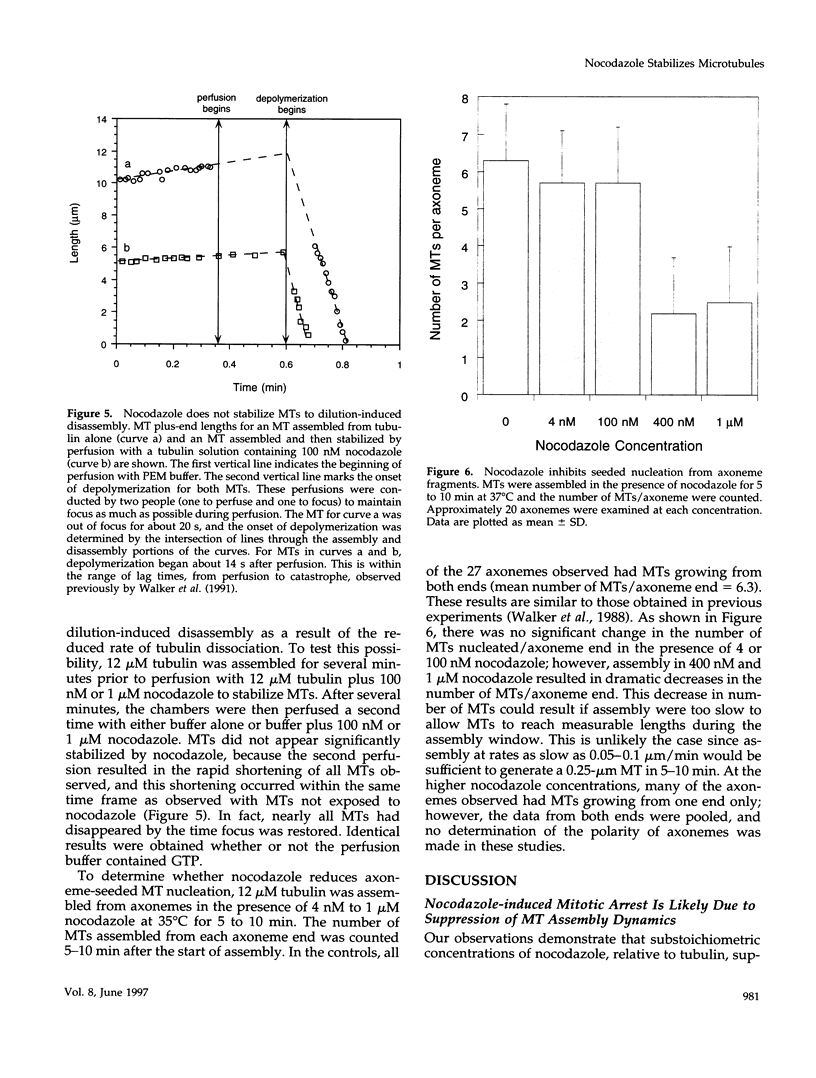
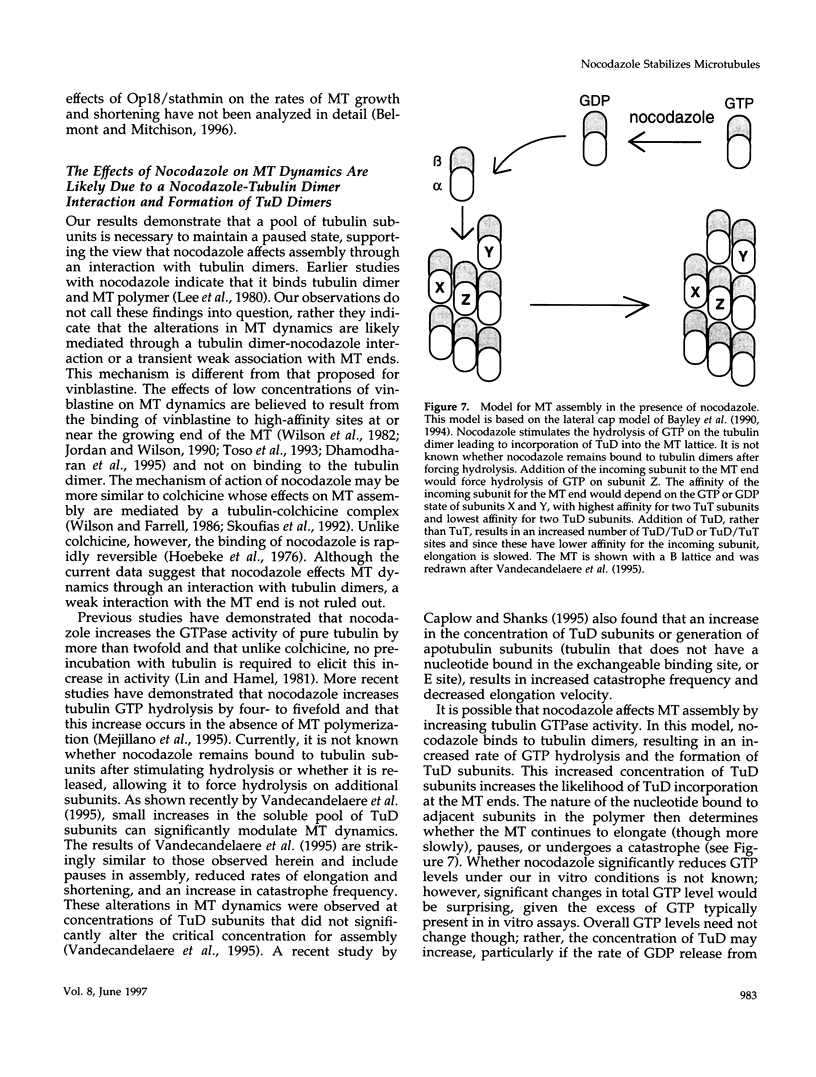
Previous studies demonstrated that nanomolar concentrations of nocodazole can block cells in mitosis without net microtubule disassembly and resulted in the hypothesis that this block was due to a nocodazole-induced stabilization of microtubules. We tested this hypothesis by examining the effects of nanomolar concentrations of nocodazole on microtubule dynamic instability in interphase cells and in vitro with purified brain tubulin. Newt lung epithelial cell microtubules were visualized by video-enhanced differential interference contrast microscopy and cells were perfused with solutions of nocodazole ranging in concentration from 4 to 400 nM. Microtubules showed a loss of the two-state behavior typical of dynamic instability as evidenced by the addition of a third state where they exhibited little net change in length (a paused state). Nocodazole perfusion also resulted in slower elongation and shortening velocities, increased catastrophe, and an overall decrease in microtubule turnover. Experiments performed on BSC-1 cells that were microinjected with rhodamine-labeled tubulin, incubated in nocodazole for 1 h, and visualized by using low-light-level fluorescence microscopy showed similar results except that nocodazole-treated BSC-1 cells showed a decrease in catastrophe. To gain insight into possible mechanisms responsible for changes in dynamic instability, we examined the effects of 4 nM to 12 microM nocodazole on the assembly of purified tubulin from axoneme seeds. At both microtubule plus and minus ends, perfusion with nocodazole resulted in a dose-dependent decrease in elongation and shortening velocities, increase in pause duration and catastrophe frequency, and decrease in rescue frequency. These effects, which result in an overall decrease in microtubule turnover after nocodazole treatment, suggest that the mitotic block observed is due to a reduction in microtubule dynamic turnover. In addition, the in vitro results are similar to the effects of increasing concentrations of GDP-tubulin (TuD) subunits on microtubule assembly. Given that nocodazole increases tubulin GTPase activity, we propose that nocodazole acts by generating TuD subunits that then alter dynamic instability.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley P. M., Schilstra M. J., Martin S. R. Microtubule dynamic instability: numerical simulation of microtubule transition properties using a Lateral Cap model. J Cell Sci. 1990 Jan;95(Pt 1):33–48. doi: 10.1242/jcs.95.1.33. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Mitchison T. J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996 Feb 23;84(4):623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- Caplow M. Microtubule dynamics. Curr Opin Cell Biol. 1992 Feb;4(1):58–65. doi: 10.1016/0955-0674(92)90059-l. [DOI] [PubMed] [Google Scholar]
- Caplow M., Shanks J. Evidence that a single monolayer tubulin-GTP cap is both necessary and sufficient to stabilize microtubules. Mol Biol Cell. 1996 Apr;7(4):663–675. doi: 10.1091/mbc.7.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplow M., Shanks J. Induction of microtubule catastrophe by formation of tubulin-GDP and apotubulin subunits at microtubule ends. Biochemistry. 1995 Dec 5;34(48):15732–15741. doi: 10.1021/bi00048a018. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Pantaloni D. Microtubule elongation and guanosine 5'-triphosphate hydrolysis. Role of guanine nucleotides in microtubule dynamics. Biochemistry. 1987 Jul 14;26(14):4428–4437. doi: 10.1021/bi00388a036. [DOI] [PubMed] [Google Scholar]
- Cassimeris L., Pryer N. K., Salmon E. D. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L. Regulation of microtubule dynamic instability. Cell Motil Cytoskeleton. 1993;26(4):275–281. doi: 10.1002/cm.970260402. [DOI] [PubMed] [Google Scholar]
- Cole N. B., Lippincott-Schwartz J. Organization of organelles and membrane traffic by microtubules. Curr Opin Cell Biol. 1995 Feb;7(1):55–64. doi: 10.1016/0955-0674(95)80045-x. [DOI] [PubMed] [Google Scholar]
- De Brabander M. J., Van de Veire R. M., Aerts F. E., Borgers M., Janssen P. A. The effects of methyl (5-(2-thienylcarbonyl)-1H-benzimidazol-2-yl) carbamate, (R 17934; NSC 238159), a new synthetic antitumoral drug interfering with microtubules, on mammalian cells cultured in vitro. Cancer Res. 1976 Mar;36(3):905–916. [PubMed] [Google Scholar]
- Dhamodharan R., Jordan M. A., Thrower D., Wilson L., Wadsworth P. Vinblastine suppresses dynamics of individual microtubules in living interphase cells. Mol Biol Cell. 1995 Sep;6(9):1215–1229. doi: 10.1091/mbc.6.9.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamodharan R., Wadsworth P. Modulation of microtubule dynamic instability in vivo by brain microtubule associated proteins. J Cell Sci. 1995 Apr;108(Pt 4):1679–1689. doi: 10.1242/jcs.108.4.1679. [DOI] [PubMed] [Google Scholar]
- Drechsel D. N., Kirschner M. W. The minimum GTP cap required to stabilize microtubules. Curr Biol. 1994 Dec 1;4(12):1053–1061. doi: 10.1016/s0960-9822(00)00243-8. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., O'Brien E. T. Microtubule dynamic instability and GTP hydrolysis. Annu Rev Biophys Biomol Struct. 1992;21:145–166. doi: 10.1146/annurev.bb.21.060192.001045. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Platzer E. G. Interaction of anthelmintic benzimidazoles and benzimidazole derivatives with bovine brain tubulin. Biochim Biophys Acta. 1978 Dec 18;544(3):605–614. doi: 10.1016/0304-4165(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Gliksman N. R., Parsons S. F., Salmon E. D. Okadaic acid induces interphase to mitotic-like microtubule dynamic instability by inactivating rescue. J Cell Biol. 1992 Dec;119(5):1271–1276. doi: 10.1083/jcb.119.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebeke J., Van Nijen G., De Brabander M. Interaction of oncodazole (R 17934), a new antitumoral drug, with rat brain tubulin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):319–324. doi: 10.1016/0006-291x(76)90524-6. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Salser S., Drechsel D. N., Unwin N., Mitchison T. J. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol Biol Cell. 1992 Oct;3(10):1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman A., Drechsel D., Kellogg D., Salser S., Sawin K., Steffen P., Wordeman L., Mitchison T. Preparation of modified tubulins. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- Inoué S., Salmon E. D. Force generation by microtubule assembly/disassembly in mitosis and related movements. Mol Biol Cell. 1995 Dec;6(12):1619–1640. doi: 10.1091/mbc.6.12.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireland C. M., Gull K., Gutteridge W. E., Pogson C. I. The interaction of benzimidazole carbamates with mammalian microtobule protein. Biochem Pharmacol. 1979 Sep 1;28(17):2680–2682. doi: 10.1016/0006-2952(79)90049-2. [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Margolis R. L., Himes R. H., Wilson L. Identification of a distinct class of vinblastine binding sites on microtubules. J Mol Biol. 1986 Jan 5;187(1):61–73. doi: 10.1016/0022-2836(86)90406-7. [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Thrower D., Wilson L. Effects of vinblastine, podophyllotoxin and nocodazole on mitotic spindles. Implications for the role of microtubule dynamics in mitosis. J Cell Sci. 1992 Jul;102(Pt 3):401–416. doi: 10.1242/jcs.102.3.401. [DOI] [PubMed] [Google Scholar]
- Jordan M. A., Thrower D., Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991 Apr 15;51(8):2212–2222. [PubMed] [Google Scholar]
- Jordan M. A., Toso R. J., Thrower D., Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. A., Wilson L. Kinetic analysis of tubulin exchange at microtubule ends at low vinblastine concentrations. Biochemistry. 1990 Mar 20;29(11):2730–2739. doi: 10.1021/bi00463a016. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Field D. J., Lee L. L. Effects of nocodazole on structures of calf brain tubulin. Biochemistry. 1980 Dec 23;19(26):6209–6215. doi: 10.1021/bi00567a041. [DOI] [PubMed] [Google Scholar]
- Lin C. M., Hamel E. Effects of inhibitors of tubulin polymerization on GTP hydrolysis. J Biol Chem. 1981 Sep 10;256(17):9242–9245. [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D. Direct evidence for GTP and GDP-Pi intermediates in microtubule assembly. Biochemistry. 1990 Sep 25;29(38):8921–8932. doi: 10.1021/bi00490a007. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Microtubule assembly nucleated by isolated centrosomes. Nature. 1984 Nov 15;312(5991):232–237. doi: 10.1038/312232a0. [DOI] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- ROSE G. G., POMERAT C. M., SHINDLER T. O., TRUNNELL J. B. A cellophane-strip technique for culturing tissue in multipurpose culture chambers. J Biophys Biochem Cytol. 1958 Nov 25;4(6):761–764. doi: 10.1083/jcb.4.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelden E., Wadsworth P. Observation and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol. 1993 Feb;120(4):935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoufias D. A., Wilson L. Mechanism of inhibition of microtubule polymerization by colchicine: inhibitory potencies of unliganded colchicine and tubulin-colchicine complexes. Biochemistry. 1992 Jan 28;31(3):738–746. doi: 10.1021/bi00118a015. [DOI] [PubMed] [Google Scholar]
- Tanaka E., Ho T., Kirschner M. W. The role of microtubule dynamics in growth cone motility and axonal growth. J Cell Biol. 1995 Jan;128(1-2):139–155. doi: 10.1083/jcb.128.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toso R. J., Jordan M. A., Farrell K. W., Matsumoto B., Wilson L. Kinetic stabilization of microtubule dynamic instability in vitro by vinblastine. Biochemistry. 1993 Feb 9;32(5):1285–1293. doi: 10.1021/bi00056a013. [DOI] [PubMed] [Google Scholar]
- Vandecandelaere A., Martin S. R., Bayley P. M. Regulation of microtubule dynamic instability by tubulin-GDP. Biochemistry. 1995 Jan 31;34(4):1332–1343. doi: 10.1021/bi00004a028. [DOI] [PubMed] [Google Scholar]
- Vasquez R. J., Gard D. L., Cassimeris L. XMAP from Xenopus eggs promotes rapid plus end assembly of microtubules and rapid microtubule polymer turnover. J Cell Biol. 1994 Nov;127(4):985–993. doi: 10.1083/jcb.127.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigers G. P., Coue M., McIntosh J. R. Fluorescent microtubules break up under illumination. J Cell Biol. 1988 Sep;107(3):1011–1024. doi: 10.1083/jcb.107.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth P., Salmon E. D. Preparation and characterization of fluorescent analogs of tubulin. Methods Enzymol. 1986;134:519–528. doi: 10.1016/0076-6879(86)34117-x. [DOI] [PubMed] [Google Scholar]
- Walczak C. E., Mitchison T. J., Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996 Jan 12;84(1):37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- Walker R. A., Inoué S., Salmon E. D. Asymmetric behavior of severed microtubule ends after ultraviolet-microbeam irradiation of individual microtubules in vitro. J Cell Biol. 1989 Mar;108(3):931–937. doi: 10.1083/jcb.108.3.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., Pryer N. K., Salmon E. D. Dilution of individual microtubules observed in real time in vitro: evidence that cap size is small and independent of elongation rate. J Cell Biol. 1991 Jul;114(1):73–81. doi: 10.1083/jcb.114.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell K. L., Wilson L., Jordan M. A. Mitotic block in HeLa cells by vinblastine: ultrastructural changes in kinetochore-microtubule attachment and in centrosomes. J Cell Sci. 1993 Feb;104(Pt 2):261–274. doi: 10.1242/jcs.104.2.261. [DOI] [PubMed] [Google Scholar]
- Wilson L., Farrell K. W. Kinetics and steady state dynamics of tubulin addition and loss at opposite microtubule ends: the mechanism of action of colchicine. Ann N Y Acad Sci. 1986;466:690–708. doi: 10.1111/j.1749-6632.1986.tb38452.x. [DOI] [PubMed] [Google Scholar]
- Wilson L., Jordan M. A. Microtubule dynamics: taking aim at a moving target. Chem Biol. 1995 Sep;2(9):569–573. doi: 10.1016/1074-5521(95)90119-1. [DOI] [PubMed] [Google Scholar]
- Wilson L., Jordan M. A., Morse A., Margolis R. L. Interaction of vinblastine with steady-state microtubules in vitro. J Mol Biol. 1982 Jul 25;159(1):125–149. doi: 10.1016/0022-2836(82)90035-3. [DOI] [PubMed] [Google Scholar]




