Abstract
We present a novel fully hydrophilic, hydrolytically degradable poly(ethylene glycol) (PEG) hydrogel suitable for soft tissue engineering and delivery of protein drugs. The gels were designed to overcome drawbacks associated with current PEG hydrogels (i.e., reaction mechanisms or degradation products that compromise protein stability): the highly selective and mild cross-linking reaction allowed for encapsulating proteins prior to gelation without altering their secondary structure as shown by circular dichroism experiments. Further, hydrogel degradation and structure, represented by mesh size, were correlated to protein release. It was determined that polymer density had the most profound effect on protein diffusivity, followed by the polymer molecular weight, and finally by the specific chemical structure of the cross-linker. By examining the diffusion of several model proteins, we confirmed that the protein diffusivity was dependent on protein size as smaller proteins (e.g., lysozyme) diffused faster than larger proteins (e.g., Ig). Furthermore, we demonstrated that the protein physical state was preserved upon encapsulation and subsequent release from the PEG hydrogels and contained negligible aggregation or protein-polymer adducts. These initial studies indicate that the developed PEG hydrogels are suitable for release of stable proteins in drug delivery and tissue engineering applications.
Keywords: Protein, Diffusion, PEG hydrogel, Hydrolytic degradation, BSA
Introduction
Hydrogels are especially promising for protein delivery in tissue engineering applications due to their high water content and physical similarity to many native tissues (Lee and Mooney 2001; Bryant SJ 2004). Hydrogels have also been extensively used as drug delivery carriers due to their biocompatibility and ability to improve the pharmacology of proteins without changing their structure and efficacy (Elbert et al. 2001; Satish et al. 2006). Degradable hydrogels in particular are desirable for a variety of applications; for example, tissue engineering scaffolds that can be degraded and remodeled as the cells migrate and synthesize new matrix are thought to allow more successful long-term regeneration (Bryant and Anseth 2002). In cell-based therapies and protein delivery, hydrogel degradation can be used to control the release rate of the delivered component and permit clearance of the device from the body when it is no longer needed (Park et al. 1993). Thus, in recent years degradable gels have been developed to combine the useful properties of hydrogels with various degradation mechanisms.
The demand continues for degradable hydrogels that can be utilized in a general manner for protein delivery or tissue engineering. The most commonly-used degradable polymers, such as copolymers of lactic and glycolic acid (e.g., PLGA), are very useful but are associated with a number of drawbacks including protein denaturation and adsorption due to their hydrophobicity (Crotts et al. 1997; Jiang et al. 2002) as well as inflammation at the site of delivery due to acidic degradation products (Anderson and Shive 1997). Another material used commonly for these purposes is the copolymer of poly(ethylene glycol) and poly(lactic acid) (PEG-PLA) (Molina et al. 2001; Caliceti et al. 2004). Because of the PEG block, this copolymer is mainly hydrophilic but it still contains regions of hydrophobicity and acidic degradation products due to the PLA block. Thus, fully hydrophilic materials with biocompatible degradation products are desired to counteract these negative effects.
Several fully hydrophilic materials have been developed as delivery systems; however, the method of loading protein into these materials limits their use. One way of encapsulating protein within the hydrogels is to add them prior to gelation and in a manner that does not compromise their physical state or lead to covalent incorporation of the protein in the polymer matrix. However, many hydrophilic polymers are cross-linked in a manner that can be potentially harmful for proteins. For example, a great number of hydrogels utilize ultraviolet irradiation of diacrylate groups to achieve a 3D structure and ultraviolet irradiation can potentially harm the physical state of the encapsulated proteins (Peppas and Reinhart 1983; Lu and Anseth 2000). In addition, proteins can bind non-specifically to the gel matrix via Michael-type addition between amino acids in the protein and acrylate and methacrylate groups in the polymers and thus results in incomplete protein release (Mellott et al. 2001). Still other approaches to cross-link gels utilize reactions that non-specifically target amine groups, which are plentiful on proteins (Wacker et al. 2006) or nucleophilic substitution between PEG-carboxylic acids and PEG nucleophiles in which case the protein is coupled to the polymer in an uncontrolled manner and can potentially result in diminished activity (Zhao and Harris 1998). Lastly, gels have been cross-linked with γ-irradiation or high temperatures which may also compromise protein structure and activity (Kamath and Park 1995; Peppas et al. 1999; Moghaddam and Matsuda 2003).
Our materials were designed to circumvent these drawbacks. We present hydrolytically-degradable hydrogels composed of 4-arm PEG-vinyl sulfone (PEG-VS) and PEG-diester-dithiol cross-linkers. The vinyl sulfone groups of the 4-arm PEG-VS react with the thiol groups of the cross-linker via a Michael-type addition reaction pioneered for use in biomaterials by Hubbell et al. (Lutolf and Hubbell 2003); their work utilized predominantly cysteine-terminated matrix metalloproteinase-degradable peptide sequences as cross-linkers which rendered the PEG gels enzymatically degradable. Some limitations are associated with these sequences, such as limited hydrophilicity, acidic products upon cleavage, instability of peptide sequences upon storage, and the expense of fabricating and purifying peptides. Another class of degradable PEG hydrogels utilizing Michael-type addition reaction were also proposed by Hubbell’s group (Elbert et al. 2001; Metters and Hubbell 2005; Wetering et al. 2005). These hydrogels, consisting of PEG-multiacrylate and PEG-dithiol or dithiothreitol (DTT) cross-linkers, were rendered hydrolytically degradable via thioether-ester linkages. However, the degradation of these hydrogels occurred on an order of days to weeks and thus may be unsuitable for applications that would require more immediate release periods such as acute delivery of anti-inflammatory drugs or vaccines.
We describe fully hydrophilic, hydrolytically degradable gels that are not associated with acidic degradation products. The degradation of the materials spans a wide temporal range, from hours to weeks, with the potential for further tuning of the degradation and release characteristics. The degradation products are of sufficiently low molecular weight as to be easily cleared by the body (Yamaoka et al. 1994). Additionally, the cross-linking reaction occurs at physiological conditions which are preferred for protein release in most tissue engineering applications. Furthermore, the Michael-type addition reaction is highly selective as thiols are very rarely unpaired in natural proteins. We characterized the mesh size of our novel degradable PEG gels and the effective diffusivity of model proteins though the system. Further, we demonstrated the versatility of the proposed hydrogels by exploring mechanisms of tuning the rate of protein diffusion and established that the physical state of the proteins was preserved during encapsulation and subsequent release from the hydrogels.
Materials and methods
Synthesis of 4-arm PEG-VS and PEG-diester-dithiol cross-linker
The synthesis of 4-arm PEG-VS was adapted from a previous protocol (Lutolf and Hubbell 2003; Zustiak and Leach 2010) where 4-arm PEG-OH was modified in the presence of excess divinyl sulfone. The product was stored under Ar at −20°C until use. Derivatization was confirmed by 1H NMR (CDCL3): 3.6 ppm (982H, PEG backbone), 6.1 ppm (4H, 1H, =CH2), 6.4 ppm (4H, 1H, =CH2), 6.8 ppm (4H, 1H, -SO2CH=). The typical yield from this procedure was 80–90% and the degree of end group conversion, as shown by NMR, was 93–98%.
The synthesis of the various PEG-diester-dithiol cross-linkers was adapted from previous protocols (Yu et al. 2006; Nie et al. 2007) and is described in detail elsewhere (Zustiak and Leach 2010). The final product was stored under Ar at −20°C until use. Derivatization was confirmed by 1H NMR (CDCl3): 4.27 ppm (4H, -CH2OC(O)-, m), 3.74-3.50 ppm (726H, -CH2CH2O-, s), 2.80-2.69 ppm (8H, -CH2CH2SH, m). The typical yield from this procedure was 70% and the end-group conversion as shown by NMR was 95–98%. The structures and abbreviated names of the PEG polymers are described in Fig. 1 and Table I. The general name PEG-diester-dithiol or the abbreviated names will be used further in the text.
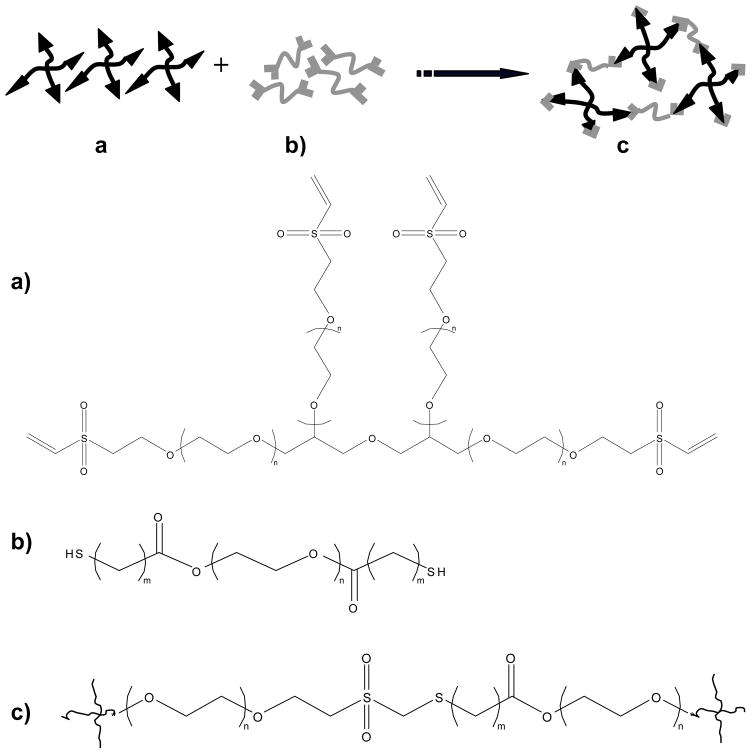
Figure 1. Schematic of the PEG hydrogel cross-linking reaction.
a) Four-arm PEG-VS where n is the number of the repeat PEG unit. b) PEG-diester-dithiol where m = 1 for PEG-dithioglacolate (i.e., one methylene group between the thiol and ester moieties) or m = 2 for PEG-dithiopropionate (i.e., two methylene groups between the thiol and ester moieties. c) Product of cross-linking reaction between PEG-VS and PEG-diester-dithiol.
Table I.
Summary of the abbreviations, characteristics and properties of the PEG-diester-dithiol and non-degradable PEG-dithiol cross-linkers. All of the degradable cross-linkers were synthesized in-house.
| Cross-linker name | Molecular weight (kDa) | Number of methylene groups between thiol and ester moieties | Degradablility |
|---|---|---|---|
| PEG-SH 3.4 | 3.4 | 0 | no |
| PEG-SH 1 3.4 | 3.4 | 1 | yes |
| PEG-SH 2 3.4 | 3.4 | 2 | yes |
| PEG-SH 2 6 | 6 | 2 | yes |
| PEG-SH 2 8 | 8 | 2 | yes |
Formation of PEG-based gels
The gels were formed by a Michael-type addition of PEG-diester-dithiol or non-degradable PEG-dithiol cross-linkers onto 4-arm PEG-VS (Zustiak and Leach 2010). Each polymer precursor was dissolved in a pH 8, 0.3 M triethanolamine (TEA) solution and the ratio of VS:SH was 1:1 for all gels. After mixing, the solution was quickly vortexed and then transferred to the center of a glass slide pre-treated with RainX (Sopus Products, Houston, TX) to provide a hydrophobic surface. Silicone spacers (2.5-mm thick CoverWell chambers, Grace Bio-Labs, Bend, OR) were placed at the ends of the slide and a second hydrophobic slide was placed on top. The slides were then transferred to a humidified 37°C incubator. Gelation occurred in several min but the gels were left in the incubator for 1–2 h to achieve maximum cross-linking.
Diffusion experiments
Three proteins were used in the diffusion studies, namely bovine serum albumin (BSA), lysozyme and bovine γ-globulins (Ig) (Table II). Diffusivity in aqueous solution at 37°C was calculated via the Stokes-Einstein equation (Eq. 1), where rs is the protein Stokes-Einstein hydrodynamic radius, kB is Boltzmann’s constant, T is temperature, η is the viscosity of water at T, and D0is the protein diffusivity in water at T:
| (1) |
Table II.
Properties of proteins used in diffusion studies.
| Protein | Molecular weight (Da) | Stokes’ radius (nm) | Solubility in water at 25°C (mg/ml) | Diffusivity at 37°C in water, Do (10−5 mm2/s) |
|---|---|---|---|---|
| Lysozyme | 14,100 | 1.60 | 10 | 2.17 |
| BSA | 65,000 | 3.48 | 40 | 1.00 |
| Ig | 150,000 | 4.70 | 25 | 0.69 |
In order to assess protein diffusivity, the hydrogels were cross-linked in the presence of protein to achieve a final protein concentration in the gel of 2% w/v. After cross-linking the gels were placed in 15-ml tubes filled with 10 mM phosphate buffer saline (PBS) of pH 7.4 and mixed end-over-end at 37°C. At specified sample collection times, 1 ml of solution was transferred to a microfuge tube and the volume was replaced in the 15-ml tube with fresh PBS. The protein content of each sample was analyzed with the Bio-Rad protein assay using the manufacturer’s microassay procedure.
Calculation of the effective diffusion coefficient
The effective diffusion coefficients were calculated via a modified form of the Fick’s law for short release times (Ritger and Peppas 1987):
| (2) |
where Mi is the concentration of released solute at time i, Minf is the concentration of solute at infinite time, Mi/Minf is the fractional release, De is the effective diffusion coefficient, t is time, δ is half of the hydrogel thickness. From the Eq. 2 it follows that Mi/Minf is directly proportional to t1/2 and a plot of Mi/Minf versus t1/2 would give De. A mass balance was performed to find Mi:
| (3) |
where Ci is the concentration of solute in the release solution at time i, V is the total volume of the release solution (15 ml) and Vs is the sample volume (1 ml). All experiments were performed at room temperature. The measured De was normalized using Do, at 37°C (see Table II). An average De/Do was reported for each solute.
Flory-Rehner calculations for determining hydrogel mesh size
Flory-Rehner calculations were used to determine hydrogel ξ. First, the molecular weight between cross-links (Mc) was calculated by Eq. 4 (Flory 1953):
| (4) |
where is the number-average molecular weight of the un-cross-linked hydrogel, V1 is the molar volume of the solvent (18 cm3/mol for water), ν2 is the polymer volume fraction in the equilibrium swollen hydrogel, which is equal to the reciprocal of QV, v̄ is the specific volume of the polymer (ρs/ρp), and χ1is the polymer-solvent interaction parameter (0.426 for PEG-water (Lu and Anseth 2000; Leach et al. 2003)) and assumed constant for our work becauseχ1 has been found to be nearly independent of PEG ν2 (for ν2= 0.04–0.2 (Merrill et al. 1993)).
To determine ξ, the root-mean-square end to end distance of the polymer chain in the unperturbed state ( ) was calculated using the following equation:
| (5) |
where l is the average bond length (0.146 nm (Cruise et al. 1998)), Cn is the characteristic ratio of the polymer (typically 4.0 for PEG (Merrill et al. 1993; Mellott et al. 2001)) and n is the number of bonds in the cross-link. Mesh size was then calculated by (Canal and Peppas 1989):
| (6) |
Note that degradation times for each experiment were reported based on visual observations and data presented in a previous publication (Zustiak and Leach 2010).
Circular dichroism (CD)
Secondary structure of BSA was measured using a PiStar-180 CD Spectrophotometer (Applied Photophysics, Leatherhead Surrey, UK) with a Xe lamp light source. Other settings were as follows: 1.0 nm bandwidth, 0.2 nm step interval, and 1.5 sec/nm scanning speed. CD spectra in the far UV range (190–260 nm) were obtained using quartz cuvette with a 1-cm path length at 22°C and 10 mM PBS pH 7.4 as a baseline. All gels were 10% w/v made of 4-arm PEG-VS 10 kDa and PEG-SH 2 3.4 cross-linker.
Size exclusion chromatography
To explore the possible effect of cross-linking conditions on protein stability we analyzed the released protein solutions using size exclusion chromatography (SEC) (Leach and Schmidt 2005). An Agilent 1100 (Agilent Technologies, Palo Alto, CA) high performance liquid chromatography system with a DAD diode array detector and a 7.8 mm × 30.0 cm TSK-Gel G3000 column (Tosoh Biosciences Corporation, Montgomeryville, PA) was used. The mobile phase was 100 mM PBS, pH 6.8, the flow rate was 1.0 ml/min, the sample concentrations were 0.03 mg/ml and the sample volume was 100 μl. The effluent was detected using absorbance at 280 nm. Triplicate measurements were made for each sample.
Statistical analysis
The results of all experiments are the mean values (± SD) of triplicate samples performed in three independent experiments. Comparisons between multiple samples were performed with single factor analysis of variance (ANOVA). Comparisons between two samples were performed with two-tailed Student’s t-test and differences between two data sets were considered significant when p< 0.05.
Results
Unless otherwise stated, hydrogels were synthesized with 10 kDa 4-arm PEG-VS and PEG-SH 2 3.4 and a total polymer density of 10% (w/v).
Mesh size
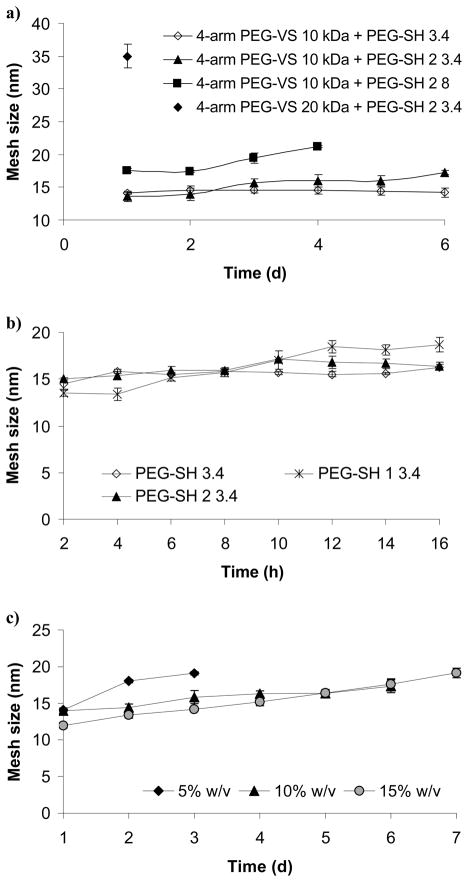
Fig. 2a shows the influence of the polymer molecular weight on ξ. For the control hydrogels made with the non-degradable cross-linker (PEG-SH 3.4), ξ remained unchanged during the course of the experiment but ranged from ~14 nm for gels made of 4-arm PEG-VS 10 kDa + PEG-SH 2 3.4 (at 1 d) to ~35 nm for gels made of 4-arm PEG-VS 20 kDa + PEG-SH 2 3.4 (at 1 d). For all degradable hydrogels ξ was measured until ~75% degradation was reached (1–6 d) when the hydrogels became too weak and hard to handle. Only one data point is presented for gels made of 4-arm PEG-VS 20 kDa + PEG-SH 2 3.4 as they degraded shortly after the 1 d time point.
Figure 2.
Influence of the following parameters on PEG hydrogel mesh size: a) polymer molecular weight, b) cross-linker’s number of methylene groups between the ester and the thiol moieties, c) polymer density. Open symbols note non-degradable gels; shaded or × symbols note degradable gels. Unless otherwise stated, hydrogels were synthesized with 10 kDa 4-arm PEG-VS and PEG-SH 2 3.4 and a total polymer density of 10% (w/v).
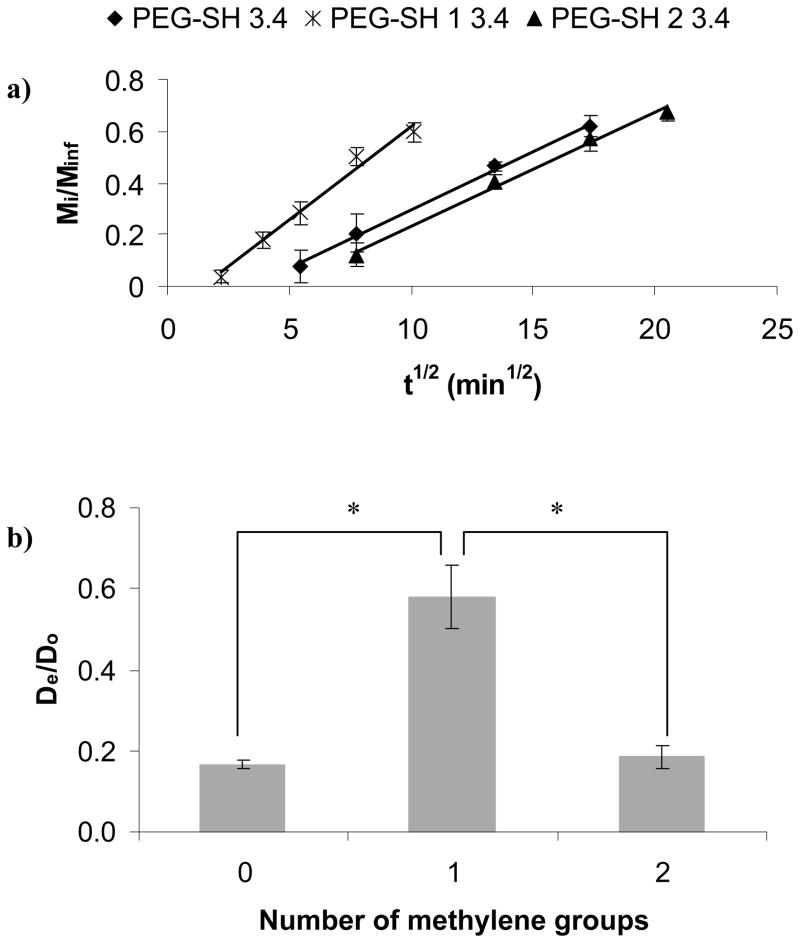
Fig. 2b shows the effect of the number of methylene groups between the ester and the thiol of the cross-linker on ξ. The hydrogels synthesized with PEG-SH 1 3.4 degraded at 16 h, but the other gels (PEG-SH and PEG-SH 2 3.4) remained intact >16h (as shown in Fig 2a). At 2 h, ξ of the hydrogels made with PEG-SH 1 3.4 was 13.5 ± 0.3 nm, which was 9% lower than the ξ of the gels made with PEG-SH 2 3.4 or PEG-SH 3.4. At 16 h, ξ of the hydrogels made with PEG-SH 1 3.4 was 18.6 ± 0.7 nm which was 12% higher than the ξ of the gels made with PEG-SH 2 3.4 or PEG-SH 3.4.
Fig. 2c shows the effect of polymer density on hydrogel ξ. We observed that initially (until day 3) ξ decreased with an increase in polymer density. The 15% w/v hydrogels exhibited the highest total change (37%) in ξ, from 11.9 ± 0.3 nm at day 1 to 19.1 ± 0.7 nm at day 7.
Effect of protein size on diffusion
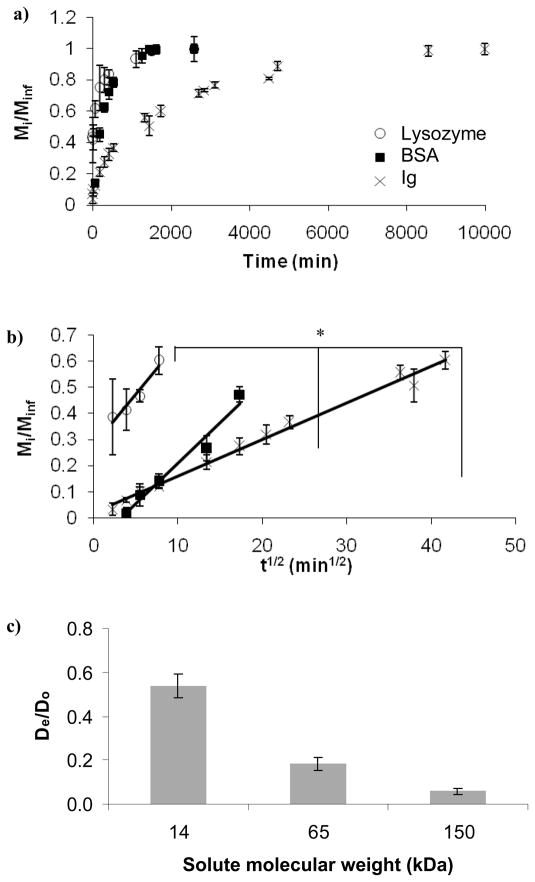
Fig. 3a shows the fractional release as a function of time for three proteins of various sizes (see Table II). It can be seen from the data that the smallest protein lysozyme was released within the first 18 hr while the largest protein Ig was released completely in less than 7 d (coincident with the complete degradation of the gel). Fig. 3b shows the protein fractional release, Mi/Minf, as a function of t1/2. According to Eq. 2, for short diffusion times (Mi/Minf < 0.6), this relationship should yield a line with a slope proportional to De. We can see that the release rate increased with a decrease in the protein size: the release rate for lysozyme was the highest, followed by BSA, and lastly by Ig. We observed that lysozyme, the smallest protein, had the highest De, ~50% of that in water. Ig, the largest protein, had the lowest De, only ~6% of that in water (Fig. 3c).
Figure 3.
Effect of protein size on protein diffusion through the PEG hydrogels: a) fractional release b) fractional release where Mi/Minf < 0.6 (R2=0.98 for Ig, R2=0.97 for BSA, and R2=0.93 for lysozyme), b) normalized diffusion coefficients. The asterisk indicates significant difference (p<0.05).
Effect of material properties on diffusion
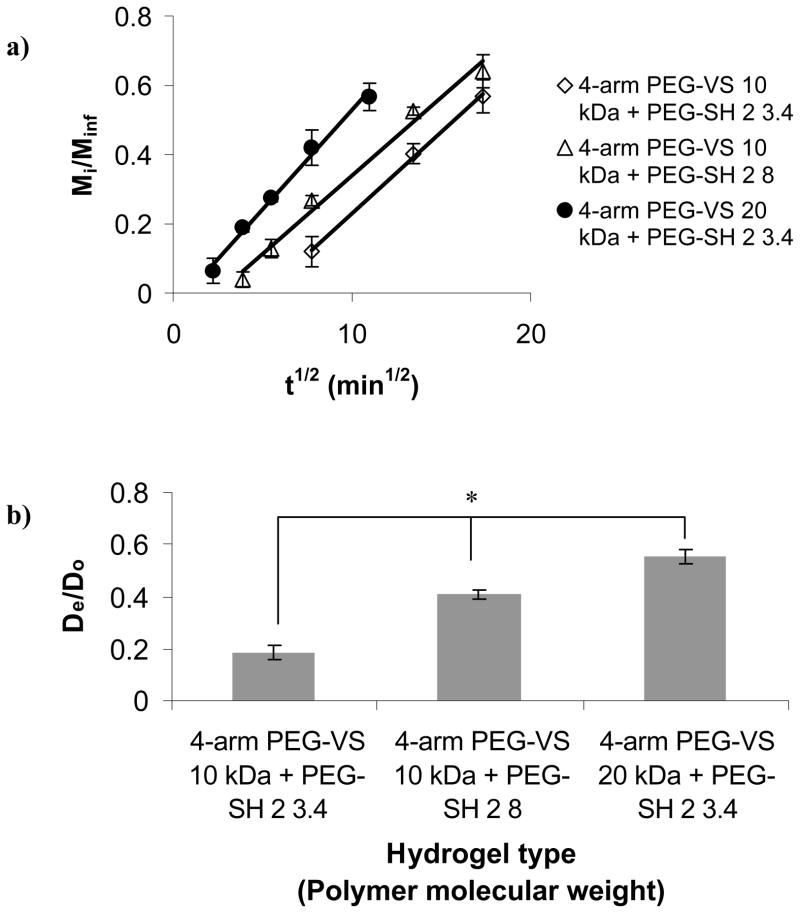
Fig. 4a shows the fractional release of BSA from gels made with cross-linkers or 4-arm PEG-VS with increasing molecular weight, as a function of t1/2. The data revealed that Mi/Minf = 0.6 occurred at the longest time, 330 min, for gels made of 4-arm PEG-VS 10 kDa + PEG-SH 2 3.4, followed by 260 min for the gels made of 4-arm PEG-VS 10 kDa + PEG-SH 2 8, and 190 min for the gels made of 4-arm PEG-VS 20 kDa + PEG-SH 2 3.4. Hence, the release rate was faster for gels made with the higher molecular weight cross-linker PEG-SH 2 8 vs gels made with the lower molecular weight cross-linker PEG-SH 2 3.4. It was also faster for gels made with the 20 kDa 4-arm PEG-VS as opposed to the 10 kDa 4-arm PEG-VS. From the data on Fig. 4a we determined De/Do as shown in Fig. 4b. Both figures show that BSA diffusivity was directly proportional to polymer molecular weight.
Figure 4.
Effect of molecular weight of cross-linker and 4-arm PEG-VS on protein diffusion through the PEG hydrogels: a) fractional release (R2=0.99 for all gel types), b) normalized diffusion coefficients for BSA. The asterisk indicates significant difference (p<0.05).
Fig. 5a shows Mi/Minf of BSA for gels made with cross-linkers of increasing number of methylene groups between the thiol and the ester as a function of t1/2. The data showed that 60% of the BSA release occurred at the longest time, 330 min, for gels made with PEG-SH 2 3.4, followed by 280 min for PEG-SH 3.4, and 100 min for PEG-SH 1 3.4. Hence, the release rate was faster for hydrogels made with the cross-linker with a lower number of methylene groups between the thiol and the ester. From Fig. 5a we determined De/Do as shown in Fig. 5b. Again, the linear relationship between Mi/Minf and t1/2 for short release times supported the diffusion release mechanism described in Eq. 2. However, for gels made with PEG-SH 1 3.4 that degraded in several h, we assumed that the release was controlled predominantly by diffusion but was also affected by gel degradation. The assumption was supported by the 70% higher BSA De/Do as compared to that of BSA in the slow-degrading gel made with PEG-SH 2 3.4. There was no significant difference between the BSA De/Do in gels made with the control PEG-SH 3.4 or the PEG-SH 2 3.4 cross-linker.
Figure 5.
Effect of number of methylene groups between the ester and the thiol moieties of the cross-linker on protein diffusion through the PEG hydrogels: a) fractional release (R2=0.99 for all gel types), b) normalized diffusion coefficients for BSA. The asterisks indicate significant differences (p<0.05).
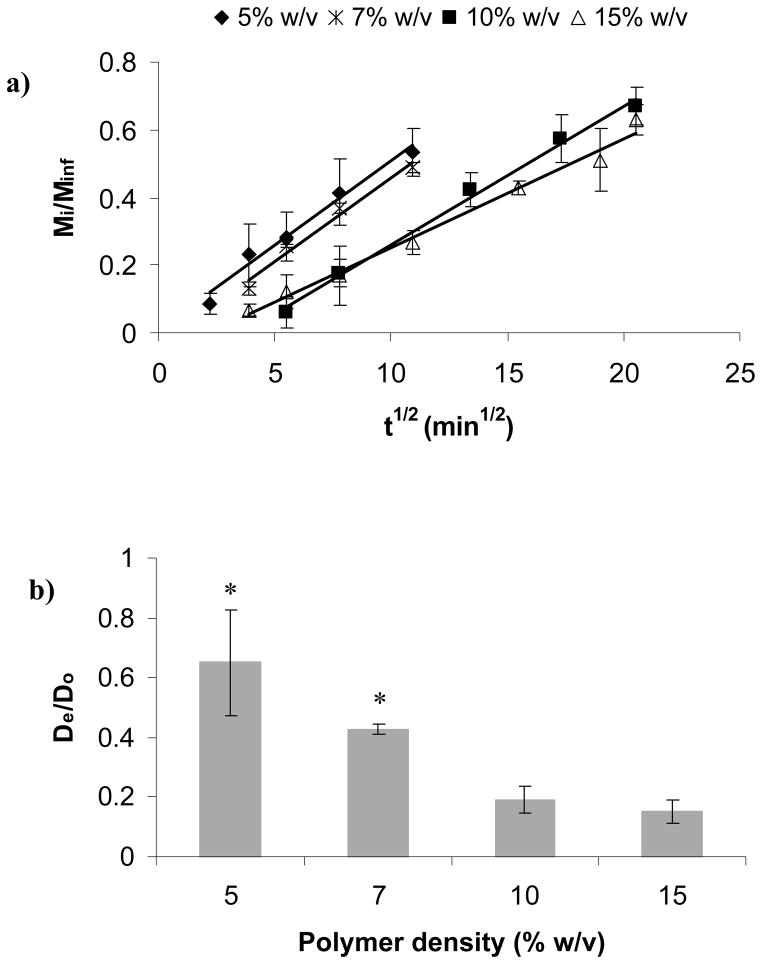
Fig. 6a shows the fractional release of BSA for different polymer density gels as a function of t1/2. Again, the linear relationship between Mi/Minf and t1/2 for short release times supported the Fickian diffusion mechanism. The data also revealed that Mi/Minf = 0.6 occurred at the longest time, 400 min, for the 15% w/v gels, followed by 330 min for 10% w/v gels, 120 min for 7% w/v gels and 110 min for 5% gels. From Fig. 6a we determined De/Do (Fig. 6b) observing that BSA normalized diffusivity was inversely proportional to polymer density.
Figure 6.
Effect polymer density on protein diffusion through the PEG hydrogels: a) fractional release (R2=0.98 for 5% w/v gel; R2= 0.99 for 7%, 10%, and 15% w/v gels), b) normalized diffusion coefficients for BSA. The asterisks indicate significant differences (p<0.05).
Analysis of the physical state of the released BSA
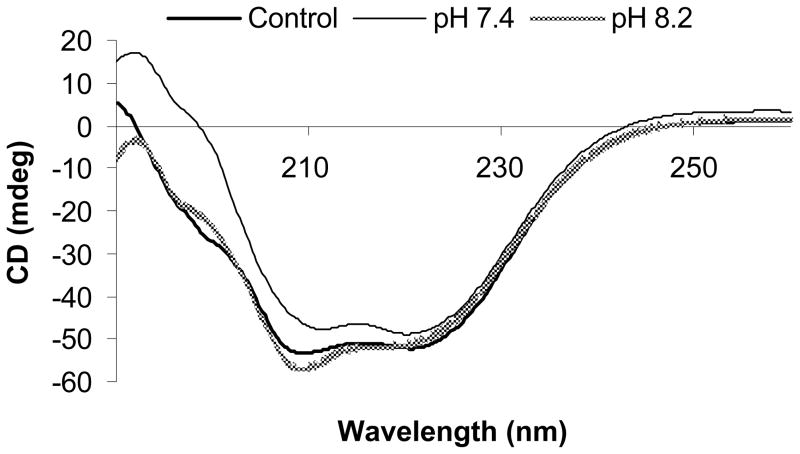
We used CD to compare the secondary structures of BSA prior to encapsulation (control) to BSA after it had been released (Fig. 7). No major differences or spectral shifts were observed between the spectra of the three samples; thus, we concluded that the secondary structure of BSA remained intact upon release from the gel and was not affected by the pH during gelation in the range studied.
Figure 7.
Effect of the encapsulation pH on the BSA secondary structure as measured via circular dichroism (CD). BSA dissolved in TEA pH 8.2 was compared to a control of BSA dissolved in PBS, pH 7.4. A TEA solution of pH 7.4 was also examined to determine the effect of PBS vs TEA (constant pH) (n = 3).
In addition, to test for possible protein aggregation, SEC was used to compare BSA molecular weight prior to encapsulation and after it was released from PEG hydrogels made with PEG-SH 2 3.4 (Table III). The BSA in each release solution contained more than 90% monomer while the BSA standard contained 83.6 ± 1.7% monomer. Therefore, the released BSA was enriched in monomer as compared to the control BSA. There was no significant difference between the % monomer in BSA released at 6 h and at complete hydrogel degradation.
Table III.
SEC analysis of the physical state of released BSA. Protein was encapsulated within the PEG hydrogels prior to gelation; the supernatant containing BSA released from the hydrogels was analyzed using SEC for monomeric content.
| Sample type | Time (h) | % monomer |
|---|---|---|
| BSA released from PEG hydrogel | 6 | 94.34 ± 1.44 |
| BSA released from PEG hydrogel | 72 (complete degradation) | 93.20 ± 0.61 |
| BSA control in PBS | 83.58 ± 1.70 | |
| BSA control in TEA, pH 7.4 | 85.30 ± 0.68 | |
| BSA control in TEA, pH 8.2 | 86.58 ± 0.20 |
Discussion
The diffusion of three model proteins, namely lysozyme, BSA and Ig, were chosen for this study for several reasons: 1) their physical properties are well characterized (Peters 1996), 2) they all have rs smaller than the calculated ξ of the hydrogels, and 3) they cover a large range of sizes. If the protein release is to be controlled by the degradation of the polymer, the comparison of protein size and ξ is very important; for example, in cases where initial gel ξ is much larger than the protein to be released, the protein diffuses out without significant influence from gel degradation. The release is influenced primarily by the hydrogel degradation when the protein size is similar or larger than the initial gel ξ. Thus, control of ξ is very important in the design of controlled release devices and scaffolds for tissue engineering applications, and for this reason, parameters that influence degradation, ξ and diffusivity were investigated in this study. All hydrogels in the study were hydrolytically degradable; hence the changes in ξ and diffusivity were monitored over time. A non-degradable control was also characterized for comparison.
The ξ and protein release were controlled by three major strategies: the molecular weight of the PEG polymer, the number of methylene groups between the ester and the thiol of the cross-linker, and the polymer density. We confirmed the findings of similar studies (Bell and Peppas 1996; Cruise et al. 1998; Lu and Anseth 2000) that all the chosen strategies were efficient in controlling ξ as increase in molecular weight of the PEG polymer, decrease in the number of methylene groups between the thiol and the ester of the cross-linker, and decrease in polymer density all lead to an overall increase in ξ as well as increase in the rate of change of ξ (Fig. 2). However, the number of methylene groups between the ester and the thiol of the cross-linker had the largest effect on ξ. To explain this result we first must note that the number of methylene groups had a profound effect on hydrogel degradation (gels with only 1 methylene group degraded in 16 h as opposed to gels with 2 methylene groups that degraded in 6 d). As the cross-linkers used in forming the hydrogel were degradable via an ester bond incorporated in the polymer backbone, the presence of water causes the ester bond to hydrolyze, resulting in breakage of a cross-link and increase in ξ. As cross-linkers with esters in close proximity to thiols hydrolyze more quickly than those with greater separation between the ester and thiol (Schoenmakers et al. 2004; Rydholm et al. 2007; Zustiak and Leach 2010), a more rapid change in ξ would be observed for gels made with fewer methylenes between the ester and thiol. In all cases, increase in ξ enhanced the diffusion of proteins not only initially but also further upon degradation.
Based on degradation stage and gel type, ξ varied from ~13 to ~35 nm. All proteins in this study had a smaller rs than the calculated ξ; therefore we assumed that all of the proteins had unobstructed diffusion in the gel. Even so, protein size proved to be an important factor in controlling diffusivity. The protein release rate was inversely proportional to protein size and was more sensitive to physical size rather than other protein properties such as charge, which was an expected result as PEG is inert (Fig. 3a). We observed that the smallest protein, lysozyme, with a diameter that was several orders of magnitude smaller than the mesh size of the PEG gel, was completely released in less than 24 h. Since the gel degradation was negligible in this time frame (Fig. 2b), the release of lysozyme was guided by simple diffusion. On the other hand, the largest protein, Ig, with a diameter less than an order of magnitude smaller than the initial mesh size of the gel, exhibited a prolonged release over several days indicating that the release was aided by gel degradation as well as diffusion. Release of this protein was steady and did not display a burst at complete gel degradation signifying the potential use of the presented gels for sustained release of large protein molecules in drug delivery or tissue engineering applications.
Note that Fig. 3b shows that the lysozyme release profiles start with a burst at early times (<200 min). Since the size of lysozyme was relatively small compared to ξ, lysozyme diffused freely and a higher initial protein concentration gradient may have contributed to the burst effect. Other factors that may have added to this burst include: 1) lysozyme molecules that were near the solvent/gel interface and escaped rapidly in the surrounding solution, and 2) faster release of lysozyme through larger pores of the gel compared to slower release from pores obstructed by polymer entanglements.
Based on Fig. 3c we observed that the application of the commonly used Fickian model (Eq. 2) resulted in De significantly different from Do (~94% for Ig). The model assumes: a) unobstructed diffusion of small solutes inside the gel, b) infinite dilution of the solute in the supernatant, and c) diffusion inside the gel based solely on Brownian motion. Nevertheless, Eq. 2 is a good approximation even when not all the assumptions are met and has been widely used for calculating diffusivities in non-ideal gel systems (Leach and Schmidt 2005; Koutsopoulos et al. 2009). The deviation of experimental results from the model, however, would increase as the size of the protein approaches ξ, as was the case of Ig which has a hydrodynamic diameter of 9.4 nm (~ 70% of the average ξ, 14 nm). Even small proteins such as lysozyme (3.2 nm diameter) were obstructed by the polymer chains which resulted in ~50% decrease in De. Furthermore, even though we assumed a homogeneous matrix, it is possible that there were some non-ideal physical entanglements in the PEG chains which additionally decreased ξ or non-specific protein/polymer interactions that contributed to decreased De (Zustiak et al. 2010). Also note that the initial concentration of all proteins inside the gels was 2% w/v; hence, protein crowding may have affected diffusion by slowing molecular motion. The rationale for using a relatively high concentration of protein was to resemble application wherein a delivery vehicle or 3D scaffold would carry a high load of bioactive molecules.
Next, we aimed to identify simple and effective ways to control protein release. We saw that at initial time points (< 16 h), degradation of the gel was negligible, and protein diffusion as represented by Mi/Minf, was correlated to ξ (Peppas et al. 1999; Tsunomori and Ushiki 1999). This relationship can be explained by Eq. 2 in conjunction with Eq. 7 which applies to neutral uncharged polymers such as PEG (Lustig and Peppas 1988):
| (7) |
This equation suggests that protein diffusivity, as calculated from Mi/Minf as per Eq.2, is dependent on ξ and will decrease with increase in solute size. As expected, based on the results from Fig. 2 and the dependence of protein diffusion on ξ, an increase in BSA diffusivity was associated with increased polymer molecular weight, decreased number of methylene groups between the thiol and the ester of the cross-linker, and decreased polymer density (Fig. 4, 5 and 6). Similar trends have been noted previously for various degradable PEG polymers (West and Hubbel 1995; Jeong et al. 2000; Schoenmakers et al. 2004). In all of the above cases we saw that the diffusion data for Mi/Minf < 0.6 was well explained by the Fickian diffusion model (Eq. 2). This finding suggested that initial protein release was guided mainly by diffusion. However, in the case of the fast-degrading hydrogels made with PEG-SH 1 3.4, we observed a ~3-fold increase in BSA De/Do as compared to the slow-degrading gels made with PEG-SH 2 3.4. Therefore, we speculate that bulk degradation of the hydrogel (due to the hydrophilic nature of the PEG polymer (Sawhney et al. 1993)) and subsequent increases in gel water content and ξ were partially responsible for this result.
It has been shown that encapsulating protein in hydrogels prior to gelation may affect protein stability and structure (Morlock et al. 1997; Eggers and Valentine 2001; Wetering et al. 2005). The effects include but are not limited to various types of degradation such as denaturation, aggregation, hydrolysis, and reaction with the cross-linkers which could lead to polymer-protein adducts. Such effects have the potential to decrease protein activity after release and limit the usefulness of such a release device. Thus, we explored the outcome of encapsulation during gelation on the physical state of BSA using CD and SEC. We examined TEA solutions of pH 7.4 and 8.2 as aggregation of BSA is usually attributed to thiol-disulfide interchange in a basic environment (Zhu and Schwendeman 2000; Maruyama et al. 2001). Our CD experiments (Fig. 7) indicated that the predominantly α-helical structure of BSA (Maruyama et al. 2001; Abeywickrama et al. 2007; Watkins et al. 2008) was preserved independently of pH during gelation as well as after release from the gels.
We also determined via SEC (Table III) that BSA did not aggregate upon release from the gel but was instead enriched in monomer solution which was also observed for hyaluronic acid-polyethylene glycol gels (Leach and Schmidt 2005). Furthermore, there was no significant difference between the % monomer in BSA released at 6 h and at complete gel degradation which suggested that the enriched monomeric content in the released solution was not due to larger aggregates being retained inside the gel. Small fragments were not detected which indicated that hydrolyzed BSA was not released. Polymer-BSA adducts were also not detected in the release solution which confirmed that BSA did not react with PEG during the cross-linking reaction as we and others have shown previously (Elbert et al. 2001; Zustiak et al. 2010). Lastly, we concluded that neither TEA nor pH had an effect on BSA aggregation under the examined conditions. This was an important result as gelation time can be controlled effectively by the pH of the gelation solution and a change in pH from 7 to 8 lead to a ~ 3-fold increase in gelation time (Elbert et al. 2001; Zustiak and Leach 2010). Control of gelation time has implications in control of the protein environment and minimization of exposure to conditions which over a long time period have the potential to lead to unwanted side reactions between the polymer and protein of interest.
Conclusions
In the present work we report the development of novel hydrolytically degradable PEG hydrogels suitable for protein drug delivery applications. Protein release from the developed hydrogels was examined in detail and related to gel mesh size, which spanned a range of ~13 nm to ~35 nm depending on gel type and degradation stage. The effects of both protein size and hydrogel structure on protein diffusivity were examined. We confirmed that protein diffusivity was related to protein size as smaller proteins (e.g., lysozyme) diffused faster than larger proteins (e.g., Ig). We also determined that polymer density had the most profound effect on protein diffusivity, followed by molecular weight of the polymer, and finally by the chemical structure of the cross-linker represented by the number of methylene groups between the thiol and ester moieties. Additionally, we confirmed that the BSA secondary structure was preserved during the encapsulation process and subsequent release and that BSA was almost entirely released in the native monomeric form. These hydrolytically degradable PEG gels thus overcome the drawbacks of compromised protein stability associated with current PEG hydrogels and therefore these initial studies indicate that the novel PEG hydrogels are a reliable and tunable system for release of stable proteins in drug delivery and soft tissue engineering applications.
Acknowledgments
We thank Rohan Durbal for technical assistance as well as Ben Keshet, Theresa Good and William Leach for helpful discussions and use of their CD and SEC systems. This work was supported by NIH-NINDS (R01NS065205), the Henry Luce Foundation and UMBC.
References
- Abeywickrama C, Matsuda H, Jockusch S, Zhou J, Jang YP, Chen BX, Itagaki Y, Erlanger BF, Nakanishi K, Turro NJ, Sparrow JR. Immunochemical recognition of A2E, a pigment in the lipofuscin of retinal pigment epithelial cells. Proc Natl Acad Sci U S A. 2007;104:14610–14615. doi: 10.1073/pnas.0706806104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Advanced Drug Delivery Reviews. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- Bell CL, Peppas NA. Water, solute and protein diffusion in physiologically responsive hydrogels of poly (methacrylic acid-g-ethylene glycol) Biomaterials. 1996;17:1203–1218. doi: 10.1016/0142-9612(96)84941-6. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- Bryant SJBR, Durant KL, Anseth KS. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol Bioeng. 2004;86:747–755. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- Caliceti P, Salmaso S, Elvassore N, Bertucco A. Effective protein release from PEG/PLA nano-particles produced by compressed gas anti-solvent precipitation techniques. J Control Release. 2004;94:195–205. doi: 10.1016/j.jconrel.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Canal T, Peppas NA. Correlation between mesh size and equilibrium degree of swelling of polymeric networks. J Biomed Mater Res. 1989;23:1183–1193. doi: 10.1002/jbm.820231007. [DOI] [PubMed] [Google Scholar]
- Crotts G, Sah H, Park tG. Adsorption determines in-vitro protein release rate from biodegradable microspheres: quantitative analysis of surface area during degradation. J Control Release. 1997;47:101–111. [Google Scholar]
- Cruise GM, Scharp DS, Hubbell JA. Characterization of permeability and network structure of interfacially photopolymerized poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 1998;19:1287–1294. doi: 10.1016/s0142-9612(98)00025-8. [DOI] [PubMed] [Google Scholar]
- Eggers DK, Valentine JS. Molecular confinement influences protein structure and enhances thermal protein stability. Protein Sci. 2001;10:250–261. doi: 10.1110/ps.36201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert DL, Pratt AB, Lutolf MP, Halstenberg S, Hubbell JA. Protein delivery from materials formed by self-selective conjugate addition reactions. J Control Release. 2001;76:11–25. doi: 10.1016/s0168-3659(01)00398-4. [DOI] [PubMed] [Google Scholar]
- Flory PJ. Principles of Polymer Chemistry. Cornell University Press; Ithaca, NY: 1953. [Google Scholar]
- Jeong B, Bae YH, Kim SW. Drug release from biodegradable injectable thermosensitive hydrogel of PEG-PLGA-PEG triblock copolymers. J Control Release. 2000;63:155–163. doi: 10.1016/s0168-3659(99)00194-7. [DOI] [PubMed] [Google Scholar]
- Jiang G, Woo BH, Kang F, Singh J, DeLuca PP. Assessment of protein release kinetics, stability and protein polymer interaction of lysozyme encapsulated poly(D,L-lactide-co-glycolide) microspheres. J Control Release. 2002;79:137–145. doi: 10.1016/s0168-3659(01)00533-8. [DOI] [PubMed] [Google Scholar]
- Kamath KR, Park K. Study on the release of invertase from enzymatically degradable dextran hydrogels. Polymer Gels and Networks. 1995;3:243–254. [Google Scholar]
- Koutsopoulos S, Unsworth LD, Nagai Y, Zhang S. Controlled release of functional proteins through designer self-assembling peptide nanofiber hydrogel scaffold. Proc Natl Acad Sci U S A. 2009;106:4623–4628. doi: 10.1073/pnas.0807506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach JB, Bivens KA, Patrick CW, Jr, Schmidt CE. Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol Bioeng. 2003;82:578–589. doi: 10.1002/bit.10605. [DOI] [PubMed] [Google Scholar]
- Leach JB, Schmidt CE. Characterization of protein release from photocrosslinkable hyaluronic acid-polyethylene glycol hydrogel tissue engineering scaffolds. Biomaterials. 2005;26:125–135. doi: 10.1016/j.biomaterials.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Lee KY, Mooney DJ. Hydrogels for tissue engineering. Chem Rev. 2001;101:1869–1879. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- Lu S, Anseth KS. Release behavior of high molecular weight solutes from poly(ethylene glycol)-based degradable networks. Macromolecules. 2000;33:2509–2515. [Google Scholar]
- Lustig SR, Peppas N. Solute diffusion in swollen membranes. IX. Scaling laws for solute diffusion in gels. Journal of Applied Polymer Science. 1988;36:735–747. [Google Scholar]
- Lutolf MP, Hubbell JA. Synthesis and pysiochemical characterization of end-linked poly(ethylene glycol)-co-peptide hydrogels formed by Michael-type addition. Biomacromolecules. 2003;4:713–722. doi: 10.1021/bm025744e. [DOI] [PubMed] [Google Scholar]
- Maruyama T, Katoh S, Nakajima M, Nabetani H. Mechanism of bovine serum albumin aggregation during ultrafiltration. Biotechnol Bioeng. 2001;75:233–238. doi: 10.1002/bit.10001. [DOI] [PubMed] [Google Scholar]
- Mellott MB, Searcy K, Pishko MV. Release of protein from highly cross-linked hydrogels of poly(ethylene glycol) diacrylate fabricated by UV polymerization. Biomaterials. 2001;22:929–941. doi: 10.1016/s0142-9612(00)00258-1. [DOI] [PubMed] [Google Scholar]
- Merrill EW, Dennison KA, Sung C. Partitioning and diffusion of solutes in hydrogels of poly(ethylene oxide) Biomaterials. 1993;14:1117–1126. doi: 10.1016/0142-9612(93)90154-t. [DOI] [PubMed] [Google Scholar]
- Metters AT, Hubbell JA. Network formation abd degradation behavior of hydrogels formed by Michael-type addition reactions. Biomacromolecules. 2005;6:290–301. doi: 10.1021/bm049607o. [DOI] [PubMed] [Google Scholar]
- Moghaddam MJ, Matsuda T. Molecular design of three-dimensional artificial extracellular matrix: photosensitive polymers containing cell adhesive peptide. J Polym Sci A: Polym Chem. 2003;31:1589–1597. [Google Scholar]
- Molina I, Li S, Martinez MB, Vert M. Protein release from physically crosslinked hydrogels of the PLA/PEO/PLA triblock copolymer-type. Biomaterials. 2001;22:363–369. doi: 10.1016/s0142-9612(00)00192-7. [DOI] [PubMed] [Google Scholar]
- Morlock M, Koll H, Winter G, Kissel T. Microencapsulation of rh-erythropoietin, using biodegradable poly(d, l-lactide-co-glycolide): protein stability and the effects of stabalizing excipients. Eur J Pharm Biopharm. 1997;43:29–36. [Google Scholar]
- Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122:287–296. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Shalaby WSW, Park H. Biodegradable hydrogels for drug delivery. Technomic Publishing; Lancaster, PA: 1993. [Google Scholar]
- Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing hydrogels in drug delivery. J Control Release. 1999;62:81–87. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Reinhart CT. Solute diffusion in swollen membranes. I: A new theory. J Membrane Sci. 1983;15:275–287. [Google Scholar]
- Peters T. All about albumin: biochemistry, genetics, and medical applications. Academic Press; San Diego, CA: 1996. [Google Scholar]
- Ritger PL, Peppas NA. A simple equation for description of solute release: I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or disks. J Control Release. 1987;5:23–36. [PubMed] [Google Scholar]
- Rydholm AE, Anseth KS, Bowman CN. Effects of neighboring sulfides and pH on ester hydrolysis in thiol-acrylate photopolymers. Acta Biomater. 2007;3:449–455. doi: 10.1016/j.actbio.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satish CS, Satish KP, Shivakumar HG. Hydrogels as controlled drug delivery systems: synthesis, crosslinking, water and drug transport mechanisms. Indian J Pharm Sci. 2006;68:133–140. [Google Scholar]
- Sawhney AS, Pathak CP, Hubbell JA. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(a-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581–587. [Google Scholar]
- Schoenmakers RG, van de Wetering P, Elbert DL, Hubbell JA. The effect of the linker on the hydrolysis rate of drug-linked ester bonds. J Control Release. 2004;95:291–300. doi: 10.1016/j.jconrel.2003.12.009. [DOI] [PubMed] [Google Scholar]
- Tsunomori F, Ushiki H. Pore size effect on diffusion coefficient of rhodamine B in PNIPA gel. Physics Letters A. 1999;258:171–176. [Google Scholar]
- Wacker BK, Scott EA, Kaneda MM, Alford SK, Elbert DL. Delivery of sphingosine 1-phosphate from poly(ethylene glycol) hydrogels. Biomacromolecules. 2006;7:1335–1343. doi: 10.1021/bm050948r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins JD, Campbell GR, Halimi H, Loret EP. Homonuclear 1H NMR and circular dichroism study of the HIV-1 Tat Eli variant. Retrovirology. 2008;5:83. doi: 10.1186/1742-4690-5-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JL, Hubbel JA. Photopolymerized hydrogel materials for drug delivery applications. Reactive Polymers. 1995;25:139–147. [Google Scholar]
- Wetering Pvd, Metters AT, Schoenmakers RG, Hubbell JA. Poly(ethylene glycol) Hydrogels Formed by Conjugate Addition with Controllable Swelling, Degradation, and Release of Pharmaceutically Active Proteins. J Control Release. 2005;102:619–627. doi: 10.1016/j.jconrel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Yamaoka T, Tabata Y, Ikada Y. Distribution and tissue uptake of poly(ethylene glycol) with different molecular weights after intravenous administration to mice. J Pharm Sci. 1994;83:601–606. doi: 10.1002/jps.2600830432. [DOI] [PubMed] [Google Scholar]
- Yu H, Feng ZG, Zhang AY, Sun LG, LQ Synthesis and characterization of three-dimensional crosslinked networks based on self-assemly of alpha-cyclodextrins with thiolated 4-arm PEG using a three-step oxidation. Soft Matter. 2006;2:343–349. doi: 10.1039/b517206c. [DOI] [PubMed] [Google Scholar]
- Zhao H, Harris JM. Novel degradable Poly(ethylene glycol) hydrogels for controlled release of protein. J Pharm Sci. 1998;87:1450–1458. doi: 10.1021/js980065o. [DOI] [PubMed] [Google Scholar]
- Zhu G, Schwendeman SP. Stabilization of proteins encapsulated in cylindrical poly(lactide-co-glycolide) implants: mechanism of stabilization by basic additives. Pharm Res. 2000;17:351–357. doi: 10.1023/a:1007513425337. [DOI] [PubMed] [Google Scholar]
- Zustiak SP, Boukari H, Leach JB. Solute diffusion and interactions in cross-linked poly(ethylene glycol) hydrogels studied by fluorescence correlation spectroscopy. Soft Matter. 2010;6:3609–3618. doi: 10.1039/C0SM00111B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zustiak SP, Leach JB. Hydrolytically degradable poly(ethylene glycol) hydrogel scaffolds with tunable degradation and mechanical properties. Biomacromolecules. 2010;11:1348–1357. doi: 10.1021/bm100137q. [DOI] [PMC free article] [PubMed] [Google Scholar]