Abstract
Oplopanax horridus is used as a folk medicine by natives in the Northern Pacific coast of North America. This experiment studied the anti-proliferative effects of the extract of O. horridus root bark and its fractions chromatographed from Dianion HP20 resin column with water, 30, 50, 70 and 100% ethanol on human breast cancer MCF-7 cells and non-small cell lung cancer (NSCLC) cells. The role of O. horridus in the cell cycle and apoptosis of MCF-7 cells was also investigated. The results showed that the 70% and 100% ethanol fractions demonstrated more potent anti-proliferative effects than the total extract on both cell lines. The anti-proliferative effects may result from the enrichment of active constituents detected by the HPLC. The IC50 of the total extract, 50, 70, and 100% ethanol fractions for anti-proliferation on MCF-7 cells were 248.4, 123.1, 44.0, and 31.5 μg/mL, respectively, and on NSCLC cells were 125.3, 271.1, 17.6, and 23.2 μg/mL, respectively. On the other hand, the water and 30% ethanol fractions significantly promoted cell proliferation on MCF-7 cells at concentrations > 100 μg/mL, suggesting that the hydrophilic fractions should be removed from the extract when used for cancer chemoprevention in order to achieve desirable activities. The effects of the total extract on cell cycle and apoptosis were similar to that of the 100% ethanol fraction because of the similarity of their chemical composition. At higher concentrations, the apoptotic effects of the 70% ethanol fraction are more significant. Data from this study suggested that the 70% and 100% ethanol fractions are active anti-proliferative fractions and that induction of apoptosis is the mechanism involved in the anti-proliferative effect observed.
Keywords: Oplopanax horridus, anti-proliferative effect, human breast cancer MCF-7 cells, non-small cell lung cancer (NSCLC) cells, apoptosis, cell cycle
INTRODUCTION
Ginsengs, which belong to the family Araliaceae, are a group of perennial aromatic herbs widely used in Oriental medicine and include Asian ginseng (Panax ginseng), North American ginseng (P. quinquefolius) and notoginseng (P. notoginseng) (Wang et al., 2009; Wang and Yuan, 2008). Oplopanax (Echinopanax) is a small genus also belonging to the family Araliaceae. In this genus, there are only three species whose ranges are rather narrow. O. elatus grows in the southern regions of Primorye, northeastern China, and north of the Korean Peninsula; O. japonicus in Japan; and O. horridus (Devil’s club) in the Northern Pacific coast of North America (Artyukova et al., 2005; Xiang and Lowry, 2007).
The three species of Oplopanax have been used in folk medicine by natives of the region that originate from ( Academy of Traditional Chinese Medicine and Traditional Chinese Herb of Ji-lin Province, 1982; Lantz et al., 2004; Takeda et al., 1966). One species, O. horridus, is even regarded to be on par with Oriental ginseng and is used as a body-balancing and system-strengthening tea (Schofield, 2000). In traditional medicinal use, the stem and root of O. elatus is used for treating neurasthenic, hypopiesis, schizophrenia, cardiovascular diseases, diabetes mellitus and rheumatism ( Academy of Traditional Chinese Medicine and Traditional Chinese Herb of Ji-lin Province, 1982); the root, bark and stem of O. japonicus are used as an antipyretic or cough medicine (Takeda et al., 1966); the bark or berries of O. horridus are often used in the form of an extract for the treatment of arthritis (inflammation), hyperglycemia, gastrointestinal disorders, infections and respiratory ailments (Johnson, 2006; Moore, 1993; Schofield, 2000). It is believed that the dammarane-type triterpenoids are related to the bioactivities of ginseng (Kang et al., 2008; Zuo et al., 2009). Based on previous observations, the anticancer components of processed ginseng are reliant on its derived ginsenosides (Wang et al., 2007a; Wang et al., 2007b; Yun et al., 1983). O. elatus, on which the phytochemical and physiological studies are reported, is found to contain triterpenoids, specifically oleanane-type and lupene-type triterpenoids whose structures differ from that of dammarane-type ginsenosides (Wang, 2006). O. horridus is reported to contain sesquiterpenes which include equinopanacene, equinopanacol (Kariyone and Morotomi, 1927), ox-cubebene,, spathulenol, oplopanone (Bloxton and Marderosian, 2002), 3,10-epoxy-11-hydroxynerolid-6-ene, and 3S,6R,7S, 10R-cis-6, 11-dihydroxy-7, 10-epoxynerolidol (Inui, 2008); 5 polyynes includes falcarinol, falcarindiol, oplopandiol, 9,17-octadecadiene-12,14-diyne-1,11,16-triol, 1-acetate, and oplopandiol acetate (Kobaisy et al., 1997); and one liginin, sesamin (Inui, 2008). In addition, lignan 1,3 benzodioxole, 5,5'-tetrahydro-1H,3H-furo[3,4-c]furan- 1,4-diyl)bis, stearic acid, stigmasterol and β-sitosterol were identified by comparison (Bloxton and Marderosian, 2002; Gruber et al., 2004). Among them, polyynes were directly identified to display significant antimycobacterial, antimicrobial, and antifungal activity (Kobaisy et al., 1997). However, other components of Oplopanax were also reported to be active. For example, trans-nerolidol with anti-colon cancer effects in rats (Wattenberg, 1991) and spasmolytic effect in mice (1966). Stigmasterol and β-sitosterol with antirheumatic and anticholesterolemic properties (1989). The bioactivities of O. horridus have been evaluated by pharmacological tests. For example, studies conducted by Large and Brocklesby exhibited marked hypoglycemic property attributable to the extract (Large and Brocklesby, 1938), though this effect can not be confirmed in a subsequent clinical study (Smith, 1983). Tests by McCutcheon have proven the extract to display antiviral effects against respiratory syncytial virus (McCutcheon et al., 1995). McCutcheon (McCutcheon et al., 1997) and Inhu (Inui, 2008; Inui et al., 2007) evaluated the inhibitory effects of O. horridus bark and root extract on Mycobacterium tuberculosis cell growth in vitro. Tai (Tai et al., 2006) reported the anti-proliferative effect of 70% ethanol extract of O. horridus root bark on several cancer cell lines, K562, HL60, MCF7, and MDA-MB-468.
The profile of O. horridus fractions responsible for the anti-cancer activities and their related mechanism, however, has not been investigated. This experiment was designed to study the anti-proliferative effects of the different fractions chromatographed from O. horridus root bark by Dianion HP20 resin on breast cancer MCF-7 cells and non-small cell lung cancer cells (NSCLC). In addition, the role played by different compositions in the MCF-7 cell cycle and apoptosis was also investigated.
EXPERIMENTAL DETAILS
Chemicals
All solvents were of high-performance liquid chromatography (HPLC) grade (Fisher Scientific, Norcross, GA). Milli Q water was supplied by a water purification system (US Filter, Palm Desert, CA). Plastic materials were purchased from Falcon Labware (Franklin Lakes, NJ). Trypsin, Leibovitz's L-15 medium, fetal bovine serum (FBS), and penicillin/streptomycin solution (200×) were obtained from Mediatech, Inc. (Herndon, VA). A CellTiter 96 Aqueous One Solution Cell Proliferation Assay kit was obtained from Promega (Madison, WI). An Annexin V-FITC Apoptosis Detection kit was obtained from BD Biosciences (San Diego, CA). Other reagents were purchased from Fisher Scientific (Pittsburgh, PA).
Plant Material
The root bark of Oplopanax horridus (Sm.) Miq was purchased from Pacific Botanicals, Grants Pass, OR. A voucher specimen (no. 20080420-1) was deposited in the Tang Center for Herbal Medicine Research at the University of Chicago.
Extraction and Isolation
Dried and ground root bark of O. horridus (2.4 kg) was extracted with 70% ethanol (1: 8, W: V) refluxed at 90°C for 4 h and filtered, the residue was extracted with 70% ethanol (1: 6, W: V) refluxed at 90°C for 2 h two more times. The filtrates were combined and evaporated at 60°C in vacuo to acquire a total extract (503.2g, 20.97%). The ethanol extract was applied to Dianion HP-20 (Sorbent Technologies, Atlanta) column chromatography (75×610 mm, Ace Glass, Inc., Vineland, NJ) eluting with a H2O-EtOH gradient system (0, 30, 50, 70,100% ethanol) to give corresponding fractions of 284.0, 38.5, 56.0, 75.0 and 53.5 g, respectively. The experiment was performed twice. Thin layer chromatography (TLC) was carried out on TLC Silica gel 60 F254 plates and TLC RP-18 F254 (EMD Chemicals Inc., Darmstadt, Germany), and spots were visualized by UVLMS38 (UVP, LLC., Upland, CA) and by spraying the plates with 10% H2SO4 of ethanol solution followed by heating.
Sample Preparation
For HPLC samples, weighted 40 mg samples were dissolved with MeOH/H2O into 10 mg/mL concentrated solutions (70% fraction into 5mg/mL), the solutions were then filtered with Millex 0.2 μm nylon membrane syringe filters (Millipore Co., Bedford, MA) to vials for HPLC analysis. For bioassay samples, weighted 30 mg samples were dissolved with DMSO or 70% ethanol into 30 mg/mL solutions, the solutions were diluted into 300, 100, 30, 10, 3, 1, 0.3 and 0.1 μg/mL for the bioassay. Stock solutions were stored at −20°C before use.
HPLC Instrumentation and Analysis
The HPLC system included a Waters 2965 instrument (Milford, MA) with a quaternary pump, an automatic injector, a photodiode array detector (Model 996), and Waters Millennium 32 software for peak identification and integration. The separation was carried out on a 250×3.2 mm i.d., 5 μ, Prodigy ODS (2) column (Phenomenex, Torrance, CA) with a 7.5× 3.2 mm i.d. guard column. For HPLC analysis, a 20 μL sample was injected into the column and eluted at room temperature with a constant flow rate of 1.0 mL/min. For the mobile phase, acetonitrile (solvent A) and water (solvent B) were used. Gradient elution started with 30% solvent A and 70% solvent B, and held for 1 min. The elution was changed to 36% A for 9 min, to 50% A for 9 min, to 68% A for 10 min, to 80% A for 3 min, to 90% A for 4 min and held for 3 min. The last elution was changed to 30% A for 5 min and held for 7 min. The detection wavelength was set to 202 nm, and the wavelength range was 196–450 nm.
Cell Culture
The human breast cancer cell lines MCF-7 (RPMI-1640) and non-small cell lung cancer cells (NSCLC, DMEM) were purchased from American Type Culture Collection, ATCC, (Manassas, VA) and grown in the indicated media supplemented with 10 % FBS and 50 IU penicillin/streptomycin in a humidified atmosphere of 5 % CO2 at 37 °C.
Cell Proliferation Analysis
Cells were seeded in a flat-bottomed 96-well plate with a multichannel pipet (1×104 cells/well). After 24 h, the medium was removed and 200 μL of fresh culture medium was added to each well. Various concentrations of extract and fractions were added to the wells. The final concentration of DMSO or ethanol tested groups was 0.1or 0.5%. Controls were exposed to culture medium containing the same quantity of DMSO or ethanol without drugs. All experiments were performed at least three times. At the end of the drug exposure period (48 h), the medium was removed from all the wells and 100 μL of fresh medium plus 20 μL of CellTiter 96 aqueous solution was added to each well. CellTiter 96 aqueous solution is composed of a tetrazolium compound, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, an electron-coupling reagent (phenazine methosulfate), and buffer. When the solution contacts viable cells, it is bioreduced by dehydrogenase enzymes in metabolically active cells into a formazan product. The quantity of formazan product, measured by the amount of absorbance at 490 nm, is directly proportional to the number of living cells in culture. The plate was then incubated for 1 h in a humidified atmosphere at 37°C. 60 μL of medium from each well was transferred to an ELISA 96-well plate, and the absorbance of the formazan product at 490 nm was measured. The blank was recorded by measuring the absorbance at 490 nm with wells containing medium but no cells. All observations were performed in triplicate. Results were expressed as percent of control (solvent vehicle set at 100%).
Cell Cycle Assay
The MCF-7 cells were seeded in 24-well tissue culture plates. On day 2, the medium was changed and the cells were treated with extract/fractions. The cells were incubated for 48 h before being harvested. The cells were fixed gently by adding 80% ethanol and placing them in a −20 °C freezer for 2 h. They were then treated with 0.25% Triton X-100 for 5 min in an ice bath. The cells were resuspended in 300 μL of PBS containing 40 μg/mL propidium iodide and 0.1 mg/mL RNase. Then the cells were incubated in a dark room for 20 min at room temperature, and analyzed with a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and FlowJo 7.1.0 software (Tree Star, Ashland, OR). For each measurement, at least 20,000 cells were counted.
Apoptosis Assay
The MCF-7 cells were seeded in 24-well tissue culture plates. After culturing for 1 day, the medium was changed and the extract/fractions were added. After treatment for 48 h, the cells floating in the medium were collected. The adherent cells were detached with 0.05% trypsin. Then the culture medium containing 10% FBS (and floating cells) was added to inactivate the trypsin. After being pipetted gently, the cells were centrifuged for 5 min at 1500 g. The supernatant was removed and the cells were stained with annexin V-FITC and PI according to the manufacturer’s instructions. Annexin V-FITC detects translocation of phosphatidylinositol from the inner to the outer cell membrane during early apoptosis, and PI can enter the cell in late apoptosis or necrosis. Untreated cells were used as control for the double staining. The cells were analyzed immediately after staining using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) and FlowJo 7.1.0 software (Tree Star, Ashland, OR). For each measurement, at least 20,000 cells were counted.
Statistical Analysis
Data are presented as mean ± standard error (SE). A one-way ANOVA determined whether the results had statistical significance. In some cases, Student's t-test was used for comparing two groups. The level of statistical significance was set at P < 0.05.
RESULTS
Column chromatography and HPLC profile
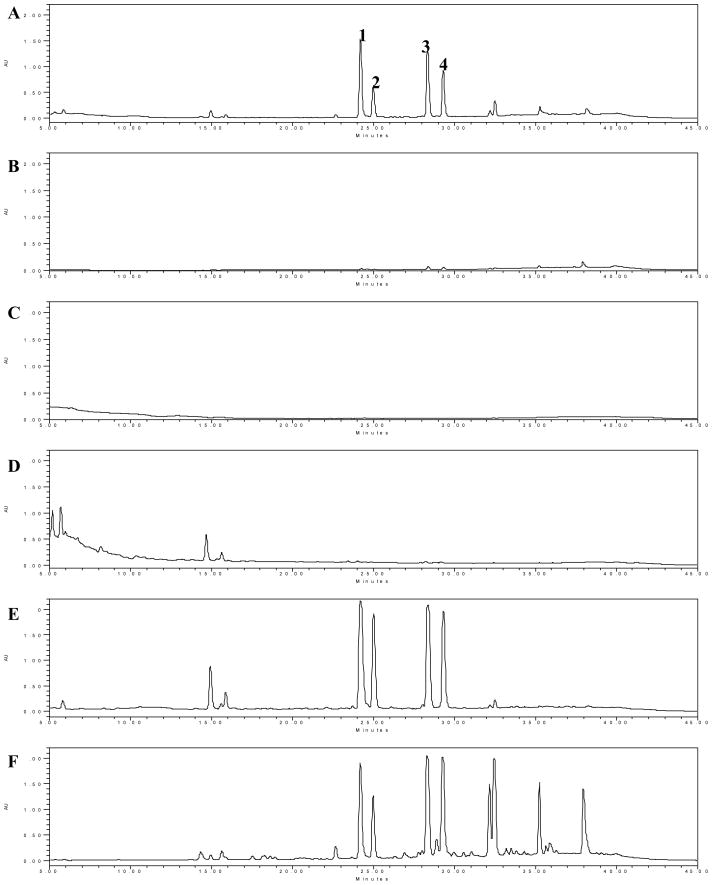
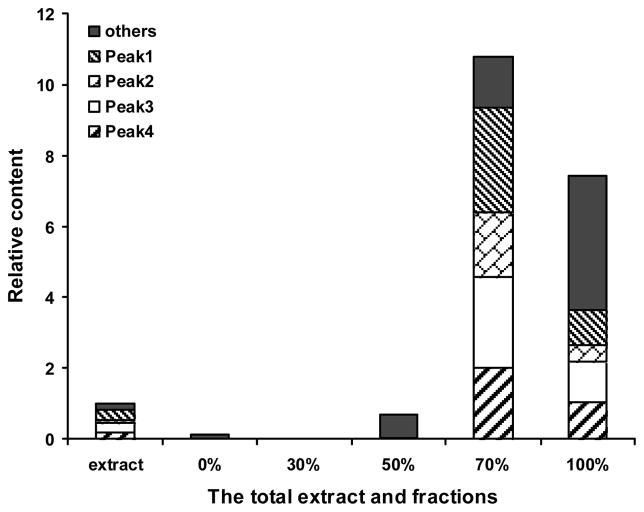
EtOH-H2O as the gradient elute was 0, 30, 50, 70, and 100% ethanol. HPLC chromatograms (Figure 1A–F) showed that 4 major and 9 minor components detectable in the root bark extract of O. horridus may be effectively enriched by the column chromatography of Dianion HP-20 resin. For 100% ethanol elute, the total number of peaks detectable under UV 202 nm was 34. These peaks not only include all components detected in the total extract, but also other components which were not observed in the extract. The 70% elute had a larger proportion of 4 major components as compared to the other fractions; There were hardly any detectable components in the water and 30% fraction in HPLC chromatogram under UV 197–450 nm. The relative content of the components in the 70% and 100% ethanol fractions increased significantly, 10.64 and 7.42 folds respectively, by the area normalization method (Figure 2). There was greater accumulation of 4 major components in the 70% fraction (5.10 folds) than in the 100% fraction (1.98 folds).
Figure 1.
HPLC-UV Chromatograms of the total extract from the root bark of O. horridus and fractions chromatographed from Dianion HP20 resin column at UV λ=202 nm. A: total extract (10mg/mL); B–F: 0% (10mg/mL), 30% (10mg/mL), 50% (10mg/mL), 70% (5mg/mL), and 100% (10mg/mL) ethanol eluted fractions from Diaion HP-20 column.
Figure 2.
The relative content of components from the root bark of O. horridus detectable in HPLC at UV λ=202 nm. The relative content values are the ratio of each peak area to the total peak areas of the total extract.
Anti-proliferative activity
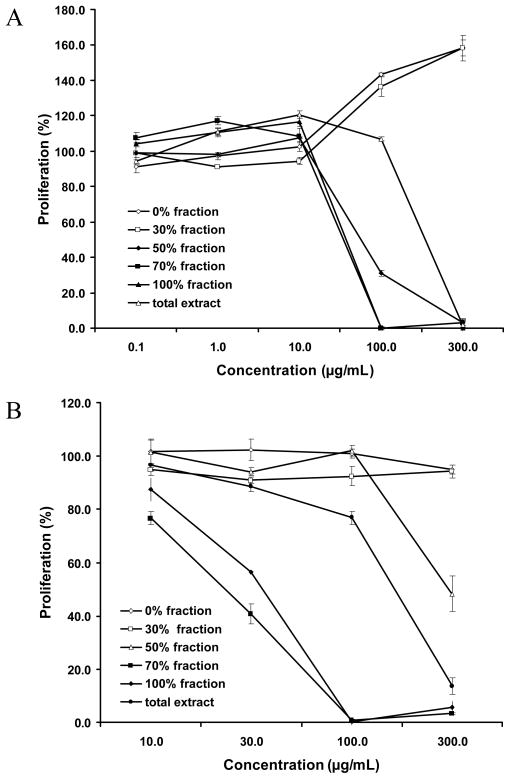
Figure 3 showed the anti-proliferative activities of the chromatographic fractions of the extract of O. horridus root bark subjected to macroporous resin on human breast cancer MCF-7 cells and non-small cell lung cancer (NSCLC) cells. The results showed that the hydrophobic fractions were more active than the hydrophilic fractions: The 70% and 100% fractions were the most active fractions. The IC50 of the total extract and 50%, 70%, and 100% fractions of anti-proliferation on MCF-7 was at 248.4, 123.1, 44.0, and 31.5 μg/mL respectively. On NSCLC cells, the IC50 was 125.3, 271.1, 17.6, and 23.2 μg/mL respectively. However, water and 30% ethanol fractions promoted MCF-7 cell proliferation at concentrations > 100 μg/mL.
Figure 3.
Percentage of proliferation of human (A) breast cancer MCF-7 cells and (B) non-small cell lung cancer (NSCLC) cells treated for 48 h with the root bark extract of O. horridus and the fractions.
Effects of extracts on MCF-7 cell cycle
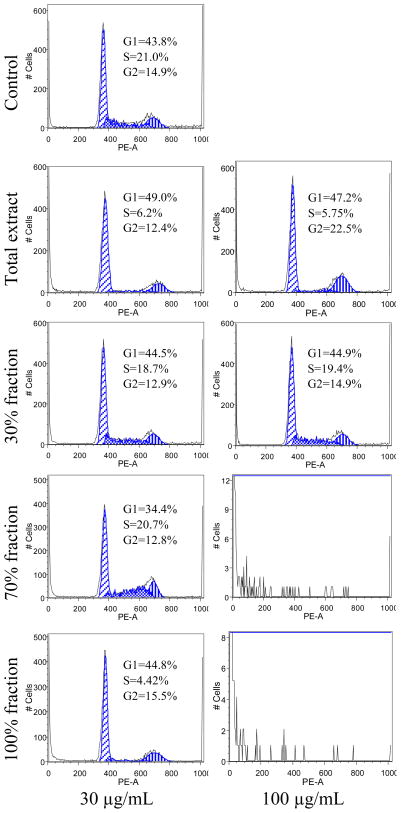
To determine whether the inhibitive mechanism of the total extract and fractions on MCF-7 cells proliferation involved cell cycle changes, cell cycle phases distribution of the treated cells were examined by flow cytometry. As shown in Figure 4, when the cells were treated with 30 μg/mL of the total extract and fractions, the total extract increased the percentage of cells in the G1-phase to 49% and decreased S-phase to 6.22%. The 70% ethanol fraction decreased the percentage of cells in the G1-phase to 34.4%. The 100% ethanol fraction decreased the percentage of cells in the S-phase to 6.22%. When the cells were treated with 100 μg/mL samples, the total extract increased the percentage of cells in the G1-phase to 47.2% and G2-phase to 22.5%. For the 70% and 100% ethanol fractions, cell cycle profiles could not be detected because there were not enough viable cells to be collected. The 30% ethanol fraction, contrasted to the control, had hardly any influence on the cell cycle.
Figure 4.
Effects of root bark extract of O. horridus and the fractions on MCF-7 cell cycle. After treatment with the total extract or fractions for 48 h, the cells were stained with PI and assayed using flow cytometry. The percentage of cells in G1-, S- and G2/M-phases are indicated.
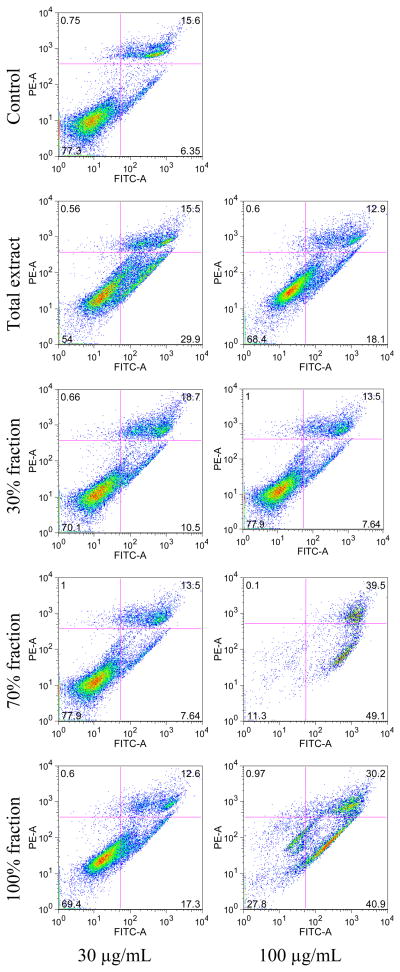
Apoptotic effect of extracts on MCF-7 cells
As shown in Figure 5, at a concentration of 30 μg/mL, only the total extract showed marked inductive effect on the apoptosis of MCF-7 cells, early apoptosis was 29.9%, late apoptosis/necrosis 15.5% after treatment for 48 h. At a concentration of 100 μg/mL, compared to the total extract (early apoptosis 18.1%, late apoptosis/necrosis 12.9%) and 30% ethanol fraction (early apoptosis 7.64%, late apoptosis/necrosis 13.5%), the 70% and 100% ethanol fractions significantly increased early apoptosis to 49.1% and 40.9%, and late apoptosis/necrosis to 39.5% and 30.2%, respectively. For the viable cells, the control was 77.3%, total extract and 30% fraction at 100 μg/mL concentration were 68.4% and 77.9%, respectively, while the 70% and 100% fractions were 11.3% and 27.8%, respectively. At a concentration of 300 μg/mL, the 30% ethanol fraction had no effect on MCF-7 cells (early apoptosis 8.15%, late apoptosis/necrosis 14.4%, and viable cell 76.9%). The total extract significantly increased apoptosis (early 45.6%, late 18.8%, viable cell 34.4%) and the 70% and 100% ethanol fractions markedly reduced the proportion of the viable cells.
Figure 5.
Apoptosis assay using flow cytometry after annexin V-FITC/propidium iodide (PI) staining. MCF-7 cells were treated for 48 h with O. horridus extract and fractions. Viable cells are in the lower left quadrant, early apoptotic cells are in the lower right quadrant, late apoptotic or necrotic cells are in the upper right quadrant and non-viable necrotic cells are in upper left quadrant.
DISCUSSION
Herbal extracts are comprised of a complicated mixture of secondary metabolites which may possess biological activities. The active components can be enriched by partitioning or chromatographic methods. In this experiment, the active components, which should be hydrophobic, were effectively enriched in 70% and 100% ethanol fractions chromatographed from Dianion HP20 column (Figures 1 and 2). From the root bark of O. horridus, Kobaisy et al (1997) showed its major components were polyacetylenes, and this observation supported by current study using HPLC-UV determination.
Previous studies demonstrated anti-proliferation effects of polyacetylenes from plants of families Araliaceae and Apiaceae (Christensen and Brandt, 2006). Naturrally occurring polyacetylenes possess cytotoxic effects on different cancer cell lines (Dembitsky, 2006), such as mouse malignant melanoma cells (B16) and fibroblast derived tumor cells (L-929), human gastric carcinoma MK-1 cells, breast carcinoma M25-SF and MCF7 cells ovarian cancer cell lines SK-OV-3, and hepatocarcinoma cell line HepG2 (Guo et al., 2009).
We assayed anti-proliferative effect, which also called cytotoxic effect in literature, of different fractions of O. horridus on human breast cancer MCF-7 cells or NSCLC cells using MTS method, and observed significant pharmacological activities in a concentration-dependent manner.
In MCF-7 cells, either proliferative or anti-proliferative effects may occur in different O. horridus fractions at different concentrations. The small or non-polar (70% and 100% ethanol) fractions significantly increased anti-proliferation, while the polar (water and 30% ethanol) fractions promoted proliferation of MCF-7 cell at over 100 μg/mL (Figure 3A). This result suggested that promoting proliferative components and anti-proliferative components may coexist in the total extract and interact to influence the total extract’s effect on the cancer cells. To improve efficiency, the two hydrophilic fractions should be removed from the root bark extract if it is to be used in the treatment of certain cancers.
Functional mechanisms involved in the anti-proliferative effects of O. horridus extract and fractions were evaluated. Cancer is frequently considered to be a disease of disruption of equilibrium by loss of cell cycle control (Sandal, 2002). Some compounds, inhibiting cell cycle progression, were developed as anti-tumor agents (Welburn and Endicott, 2005). In this study, we observed the total extract increase the percentage of cells in G2/M-phase, and reduce the cell percentage in S-phase. For the active fractions, 70% fraction reduced the percentage of cells in G1-phase, while 100% fraction reduced cells in S-phase (Figure 4). Since diverse cell cycle profile changes were observed among extract and active fractions, cell cycle arrest may not play the key role in the cancer cell inhibition of the extract and fractions.
Apoptosis is programmed cell death, a highly regulated process used to eliminate unwanted or defective cells (Pucci et al., 2000). Many chemotherapeutic agents and natural compounds, radiation, immunotherapy, and cytokines induce cancer cell death via the apoptotic pathway (Yun et al., 1983; Lowe and Lin, 2000). We observed O. horridus extracts and fractions on the apoptotic induction of MCF-7 cells. The total extract and the two active fractions obviously induced cell apoptosis. The 70% fraction, which possessed most potent anti-proliferative activity, showed the strongest apoptotic induction activity (Figure 5). This result suggested that the anti-proliferative effects of O. horridus extracts and active fractions were mediated by the induction of apoptosis.
Data from this study showed that the root bark extract of O. horridus and 70% and 100% ethanol fractions chromatographed from Dianion HP20 resin column presented significant inhibitory effects on human breast cancer MCF-7 cells and non-small cell lung cancer (NSCLC) cells. The anti-proliferative activities increased with the accumulation of the active secondary metabolites in the non-polar fractions. Since the water and 30% ethanol fractions promoted cell growth in the MCF-7 cells at certain concentrations, these fractions should be removed from the extract to ensure desirable chemopreventive activities. The responsible constituents and the detailed functional mechanisms remain to be further investigated.
Acknowledgments
This work was supported in part by the NIH/NCCAM grants AT003255 and AT004418.
References
- (Laboratoires Meram). assignee. Terpene alcohol sedatives and spasmolytics. 19640120 Application: FR patent M4055. 1966
- Budavari S, editor. The Merck Index. Merck & Co; Rabway: [Google Scholar]
- Academy of Traditional Chinese Medicine and Traditional Chinese Herb of Ji-lin Province. Changbaishan Plant Materia Medica. People's Medical Publishing House of Ji-lin; Changchun: 1982. p. 785. [Google Scholar]
- Artyukova EV, Gontcharov AA, Kozyrenko MM, Reunova GD, Zhuravlev YN. Phylogenetic Relationships of the Far Eastern Araliaceae Inferred from ITS Sequences of Nuclear rDNA. Russ J Gene. 2005;41:649–658. [PubMed] [Google Scholar]
- Bloxton JD, II, Marderosian AD. Bioactive Constituents of Alaskan Devil's Root (Oplopanax horridus, Araliaceae) Economic Botany. 2002;56:285–287. [Google Scholar]
- Christensen LP, Brandt K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity, and analysis. J Pharma Biomed Anal. 2006;41:683–693. doi: 10.1016/j.jpba.2006.01.057. [DOI] [PubMed] [Google Scholar]
- Gruber JW, Kittipongpatana N, Bloxton JD, II, Der Marderosian A, Schaefer FT, Gibbs R. High-performance liquid chromatography and thin-layer chromatography assays for devil's club (Oplopanax horridus) J Chromatogr Sci. 2004;42:196–199. doi: 10.1093/chromsci/42.4.196. [DOI] [PubMed] [Google Scholar]
- Guo L, Song L, Wang Z, Zhao W, Mao W, Yin M. Panaxydol inhibits the proliferation and induces the differentiation of human hepatocarcinoma cell line HepG2. Chem Biol Interact. 2009;181:138–43. doi: 10.1016/j.cbi.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Inui T. Phytochemical and Biochemometric Evaluation of the Alaskan Anti-TB Ethnobotanical: Oplopanax horridus. Chicago: University of Illinois at Chicago; 2008. p. 187. [Google Scholar]
- Inui T, Wang Y, Deng S, Smith DC, Franzblau SG, Pauli GF. Counter-current chromatography based analysis of synergy in an anti-tuberculosis ethnobotanical. J Chromatogr A. 2007;1151:211–5. doi: 10.1016/j.chroma.2007.01.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LM. Gitksan medicinal plants--cultural choice and efficacy. J Ethnobiol Ethnomed. 2006;2:29. doi: 10.1186/1746-4269-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang KA, Kang JH, Yang MP. Ginseng total saponin enhances the phagocytic capacity of canine peripheral blood phagocytes in vitro. Am J Chin Med. 2008;36:329–41. doi: 10.1142/S0192415X08005801. [DOI] [PubMed] [Google Scholar]
- Kariyone T, Morotomi S. The essential oil of Echinopanax horridus, Decne et Planch. Yakugaku Zasshi. 1927;546:671–4. [Google Scholar]
- Kobaisy M, Abramowski Z, Lermer L, Saxena G, Hancock RE, Towers GH, Doxsee D, Stokes RW. Antimycobacterial polyynes of Devil's Club (Oplopanax horridus), a North American native medicinal plant. J Nat Prod. 1997;60:1210–3. doi: 10.1021/np970182j. [DOI] [PubMed] [Google Scholar]
- Lantz TC, Swerhun K, Turner NJ. Devil’s Club (Oplopanax horridus): An Ethnobotanical Review. HerbalGram: J Am Bot Counc. 2004;62:33–48. [Google Scholar]
- Large RG, Brocklesby HN. A hypoglucemic substance from the roots of the devil's club (Fatsia horrida) Cana Med Assoc J. 1938;39:32–5. [PMC free article] [PubMed] [Google Scholar]
- Lowe SW, Lin AW. Apoptosis in cancer. Carcinogenesis. 2000;21:485–95. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- McCutcheon AR, Roberts TE, Gibbons E, Ellis SM, Babiuk LA, Hancock RE, Towers GH. Antiviral screening of British Columbian medicinal plants. J Ethnopharmacol. 1995;49:101–10. doi: 10.1016/0378-8741(95)90037-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon AR, Stokes RW, Thorson LM, Ellis SM, Hancock REW, Towers GHN. Anti-Mycobacterial Screening of British Columbian Medicinal Plants. Pharmaceut Biol. 1997;35:77–83. [Google Scholar]
- Moore M. Medicinal Plants of the Pacific West. Red Crane Books; Santa Fe: 1993. pp. 125–128. [Google Scholar]
- Pucci B, Kasten M, Giordano A. Cell cycle and apoptosis. Neoplasia (New York) 2000;2:291–299. doi: 10.1038/sj.neo.7900101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandal T. Molecular aspects of the mammalian cell cycle and cancer. Oncologist. 2002;7:73–81. doi: 10.1634/theoncologist.7-1-73. [DOI] [PubMed] [Google Scholar]
- Schofield JJ. Discovering Wild Plants: Alaska, Western Canada, The Northwest. Alaska Northwest Books; Portland: 2000. pp. 87–89. [Google Scholar]
- Smith GW. Arctic pharmacognosia II. Devil's Club, Oplopanax horridus. J Ethnopharmacol. 1983;7:313–20. doi: 10.1016/0378-8741(83)90005-3. [DOI] [PubMed] [Google Scholar]
- Tai J, Cheung S, Cheah S, Chan E, Hasman D. In vitro anti-proliferative and antioxidant studies on Devil's Club Oplopanax horridus. J Ethnopharmacol. 2006;108:228–35. doi: 10.1016/j.jep.2006.05.018. [DOI] [PubMed] [Google Scholar]
- Takeda K, Minato H, Ishikawa M. Studies on sesquiterpenoids. XII. Structure and absolute configuration of oplopanone, a new sesquiterpene from Oplopanax japonicus. Tetrahedron, Supplement. 1966;11:219–25. [Google Scholar]
- Wang CZ, Aung HH, Ni M, Wu JA, Tong R, Wicks S, He TC, Yuan CS. Red American ginseng: ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007a;73:669–74. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Ni M, Sun S, Li XL, He H, Mehendale SR, Yuan CS. Detection of adulteration of notoginseng root extract with other panax species by quantitative HPLC coupled with PCA. J Agric Food Chem. 2009;57:2363–7. doi: 10.1021/jf803320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Yuan CS. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–28. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. The Studies on the Oleanane-type Triterpenoid Saponins and Lupene-type Triterpenoid Saponins in Chinese Herbs Oplopanax Elatus and Achyranthes Bidentata. Changchun: Jilin University; 2006. [Google Scholar]
- Wang W, Zhao Y, Rayburn ER, Hill DL, Wang H, Zhang R. In vitro anti-cancer activity and structure-activity relationships of natural products isolated from fruits of Panax ginseng. Cancer Chemother Pharmacol. 2007b;59:589–601. doi: 10.1007/s00280-006-0300-z. [DOI] [PubMed] [Google Scholar]
- Wattenberg LW. Inhibition of azoxymethane-induced neoplasia of the large bowel by 3-hydroxy-3,7,11-trimethyl-1,6,10-dodecatriene (nerolidol) Carcinogenesis. 1991;12:151–2. doi: 10.1093/carcin/12.1.151. [DOI] [PubMed] [Google Scholar]
- Welburn JPI, Endicott JA. Inhibition of the cell cycle with chemical inhibitors: A targeted approach. Seminars in Cell & Developmental Biology. 2005;16:369–381. doi: 10.1016/j.semcdb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Xiang Q, Lowry PP. Araliaceae. In: Wu Z, Raven PH, Hong D, editors. Flora of China. Science Press & Missouri Botanical Garden; Beijing & St. Louis: 2007. p. 441. [Google Scholar]
- Yun TK, Yun YS, Han IW. Anticarcinogenic effect of long-term oral administration of red ginseng on newborn mice exposed to various chemical carcinogens. Cancer Detect Prev. 1983;6:515–25. [PubMed] [Google Scholar]
- Zuo G, Guan T, Chen D, Li C, Jiang R, Luo C, Hu X, Wang Y, Wang J. Total saponins of Panax ginseng induces K562 cell differentiation by promoting internalization of the erythropoietin receptor. Am J Chin Med. 2009;37:747–57. doi: 10.1142/S0192415X09007211. [DOI] [PubMed] [Google Scholar]