Abstract
The high stiffness of periarticular locked plating constructs can suppress callus formation and fracture healing. Replacing standard locking screws with Far Cortical Locking (FCL) screws can decrease construct stiffness and can improve fracture healing in diaphyseal plating constructs. However, FCL function has not been tested in conjunction with periarticular plating constructs in which FCL screws are confined to the diaphyseal segment. This biomechanical study evaluated if diaphyseal fixation of a periarticular locking plate with FCL screws reduces construct stiffness and induces parallel interfragmentary motion without decreasing construct strength.
Periarticular locking plates were applied to stabilize distal femur fractures in 22 paired femurs, using either a standard locked plating approach (LP group) or FCL for diaphyseal fixation (FCL group) using MotionLoc screws (Zimmer, Warsaw, IN). Each specimen was evaluated under quasi-physiological loading to assess construct stiffness, construct durability under dynamic loading, and residual strength after dynamic loading.
FCL constructs had an 81% lower initial stiffness than LP constructs. They induced nearly five times more interfragmentary motion than LP constructs under one body-weight loading (p<0.001). FCL constructs generated parallel interfragmentary motion, while LP constructs exhibited 48% less motion at the near cortex than at the far cortex (p=0.002). Seven LP constructs and eight FCL constructs survived 100,000 loading cycles. The residual strength of surviving constructs was 5.0±1.6 kN (LP group) and 5.3±1.1 kN (FCL group, p=0.73).
In summary, FCL screws reduce stiffness, generate parallel interfragmentary motion and retain the strength of a periarticular locked plating construct. Therefore, FCL fixation may be advisable for stiffness reduction of periarticular plating constructs to promote fracture healing by callus formation.
Introduction
By delivering improved fixation strength with fixed-angle locking screws, periarticular locking plates have been rapidly adopted as an alternative to intramedullary nails and non-locking buttress plates for distal femur fractures (1). However, the inherently high stiffness of locked constructs is increasingly recognized as a potential cause of deficient healing observed in fractures stabilized with periarticular locking plates (2–5). The near cortex adjacent to the plate is particularly predisposed to deficient healing, since interfragmentary motion of locked plating constructs is asymmetric and minimal at the near cortex (5). A recent clinical study documented asymmetric callus formation and reported a non-union rate of 18.6% for supracondylar femur fractures stabilized with periarticular locking plates (4).
In response to the clinical concern that locked plating constructs may be too stiff, several approaches to decrease the stiffness of locked plating constructs were proposed (6–8). One of these approaches, termed Far Cortical Locking (FCL), has demonstrated improved fracture healing compared to standard locked plating in an ovine study by providing flexible fixation and parallel interfragmentary motion (2, 9). FCL employs locking screws that have a shaft section with a reduced diameter. These FCL screws lock into the plate and into the far cortex of the diaphysis. The reduced screw shaft diameter allows for elastic flexion of the screw within a controlled motion envelope at the near cortex (9). This flexion induces parallel interfragmentary motion for mediation of fracture healing by callus formation.
In a biomechanical study, the use of FCL screws in place of standard locked screws reduced the axial stiffness of a diaphyseal plating construct by 88% while retaining construct strength and producing nearly parallel motion at the fracture gap (9). The effect of diaphyseal FCL plating constructs on fracture healing was also evaluated in a tibial osteotomy model in sheep (2). This in vivo study demonstrated that FCL constructs healed to be 54% stronger and tolerated 157% more energy to failure than a control group stabilized using standard locking screws.
However, both the biomechanical as well as the ovine FCL study were limited to the evaluation of FCL function in diaphyseal bridge plating constructs, whereby plates were applied with FCL screws that were symmetrically placed on both sides of the fracture. For periarticular plating, FCL screws can only be applied to the diaphyseal side of the fracture, since they rely on fixation in the far cortex. It therefore remains hypothetical that adequate stiffness reduction and parallel motion can be obtained in a hybrid periarticular construct that employs FCL screws for diaphyseal fixation and conventional locking screws for metaphyseal fixation.
The present study evaluated the effects of diaphyseal FCL fixation on construct stiffness and interfragmentary motion for periarticular locking plates applied to stabilize distal femur fractures in human cadaveric specimens. Results of the present study tested the hypothesis that diaphyseal fixation of a periarticular femur plate with FCL screws will reduce construct stiffness and will induce parallel interfragmentary motion without decreasing construct strength compared to plate application using standard locking screws.
Material and Methods
In a biomechanical study, periarticular locking plates were applied to stabilized distal femur fractures in 22 paired human femurs. One femur of each pair was stabilised in a standard locked plating approach (LP group). In contralateral femurs, FCL screws were used in place of standard locking screws for diaphyseal fixation (FCL group). Each specimen was subjected to three tests under quasi-physiological loading. First, specimens were loaded statically to 1,200 N for assessment of construct stiffness. Second, 100,000 dynamic loading cycles representative of level walking were simulated to assess construct durability. Finally, those specimens that survived dynamic loading were loaded to failure to assess their residual strength and failure mode.
Specimens
Twenty-two paired human femurs were harvested from four male and seven female fresh-frozen body donors with an average age of 76.8 ± 9.6 years. Dual energy X-ray absorption (DEXA) scans of the proximal femur were obtained to quantify bone quality in terms of T-scores. An unstable distal femur fracture (AO/OTA 33-A3) was modeled by introducing a 10-mm gap osteotomy located 60 mm proximal to the intercondylar notch (10).
Fixation Constructs
Gap osteotomies were stabilized with periarticular locking plates (9-hole NCB Femoral Plate, Zimmer, Warsaw, IN) of 246 mm length. The distal plate segment was applied to the metaphysis using six 5.0 mm cancellous locking screws in accordance with the manufacturer’s technique guide. The proximal plate segment was applied to the diaphysis using four evenly spaced locking screws in a 1-3-5-7 configuration. The plate was applied at 1 mm elevation from the diaphysis using temporary spacers to simulate biological fixation.
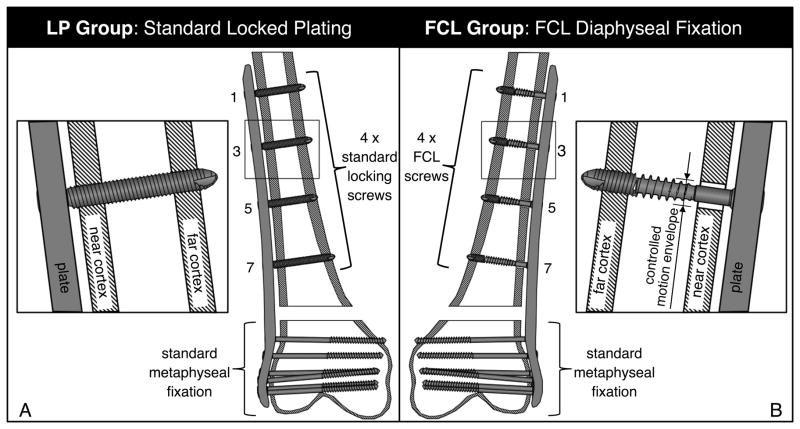
One femur of each pair was randomly assigned to the LP group, which utilized standard 5.0 mm bi-cortical locking screws for diaphyseal fixation (Figure 1A). The contralateral femur of each pair was assigned to the FCL group, which utilized 5.0 mm FCL screws (MotionLoc, Zimmer, Warsaw, IN) for diaphyseal fixation in place of standard bi-cortical locking screws (Figure 1B). FCL screws had a flexible mid-shaft section of reduced core diameter for elastic connection between the plate and far cortex. By avoiding rigid constraint in the near cortex, the FCL screw design increased the working length of the screw, allowing for elastic flexion of the screw shaft within a controlled motion envelope in the near cortex. A detailed description and formal biomechanical evaluation of FCL constructs has been published (9). Antero-posterior radiographs of all specimens were obtained before mechanical testing to document reduction and implant placement.
Figure 1.
A) In the LP group, periarticular femur plates were applied with standard locking screws; B) In the FCL group, plates were applied to the diaphysis using four FCL screws that provide a controlled motion enveloped in the near cortex.
Test Setup
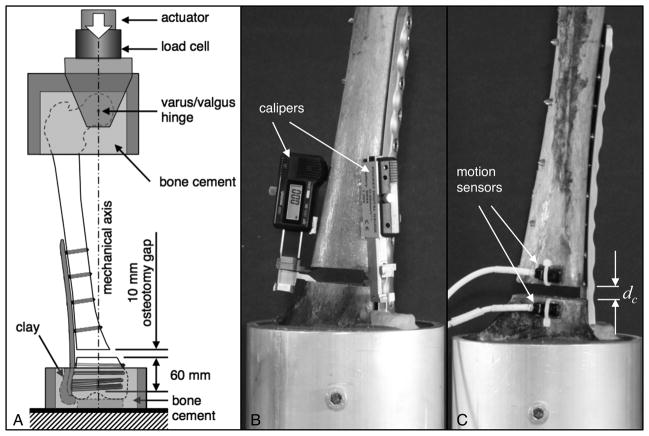
The proximal femur and the condyles were secured in mounting fixtures for application of quasi-physiologic loading in accordance with an established loading procedure (11, 12). The proximal fixture was connected to the actuator of a material test system (8874, Instron, Canton, MA) via a hinge joint that was aligned with the femoral head center to allow unconstrained varus/valgus rotation (Figure 2A). The distal fixture was rigidly mounted to the base of the test system. The distal tip of the plate was covered with a clay layer to prevent artificial support by bone cement in the distal fixture (12, 13). Load was induced along the mechanical axis of the femur, with the load vector intersecting the femoral head and the epicondylar center.
Figure 2.
A) Fixation constructs were loaded along the mechanical axis of the femur. B) Construct stiffness was assessed by measuring interfragmentary motion at the near and far cortex with digital calipers in response to femur loading. C) Construct durability was assessed under dynamic loading, whereby motion sensors continuously captured the gradual collapse dc at the osteotomy gap.
Loading Protocol
First, construct stiffness was measured by loading constructs in 50 N increments up to 1,200 N. After each load increment, the resulting compression of the osteotomy gap was measured at the lateral (near) cortex and medial (far) cortex with digital calipers (Blitz, Jeffersonville, IN) at a resolution of 0.01 mm (Figure 2B). Construct stiffness was calculated by dividing each load step by the average change in gap width measured at the near and far cortex.
Subsequently, construct durability was assessed by applying 100,000 load cycles of 1,870 N amplitude. This loading regime simulated level walking forces (14) accumulated over a two month period (15). Progressive collapse dC of the osteotomy gap during dynamic loading was monitored in real time with two electromagnetic motion sensors (PC-Bird, Ascension Technology, Burlington, VT) (Figure 2C). Construct durability was defined by the number of loading cycles that constructs endured before collapse dC at the osteotomy gap exceeded 5 mm.
Finally, the residual strength of those constructs that survived dynamic loading (dC < 5 mm) was determined by stepwise loading to failure in 500 N increments. Failure load was defined as the load step which caused either catastrophic fracture or osteotomy collapse dC > 5 mm. After failure, each specimen was visually and radiographically analyzed to determine the failure mode.
For statistical analysis, results between the LP and FCL group were compared using paired (T-score, stiffness) or unpaired (durability, residual strength) two-tailed Student’s T-tests at a level of significance of α = 0.05.
Results
Bone quality did not differ between specimens of the LP group (−1.1 ± 1.7 T-score) and the contralateral specimens assigned to the FCL group (−1.0 ± 1.7 T-score, p = 0.6). T-scores ranged from −3.4 to 2.5, demonstrating that specimens spanned the range from osteoporotic to normal bone quality.
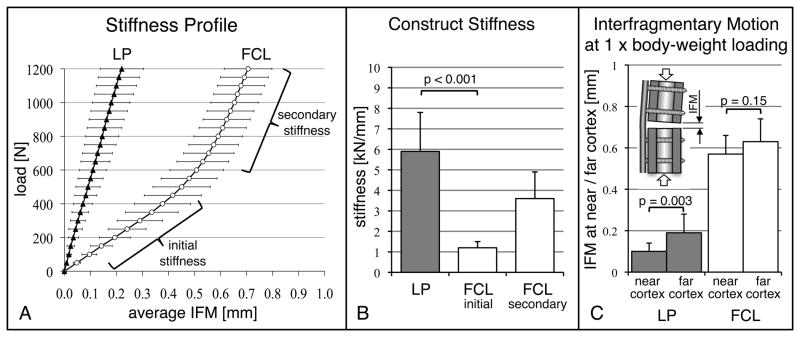
In LP constructs, the average interfragmentary motion increased linearly with increasing load (Figure 3a). In contrast, FCL constructs exhibited a bi-phasic stiffness profile with an initial stiffness and a secondary stiffness. The initial stiffness of FCL constructs (1.2 ± 0.3 kN/mm) was 81% lower than the stiffness of LP constructs (Figure 3b). Under elevated loading, the stiffness of FCL constructs increased to 3.7 ± 1.2 kN/mm due to additional support of the FCL screw shaft at the near cortex. The average interfragmentary motion in response to 800 N (one bodyweight) was four times smaller in the LP group (0.15 ± 0.06 mm) than in the FCL group (0.6 ± 0.1 mm, p < 0.001). Due to asymmetric gap closure in the LP group, 800 N loading induced 48% less motion at the near cortex (0.10 ± 0.04 mm) than at the far cortex (0.19 ± 0.09 mm, p = 0.003) (Figure 3c). FCL constructs exhibited substantially parallel gap motion, whereby 800 N induced similar amounts of motion at the near cortex (0.57 ± 0.09 mm) and far cortex (0.63 ± 0.11 mm, p = 0.15).
Figure 3.
A) Average interfragmentary motion in response to femur loading. FCL constructs exhibited a bi-phasic stiffness profile, whereby elevated loading induced near cortex support of FCL screws and yielded progressive construct stiffening. B) The initial stiffness of FCL constructs was 81% lower than the stiffness of LP constructs (p < 0.001). C) Due to asymmetric gap closure in the LP group, interfragmentary motion (IFM) under one body weight (800 N) loading was 48% smaller at the near cortex than at the far cortex. FCL constructs exhibited substantially parallel interfragmentary motion (p = 0.15), which was on average four times larger than in the LP group (p = 0.003).
During dynamic loading, four of eleven LP constructs failed and three of eleven FCL constructs failed (Table 1). All other constructs survived 100,000 loading cycles.
Table 1.
Durability and residual strength
| Specimen | Construct | side | T-score | Durability [cycles] | Residual strength [kN] | Failure mode |
|---|---|---|---|---|---|---|
| 1 | FCL LP |
left right |
−1.3 −1.4 |
100,000* 100,000* |
6.5 7.0 |
distal fixation distal fixation |
| 2 | FCL LP |
left right |
2.5 2.5 |
100,000* 100,000* |
7.0 7.0 |
diaphyseal fracture diaphyseal fracture |
| 3 | FCL LP |
right left |
0.1 0.1 |
100,000* 100,000* |
5.0 4.0 |
distal fixation distal fixation |
| 4 | FCL LP |
right left |
0.7 0.8 |
100,000* 100,000* |
5.0 4.5 |
distal fixation distal fixation |
| 5 | FCL LP |
left right |
−2.2 −2.7 |
100,000* 74,749 |
5.0 n/a |
distal fixation distal fixation |
| 6 | FCL LP |
left right |
−3.4 −3.2 |
316 138 |
n/a n/a |
distal fixation distal fixation |
| 7 | FCL LP |
right left |
2.5 −2.7 |
11,938 8,240 |
n/a n/a |
distal fixation distal fixation |
| 8 | FCL LP |
right left |
−2.8 −2.2 |
100,000* 100,000* |
5.0 3.5 |
distal fixation distal fixation |
| 9 | FCL LP |
left right |
−1.7 −2.1 |
100,000* 100,000* |
3.5 3.5 |
distal fixation distal fixation |
| 10 | FCL LP |
left right |
−0.4 −0.4 |
100,000* 100,000* |
5.0 5.0 |
distal fixation distal fixation |
| 11 | FCL LP |
right left |
−0.5 −0.7 |
25,845 22,073 |
n/a n/a |
distal fixation distal fixation |
Specimen sustained 100,000 loading cycles without failure
Subsequent testing to failure of the surviving constructs demonstrated that LP and FCL constructs retained comparable strength. The seven surviving LP constructs failed at 5.0 ± 1.6 kN and the eight surviving FCL constructs failed at 5.3 ± 1.1 kN (p = 0.73).
Radiographs demonstrated the same failure modes in the LP and FCL group. Ten of the 11 femur pairs sustained metaphyseal fixation failure, whereby gradual migration of distal screws caused a collapse of the osteotomy gap in excess of 5 mm (Figure 4). In the remaining femur pair, failure occurred by diaphyseal fracture in vicinity of the proximal plate tip. This femur pair had the highest bone quality (T-score: 2.5). None of the constructs sustained diaphyseal fixation failure, and no FCL screw sustained permanent bending or fixation failure.
Figure 4.
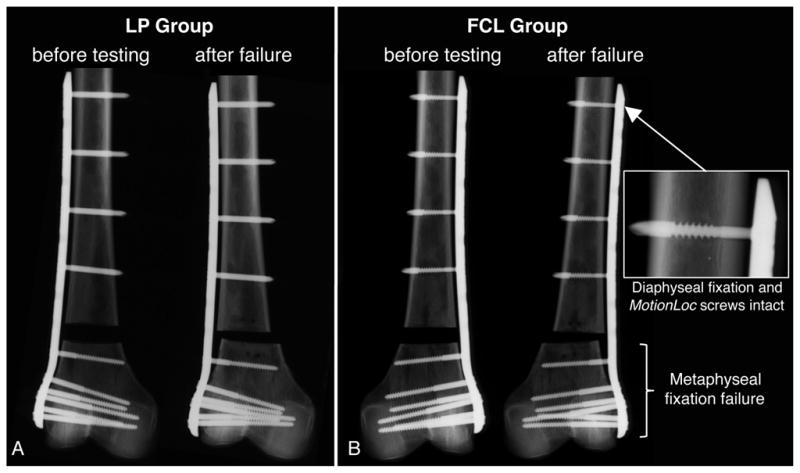
Radiographs of a representative LP (A) and FCL constructs (B), obtained before and after loading to failure. In each group, 10 of the 11 specimens sustained metaphyseal fixation failure. None of the constructs sustained diaphyseal fixation failure. No FCL screw sustained permanent bending or fixation failure.
Discussion
The present study described the efficacy of FCL in conjunction with periarticular locking plates, where FCL screws are confined to diaphyseal fixation. Results support the hypothesis that FCL screws reduce the stiffness of a periarticular locking construct and induce nominally parallel interfragmentary motion while retaining construct strength.
A stiffness reduction of 81% was achieved by FCL fixation in the proximal segment of a periarticular locked plating construct. It approached the 88% stiffness reduction previously reported for diaphyseal plating constructs in which three FCL screws were applied on each side of a diaphyseal plating construct (9). The comparably high stiffness reduction achieved by periarticular FCL constructs despite limiting FCL fixation to only one fracture side can largely be attributed to differences in screw length between the studies. The previous study evaluated diaphyseal fixation constructs with a constant screw length of 32 mm in synthetic bone cylinders. The average screw length required for cadaveric femurs in the present study was 40 mm (range: 38 – 50mm), whereby a 40 mm FCL screw has an approximately 95% higher bending flexibility than a 32 mm long FCL screw. The 81% stiffness reduction provided by FCL with MotionLoc screws in the present study was furthermore higher than the 16% stiffness reduction reported for dynamic locking screws (DLS) (6).
The present study measured a far greater stiffness for the locked plating construct (5,919 N/mm) than previously reported for the same implant (168 N/mm (16) to 1,137 N/mm (17)). While stiffness is simply calculated by dividing an applied load by the resulting displacement, comparing stiffness reports between studies deserves further attention. Reports of construct stiffness can vary by over one order of magnitude for the same implant since they are highly influenced by the test setup, especially when stiffness is calculated from the displacement of the loading actuator. Actuator displacement represents deformation along the entire test specimen and can grossly overestimate the actual motion at the fracture site. Based on actuator displacement, construct stiffness of femoral locking plates has been reported in the range of 63 N/mm to 1,137 N/mm (8, 10, 11, 16, 17). This stiffness range overlaps with that of external fixators (50–400 N/mm) (18–20), suggesting that locked plating constructs would provide sufficient interfragmentary motion to promote fracture healing by callus formation. In the present study, construct stiffness based on actuator displacement was 1,100 N/mm (LP group) and 490 N/mm (FCL group). However, studies that measure the actual displacement at the fracture site with sensors applied to the far cortex report one order of magnitude greater stiffness (2,100 N/mm to 5,000 N/mm) for femoral bridge plating constructs (9, 21–23). Furthermore, flexion of a bridging plate causes asymmetric gap closure, whereby the near cortex adjacent the plate experiences less than half the interfragmentary motion seen by the far cortex (9). The present study therefore captured interfragmentary motion at both the near and far cortex to assess asymmetric gap closure, and to calculate construct stiffness that accurately represents the effective interfragmentary motion experienced at the fracture gap.
Interfragmentary motion in LP constructs was asymmetric and was attenuated towards the near cortex adjacent to the plate. Under one body-weight postoperative weight-bearing, interfragmentary motion at the near cortex of LP constructs was less than 0.1 mm and remained below the 0.2–1.0 mm stimulus range of motion known to promote secondary bone healing (24–26). This deficient interfragmentary motion at the near cortex corresponds to findings of a recent ovine in vivo study, in which 50% of tibial osteotomies stabilized with a locking plate failed to heal at the near cortex (2).
Interfragmentary motion of FCL constructs was nearly parallel, whereby one bodyweight loading yielded 0.57 mm and 0.63 mm motion at the near and far cortex, respectively. This interfragmentary motion corresponds to an 0.6 mm motion envelope of FCL constructs used in an ovine fracture healing model to assess the effect of FCL on fracture healing (2). This in vivo study demonstrated that parallel motion provided by FCL constructs yielded symmetric callus formation and consistent bridging of a gap osteotomy, with FCL specimens healing stronger and tolerating 154% more energy to failure than a control group stabilized with standard locking constructs.
Construct strength was assessed in terms of durability during dynamic loading, and residual strength after dynamic loading. Dynamic load simulation with walking forces of 1,870 N represented exaggerated post-operative loading and did not account for load sharing by progressive callus formation. In addition, 12 of the 22 specimens were either osteopenic or osteoporotic (T-score < −1). Despite this “worst case” combination of exaggerated loading in presence of poor bone quality, only four LP constructs and three FCL constructs failed during durability testing to 100,000 loading cycles. All of these failures were isolated to loss of distal fixation while proximal fixation remained fully intact. Similarly, Stoffel et al. reported that locked plating constructs applied to bridge distal femur fracture in cadaveric specimens consistently failed by loss of distal screw fixation and cut-out (23). Subsequent evaluation of residual strength of the surviving specimens required on average a load of 5 kN in LP constructs and 5.3 kN in FCL constructs to induce failure. Despite these large failure loads, construct failure remained isolated to distal fixation in all but the strongest specimen pair (T-score: 2.5), which failed by diaphyseal fracture. Diaphyseal screws sustained neither bending nor fixation failure in any specimen. The finding that FCL constructs were as strong and durable as LP constructs despite being 81% less stiff may be attributed to two principal characteristics of FCL constructs: First, elastic cantilever bending of FCL screws provides even load distribution to all four FCL screws and thereby reduces stress risers (27). Second, elastic flexion of FCL screws provides added support at the near cortex under elevated loading, enabling load transfer at the near and far cortices (9).
Results of this study are limited to a particular physiologic loading mode representing the stance phase of level walking in a simplified and controlled manner (12, 14). Results are specific to a particular titanium locking plate system and a representative diaphyseal screw configuration. It is important to understand that the ability of FCL screws to reduce construct stiffness and to induce controlled interfragmentary motion relies on establishing a specific motion envelope in the near cortex. This is assured by placing the screw shaft concentrically in the near cortex drill hole. In absence of a concentric placement of the screw shaft, FCL functionality may be diminished or lost. FCL screws in the present study were inserted manually in a standard procedure, demonstrating that FCL functionality can be reliably achieved without the need for additional procedures to ensure concentric screw alignment. Most importantly, while results of the present study document the biomechanical benefits of diaphyseal FCL fixation, clinical studies will be required to document if FCL fixation using periarticular locking plates can improve fracture healing compared to standard locked plating.
In conclusion, the use of FCL screws in place of standard locking screws for diaphyseal fixation of periarticular locking plates significantly reduced construct stiffness and provided controlled interfragmentary motion for promotion of fracture healing by callus formation. FCL constructs delivered nominally parallel interfragmentary motion, whereby the magnitude of interfragmentary motion in response to one body-weight loading corresponded to the range of interfragmentary motion known to promote secondary bone healing (24–26). Finally, FCL constructs were as durable and strong as standard locked constructs. Therefore, FCL screws provide a simple and effective approach to reduce the stiffness of periarticular locking constructs without requiring additional procedures. Future studies are required to determine the effect of periarticular FCL plating constructs on fracture healing.
Acknowledgments
The institution of the authors (JD, SMM, MB) received funding from Zimmer (Warsaw, IN) in support of this study. Several authors (DCF, SMM, MB) have a licensing agreement with Zimmer and may receive payments from Zimmer related to Far Cortical Locking technology.
References
- 1.Kubiak EN, Fulkerson E, Strauss E, et al. The evolution of locked plates. The Journal of bone and joint surgery. 2006;88 (Suppl 4):189–200. doi: 10.2106/JBJS.F.00703. [DOI] [PubMed] [Google Scholar]
- 2.Bottlang M, Lesser M, Koerber J, et al. Far cortical locking can improve healing of fractures stabilized with locking plates. The Journal of bone and joint surgery. 2010;92:1652–1660. doi: 10.2106/JBJS.I.01111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henderson CE, Bottlang M, Marsh JL, et al. Does locked plating of periprosthetic supracondylar femur fractures promote bone healing by callus formation? Two cases with opposite outcomes. The Iowa orthopaedic journal. 2008;28:73–76. [PMC free article] [PubMed] [Google Scholar]
- 4.Lujan TJ, Henderson CE, Madey SM, et al. Locked plating of distal femur fractures leads to inconsistent and asymmetric callus formation. Journal of orthopaedic trauma. 2010;24:156–162. doi: 10.1097/BOT.0b013e3181be6720. [DOI] [PubMed] [Google Scholar]
- 5.Uhthoff HK, Poitras P, Backman DS. Internal plate fixation of fractures: short history and recent developments. J Orthop Sci. 2006;11:118–126. doi: 10.1007/s00776-005-0984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dobele S, Horn C, Eichhorn S, et al. The dynamic locking screw (DLS) can increase interfragmentary motion on the near cortex of locked plating constructs by reducing the axial stiffness. Langenbeck’s archives of surgery/Deutsche Gesellschaft fur Chirurgie. 2010;395:421–428. doi: 10.1007/s00423-010-0636-z. [DOI] [PubMed] [Google Scholar]
- 7.Gardner MJ, Nork SE, Huber P, et al. Less rigid stable fracture fixation in osteoporotic bone using locked plates with near cortical slots. Injury. 2010;41:652–656. doi: 10.1016/j.injury.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Stoffel K, Dieter U, Stachowiak G, et al. Biomechanical testing of the LCP--how can stability in locked internal fixators be controlled? Injury. 2003;34 (Suppl 2):B11–19. doi: 10.1016/j.injury.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Bottlang M, Doornink J, Fitzpatrick DC, et al. Far Cortical Locking can reduce the stiffness of locked plating constructs while retaining construct strength. The Journal of bone and joint surgery. 2009;92 doi: 10.2106/JBJS.H.01038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khalafi A, Curtiss S, Hazelwood S, et al. The effect of plate rotation on the stiffness of femoral LISS: a mechanical study. Journal of orthopaedic trauma. 2006;20:542–546. doi: 10.1097/01.bot.0000244996.45127.ad. [DOI] [PubMed] [Google Scholar]
- 11.Prayson MJ, Datta DK, Marshall MP. Mechanical comparison of endosteal substitution and lateral plate fixation in supracondylar fractures of the femur. Journal of orthopaedic trauma. 2001;15:96–100. doi: 10.1097/00005131-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Zlowodzki M, Williamson S, Cole PA, et al. Biomechanical evaluation of the less invasive stabilization system, angled blade plate, and retrograde intramedullary nail for the internal fixation of distal femur fractures. Journal of orthopaedic trauma. 2004;18:494–502. doi: 10.1097/00005131-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Meyer RW, Plaxton NA, Postak PD, et al. Mechanical comparison of a distal femoral side plate and a retrograde intramedullary nail. Journal of orthopaedic trauma. 2000;14:398–404. doi: 10.1097/00005131-200008000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Bergmann G, Deuretzbacher G, Heller M, et al. Hip contact forces and gait patterns from routine activities. Journal of biomechanics. 2001;34:859–871. doi: 10.1016/s0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 15.Egol KA, Kubiak EN, Fulkerson E, et al. Biomechanics of locked plates and screws. Journal of orthopaedic trauma. 2004;18:488–493. doi: 10.1097/00005131-200409000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Wilkens KJ, Curtiss S, Lee MA. Polyaxial locking plate fixation in distal femur fractures: a biomechanical comparison. Journal of orthopaedic trauma. 2008;22:624–628. doi: 10.1097/BOT.0b013e31818896b3. [DOI] [PubMed] [Google Scholar]
- 17.Otto RJ, Moed BR, Bledsoe JG. Biomechanical comparison of polyaxial-type locking plates and a fixed-angle locking plate for internal fixation of distal femur fractures. Journal of orthopaedic trauma. 2009;23:645–652. doi: 10.1097/BOT.0b013e3181a567c8. [DOI] [PubMed] [Google Scholar]
- 18.Caja V, Kim W, Larsson S, et al. Comparison of the mechanical performance of three types of external fixators: linear, circular and hybrid. Clinical biomechanics (Bristol, Avon) 1995;10:401–406. doi: 10.1016/0268-0033(95)00014-3. [DOI] [PubMed] [Google Scholar]
- 19.Finlay JB, Moroz TK, Rorabeck CH, et al. Stability of ten configurations of the Hoffmann external-fixation frame. The Journal of bone and joint surgery. 1987;69:734–744. [PubMed] [Google Scholar]
- 20.Moroz TK, Finlay JB, Rorabeck CH, et al. External skeletal fixation: choosing a system based on biomechanical stability. Journal of orthopaedic trauma. 1988;2:284–296. [PubMed] [Google Scholar]
- 21.Bong MR, Egol KA, Koval KJ, et al. Comparison of the LISS and a retrograde-inserted supracondylar intramedullary nail for fixation of a periprosthetic distal femur fracture proximal to a total knee arthroplasty. The Journal of arthroplasty. 2002;17:876–881. doi: 10.1054/arth.2002.34817. [DOI] [PubMed] [Google Scholar]
- 22.Fitzpatrick DC, Doornink J, Madey SM, et al. Relative stability of conventional and locked plating fixation in a model of the osteoporotic femoral diaphysis. Clinical biomechanics (Bristol, Avon) 2009;24:203–209. doi: 10.1016/j.clinbiomech.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoffel K, Lorenz KU, Kuster MS. Biomechanical considerations in plate osteosynthesis: the effect of plate-to-bone compression with and without angular screw stability. Journal of orthopaedic trauma. 2007;21:362–368. doi: 10.1097/BOT.0b013e31806dd921. [DOI] [PubMed] [Google Scholar]
- 24.Claes LE, Heigele CA, Neidlinger-Wilke C, et al. Effects of mechanical factors on the fracture healing process. Clinical orthopaedics and related research. 1998:S132–147. doi: 10.1097/00003086-199810001-00015. [DOI] [PubMed] [Google Scholar]
- 25.Goodship AE, Kenwright J. The influence of induced micromovement upon the healing of experimental tibial fractures. J Bone Joint Surg Br. 1985;67:650–655. doi: 10.1302/0301-620X.67B4.4030869. [DOI] [PubMed] [Google Scholar]
- 26.Kenwright J, Richardson JB, Cunningham JL, et al. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br. 1991;73:654–659. doi: 10.1302/0301-620X.73B4.2071654. [DOI] [PubMed] [Google Scholar]
- 27.Bottlang M, Doornink J, Byrd GD, et al. A nonlocking end screw can decrease fracture risk caused by locked plating in the osteoporotic diaphysis. The Journal of bone and joint surgery. 2009;91:620–627. doi: 10.2106/JBJS.H.00408. [DOI] [PubMed] [Google Scholar]