Abstract
In continuing our search of potent antimalarials based on 8-aminoquinoline structural framework, three series of novel bis(8-aminoquinolines) using convenient one to four steps synthetic procedures were synthesized. The bisquinolines were evaluated for in vitro antimalarial (P. falciparum), antileishmanial (L. donovani), antimicrobial (a panel of pathogenic bacteria and fungi), cytotoxicity, β-hematin inhibitory and methemoglobin (MetHb) formation activities. Several compounds exhibited superior antimalarial activities compared to parent drug primaquine. Selected compounds (41, 61 and 79) when tested for in vivo blood-schizontocidal antimalarial activity (P. berghei) displayed potent blood-schizontocial activities. The bisquinolines showed negligible MetHb formation (0.2 – 1.2%) underlining their potential in the treatment of glucose-6-phosphate dehydrogenase deficient patients. The bisquinoline analogues (36, 73 and 79) also exhibited promising in vitro antileishmanial activity, and antimicrobial activities (43, 44 and 76) against a panel of pathogenic bacteria and fungi. The results of this study provide evidence that bis(8-aminoquinolines), like their bis(4-aminoquinolines) and artemisinin dimers counterparts, are a promising class of antimalarial agents.
Introduction
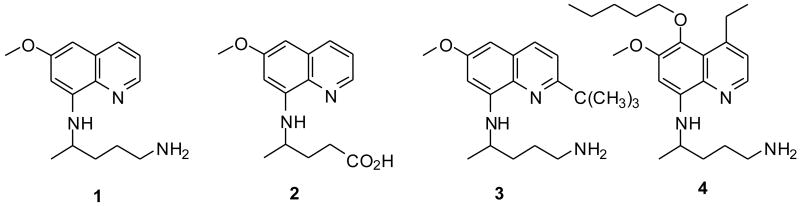
Malaria is a serious parasitic disease, which ranks third among the major infectious diseases in causing deaths after pneumococcal acute respiratory infections, and tuberculosis. Approximately 1.5–2.5 million people die of malaria every year, accounting for about 5% of all fatalities in the world.1 Uncontrolled and irrational use of existing blood-schizontocidal (for example, chloroquine) and limited use of newer blood-schizontocidal antimalarials (for example, artemisinin and its derivatives) has limited the clinical options to treat malaria.2 8-Aminoquinolines exemplified by primaquine (PQ, 1, Fig. 1) display activity against all four species of Plasmodium that infect human. PQ, a tissue schizontocidal antimalarial, is the only clinical drug available for the treatment of relapsing cases of malaria. Although PQ is effective against all life cycle stages of human malaria parasite, its use is often associated with serious adverse effects as a consequence of its toxic metabolites.3-6 Its clinical use as a tissue-schizontocide is limited by side effects such as severe hematotoxicities in patients with the glucose-6-phosphate dehydrogenase (G6PD) deficiency.7 PQ is also known to have poor pharmacokinetic properties with a short half life of 4-6 h.8 It has been shown that in rodents, the PQ is rapidly metabolized, and two major metabolites start appearing in the blood in about 30 min, one of which has been identified as 4-(6-methoxyquinolin-8-ylamino)pentanoic acid (carboxyprimaquine, 2, Fig. 1), a product also reported by the microbiological degradation of PQ.9 Modulation of PQ structure has resulted in less toxic and promising blood-schizontocides.3,9,10 We have recently shown that appropriate substitution on the quinoline ring and on the side-chain results in PQ analogues exhibiting promising antimicrobial and antileishmanial activities, in addition to potent antimalarial activities.11-17
Figure 1.
8-Aminoquinolines
Dimer of known antimalarial agents has been an area of active interest. For example, a number of bis(4-aminoquinolines)18-21 and bis-artemisinins22-24 are known to display activities superior to their monomeric counterparts. Superior activity of artemisinin dimers is attributed to their dual functionality and increased number of peroxide linkage which is essential for expression of activity. The higher efficacy of the bis(4-aminoquinolines) against chloroquine resistant parasites is explained by the greater number of protonation sites compared with chloroquine resulting in their accumulation to a higher degree in the face of a decreased pH gradient in the chloroquine resistant parasites. Although, dimers of 4-aminquinolines and artemisinin are extensively investigated, the potential of bis(8-aminoquinolines) in antimalarial drug discovery is not fully explored. Blanton et al. reported the synthesis of varied chain length amino group containing dimeric 8-aminoquinolines; however, all analogues were inactive as blood-schizontocides.25,26
The importance of dimers in 4-aminoquinoline and artemisinin classes prompted us to examine the unexplored potential of bis(8-aminoquinolines) in antimalarial chemotherapy. The synthesis of bis(8-aminoquinolines) can be justified due to following facts: i) It is known that rapid metabolism of 8-aminoquinolines results in the removal of side-chain amino group to yield inactive metabolites, including 2. We believe that the side-chain primary amino group present as an amide or secondary amine in the synthesized bis(8-aminoquinolines) could prevent metabolic degradation resulting in increased activity; ii) due to increase in steric bulk, the bis(8-aminoquinolines) are expected to penetrate less into the red blood cell that may not allow destabilization of red cell membrane inducing hemolysis, the main cause of toxicity. Thus, synthesis of bis(8-aminoquinolines) appears to be an attractive strategy to develop analogues with improved blood-schizontocidal activity and reduced MetHb toxicity. We report herein, synthesis, detailed antiprotozoal, and antimicrobial activities of bis(8-aminoquinolines) linked through their side-chain using a set of linkers, including amino acids.
Results and Discussion
Chemistry
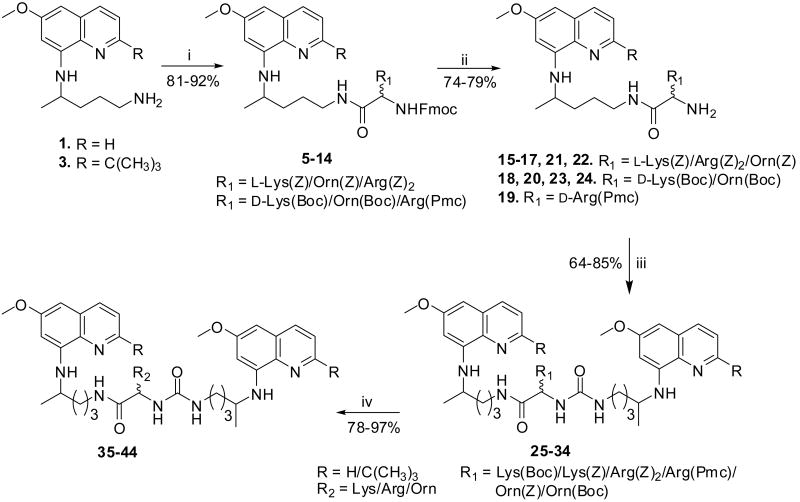
We have earlier observed that the cationic amino acid conjugates of 8-aminoquinolines exhibit promising biological activities.14 The 8-aminoquinolines conjugated to Lys, Arg, and Orn provide two free amino groups (α- and side-chain NH2 or guanidino group), which can be manipulated to synthesize a series of bis(8-aminoquinolines). In order to maintain the structural similarity with earlier reported monomers,14 conjugates of 8-aminoquinolines were coupled through their free α-NH2 group with PQ (1), 2-tert-butylprimaquine (3, Fig. 1) to obtain bis(8-aminoquinolines) linked through cationic amino acids (35-44, Scheme 1). Starting material, PQ (1) was obtained commercially, while its analogues, 3 and (4-ethyl-5-pentyloxy)primaquine (4, Fig. 1) were synthesized following previously published procedure.12,13 The reaction of 1 or 3 with suitably orthogonally protected d/l-amino acids in the presence of 1,3-diisopropylcarbodiimide (DIC) readily provided α- and side-chain NH2 protected amino acid conjugates 5-14. Depending upon its nature, α-NH2 protecting group in 5-14 was removed under acidic or basic conditions to afford 15-24. The compounds 15-24 upon coupling reaction with 1 or 3 in the presence of 1,1′-carbonyldiimidazole (CDI) afforded protected bis(8-quinolinmaines) 25-34. The side-chain amino protecting groups in the compounds 25-34 were removed by either acidolysis or hydrogenolysis to afford desired bis(8-aminoquinolines) 35-44.
Scheme 1.
Reagents and conditions: (i) DIC, Fmoc-NH-CH(R1)-CO2H, DCM, 0 °C - rt, 4 h; (ii) 4N HCl in MeOH, rt, 45 min, 20% NH4OH or 20% piperidine in DCM, 20 min, rt; (iii) CDI, 1 or 3, DCM, rt, 5 h; (iv) Pd-C/H2, MeOH, rt, 4h or 4N HCl in MeOH, rt, 45 min or 8N HCl in MeOH, rt, 8 h.
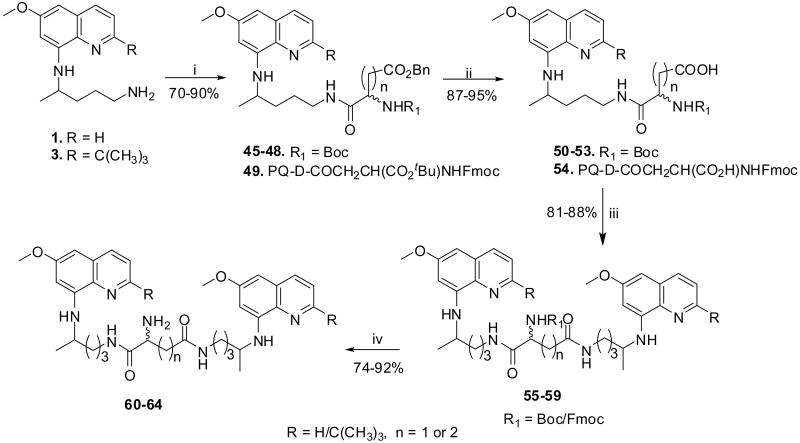
The anionic amino acids, Asp and Glu contain three groups, which provide suitable functionalities for the synthesis of bis(8-aminoquinolines). We have earlier observed that the presence of free NH2 group in the side-chain is essential for the activity of 8-aminoquinolines.14 Therefore, in this series we decided to synthesize bis(8-aminoquinolines) (60-64, Scheme 2) by first coupling 8-aminoquinolines with Asp or Glu residues through their α-CO2H group followed by linking another molecule of 8-aminoquinolines to the side-chain β/γ-CO2H group. The coupling reaction of 1 or 3 with suitable orthogonally protected d/l-amino acids using DIC afforded 45-49. The benzyl ester or tert-butyl ester group in compounds 45-49 was cleaved using Pd-C/H2 or acidolysis to provide analogues 50-54. The latter compounds, 50-54 upon coupling reaction with 1 or 3 using DIC provided side-chain protected analogues 55-59. The t-Boc group in compounds 55-58 was removed by acidolysis to provide 60-63, while Fmoc group in the compound 59 was cleaved with a 20% solution of piperidine to give analogue 64.
Scheme 2.
Reagents and conditions: (i) DIC, R1NH-CHCOOH(CH2)n-CO2Bn, DCM, 0 °C - rt, 4 h; (ii) Pd-C/H2, MeOH, rt, 4h or 6N HCl, rt, 5h, 20%NH4OH; (iii) DIC, 1 or 3, DCM, 0 °C - rt, 4 h; (iv) 4N HCl in MeOH, rt, 45 min or 20% piperidine in DCM, rt, 20 min.
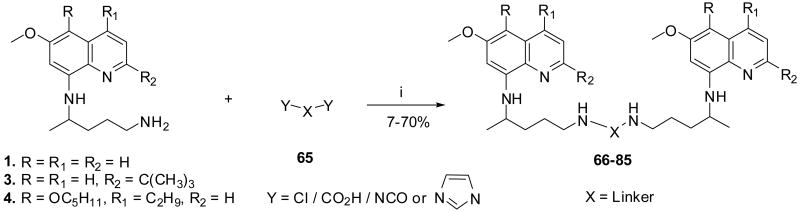
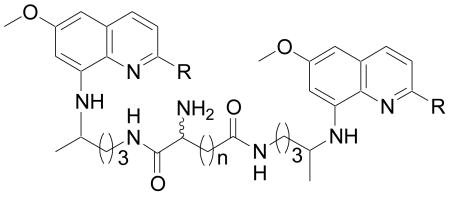
We also report one-pot synthesis of bis(8-aminoquinolines) 66-85 attached via both aliphatic and aromatic linkers to examine their effect on the antimalarial activity (Scheme 3). Bisquinolines 66-85 were obtained by covalent attachment of 1, 3 and 4 through the linker at the side-chain primary amino group. The 8-aminoquinolines 1, 3 and 4 upon reaction with various electrophiles 65 either under neat conditions in the presence of excess triethylamine (Et3N), or in anhydrous tetrahydrofuran (THF), or dichloromethane (DCM) in the presence of catalytic Et3N afforded analogues 66-85 in good yields. Depending upon the relative reactivities of the electrophile, temperature varying from 0 °C to 70 °C and reaction time of 3 h to 24 h was used. Coupling reactions with 1,1′-carbonyldiimidazole (CDI), 1,1′-thiocarbonyldiimidazole (TCDI), bis(2-chloroethyl)amine and 2,6/3,4/3,5-pyridinedicarbonyl chloride were carried out at ambient temperature. The electrophiles like N-(chlorocarbonyl)isocyanate, chloromethyl chloroformate, oxalyl chloride and chlorocarbonylsulfenyl chloride gave products at 0 °C; while, condensation reaction using chloroacetic acid was achieved in THF at 70 °C.
Scheme 3.
Reagents and conditions: (i) Et3N, 0-70 °C, 4-24 h or Et3N, 0-70 °C, THF/CH2Cl2, 4-24 h or CH2Cl2, rt, 24 h.
Antimalarial activity, cytotoxicity, inhibition of β-hematin (BH) and MetHb formation
Determination of in vitro antimalarial activity was based on the assay of plasmodial LDH activity.27 The antimalarial activities of all synthesized analogues are reported as IC50 values against chloroquine-sensitive (D6) and chloroquine-resistant (W2) strains of P. falciparum in Tables 1-3.
Table 1.
In vitro antimalarial activity (P. falciparum), cytotoxicity, β-hematin (BH) inhibition, methemoglobin (MetHb) formation and in vitro antileishmanial activity (L. donovani) of bis(8-aminoquinolines) (35-44) (Series 1)
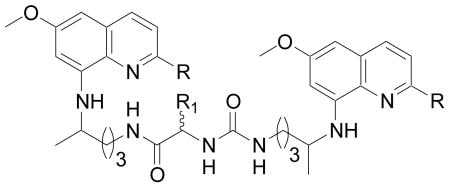 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compd. No. | R | R1 | P. falciparum (D6) | P. falciparum (W2) | Cytotoxicity (Vero) | BH inhibition | MetHb toxicity (% MetHb formation at 20μg/mL) | L. donovani | ||
| IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | IC50(μM) | IC50 (μg/mL) | IC90 (μg/mL) | |||||
| 35 | H | Lys | (L) | 2.7 | 2.7 | NC | 80 | 3.75 | 18 | >40 |
| 36 | H | Arg | (L) | 3.2 | 1.8 | NC | 90 | 3.1 | 3.1 | 7.2 |
| 37 | H | Orn | (L) | 2.8 | 2.0 | NC | 88 | 7.9 | 14 | 40 |
| 38 | H | Lys | (D) | 3.6 | 2.8 | NC | 162 | 2.8 | 18.5 | 36 |
| 39 | H | Arg | (D) | 2.4 | 1.5 | NC | 88 | 5.5 | 19 | 36 |
| 40 | H | Orn | (D) | 4.2 | 2.8 | NC | 154 | 6.10 | 18 | 39 |
| 41 | C(CH3)3 | Lys | (L) | NA | NA | NC | >1000 | 1.1 | 19 | 38 |
| 42 | C(CH3)3 | Orn | (L) | 4.0 | 3.2 | NC | 152 | 0.2 | 18 | 33 |
| 43 | C(CH3)3 | Lys | (D) | 1.6 | 1.3 | NC | 45 | 2.25 | 21 | 36 |
| 44 | C(CH3)3 | Orn | (D) | 0.34 | 0.3 | NC | 10.8 | 2.3 | 20 | 34 |
| PQ | 2.0 | 2.8 | NC | >1000 | 10 | 19.9 | NA | |||
IC50 and IC90 are the sample concentration that kills 50% and 90% cells compared to vehicle control. NC, not cytotoxic up to 10 μg/mL. NA, Not active. Chloroquine: IC50 = 0.014 μg/mL (D6 clone); IC50 = 0.1 μg/mL, (W2 clone). Artemisinin: IC50 = 0.015 μg/mL (D6 clone); IC50 = 0.009 μg/mL (W2 clone). BH inhibition activity: Chloroquine: IC50 = 80 μM, BPQ: IC50 = 2.9 μM, PQ: IC50 > 1000 μM. Antileishmanial activity: Pentamidine: IC50 = 1 μg/mL, IC90 = 3.8 μg/mL. Amphotericin B: IC50 = 0.19 μg/mL, IC90 = 0.35 μg/mL.
Table 3.
In vitro antimalarial activity (P. falciparum), cytotoxicity, β-hematin (BH) inhibition, methemoglobin (MetHb) formation and in vitro antileishmanial activity (L. donovani) of bis(8-aminoquinolines) (66-85) (Series 3)
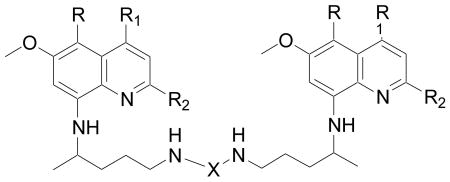 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Compd. No. | R | R1 | R2 | X | P. falciparum (D6) | P. falciparum (W2) | Cytotoxicity (Vero) | BH Inhibition | MetHb toxicity (% MetHb formation at 20μg/mL) | L. donovani | |
| IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μM) | IC50 (μg/mL) | IC90 (μg/mL) | ||||||
| 66 | H | H | H | CO | 4.76 | 4.76 | NC | >1000 | 9.8 | NA | NA |
| 67 | H | H | H | CS | 2.3 | 2.4 | NC | >1000 | 3.7 | 4 | 20 |
| 68 | H | H | C(CH3)3 | CO | 3.0 | 3.7 | NC | >1000 | 1.0 | NA | NA |
| 69 | H | H | C(CH3)3 | CS | 3.0 | 2.3 | NC | >1000 | 0.75 | 9 | 31 |
| 70 | OC5H11 | C2H5 | H | CO | 1.7 | 1.3 | NC | 80 | 4.7 | 3.5 | 7 |
| 71 | OC5H11 | C2H5 | H | CS | NA | 4.76 | NC | >1000 | 1.7 | 40 | NA |
| 72 | H | H | H | COCH2 | NA | NA | NC | >1000 | 20.3 | 20 | NA |
| 73 | H | H | C(CH3)3 | COCH2 | 1.5 | 1.3 | NC | 75 | 1.1 | 2.9 | 7.8 |
| 74 | H | H | H | CONHCO | 4.76 | 3.4 | NC | 155 | 2.25 | NA | NA |
| 75 | H | H | C(CH3)3 | CONHCO | NA | NA | NC | >1000 | 8.8 | 25 | >40 |
| 76 | H | H | H | COOCH2 | 2.6 | 2.7 | NC | 101 | 5.3 | 20 | 38 |
| 77 | H | H | H | COS | NA | 4.76 | NC | 153 | 10.9 | 20 | 38 |
| 78 | H | H | H | COCO | NA | NA | NC | >1000 | 6.45 | 15 | >40 |
| 79 | H | H | H | CH2CH2NHCH2CH2 | 2.6 | 1.5 | NC | 180 | 8.4 | 2.99 | 27 |
| 80 | H | H | H |  |
2.2 | 2.3 | NC | 123 | 5.7 | 6.5 | NA |
| 81 | H | H | H |  |
3.0 | 1.7 | NC | 108 | 3.55 | NA | NA |
| 82 | H | H | H | 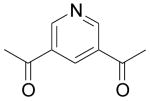 |
3.3 | 3.0 | NC | 110 | 5.5 | 18 | NA |
| 83 | H | H | C(CH3)3 | 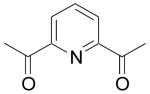 |
NA | 4.5 | NC | >1000 | 0.25 | NA | NA |
| 84 | H | H | C(CH3)3 | 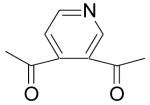 |
1.5 | 0.87 | NC | 70 | 1.2 | 16 | 31 |
| 85 | H | H | C(CH3)3 | 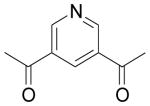 |
2.7 | 1.8 | NC | 120 | 0.55 | 19 | NA |
| PQ | 2.0 | 2.8 | NC | >1000 | 10 | 19.9 | NA | ||||
IC50 and IC90 are the sample concentration that kills 50% and 90% cells compared to vehicle control. NC, not cytotoxic up to 10 μg/mL. NA, Not active. Chloroquine: IC50 = 0.014 μg/mL (D6 clone); IC50 = 0.1 μg/mL (W2 clone). Artemisinin: IC50 = 0.015 μg/mL (D6 clone); IC50 = 0.009 μg/mL (W2 clone). BH inhibition activity: Chloroquine: IC50 = 80 μM, BPQ: IC50 = 2.9 μM, PQ: IC50 > 1000 μM. Antileishmanial activity: Pentamidine: IC50 = 1 μg/mL, IC90 = 3.8 μg/mL. Amphotericin B: IC50 = 0.19 μg/mL, IC90 = 0.35 μg/mL.
The bis(8-aminoquinolines) 35-44 linked through amino acids (Series 1) were active against both strains of plasmodium, except 41. Among the series, 44 [R = C(CH3)3, R1 = d-Orn] was the most active and exhibited IC50 values of 0.34 and 0.30 μg/mL against D6 and W2 strains, respectively. While, analogue 43 showed IC50 of 1.6 and 1.3 μg/mL against D6 and W2 strains, respectively. The IC50 values of remaining analogues were in the range of 2.4 – 4.2 μg/mL for D6 strain and 1.5-3.2 μg/mL for W2 strain. The bis(8-aminoquinolines) 60-64 (Series 2) were less active with IC50 values in the range of 2.7 – 4.76 μg/mL (D6 strain) and 2.3 – 3.8 μg/mL (W2 strain). In Series 3, the most promising analogue 84, a bisquinoline derivative linked through pyridine-3,4-dicarbonyl linker displayed IC50 of 1.5 μg/mL for D6 clone and 0.87 μg/mL for W2 clone. Analogues 70 and 73 showed IC50 of 1.7 and 1.5 μg/mL for D6 clone, respectively, and 1.3 μg/mL for W2 clone. While, analogue 79, displayed IC50 of 2.6 μg/mL, and 1.5 μg/mL for D6 and W2 strains, respectively. Remaining analogues were also active (IC50 values in the range of 1.7 – 4.76 μg/mL) with the exception of 41, 62, 71-72, 75, 77-78, and 83.
In vitro cytotoxicity of all the analogues was determined against mammalian kidney cell line (Vero) up to a highest concentration of 10 μg/mL by neutral red assay.28,29 None of the compounds were found cytotoxic indicating a selectivity of antimalarial action.
Blocking heme detoxification is one of the main mechanism of antimalarial action of quinolines, and we have recently reported that potent blood-schizontocidal antimalarial activity of 3 is possibly due to its inhibition of heme crystallization.30 In another report, effects of amino acids on the formation of β-hematin (BH) was investigated, and results showed that BH formation was significantly inhibited by basic amino acids due to their abilities to complex with heme.31 Therefore, synthesized bisquinolines were assayed for inhibition of BH formation according to a procedure reported earlier.32 Analogue 44 (Series 1) inhibited BH formation with a lower IC50 value (10.8 μM) in comparison to several other analogues of the series (IC50 values ranging between 45 and 162 μM). The compounds 60-64 of the Series 2 inhibited BH formation with IC50 values in the range of 85 – 185 μM. Among bisquinolines 66-85 of Series 3, analogues 70, 73 and 84 displayed IC50 values of 80, 75 and 70 μM, respectively compared to IC50 of 80 μM for standard drug chloroquine (Table 1-3). It has been proposed that pKa of the side chain amino function contributes substantially to antimalarial activity of chloroquine. Therefore, it is possible that reduced basicity of these bisquinolines due to decreased pKa values is responsible for moderate β-hematin inhibition.
Hematotoxicity by 8-aminoquinolines is caused due to their metabolism to the toxic metabolites, which are unstable and difficult to isolate. The synthesized analogues were also tested for metabolism-linked methemoglobin (MetHb) toxicity in vitro and % MetHb formation was calculated at 20 μg/mL of the test compound, in comparison to vehicle control.33 A number of analogues showed lower MetHb formation (Series1: 0.2 – 7.9%, Series 2: 1.55 – 13.35%, and Series 3: 0.25 – 20.3%) compared to PQ (10%) as shown in Tables 1-3. Analogues 42 (Series 1), 62 (Series 2), 68, 69, 83, and 85 (Series 3) induced substantially lower MetHb formation (0.2 – 1.2%) than others, including standard drug, PQ.
Selected analogues were evaluated in vivo for the blood-schizontocidal antimalarial activity against P. berghei (sensitive strain) in a rodent malaria model using procedure described earlier.12 The analogues 44, 61 and 79 exhibited significantly high activity and cured 100% mice at a dose of 25 mg/kg, and were suppressive at the lowest tested dose of 10 mg/kg. The PQ dimer, 36, linked through l-Arg was curative at 100 mg/kg, while analogues 43 and 76 were suppressive at the same concentration with 5/6 mice surviving on day 60. Remaining of the tested analogues 37, 62, 63, 66, 68, and 74 were inactive at the highest test dose of 100 mg/kg.
Antileishmanial activities
Antileishmanial activities of the bisquinolines were evaluated in vitro against L. donovani promastigotes by Alamar Blue assay.34,35 From Series 1, 36 (R = H, R1 = l-Arg) exhibited better antileishmanial activity (IC50 = 3.1 μg/mL, and IC90 = 7.2 μg/mL) compared to other compounds of the series (IC50 in the range of 14 – 21 μg/mL, and IC90 in the range of 33 – 40 μg/mL) as shown in Table 1. Among the analogues of Series 2, 60 (R = H, n = 1) and 63 [R = C(CH3)3, n = 2] were more active (IC50 = 6.2 and 3.9 μg/mL and IC90 = 30 and 10.7 μg/mL, respectively) than others (Table 2). Several bisquinolines of Series 3 also exhibited antileishmanial activities to a considerable extent (Table 3). Of these analogues, 70, 73 and 79 were most potent with IC50 values of 3.5, 2.9, and 2.99 μg/mL and IC90 of 7.0, 7.8, and 27 μg/mL, respectively, compared to the IC50 of 1 μg/mL and IC90 of 3.8 μg/mL for standard drug, pentamidine. Analogues 67, and 69 were also promising (IC50 = 4 – 9 μg/mL and IC90 = 20 – 31 μg/mL).
Table 2.
In vitro antimalarial activity (P. falciparum), cytotoxicity, β-hematin (BH) inhibition, methemoglobin (MetHb) formation and in vitro antileishmanial activity (L. donovani) of bis(8-aminoquinolines) (60-64) (Series 2)
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compd. No. | R | n | P. falciparum (D6) | P. falciparum (W2) | Cytotoxicity (Vero) | BH inhibition | MetHb toxicity (% MetHb formation at 20μg/mL) | L. donovani | ||
| IC50 (μg/mL) | IC50 (μg/mL) | IC50 (μg/mL) | IC50(μM) | IC50 (μg/mL) | IC90 (μg/mL) | |||||
| 60 | H | 1 | (L) | 4.76 | 3.8 | NC | 185 | 5.4 | 6.2 | 30 |
| 61 | H | 2 | (L) | 2.7 | 2.2 | NC | 85 | 13.35 | 16 | 35 |
| 62 | C(CH3)3 | 1 | (L) | NA | 3.2 | NC | 140 | 1.55 | 15 | 33 |
| 63 | C(CH3)3 | 2 | (L) | 4.7 | 2.3 | NC | >1000 | 3.3 | 3.9 | 10.7 |
| 64 | H | 1 | (D) | 3.3 | 3.6 | NC | 120 | 7.25 | 11.5 | 31 |
| PQ | 2.0 | 2.8 | NC | >1000 | 10 | 19.9 | NA | |||
IC50 and IC90 are the sample concentration that kills 50% and 90% cells compared to vehicle control. NC, not cytotoxic up to 10 μg/mL. NA, Not active. Chloroquine: IC50 = 0.014 μg/mL (D6 clone); IC50 = 0.1 μg/mL (W2 clone). Artemisinin: IC50 = 0.015 μg/mL (D6 clone); IC50 = 0.009 μg/mL (W2 clone). BH inhibition activity: Chloroquine: IC50 = 80 μM, BPQ: IC50 = 2.9 μM, PQ: IC50 > 1000 μM. Antileishmanial activity: Pentamidine: IC50 = 1 μg/mL, IC90 = 3.8 μg/mL. Amphotericin B: IC50 = 0.19 μg/mL, IC90 = 0.35 μg/mL.
Antimicrobial activities
Bisquinolines were also tested for their antibacterial properties against Staphylococcus aureus, methicillin-resistant Staphylococcus aureus ATCC 43300 (MRSA), Mycobacterium intracellulare ATCC 23068, Escherichia coli ATCC 35218, and Pseudomonas aeruginosa ATCC 27853. Susceptibility testing is performed using a modified version of the CLSI (formerly NCCLS) methods.36-40 M. intracellulare is tested using a modified method of Franzblau et al.41 None of the analogues were active against E. coli and P. aeruginosa (data not shown). Analogues 43 and 44 were active against S. aureus. Their IC50 values were 1.78 – 2.73 μg/mL and they showed a bactericidal activity at 5 μg/mL, for both strains of S. aureus. Analogues 36-42 also possessed moderate activity against MRSA with IC50 values in the range of 6.5 – 15 μg/mL and MIC of 20 μg/mL. All were bactericidal at 20 μg/mL, except analogues 38 and 40. Activities were also observed against M. intracellulare with 36, 42, 43, and 44 (IC50 ranged between 7.12 – 15 μg/mL, MIC = 20 μg/mL, and MBC = 20 μg/mL, for analogue 36). The bis(8-aminoquinolines) of Series 2 were also active against MRSA; 62 and 63 being most active with IC50 values of 6.5 and 8.5 μg/mL, MIC of 10 and 20 μg/mL, respectively, and bactericidal at 20 μg/mL. Analogues of Series 3 also exhibited antibacterial activity against MRSA with the exception of 79. Of these, 67, 71 and 84 were bactericidal (IC50 = 3 – 15 μg/mL, MIC = 5 – 20 μg/mL, and MBC = 10 – 20 μg/mL). Analogue 67 also exhibited promising activity against M. intracellulare (IC50, MIC and MBC of 4.5, 10 and 20 μg/mL, respectively) (Table 5).
Table 5.
In vitro antibacterial activities of bis(8-aminoquinolines)
| Compd. No. | S. aureus | MRSA | M. intracellulare | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IC50 (μg/mL) |
MIC (μg/mL) |
MBC (μg/mL) |
IC50 (μg/mL) |
MIC (μg/mL) |
MBC (μg/mL) |
IC50 (μg/mL) |
MIC (μg/mL) |
MBC (μg/mL) |
|
| 36 | – | – | – | 15 | 20 | 20 | 15 | 20 | 20 |
| 38 | – | – | – | 10 | 20 | NA | NA | NA | NA |
| 39 | – | – | – | 15 | 20 | 20 | NA | NA | NA |
| 40 | – | – | – | 10 | 20 | NA | NA | NA | NA |
| 41 | – | – | – | 8 | 20 | 20 | NA | NA | NA |
| 42 | – | – | – | 6.5 | 20 | 20 | 15 | 20 | NA |
| 43 | 2.58 | 5 | 5 | 1.97 | 5 | 5 | 7.12 | 20 | NA |
| 44 | 2.73 | 5 | 5 | 1.78 | 5 | 5 | 10.05 | 20 | NA |
| 60 | – | – | – | 15 | 20 | NA | 20 | NA | NA |
| 61 | – | – | – | 15 | 20 | NA | 15 | 20 | NA |
| 62 | – | – | – | 6.5 | 10 | 20 | NA | NA | NA |
| 63 | – | – | – | 8.5 | 20 | 20 | NA | NA | NA |
| 64 | – | – | – | 15 | NA | NA | 15 | 20 | NA |
| 67 | – | – | – | 3.0 | 5.0 | 10 | 4.5 | 10 | 20 |
| 70 | – | – | – | 8.5 | 20 | NA | NA | NA | NA |
| 71 | – | – | – | 3.5 | 5.0 | 10 | 15 | 20 | NA |
| 73 | – | – | – | 15 | 20 | NA | 15 | NA | NA |
| 79 | – | – | – | NA | NA | NA | 10 | 20 | 20 |
| 84 | – | – | – | 15 | 20 | 20 | NA | NA | NA |
IC50 = the concentration (μg/mL) that affords 50% growth inhibition. MIC, minimum inhibitory concentration (the lowest concentration in μg/mL that allows no detectable growth). MBC, minimum bactericidal concentration (the lowest concentration in μg/mL that kills the organism). “–” not tested. NA, no activity at the highest test concentration of 20 μg/mL. Ciprofloxacin: IC50 = 0.12 μg/mL, MIC = 0.50 μg/mL, MBC = 50 μg/mL (Sa); IC50 = 0.09 μg/mL, MIC = 0.31 μg/mL, MBC = 2.5 μg/mL (MRSA); IC50 = 0.3 μg/mL, MIC = 0.63 μg/mL, MBC = 2.5 μg/mL (Mi).
The antifungal activities of the bis(8-aminoquinolines) against the opportunistic fungi Candida albicans ATCC 90028, Cryptococcus neoformans ATCC 90113, and Aspergillus fumigatus ATCC 90906, along with the positive control amphotericin B are reported in Table 6. All compounds were inactive at 20 μg/mL against C. glabrata, C. krusei, and A. fumigatus (data not shown). The bis(8-aminoquinolines) of all three series showed activities against C. neoformans to a variable extent and were found to be fungicidal except 67 (Table 6). The bis(8-aminoquinolines) 60, 61, and 62 (Series 2) showed moderate activity against C. neoformans. The analogue 60 produced IC50 value of 7.5 μg/mL, and MIC and MFC values of 10 μg/mL; while, analogues 61 and 62 exhibited the IC50 values of 15 and 10 μg/mL, respectively, and were fungicidal at 20 μg/mL. Among the bis(8-aminoquinolines) of Series 3, analogues 67, 71, 76 and 79 were active against C. neoformans with IC50 values ranging between 4.5 and 15 μg/mL. The promising analogue 76 exhibited IC50 of 4.5 μg/mL, MIC of 5 μg/mL, and was also fungicidal at 5 μg/mL.
Table 6.
In vitro antifungal activities of bis(8-aminoquinolines)
| Compd. No. | C. albicans | C. neoformans | ||||
|---|---|---|---|---|---|---|
| IC50 (μg/mL) |
MIC (μg/mL) |
MFC (μg/mL) |
IC50 (μg/mL) | MIC (μg/mL) |
MFC (μg/mL) |
|
| 36 | NA | NA | NA | 6.5 | 10 | 10 |
| 37 | NA | NA | NA | 15 | 20 | 20 |
| 38 | NA | NA | NA | 10 | 20 | 20 |
| 39 | NA | NA | NA | 15 | 20 | 20 |
| 40 | NA | NA | NA | 7 | 10 | 10 |
| 42 | 15 | NA | NA | 15 | 20 | 20 |
| 43 | 17.86 | 20 | NA | 6.45 | 10 | 10 |
| 44 | >20 | NA | NA | 9.97 | 20 | 20 |
| 60 | NA | NA | NA | 7.5 | 10 | 10 |
| 61 | NA | NA | NA | 15 | 20 | 20 |
| 62 | NA | NA | NA | 10 | 20 | 20 |
| 67 | 10 | 20 | NA | 15 | NA | NA |
| 71 | 10 | 20 | NA | 10 | 20 | 20 |
| 76 | NA | NA | NA | 4.5 | 5 | 5 |
| 79 | NA | NA | NA | 15 | 20 | 20 |
IC50 = the concentration (μg/mL) that affords 50% growth inhibition. MIC, minimum inhibitory concentration (the lowest concentration in μg/mL that allows no detectable growth). MFC, minimum fungicidal concentration (the lowest concentration in μg/mL that kills the organism). NA, no activity at the highest test concentration of 20 μg/mL. Amphotericin B: IC50 = 0.25 μg/mL, MIC = 0.63 μg/mL, MFC = 1.25 μg/mL (Ca); IC50 = 0.75 μg/mL, MIC = 1.25 μg/mL, MFC = 1.5 μg/mL (Cn).
Conclusions
We have synthesized three series of bisquinolines based upon 8-aminoquinolines structural framework. The bis(8-aminoquinolines) produced promising antimalarial activity in vitro against drug-sensitive and drug-resistant strains of P. falciparum, and potent activity in the rodent malaria model in vivo. Inhibition of β-hematin formation by bisquinolines, although moderate, indicated it as a plausible pathway of their antimalarial activity. MetHb formation was observed for a considerably lower extent by several analogues compared to PQ, thereby offering new avenues for the treatment of patients suffering from severe hematotoxicities due to the deficiency of cytosolic enzyme G6PD. Most promising analogue 44 not only produced promising in vitro and in vivo antimalarial activity but also exhibited promising in vitro antimicrobial activities. Analogues 36, 70 and 73 exhibited best antileishmanial activities, while analogue 76 exhibited highest antifungal activity (against C. neoformans). The combination of broad spectrum of activities without any cytotoxic effects to the mammalian cells added to substantially reduced MetHb formation capabilities makes bis(8-aminoquinolines) as a promising new structural class of compounds.
Experimental
Material and Methods
The synthesized bisquinolines were checked for their purity on pre-coated silica gel G254 TLC plates (Merck) and the spots were visualized under UV light and by exposing them to iodine vapors. Column chromatographic purification was carried out on Merck silica gel (100-200 mesh). Melting points were recorded on a capillary melting point apparatus and are uncorrected. All solvents used for synthesis were of analytical grade and used without any further purification unless otherwise stated. 1H and 13C NMR spectra were recorded on a 300 MHz Bruker FT-NMR (Avance DPX 300) spectrometer using tetramethylsilane as internal standard and the chemical shifts are reported in δ units. Mass spectra were recorded on a Finnigan Mat LCQ spectrometer (APCI/ESI). Elemental analyses were recorded on Elementar Vario EL spectrometer. The elemental analyses of all final compounds were within ± 0.4% of the expected values, unless otherwise stated. All reagents were purchased from Aldrich Chemicals Ltd.
General method for the synthesis of protected bis(8-quinolinamines) (25-34)
A mixture of 15-24 (0.58 mmol) and CDI (0.64 mmol) in anhydrous CH2Cl2 (5 mL) was stirred at ambient temperature for 30 min. At that time, TLC showed the absence of starting material. 8-Quinolinamine (1 or 3, 0.58 mmol) was added and the reaction mixture was stirred for another 5 h. The reaction mixture was diluted with CH2Cl2 (20 mL) and washed with water (3 × 10 mL) followed by brine solution (5 mL). The organic layer was dried (Na2SO4) and concentrated to afford crude product. Purified by column chromatography on silica gel (100-200 mesh) using 1.5% CH3OH in CH2Cl2 to give 25-34 as viscous oil.
Benzyl{(5R)-6-({4-[(6-methoxyquinolin-8-yl)amino]pentyl}amino)-5-[({4-[(6-methoxy-quinolin-8-yl)amino]pentyl}carbamoyl)amino]-6-oxohexyl}carbamate (25)
Yield: 85%; oil; IR (CH2Cl2): 3338, 1696 cm-1; 1H NMR (CDCl3): δ 8.51 (d, 2H, J = 4.4 Hz), 7.89 (d, 2H, J = 8.1 Hz), 7.30 (m, 7H), 6.87 (bs, 2H), 6.32 (m, 2H), 6.25 (m, 2H), 6.02 (bs, 2H), 5.05 (s, 2H), 4.96 (bs, 2H), 4.17 (t, 1H, J = 5.1 Hz), 3.87 (s, 6H), 3.55 (m, 2H), 3.26 (m, 6H), 1.61 (m, 14H), 1.25 (d, 6H, J = 6.0 Hz); 13C NMR (CDCl3): δ 174.8, 159.4, 156.4, 144.9, 144.3, 136.6, 135.3, 129.8, 128.4, 128.0, 121.8, 96.7, 91.6, 66.5, 55.2, 54.9, 51.9, 47.8, 42.1, 40.6, 34.6, 29.6, 23.4, 22.7, 20.5; MS (ESI): m/z 807 (M+1); anal. for C45H58N8O6 (806.5), calcd: C, 66.97; H, 7.24; N, 13.89; found: C, 67.07; H, 7.15; N, 13.73.
Benzyl(N′-[(benzyloxy)carbonyl]-N-{(4R)-5-({4-[(6-methoxyquinolin-8-yl)amino]-pentyl}amino)-4-[({4-[(6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-5-oxopentyl}carbamimidoyl)carbamate (26)
Yield: 77%; oil; IR (CH2Cl2): 3436, 1693 cm-1; 1H NMR (CDCl3): δ 9.32 (bs, 2H), 8.49 (d, 2H, J = 4.1 Hz), 7.87 (d, 2H, J = 8.5 Hz), 7.31 (m, 12H), 6.83 (bs, 2H), 6.35 (m, 2H), 6.28 (m, 2H), 5.98 (bs, 2H), 5.11 (s, 2H), 5.09 (s, 2H), 5.02 (bs, 2H), 4.27 (t, 1H, J = 5.8 Hz), 3.86 (s, 6H), 3.62 (m, 2H), 3.22 (m, 6H), 1.69 (m, 12H), 1.30 (d, 6H, J = 6.5 Hz); 13C NMR (CDCl3): δ 171.2, 160.1, 159.6, 156.8, 144.9, 143.9, 135.6, 134.8, 129.9, 128.8, 128.5, 127.9, 122.2, 120.5, 96.8, 92.1, 65.7, 55.1, 47.9, 45.8, 41.3, 39.7, 39.2, 33.7, 32.8, 28.3, 25.2, 21.7; MS (APCI): m/z 969 (M+1); anal. for C53H64N10O8 (968.4), calcd: C, 65.68; H, 6.66; N, 14.45; found: C, 65.75; H, 6.72; N, 14.32.
Benzyl{(4R)-5-({4-[(6-methoxyquinolin-8-yl)amino]pentyl}amino)-4-[({4-[(6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-5-oxopentyl}carbamate (27)
Yield: 79%; oil; IR (CH2Cl2): 3338, 1695 cm-1; 1H NMR (CDCl3): δ 8.52 (d, 2H, J = 4.2 Hz), 7.92 (d, 2H, J = 8.6 Hz), 7.34 (m, 7H), 6.70 (bs, 2H), 6.31 (m, 2H), 6.25 (m, 2H), 5.95 (bs, 2H), 5.11 (s, 2H), 4.78 (bs, 1H), 4.68 (bs, 1H), 4.30 (t, 1H, J = 5.3 Hz), 3.88 (s, 6H), 3.53 (m, 2H), 3.21 (m, 4H), 3.14 (t, 2H, J = 5.9 Hz), 1.78-1.53 (m, 12H), 1.32 (d, 6H, J = 6.3 Hz); 13C NMR (CDCl3): δ 173.0, 159.4, 159.3, 156.5, 144.9, 144.4, 135.8, 135.3, 134.8, 129.9, 122.0, 121.8, 96.7, 92.1, 91.6, 65.2, 55.1, 47.9, 40.8, 40.3, 33.8, 33.7, 31.2, 30.9, 28.3, 26.0, 20.7; MS (APCI): m/z 793 (M+1); anal. for C44H56N8O6 (792.3), calcd: C, 66.64; H, 7.12; N, 14.13; found: C, 66.58; H, 7.24; N, 14.27.
tert-Butyl{(5S)-6-({4-[(6-methoxyquinolin-8-yl)amino]pentyl}amino)-5-[({4-[(6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-6-oxohexyl}carbamate (28)
Yield: 75%; oil; IR (CH2Cl2): 3334, 1705, 1665 cm-1; 1H NMR (CDCl3): δ 8.69 (m, 2H), 8.08 (m, 2H), 7.47 (m, 2H), 7.00 (bs, 1H), 6.49 (m, 2H), 6.42 (m, 2H), 5.87 (bs, 1H), 5.78 (bs, 1H), 5.33 (bs, 1H), 5.23 (bs, 1H), 4.39 (t, 1H, J = 5.7 Hz), 4.04 (s, 6H), 3.70 (m, 2H), 3.36 (m, 6H), 1.91 (m, 14H), 1.57 (s, 9H), 1.41 (m, 6H); 13C NMR (CDCl3): δ 173.7, 158.8, 156.8, 145.4, 144.8, 135.8, 135.4, 122.4, 97.3, 92.1, 81.2, 55.7, 54.2, 48.3, 48.2, 40.7, 39.7, 34.5, 34.3, 32.7, 32.4, 30.1, 28.9, 27.4, 26.6, 23.2, 21.0; MS (APCI): m/z 773 (M+1); anal. for C42H60N8O6 (772.4), calcd: C, 65.26; H, 7.82; N, 14.50; found: C, 65.34; H, 7.74; N, 14.63.
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)-N5-{N-[(2,2,5,7,8-pentamethyl-3,4-dihydro-2H-chromen-6-yl)sulfonyl]carbamimidoyl}-l-ornithinamide (29)
Yield: 64%; oil; IR (CH2Cl2): 3031, 1665, 1386 cm-1; 1H NMR (CDCl3): δ 8.72 (bs, 2H), 8.42 (d, 2H, J = 2.8 Hz), 7.84 (d, 2H, J = 7.8 Hz), 7.22 (dd, 2H, J = 2.8 and 7.8 Hz), 6.25 (d, 2H, J = 2.1 Hz), 6.18 (d, 2H, J = 2.1 Hz), 5.62 (bs, 2H), 4.25 (t, 1H, J = 6.4 Hz), 3.91 (s, 6H), 3.50 (m, 2H), 3.21 (m, 6H), 2.49 (m, 11H), 1.72 (m, 12H), 1.21 (m, 12H); 13C NMR (CDCl3): δ 172.1, 158.3, 156.6, 155.4, 152.6, 143.8, 134.4, 134.1, 133.8, 128.9, 123.1, 120.8, 117.0, 95.8, 90.7, 54.1, 46.7, 43.9, 39.6, 38.5, 32.9, 31.7, 25.7, 25.0, 24.5, 24.1, 23.2, 20.3, 19.3, 17.5, 16.4; MS (ESI): m/z 989.1 (M+23); anal. for C51H70N10O7S (966.5), calcd: C, 63.33; H, 7.29; N, 14.48; found: C, 63.24; H, 7.35; N, 14.65.
tert-Butyl{(4S)-5-({4-[(6-methoxyquinolin-8-yl)amino]pentyl}amino)-4-[({4-[(6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-5-oxopentyl}carbamate (30)
Yield: 71%; oil; IR (CH2Cl2): 3436, 1681 cm-1; 1H NMR (CDCl3): δ 8.51 (m, 2H), 7.91 (m, 2H), 7.30 (m, 2H), 6.32 (m, 2H), 6.24 (m, 2H), 4.31 (t, 1H, J = 6.1 Hz), 3.86 (s, 6H), 3.51 (m, 2H), 3.16 (m, 6H), 1.75 (m, 12H), 1.38 (s, 9H), 1.28 (m, 6H); 13C NMR (CDCl3): δ 172.3, 159.2, 157.8, 145.6, 143.8, 135.3, 135.0, 122.7, 97.2, 92.1, 80.8, 55.7, 54.2, 48.8, 48.2, 40.6, 38.8, 34.7, 32.8, 32.4, 30.1, 28.9, 27.4, 23.6, 23.2, 21.0; MS (APCI): m/z 759 (M+1); anal. for C41H58N8O6 (758.4), calcd: C, 64.88; H, 7.70; N, 14.76; found: C, 64.75; H, 7.64; N, 14.81.
Benzyl{(5R)-6-({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}amino)-5-[({4-[(2-tert-but-yl-6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-6-oxohexyl}carbamate (31)
Yield: 80%; oil; IR (CH2Cl2): 3436, 1691 cm-1; 1H NMR (CDCl3): δ 7.85 (d, 2H, J = 8.5 Hz), 7.42 (d, 2H, J = 8.5 Hz), 7.37 (m, 5H), 6.29 (d, 2H, J = 1.5 Hz), 6.24 (d, 2H, J = 1.5 Hz), 5.07 (s, 2H), 4.11 (t, 1H, J = 6.0 Hz), 3.85 (s, 6H), 3.51 (m, 2H), 3.22 (m, 6H), 1.84 (m, 14H), 1.41 (s, 18H), 1.28 (d, 6H, J = 6.2 Hz); 13C NMR (CDCl3): δ 173.8, 163.8, 159.3, 158.8, 158.4, 157.2, 145.4, 137.1, 135.5, 134.1, 129.0, 128.6, 128.5, 128.0, 119.3, 97.1, 91.9, 67.0, 57.4, 55.6, 54.2, 48.4, 48.3, 41.0, 40.8, 39.9, 38.2, 34.6, 32.8, 30.8, 29.9, 29.7, 27.4, 26.4, 23.1, 21.1; MS (APCI): m/z 919 (M+1); anal. for C53H74N8O6 (918.5), calcd: C, 69.25; H, 8.11; N, 12.19; found: C, 69.33; H, 8.20; N, 12.05.
Benzyl{(4R)-5-({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}amino)-4-[({4-[(2-tert-but-yl-6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-5-oxopentyl}carbamate (32)
Yield: 77%; oil; IR (CH2Cl2): 3385, 1663 cm-1; 1H NMR (CDCl3): δ 7.85 (d, 2H, J = 8.5 Hz), 7.41 (d, 2H, J = 8.5 Hz), 7.29 (m, 5H), 6.54 (bs, 2H), 6.29 (d, 2H, J = 2.7 Hz), 6.23 (d, 2H, J = 2.7 Hz), 6.09 (bs, 2H), 5.85 (bs, 1H), 5.04 (s, 2H), 4.87 (bs, 1H), 3.85 (s, 6H), 3.54 (m, 2H), 3.20 (m, 7H), 1.61 (m, 12H), 1.40 (s, 18H), 1.28 (m, 6H); 13C NMR (CDCl3): δ 172.8, 163.4, 163.3, 158.8, 158.7, 144.8, 139.2, 134.9, 133.5, 128.4, 128.0, 127.4, 118.7, 114.0, 96.6, 91.4, 66.6, 55.1, 53.1, 47.9, 47.6, 40.0, 39.3, 37.6, 34.0, 33.7, 31.6, 30.2, 29.6, 29.3, 29.1, 28.9, 26.7, 25.9, 22.6, 20.6; MS (MALDI): m/z 905 (M+1); anal. for C52H72N8O6 (904.5), calcd: C, 69.00; H, 8.02; N, 12.38; found: C, 69.07; H, 8.17; N, 12.51.
tert-Butyl{(5S)-6-({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}amino)-5-[({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-6-oxohexyl}carbamate (33)
Yield: 84%; oil; IR (CH2Cl2): 3360, 1673 cm-1; 1H NMR (CDCl3): δ 7.85 (d, 2H, J = 8.5 Hz), 7.42 (d, 2H, J = 8.5 Hz), 6.48 (bs, 1H), 6.30 (m, 2H), 6.22 (m, 2H), 6.11 (bs, 2H), 5.06 (bs, 2H), 4.21 (t, 1H, J = 5.8 Hz), 3.87 (s, 6H), 3.56 (m, 2H), 3.19 (m, 6H), 1.65 (m, 14H), 1.41 (m, 27H), 1.28 (d, 6H, J = 6.2 Hz); 13C NMR (CDCl3): δ 174.3, 169.0, 163.4, 157.5, 145.4, 135.5, 134.1, 128.0, 119.3, 97.1, 91.9, 71.2, 55.7, 48.4, 38.2, 34.2, 30.8, 30.1, 28.9, 21.2, 21.1; MS (APCI): m/z 885 (M+1); anal. for C50H76N8O6 (884.5), calcd: C, 67.84; H, 8.65; N, 12.66; found: C, 67.79; H, 8.74; N, 12.54.
tert-Butyl{(4S)-5-({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}amino)-4-[({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}carbamoyl)amino]-5-oxopen-tyl}carbamate (34)
Yield: 80%; oil; IR (CH2Cl2): 3378, 1686 cm-1; 1H NMR (CDCl3): δ 7.86 (d, 2H, J = 8.5 Hz), 7.42 (d, 2H, J = 8.5 Hz), 6.66 (bs, 1H), 6.30 (m, 4H), 6.11 (bs, 2H), 5.20 (bs, 1H), 4.73 (bs, 1H), 3.86 (s, 6H), 3.56 (m, 3H), 3.18 (m, 6H), 1.64 (m, 12H), 1.41 (m, 27H), 1.28 (m, 6H); 13C NMR (CDCl3): δ 172.9, 163.4, 163.3, 158.8, 158.7, 144.9, 139.3, 134.9, 133.5, 118.7, 114.0, 96.6, 91.4, 80.6, 66.6, 55.1, 53.1, 47.9, 40.0, 39.3, 37.6, 33.7, 31.6, 29.3, 22.6, 21.2; MS (APCI): m/z 871 (M+1); anal. for C49H74N8O6 (870.5), calcd: C, 67.56; H, 8.56; N, 12.86; found: C, 67.46; H, 8.51; N, 12.94.
General method for the synthesis of N-{4-[(6-methoxy-2-substituted-quinolin-8-yl)amino]-pentyl}-N2-({4-[(6-methoxy-2-substituted-quinolin-8-yl)amino]pentyl}-carbamoyl)-d/l-lysinamide/argininamide/ornithinamide (35-44)
a) Removal of Z group
To a mixture of 25-27, 31 and 32 (0.12 mmol) and 10% Pd-C (0.04 g) in glacial acetic acid (1 mL) and CH3OH (20 mL) was bubbled H2 gas for 4 h. The catalyst was removed and solvent evaporated to obtain the product as oily syrup, which upon treatment with ethereal HCl (2N solution) provided 35-37, 41 and 42 as hydrochloride salts.
b) Removal of t-Boc and Pmc groups
A solution of 28-30, 31 and 32 (0.43 mmol) in 6N methanolic HCl (10 mL) was stirred at ambient temperature for 45 min (for t-Boc group) or 8N HCl in MeOH (10 mL) for 8 h (for Pmc group). The solvent was removed to afford 38-40, 41 and 42 as hygroscopic salts.
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)-amino]pentyl}carbamoyl)-d-lysinamide·3HCl (35)
Yield: 81%; hygroscopic solid; IR (KBr): 3338, 1664 cm-1; 1H NMR (CD3OD): δ 8.84 (m, 4H), 7.90 (m, 2H), 6.81 (m, 4H), 4.25 (t, 1H, J = 5.1 Hz), 3.97 (s, 6H), 3.76 (m, 2H), 3.35 (m, 4H), 2.96 (t, 2H, J = 5.6 Hz), 1.74 (m, 14H), 1.25 (d, 6H, J = 6.5 Hz); 13C NMR (CD3OD): δ 172.1, 161.9, 158.9, 145.3, 140.2, 139.3, 133.0, 122.6, 106.7, 97.4, 56.1, 50.9, 41.0, 39.9, 33.3, 32.1, 27.4, 26.8, 26.1, 23.3, 19.8; MS (APCI): m/z 673 (M+1).
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)-amino]pentyl}carbamoyl)-d-argininamide·3HCl (36)
Yield: 78%; hygroscopic solid; IR (KBr): 3435 cm-1; 1H NMR (free base, CDCl3): δ 8.48 (d, 2H, J = 4.0 Hz), 7.87 (d, 2H, J = 8.2 Hz), 7.71 (bs, 1H), 7.42 (bs, 1H), 7.24 (dd, 2H, J = 4.0 and 8.2 Hz), 6.98 (bs, 1H), 6.28 (m, 2H), 6.21 (m, 2H), 5.89 (bs, 2H), 4.13 (t, 1H, J = 6.0 Hz), 3.82 (s, 6H), 3.46 (m, 2H), 3.05 (m, 6H), 1.51 (m, 12H), 1.25 (d, 6H, J = 6.0 Hz); 13C NMR (free base, CDCl3): δ 173.5, 159.3, 158.9, 157.3, 144.8, 144.3, 135.2, 134.9, 129.9, 121.9, 96.9, 91.7, 55.2, 53.4, 47.4, 40.8, 40.2, 39.4, 33.9, 29.7, 26.8, 25.9, 25.0, 20.3; MS (APCI): m/z 701 (M+1).
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)-amino]pentyl}carbamoyl)-d-ornithinamide·3HCl (37)
Yield: 83%; hygroscopic solid; IR (KBr): 3383 cm-1; 1H NMR (CD3OD): δ 8.86 (m, 2H), 8.80 (m, 2H), 7.92 (m, 2H), 7.04 (m, 2H), 6.91 (m, 2H), 4.10 (t, 1H, J = 5.7 Hz), 3.98 (m, 6H), 3.81 (m, 2H), 2.99 (m, 6H), 1.98-1.74 (m, 12H), 1.36 (m, 6H); 13C NMR (CD3OD): δ 173.8, 170.1, 158.6, 145.9, 141.3, 134.0, 123.5, 98.1, 92.7, 56.7, 54.9, 50.2, 40.8, 40.4, 34.6, 30.4, 29.9, 27.2, 25.4, 24.2, 20.1; MS (APCI): m/z 659 (M+1).
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)-amino]pentyl}carbamoyl)-l-lysinamide·3HCl (38)
Yield: 97%; hygroscopic solid; IR (KBr): 3404, 1648 cm-1; 1H NMR (CD3OD): δ 8.96 (m, 6H), 7.89 (m, 2H), 7.61 (m, 2H), 4.19 (t, 1H, J = 5.2 Hz), 3.96 (s, 6H), 3.73 (m, 2H), 3.35 (m, 4H), 3.01 (t, 2H, J = 6.0 Hz), 1.71 (m, 14H), 1.32 (m, 6H); 13C NMR (CD3OD): δ 176.0, 162.8, 161.1, 145.9, 141.3, 140.3, 135.7, 133.9, 120.9, 107.1, 97.4, 57.0, 54.2, 41.4, 40.9, 34.3, 28.4, 28.2, 27.3, 24.2, 20.1; MS (APCI): m/z 673 (M+1).
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)-amino]pentyl}carbamoyl)-l-argininamide·3HCl (39)
Yield: 86%; hygroscopic solid; IR (KBr): 3433 cm-1; 1H NMR (CD3OD): δ 8.88 (m, 2H), 8.73 (m, 2H), 7.81 (m, 2H), 7.52 (m, 4H), 4.15 (t, 1H, J = 5.7 Hz), 3.87 (s, 6H), 3.64 (m, 2H), 3.15 (m, 6H), 2.02-1.75 (m, 12H), 1.24 (d, 6H, J = 5.3 Hz); 13C NMR (CD3OD): δ 175.6, 162.9, 161.9, 161.5, 159.0, 146.7, 146.2, 141.2, 140.4, 139.2, 138.8, 135.7, 134.0, 120.9, 97.4, 92.1, 57.1, 42.4, 41.6, 34.4, 34.1, 33.9, 31.1, 28.1, 27.5, 26.8, 22.7, 20.3; MS (APCI): m/z 701 (M+1).
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)-amino]pentyl}carbamoyl)-l-ornithinamide·3HCl (40)
Yield: 91%; hygroscopic solid; IR (KBr): 3400, 1631 cm-1; 1H NMR (CD3OD): δ 8.86 (m, 2H), 8.74 (m, 2H), 7.81 (m, 2H), 6.90 (m, 2H), 6.85 (m, 2H), 4.13 (t, 1H, J = 6.0 Hz), 3.87 (s, 6H), 3.65 (m, 2H), 3.22 (m, 4H), 2.90 (t, 2H, J = 6.4 Hz), 1.62 (m, 12H), 1.29 (m, 6H); 13C NMR (CD3OD): δ 171.4, 160.9, 144.6, 143.8, 139.7, 138.5, 133.9, 132.1, 121.7, 121.6, 119.1, 97.6, 93.1, 55.2, 53.4, 39.5, 38.7, 32.5, 29.5, 29.1, 28.7, 26.5, 25.6, 23.9, 23.6, 21.5; MS (APCI): m/z 659 (M+1).
N-{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(2-tert-butyl-6-methoxyquinlin-8-yl)amino]pentyl}carbamoyl)-d-lysinamide·3HCl (41)
Yield: 84%; hygroscopic solid; IR (KBr): 3391, 1643 cm-1; 1H NMR (free base, CDCl3): δ 7.85 (d, 2H, J = 7.8 Hz), 7.42 (d, 2H, J = 7.8 Hz), 6.76 (bs, 1H), 6.29 (m, 2H), 6.22 (m, 2H), 6.10 (bs, 2H), 4.67 (bs, 2H), 4.23 (t, 1H, J = 6.4 Hz), 3.85 (s, 6H), 3.53 (m, 2H), 3.21 (m, 4H), 2.67 (t, 2H, J = 5.3 Hz), 2.22 (bs, 2H), 1.60 (m, 14H), 1.40 (s, 18H), 1.27 (d, 6H, J = 5.7 Hz); 13C NMR (free base, CDCl3): δ 174.3, 169.0, 163.2, 157.7, 145.4, 144.0, 135.5, 134.1, 128.0, 119.3, 97.1, 91.9, 55.7, 53.0, 48.4, 38.2, 34.6, 30.8, 30.2, 21.1; MS (APCI): m/z 785 (M+1).
N-{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(2-tert-butyl-6methoxyquinolin-8-yl)amino]pentyl}carbamoyl)-d-ornithinamide·3HCl (42)
Yield: 79%; hygroscopic solid; IR (KBr): 3403, 1678 cm-1; 1H NMR (CD3OD): δ 8.37 (d, 2H, J = 8.1 Hz), 7.81 (d, 2H, J = 8.1 Hz), 7.20 (m, 2H), 7.18 (m, 2H), 4.10 (t, 1H, J = 5.1 Hz), 3.98 (s, 6H), 3.86 (m, 2H), 3.25 (m, 6H), 1.77 (m, 12H), 1.49 (s, 18H), 1.28 (d, 6H, J = 6.1 Hz); 13C NMR (CD3OD): δ 175.1, 169.3, 158.2, 145.0, 144.8, 137.9, 132.4, 121.9, 119.3, 97.1, 91.9, 60.1, 56.8, 54.9, 40.7, 39.3, 32.1, 30.8, 30.4, 27.5, 26.6, 25.0, 21.1; MS (APCI): m/z 771 (M+1).
N-{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(2-tert-butyl-6methoxyquinolin-8-yl)amino]pentyl}carbamoyl)-l-lysinamide·3HCl (43)
Yield: 92%; hygroscopic solid; IR (KBr): 3360, 1686 cm-1; 1H NMR (CD3OD): δ 8.34 (d, 2H, J = 8.8 Hz), 7.81 (d, 2H, J = 8.8 Hz), 7.71 (m, 2H), 7.59 (m, 2H), 4.12 (t, 1H, J = 7.2 Hz), 3.99 (s, 6H), 3.23 (m, 2H), 3.14 (m, 4H), 2.96 (t, 2H, J = 7.2 Hz), 1.81-1.69 (m, 14H), 1.49 (s, 18H), 1.37 (d, 6H, J = 6.4 Hz); 13C NMR (CD3OD): δ 176.0, 169.4, 160.9, 158.5, 144.7, 137.9, 136.6, 130.2, 122.2, 97.3, 91.6, 57.0, 41.0, 40.9, 39.6, 32.4, 30.8, 30.5, 28.5, 27.9, 24.2, 21.3; MS (APCI): m/z 785 (M+1).
N-{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-N2-({4-[(6-methoxyquinolin-8-yl)-amino]pentyl}carbamoyl)-l-lysinamide·3HCl (44)
Yield: 94%; hygroscopic solid; IR (KBr): 3398, 1678 cm-1; 1H NMR (CD3OD): δ 8.34 (d, 2H, J = 8.7 Hz), 7.81 (d, 2H, J = 8.7 Hz), 7.56 (m, 2H), 7.46 (m, 2H), 4.08 (t, 1H, J = 5.7 Hz), 3.98 (s, 6H), 3.22 (m, 2H), 3.00 (m, 4H), 2.70 (t, 2H, J = 7.0 Hz), 1.79 (m, 12H), 1.48 (s, 18H), 1.36 (m, 6H); 13C NMR (CD3OD): δ 172.3, 162.3, 161.8, 154.3, 145.6, 135.2, 134.7, 128.3, 120.5, 98.2, 91.6, 55.5, 39.2, 38.9, 37.9, 30.5, 29.0, 26.1, 25.2, 23.6, 21.6; MS (APCI): m/z 771 (M+1).
General method for the synthesis of protected bis(8-quinolinamines) (55-59)
a) Removal of benzyl ester group
To a mixture of 45-48 (0.1 mmol), 10% Pd-C (0.04 g) in glacial acetic acid (1 mL) and CH3OH (20 mL), H2 gas was bubbled for 4 h. The catalyst was filtered and solvent removed to afford 50-53 as oily syrup
b) Removal of tert-butyl ester group
A solution of 49 in 6N HCl (5 mL) was stirred for 5 h at ambient temperature. The solvent was removed to afford HCl salt, which was dissolved in water (5 mL) and neutralized by drop wise addition of 25% NH4OH solution. The mixture was extracted with CH2Cl2 (3 × 20 mL). The organic layer washed with brine (5 mL) and dried (Na2SO4). The solvent was removed to afford 54 as oil. The formation of intermediate products 50-54 was confirmed by TLC and mass spectral analysis, which were used for the next step without any purification.
To an ice cooled stirred solution of 8-quinolinamine (1 or 3, 0.24 mmol) and 50-54 (0.24 mmol) in anhydrous CH2Cl2 (5 mL), DIC (0.26 mmol) was added. The reaction mixture was allowed to attain room temperature and stirring was continued for another 4 h. The solvent was removed yielding the crude product, which was purified by column chromatography on silica gel (100-200 mesh) using 1.2-2% CH3OH in CH2Cl2 to afford 55-59 as oil.
tert-Butyl[(2S)-1,4-bis({4-[(6-methoxyquinolin-8-yl)amino]pentyl}amino)-1,4-dioxobutan-2-yl]carbamate (55)
Yield: 81%; oil; IR (CH2Cl2): 3338, 1656 cm-1; 1H NMR (CDCl3): δ 8.52 (d, 2H, J = 3.9 Hz), 7.92 (d, 2H, J = 8.0 Hz), 7.31 (dd, 2H, J = 3.9 and 8.0 Hz), 6.86 (bs, 2H), 6.54 (bs, 2H), 6.32 (m, 2H), 6.25 (m, 2H), 5.98 (bs, 1H), 4.38 (m, 1H), 3.87 (s, 6H), 3.57 (m, 2H), 3.22 (m, 4H), 2.65 (m, 2H), 1.58 (m, 8H), 1.42 (s, 9H), 1.27 (d, 6H, J = 6.1 Hz); 13C NMR (CDCl3): δ 171.7, 170.5, 159.4, 155.5, 144.9, 144.3, 135.3, 134.7, 121.8, 114.0, 96.7, 91.6, 79.3, 55.2, 50.6, 47.7, 42.2, 39.5, 39.4, 36.1, 33.7, 31.9, 29.7, 28.2, 26.1, 22.6; MS (APCI): m/z 716 (M+1).
tert-Butyl[(2S)-1,5-bis({4-[(6-methoxyquinolin-8-yl)amino]pentyl}amino)-1,5-dioxopentan-2-yl]carbamate (56)
Yield: 83%; oil; IR (CH2Cl2): 3323, 1735, 1657 cm-1; 1H NMR (CDCl3): δ 8.51 (d, 2H, J = 3.9 Hz), 7.92 (d, 2H, J = 8.2 Hz), 7.29 (dd, 2H, J = 3.9 and 8.2 Hz), 6.32 (m, 3H), 6.27 (m, 2H), 5.81 (bs, 2H), 5.74 (bs, 2H), 4.07 (t, 1H, J = 5.9 Hz), 3.87 (s, 6H), 3.59 (m, 2H), 3.23 (m, 4H), 2.88 (t, 2H, J = 6.3 Hz), 2.17 (m, 2H), 1.89 (m, 8H), 1.39 (s, 9H), 1.27 (d, 6H, J = 6.0 Hz); 13C NMR (CDCl3): δ 173.4, 172.1, 163.1, 159.9, 145.4, 144.8, 135.8, 135.4, 130.4, 122.4, 97.4, 92.4, 80.4, 55.7, 54.1, 48.3, 40.1, 37.0, 34.5, 33.2, 31.9, 30.2, 28.8, 26.6, 21.0; MS (APCI): m/z 730 (M+1).
tert-Butyl[(2S)-1,4-bis({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-amino)-1,4-dioxobutan-2-yl]carbamate (57)
Yield: 88%; oil; IR (CH2Cl2): 3308, 1645 cm-1; 1H NMR (CDCl3): δ 7.85 (d, 2H, J = 7.8 Hz), 7.42 (d, 2H, J = 7.8 Hz), 6.99 (bs, 2H), 6.30 (m, 2H), 6.23 (m, 2H), 6.11 (bs, 1H), 5.87 (bs, 2H), 4.39 (m, 1H), 3.86 (s, 6H), 3.56 (m, 2H), 3.24 (m, 4H, J = 5.9 Hz), 2.63 (m, 2H), 1.58 (m, 8H), 1.41 (m, 27H), 1.28 (d, 6H, J = 5.7 Hz); 13C NMR (CDCl3): δ 170.9, 163.2, 158.7, 144.8, 134.9, 133.5, 127.4, 118.8, 96.6, 91.4, 80.6, 55.1, 47.8, 39.5, 37.6, 33.8, 30.2, 28.3, 26.1, 23.5, 20.5; MS (APCI): m/z 828 (M+1).
tert-Butyl[(2S)-1,5-bis({4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-amino)-1,5-dioxopentan-2-yl]carbamate (58)
Yield: 85%; oil; IR (CH2Cl2): 3383, 1700, 1657 cm-1; 1H NMR (CDCl3): δ 7.86 (d, 2H, J = 8.6 Hz), 7.43 (d, 2H, J = 8.6 Hz), 6.80 (bs, 1H), 6.66 (bs, 1H), 6.31 (d, 2H, J = 2.1 Hz), 6.25 (d, 2H, J = 2.1 Hz), 6.12 (bs, 1H), 5.99 (bs, 1H), 5.68 (bs, 1H), 4.01 (t, 1H, J = 6.0 Hz), 3.87 (s, 6H), 3.56 (m, 2H), 3.29 (m, 4H), 2.02 (t, 2H, J = 6.5 Hz), 1.78-1.62 (m, 10H), 1.42 (m, 27H), 1.31 (d, 6H, J = 6.1 Hz); 13C NMR (CDCl3): δ 171.4, 163.3, 158.8, 153.6, 144.8, 134.9, 133.5, 127.4, 118.7, 96.7, 91.4, 79.7, 55.1, 53.1, 47.8, 47.1, 43.1, 39.7, 37.6, 34.0, 31.6, 30.2, 28.9, 28.2, 26.1, 22.3, 20.7; MS (ESI): m/z 842 (M+1).
9H-Fluoren-9-ylmethyl[(2R)-1,4-bis({4-[(6-methoxyquinolin-8-yl)amino]pentyl}-amino)-1,4-dioxobutan-2-yl]carbamate (59)
Yield: 87%; oil; IR (CH2Cl2): 3356, 1709, 1675 cm-1; 1H NMR (CDCl3): δ 8.51 (d, 2H, J = 3.9 Hz), 7.91 (d, 2H, J = 7.5 Hz), 7.74 (d, 2H, J = 7.4 Hz), 7.57 (d, 2H, J = 6.6 Hz), 7.39 (m, 6H), 6.93 (bs, 2H), 6.38 (bs, 2H), 6.32 (m, 2H), 6.25 (m, 2H), 5.97 (bs, 1H), 4.39 (d, 2H, J = 6.5 Hz), 4.18 (m, 2H), 3.86 (s, 6H), 3.57 (m, 2H), 3.21 (m, 4H), 2.73 (m, 2H), 1.59 (m, 8H), 1.26 (d, 6H, J = 6.1 Hz); 13C NMR (CDCl3): δ 172.1, 169.9, 169.6, 158.4, 153.1, 143.9, 142.7, 140.2, 134.3, 133.8, 128.9, 126.7, 124.0, 118.9, 95.8, 95.7, 90.7, 90.6, 66.1, 54.1, 50.7, 46.7, 46.1, 38.5, 37.0, 32.7, 29.8, 28.6, 25.1, 25.0, 24.9, 21.0; MS (APCI): m/z 838 (M+1).
General method for the synthesis of N1,N4-bis{4-[(6-methoxy-2-substituted-quinolin-8-yl)amino]pentyl}-d/l-aspartamide/glutamamide (60-64)
A solution of t-Boc group protected bis(8-aminoquinolines) (55-58, 0.30 mmol) was stirred at ambient temperature in HCl (5 mL, 6N solution) for 45 min. The removal of solvent provides salts of 60-63 in good yields. In case of Fmoc group, to 8-aminoquinoline (59, 0.54 mmol) a solution of 20% piperidine in CH2Cl2 (10 mL) was added and reaction mixture was stirred for 20 min. The solvent was removed and the crude product was purified by silica gel (100-200 mesh) column chromatography eluting with 3% CH3OH in CH2Cl2 to afford 64, which upon treatment with HCl (2N in ether) provided its hydrochloride salt.
N1,N4-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-l-aspartamide·3HCl (60)
Yield: 88%; hygroscopic solid; IR (KBr): 3435, 1638 cm-1; 1H NMR (CD3OD): δ 8.83 (m, 4H), 7.88 (m, 2H), 7.00 (m, 2H), 6.86 (m, 2H), 4.24 (m, 1H), 3.97 (m, 6H), 3.76 (m, 2H), 3.33 (m, 4H), 2.43 (m, 2H), 1.87 (m, 8H), 1.32 (m, 6H); 13C NMR (CD3OD): δ 172.3, 171.3, 161.9, 145.3, 140.3, 139.4, 133.0, 122.6, 96.4, 91.8, 66.3, 56.2, 39.9, 39.7, 36.3, 33.5, 26.4, 21.2; MS (APCI): m/z 616 (M+1).
N1,N5-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-l-glutamamide·3HCl (61)
Yield: 86%; hygroscopic solid; IR (KBr): 3391, 1673 cm-1; 1H NMR (CD3OD): δ 8.87 (m, 4H), 7.91 (m, 2H), 7.02 (m, 2H), 6.85 (m, 2H), 3.97 (m, 6H), 3.78 (t, 3H), 3.04 (m, 4H), 2.56 (t, 2H, J = 5.6 Hz), 2.19 (m, 2H), 1.89 (m, 8H), 1.26 (m, 6H); 13C NMR (CD3OD): δ 174.2, 169.0, 161.9, 145.3, 140.1, 139.1, 133.0, 122.6, 96.5, 92.4, 66.2, 56.2, 53.5, 51.0, 49.3, 49.0, 40.3, 39.9, 34.9, 33.4, 31.6, 27.9, 26.3, 26.1, 19.1; MS (APCI): m/z 630 (M+1).
N1,N4-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-l-aspartamide·3HCl (62)
Yield: 90%; hygroscopic solid; IR (KBr): 3401, 1646 cm-1; 1H NMR (CD3OD): δ 8.40 (d, 2H, J = 7.8 Hz), 7.83 (d, 2H, J = 7.8 Hz), 7.71 (m, 2H), 7.50 (m, 2H), 4.12 (m, 1H), 3.97 (m, 6H), 3.51 (m, 2H), 3.36 (m, 4H), 2.05 (m, 2H), 1.76 (m, 8H), 1.51 (m, 18H), 1.32 (m, 6H); 13C NMR (CD3OD): δ 170.4, 168.7, 161.3, 157.4, 145.6, 144.3, 137.6, 135.3, 121.3, 118.4, 107.4, 94.5, 66.3, 59.6, 56.3, 51.1, 39.5, 38.7, 36.2, 31.3, 29.9, 26.0, 25.9, 22.0; MS (ESI): m/z 728 (M+1).
N1,N5-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-l-glutamamide·3HCl (63)
Yield: 92%; hygroscopic solid; IR (KBr): 3438, 1658 cm-1; 1H NMR (CD3OD): δ 8.34 (d, 2H, J = 8.6 Hz), 7.80 (d, 2H, J = 8.6 Hz), 7.59 (m, 2H), 7.42 (m, 2H), 4.10 (m, 1H), 3.97 (m, 6H), 3.78 (m, 2H), 3.31 (m, 4H), 2.00 (t, 2H, J = 5.6 Hz), 1.74 (m, 2H), 1.65 (m, 8H), 1.49 (s, 18H), 1.37 (m, 6H); 13C NMR (CD3OD): δ 170.9, 169.8, 161.5, 157.5, 145.7, 138.0, 131.4, 129.4, 121.4, 118.3, 107.5, 93.1, 66.2, 59.5, 56.4, 53.4, 44.1, 38.7, 31.3, 29.9, 21.7, 20.4; MS (ESI): m/z 742 (M+1).
N1,N4-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}-d-aspartamide·3HCl (64)
Yield: 74%; hygroscopic solid; IR (KBr): 3405, 1687 cm-1; 1H NMR (free base, CDCl3): δ 8.53 (dd, 2H, J = 1.4 and 4.1 Hz), 7.92 (dd, 2H, J = 1.4 and 8.2 Hz), 7.30 (dd, 2H, J = 4.1 and 8.2 Hz), 6.82 (bs, 2H), 6.32 (d, 2H, J = 2.3 Hz), 6.26 (d, 2H, J = 2.3 Hz), 5.99 (bs, 2H), 3.88 (s, 6H), 3.59 (m, 3H), 3.25 (m, 4H), 2.59 (m, 2H), 2.09 (bs, 2H), 1.69 (m, 8H), 1.28 (d, 6H, J = 6.3 Hz); 13C NMR (free base, CDCl3): δ 170.9, 169.2, 162.6, 140.9, 135.7, 123.0, 118.9, 97.1, 92.3, 56.5, 40.5, 40.3, 36.8, 34.2, 26.9, 23.7, 19.8; MS (APCI): m/z 616 (M+1).
General method for the synthesis of 1,3-bis{4-[(6-methoxy-2/4,5-substituted-quinolin-8-yl)-amino]pentyl}urea/thiourea·2HCl (66-71)
A solution of CDI or TCDI (0.19 mmol) and 8-aminoquinoline (1, 3, or 4, 0.39 mmol) in dry CH2Cl2 (5 mL) was stirred at ambient temperature for 24 h. The solvent was distilled off. The reaction mixture was dissolved in CH2Cl2 (20 mL) and washed with water (3 × 5 mL) followed by brine solution (5 mL). The organic layer was dried (Na2SO4) and concentrated to afford crude product, which was purified by column chromatography on silica gel (100-200 mesh) using 1.5% CH3OH in CH2Cl2 to provide product as viscous oil. Treatment with HCl solution (2N in ether) provided their HCl salts.
1,3-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}urea·2HCl (66)
Yield: 55%; hygroscopic solid; IR (free base, CH2Cl2): 3378, 1680 cm-1; 1H NMR (free base, CDCl3): δ 8.52 (dd, 2H, J = 1.3 and 4.1 Hz), 7.92 (d, 2H, J = 8.0 Hz), 7.30 (dd, 2H, J = 4.1 and 8.0 Hz), 6.31 (d, 2H, J = 2.3 Hz), 6.27 (d, 2H, J = 2.3 Hz), 5.98 (bs, 2H), 3.86 (s, 6H), 3.52 (m, 4H), 3.14 (m, 2H), 1.67-1.59 (m, 8H), 1.27 (d, 6H, J = 6.3 Hz); 13C NMR (free base, CDCl3): δ 172.2, 159.9, 145.4, 144.8, 135.8, 130.4, 128.2, 120.5, 97.3, 92.2, 55.7, 48.3, 42.7, 34.5, 26.7, 21.8; MS (APCI): m/z 545 (M+1).
1,3-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}thiourea·2HCl (67)
Yield: 52%; hygroscopic solid; IR (free base, CH2Cl2): 3374, 1615 cm-1; 1H NMR (free base, CDCl3): δ 8.50 (d, 2H, J = 3.9 Hz), 7.89 (d, 2H, J = 7.8 Hz), 7.30 (dd, 2H, J = 3.9 and 7.8 Hz), 6.51 (d, 2H, J = 2.1 Hz), 6.45 (d, 2H, J = 2.1 Hz), 6.00 (bs, 2H), 3.87 (s, 6H), 3.55 (m, 4H), 3.14 (m, 2H), 1.65 (m, 8H), 1.25 (d, 6H, J = 6.1 Hz); 13C NMR (free base, CDCl3): δ 183.6, 158.4, 145.8, 144.7, 134.8, 131.4, 128.6, 121.8, 97.8, 92.3, 55.1, 48.9, 41.2, 34.1, 26.8, 21.6; MS (ESI): m/z 583.1 (M+23).
1,3-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}urea·2HCl (68)
Yield: 62%; hygroscopic solid; IR (free base, CH2Cl2): 3316, 1660 cm-1; 1H NMR (free base, CDCl3): δ 7.84 (d, 2H, J = 8.6 Hz), 7.41 (d, 2H, J = 8.6 Hz), 6.30 (d, 2H, J = 1.9 Hz), 6.25 (d, 2H, J = 1.9 Hz), 6.12 (bs, 2H), 3.85 (s, 6H), 3.58 (m, 2H), 3.12 (m, 4H), 1.59 (m, 8H), 1.41 (s, 18H), 1.28 (d, 6H, J = 6.3 Hz); 13C NMR (free base, CDCl3): δ 171.7, 162.5, 157.9, 143.8, 135.5, 129.0, 128.4, 119.3, 97.1, 92.0, 55.7, 48.4, 40.0, 34.4, 30.8, 28.9, 26.7, 21.1; MS (APCI): m/z 657 (M+1).
1,3-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}thiourea·2HCl (69)
Yield: 60%; hygroscopic solid; IR (free base, CH2Cl2): 3363, 1651 cm-1; 1H NMR (free base, CDCl3): δ 7.83 (d, 2H, J = 8.5 Hz), 7.41 (d, 2H, J = 8.5 Hz), 6.30-6.25 (m, 4H), 6.08 (bs, 1H), 5.64 (bs, 1H), 3.85 (m, 6H), 3.55 (m, 2H), 3.25 (m, 4H), 1.59 (m, 8H), 1.41 (s, 18H), 1.26 (d, 6H, J = 6.3 Hz); 13C NMR (free base, CDCl3): δ 181.4, 163.5, 158.8, 144.7, 135.0, 133.5, 127.5, 118.9, 97.0, 91.8, 55.2, 47.9, 44.3, 37.7, 33.8, 30.2, 25.4, 20.7; MS (APCI): m/z 673 (M+1).
1,3-Bis{4-[4-ethyl-6-methoxy-5-(pentyloxy)quinolin-8-ylamino]pentyl}urea·2HCl (70)
Yield: 39%; hygroscopic solid; IR (free base, CH2Cl2): 3376, 1669 cm-1; 1H NMR (free base, CDCl3): δ 8.32 (d, 2H, J = 4.2 Hz), 7.55 (d, 2H, J = 4.2 Hz), 6.85 (bs, 2H), 6.37 (s, 2H), 3.89 (s, 6H), 3.83 (t, 4H, J = 6.9 Hz), 3.57 (m, 2H), 3.43 (m, 4H), 3.21 (q, 4H, J = 7.2 Hz), 1.98 (m, 20H), 1.55 (m, 18H); 13C NMR (free base, CDCl3): δ 169.3, 151.1, 141.8, 134.6, 134.5, 134.0, 128.5, 128.1, 122.4, 94.5, 56.9, 53.3, 50.8, 48.1, 38.1, 34.2, 29.6, 26.4, 26.2, 22.5, 20.7, 14.0; MS (APCI): m/z 773 (M+1).
1,3-Bis{4-[4-ethyl-6-methoxy-5-(pentyloxy)quinolin-8-ylamino]pentyl}-thiourea·2HCl (71)
Yield: 42%; hygroscopic solid; IR (free base, CH2Cl2): 3365, 1606 cm-1; 1H NMR (free base, CDCl3): δ 8.36 (d, 2H, J = 4.2 Hz), 7.09 (d, 2H, J = 4.2 Hz), 6.44 (s, 2H), 5.88 (bs, 2H), 3.94 (s, 6H), 3.89 (t, 4H, J = 6.8 Hz), 3.61 (m, 2H), 3.33 (m, 4H), 3.25 (q, 4H, J = 7.2 Hz), 1.83 (t, 6H, J = 7.2 Hz), 1.64-1.55 (m, 20H), 1.43 (m, 12H); 13C NMR (free base, CDCl3): δ 182.3, 151.1, 144.3, 134.5, 134.1, 128.5, 128.1, 122.4, 94.6, 56.9, 50.8, 48.1, 39.3, 32.1, 28.5, 26.2, 20.7, 19.2, 13.9; MS (APCI): m/z 789 (M+1).
General method for the synthesis of N,N2-bis{4-[(6-methoxy-2-substituted-quinolin-8-yl)amino]pentyl}glycinamide·2HCl (72 and 73)
A mixture of 8-aminoquinoline (1 or 3, 1.08 mmol), chloroacetic acid (4.32 mmol) and Et3N (4.32 mmol) in anhydrous THF (15 mL) was refluxed for 24 h. The solvent was removed under reduced pressure. The residue was dissolved in CH2Cl2 (50 mL) and washed with water (3 × 10 mL) followed by brine solution (5 mL). The organic layer was dried (Na2SO4) and solvent was removed under reduced pressure. Column chromatographic purification of crude product on silica gel (100-200 mesh) using 2% CH3OH in CH2Cl2 gave product as viscous oil, which upon treatment with HCl solution (2N in ether) provided 72 and 73 as HCl salts.
N,N2-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}glycinamide·2HCl (72)
Yield: 21%; hygroscopic solid; IR (free base, CH2Cl2): 3436, 1637 cm-1; 1H NMR (free base, CDCl3): δ 8.53 (dd, 2H, J = 1.5 and 4.2 Hz), 7.94 (dd, 2H, J = 1.5 and 8.2 Hz), 7.33 (dd, 2H, J = 4.2 and 8.2 Hz), 6.34 (d, 2H, J = 2.3 Hz), 6.28 (d, 2H, J = 2.3 Hz), 5.96 (bs, 2H), 3.88 (s, 6H), 3.68 (m, 4H), 3.27 (t, 2H, J = 5.6 Hz), 2.30 (t, 2H, J = 6.4 Hz), 1.87-1.63 (m, 8H), 1.30 (d, 6H, J = 6.3 Hz); 13C NMR (free base, CDCl3): δ 173.4, 159.4, 144.9, 144.3, 135.3, 134.9, 129.9, 121.9, 96.9, 91.8, 62.3, 55.2, 47.8, 39.5, 34.0, 26.1, 20.6; MS (APCI): m/z 559 (M+1).
N,N2-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}-glycinamide·2HCl (73)
Yield: 18%; hygroscopic solid; IR (free base, CH2Cl2): 3391, 1648 cm-1; 1H NMR (free base, CDCl3): δ 7.87 (d, 2H, J = 8.5 Hz), 7.43 (d, 2H, J = 8.5 Hz), 6.50 (bs, 2H), 6.32 (s, 2H), 6.34 (s, 2H), 4.02 (s, 2H), 3.87 (s, 6H), 3.60 (m, 2H), 3.36 (m, 2H), 2.58 (t, 2H, J = 5.9 Hz), 1.79-1.60 (m, 8H), 1.42 (s, 18H), 1.30 (d, 6H, J = 6.1 Hz); 13C NMR (free base, CDCl3): δ 170.4, 162.5, 157.6, 143.8, 134.0, 132.6, 126.4, 117.0, 95.6, 90.5, 61.1, 54.2, 46.8, 37.8, 36.6, 32.5, 29.2, 24.9, 20.9; MS (APCI): m/z 671 (M+1).
General method for the synthesis of N,N′-Bis{4-[(6-methoxy-2-substituted-quinolin-8-yl)amino]pentyl}dicarbonimidic diamide·2HCl (74 and 75)
To an ice cooled stirred solution of 1 or 3 (1.31 mmol) and Et3N (0.65 mmol) in anhydrous CH2Cl2 (10 mL), N-(chlorocarbonyl)isocyanate (0.65 mmol) was added drop wise. The reaction mixture was allowed to attain room temperature and stirring continued for another 3 h. The solvent was then removed under reduced pressure. The residue was dissolved in CH2Cl2 (20 mL), and organic layer was washed with water (3 × 10 mL) followed by brine solution (5 mL). The organic layer was dried over Na2SO4 and concentrated to obtain crude product, which was purified by column chromatography on silica gel (100-200 mesh) using 2.5% CH3OH in CH2Cl2 to afford products, which were converted to their hydrogen chloride salts upon treatment with HCl solution (2N in ether).
N,N′-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}dicarbonimidic diamide·2HCl (74)
Yield: 70%; hygroscopic solid; IR (free base, CH2Cl2): 3382, 1689, 1668 cm-1; 1H NMR (free base, CDCl3): δ 8.77 (bs, 1H), 8.52 (bs, 1H), 8.06 (d, 2H, J = 4.2 Hz), 7.91 (d, 2H, J = 8.5 Hz), 7.22 (dd, 2H, J = 4.2 and 8.5 Hz), 6.32-6.27 (m, 4H), 3.87 (s, 6H), 3.62 (m, 2H), 3.24 (t, 4H, J = 6.1 Hz), 1.64 (m, 8H), 1.28 (d, 6H, J = 5.7 Hz); 13C NMR (free base, CDCl3): δ 160.0, 157.6, 149.4, 145.5, 135.9, 135.2, 130.4, 122.3, 107.6, 97.3, 92.2, 55.7, 53.2, 48.4, 34.6, 27.1, 21.0; MS (APCI): m/z 588 (M+1).
N,N′-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}dicarbonimidic diamide·2HCl (75)
Yield: 38%; hygroscopic solid; IR (free base, CH2Cl2): 3374, 1682, 1665 cm-1; 1H NMR (free base, CDCl3): δ 7.98 (d, 2H, J = 8.6 Hz), 7.85 (d, 2H, J = 8.6 Hz), 6.30 (s, 2H), 6.25 (s, 2H), 6.15 (bs, 2H), 3.86 (s, 6H), 3.59 (m, 2H), 3.27 (t, 4H, J = 6.2 Hz), 1.64 (m, 8H), 1.42 (s, 18H), 1.31 (d, 6H, J = 6.1 Hz); 13C NMR (free base, CDCl3): δ 168.2, 159.4, 157.0, 145.5, 135.8, 135.4, 128.9, 123.7, 107.0, 96.9, 55.7, 48.5, 40.3, 34.7, 32.8, 30.5, 27.0, 21.1; MS (APCI): m/z 700 (M+1).
General method for the synthesis of bisquinolines 76-78
To an ice cooled stirred solution of 1 (0.772 mmol) and Et3N (0.772 mmol) in anhydrous CH2Cl2 (5 mL), chloromethyl chloroformate or chlorocarbonylsulfenyl chloride or oxalyl chloride (0.386 mmol) was added drop wise. The reaction mixture was allowed to warm to ambient temperature and stirring continued for another 6 h. The reaction mixture was concentrated, and residue was dissolved in CH2Cl2 (20 mL). The organic layer was washed with water (3 × 10 mL) followed by brine solution (5 mL). Organic layer was dried over Na2SO4 and concentrated to obtain crude product. Pure product was isolated as viscous oil by column chromatography on silica gel (100-200 mesh) using 1.5% CH3OH in CH2Cl2. Treatment with HCl solution (2N in ether) provided 76-78 as HCl salts.
({4-[(6-Methoxyquinolin-8-yl)amino]pentyl}amino)methyl{4-[(6-methoxy-quinolin-8-yl)amino]pentyl}carbamate·2HCl (76)
Yield: 63%; hygroscopic solid; IR (free base, CH2Cl2): 3375, 1742 cm-1; 1H NMR (free base, CDCl3): δ 8.53 (dd, 2H, J = 1.4 and 4.2 Hz), 7.93 (dd, 2H, J = 1.4 and 8.2 Hz), 7.32 (dd, 2H, J = 4.2 and 8.2 Hz), 6.34 (d, 2H, J = 2.3 Hz), 6.28 (d, 2H, J = 2.3 Hz), 6.00 (bs, 1H), 5.74 (s, 2H), 4.90 (bs, 1H), 3.88 (s, 6H), 3.64 (m, 2H), 3.26 (m, 4H), 1.72-1.62 (m, 8H), 1.31 (d, 6H, J = 6.3 Hz); 13C NMR (free base, CDCl3): δ 159.9, 154.1, 145.4, 144.9, 135.8, 135.3, 130.4, 122.4, 97.3, 92.3, 71.0, 55.7, 48.3, 41.7, 34.3, 26.9, 21.1; MS (APCI): m/z 574.8 (M+1).
({4-[(6-Methoxyquinolin-8-yl)amino]pentyl}amino)[({4-[(6-methoxyquinolin-8-yl)amino]pentyl}amino)sulfanyl]methanone·2HCl (77)
Yield: 66%; hygroscopic solid; IR (free base, CH2Cl2): 3370, 1659 cm-1; 1H NMR (free base, CDCl3): δ 8.51 (m, 4H), 7.91 (m, 2H), 6.33 (m, 4H), 5.98 (bs, 1H), 5.49 (bs, 1H), 3.89 (s, 6H), 3.57 (m, 2H), 3.34 (m, 4H), 1.61 (m, 8H), 1.25 (d, 6H, J = 6.1 Hz); 13C NMR (free base, CDCl3): δ 162.1, 160.0, 148.4, 145.1, 135.4, 133.8, 130.5, 123.3, 97.4, 92.3, 55.7, 48.3, 41.6, 34.3, 26.9, 21.0; MS (APCI): m/z 577 (M+1).
N,N′-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}ethanediamide·2HCl (78)
Yield: 7%; hygroscopic solid; IR (free base, CH2Cl2): 3381, 1659 cm-1; 1H NMR (free base, CDCl3): δ 8.52 (d, 2H, J = 3.2 Hz), 7.91 (d, 2H, J = 8.0 Hz), 7.30 (dd, 2H, J = 3.2 and 8.0 Hz), 6.32 (s, 2H), 6.27 (s, 2H), 3.87 (s, 6H), 3.63 (m, 2H), 3.33 (t, 4H, J = 5.5 Hz), 1.71 (m, 8H), 1.29 (d, 6H, J = 6.2 Hz); 13C NMR (free base, CDCl3): δ 160.4, 159.9, 145.4, 144.8, 135.9, 135.2, 130.4, 122.3, 97.3, 92.3, 55.7, 48.3, 40.2, 34.5, 26.6, 21.1; MS (APCI): m/z 573 (M+1).
Synthesis of N1,N1′-(iminodiethane-2,1-diyl)bis[N4-(6-methoxyquinolin-8-yl)pentane-1,4-diamine]·2HCl (79)
A mixture of 1 (0.965 mmol), Et3N (0.965 mmol) and bis(2-chloroethyl)amine (0.482 mmol) was stirred at room temperature for 14 h. At this stage, EtOAc (20 mL) was added and the separated Et3N·HCl salt was filtered. The filtrate was concentrated and residue was purified by column chromatography on silica gel (100-200 mesh) using 7% CH3OH in CH2Cl2 to afford pure product, which was converted to HCl salt upon treating with 2N solution of HCl in ether. Yield: 82%; hygroscopic solid; IR (free base, CH2Cl2): 3428 cm-1; 1H NMR (free base, CDCl3): δ 8.52 (d, 2H, J = 3.2 Hz), 7.91 (d, 2H, J = 8.0 Hz), 7.32 (dd, 2H, J = 3.2 and 8.0 Hz), 6.32 (s, 2H), 6.26 (s, 2H), 5.97 (bs, 1H), 4.04 (bs, 1H), 3.87 (s, 6H), 3.63 (m, 2H), 3.33 (m, 12H), 1.71 (m, 8H), 1.29 (d, 6H, J = 6.2 Hz); 13C NMR (free base, CDCl3): δ 159.9, 145.3, 145.8, 135.8, 135.3, 130.4, 122.4, 97.5, 92.4, 55.7, 51.7, 48.2, 41.0, 34.1, 26.6, 20.8; MS (APCI): m/z 588 (M+1).
General method for the synthesis of N,N′-bis{4-[(6-methoxy-2-substituted-quinolin-8-yl)amino]pentyl}pyridine-2,6/3,4/3,5-dicarboxamide·2HCl (80-85)
A mixture of 8-aminoquinoline (1 or 3, 0.980 mmol), Et3N (0.980 mmol) and pyridine-2,6/3,4/3,5-dicarbonyl chloride (0.490 mmol) was stirred in anhydrous THF (15 mL) at room temperature for 12 h and filtered. The filtrate was concentrated and residue was purified by column chromatography on silica gel (100-200 mesh) using 1% CH3OH in CH2Cl2 to afford 80-85 as viscous oil, which were converted to HCl salts upon treatment with a 2N solution of ethereal HCl.
N,N′-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}pyridine-2,6-dicarboxamide·3HCl (80)
Yield: 63%; hygroscopic solid; IR (free base, CH2Cl2): 3391, 1659 cm-1; 1H NMR (free base, CDCl3): δ 8.48 (d, 2H, J = 4.0 Hz), 8.32 (d, 2H, J = 7.7 Hz), 7.97 (m, 3H), 7.28 (dd, 2H, J = 4.0 and 7.8 Hz), 6.30 (d, 2H, J = 2.1 Hz), 6.20 (d, 2H, J = 2.1 Hz), 5.93 (bs, 2H), 3.84 (s, 6H), 3.53 (m, 2H), 3.42 (t, 4H, J = 6.8 Hz), 1.60 (m, 8H), 1.26 (d, 6H, J = 6.2 Hz); 13C NMR (free base, CDCl3): δ 164.0, 159.9, 149.4, 145.3, 144.7, 139.3, 135.8, 135.4, 130.4, 125.4, 122.3, 97.4, 92.2, 55.7, 48.2, 40.0, 34.5, 26.8, 21.0; MS (APCI): m/z 650 (M+1).
N,N′-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}pyridine-3,4-dicarboxamide·3HCl (81)
Yield: 35%; hygroscopic solid; IR (free base, CH2Cl2): 3392, 1634 cm-1; 1H NMR (free base, CDCl3): δ 8.76 (s, 1H), 8.63 (d, 1H, J = 5.0 Hz), 8.48 (d, 2H, J = 3.9 Hz), 7.91 (d, 2H, J = 7.4 Hz), 7.40 (d, 1H, J = 5.0 Hz), 7.29 (dd, 2H, J = 3.9 and 7.4 Hz), 6.31 (d, 2H, J = 1.8 Hz), 6.26 (d, 2H, J = 1.8 Hz), 5.98 (bs, 2H), 3.85 (s, 6H), 3.59 (m, 2H), 3.37 (t, 4H, J = 6.1 Hz), 1.70 (m, 8H), 1.28 (d, 6H, J = 6.3 Hz); 13C NMR (free base, CDCl3): δ 167.5, 159.9, 152.0, 149.9, 144.8, 142.0, 135.8, 135.3, 129.4, 122.6, 122.4, 97.4, 55.7, 48.3, 40.7, 34.3, 26.5, 21.1; MS (APCI): m/z 650 (M+1).
N,N′-Bis{4-[(6-methoxyquinolin-8-yl)amino]pentyl}pyridine-3,5-dicarboxamide·3HCl (82)
Yield: 68%; hygroscopic solid; IR (free base, CH2Cl2): 3369, 1645 cm-1; 1H NMR (free base, CDCl3): δ 9.00 (s, 2H), 8.49 (d, 2H, J = 4.0 Hz), 8.35 (s, 1H), 7.90 (d, 2H, J = 8.2 Hz), 7.29 (dd, 2H, J = 4.0 and 8.2 Hz), 6.30 (s, 2H), 6.25 (s, 2H), 5.94 (bs, 2H), 3.84 (s, 6H), 3.60-3.42 (m, 6H), 1.70 (m, 8H), 1.27 (d, 6H, J = 6.1 Hz); 13C NMR (free base, CDCl3): δ 165.5, 159.9, 151.0, 145.3, 144.8, 135.8, 135.4, 133.7, 130.4, 130.3, 122.4, 97.5, 92.4, 55.7, 48.3, 40.7, 34.5, 26.6, 21.1; MS (APCI): m/z 650 (M+1).
N,N′-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}pyridine-2,6-dicarboxamide.3HCl (83)
Yield: 55%; hygroscopic solid; IR (free base, CH2Cl2): 3391, 1661 cm-1; 1H NMR (free base, CDCl3): δ 8.32 (d, 2H, J = 7.7 Hz), 8.01 (d, 1H, J = 7.7 Hz), 7.84 (d, 2H, J = 8.6 Hz), 7.41 (d, 2H, J = 8.6 Hz), 6.28 (d, 2H, J = 2.4 Hz), 6.22 (d, 2H, J = 2.3 Hz), 3.82 (s, 6H), 3.48 (m, 6H), 1.76 (m, 8H), 1.39 (s, 18H), 1.27 (d, 6H, J = 6.6 Hz); 13C NMR (free base, CDCl3): δ 162.8, 162.3, 157.7, 147.8, 143.8, 137.7, 133.9, 133.5, 126.4, 125.5, 117.7, 95.5, 90.4, 55.9, 54.0, 46.8, 36.6, 33.2, 25.8, 21.6; MS (APCI): m/z 762 (M+1).
N,N′-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}pyridine-3,4-dicarboxamide·3HCl (84)
Yield: 22%; hygroscopic solid; IR (free base, CH2Cl2): 3435, 1655 cm-1; 1H NMR (free base, CDCl3): δ 8.72 (s, 1H), 8.57 (d, 1H, J = 2.8 Hz), 7.83 (d, 2H, J = 8.5 Hz), 7.41 (d, 2H, J = 8.5 Hz), 7.33 (d, 1H, J = 2.8 Hz), 6.28 (s, 2H), 6.22 (s, 2H), 6.11 (bs, 2H), 3.86 (s, 6H), 3.67 (m, 6H), 1.70 (m, 8H), 1.40 (s, 18H), 1.29 (d, 6H, J = 6.2 Hz); 13C NMR (free base, CDCl3): δ 169.0, 167.9, 157.7, 152.9, 144.1, 143.4, 134.4, 134.0, 128.3, 124.1, 118.0, 96.0, 90.4, 56.0, 43.6, 35.8, 34.2, 30.9, 26.6, 21.5; MS (MALDI): m/z 762 (M+1).
N,N′-Bis{4-[(2-tert-butyl-6-methoxyquinolin-8-yl)amino]pentyl}pyridine-3,5-dicarboxamide·3HCl (85)
Yield: 45%; hygroscopic solid; IR (free base, CH2Cl2): 3391, 1660 cm-1; 1H NMR (free base, CDCl3): δ 9.12 (d, 2H, J = 1.9 Hz), 8.43 (d, 1H, J = 1.9 Hz), 8.02 (d, 2H, J = 8.5 Hz), 7.60 (d, 2H, J = 8.5 Hz), 6.47 (d, 2H, J = 2.1 Hz), 6.42 (d, 2H, J = 2.1 Hz), 6.31 (bs, 2H), 4.02 (s, 6H), 3.69 (m, 6H), 1.95 (m, 8H), 1.59 (s, 18H), 1.50 (d, 6H, J = 6.6 Hz); 13C NMR (free base, CDCl3): δ 163.8, 162.4, 157.7, 149.2, 143.7, 134.0, 133.5, 132.0, 128.8, 117.8, 95.8, 90.7, 56.0, 54.1, 46.8, 36.6, 33.1, 30.5, 25.0, 21.6; MS (APCI): m/z 762 (M+1).
Supplementary Material
Table 4.
In vivo (P. berghei) antimalarial activity of selected bis(8-aminoquinolines)
| Compd. No. | P. berghei | |||
|---|---|---|---|---|
| (10 mg/kg/day × 4, oral) | (25 mg/kg/day × 4, oral) | (50 mg/kg/day × 4, oral) | (100 mg/kg/day × 4, oral) | |
| 36 | – | – | (0/6) Inactive | (6/6) Curative |
| 37 | – | – | – | (0/6) Inactive |
| 43 | – | – | – | (5/6) Suppressive |
| 44 | (5/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 61 | (4/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| 62 | – | – | – | (0/6) Inactive |
| 63 | – | – | – | (0/6) Inactive |
| 66 | – | – | – | (0/6) Inactive |
| 68 | – | – | – | (0/6) Inactive |
| 74 | – | – | – | (0/6) Inactive |
| 76 | – | – | – | (5/6) Suppressive |
| 79 | (3/6) Suppressive | (6/6) Curative | (6/6) Curative | (6/6) Curative |
| PQ | – | – | – | (0/6) Inactive |
The term ‘curative’ indicates complete elimination of malaria parasites from the body and animals survive up to day D+60. The term ‘suppressive’ indicates that all of the treated animals show negative parasitemia up to D+7. However, by D+60, some mice die, and some survive with complete elimination of parasitemia as indicated by numbers given in parentheses. The term ‘inactive’ indicates that the treated animals show positive parasitemia either on D+4 or D+7 and usually die by D+14. “–”, not tested.
Acknowledgments
Kirandeep Kaur thanks the Council of Scientific and Industrial Research (CSIR), New Delhi for the award of Senior Research Fellowship. Mr John Trott, Ms Marsha Wright and Mr Rajnish Sahu are acknowledged for their excellent technical help in the in vitro screening of biological activity. Antimicrobial screening was supported by the NIH, NIAID, Division of AIDS, grant no. AI 27094, and the USDA Agricultural Research Service Specific Cooperative Agreement No. 58-6408-2-0009.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization, Malaria Fact Sheet, No. 94. 2010 please see: http://www.who.int/mediacentre/factsheets/fs094/en/
- 2.Vangapandu S, Jain M, Kaur K, Patel S, Patil P, Jain R. Med Res Rev. 2007;27:65–107. doi: 10.1002/med.20062. [DOI] [PubMed] [Google Scholar]
- 3.Vale N, Moreira R, Gomes P. Eur J Med Chem. 2009;44:937–953. doi: 10.1016/j.ejmech.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Hill DR, Baird JK, Parise ME, Lewis LS, Ryan ET, Magill A. Am J Trop Med Hyg. 2006;75:402–415. [PubMed] [Google Scholar]
- 5.Srivastava P, Singh S, Jain GK, Puri SK, Pandey VC. Ecotoxicol Environ Saf. 2000;45:236–239. doi: 10.1006/eesa.1999.1868. [DOI] [PubMed] [Google Scholar]
- 6.Bowman ZS, Oatis JE, Whelan JL, Jollow DJ, McMillan DC. J Pharmacol Exp Ther. 2004;309:79–85. doi: 10.1124/jpet.103.062984. [DOI] [PubMed] [Google Scholar]
- 7.Baird KJ, Hoffman SL. Clin Infect Dis. 2004;39:1336–1345. doi: 10.1086/424663. [DOI] [PubMed] [Google Scholar]
- 8.Constantino L, Paixão P, Moreira R, Portela MJ, Rosário VE, Iley J. Exp Toxicol Pathol. 1999;51:299–303. doi: 10.1016/S0940-2993(99)80010-4. [DOI] [PubMed] [Google Scholar]
- 9.Nodiff EA, Chatterjee S, Musallam HA. Prog Med Chem. 1991;28:1–40. doi: 10.1016/s0079-6468(08)70362-x. [DOI] [PubMed] [Google Scholar]
- 10.Kaur K, Jain M, Reddy RP, Jain R. Eur J Med Chem. 2010;45:3245–3264. doi: 10.1016/j.ejmech.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Jain R, Jain S, Gupta RC, Anand N, Dutta GP, Puri SK. Indian J Chem. 1994;33B:251–254. [Google Scholar]
- 12.Vangapandu S, Sachdeva S, Jain M, Singh S, Singh PP, Kaul CL, Jain R. Bioorg Med Chem. 2003;11:4557–4568. doi: 10.1016/j.bmc.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Jain M, Vangapandu S, Sachdeva S, Singh S, Singh PP, Gena GB, Tikoo K, Ramarao P, Kaul CL, Jain R. J Med Chem. 2004;47:285–287. doi: 10.1021/jm0304562. [DOI] [PubMed] [Google Scholar]
- 14.Vangapandu S, Sachdeva S, Jain M, Singh S, Singh PP, Kaul CL, Jain R. Bioorg Med Chem. 2004;12:239–247. doi: 10.1016/j.bmc.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Jain M, Vangapandu S, Sachdeva S, Jain R. Bioorg Med Chem. 2004;12:1003–1010. doi: 10.1016/j.bmc.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 16.Jain M, Khan SI, Tekwani BL, Jacob MR, Singh S, Singh PP, Jain R. Bioorg Med Chem. 2005;13:4458–4466. doi: 10.1016/j.bmc.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 17.Kaur K, Patel S, Patil P, Jain M, Khan SI, Jacobs MR, Ganesan S, Tekwani BL, Jain R. Bioorg Med Chem. 2007;15:915–930. doi: 10.1016/j.bmc.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vennerstrom JL, Ellis WY, Ager LA. J Med Chem. 1992;35:2129–2134. doi: 10.1021/jm00089a025. [DOI] [PubMed] [Google Scholar]
- 19.Girault S, Grellier P, Berecibar A, Maes L, Lemiere P, Mouray E, Elisabeth DC, Sergheraert C. J Med Chem. 2001;44:1658–1665. doi: 10.1021/jm001096a. [DOI] [PubMed] [Google Scholar]
- 20.Vennerstorm JL, Ager LA, Dorn A, Anderson SL, Gerena L, Ridley RG, Milhous WK. J Med Chem. 1998;41:4360–4364. doi: 10.1021/jm9803828. [DOI] [PubMed] [Google Scholar]
- 21.Raynes K, Galatis D, Cowman AF, Tilley L, Deady L. J Med Chem. 1995;38:204–206. doi: 10.1021/jm00001a026. [DOI] [PubMed] [Google Scholar]
- 22.Posner GH, Paik IH, Sur S, McRiner AJ, Borstnik K, Xie S, Shapiro TA. J Med Chem. 2003;46:1060–1065. doi: 10.1021/jm020461q. [DOI] [PubMed] [Google Scholar]
- 23.Jeyadevan JP, Bray PG, Chadwick J, Ward SA, O'Neill PM. J Med Chem. 2004;47:1290–1298. doi: 10.1021/jm030974c. [DOI] [PubMed] [Google Scholar]
- 24.Posner GH, McRiner AJ, Paik IH, Sur S, Borstnik K, Xie S, Shapiro TA, Alsgbala A, Foster B. J Med Chem. 2004;47:1299–1301. doi: 10.1021/jm0303711. [DOI] [PubMed] [Google Scholar]
- 25.Paul K, Blanton C, DeW J Med Chem. 1973;16:1391–1394. doi: 10.1021/jm00270a016. [DOI] [PubMed] [Google Scholar]
- 26.Shetty RV, Blanton CD., Jr Eur J Med Chem. 1979;4:353–356. [Google Scholar]
- 27.Makler MT, Hinrichs DJ. Am J Trop Med Hyg. 1993;48:205–210. doi: 10.4269/ajtmh.1993.48.205. [DOI] [PubMed] [Google Scholar]
- 28.Borenfreund E, Babich H, Martin-Alguacil N. In Vitro Cell Dev Biol. 1990;26:1030–1034. doi: 10.1007/BF02624436. [DOI] [PubMed] [Google Scholar]
- 29.Mustafa J, Khan SI, Ma G, Walker LA, Khan IA. Lipids. 2004;39:167–172. doi: 10.1007/s11745-004-1215-5. [DOI] [PubMed] [Google Scholar]
- 30.Huy NT, Mizunuma K, Kaur K, Nhien NTT, Jain M, Uyen DH, Harada S, Jain R, Kamei K. Antimicrob Agents Chemother. 2007;51:2842–2847. doi: 10.1128/AAC.00288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uyen DT, Huy NT, Trang DTX, Nhien NTT, Oida T, Hirayama K, Harada S, Kamei K. Biol Pharm Bull. 2008;31:1483–1488. doi: 10.1248/bpb.31.1483. [DOI] [PubMed] [Google Scholar]
- 32.Trang DT, Huy NT, Uyen DT, Sasai M, Shiono T, Harada S, Kamei K. Anal Biochem. 2006;349:292–296. doi: 10.1016/j.ab.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 33.Fasanmade AA, Jusko WJ. Drug Metab Dispos. 1995;23:573–576. [PubMed] [Google Scholar]
- 34.Harrison JH, Jollow DJ. Mol Pharmacol. 1987;32:423–431. [PubMed] [Google Scholar]
- 35.Mikus J, Steverding D. Parasitol Int. 2000;48:265–269. doi: 10.1016/s1383-5769(99)00020-3. [DOI] [PubMed] [Google Scholar]
- 36.Ma G, Khan SI, Jacob MR, Tekwani BL, Li Z, Pasco DS, Walker LA, Khan IA. Antimicrob Agents Chemother. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.NCCLS. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically M7-A5. 2 Vol. 20. National Committee on Clinical Laboratory Standards; 2000. [Google Scholar]
- 38.NCCLS. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard M27-A2. 15 Vol. 22. National Committee on Clinical Laboratory Standards; 2002. [Google Scholar]
- 39.NCCLS. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard, M38-A. 16 Vol. 22. National Committee on Clinical Laboratory Standards; 2002. [Google Scholar]
- 40.NCCLS. Susceptibility testing of mycobacteria, nocardia, and other aerobic actinomycetes; tentative standard—second edition, M24-T2. 26. Vol. 20. National Committee on Clinical Laboratory Standards; 2000. [PubMed] [Google Scholar]
- 41.Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, Hernandez A, Degnan MT, Cook MB, Quenzer VK, Ferguson RM, Gilman RH. J Clin Microbiol. 1998;36:362–366. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.