Abstract
Purpose
To update information on cytomegalovirus (CMV) retinitis in patients with the acquired immune deficiency syndrome (AIDS) and to integrate information on its pathogenesis and clinical outcomes.
Design
Literature review.
Methods
Selected articles from the medical literature, particularly large epidemiologic studies, including the Johns Hopkins Cytomegalovirus Retinitis Cohort Study, the Longitudinal Study of the Ocular Complications of AIDS, and the Cytomegalovirus Retinitis and Viral Resistance Study, were reviewed. Clinical information is discussed in light of knowledge on CMV, its pathogenesis, and its interactions with human immunodeficiency virus (HIV).
Results
Cytomegalovirus uses several mechanisms to evade the immune system and establish latent infection in immunologically normal hosts. With immune deficiency, such as late-stage AIDS, CMV reactivates, is disseminated to the eye, and establishes a productive infection, resulting in retinal necrosis. HIV and CMV potentiate each other: CMV accelerates HIV disease, and CMV retinitis is associated with increased mortality. Randomized clinical trials have demonstrated the efficacy of treatments for CMV retinitis. Systemically-administered treatment for CMV retinitis decreases AIDS mortality. Highly active antiretroviral therapy (HAART), effectively suppresses HIV replication, resulting in immune recovery, which, if sufficient, controls retinitis without anti-CMV therapy. Resistant CMV, detected in the blood, correlates with resistant virus in the eye and is associated with worse clinical outcomes, including mortality. Host factors, including host genetics and access to care, play a role in the development of CMV retinitis.
Conclusions
Clinical outcomes of CMV retinitis in patients with AIDS are dependent on characteristics of the virus and host and on HIV–CMV interactions.
Epidemiology of Cytomegalovirus Retinitis
Prior to the advent of the acquired immune deficiency syndrome (AIDS), cytomegalovirus (CMV) retinitis (Figure 1) was a rare disease seen primarily among transplant patients, but also among those with congenital CMV infection and other forms of immune compromise.1-4 It was estimated to affect ~1% of patients undergoing renal transplant and ~0.5% of those undergoing bone marrow transplant.1-4 It became evident early in the AIDS epidemic that CMV retinitis was a frequent opportunistic infection among patients with AIDS, and that it typically occurred in patients with CD4+ T cells (helper T cells) <50 cells/μL. 5,6 Epidemiological studies in the era before highly active antiretroviral therapy (HAART) reported that the incidence of CMV retinitis among patients with CD4+ T cells <50 cells/μL was 0.20/person-year (PY), and that the lifetime probability of a patient with AIDS getting CMV retinitis was ~30%.7,8 Although other end-organ CMV disease, such as colitis, esophagitis, cerebritis, and pneumonitis, occurred in patients with AIDS, the eye was the organ affected in ~80% of patients with AIDS and CMV disease.5,6 By the mid 1990's CMV retinitis was the most frequently encountered intraocular infection at major urban medical centers in the United States.
Figure 1.
Cytomegalovirus retinitis.
The primary risk factor for CMV retinitis appeared to be the level of immune deficiency, as determined by CD4+ T cells, but other risk factors contributed. These other factors included the level of CD8+ T cells (cytotoxic T cells), retinal vascular damage, as evidenced by the microangiopathy in the retina (known as AIDS retinopathy or HIV retinopathy), and bacteremia from Mycobacterium avium complex (MAC).5, 9-11 In a case control study of patients with AIDS matched for CD4+ T cells, those with CMV retinitis had significantly lower CD8+ T cells (152 cells/μL) than did those without CMV retinitis (296 cells/μL, P<0.001).9 In the Johns Hopkins CMV Retinitis Cohort Study, the presence of HIV microangiopathy, as evidenced by cotton wool spots, increased the odds of CMV retinitis 1.46-fold (P<0.05). In a study by the UCLA CMV Retinitis Study Group, the large majority of small (and presumably early) CMV lesions (73%) were adjacent of retinal blood vessels.10 These data suggest the importance of vascular damage to the risk of CMV retinitis in patients with AIDS and are consistent with data suggesting that the method of entry of CMV into the retina is through vascular endothelial cells (see below). In the Multicenter AIDS Cohort Study, the relative risk for CMV retinitis among patients with MAC bacteremia was 3.94 (P<0.0001), and in the Johns Hopkins CMV Retinitis Cohort the relative odds were 3.04 (P<0.05).5,11
The development of highly active antiretroviral therapy (HAART) profoundly altered the AIDS epidemic as it resulted in control of replication by the human immunodeficiency virus (HIV), improved immune function (as evidenced by rises in CD4+ T cells), a marked decrease in the incidence of opportunistic infections, and improved survival.12 With the advent of HAART, the incidence of CMV retinitis has decreased by 80-90%, although that decrease has leveled off (Figure 2), and CMV retinitis continues to occur.12-16 HIV infection now is a chronic disease with survival after infection with HIV estimated at >14 years.17 However, because of late testing among many high-risk patients, approximately one-third of patients with newly-diagnosed HIV infection in the United States still will progress to AIDS within one year of the diagnosis of HIV infection,18 resulting in an ongoing population at risk for CMV retinitis.
Figure 2.
Annual number of new cases of cytomegalovirus (CMV) retinitis at the Johns Hopkins Medical Institutions (JHMI). Informal surveys suggest that these data represent ~90% of the new cases of CMV retinitis in the Baltimore metropolitan area.
Pathogenesis of Cytomegalovirus Disease
Cytomegalovirus is a ubiquitous herpes family virus, and sero-prevalence surveys estimate that ~ 50% of the general population is latently infected.19,20 Certain populations, such as men who have sex with men, have a higher rate of latent infection (estimated at >90%).21 In immunologically normal hosts, acquired CMV typically manifests as a mild, self-limited, flu-like illness, followed by a latent infection. The site of latency is thought to be granulocytes.1 Cytomegalovirus uses several of its protein products to prevent the presentation of CMV antigens in conjunction with HLA class I molecules and thereby prevent clearance of CMV-infected cells by CD8+ T cells. Although natural killer (NK) cells recognize down-regulation of HLA class I by virally-infected cells and thereby clear them, CMV produces an HLA class I homolog, which allows CMV-infected cells to evade NK cell surveillance.19 With immune compromise, CMV can reactivate and be hematogenously disseminated to target organs. The large majority of patients with newly-diagnosed CMV retinitis have evidence of systemic CMV replication, detected either by culture in either the blood or urine (~80%) or by polymerase chain reaction (PCR) amplification of blood specimens to detect CMV DNA in the blood (~60%).22,23 The entry of CMV into the eye appears to be through retinal blood vessels, and pathology studies have shown infection of vascular endothelial cells at the edge of the lesion.24 Cytomegalovirus retinitis results in full-thickness retinal necrosis of infected areas. The end result of infection is retinal destruction of infected areas. Cytomegalovirus resides at the edge of the lesion, and unless there is immune recovery or anti-CMV therapy is administered, CMV spreads into contiguous retina, resulting in enlarging lesions.25 As lesions enlarge ever-increasing amounts of visual field are lost, and when the optic nerve or fovea is damaged there is a loss of visual acuity.
Cytomegalovirus and HIV interactions
Cytomegalovirus and HIV interact to promote both infections. Cytomegalovirus and HIV trans-activate each other in vitro resulting in increased yields of both viruses.26,27 Dual infection of individual retinal cells of autopsy eyes from patients AIDS and CMV retinitis with both CMV and HIV has been demonstrated.28 Cytomegalovirus produces cytokine homologues that bind to host receptors and down-regulate the immune system.29-32 One key cytokine homologue appears to be an interleukin (IL)-10 homologue (cmv IL-10h). Interleukin-10 is a human cytokine that inhibits T helper type-1 (Th1) immune responses, which are key to cell-mediated immunity.29,30 Cytomegalovirus also produces chemokine receptors, which bind chemokines, and inhibit the recruitment of inflammatory and immune cells, and (as noted above) it interferes with natural killer cells, thereby inhibiting the host's ability to clear viruses.31,32 Studies of populations with low sero-prevalence of CMV infection, such as neonatal and transfusion acquired HIV infection, demonstrate that CMV co-infection (as evidenced by positive serology), even in the absence of end-organ CMV disease, accelerates the development of AIDS and shortens the time from acquisition of HIV to AIDS.33-35
Mechanisms of visual loss
The primary cause of visual loss in patients with CMV retinitis is necrotic damage to the fovea or optic nerve from CMV infection (Table 1).36-41 In the era before HAART, the second leading cause of visual loss was retinal detachment, whereas in the HAART era it was cataract, in part due to the reduced incidence of retinal detachment in the HAART era.41 Macular edema was uncommon in the pre-HAART era, but is a more common cause of visual impairment in the HAART era, particularly among patients with immune recovery uveitis (see below).41
Table 1.
Causes of Visual Loss from Cytomegalovirus Retinitis
| Pre-HAARTa era | HAART era | ||
|---|---|---|---|
| Cause (% of eyes) | 20/200 or worse | 20/50 or worse | 20/200 or worse |
| Macula or optic nerve disease | 54-84 | 63.1 | 69.8 |
| Cataract | No data | 22.0 | 28.6 |
| Retinal detachment | 36-63 | 12.9 | 16.7 |
| Macular edema | <5 | 12.4 | 11.1 |
| Immune recovery uveitis | Not applicable | 4.3 | 3.2 |
| Epiretinal membrane | <5 | 3.3 | 4.1 |
HAART = highly active antiretroviral therapy.
From Thorne JE, Jabs DA, Kempen JH, Holbrook JT, Nichols C, Meinert CL for the Studies of Ocular Complications of AIDS Research Group. Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology 2006;113(8):1432-40 and Thorne JE, Jabs DA, Kempen JH, Holbrook JT, Nichols C, Meinert CL for the Studies of Ocular Complications of AIDS Research Group. Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology 2006;113(8):1441-5. Used with permission. Only data on causes of blindness (20/200 or worse) are available from the pre-HAART era.
Immune Recovery and Immune Recovery Uveitis
Highly active antiretroviral therapy consists of combination therapy, generally with three or more drugs, including at least one potent antiretroviral drug, such as a protease inhibitor. In the industrialized world HAART became widely used in the mid-1990s and changed the course of HIV management. For the first time, clinicians were able to obtain substantial and sustained suppression of HIV replication, typically measured as the amount of HIV RNA in the blood, also known as HIV “load” or “viral load”.42 With suppression of HIV replication, the decline in immunity, measured as the decline in CD4+ T cells could be forestalled, and those with impaired immunity could experience immune recovery, measured as a rise in CD4+ T cells. The incidence of AIDS among patients with HIV infection declined, as fewer patients progressed to end-stage HIV infection, and the incidence of opportunistic infections also declined. Immunologic studies demonstrated the restoration of specific immunity to pathogens, such as CMV, with immune recovery,45 although the recovery generally took three to six months. AIDS mortality declined, and the prevalence of patients alive with AIDS and earlier stages of HIV infection increased.12 Resistance of HIV to the drugs in the HAART regimen, particularly among patients heavily pretreated with single drugs in the pre-HAART era, could limit immune recovery. In addition failure to fully reconstitute the immune system, resulting in partial restoration of immunity or lacunae in the immune repertoire, has been demonstrated.43,44 These problems would result in some patients remaining at risk for opportunistic infections, but they appear to be decreasing as HAART use expanded and with the introduction of additional antiretroviral drugs.
Patients with CMV retinitis started on HAART, restarted on HAART, or having their regimen changed often experienced sufficient immune recovery to stop anti-CMV therapy, without the CMV retinitis relapsing, documented by numerous case series.46-51 The recommended threshold for interrupting anti-CMV therapy is a CD4+ T cell count over 100 cells/μL.51 Because there is a delay in restoring specific immunity after a rise in CD4+ T cells, the CD4+ T cell count should be above 100 cells/μL for a minimum of three to six months before stopping anti-CMV therapy. 51 Case series have suggested that among patients with immune recovery, discontinuation of anti-CMV therapy, and subsequent failure of immune recovery, relapse of the CMV retinitis occurs when CD4+ T cells have declined again to <50 cells/μL.50
With the advent of HAART and immune recovery, a new problem arose, immune recovery uveitis (IRU). Immune recovery uveitis is one of the Immune Recovery Inflammatory Syndromes (IRIS), which have been described in other organs and with other opportunistic infections (such as mycobacterial infections). Patients with immune compromise and CMV retinitis usually have either no or mild anterior chamber and vitreous cellular reactions, which are usually less among patients treated with anti-CMV therapy.52-54 Immune recovery uveitis is characterized by the new occurrence of or an increase in anterior chamber or vitreous inflammatory reaction (cells) in the face of immune recovery, most often a vitritis.54-57 Uveitis-related structural complications, such as macular edema, also occur and can compromise vision.54- 57 Immediately after the introduction of HAART, widely disparate rates of IRU were reported, from 0.83/person-year (PY)56 to 0.11/PY.54 Initially the reasons for this disparity were unclear, but subsequent investigations suggested a substantial role for intravitreal cidofovir in these disparate rates. The use of intravitreal cidofovir is a major risk factor for IRU (relative odds =19),57 and the highest rate of IRU was reported from the clinic that pioneered the investigation of intravitreal cidofovir therapy as treatment for CMV retinitis.58 In the late 1990s to early 2000s the prevalence of IRU among patients with CMV retinitis was reported as 14% in the Longitudinal Study of the Ocular Complications of AIDS (LSOCA) cohort.57 It was hypothesized that IRU might represent a partial restoration of immunity, particularly among those who could not develop full benefit from HAART due to prior antiretroviral treatment or prolonged sustained immune deficiency with resultant partial immune restoration from HAART.59 Supporting this latter concept is the apparent decrease in the incidence of IRU among patients diagnosed with CMV retinitis and then experiencing immune recovery in the HAART era (Figure 3), which now is estimated to be 0.04/PY.60
Figure 3.
Immune recovery uveitis among patients with cytomegalovirus retinitis. Adapted from Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S for the Studies of the Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology (in press). Used with permission.
There is an issue on the timing of starting a patient on HAART who has active CMV retinitis. In general HAART treatment is started as soon as possible in patients with opportunistic infections. However, one historically-controlled study suggested that early introduction of HAART in a patient with CMV retinitis before completing induction therapy for CMV resulted in a higher incidence of IRU (71%) than among those who had suppressed retinitis before starting HAART (31%, P=0.01), and a greater severity of IRU.61 These data suggest that all patients with CMV retinitis, even HAART-naive patients with small peripheral lesions, should be treated for the CMV to reduce the incidence of IRU. Furthermore, these data suggest that a delay in initiating HAART until after the retinitis had been controlled might be beneficial. However, this approach must be counterbalanced by the risk of other opportunistic infections if antiretroviral therapy is delayed. One randomized trial in which HAART was started approximately two weeks after opportunistic infection therapy vs. approximately six weeks demonstrated a significantly greater risk of AIDS-related events in the latter group.62 Furthermore, there was no increase in IRIS events in the latter group, although CMV retinitis was not specifically studied. Because the rate of IRU is low (0.04/PY) and because CMV replication is controlled within 1-2 weeks, a delay of no more than two weeks would seem prudent.
Treatment of Cytomegalovirus Retinitis
Treatment of CMV retinitis is influenced by the fact that all available treatments are virostatic and do not eliminate the virus. A histopathologic study of treated CMV retinitis demonstrated the presence of viral DNA at the border of the lesion, but ineffective assembly of intact virions.25 With cessation of therapy viral assembly resumes, as does clinical retinitis. Therefore, unless there is immune recovery, chronic, life-long therapy to prevent relapse is required for patients with AIDS. Three United States Food and Drug Administration (FDA)-approved drugs are available for the treatment of CMV retinitis: ganciclovir (Cytovene, Roche Pharmaceuticals, Nutley, NJ), foscarnet (Foscavir, AstraZeneca Pharmaceuticals LP, Wilmington, DE), and cidofovir (Vistide, Gilead, Foster City, CA). A fourth drug, fomivirsen (Vitravene, Isis Pharmaceuticals, Inc., Carlsbad, CA), an anti-sense aptamer, was FDA-approved and then withdrawn from the market when the incidence of CMV retinitis declined after the introduction of HAART. Ganciclovir can be administered by IV infusion, surgical placement of a sustained-release implant into the vitreous (Vitrasert, Bausch & Lomb Pharmaceuticals, Inc., Tampa, FL), which typically lasts at least six months, or orally with the pro-drug valganciclovir (Valcyte, Roche Pharmaceuticals). An oral formulation of ganciclovir, with poor bioavailability, largely has been replaced by valganciclovir. Foscarnet and cidofovir are administered intravenously. Ganciclovir and foscarnet also are administered off-label as intravitreal injections.16 Cidofovir was investigated as an intravitreal drug, but its use largely has been discontinued due to a narrow therapeutic-toxic window and problems with uveitis and hypotony.58 The treatment of CMV retinitis has been the subject of numerous clinical trials (Table 2), sponsored both by the pharmaceutical industry and by the National Institutes of Health (NIH).36,38,63-73
Table 2.
Clinical Trials of Treatments for Cytomegalovirus Retinitis.
| Trial name/design | Year published | Treatments | Main results |
|---|---|---|---|
| Foscarnet v observation63 | 1991 | foscarnet | foscarnet effective |
| Ganciclovir v observation for peripheral retinitis64 | 1993 | ganciclovir | ganciclovir effective |
| Foscarnet Ganciclovir CMVa Retinitis Trial (FGCRT)36,65 | 1992 | foscarnet v ganciclovir | foscarnet and ganciclovir equivalent for controlling CMV retinitis; foscarnet associated with a lower mortality |
| CMV Retinitis Retreatment Trial (CRRT)37 | 1996 | foscarnet v ganciclovir v combination | combination treatment superior to monotherapy for relapsed retinitis |
| Monoclonal Antibody CMV Retinitis Trial (MACRT)38 | 1997 | anti-CMV monoclonal antibody v placebo as adjunct to drug therapy | antibody ineffective and associated with increased mortality |
| HPMPC Peripheral CMV Retinitis Trial (HPCRT)66 | 1997 | cidofovir v observation for peripheral retinitis | cidofovir effective |
| Cidofovir peripheral CMV retinitis trial67 | 1997 | cidofovir v observation for peripheral retinitis | cidofovir effective |
| Ganciclovir implant trial68 | 1994 | ganciclovir implant v observation for peripheral CMV retinitis | ganciclovir implant effective |
| Ganciclovir implant v ganciclovir trial69 | 1997 | ganciclovir implant v intravenous ganciclovir | implant more effective for controlling retinitis but higher rate 2nd eye & visceral disease |
| Ganciclovir implant v implant & oral ganciclovir v intravenous ganciclovir70 | 1999 | ganciclovir implant v implant & oral ganciclovir v intravenous ganciclovir | implant & oral ganciclovir more effective than implant alone for preventing 2nd eyed & visceral disease & similar to intravenous ganciclovir |
| Ganciclovir Cidofovir CMV Retinitis Trial (GCCRT)71 | 2001 | Ganciclovir implant & oral ganciclovir v cidofovir | two regimens equivalent for controlling retinitis |
| Valganciclovir v intravenous ganciclovir for induction72 | 2002 | valganciclovir v intravenous ganciclovir | valganciclovir similar to intravenous ganciclovir for induction therapy |
CMV = cytomegalovirus.
Data from Jabs DA. Clinical research in uveitis. In Zierhut M, ed. Uveitis (in press) with permission.
Systemically administered anti-CMV therapies are given initially at higher doses to control the infection (known as induction) for two to three weeks and then at lower doses to prevent relapse of the clinical disease (maintenance or secondary prophylaxis). Although systemically-administered therapies initially control the retinitis, with time, the retinitis relapses, requiring repeated cycles of induction and maintenance, and unless there is immune recovery, there is a progressive shortening of the time to relapse.36
The evaluation of the efficacy of anti-CMV therapies required the development of methodologies and outcomes suitable for clinical trials. The primary outcome of nearly all trials of anti-CMV therapies has been the time to progression, defined as the movement of the border of a CMV lesion ½-disc diameter in distance, along a front ½-disc diameter in size, or the occurrence of a new lesion ¼-disc area in size.74 Meta-analysis of clinical trials with long-term follow-up demonstrated that retinitis progression is a reasonable surrogate marker for subsequent visual loss.75 Because the administration of intravenous therapies (the first ones developed) required a central line and because of the differential methods of administering the different drugs, masked treatment administration typically was not feasible, so clinical trials employed a reading center to read standardized retinal photographs with graders masked as to treatment assignment. Studies of reading center performance demonstrated that reading center grading of retinal photographs detected retinitis progression earlier than clinicians.36 A study of two reading centers’ performance grading the same set of photographs demonstrated that although the two reading centers might differ on an occasional grading (κ=0.55), overall they gave very similar results for time to progression analyses of the entire study population (medians 65 v 69 days).76
In order to determine if a new treatment for CMV retinitis was effective, clinical trials often randomized patients with “small peripheral lesions” to the new treatment or to observation for a brief period of time until there was retinitis progression.64-67,77 Formalization of this methodology included the definition of retinal zones: zone 1 encompassed an area one disc-diameter from the edge of the optic nerve or two disc-diameters from the center of the fovea (these lesions are considered immediately vision threatening); zone 2 extended from the edge of zone 1 approximately to the equator as marked by a circle identified by the vortex vein ampullae, and zone 3 extended anteriorly from the edge of zone 2 to the ora serrata. Clinical trials of “small peripheral lesions” enrolled patients with lesions only in zones 2 or 3 which occupied less than 25% of the retinal area.76 A long-term follow-up study of patients enrolled in one such trial demonstrated no evident long-term adverse outcomes in terms of mortality, vision loss or structural complications from the brief period of observation.66,78
The second major type of trial was a comparative trial between two drugs. All of the systemically-administered drugs appear to have similar efficacy. Comparative trials of intravenous ganciclovir and intravenous foscarnet and of intravenous ganciclovir and oral valganciclovir have demonstrated similar efficacy. Because of the ease of administration and absence of catheter-related complications, valganciclovir now is used nearly always for systemic therapy.36,65,71,72
The ganciclovir implant produces intraocular levels of ganciclovir five times that of systemically administered ganciclovir and generally controls new cases of CMV retinitis until it runs out of drug, on average in seven months.68 Comparative studies have shown that the implant is associated with longer times to retinitis progression than is systemic ganciclovir therapy.69 The implant can be replaced, and studies showed that the same site could be used twice relatively easily, but that the third surgery in a quadrant was associated with a higher rate of surgical complications, suggesting that eight implants could be placed in sequence (two in each quadrant) over a period of four years with a reasonably low level of surgical complications.79 However, because CMV retinitis is part of a systemic infection, and local intraocular therapy does not control the systemic part of the disease, local only therapy was associated with high rates of contralateral or “second eye” disease (in those presenting with unilateral disease) and visceral disease, substantially higher than those seen with systemic therapy.69,70 Given the benefit of systemic therapy on mortality (see below) and on dissemination of disease, most patients treated with an implant also receive systemic therapy with valganciclovir.16,70,80
Intravitreal injections of ganciclovir and foscarnet have to be given two to three times weekly as induction therapy and weekly as maintenance therapy and have relapse rates similar to those of systemic therapy.81,82 Therefore, in the industrialized world, injections generally are used as initial therapy until an implant can be placed or to determine if relapsed retinitis can be controlled by higher doses of the drug (e.g. an implant in a patient previously treated systemically) or the alternative drug (e.g. in a patient with presumed resistant CMV).16 In resource poor countries, such as sub-Saharan Africa, for economic reasons, repetitive intravitreal injections often are the primary mode of therapy.83
Because immune recovery can result in control of the retinitis without concomitant anti-CMV therapy, the United States Department of Health and Human Services recommendations for managing patients with CMV retinitis recommend that patients with immune recovery to >100 cells/μL for at least three to six months have anti-CMV therapy discontinued 51. However, because not all patients with immune recovery will control the retinitis without anti-CMV therapy, and because the risk of relapse continues for at least five years (Table 3, Figure 4), all such patients require regular ophthalmologic follow-up, which has been recommended to occur at three-month intervals.60,84 ,85
Table 3.
Outcomes of Cytomegalovirus Retinitis.
| HAARTa era |
|||
|---|---|---|---|
| Outcome | Pre-HAART | 2-3 year rate | 5-year rate |
| Mortality (/PY)b | 0.12 | 0.10 | |
| Retinitis progression (/PY) | |||
| Overall | ~3.0 | 0.10 | 0.07 |
| CD4+ T cells <50 cells/μL | 0.27 | 0.23 | |
| CD4+ T cells >100 cells/μL | 0.03 | 0.02 | |
| Retinal detachment (/EY)c | |||
| Overall | 0.33 | ~0.04 | 0.02 |
| CD4+ T cells <50 cells/μL | ~0.10 | 0.04 | |
| CD4+ T cells >100 cells/μL | ~0.03 | 0.01 | |
| 2nd eye involvement (/PY) | |||
| Overall | 0.40 | 0.07 | 0.03 |
| CD4+ T cells <50 cells/μL | 0.16 | 0.07 | |
| CD4+ T cells >100 cells/μL | 0.02 | 0.01 | |
| Visual acuity loss to | |||
| Worse than 20/40(/EY) | |||
| Overall | ~0.96 | 0.10 | 0.08 |
| CD4+ T cells <50 cells/μL | 0.19 | 0.09 | |
| CD4+ T cells >100 cells/μL | 0.10 | 0.05 | |
| 20/200 or worse (/EY) | |||
| Overall | ~0.48 | 0.06 | 0.03 |
| CD4+ T cells <50 cells/μL | 0.07 | 0.05 | |
| CD4+ T cells >100 cells/μL | 0.04 | 0.025 | |
HAART = highly active antiretroviral therapy.
PY = person-year.
EY = eye-year.
From Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S for the Studies of the Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology (in press); Jabs DA, Van Natta ML, Thorne JE, et al. for the Studies of Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 1. retinitis progresion. Ophthalmology 2004;111(12):2224-31; and Jabs DA, Van Natta ML, Thorne JE, et al. for the Studies of Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2. second eye involvement and retinal detachment. Ophthalmology 2004;111(12):2232-9. Used with permission.
Figure 4.
Retinitis progression among patients with cytomegalovirus retinitis. Adapted from Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S for the Studies of the Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology (in press). Used with permission.
Treatment for CMV retinitis is dependent on the location of the retinitis and the likelihood of immune recovery (Table 4), as well as treatment adherence considerations.16 Because there is a three- to six-month lag between initiation of HAART and recovery of specific immunity to CMV, and because the likelihood of complications of CMV (e.g. retinal detachment, IRU) is related to the size of the CMV lesion 57,86 nearly all patients with CMV retinitis, even those expected to experience immune recovery, should be treated with anti-CMV therapy for at least six months.16 Patients without immune recovery will require life-long therapy. Because of the improvement in survival and decrease in the incidence of second eye and visceral disease with systemic therapy, most patients should receive systemic anti-CMV therapy.70,80 Because the implant controls retinitis progression better than systemic anti-CMV therapy, it generally is the preferred treatment for zone 1 lesions. Small peripheral lesions in a HAART-naïve patient often are treated with systemic therapy alone, as immune recovery is expected.16
Table 4.
Proposed Initial Treatment Algorithm for Patients with Cytomegalovirus Retinitis.
| Highly active antiretroviral therapy |
||
|---|---|---|
| Retinitis locationa | Experienced | Naive |
| Zone 1 | Valganciclovir + ganciclovir implant | Valganciclovir + ganciclovir implant |
| Zones 2 and/or 3 only | Valganciclovir ± ganciclovir implant | Valganciclovir |
Zone 1 refers to an area of the retina extending 2 disc diameters from the center of the fovea and one disc diameter from the edge of the optic nerve. Zone 2 extends from the edge of zone 1 to a circle identified by the vortex vein ampullae, and zone 3 extends from the anterior edge of zone 2 to the ora serrata.
From Jabs DA. AIDS and ophthalmology, 2008. Arch Ophthalmol 2008;126(8):1143-6. Used with permission.
Cytomegalovirus Resistance to Anti-cytomegalovirus Therapy
Unless there is immune recovery, patients with CMV retinitis need chronic, lifelong treatment, as, without immune recovery, CMV retinitis typically relapses within three to four weeks of stopping therapy.87 Even with chronic systemic therapy, without immune recovery, patients nearly always relapse given sufficient time (albeit at much longer times than without treatment). Early relapses appear to be due to the limited penetration of systemically-administered drugs.88,89 In patients with newly-diagnosed CMV retinitis, the ganciclovir implant tends to control the retinitis until it runs out of drug, approximately six months after implantation.68,69
With chronic treatment for CMV, resistance of the CMV to the drug may occur. Resistance is uncommon in untreated patients (<5%) and tends to occur only after three months of anti-CMV therapy.90, 91 In the pre-HAART era, the incidences of resistance to ganciclovir and to foscarnet were estimated at 0.25/PY for each drug.92-94 The data on cidofovir were less robust than those on ganciclovir and foscarnet, but suggested that the resistance rate was similar to those for ganciclovir and foscarnet.
Low-level ganciclovir resistance is due to mutations in the CMV UL97 gene, which encodes for a phosphotransferase, which phosphorylates ganciclovir. Cellular enzymes complete two more phosphorylations to ganciclovir tri-phosphate, which then inhibits the CMV DNA polymerase, encoded by the CMV UL54 gene, and thereby CMV replication. High-level ganciclovir resistance is due to mutations in both the CMV UL97 and UL54 genes. Cidofovir is a nucleotide analog with one phosphate group already present. It is phosphorylated by cellular enzymes to cidofovir di-phosphate, which inhibits the CMV DNA polymerase. Mutations in the same regions of the CMV UL54 gene which are associated with high-level ganciclovir resistance cause cidofovir resistance. Foscarnet directly inhibits the CMV DNA polymerase, and mutations in regions of the CMV UL54 disease different from those causing high-level ganciclovir resistance cause foscarnet resistance.95-106 The implications of these data are that low-level ganciclovir-resistant CMV should be susceptible to cidofovir, whereas high-level ganciclovir-resistant CMV is unlikely to be susceptible to cidofovir, and that ganciclovir-resistant CMV should be susceptible to foscarnet. Clinical case series have demonstrated renewed control of rapidly-relapsing, ganciclovir-resistant, CMV retinitis with changing therapy to foscarnet.107
In patients with CMV retinitis, CMV can be cultured from the blood or urine in ~80% of patients at the time of diagnosis of the retinitis.22 Cultured virus can be assessed for resistance to anti-CMV drugs using two different approaches. Phenotypic methods assess the ability of the virus to replicate in the presence of the drug, and they are measured as the inhibitory concentration 50% (IC50). Genotypic methods, sequence the CMV culture isolate for known resistance-conferring mutations.92-108 The Cytomegalovirus Retinitis and Viral Resistance (CRVR) Study, a prospective cohort study of patients with CMV retinitis for the occurrence of resistance and its consequences, demonstrated excellent correlation between phenotypic and genotypic measures of resistance.92-94,108 Blood and ocular (vitreous) specimens can be directly polymerase chain reaction (PCR) amplified and sequenced to detect resistance conferring mutations (genotypic assays). In the CRVR Study there was excellent correlation (~95%) for the detection of resistance-conferring mutations in the CMV UL97 genome between the virus found in the eye and the blood, suggesting that assessing the blood provides data on the virus in the eye.109 In addition, it was observed that resistant virus typically was detected in the blood simultaneously with or before it was detected in the eye, and that when the eyes of patients with bilateral retinitis gave discordant results, both viruses typically (though not always) were present in the blood.109 These data suggest that, although compartmentalized evolution of resistance may occur in the eye, the primary source of resistant virus may the blood with re-infection of the eye. Stochastically, the much greater volume of the blood and, therefore, number of virions, makes the blood a more likely site for the evolution of resistant virus. Furthermore, the detection of resistance in the blood or urine, using either phenotypic methods or sequencing the UL97 gene of culture isolates, correlated well with clinical outcomes, including retinitis progression, destruction of retinal area, and loss of vision. These data suggest that detection of resistant virus in the blood or urine could be clinically useful.94,110 Longitudinal studies demonstrated that as long as anti-CMV treatment was unchanged, resistant CMV persisted and that there were increasing levels of resistance, as measured by increasing IC50's.96 Changing the anti-CMV drug to one to which the virus was sensitive would become necessary to control the retinitis.107
Conventional resistance testing requires up to eight weeks to perform, as the virus is grown from the blood or urine specimen, and then the culture isolate is tested for resistance as outlined above. This delay is too long to be used clinically to manage patients. The CRVR Study explored two approaches to rapidly diagnose whether a patient harbors resistant CMV. The first was the use of the amount of CMV DNA in the blood (CMV “load” or “viral load”).23 Cytomegalovirus DNA is detectable in the blood from ~60-70% of patients on diagnosis of CMV retinitis and in ~20% during follow-up while on treatment, fairly consistently over time. The assessment of CMV load is drug independent and can be performed in one to two days. Although CMV load correlated with the occurrence of resistance and with retinitis progression, it poor positive predictive value (0.07-0.09) limited its use for detecting resistance. However, its excellent negative predictive value (0.99), suggested it could be used to exclude resistant virus in a patient with rapidly-relapsing CMV retinitis.23
The second method for detecting ganciclovir-resistant virus rapidly is PCR amplification of blood specimens (without culturing) and sequencing of the CMV UL97 gene. Because the resistance-conferring mutations in the CMV UL97 gene have been characterized (Table 5), and because both low- and high-level ganciclovir resistance will have a CMV UL97 gene mutation, this method allows for rapid detection of ganciclovir resistance (one to two days). The CRVR Study compared the detection of resistance using both culture specimens and directly PCR-amplified and UL97 gene sequenced blood specimens. The agreement between the two methods was >92%, suggesting that the rapid method would be clinically useful.111 Furthermore, the more rapid PCR-amplification and UL97 gene sequencing of blood specimens also correlated with clinical outcomes, such as retinitis progression.111 Because ganciclovir (now generally valganciclovir with or without a ganciclovir implant) is the initial therapy for >80% of patients, this approach would have broad utility, and the detection of ganciclovir resistance would allow for switching to an alternative drug to control the replicating virus once again. Unfortunately, this test remains largely investigational and not widely available.
Table 5.
Cytomegalovirus UL97 gene mutations causing ganciclovir resistance
| UL97 mutation | Amino acid change |
|---|---|
| M460V | Methionine to valine |
| M460I | Methionine to isoleucine |
| N510S | Asparagine acid to serine |
| H520Q | Histidine to glutamine |
| A590T | Alanine to threonine |
| A591D | Alanine to aspartic acid |
| A591V | Alanine to valine |
| C592G | Cysteine to glysine |
| A594V | Alanine to valine |
| A594T | Alanine to threonine |
| del591-594 | Deletion of alanine-alanine-cysteine-arginine |
| L595S | Leucine to serine |
| L595F | Leucine to phenylalanine |
| L595T | Leucine to threonine |
| del595 | Deletion of leucine |
| L595W | Leucine to tryptophan |
| E596G | Glutamic acid to glysine |
| E596D | Glutamic acid to aspartic acid |
| N597I | Asparagine to isoleucine |
| G598V | Glysine to valine |
| K599M | Lysine to methionine |
| Del600 | Deletion of leucine |
| C603W | Cysteine to tryptophan |
| C607Y | Cysteine to tyrosine |
| C603Y | Cysteine to tyrosine |
| A606D | Alanine to aspartic acid |
| V665I | Valine to isoleucine |
Data from Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Reviews. 1999;12:286-97.
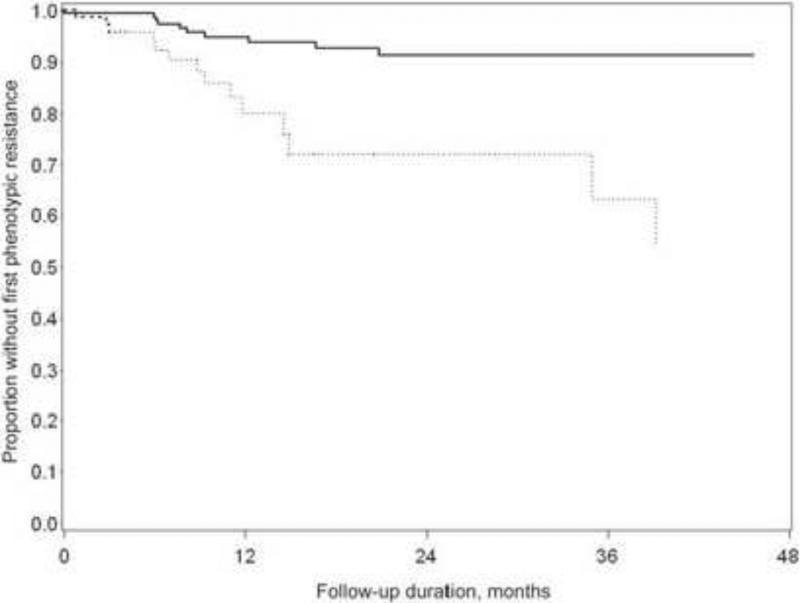
The introduction of HAART in the mid-1990s altered the incidence of resistance (Figure 5). The occurrence of immune recovery and the ability to discontinue anti-CMV therapy would remove the selective pressure for the development of resistant virus. The nearly simultaneous advent of better delivery of therapies for CMV (the ganciclovir implant and then valganciclovir) also might result in better control of viral replication and less resistance. The CRVR Study evaluated the change in incidence of resistant CMV among those being treated for CMV retinitis in the era before 1996 (pre-HAART) and after (HAART era). The two-year incidence of ganciclovir-resistant CMV declined significantly from 28% to 9%. All patients who developed resistant CMV had CD4+ T cell counts of <50 cells/μL at the time of diagnosis of the retinitis. The one- and two-year incidence of resistance in a small subgroup of patients who did not receive HAART in the HAART era were 16% and 50%, respectively, rates similar to those in the pre-HAART era.112 These data suggest an indirect effect of HAART on the incidence of resistant CMV. It can be speculated that HAART, by decreasing HIV replication, secondarily decreases CMV replication (through less transactivation), thereby decreasing statistically the likelihood of developing a resistant virus.
Figure 5.
Incidence of resistant cytomegalovirus among patients with cytomegalovirus retinitis, comparing patients diagnosed pre-1996 (hatched line) to those diagnosed in 1996 or later (solid line). From Martin BK, Ricks MO, Forman MS, Jabs DA for the Cytomegalovirus Retinitis and Viral Resistance Research Group. Change over time in incidence of ganciclovir resistance in patients with cytomegalovirus retinitis. Clin Infect Dis 2007;44(7):1001-8. Used with permission.
Visual Outcomes of Cytomegalovirus Retinitis
When data were correctly analyzed, it was clear that the visual outcomes in the pre-HAART era were poor. Despite treatment with anti-CMV agents, rates of visual loss to 20/50 or worse (visual impairment) were 0.94-0.98/EY and to 20/200 or worse (blindness) 0.47-0.49/EY (Table 3). The median time to bilateral blindness was ~21 months.5 The primary causes of visual loss were optic nerve or foveal damage directly from the necrosis caused by CMV and retinal detachment.5,39 Retinal detachments occurred at the rate of 0.33/EY.5,86 Although surgical repair of the detachments could be accomplished by vitreoretinal surgery with silicone oil, visual recovery after detachment was limited by the refractive problems with silicone oil and the subsequent development of cataract.86,113,114 In the Johns Hopkins CMV Retinitis Cohort Study, nearly all patients undergoing surgical repair of CMV retinitis-related retinal detachments subsequently experienced cataract formation, and the median time from retinal surgery to cataract formation was 1.8 months. Although phacoemulsification and intraocular lens implantation could be performed successfully, posterior capsule opacification occurred rapidly, a median of 7 days after cataract surgery 114. The introduction of HAART coincided with the introduction of the ganciclovir implant and was followed soon by the introduction of valganciclovir. Thus in the mid-1990's improved control of CMV retinitis and immune recovery led to better control of CMV retinitis and lower rates of retinitis progression, retinal detachment, and visual loss than in the pre-HAART era (Table 3). The overall detachment rate dropped eight-fold, and among patients with persistent immune deficiency (CD4+ T cells <50 cells/μL) the rate dropped by nearly ~70%. However, even among patients with immune recovery, the incidence of retinal detachment in at least one eye (0.016/PY) is over 100-fold greater than that in the general population (~10/100,000/PY).115 In the HAART era, rates of visual impairment and blindness decreased by >90%, and even among those with CD4+ T cells <50 cells/μL, the rates were decreased by 90%. Although the rates of visual impairment and blindness decline over time (Table 3 and Figures 6 and 7), at no time do they plateau to zero, suggesting ongoing need for ophthalmologic management.40,60,84,85
Figure 6.
Visual impairment (worse than 20/40 visual acuity) in eyes of patients with newly-diagnosed cytomegalovirus retinitis in the HAART era. Adapted from Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S for the Studies of the Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology (in press). Used with permission.
Figure 7.
Blindness (visual acuity 20/200 or worse) in eyes of patients with newly-diagnosed cytomegalovirus retinitis in the HAART era. Adapted from Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S for the Studies of the Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology (in press). Used with permission.
Mortality
In patients with AIDS in the HAART era, CMV disease, as manifested by retinitis, is associated with a 60% increase in mortality (Figure 8) 116. This increase in mortality is abrogated by immune recovery from HAART. Available data on the causes of death suggest no increase in any specific cause of death from co-infection with CMV but rather a generalized increase in mortality due to the usual causes of death among patients with AIDS.116 In the HAART era, the mortality rate for patients newly-diagnosed with CMV retinitis (Figure 9) is 0.26/person-year (PY).60 The subsequent development of immune recovery dramatically alters this outlook; the one- and five-year survivals from the diagnosis of CMV retinitis are 43.6% and 1.4% for those without immune recovery, compared to 97.9% and 63.7% for those with immune recovery.60 However, only 50% of patients with newly-diagnosed CMV retinitis diagnosed in the HAART era experience immune recovery to a level of CD4+ T cells >100 cells/μL (Figure 10), so that mortality remains substantial (Figure 9).60
Figure 8.
Mortality among patients with AIDS in the era of highly active antiretroviral therapy. CMVR = cytomegalovirus retinitis. From Jabs DA, Holbrook JT, Van Natta ML, et al. for the Studies of the Ocular Complications of AIDS Research Group. Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology 2005;112(5):771-9. Used with permission.
Figure 9.
Mortality among patients with newly-diagnosed cytomegalovirus retinitis in the HAART era. Adapted from Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S for the Studies of the Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: five-year outcomes. Ophthalmology (in press). Used with permission.
Figure 10.
Immune recovery among patients with newly-diagnosed cytomegalovirus retinitis. Adapted from Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S for the Studies of the Ocular Complications of AIDS Research Group. Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: Five-year outcomes. Ophthalmology (in press). Used with permission.
Among patients with CMV retinitis, an increased CMV burden also is associated with an increased mortality. At the time of diagnosis of CMV retinitis the detection of replicating CMV in the blood either by culture or by PCR amplification and detection of CMV DNA in the blood (“CMV viral load”) is associated with an increased mortality; higher CMV viral loads are associated with greater mortality (Figure 11).23 Among patients with CMV retinitis the use of systemic anti-CMV therapy (as opposed to only local intraocular therapy) is associated with a 28% reduction in mortality (Figure 12).80 Consistent with the beneficial effect of systemic anti-CMV therapy on survival are data from the pre-HAART era demonstrating that treatment for CMV retinitis, regardless of which drug is used, resulted in a decrease in HIV in the blood.117 Finally, the occurrence of resistant CMV while on treatment, which results in CMV replicating in the blood, also is associated with a 65% increase in mortality (Figure 13).118 The likely explanations for these observations are the bidirectional interactions of CMV and HIV and the immunosuppressive effect of CMV, both of which result in greater impairment of the immune system and thereby greater mortality.
Figure 11.
Effect of cytomegalovirus “viral load” on mortality among patients with newly-diagnosed cytomegalovirus retinitis. From Jabs DA, Martin BK, Forman MS, Ricks MO for the Cytomegalovirus Retinitis and Viral Resistance Research Group. Cytomegalovirus (CMV) blood DNA load, CMV retinitis progression, and occurrence of resistant CMV in patients with CMV retinitis. J Infect Dis 2005;192(4):640-9. Used with permission.
Figure 12.
Effect of systemic anti-cytomegalovirus therapy on mortality among patients with cytomegalovirus retinitis. From Kempen JH, Jabs DA, Wilson LA, Dunn JP, West SK, Tonascia J. Mortality risk for patients with cytomegalovirus retinitis and acquired immune deficiency syndrome. Clin Infect Dis 2003;37(10):1365-73. Used with permission.
Figure 13.
Effect of resistant cytomegalovirus on mortality among patients with cytomegalovirus retinitis. From Jabs DA, Martin BK, Forman MS for the Cytomegalovirus Retinitis and Viral Resistance Research Group. Mortality associated with resistant cytomegalovirus among patients with cytomegalovirus retinitis and AIDS. Ophthalmology 2010;117(1):128-132. Used with permission.
Host Factors Affecting Cytomegalovirus Retinitis
Genetic susceptibility to cytomegalovirus retinitis
Host genetic factors play an important role in the course of HIV infection. Polymorphisms in a limited number of genes, typically related to the immune response, accounted for ~50% of the variability in the time from acquisition of HIV to the development of AIDS in the pre-HAART era and influence the response to HAART.119-121 A collaborative study between the National Cancer Institute Laboratory of Genetic Diversity and the Studies of the Ocular Complications of AIDS Research Group investigated the effect of host genetics on the development of CMV retinitis among patients with AIDS.122 The IL-10 receptor has two subunits (IL-10R1 and IL-10R2), and polymorphisms in the IL-10R1 gene are associated with the development of CMV retinitis in patients with AIDS. The amino acid changing polymorphism rs2229114 in the IL-10R1 gene was protective against CMV retinitis (odds ratio [OR] = 0.44, P=0.03), and no patient homozygous for this polymorphism had developed CMV retinitis in this study. Haplotype analyses showed that the sequence AACAGGT was protective against CMV retinitis (OR=0.14, P=0.04), whereas the sequence AGCAGGC was associated with CMV retinitis (OR=6.21, P=0.03), and these haplotype analyses agreed with the single nucleotide polymorphism analyses.122 Because polymorphisms in the IL-10R1 gene have been shown to diminish the inhibitory effect of IL-10 on monocytes and to decrease the signaling activity of the cmvIL-10h, these data have biologic plausibility.123-126
Access to health care
In the United States, patients diagnosed with CMV retinitis in the HAART era are significantly more likely to be uninsured than in the pre-HAART era, suggesting that access to care is an important factor in the occurrence of CMV retinitis.127 The racial and ethnic distributions of patients with CMV retinitis and AIDS are similar to those for AIDS overall, reflecting the well known impact of access to care on HIV/AIDS,128 and, in addition, non-white patients appear to have a worse visual prognosis.129 Although CMV infection is worldwide in its distribution, CMV retinitis has occurred primarily in those countries with sufficient health-care resources to keep patients with AIDS alive long enough to develop low CD4+ T cell counts. In countries where substantial mortality from other infections (e.g. tuberculosis) occurs at higher CD4+ T cells, there is a limited population of patients with AIDS at risk for CMV retinitis (i.e. with CD4+ T cells < 50 cells/μL). Furthermore, in those countries, high rates of mortality after CMV retinitis occurs result in lower estimates of the cumulative risk of CMV retinitis.130 In those countries with intermediate levels of health care delivery, but not full access to HAART, the incidence of CMV retinitis is similar to that in the United States in the pre-HAART era. Hence, the frequency of CMV retinitis traditionally has been reported as low in sub-Saharan Africa (<10% and often 1-2%) but higher in South Asia and Southeast Asia (~20-30%).130-140 These studies often are hampered by design issues, such as no or incomplete data on CD4+ T cells, so that direct comparability to studies in the industrialized world can be difficult. Nevertheless, given the large number of HIV-infected patients in these areas, the potential number of cases of CMV retinitis could be substantial, even at low disease frequencies. Moreover, more recent studies suggest an increasing frequency of CMV retinitis in Sub-Saharan Africa (15-20%), in part attributed to better treatment of other opportunistic infections.83 In a study of 200 patients from Togo the frequency of CMV retinitis among patients with AIDS was 21.5%.137 Furthermore, given the limitations on access to more expensive treatments, there is the potential for substantial visual loss due to CMV retinitis.139 In a publication from a hospital in Chiang Mai, Thailand in 2007, CMV retinitis was the second leading cause of blindness (34.6%) behind cataract (36.3%) and ahead of glaucoma (3.0%), diabetic retinopathy (2.4%), and age-related macular degeneration (0.8%).140
Conclusions
Cytomegalovirus represents a paradigm for the understanding of opportunistic infections. A ubiquitous virus, it causes retinitis in immune compromised hosts, and the likelihood of disease is related to the level of immune compromise. Immune recovery can control the retinitis (in most patients) without anti-CMV drugs, demonstrating the importance of the immune system in viral control. The interactions between CMV and HIV observed in vitro and the immunosuppressive effects of CMV are consistent with the clinically observed effects of CMV on the course of HIV infection and on mortality. Treatment algorithms are based on numerous clinical trials, which have defined the efficacy and relative efficacy of the available treatment choices. The molecular biology of the virus as regards the development of resistance is known and it correlates with clinical outcomes. Host variations in the incidence of CMV retinitis are affected by host genetics and by sociologic factors, such as level of and access to care. Information gathered from patients with AIDS is applicable to patients with CMV retinitis and other forms of immune compromise, who behave similarly to patients with AIDS in the HAART era, in terms of retinitis progression, structural complications, the ability to stop anti-CMV therapy if immune compromise is improved, and the occurrence of IRU.4 The increasing use of immunosuppression for treatment of autoimmune and auto-inflammatory diseases and transplantation, and the continued occurrence of AIDS18 means that CMV retinitis will continue to occur and require long-term management.
ACKNOWLEDGEMENT
A) Funding/Support: Supported in part by cooperative agreement U10 EY08052 from the National Eye Institute, the National Institutes of Health, Bethesda, MD to the Mount Sinai School of Medicine, New York, NY and grants RO1 EY10268 and R03 EY015643 to the Johns Hopkins University School of Medicine, Baltimore, NY.
B) Financial Disclosures: Dr. Jabs has acted as a consultant for Allergan Inc., Abbott Laboratories, Genzyme Corporation, Novartis Pharmaceutical Corporation, Roche Pharmaceuticals, GlaxoSmithKline, Alcon Laboratories, and GenenTech. Dr. Jabs serves on the Data and Safety Monitoring Board for Applied Genetic Technologies Corporation Roche Diagnostics provided partial support for some of the Cytomegalovirus Retinitis and Viral Resistance research via an unrestricted grant.
C) Contributions to Authors in each of these areas: Design of the study (DAJ); Conduct of the study (DAJ); Collection of the data (DAJ); Management of the data (DAJ); Analysis of the data (DAJ); Interpretation of the data (DAJ); Preparation of the manuscript (DAJ); Review of the manuscript (DAJ); Approval of the manuscript (DAJ).
D) Statement about Conformity with Author Information: IRB waived need for approval of this type of study.
E) Other Acknowledgements: none
Biosketch

Douglas A. Jabs, MD, MBA is the Chief Executive Office of the Mount Sinai Faculty Practice Associates, Dean for Clinical Affairs, and Professor and Chairman of the Department of Ophthalmology at Mount Sinai School of Medicine. He is the study chair of The Studies of the Ocular Complications of AIDS, the Multicenter Uveitis Steroid Treatment Trial, and the Standardization of Uveitis Nomenclature project. His interests include uveitis, epidemiology, and clinical trials.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Off label use of drugs: Discussion of intravitreal injection of antiviral drugs ganciclovir, foscarnet, and cidofovir.
REFERENCES
- 1.Fiala M, Chatterjee SN, Carson S, et al. Cytomegalovirus retinitis secondary to chronic viremia in phagocytic leukocytes. Am J Ophthalmol. 1977;84(4):567–573. doi: 10.1016/0002-9394(77)90454-8. [DOI] [PubMed] [Google Scholar]
- 2.Fiala M, Payne JE, Berne TV, et al. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- 3.Coskuncan NM, Jabs DA, Dunn JP, et al. The eye in bone marrow transplantation: VI. Retinal complications. Arch Ophthalmol. 1994;112(3):1372–1379. doi: 10.1001/archopht.1994.01090150102031. [DOI] [PubMed] [Google Scholar]
- 4.Kuo IC, Kempen JH, Dunn JP, Vogelsang G, Jabs DA. Clinical characteristics and outcomes of cytomegalovirus retinitis in persons without human immunodeficiency virus infection. Am J Ophthalmol. 2004;138(3):338–346. doi: 10.1016/j.ajo.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 5.Jabs DA. Ocular manifestations of HIV infection. Trans Am Ophthalmol Soc. 1995;93:623–683. [PMC free article] [PubMed] [Google Scholar]
- 6.Gallant JE, Moore RD, Richman DD, Keruly J, Chaisson RE. Incidence and natural history of cytomegalovirus disease in patients with advanced human immunodeficiency virus disease treated with zidovudine. The Zidovudine Epidemiology Study Group. J Infect Dis. 1992;166(6):1223–1227. doi: 10.1093/infdis/166.6.1223. [DOI] [PubMed] [Google Scholar]
- 7.Pertel P, Hirschtick RE, Phair J, Chmiel JS, Poggensee L, Murphy R. Risk of developing cytomegalovirus retinitis in persons infected with the human immunodeficiency virus. J Acquir Immune Defic Syndr. 1992;5(11):1069–1074. [PubMed] [Google Scholar]
- 8.Hoover DR, Peng Y, Saah A, et al. Occurrence of cytomegalovirus retinitis after human immunodeficiency virus immunosuppression. Arch Ophthalmol. 1996;114(7):821–827. doi: 10.1001/archopht.1996.01100140035004. [DOI] [PubMed] [Google Scholar]
- 9.Tay-Kearney ML, Enger C, Semba RD, Royal W, III, Dunn JP, Jabs DA. T cell subsets and cytomegalovirus retinitis in human immunodeficiency virus-infected patients. J Infect Dis. 1997;176(3):790–794. doi: 10.1086/517303. [DOI] [PubMed] [Google Scholar]
- 10.Holland GN, Vaudaux JD, Jeng SM, et al. Characteristics of untreated AIDS-related cytomegalovirus retinitis. I. Findings before the era of highly active antiretroviral therapy (1988-1994). Am J Ophthalmol. 2008;145(1):5–11. doi: 10.1016/j.ajo.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 11.Hoover DR, Neil MH, Graham HB, et al. An epidemiologic analysis of Mycobacterium avium complex disease in homosexual men infected with human immunodeficiency virus type 1. Clin Infect Dis. 1995;20:1250–1258. doi: 10.1093/clinids/20.5.1250. [DOI] [PubMed] [Google Scholar]
- 12.Palella FJ, Jr., Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 13.Holtzer CD, Jacobson MA, Hadley WK, et al. Decline in the rate of specific opportunistic infections at San Francisco General Hospital, 1994-1997. AIDS. 1998;12(14):1931–1933. [PubMed] [Google Scholar]
- 14.Jacobson MA, Stanley H, Holtzer C, Margolis TP, Cunningham ET. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30(1):231–233. doi: 10.1086/313612. [DOI] [PubMed] [Google Scholar]
- 15.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD, the Studies of the Ocualr Complications of AIDS Research Group Longitudinal study of the ocular complications of AIDS: 1. Ocular diagnoses at enrollment 1. Ophthalmology. 2007;114(4):780–786. doi: 10.1016/j.ophtha.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 16.Jabs DA. AIDS and ophthalmology, 2008. Arch Ophthalmol. 2008;126(8):1143–1146. doi: 10.1001/archopht.126.8.1143. [DOI] [PubMed] [Google Scholar]
- 17.Braithwaite RS, Roberts MS, Chang CC, et al. Influence of alternative thresholds for initiating HIV treatment on quality-adjusted life expectancy: a decision model. Ann Intern Med. 2008;148(3):178–185. doi: 10.7326/0003-4819-148-3-200802050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Late HIV testing - 34 states, 1996-2005. MMWR Morb Mortal Wkly Rep. 2009;58(24):661–665. [PubMed] [Google Scholar]
- 19.Griffiths PD, Polis MA, Dolin R, Masur H, Saag M. Cytomegalovirus disease. AIDS Therapy 3rd edition Elsevier; Philadelphia: 2008. pp. 855–884. [Google Scholar]
- 20.Wentworth BB, Alexander ER. Seroepidemiology of infections due to members of the herpesvirus group. Am J Epidemiol. 1971;94(5):496–507. doi: 10.1093/oxfordjournals.aje.a121347. [DOI] [PubMed] [Google Scholar]
- 21.Drew WL, Mintz L, Miner RC, et al. Prevalence of cytomegalovirus infection in homosexual men. J Infect Dis. 1981;143(2):188–192. doi: 10.1093/infdis/143.2.188. [DOI] [PubMed] [Google Scholar]
- 22.Jabs DA, Enger C, Dunn JP, Forman M, Hubbard L, the Cytomegalovirus Retinitis and Viral Resistance Research Group Cytomegalovirus retinitis and viral resistance: 3. Culture results. Am J Ophthalmol. 1998;126(4):543–549. doi: 10.1016/s0002-9394(98)00134-2. [DOI] [PubMed] [Google Scholar]
- 23.Jabs DA, Martin BK, Forman MS, Ricks MO, the Cytomegalovirus Retinitis and Viral Resistance Research Group Cytomegalovirus (CMV) blood DNA load, CMV retinitis progression, and occurrence of resistant CMV in patients with CMV retinitis. J Infect Dis. 2005;192(4):640–649. doi: 10.1086/432012. [DOI] [PubMed] [Google Scholar]
- 24.Rao NA, Zhang J, Ishimoto S. Role of retinal vascular endothelial cells in development of CMV retinitis. Trans Am Ophthalmol Soc. 1998;96:111–123. discussion 124-126. [PMC free article] [PubMed] [Google Scholar]
- 25.Pepose JS, Holland GN, Nestor MS, Cochran AJ, Foos RY. Acquired immune deficiency syndrome. Pathogenic mechanisms of ocular disease. Ophthalmology. 1985;92(4):472–484. doi: 10.1016/s0161-6420(85)34008-3. [DOI] [PubMed] [Google Scholar]
- 26.Davis MG, Kenney SC, Kamine J, Pagano JS, Huang ES. Immediate-early gene region of human cytomegalovirus trans-activates the promoter of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1987;84(23):8642–8646. doi: 10.1073/pnas.84.23.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Skolnik PR, Kosloff BR, Hirsch MS. Bidirectional interactions between human immunodeficiency virus type 1 and cytomegalovirus. J Infect Dis. 1988;157(3):508–514. doi: 10.1093/infdis/157.3.508. [DOI] [PubMed] [Google Scholar]
- 28.Skolnik PR, Pomerantz RJ, de la Monte SM, et al. Dual infection of retina with human immunodeficiency virus type 1 and cytomegalovirus. Am J Ophthalmol. 1989;107:261–272. doi: 10.1016/0002-9394(89)90659-4. [DOI] [PubMed] [Google Scholar]
- 29.Kotenko SV, Saccani S, Izotova LS, Mirochnitchenko OV, Pestka S. Human cytomegalovirus harbors its own unique IL-10 homolog (cmvIL-10). Proc Natl Acad Sci U S A. 2000;97(4):1695–1700. doi: 10.1073/pnas.97.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spencer JV, Lockridge KM, Barry PA, et al. Potent immunosuppressive activities of cytomegalovirus-encoded interleukin-10. J Virol. 2002;76(3):1285–1292. doi: 10.1128/JVI.76.3.1285-1292.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seow HF. Pathogen interactions with cytokines and host defence: an overview. Vet Immunol Immunopathol. 1998;63(1-2):139–148. doi: 10.1016/s0165-2427(98)00090-7. [DOI] [PubMed] [Google Scholar]
- 32.Vink C, Beisser PS, Bruggeman CA. Molecular mimicry by cytomegaloviruses. Function of cytomegalovirus-encoded homologues of G protein-coupled receptors, MHC class I heavy chains and chemokines. Intervirology. 1999;42(5-6):342–349. doi: 10.1159/000053970. [DOI] [PubMed] [Google Scholar]
- 33.Kovacs A, Schluchter M, Easley K, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341(2):77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Webster A, Lee CA, Cook DG, et al. Cytomegalovirus infection and progression towards AIDS in haemophiliacs with human immunodeficiency virus infection. Lancet. 1989;2(8654):63–66. doi: 10.1016/s0140-6736(89)90312-7. [DOI] [PubMed] [Google Scholar]
- 35.Sabin CA, Phillips AN, Lee CA, Janossy G, Emery V, Griffiths PD. The effect of CMV infection on progression of human immunodeficiency virus disease is a cohort of haemophilic men followed for up to 13 years from seroconversion. Epidemiol Infect. 1995;114(2):361–372. doi: 10.1017/s095026880005799x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial. 4. Visual outcomes. Ophthalmology. 1994;101(7):1250–1261. [PubMed] [Google Scholar]
- 37.Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group Combination foscarnet and ganciclovir therapy vs monotherapy for the treatment of relapsed cytomegalovirus retinitis in patients with AIDS. The Cytomegalovirus Retreatment Trial. Arch Ophthalmol. 1996;114(1):23–33. doi: 10.1001/archopht.1996.01100130021004. [DOI] [PubMed] [Google Scholar]
- 38.Studies of Ocular Complications of AIDS Research Group AIDS Clinical Trials Group. MSL-109 adjuvant therapy for cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: the Monoclonal Antibody Cytomegalovirus Retinitis Trial. Arch Ophthalmol. 1997;115(12):1528–1536. [PubMed] [Google Scholar]
- 39.Holbrook JT, Jabs DA, Weinberg DV, Lewis RA, Davis MD, Friedberg D, the Studies of Ocular Complications of AIDS Research Group Visual loss in patients with cytomegalovirus retinitis and acquired immunodeficiency syndrome before widespread availability of highly active antiretroviral therapy. Arch Ophthalmol. 2003;121(1):99–107. doi: 10.1001/archopht.121.1.99. [DOI] [PubMed] [Google Scholar]
- 40.Thorne JE, Jabs DA, Kempen JH, Holbrook JT, Nichols C, Meinert CL, the Studies of Ocular Complications of AIDS Research Group Incidence of and risk factors for visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113(8):1432–1440. doi: 10.1016/j.ophtha.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Thorne JE, Jabs DA, Kempen JH, Holbrook JT, Nichols C, Meinert CL, the Studies of Ocular Complications of AIDS Research Group Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113(8):1441–1445. doi: 10.1016/j.ophtha.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 42.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 43.Johnson SC, Benson CA, Johnson DW, Weinberg A. Recurrences of cytomegalovirus retinitis in a human immunodeficiency virus-infected patient, despite potent antiretroviral therapy and apparent immune reconstitution. Clin Infect Dis. 2001;32(5):815–819. doi: 10.1086/319219. [DOI] [PubMed] [Google Scholar]
- 44.Komanduri KV, Feinberg J, Hutchins RK, et al. Loss of cytomegalovirus-specific CD4+ T cell responses in human immunodeficiency virus type 1-infected patients with high CD4+ T cell counts and recurrent retinitis. J Infect Dis. 2001;183(8):1285–1289. doi: 10.1086/319683. [DOI] [PubMed] [Google Scholar]
- 45.Komanduri KV, Viswathanan MN, Wieder ED, et al. Restoration of cytomegalovirus specific CD4+ T lymphocyte responses after ganciclovir and highly active antiretroviral therapy in individuals infected with HIV-1. Nat Med. 1998;4(8):953–956. doi: 10.1038/nm0898-953. [DOI] [PubMed] [Google Scholar]
- 46.Jabs DA, Bolton SG, Dunn JP, Palestine AG. Discontinuing anticytomegalovirus therapy in patients with immune reconstitution after combination antiretroviral therapy. Am J Ophthalmol. 1998;126(6):817–822. doi: 10.1016/s0002-9394(98)00285-2. [DOI] [PubMed] [Google Scholar]
- 47.Macdonald JC, Torriani FJ, Morse LS, Karavellas MP, Reed JB, Freeman WR. Lack of reactivation of cytomegalovirus (CMV) retinitis after stopping CMV maintenance therapy in AIDS patients with sustained elevations in CD4 T cells in response to highly active antiretroviral therapy. J Infect Dis. 1998;177(5):1182–1187. doi: 10.1086/515281. [DOI] [PubMed] [Google Scholar]
- 48.Whitcup SM, Fortin E, Lindblad AS, et al. Discontinuation of anticytomegalovirus therapy in patients with HIV infection and cytomegalovirus retinitis. JAMA. 1999;282(17):1633–1637. doi: 10.1001/jama.282.17.1633. [DOI] [PubMed] [Google Scholar]
- 49.Kirk O, Reiss P, Uberti-Foppa C, et al. Safe interruption of maintenance therapy against previous infection with four common HIV-associated opportunistic pathogens during potent antiretroviral therapy. Ann Intern Med. 2002;137(4):239–250. doi: 10.7326/0003-4819-137-4-200208200-00008. [DOI] [PubMed] [Google Scholar]
- 50.Torriani FJ, Freeman WR, Macdonald JC, et al. CMV retinitis recurs after stopping treatment in virological and immunological failures of potent antiretroviral therapy. AIDS. 2000;14(2):173–180. doi: 10.1097/00002030-200001280-00013. [DOI] [PubMed] [Google Scholar]
- 51.Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. MMWR Morb Mortal Wkly Rep. 2009;58(RR-4):55. [Google Scholar]
- 52.Studies of ocular complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial: 5. Clinical features of cytomegalovirus retinitis at diagnosis. Am J Ophthalmology. 1997;124(2):141–157. [PubMed] [Google Scholar]
- 53.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD, the Studies of Ocular Complications of AIDS Research Group Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114(4):787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen QD, Kempen JH, Bolton SG, Dunn JP, Jabs DA. Immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis after highly active antiretroviral therapy. Am J Ophthalmol. 2000;129(5):634–639. doi: 10.1016/s0002-9394(00)00356-1. [DOI] [PubMed] [Google Scholar]
- 55.Robinson MR, Reed G, Csaky KG, Polis MA, Whitcup SM. Immune-recovery uveitis in patients with cytomegalovirus retinitis taking highly active antiretroviral therapy. Am J Ophthalmol. 2000;130(1):49–56. doi: 10.1016/s0002-9394(00)00530-4. [DOI] [PubMed] [Google Scholar]
- 56.Karavellas MP, Plummer DJ, Macdonald JC, et al. Incidence of immune recovery vitritis in cytomegalovirus retinitis patients following institution of successful highly active antiretroviral therapy. J Infect Dis. 1999;179(3):697–700. doi: 10.1086/314639. [DOI] [PubMed] [Google Scholar]
- 57.Kempen JH, Min YI, Freeman WR, et al. the Studies of Ocular Complications of AIDS Research Group Risk of immune recovery uveitis in patients with AIDS and cytomegalovirus retinitis. Ophthalmology. 2006;113(4):684–694. doi: 10.1016/j.ophtha.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 58.Taskintuna I, Rahhal FM, Arevalo JF, et al. Low-dose intravitreal cidofovir (HPMPC) therapy of cytomegalovirus retinitis in patients with acquired immune deficiency syndrome. Ophthalmology. 1997;104(6):1049–1057. doi: 10.1016/s0161-6420(97)30188-2. [DOI] [PubMed] [Google Scholar]
- 59.Nussenblatt RB, Lane HC. Human immunodeficiency virus disease: changing patterns of intraocular inflammation. Am J Ophthalmol. 1998;125(3):374–382. doi: 10.1016/s0002-9394(99)80149-4. [DOI] [PubMed] [Google Scholar]
- 60.Jabs DA, Ahuja A, VanNatta M, Lyon A, Srivastava S, Gangaputra S, the Studies of the Ocular Complications of AIDS Research Group Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: Five-year outcomes. Ophthalmology. doi: 10.1016/j.ophtha.2010.03.031. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ortega-Larrocea G, Espinosa E, Reyes-Teran G. Lower incidence and severity of cytomegalovirus-associated immune recovery uveitis in HIV-infected patients with delayed highly active antiretroviral therapy. AIDS. 2005;19(7):735–738. doi: 10.1097/01.aids.0000166100.36638.97. [DOI] [PubMed] [Google Scholar]
- 62.Zolopa A, Anderson J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: a multicenter randomized strategy trial. PLoS One. 2009;4(5):e5575. doi: 10.1371/journal.pone.0005575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palestine AG, Polis MA, De Smet MD, et al. A randomized, controlled trial of Foscarnet in the treatment of cytomegalovirus retinitis in patients with AIDS. Annals Intern Med. 1991;115(9):665–673. doi: 10.7326/0003-4819-115-9-665. [DOI] [PubMed] [Google Scholar]
- 64.Spector SA, Weingeist T, Pollard RB, et al. A randomized controlled study of intravenous ganciclovir therapy for cytomegalovirus peripheral retinitis in patients with AIDS. J Infect Dis. 1993;168(3):557–563. doi: 10.1093/infdis/168.3.557. [DOI] [PubMed] [Google Scholar]
- 65.Studies of Ocular Complications of AIDS Research Group, in collaboration with the AIDS Clinical Trials Group Mortality in patients with the acquired immunodeficiency syndrome treated with either foscarnet or ganciclovir for cytomegalovirus retinitis. N Engl J Med. 1992;326(4):213–220. doi: 10.1056/NEJM199201233260401. [DOI] [PubMed] [Google Scholar]
- 66.Studies of Ocular Complications of AIDS Research Group in Collaboration with the AIDS Clinical Trials Group Parenteral Cidofovir for Cytomegalovirus Retinitis in Patients with AIDS: The HPMPC Peripheral Cytomegalovirus Retinitis Trial. Annals Intern Med. 1997;126(4):264–274. doi: 10.7326/0003-4819-126-4-199702150-00002. [DOI] [PubMed] [Google Scholar]
- 67.Lalezari JP, Stagg RJ, Kuppermann BD, et al. Intravenous Cidofovir for Peripheral Cytomegalovirus Retinitis in Patients with AIDS: A Randomized, Controlled Trial. Annals Intern Med. 1997;126(4):257–263. doi: 10.7326/0003-4819-126-4-199702150-00001. [DOI] [PubMed] [Google Scholar]
- 68.Martin DF, Parks DJ, Mellow SD, et al. Treatment of cytomegalovirus retinitis with an intraocular sustained-release ganciclovir implant. A randomized controlled clinical trial. Arch Ophthalmol. 1994;112(12):1531–1539. doi: 10.1001/archopht.1994.01090240037023. [DOI] [PubMed] [Google Scholar]
- 69.Musch DC, Martin DF, Gordon JF, Davis MD, Kuppermann BD. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med. 1997;337(2):83–90. doi: 10.1056/NEJM199707103370203. [DOI] [PubMed] [Google Scholar]
- 70.Martin DF, Kuppermann BD, Wolitz RA, Palestine AG, Li H, Robinson CA. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. Roche Ganciclovir Study Group. N Engl J Med. 1999;340(14):1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 71.The Studies of Ocular Complications of AIDS Research Group The ganciclovir implant plus oral ganciclovir versus parenteral cidofovir for the treatment of cytomegalovirus retinitis in patients with acquired immunodeficiency syndrome: The Ganciclovir Cidofovir Cytomegalovirus Retinitis Trial. Am J Ophthalmol. 2001;131(4):457–467. doi: 10.1016/s0002-9394(01)00840-6. [DOI] [PubMed] [Google Scholar]
- 72.Martin DF, Sierra-Madero J, Walmsley S, et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. N Engl J Med. 2002;346(15):1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 73.Jabs DA. In: Clinical research in uveitis. Zierhut M, editor. Uveitis; in press. [Google Scholar]
- 74.AIDS Clinical Trials Group (ACTG) Studies of ocular complications of AIDS Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial: 1. Rationale, design, and methods. Control Clin Trials. 1992;13(1):22–39. doi: 10.1016/0197-2456(92)90027-w. [DOI] [PubMed] [Google Scholar]
- 75.Holbrook JT, Meinert CL, Van Natta ML, Davis M, Hubbard L, Jabs DA, the Studies of Ocular Complications of AIDS Research Group Photographic measures of cytomegalovirus retinitis as surrogates for visual outcomes in treated patients. Arch Ophthalmol. 2001;119(4):554–563. doi: 10.1001/archopht.119.4.554. [DOI] [PubMed] [Google Scholar]
- 76.Hubbard LD, Ricks MO, Martin BK, Bressler NM, Kempen JH, Dunn JP, Jabs DA, Cytomegalovirus Retinitis and Viral Resistance Study Group Comparability of two fundus photograph reading centers in grading cytomegalovirus retinitis progression. Am J Ophthalmol. 2004;137(3):426–434. doi: 10.1016/j.ajo.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 77.Holland GN, Buhles WC, Jr., Mastre BJ, Kaplan HJ, Group UCRS A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy: use of a standardized system for the assessment of disease outcome. Archives of Ophthalmology. 1989;107(12):1759–1766. doi: 10.1001/archopht.1989.01070020841024. [DOI] [PubMed] [Google Scholar]
- 78.Studies of Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group Long-term follow-up of patients with AIDS treated with parenteral cidofovir for cytomegalovirus retinitis: the HPMPC Peripheral Cytomegalovirus Retinitis Trial. AIDS. 2000;14(11):1571–1581. [PubMed] [Google Scholar]
- 79.Martin DF, Ferris FL, Parks DJ, et al. Ganciclovir implant exchange: timing, surgical procedure, and complications. Arch Ophthalmol. 1997;115(11):1389–1394. doi: 10.1001/archopht.1997.01100160559005. [DOI] [PubMed] [Google Scholar]
- 80.Kempen JH, Jabs DA, Wilson LA, Dunn JP, West SK, Tonascia J. Mortality risk for patients with cytomegalovirus retinitis and acquired immune deficiency syndrome. Clin Infect Dis. 2003;37(10):1365–1373. doi: 10.1086/379077. [DOI] [PubMed] [Google Scholar]
- 81.Diaz-Llopis M, España E, Muñoz G, et al. High dose intravitreal foscarnet in the treatment of cytomegalovirus retinitis in AIDS. Br J Ophthalmol. 1994;78(2):120–124. doi: 10.1136/bjo.78.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young S, Morlet N, Besen G, et al. High-dose (2000-μg) intravitreous ganciclovir in the treatment of cytomegalovirus retinitis. Ophthalmology. 1998;105:1404–1410. doi: 10.1016/s0161-6420(98)98020-4. [DOI] [PubMed] [Google Scholar]
- 83.Visser L. Managing CMV retinitis in the developing world. Community Eye Health. 2003;16(47):38–39. [PMC free article] [PubMed] [Google Scholar]
- 84.Jabs DA, Van Natta ML, Thorne JE, et al. the Studies of Ocular Complications of AIDS Research Group Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 1. retinitis progresion. Ophthalmology. 2004;111(12):2224–2231. doi: 10.1016/j.ophtha.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 85.Jabs DA, Van Natta ML, Thorne JE, et al. the Studies of Ocular Complications of AIDS Research Group Course of cytomegalovirus retinitis in the era of highly active antiretroviral therapy: 2. second eye involvement and retinal detachment. Ophthalmology. 2004;111(12):2232–2239. doi: 10.1016/j.ophtha.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 86.The Studies of Ocular Complications of AIDS (SOCA) Research Group in Collaboration with the AIDS Clinical Trials Group (ACTG) Rhegmatogenous retinal detachment in patients with cytomegalovirus retinitis: the Foscarnet-Ganciclovir Cytomegalovirus Retinitis Trial. Am J Ophthalmol. 1997;124(1):61–70. [PubMed] [Google Scholar]
- 87.Jacobson MA, O'Donnell JJ, Brodie HR, Wofsy C, Mills J. Randomized prospective trial of ganciclovir maintenance therapy for cytomegalovirus retinitis. J Med Virol. 1988;25(3):339–349. doi: 10.1002/jmv.1890250311. [DOI] [PubMed] [Google Scholar]
- 88.Kuppermann BD, Quiceno JI, Flores-Aguilar M, et al. Intravitreal ganciclovir concentration after intravenous administration in AIDS patients with cytomegalovirus retinitis: implications for therapy. J Infect Dis. 1993;168(6):1506–1509. doi: 10.1093/infdis/168.6.1506. [DOI] [PubMed] [Google Scholar]
- 89.Arevalo JF, Gonzalez C, Capparelli EV, et al. Intravitreous and plasma concentrations of ganciclovir and foscarnet after intravenous therapy in patients with AIDS and cytomegalovirus retinitis. J Infect Dis. 1995;172(4):951–956. doi: 10.1093/infdis/172.4.951. [DOI] [PubMed] [Google Scholar]
- 90.Jabs DA, Dunn JP, Enger C, et al. the Cytomegalovirus Retinitis and Viral Resistance Study Group Cytomegalovirus Retinitis and Viral Resistance: prevalence of resistance at diagnosis, 1994. Arch Ophthalmol. 1996;114(7):809–814. doi: 10.1001/archopht.1996.01100140023002. [DOI] [PubMed] [Google Scholar]
- 91.Drew WL, Miner RC, et al. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991;163(4):716–719. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- 92.Jabs DA, Enger C, Dunn JP, Forman M, the Cytomegalovirus Retinitis and Viral Resistance Research Group Cytomegalovirus retinitis and viral resistance: ganciclovir resistance. CMV Retinitis and Viral Resistance Study Group. J Infect Dis. 1998;177(3):770–773. doi: 10.1086/514249. [DOI] [PubMed] [Google Scholar]
- 93.Jabs DA, Enger C, Forman M, Dunn JP, the Cytomegalovirus Retinitis and Viral Resistance Research Group Incidence of foscarnet resistance and cidofovir resistance in patients treated for cytomegalovirus retinitis. The Cytomegalovirus Retinitis and Viral Resistance Study Group. Antimicrob Agents Chemother. 1998;42(9):2240–2244. doi: 10.1128/aac.42.9.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Weinberg A, Jabs DA, Chou S, et al. the Cytomegalovirus Retinitis and Viral Resistance Research Group Mutations conferring foscarnet resistance in a cohort of patients with the acquired immunodeficiency syndrome and cytomegalovirus retinitis. J Infect Dis. 2003;187(5):777–784. doi: 10.1086/368385. [DOI] [PubMed] [Google Scholar]
- 95.Jabs DA, Martin BK, Forman MS, et al. the Cytomegalovirus Retinitis and Viral Resistance Research Group Mutations conferring ganciclovir resistance in a cohort of patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis. J Infect Dis. 2001;183(2):333–337. doi: 10.1086/317931. [DOI] [PubMed] [Google Scholar]
- 96.Jabs DA, Martin BK, Forman MS, et al. the Cytomegalovirus Retinitis and Viral Resistance Research Group Longitudinal observations on mutations conferring ganciclovir resistance in patients with acquired immunodeficiency syndrome and cytomegalovirus retinitis: The Cytomegalovirus and Viral Resistance Study Group Report Number 8. Am J Ophthalmol. 2001;132(5):700–710. doi: 10.1016/s0002-9394(01)01161-8. [DOI] [PubMed] [Google Scholar]
- 97.Chou S, Marousek G, Guentzel S, et al. Evolution of mutations conferring multidrug resistance during prophylaxis and therapy for cytomegalovirus disease. J Infect Dis. 1997;176(3):786–789. doi: 10.1086/517302. [DOI] [PubMed] [Google Scholar]
- 98.Erice A, Gil-Roda C, Perez JL, et al. Antiviral susceptibilities and analysis of UL97 and DNA polymerase sequences of clinical cytomegalovirus isolates from immunocompromised patients. J Infect Dis. 1997;175(5):1087–1092. doi: 10.1086/516446. [DOI] [PubMed] [Google Scholar]
- 99.Chou S, Lurain NS, Weinberg A, Cai GY, Sharma PL, Crumpacker CS. Interstrain variation in the human cytomegalovirus DNA polymerase sequence and its effect on genotypic diagnosis of antiviral drug resistance. Adult AIDS Clinical Trials Group CMV Laboratories. Antimicrob Agents Chemother. 1999;43(6):1500–1502. doi: 10.1128/aac.43.6.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lurain NS, Weinberg A, Crumpacker CS, Chou S. Sequencing of cytomegalovirus UL97 gene for genotypic antiviral resistance testing. Antimicrob Agents Chemother. 2001;45(10):2775–2780. doi: 10.1128/AAC.45.10.2775-2780.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chou S, Waldemer RH, Senters AE, et al. Cytomegalovirus UL97 phosphotransferase mutations that affect susceptibility to ganciclovir. J Infect Dis. 2002;185(2):162–169. doi: 10.1086/338362. [DOI] [PubMed] [Google Scholar]
- 102.Erice A. Resistance of human cytomegalovirus to antiviral drugs. Clin Microbiol Reviews. 1999;12(2):286–297. doi: 10.1128/cmr.12.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chou S, Lurain NS, Thompson KD, Miner RC, Drew WL. Viral DNA polymerase mutations associated with drug resistance in human cytomegalovirus. J Infect Dis. 2003;188(1):32–39. doi: 10.1086/375743. [DOI] [PubMed] [Google Scholar]
- 104.Smith IL, Cherrington JM, Jiles RE, Fuller MD, Freeman WR, Spector SA. High-level resistance of cytomegalovirus to ganciclovir is associated with alterations in both the UL97 and DNA polymerase genes. J Infect Dis. 1997;176(1):69–77. doi: 10.1086/514041. [DOI] [PubMed] [Google Scholar]
- 105.Spector SA, Hsia K, Wolf D, Shinkai M, Smith I. Molecular detection of human cytomegalovirus and determination of genotypic ganciclovir resistance in clinical specimens. Clin Infect Dis. 1995;21(Suppl 2):S170–S173. doi: 10.1093/clinids/21.supplement_2.s170. [DOI] [PubMed] [Google Scholar]
- 106.Gerna G, Sarasini A, Percivalle E, Zavattoni M, Baldanti F, Revello MG. Rapid screening for resistance to ganciclovir and foscarnet of primary isolates of human cytomegalovirus from culture-positive blood samples. J Clin Microbiol. 1995;33(3):738–741. doi: 10.1128/jcm.33.3.738-741.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dunn JP, MacCumber MW, Forman MS, Charache P, Apuzzo L, Jabs DA. Viral sensitivity testing in patients with cytomegalovirus retinitis clinically resistant to foscarnet or ganciclovir. Am J Ophthalmol. 1995;119(5):587–596. doi: 10.1016/s0002-9394(14)70217-x. [DOI] [PubMed] [Google Scholar]
- 108.Enger C, Jabs DA, Dunn JP, et al. the Cytomegalovirus Retinitis and Viral Resistance Research Group Viral resistance and CMV retinitis: design and methods of a prospective study. CRVR Research Groups. Cytomegalovirus Retinitis Viral Resistance Research Group. Ophthalmic Epidemiol. 1997;4(1):41–48. doi: 10.3109/09286589709058060. [DOI] [PubMed] [Google Scholar]
- 109.Hu H, Jabs DA, Forman MS, et al. the Cytomegalovirus Retinitis and Viral Resistance Research Group Comparison of cytomegalovirus (CMV) UL97 gene sequences in the blood and vitreous of patients with acquired immunodeficiency syndrome and CMV retinitis. J Infect Dis. 2002;185(7):861–867. doi: 10.1086/339603. [DOI] [PubMed] [Google Scholar]
- 110.Jabs DA, Martin BK, Forman MS, et al. the Cytomegalovirus Retinitis and Viral Resistance Research Group Cytomegalovirus resistance to ganciclovir and clinical outcomes of patients with cytomegalovirus retinitis. Am J Ophthalmol. 2003;135(1):26–34. doi: 10.1016/s0002-9394(02)01759-2. [DOI] [PubMed] [Google Scholar]
- 111.Jabs DA, Martin BK, Ricks MO, Forman MS, the Cytomegalovirus Retinitis and Viral Resistance Research Group Detection of ganciclovir resistance in patients with AIDS and cytomegalovirus retinitis: correlation of genotypic methods with viral phenotype and clinical outcome. J Infect Dis. 2006;193(12):1728–1737. doi: 10.1086/504270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Martin BK, Ricks MO, Forman MS, Jabs DA, the Cytomegalovirus Retinitis and Viral Resistance Research Group Change over time in incidence of ganciclovir resistance in patients with cytomegalovirus retinitis. Clin Infect Dis. 2007;44(7):1001–1008. doi: 10.1086/512368. [DOI] [PubMed] [Google Scholar]
- 113.Lim JI, Enger C, Haller JA, et al. Improved visual results after surgical repair of cytomegalovirus-related retinal detachments. Ophthalmology. 1994;101(2):264–269. doi: 10.1016/s0161-6420(13)31339-6. [DOI] [PubMed] [Google Scholar]
- 114.Tanna AP, Kempen JH, Dunn JP, Haller JA, Jabs DA. Incidence and management of cataract after retinal detachment repair with silicone oil in immune compromised patients with cytomegalovirus retinitis. Am J Ophthalmol. 2003;136(6):1009–1015. doi: 10.1016/s0002-9394(03)00724-4. [DOI] [PubMed] [Google Scholar]
- 115.Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. Br J Ophthalmol. 2010;94(6):678–684. doi: 10.1136/bjo.2009.157727. [DOI] [PubMed] [Google Scholar]
- 116.Jabs DA, Holbrook JT, Van Natta ML, et al. the Studies of the Ocular Complications of AIDS Research Group Risk factors for mortality in patients with AIDS in the era of highly active antiretroviral therapy. Ophthalmology. 2005;112(5):771–779. doi: 10.1016/j.ophtha.2004.10.049. [DOI] [PubMed] [Google Scholar]
- 117.Studies of the Ocular Complications of AIDS Research Group in collaboration with the AIDS Clinical Trials Group Antiviral effects of foscarnet and ganciclovir therapy on human immunodeficiency virus p24 antigen in patients with AIDS and cytomegalovirus retinitis. J Infect Dis. 1995;172(3):613–621. doi: 10.1093/infdis/172.3.613. [DOI] [PubMed] [Google Scholar]
- 118.Jabs DA, Martin BK, Forman MS, the Cytomegalovirus Retinitis and Viral Resistance Research Group Mortality associated with resistant cytomegalovirus among patients with cytomegalovirus retinitis and AIDS. Ophthalmology. 2010;117(1):128–132. doi: 10.1016/j.ophtha.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.O'Brien SJ, Moore JP. The effect of genetic variation in chemokines and their receptors on HIV transmission and progression to AIDS. Immunol Rev. 2000;177:99–111. doi: 10.1034/j.1600-065x.2000.17710.x. [DOI] [PubMed] [Google Scholar]
- 120.O'Brien SJ, Nelson GW. Human genes that limit AIDS. Nat Genet. 2004;36(6):565–574. doi: 10.1038/ng1369. [DOI] [PubMed] [Google Scholar]
- 121.Hendrickson SL, Jacobson LP, Nelson GW, et al. Host genetic influences on highly active antiretroviral therapy efficacy and AIDS-free survival. J Acquir Immune Defic Syndr. 2008;48(3):263–271. doi: 10.1097/QAI.0b013e31816fdc5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sezgin E, Jabs DA, Hendrickson SL, Van Natta ML, Zdanov A, Lewis RA, et al. the Studies of the Ocular Complications of AIDS Research Group Effect of host genetics on cytomegalovirus retinitis occurrence in patients with AIDS. J Infect Dis. 2010;202(4):606–613. doi: 10.1086/654814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gasche C, Grundtner P, Zwirn P, et al. Novel variants of the IL-10 receptor 1 affect inhibition of monocyte TNF-alpha production. J Immunol. 2003;170(11):5578–5582. doi: 10.4049/jimmunol.170.11.5578. [DOI] [PubMed] [Google Scholar]
- 124.Gruber SG, Gloria Luciani M, Grundtner P, Zdanov A, Gasche C. Differential signaling of cmvIL-10 through common variants of the IL-10 receptor 1. Eur J Immunol. 2008;38(12):3365–3375. doi: 10.1002/eji.200837718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nat Med. 2006;12(11):1301–1309. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ejrnaes M, Filippi CM, Martinic MM, et al. Resolution of a chronic viral infection after interleukin-10 receptor blockade. J Exp Med. 2006;203(11):2461–2472. doi: 10.1084/jem.20061462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jabs DA, Van Natta ML, Kempen JH, et al. Characteristics of patients with cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Am J Ophthalmol. 2002;133(1):48–61. doi: 10.1016/s0002-9394(01)01322-8. [DOI] [PubMed] [Google Scholar]
- 128.Holland GN, Vaudaux JD, Shiramizu, et al. Characteristics of untreated AIDS-related cytomegalovirus retinitis. II. Findings in the era of highly active antiretroviral therapy (1997 to 2000). Am J Ophthalmol. 2008;145(1):12–22. doi: 10.1016/j.ajo.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 129.Kempen JH, Jabs DA, Wilson LA, et al. Risk of vision loss in patients with cytomegalovirus retinitis and the acquired immunodeficiency syndrome. Arch Ophthalmol. 2003;121(4):466–476. doi: 10.1001/archopht.121.4.466. [DOI] [PubMed] [Google Scholar]
- 130.Heiden D, Ford N, Wilson D, et al. Cytomegalovirus retinitis: The neglected disease of the AIDS pandemic. PLoS Med. 2007;4(12):e334. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kestelyn P. The epidemiology of CMV retinitis in Africa. Ocular Immunol Inflamm. 1999;7:173–177. doi: 10.1076/ocii.7.3.173.4002. [DOI] [PubMed] [Google Scholar]
- 132.Mansouri Y, Zaghoul K, Himmich H, Amraoui Les attentes oculaires au cours l'infection par le VIH au CHU de Casablanca. (A propos de 400 cas). Bull Soc Pathol Expt. 2000;93(1):14–16. [PubMed] [Google Scholar]
- 133.Giorgis AT, Melka F, Mariam AG. Ophthalmic manifestations of AIDS in Armed Forces General Teaching Hospital, Addis Ababa. Ethiop Med J. 2007;45(4):327–333. [PubMed] [Google Scholar]
- 134.Ebana Mvogo C, Ellong A, Bella AL, Luma H, Achu Joko H. Complications oculaires de l'infection a VIH-SIDA en milieu Camerounais: Y a-t-il une corrélation avec le taux de CD4? Bull Soc blege Ophtalmol. 2007;305:7–12. [PubMed] [Google Scholar]
- 135.Nwosu SN. HIV/AIDS in ophthalmic patients: The Guiness Eye Centre Onitsha experience. Nigerian Postgrad Med J. 2008;15(1):24–27. [PubMed] [Google Scholar]
- 136.Nkomazand O, Tshitswana D. Ocular complications of HIV infection in Sub-Saharan Africa. Current HIV/AIDS Reports. 2008;5(3):120–125. doi: 10.1007/s11904-008-0019-z. [DOI] [PubMed] [Google Scholar]
- 137.Balo K-P, Amoussou Y-P, Béchetoille A, et al. Rétinites à cytomégalovirus et complications oculaires du SIDA au Togo. J Fr Ophtalmol. 1999;22(10):1042–1046. [PubMed] [Google Scholar]
- 138.Otitti-Sengeri J, Colebunders R, Kempen JH, Ronald A, Sande M, Katabira E. The prevalence and causes of visual loss among HIV-infected individuals in Uganda. J Acquir Immune Def Syndr. 2010;53(1):95–101. doi: 10.1097/QAI.0b013e3181c313f0. [DOI] [PubMed] [Google Scholar]
- 139.Biswas J, Madhavan HN, George AE, Kumarasamy N, Solomon S. Ocular lesions associated with HIV infection in India: a series of 100 consecutive patients evaluated at a referral center. Am J Ophthalmol. 2000;129(1):9–15. doi: 10.1016/s0002-9394(99)00415-8. [DOI] [PubMed] [Google Scholar]
- 140.Pathanapitoon K, Ausayakhun S, Kunavisarut P, et al. Blindness and low vision in a tertiary ophthalmologic center in thailand: The importance of cytomegalovirus retinitis. Retina. 2007;27(5):635–640. doi: 10.1097/01.iae.0000249575.38830.45. [DOI] [PubMed] [Google Scholar]