Abstract
Background
Arterial stiffening or reduced compliance of proximal pulmonary vessels has been shown to be an important predictor of outcomes in patients with pulmonary hypertension. Though current evidence indicates that arterial stiffening modulates flow pulsatility in downstream vessels and is likely related to microvascular damage in organs without extensive distributing arteries, the cellular mechanisms underlying this relationship in the pulmonary circulation are unexplored. Thus, this study was designed to examine the responses of the microvascular pulmonary endothelium to changes in flow pulsatility.
Methods
A flow system was developed to reproduce arterial-like pulse flow waves with the capability of modulating flow pulsatility through regulation of upstream compliance. Pulmonary microvascular endothelial cells (PMVECs) were exposed to steady flow and pulse flow waves of varied pulsatility with varied hemodynamic energy (low: pulsatility index or PI=1.0; medium: PI=1.7; high: PI=2.6) at flow frequency of 1Hz or 2Hz for different durations (1 and 6 hours). The mean flow rates in all the conditions were kept the same with shear stress at 14 dynes/cm2. Gene expression was evaluated by analyzing mRNA levels of adhesion molecules (ICAM-1, E-selectin), chemokine (MCP-1) and growth factor/receptor (VEGF, Flt-1) in PMVECs. Functional changes were observed with monocyte adhesion assay.
Results
1) Compared to either steady flow or low pulsatility flow, increased flow pulsatility for 1hr induced significant increases in mRNA levels of ICAM-1, E-selectin and MCP-1. 2) Sustained high pulsatility flow perfusion induced increases in ICAM, E-selectin, MCP-1, VEGF and its receptor Flt-1 expression. 3) Flow pulsatility effects on PMVECs were frequency-dependent with greater responses at 2Hz and likely associated with the hemodynamic energy level. 4) Pulse flow waves with high flow pulsatility at 2Hz induced leukocyte adhesion and recruitment to PMVECs.
Conclusion
Increased upstream pulmonary arterial stiffness increases flow pulsatility in distal arteries and induces inflammatory gene expression, leukocyte adhesion and cell proliferation in the downstream PMVECs.
INTRODUCTION
It is increasingly appreciated that arterial stiffening is an important factor in determining adverse cardiovascular events1-7. In addition, increased arterial pulse pressure, a direct consequence of stiffening, has been used to guide pharmaceutical treatment for a variety of systemic vascular diseases8-13. The large elastic arteries, although often considered simply passive conduits, are known to participate in the regulation of pulsatile arterial flow. When blood is pumped into elastic arteries during systole, they expand with the pressure increase to accommodate the entire fraction of ejected blood; when the heart enters diastole, the arteries recoil, propelling the blood forward. This so-called “windkessel” effect prevents excess rise of pressure during systole and maintains flow during diastole. Large arteries thus constitute a hydraulic buffer, converting high pulsatility flow into continuous steady flow and alleviating dissipation of high hemodynamic energy of pulsatile flow in perfused organs8. When the wall of elastic arteries becomes stiffened as occurs, for example, with aging, diabetes or hypertension, systolic pressure increases and diastolic pressure decreases. Consequently, arterial stiffening causes an increase in flow pulsatility14,15; if extensive distributing arteries do not exist to dampen the flow, flow pulsatility in small arteries or even in the microvasculature may increase, exerting detrimental effects on these undeformable vessels and ultimately organ function. Previous studies have associated arterial stiffening with microvascular damage in kidney4 and brain16, though the mechanisms responsible for this association remain unclear. Recently, arterial stiffening effects have also been recognized in the pulmonary circulation, particularly in pulmonary arterial hypertension (PAH)17-20. However, it is unknown whether high flow pulsatility in the pulmonary circulation activates pathologic signaling pathways in the distal endothelial cells and thus contributes to or perpetuates the distal vascular remodeling that characterizes PAH.
In small arteries, flow shear stress, a frictional shear force, is the major mechanical force that influences cells because circumferential stress, a consequence of pressure-induced arterial wall motion, is minimal. Previous studies have examined vascular endothelial responses to a variety of flow conditions including steady laminar flow, oscillatory turbulent flow and pulsatile laminar flow. It was found that endothelial cells are able to not only sense shear stress, but also discriminate among distinct patterns of flow21-29. It is also believed that hemodynamic stresses in the vascular system are under strict regulation, and that there exists a narrow range of homeostatic flow stress. Stress outside the physiologic range leads to activation of the endothelium with resultant changes in vasomotor, inflammatory and thrombotic properties. Interestingly, though much is known about endothelial responses to flow shear stress, little is known about the effect of flow pulsatility on microvascular endothelial cells. We therefore investigated this effect on the pulmonary microvascular endothelium by utilizing a newly developed flow system. The flow system mimics changes in arterial stiffness by a custom-made compliance-adjustable chamber that modulates flow pulsatility of arterial-like pulse flow waves generated by a blood pump. Responses of pulmonary microvascular endothelial cells (PMVEC) to flow pulsatility changes were evaluated by changes in their mRNA expression of pro-inflammatory or pro-proliferative molecules, given the major role these molecules are thought to play in PAH.
MATERIALS AND METHODS
Cell Culture
Bovine distal pulmonary microvascular endothelial cells were isolated from neonatal calves as previously described30. PMVECs were cultured in a growth medium (D-Valine MEM medium; Mediatech, Inc.; Herndon, VA) containing 10% fetal bovine serum (FBS, Gemini Bio-products; West Sacramento, CA), 2% L-glutamine (Invitrogen; Carlsbad, CA) and 2% penicillin/streptomycin (Invitrogen/Gibco; Carlsbad, CA). For flow experiments, medium with 2% FBS was used. Cells at passages 4-8 were used for all experiments. THP-1 monocytes were purchased from ATCC (Manassas, VA) and maintained in 10% FBS medium.
Flow system setup
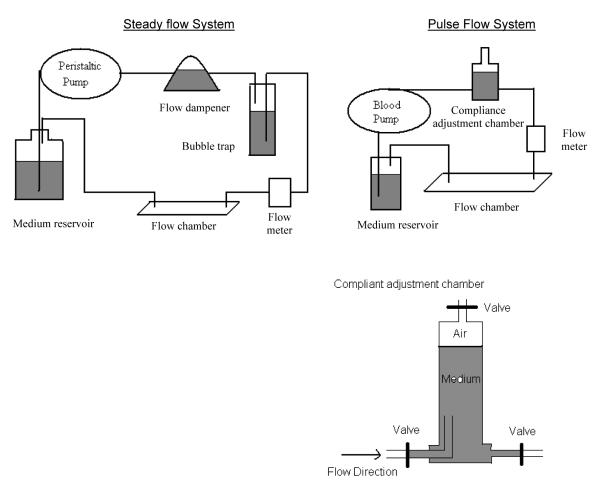
Systems used for examining endothelial cells under steady flow conditions and under mimetic pulse flow conditions are demonstrated in Figure 1. Briefly, for pulse flow studies, fluid is pumped by a pulsatile blood pump (Harvard Apparatus; Holliston, MA) to a compliance-adjustment chamber, a flow chamber, a medium reservoir, and then back to the pump. The upstream custom-designed compliance chamber simulated the elastic function of an artery. It consists of a chamber, an inlet valve, an outlet valve and a compressibility valve to achieve its pressure adjustment function. The modulation of flow pulsatility is done by adjusting the ratio between air and liquid in the compliance-adjustment chamber. To begin the experiment the pressure value is open to allow the entrance of the fluid, and fluid continues to enter until the liquid reaches a pre-determined level. For measurements, a digital flow meter (Alicat Scientific, Tuscon, AZ) or a digital pressure gauge (Coleparmer, Vernon Hills, IL) is placed between the compliance-adjustment chamber and the flow chamber to determine flow rate or pressure. The flow meter is connected to an oscilloscope (Agilent Technologies, Santa Clara, CA) which is connected to a computer to visualize the flow wave and to quantitatively determine average flow rate and dynamic flow rate at specific time points. The temporal and spatial distribution of flow dynamics in the flow chamber has been fully characterized with computational flow dynamics, and sampling area for cells exposed to high-fidelity homogenous pulse flow waves was determined with flow measurements and gene analysis31. The system used for steady flow studies consists of a peristaltic pump (Manostat pump, Barnant Company; Barrington, IL) which is used to pump endothelial cell medium through a flow dampener, a bubble trap, a flow chamber, a medium reservoir and then back to the pump. The steady flow and pulse flow conditions in this study have the same mean flow rate (or shear stress level). The waveform of the steady flow was also measured.
Figure 1.
The system setups for studying effects of steady flow and arterial-like pulse flow conditions
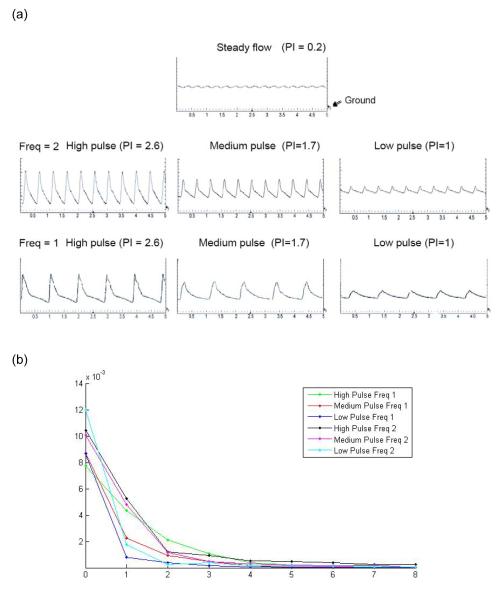
Figure 2a demonstrates the recorded pulse flow waves from an oscilloscope. We have included three different pulse flow waves generated from a blood pump (named low pulse, medium pulse and high pulse) at different frequencies (F=1: frequency at 1Hz; F=2: frequency at 2Hz.). All the pulse flow waves have the same mean flow with the shear stress of 14 dynes/cm2. The blood pump generates the initial stroke peak flow and thus the flow waveforms showing systolic and diastolic phases closely resemble those observed in vivo. This pump is often used to circulate blood flow in living animals simulating the action of the heart. With this system, the individual component of flow (i.e. magnitude, pulsatility and frequency) can be separately controlled and studied. The steady flow is considered as a condition in which upstream compliance is high enough to dampen nearly all flow pulsation. But in agreement with many others, we noticed that one single dampener can not completely dampen the pulse flow, and thus the steady flow has a very low pulsatility with index of 0.2.
Figure 2.
(a) Flow waveforms demonstrated experimental conditions including the steady flow and the arterial-like pulse flow waves with varied pulsatility, i.e. low pulsatility (PI=1), medium pulsatility (PI=1.7) and high pulsatility (PI=2.6), at the frequency of 1Hz or 2Hz. The flow waves were plotted by oscilloscope showing the relationship between voltage representing the flow rate and time. The graphs had time (unit: second) on the x-axis and voltage on the y-axis with minimal division set at 200mV or 2ml/min. Ground shows the point of 0mV. (b) Spectral analysis of the high, medium and low pulse flow waveforms.
To quantitatively describe and compare the pulsatile flow waveforms or the flow pulsatility levels, the flow waveforms used in the study were characterized by pulsatility index, spectral analysis, and hemodynamic energy equivalent pressure (EEP). The flow pulsatility index is commonly used in the evaluation of vascular stiffening and is defined by: PI = (Vmax − Vmin) / Vmean, where Vmax is the peak systolic velocity, Vmin is the minimum forward diastolic velocity and Vmean is the average velocity. Calculations show that the pulsatility index for the high, medium, and low pulse flow waves are 2.6, 1.7, and 1, respectively. The flow waveforms were also subjected to harmonic analysis (or spectral analysis) and the moduli of their harmonic components were compared. A discrete fourier transform (DFT) was performed using MATLAB software (Matworks, Natick, MA) to yield the moduli of harmonic components (Figure 2b). The analysis is emphasized on the first harmonic as it largely determines flow pulsatility. The analysis results showed that the high pulse flow wave at 2Hz had the highest modulus at the first harmonics, followed by medium pulse at 2Hz, high pulse at 1Hz, medium pulse at 1Hz, low pulse at 2Hz and low pulse at 1Hz. In order to compare the energy dissipated by each flow waveform, EEP was calculated 32-34. This was given by the formula: EEP = (∫ pfdt) /(∫ fdt), where f is the pump flow rate, and p is the flow pressure. The EEP values calculated for the high, medium and low pulse flow waves at frequency of 1Hz are 340, 240, and 83 mmHg, respectively; and the EEP values for the high, medium and low pulse flow waves at frequency of 2Hz are 467, 390, 151 mmHg, respectively. In addition to flow velocity waves, pressure waves are obtained from the flow meter for calculation of EEP. The pressure waves and flow waves are very similar in the wave contour, but a phase difference between them exists. It was found that when the mean flow rate and the frequency are constant, the flow condition with higher pulsatility index and higher modulus at the first harmonics has greater amount of energy associated with it.
Experimental protocols for flow shear stress studies
To examine the response of PMVEC to flow conditions, plain microscope slides were coated with 100μg/ml fibronectin. PMVECs at a concentration of 4.0×105 per ml were seeded on the fibronectin-coated slides and grown to confluence. Then PMVECs were exposed to steady flow as well as low, medium and high pulse flow conditions (at the frequency of 2Hz) for 1hr to study the effect of flow pulsatility on gene expressions. Additionally, the cells were exposed to either steady flow or medium pulse flow for 6 hours with 1 hour preconditioning of steady flow to study the effect of extended flow perfusion on gene expression. Furthermore, PMVECs were exposed to different pulse flow conditions for 1hr at a frequency of 1Hz to study the effect of flow frequency on gene expression. To study the effect of preconditioning on gene expression, PMVECs were exposed to either steady or medium pulse flow for 1hr at 2Hz after cells were exposed to 1hr preconditioning of steady flow. All of the flow conditions used in this study had the same mean flow with flow shear stress (FSS) at 14 dynes/cm2, which falls in the range of physiological FSS for the arterial vascular network. PMVECs grown in the absence of flow (the static condition) were used as a control. After PMVECs were exposed to different flow conditions, they were collected and analyzed for mRNA expression related to adhesion molecules (ICAM-1 and E-selectin), chemokine (MCP-1) and growth factor/receptor (VEGF and Flt-1) using the method of real-time RT-PCR.
Real-time RT-PCR
Total cellular RNA from each sample was extracted using RNeasy Mini Kit (Qiagen; Hilden, Germany) according to the manufacturer’s instructions. Complementary DNA was synthesized from 1μg of total cellular RNA using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Real-time quantitative RT-PCR primers are designed using Primer 3 Software to target bovine 1) inflammation related adhesion molecules: ICAM-1 and E-selectin; 2) chemokine: MCP-1; 3) growth factors: VEGF and Flt-1 (VEGF receptor). The SYBR Green I assay and the iCycler iQ real-time PCR detection system (Bio-Rad MyiQ Real-Time PCR System; Hercules, CA) were used for detecting real-time quantitative PCR products from 2 ng of reverse-transcribed cDNA. PCR thermal profile consisted of 95 °C for 10 min followed by 40 cycles of 95 °C for 15 sec, 60 °C for 30 s and 95 °C for 1 min. Genes were normalized to the housekeeping gene hypoxanthine-xanthine phosphoribosyl transferase (HPRT) and fold change relative to static condition was calculated using the ΔCT method 35. The sequences of these genes are listed in Table 1.
TABLE 1.
Primer sequences of bovine genes for real-time PCR analysis.
| Genes | Forward primer | Reverse primer |
|---|---|---|
| ICAM | GACTTCTTCAGCTCCCCAAG | CCCACATGCTATTTGTCCTG |
| E-selectin | CTCCCCGTCCAAGAACTACA | CGCCTCTACCTGTCCTTGAG |
| MCP-1 | CGCCTGCTGCTATACATTCA | ACACTTGCTGCTGGTGACTC |
| VEGF | TCACCAAAGCCAGCACATAG | AAATGCTTTCTCCGCTCTGA |
| Flt-1 | CTCCCGAGTCCATCTTTGAC | GGGACCCACCTAAGGAGAAG |
| HPRT | CTGGCTCGAGATGTGATGAA | CAACAGGTCGGCAAAGAACT |
Monocyte adhesion assay
In order to confirm the expression of adhesion molecules after PMVECs were exposed to different FSS conditions, we examined whether monocyte adhesion to PMVEC was affected by FSS. Briefly, after PMVECs were exposed to different flow conditions for 6hrs, monocytic THP-1 cells (5 × 105 cells/ml) were incubated with PMVECs that were still attached to slides for 30 min. Non-adherent THP-1 cells were removed by washing. The adherent THP-1 cells on the PMVECs were visualized under microscope and pictures were taken for six randomly selected microscopic fields on each slide. The numbers of monocyte in each field were counted with the Image J software.
Data analysis
All data are expressed as mean ± SEM, and n indicates the number of sample studied. One-Way ANOVA was used to determine effects of flow pulsatility on gene expression. If significantly difference exists, Student’s t-test is used to compare means of each individual group. A P value < 0.05 was considered significantly different.
RESULTS
High Flow Pulsatility Increases Gene Expression for Adhesion Molecules and Inflammatory Cytokines
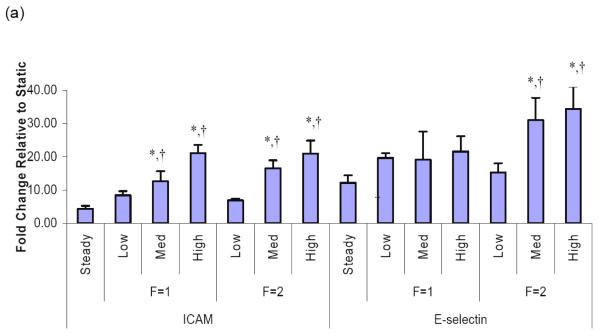
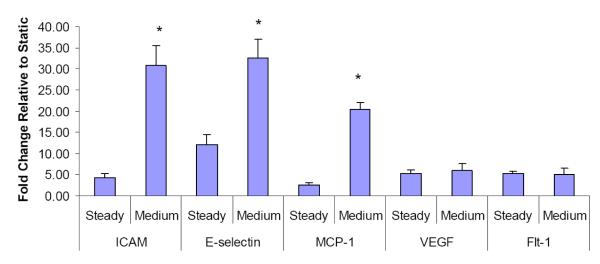
Because the role of inflammation in vascular remodeling and PAH is increasingly recognized, we sought to determine whether flow conditions with increased pulsatility, as opposed to steady flow, would induce proinflammatory changes in PMVEC. Additionally, to examine whether the effects of flow pulsatility on PMVECs are influenced by flow frequency, we examined responses of PMVECs to pulse flow at the frequencies of 1Hz and 2Hz. We found that both medium and high pulse flow conditions significantly upregulated the ICAM-1 mRNA levels in PMVECs at both 1Hz and 2Hz. We also found that, compared to steady flow, medium and high pulse flow conditions at a frequency of 2Hz but not 1Hz significantly upregulated the E-selectin mRNA levels (Figure 3a).
Figure 3.
Pulsation effects of flows on the mRNA expression in distal PMVECs. Effects of pulsatile flow conditions including peristaltic flow and arterial-like flows with varied pulse magnitudes were compared to those of the steady flow. All the flow conditions had the same mean flow rate with shear stress at 14 dynes/cm2 and the same perfusion time (1 hour). The mRNA expression of (a) adhesion molecules, ICAM-1 and E-selectin, (b) chemokine, monocyte chemoattractant protein (MCP-1), and (c) molecules that regulate cell proliferation in distal PMVECs were examined under various pulsatile flow conditions. The mRNA expression was detected using real-time RT-PCR and expressed as fold change relative to the static condition. Data represent mean ± SEM. “F” means frequency of flow. All the conditions are significantly different from the static condition; ‘*’: significantly different from the steady flow; ‘†’: significantly different from the low pulse flow at the same frequency (P ≤ 0.05).
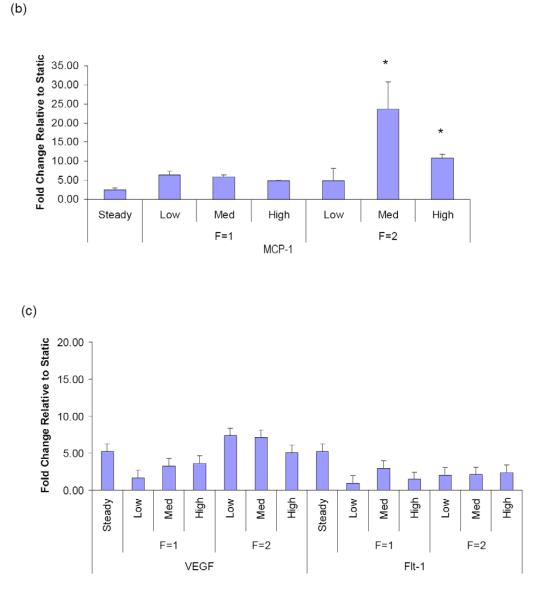
In addition to the adhesion molecules, chemokines and growth factors are also involved in vascular inflammation through their effects on leukocyte recruitment and on resident wall cell proliferation. We therefore examined the effects of high pulse flow on monocyte chemoattractant protein-1 (MCP-1) expression because of its pivotal role in the accumulation of monocytes in the injured as well as hypertensive lung vasculature, and VEGF and Flt-1. At 2Hz we observed that medium and high pulse flow significantly increased MCP-1 mRNA compared to steady flow (Figure 3b). In contrast, high pulsatility flow did not exert significant effects on VEGF or Flt-1 expression compared to steady flow (Figure 3c).
To examine whether preconditioning influences the cell responses, cells were exposed to the flow after 1-hour steady flow preconditioning. We found that preconditioning with steady flow did not affect cell responses to flow pulsatility with significant induction of ICAM, E-selectin, and MCP-1 still observed (Figure 4).
Figure 4.
Effects of the medium pulse flow with steady flow preconditioning on gene expressions by distal PMVECs were compared to those of the steady flow. PMVECs were exposed to steady flow before they were exposed to the pulse flow at frequency of 2Hz. Both of the flow conditions had the same mean flow rate with shear stress at 14 dynes/cm2 and the same perfusion time. The mRNA expression was detected using real-time RT-PCR and expressed as fold change relative to the static condition. Data represent mean ± SEM. “F” means frequency of flow. ‘*’: significantly different from the steady flow (P ≤ 0.05).
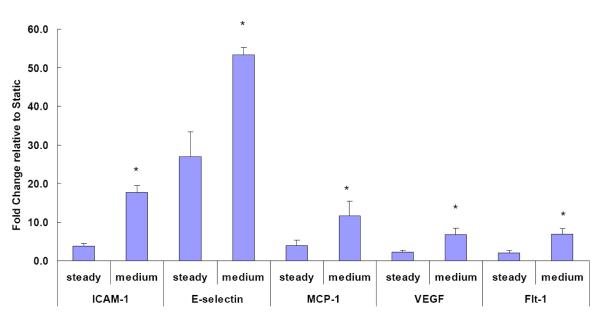
Extended Exposure to Increased Flow Pulsatility Results in Sustained Increases in Adhesion Molecule and Cytokine mRNA Expression
To further examine the relationship between flow pulsatility and endothelial gene expression, the effects of more sustained pulse flow (6 hours) were evaluated. Because the medium pulse flow at 2Hz exerted significant effects on pro-inflammatory gene expression compared to steady flow, we examined the effects of prolonged flow on adhesion molecule and cytokine mRNA expressions using this flow pattern and steady flow. We found that extended perfusion with medium pulse flow significantly increased the expression of the adhesion molecules (ICAM-1 and E-selectin) and MCP-1 compared to steady flow (Figure 5). In addition, we observed upregulation of VEGF and Flt-1 mRNA expression in response to 6hr of high pulse flow. Interestingly, PDGF-α and PDGF-β, molecules frequently upregulated in the remodeling process, were not affected by high pulse flow (data not shown).
Figure 5.
Effects of the medium pulse flow with extended perfusion at frequency of 2Hz on the gene expression by distal PMVECs were compared to those of the steady flow. The flow perfusion time was extended to 6 hours. Both of the flow conditions had the same mean flow rate with shear stress at 14 dynes/cm2 and the same perfusion time. The mRNA expression was detected using real-time RT-PCR and expressed as fold change relative to the static condition. Data represent mean ± SEM. ‘*’: significantly different from the steady flow (P ≤ 0.05).
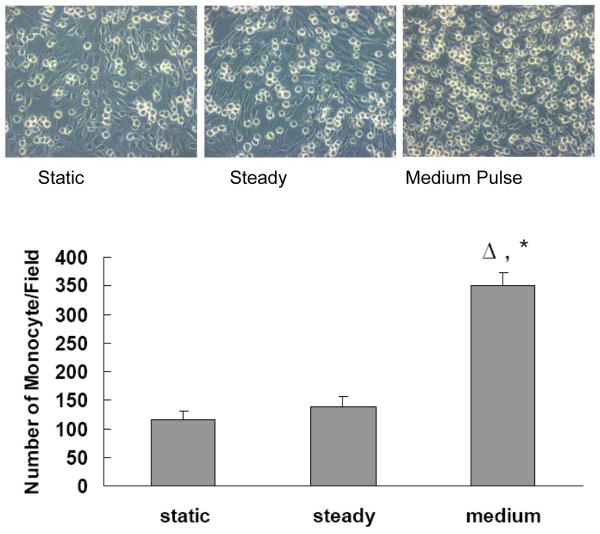
Increased Flow Pulsatility Stimulates Monocyte Adhesion to Distal PMVECs
To confirm the results from gene assays that pro-inflammatory related adhesion molecules on the surface of endothelial cells are functionally upregulated by pulse flow conditions, we carried out monocyte adhesion assays on the PMVECs after they were exposed to different flow conditions for extended perfusion times (Figure 6). Compared to the static conditions, steady flow did not increase monocyte adhesion to PMVECs (P>0.05). However, the medium pulse flow significantly increased (P<0.05) monocyte adhesion compared to both the static condition and the steady flow condition.
Figure 6.
Effect of the medium pulse flow on monocyte adhesion: PMVECs were exposed to either the steady flow or the medium pulse flow for 6 hours at frequency of 2Hz with the mean flow shear stress at 14 dynes/cm2. Monocyte adhesion assays were performed by determining the number of monocytes adhered to PMVEC per microscope field. Data represent mean ± SEM, n=6 for each group. ‘Δ’: significantly different from static; ‘*’: significantly different from steady (P ≤ 0.05).
DISCUSSION
The present study examined the effects of flow pulsatility on pulmonary microvascular endothelial function using a new flow system. Our results clearly showed that high pulsatility flow activates pro-inflammatory genes including ICAM, E-selectin and MCP-1 in pulmonary microvascular endothelial cells, and upregulates expression of the pro-proliferative genes VEGF and Flt-1 as well. The endothelial activation of pro-inflammatory genes by flow pulsatility was further confirmed by increased monocyte adhesion to the endothelium. To our knowledge, this is the first demonstration of the effects of pulse flow on vascular endothelial gene expression.
PMVECs were used as a cell model to elucidate the potential effects of large pulmonary vascular stiffening and thus high pulse flow on distal microvessel function abnormalities, which currently characterize pulmonary arterial hypertension (PAH). Recent studies showed decreased proximal vessel compliance led to increased flow pulsatility in distal arteries5,14,15. Therefore, this study was designed to test the hypothesis that decreases in upstream compliance, by altering flow pulsatility while sustaining mean flow rate and laminar flow conditions, activates inflammatory signaling in the pulmonary microvascular endothelium. In addition to pulsatility index and spectral analysis, this study also used hemodynamic energy equivalent pressure (EEP) to quantitatively describe flow pulsatility. EEP provides explanation of the flow pulsatility effects on PMVEC functions. Additionally, EEP accounts for the frequency influence on gene expression. Compared to the steady flow, medium and high pulse flow conditions at 1Hz has less of an effect on ICAM-1, E-selectin and MCP-1 mRNA expression than those at 2Hz. According to the energy level (i.e. EEP), the medium or high pulse flow waves at 2Hz have higher energy to dissipate, causing PMVECs exposed to flow at 2Hz to be more susceptible to inflammation. The spectral analysis also showed corresponding differences in moduli of the first harmonic component. These results are consistent with others’ findings28,36,37.
The results from this study may not apply to the conditions when vascular endothelial cells experience other forces in addition to flow shear stress. Considering influences of hemodynamic forces besides shear stress is important for larger vessels. Blood flow imposes three mechanical stresses: normal stress, circumferential tensile stress and wall shear stress. In large elastic arteries, these stresses synergistically regulate vascular cell functions. In distal small arteries or microvasculature, flow shear stress is the most important mechanical force and the route to dissipate hemodynamic energy because the wall motion is minimal and the pressure is relatively small 38,39. The perfusion time is yet another consideration in flow-induced activation of endothelium. Previous studies suggested that expression of many endothelial genes including proinflammatory genes changed within seconds at the onset of flow and tend to reduce or become stable after one hour of flow perfusion40,41. Several genes or molecules continue to be modulated by long-time perfusion even after 72 hours41. Thus, we chose to examine gene expression after one-hour and six-hour perfusion durations. We demonstrated increased pulsatility of pulse flow waves upregulated several pro-inflammatory molecules after both one-hour and six-hour perfusion with steady flow preconditioning. Up-regulation of growth factor VEGF and its receptor was not observed after one-hour perfusion but was found after six-hour perfusion. This might be due to slower response of genes regulating cell proliferation to flow alterations.
The specific effects of altered flow pulsatility or waveform, conditions associated with vascular stiffening, on endothelial function have received little attention. Separation of the effects of flow pulsatility (i.e. temporal variation of flow) from the effects of flow magnitude or flow turbulence on cells represents a major departure from many previous flow mechanobiology studies. For this reason, we developed a customized flow system with several unique features: First, we built a unique compliant-adjustment chamber to mimic the function of large arteries. By regulating the air/flow ratio of the chamber, we modulated the compliance of the flow system. Second, we were capable of changing flow pulsatility while keeping mean flow shear stress at the same level. Thirdly, we used a blood pump to generate physiologic arterial-like flows which simulate changes in systolic and diastolic phases of arterial flow. Finally, the pulse wave contour analysis methods, such as pulsatility index and spectral analysis, which are used to evaluate arterial compliance were adopted here to quantify the flow waveforms imposed on endothelial cells. Previous studies reported results using pulsatile flow from different types of pumps or sinusoid pulse flow waves; comparing results from these flow mechanobiology studies is difficult but may be possible by using the waveform analysis methods introduced in this study. For example, several previous studies employed peristaltic pumps to explore effects of pulsatile flow on cells42,43. We have also used different peristaltic pumps (Manostat pump, Barnant Company; Barrington, IL; and Control Company, Friendswood, TX) and found that the waveforms change with manufacturers’ pump designs. According to the waveform analysis, these peristaltic flows generated less pulsatililty than the arterial-like pulse flow conditions shown in the study. As a result, the peristaltic flows reduced mRNA expression of ICAM, E-selectin, VEGF and Flt-1, in comparison to the steady flow condition.
CONCLUSION
In conclusion, this study has demonstrated that flow pulsatility significantly upregulates inflammation and proliferation associated gene expression in distal pulmonary microvascular endothelial cells. Increased flow pulsatility with higher hemodynamic energy upregulated mRNA expression for pro-inflammatory molecules, which likely leads to monocyte recruitment. The pulsatility effects are dependent on flow frequency, perfusion time and flow waveform. Results from this study may have significant implications when evaluating hemodynamic sequelae in pulmonary hypertension, especially as related to proximal vascular stiffening: lack of flow dampening in upstream arteries likely results in increased inflammation and cell proliferation in the downstream microvascular endothelial cells.
ACKNOWLEDGEMENT
This study was funded in part by grants from the Children’s Hospital at Denver (Research Scholar Development Award and CCTSI-K12 Award to W.T.) and the NIH (HL 067397, HL 072738, K24 HL051506 to R.S., HL-14985-36 and HL084923-03 to K.R.S.).
Reference
- 1.McVeigh GE, Hamilton PK, Morgan DR. Evaluation of mechanical arterial properties: clinical, experimental and therapeutic aspects. Clin Sci (Lond) 2002;102:51–67. [PubMed] [Google Scholar]
- 2.Mattace-Raso F, van der Cammen T, Hofman A. Arterial Stiffness and Risk of Coronary Heart Disease and Stroke: The Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. al. e. [DOI] [PubMed] [Google Scholar]
- 3.Cohn JN. Arterial stiffness, vascular disease, and risk of cardiovascular events. Circulation. 2006;113:601–3. doi: 10.1161/CIRCULATIONAHA.105.600866. [DOI] [PubMed] [Google Scholar]
- 4.Goldsmith D, MacGinley R, Smith A, Covic A. How important and how treatable is vascular stiffness as a cardiovascular risk factor in renal failure? Nephrol Dial Transplant. 2002;17:965–969. doi: 10.1093/ndt/17.6.965. [DOI] [PubMed] [Google Scholar]
- 5.O’Rourke MF. Arterial pressure waveforms in hypertension. Minerva Med. 2003;94:229–50. [PubMed] [Google Scholar]
- 6.Mitchell GF, Conlin PR, Dunlap ME, Lacourciere Y, Arnold JM, Ogilvie RI, Neutel J, Izzo JL., Jr. Pfeffer MA. Aortic diameter, wall stiffness, and wave reflection in systolic hypertension. Hypertension. 2008;51:105–11. doi: 10.1161/HYPERTENSIONAHA.107.099721. [DOI] [PubMed] [Google Scholar]
- 7.Kingwell BA, Ahimastos AA. Arterial stiffness and coronary ischemic disease. Adv Cardiol. 2007;44:125–38. doi: 10.1159/000096725. [DOI] [PubMed] [Google Scholar]
- 8.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107:2864–9. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 9.Zoungas S, Asmar RP. Arterial stiffness and cardiovascular outcome. Clin Exp Pharmacol Physiol. 2007;34:647–51. doi: 10.1111/j.1440-1681.2007.04654.x. [DOI] [PubMed] [Google Scholar]
- 10.McEniery CM, Wilkinson IB, Avolio AP. Age, hypertension and arterial function. Clin Exp Pharmacol Physiol. 2007;34:665–71. doi: 10.1111/j.1440-1681.2007.04657.x. [DOI] [PubMed] [Google Scholar]
- 11.Asmar R, Safar M, Queneau P. Pulse pressure: an important tool in cardiovascular pharmacology and therapeutics. Drugs. 2003;63:927–32. doi: 10.2165/00003495-200363100-00001. [DOI] [PubMed] [Google Scholar]
- 12.Cattan V, Kakou A, Louis H, Lacolley P. Pathophysiology, genetic, and therapy of arterial stiffness. Biomed Mater Eng. 2006;16:S155–61. [PubMed] [Google Scholar]
- 13.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell G. Cross-Sectional Relations of Peripheral Microvascular Function, Cardiovascular Disease Risk Factors, and Aortic Stiffness: The Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. al. e. [DOI] [PubMed] [Google Scholar]
- 15.Safar ME. Peripheral pulse pressure, large arteries, and microvessels. Hypertension. 2004;44:121–2. doi: 10.1161/01.HYP.0000135448.73199.75. [DOI] [PubMed] [Google Scholar]
- 16.O’Rourke MF. Brain microbleeds, amyloid plaques, intellectual deterioration, and arterial stiffness. Hypertension. 2008;51:e20. doi: 10.1161/HYPERTENSIONAHA.107.109199. author reply e21. [DOI] [PubMed] [Google Scholar]
- 17.Gorgulu S, Eren M, Yildirim A, Ozer O, Uslu N, Celik S, Dagdeviren B, Nurkalem Z, Bagirtan B, Tezel T. A new echocardiographic approach in assessing pulmonary vascular bed in patients with congenital heart disease: pulmonary artery stiffness. Anadolu Kardiyol Derg. 2003;3:92–7. [PubMed] [Google Scholar]
- 18.Chesler NC, Thompson-Figueroa J, Millburne K. Measurements of mouse pulmonary artery biomechanics. J Biomech Eng. 2004;126:309–14. doi: 10.1115/1.1695578. [DOI] [PubMed] [Google Scholar]
- 19.Hunter KS, Gross JK, Lanning CJ, Kirby KS, Dyer KL, Ivy DD, Shandas R. Noninvasive methods for determining pulmonary vascular function in children with pulmonary arterial hypertension: application of a mechanical oscillator model. Congenit Heart Dis. 2008;3:106–16. doi: 10.1111/j.1747-0803.2008.00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunter K, Lee P, Lanning C, Ivy D, Kirby K, Claussen L, Chan K, Shandas R. Pulmonary vascular input impedance is a combined measure of pulmonary vascular resistance and stiffness and predicts clinical outcomes better than pulmonary vascular resistance alone in pediatric patients with pulmonary hypertension. Am Heart J. 2008;155:166–74. doi: 10.1016/j.ahj.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher AB, Chien S, Barakat AI, Nerem RM. Endothelial cellular response to altered shear stress. Am J Physiol Lung Cell Mol Physiol. 2001;281:L529–33. doi: 10.1152/ajplung.2001.281.3.L529. [DOI] [PubMed] [Google Scholar]
- 22.Dai G, Kaazempur-Mofrad MR, Natarajan S, Zhang Y, Vaughn S, Blackman BR, Kamm RD, Garcia-Cardena G, Gimbrone MA., Jr. Distinct endothelial phenotypes evoked by arterial waveforms derived from atherosclerosis-susceptible and - resistant regions of human vasculature. Proc Natl Acad Sci U S A. 2004;101:14871–6. doi: 10.1073/pnas.0406073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gimbrone MA, Jr., Topper JN, Nagel T, Anderson KR, Garcia-Cardena G. Endothelial dysfunction, hemodynamic forces, and atherogenesis. Ann N Y Acad Sci. 2000;902:230–9. doi: 10.1111/j.1749-6632.2000.tb06318.x. discussion 239-40. [DOI] [PubMed] [Google Scholar]
- 24.Davies PF, Spaan JA, Krams R. Shear stress biology of the endothelium. Ann Biomed Eng. 2005;33:1714–8. doi: 10.1007/s10439-005-8774-0. [DOI] [PubMed] [Google Scholar]
- 25.Blackman BR, Garcia-Cardena G, Gimbrone MA., Jr. A new in vitro model to evaluate differential responses of endothelial cells to simulated arterial shear stress waveforms. J Biomech Eng. 2002;124:397–407. doi: 10.1115/1.1486468. [DOI] [PubMed] [Google Scholar]
- 26.Hastings NE, Simmers MB, McDonald OG, Wamhoff BR, Blackman BR. Atherosclerosis-prone hemodynamics differentially regulates endothelial and smooth muscle cell phenotypes and promotes pro-inflammatory priming. Am J Physiol Cell Physiol. 2007;293:C1824–33. doi: 10.1152/ajpcell.00385.2007. [DOI] [PubMed] [Google Scholar]
- 27.Yee A, Bosworth KA, Conway DE, Eskin SG, McIntire LV. Gene expression of endothelial cells under pulsatile non-reversing vs. steady shear stress; comparison of nitric oxide production. Ann Biomed Eng. 2008;36:571–9. doi: 10.1007/s10439-008-9452-9. [DOI] [PubMed] [Google Scholar]
- 28.Balcells M, Suarez M Fernandez, Vazquez M, Edelman ER. Cells in fluidic environments are sensitive to flow frequency. J Cell Physiol. 2005;204:329–35. doi: 10.1002/jcp.20281. [DOI] [PubMed] [Google Scholar]
- 29.Barakat AI, Lieu DK, Gojova A. Secrets of the code: do vascular endothelial cells use ion channels to decipher complex flow signals? Biomaterials. 2006;27:671–8. doi: 10.1016/j.biomaterials.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 30.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–96. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 31.Scott D, Hunter K, Li M, Shandas R, Tan W. Experimental and computational characterization of flow device for studying cell mechanobiology under pulsatile flow. BMES proceedings. 2008 [Google Scholar]
- 32.Undar A, Zapanta CM, Reibson JD, Souba M, Lukic B, Weiss WJ, Snyder AJ, Kunselman AR, Pierce WS, Rosenberg G, Myers JL. Precise quantification of pressure flow waveforms of a pulsatile ventricular assist device. Asaio J. 2005;51:56–9. doi: 10.1097/01.mat.0000150326.51377.a0. [DOI] [PubMed] [Google Scholar]
- 33.Painter PR, Eden P, Bengtsson HU. Pulsatile blood flow, shear force, energy dissipation and Murray’s Law. Theor Biol Med Model. 2006;3:31. doi: 10.1186/1742-4682-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiss WJ, Lukic B, Undar A. Energy equivalent pressure and total hemodynamic energy associated with the pressure-flow waveforms of a pediatric pulsatile ventricular assist device. Asaio J. 2005;51:614–7. doi: 10.1097/01.mat.0000179341.95404.f8. [DOI] [PubMed] [Google Scholar]
- 35.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 36.Himburg HA, Dowd SE, Friedman MH. Frequency-dependent response of the vascular endothelium to pulsatile shear stress. Am J Physiol Heart Circ Physiol. 2007;293:H645–53. doi: 10.1152/ajpheart.01087.2006. [DOI] [PubMed] [Google Scholar]
- 37.Himburg HA, Friedman MH. Correspondence of low mean shear and high harmonic content in the porcine iliac arteries. J Biomech Eng. 2006;128:852–6. doi: 10.1115/1.2354211. [DOI] [PubMed] [Google Scholar]
- 38.Peng X, Recchia FA, Byrne BJ, Wittstein IS, Ziegelstein RC, Kass DA. In vitro system to study realistic pulsatile flow and stretch signaling in cultured vascular cells. Am J Physiol Cell Physiol. 2000;279:C797–805. doi: 10.1152/ajpcell.2000.279.3.C797. [DOI] [PubMed] [Google Scholar]
- 39.Peng X, Haldar S, Deshpande S, Irani K, Kass DA. Wall stiffness suppresses Akt/eNOS and cytoprotection in pulse-perfused endothelium. Hypertension. 2003;41:378–81. doi: 10.1161/01.hyp.0000049624.99844.3d. [DOI] [PubMed] [Google Scholar]
- 40.Davies PF, Barbee KA, Volin MV, Robotewskyj A, Chen J, Joseph L, Griem ML, Wernick MN, Jacobs E, Polacek DC, dePaola N, Barakat AI. Spatial relationships in early signaling events of flow-mediated endothelial mechanotransduction. Annu Rev Physiol. 1997;59:527–49. doi: 10.1146/annurev.physiol.59.1.527. [DOI] [PubMed] [Google Scholar]
- 41.Dekker RJ, van Soest S, Fontijn RD, Salamanca S, de Groot PG, VanBavel E, Pannekoek H, Horrevoets AJ. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–98. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Sokabe T, Watabe T, Miyazono K, Yamashita JK, Obi S, Ohura N, Matsushita A, Kamiya A, Ando J. Fluid shear stress induces differentiation of Flk-1-positive embryonic stem cells into vascular endothelial cells in vitro. Am J Physiol Heart Circ Physiol. 2005;288:H1915–H1924. doi: 10.1152/ajpheart.00956.2004. [DOI] [PubMed] [Google Scholar]
- 43.Go Y-M, Park H, Maland MC, Darley-Usmar VM, Stoyanov B, Wetzker R, Jo H. Phosphatidylinositol 3-kinase mediates shear stress-dependent activation of JNK in endothelial cells. Am J Physiol Heart Circ Physiol. 1998;275:H1898–H1904. doi: 10.1152/ajpheart.1998.275.5.H1898. [DOI] [PubMed] [Google Scholar]