Abstract
Protein tyrosine kinases are key biochemical effectors of the signaling pathways that drive both normal and aberrant cell behavior. The ability to visualize the activity of tyrosine kinases in both a continuous and sensitive fashion will have a dramatic impact on the identification and characterization of inhibitors, the elucidation of the biochemical role of protein tyrosine kinases in various biological processes, and the imaging of kinase action in cells, tissues, and whole organisms. Several chemical strategies have recently been described that translate the formation of a phosphorylated tyrosine residue into a fluorescent readout. The challenges associated with the design of protein tyrosine kinase sensors, as well as the scope and limitations of the currently available sensors, are described.
Keywords: biosensors, fluorescent probes, peptides, protein tyrosine kinases, signal transduction
1. Protein Tyrosine Kinases, Signal Transduction, Biology, and Disease
Protein tyrosine kinases (PTKs) are a family of signaling proteins that exert influence over a multiplicity of cellular behaviors. These enzymes are generally organized into two subfamilies: 1) the receptor tyrosine kinases, integral membrane proteins activated by extracellular ligands, which serve as gatekeepers to many signaling pathways and 2) the nonreceptor tyrosine kinases, which transmit the extracellular signal received by receptors to effectors that mount the cellular response. Members of both families catalyze the phosphorylation of tyrosine residues in proteins (as well as short peptides) using ATP as the phosphoryl donor. From the organic chemistry perspective, this transformation borders on the pedestrian: phosphorylation converts a neutral phenol moiety into a negatively charged species. However, in terms of protein chemistry, the phosphate monoester of tyrosine can have a dramatic impact on structure and activity. For example, many PTKs are themselves phosphorylated at a key residue near the catalytic site; this results in the activation of these enzymes.[1] On the other hand, several PTKs are also phosphorylated near their C-terminal tails; this leads to a dramatic conformational change and a loss of catalytic activity.[2] In addition, PTKs catalyze the phosphorylation of a wide array of proteins, including many with no enzymatic function. The sudden appearance of a phosphotyrosine moiety can serve as a homing beacon to initiate the formation of activated multimeric signaling complexes. In short, although the chemistry of phosphoryl transfer is relatively straightforward, the biochemical and biological consequences are dramatic.
PTKs play an important role in maintaining cellular homeostasis. Furthermore, the aberrant activity of many of these kinases has been linked to a variety of diseases.[3] Indeed, v-Src, a viral analogue of the wild-type eukaryotic tyrosine kinase c-Src, is the first reported example of an oncoprotein.[4] The Rous sarcoma virus induces connective tissue tumors (sarcomas) in chickens. At some point during the evolution of this virus, the gene coding for eukaryotic Src was inadvertently highjacked by the virus during infection. The gene subsequently mutated to code for a constitutively active, and therefore cancer-causing, enzyme. Mutations involving tyrosine kinases have also been observed in human diseases. The Philadelphia translocation is a chromosomal abnormality found in 95 % of all patients with chronic myelogenous leukemia (CML).[5] The swap of genetic material between chromosomes 9 and 22 generates a gene that codes for a fused protein: Bcr-Abl. Abl is a PTK that, when fused with Bcr, is rendered constitutively active, thereby driving unregulated cell growth.[6] An inhibitor of Abl (Gleevec) has been successfully used to treat some forms of CML.[6]
Since protein tyrosine kinases are key contributors to and markers of various biological states, the ability to visualize PTK activity in biological samples would represent a boon to scientific discovery and translational medicine. Imaging technology (X-rays, MRI, etc.) has served as a powerful tool for exploring the inner workings of the body, tissues, and cells. This technology has and will continue to evolve as we gain an ever-increasing understanding of the underlying biochemical mechanisms responsible for normal and disease biology. If PTK action is to become an integral component of imaging for biological research and diagnostic medicine, then innovations at the chemical level will be required in order to translate tyrosine phosphomonoester formation into a visual readout.
2. Visualizing the Action of Tyrosine Protein Kinases: Challenges and Design Concepts
20th century enzymology focused on the behavior of enzymes outside of their cellular environment. Although conditions in a cuvette can be adjusted to recreate some general features of the intracellular milieu (pH, ionic strength, temperature, etc.), this artificial environment generally does not provide much insight into the contribution of enzymatic activity to cellular behavior. In short, enzymatic activity as a function of cell cycle, intracellular location, or receptor activation is best discerned in living cells. In order to realize this goal, one must be able to observe the activity of the enzyme in question in a highly sensitive, selective, and continuous fashion. The use of fluorescent assays, in particular, has a number of potentially useful attributes, including sensitivity, access to a wide array of fluorophores with different photophysical properties, multiplexing capability, and the ability to monitor enzymatic activity in a continuous mode and in various formats (from plate readers to microscopes).
The key issue concerning the design of fluorescent protein kinase sensors is how to link the phosphorylation event to a fluorescent change. Tyrosine phosphorylation creates a number of local and potentially global structural alterations that could and have been used to design sensors. For example, the free phenol of tyrosine is neutral, planar, and electron rich, whereas its phosphorylated counterpart is negatively charged, contains a modestly sterically demanding phosphate substituent, and a moderately less electron rich aromatic ring system. The phosphate moiety is more strongly solvated than its uncharged phenolic counterpart; this could potentially render a phosphopeptide product more water-soluble than the starting substrate, especially if the substrate were a relatively short peptide. Finally, tyrosine phosphorylation can also generate large-scale conformational changes, as exemplified by the members of the Src tyrosine kinase family when they suffer phosphorylation at key sites.[7]
3. Peptide-Based Sensors of Protein Tyrosine Kinases
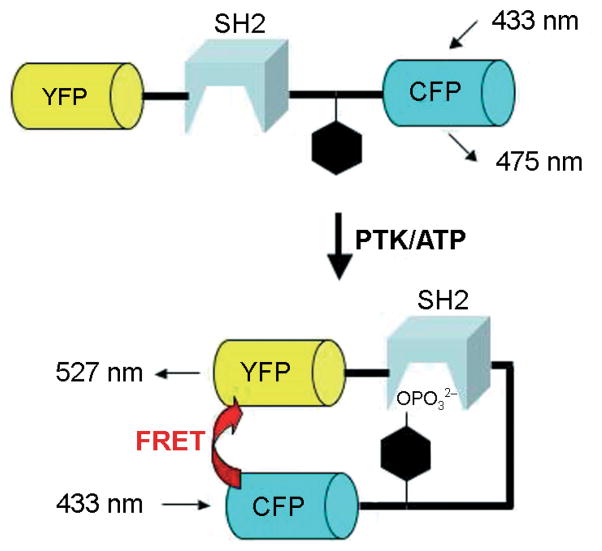
Many of the PTK sensors reported to date are protein-based constructs.[8] The primary advantage associated with the latter is that they are genetically encoded and thus readily expressed within living cells. Nearly all protein-based PTK sensors employ a fluorescence resonance energy transfer (FRET) mechanism to report phosphorylation.[9] The major breakthrough responsible for the design of these sensors was the creation of GFP analogues containing complementary photophysical properties for FRET purposes.[10] In general, the PTK sensor constructs are composed of a tyrosine-phosphorylation sequence, a phosphotyrosine-binding domain (e.g., SH2 domain), and two GFP analogues (e.g., CFP and YFP) that serve as a FRET pair (Scheme 1). Phosphorylation of the tyrosine-bearing sequence by a PTK furnishes a phosphotyrosine moiety that is intramolecularly captured by the SH2 domain; this results in a global conformational change that alters the spatial relationship between the FRET pair, which in turn changes their emission ratio (generally from a few percent up to 50 %, depending upon the system). However, this approach has two key limitations. First, FRET sensors monopolize two fluorescent windows and thus do not lend themselves as readily to multiplexing (simultaneously monitoring two or more kinases at separate wavelengths) as do single color sensors. Second, the FRET readout is constrained to a fluorescence change of less than 100 %.
Scheme 1.
Design of FRET reporter-protein constructs (adapted from refs. [8a] and [8b]).
Peptide-derived sensors offer a number of unique advantages, including ease of large-scale synthesis, handling, and storage, as well as ready access to multiply modified analogues containing an array of unnatural substituents. In addition, peptide sensors generally utilize mechanisms other than FRET to report the phosphorylation event, mechanisms that can generate a fluorescent response well in excess of 100 %. Why is this important? In addition to the brightness of the fluorophore (defined as quantum yield 0 extinction coefficient) and selectivity of the substrate, the magnitude of the fluorescence change is an essential contributor to sensor sensitivity. Sensitivity can be critical if the protein kinase of interest is present at a comparatively low concentration or is an inefficient catalyst.
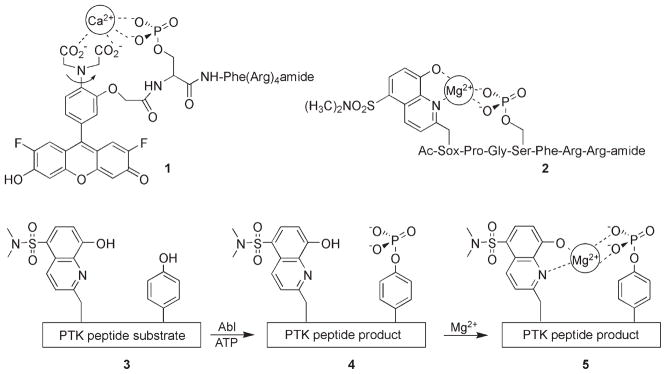
The number of protein kinase peptide-based fluorescent probes reported to date is surprisingly small, especially when one considers the large number of fluorescent substrates developed for other protein families (e.g., proteases). Furthermore, of the few kinase reporters that have been described, the vast majority serve as serine/threonine protein kinase sensors. To the best of our knowledge, Shults and Imperiali reported the first example of a fluorescently responsive tyrosine kinase peptide substrate. [11] These investigators viewed the newly introduced phosphate moiety as a potential metal chelator, a notion that has been applied to the serine/threonine protein kinases as well (e.g., 1[12] and 2;[11] Scheme 2). Upon metal ion coordination, the photophysical properties of a fluorophore interacting with the metal, in this instance Mg2+, are perturbed. Indeed, a fivefold enhancement in fluorescent intensity is reported for a peptide phosphorylated by the Abl tyrosine kinase. The Sox fluorophore in peptide product 5 displays an impressive Stokes’ shift of 125 nm as well as a phosphorylation-induced shift of λex from 316 (substrate) to 360 nm (phospho product). Some of the limitations with respect to intracellular imaging of tyrosine kinase action include the need for nonphysiological concentrations of Mg2+ (10 mM) in order to observe the greatest fluorescent change, the modest extinction coefficient (ε =6200 M−1 cm−1), and the short excitation wavelength (λex = 360 nm) of the Sox fluorophore.
Scheme 2.
Structure of metal-ion-dependent serine/threonine protein kinase sensors 1[12] and 2.[11] The PTK sensor 3 is phosphorylated by the PTK Abl to generate 4. Upon binding Mg2+, the latter elicits a fivefold enhancement in the fluorescence emission of the quinoline Sox-based fluorophore.[11]
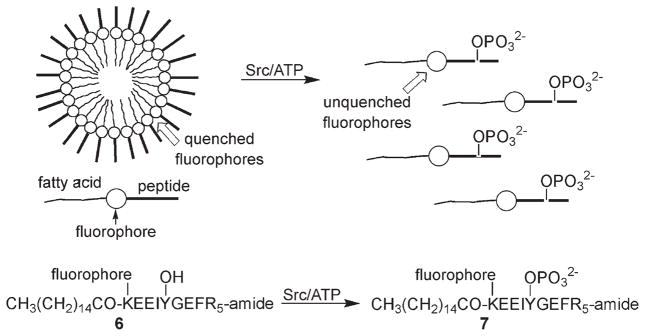
Lee and colleagues designed an assay based on the idea that the negatively charged phosphate moiety could have a dramatic effect on the structure of micellar peptide aggregates (Scheme 3).[13] Specifically, peptide 6 contains three key structural features: 1) an N-terminal fatty acid that promotes micelle formation, 2) an overall neutral charge, and 3) a fluorophore (a fluorescein derivative) whose fluorescence is quenched within the micelle. Phosphorylation disrupts the peptide aggregate by altering charge neutrality; this results in an increase in fluorescence. In practice, both the substrate and phospho-product peptides form micelles, with a somewhat lower critical micelle concentration (3 μM) for the former than for the latter (8 μM). The largest PTK-induced fluorescence change (fivefold) is observed when the concentration of the starting substrate is fixed (6 μM) just above its critical micelle concentration, but below that of the phosphorylated product. Peptide 6 serves as a substrate for Src and several other members of the Src kinase family. This assay is most likely limited to in vitro conditions given the array of intracellular membranous sites that would compromise micellar structural integrity.
Scheme 3.
The fluorescein fluorophore in 6 is self-quenched in the micelle. Phosphorylation of 6 furnishes 7, which is less prone to aggregate formation, thereby generating a fluorescent response. (Adapted from ref. [13].)
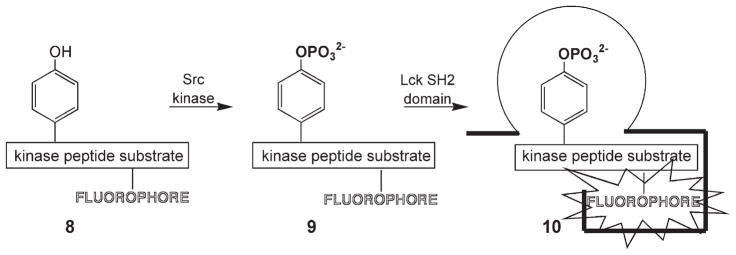
Hahn and his colleagues have described protein biosensors that fluorescently report the formation of protein–protein complexes.[14] These biosensors are designed by positioning an environmentally sensitive fluorophore at a key site so that, upon formation of the biosensor–protein complex, the fluorophore is enveloped within a hydrophobic pocket, thereby generating a fluorescent response. As noted in Section 1, phosphotyrosine moieties serve as homing beacons that drive the formation of multimeric protein complexes. Is it possible to take advantage of this fundamental mechanism of signaling pathways, by utilizing the Hahn approach, to elicit a fluorescent readout? PTK-catalyzed phosphorylation of a fluorophore-substituted peptide, 8, generates a bio-sensor, 9, in situ that, upon interaction with a phosphotyrosine-binding domain (e.g., SH2, PTB), furnishes the desired readout (Scheme 4).[15] Indeed, a greater than sevenfold enhancement in fluorescence is observed with a Src kinase peptide substrate in the presence of an SH2 domain. This study employed several environmentally sensitive fluorophores, including dapoxyl (λex = 390 nm; λem = 520 nm), NBD (λex = 470 nm; λem = 530 nm), and cascade yellow (λex = 400 nm; λem = 535 nm). Recently, several long-wavelength environmentally sensitive fluorophores have been described,[16] and these might also prove useful for the strategy outlined in Scheme 4. However, this approach has not yet been conducted in an intracellular setting, and so it remains to be demonstrated whether in situ-generated biosensor 9 will be able to productively engage endogenous phosphotyrosine binding domains.
Scheme 4.
Src kinase phosphorylates peptide 8 to create biosensor 9 in situ. A pyrene analogue of the latter gives a sevenfold fluorescent response upon binding to an SH2 domain. (Adapted from ref. [15].)
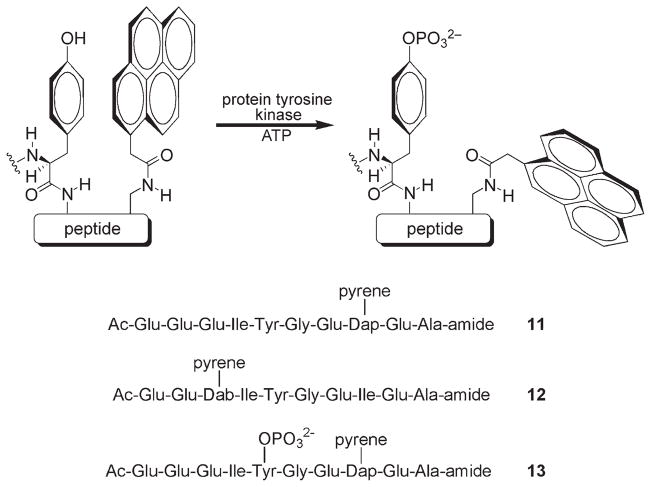
Tryptophan and, to a lesser extent, tyrosine are known to quench the fluorescence of a variety of fluorophores.[17] Quenching appears to require close contact between the aromatic moiety of these residues and the fluorescent dye.[18] We have examined the ability of tyrosine to intramolecularly quench fluorophores appended to a Src kinase peptide substrate.[19] The phosphotyrosine residue, which is both charged and more heavily solvated than the corresponding free phenol, was predicted to interact less efficiently with the aromatic rings of a nearby fluorophore, and therefore serve less effectively as a quencher (Scheme 5). In addition, studies with tryptophan reveal that electron transfer from the indole ring to the fluorophore is responsible for quenching.[20] Since the aromatic ring system of phosphotyrosine is less electron rich than that of its unphosphorylated counterpart, we reasoned that the former should be a poorer quencher than the latter. A small library of pyrene-substituted peptides was prepared, and individual peptides were examined for their ability to serve as fluorescent reporters of tyrosine phosphorylation. Peptides 11 and 12 are Src kinase substrates that exhibit 4.3- and 4.7-fold fluorescence enhancements, respectively, upon phosphorylation. Both peptides possess a relatively general PTK consensus sequence and thus serve as substrates for a wide variety of tyrosine kinases including members of the Src kinase subfamily (Fyn, Fgr, Hck, Lck, Yes, LynA, and LynB), other non-receptor tyrosine kinases (Abl, Csk, and Fes/Fps), and various receptor tyrosine kinases (FGFR, TrkA, and Flt3). Furthermore, the NOESY spectrum of starting peptide 11 revealed significant through-space interactions between the pyrene and tyrosine protons. By contrast, the corresponding NOEs in phosphorylated peptide 13 are either extraordinarily weak or nonexistent. These results are consistent with the idea that phosphorylation disrupts interactions between the quenching tyrosine aromatic ring and the appended fluorophore. However, the photophysical properties of pyrene, notably its short excitation wavelength, render it an inappropriate choice as a fluorophore for cellular studies [ε =26 000 M−1 cm−1 (MeOH), λex = 340 nm, λem = 380 nm]. Recently, the strategy outlined in Scheme 5 has been applied to fluorophores with “cell-friendly” properties including cascade yellow [ε = 24 000 M−1 cm−1 (MeOH), λex = 400 nm, λem = 550 nm], cascade blue [ε =30 000 M−1 cm−1 (H2O), λex = 400 nm, λem = 422 nm), Oregon green [ε = 87000 M−1 cm−1 (H2O), λex = 495 nm, λem = 520 nm), and several far-red dyes (ε >100 000 M−1 cm−1, λex > 650 nm).[21–22]
Scheme 5.
Fluorescence quenching by the formation of an intramolecular fluorophore–tyrosine complex is relieved upon phosphorylation. Substrates 11 (where Dap = L-2,3-diaminopropionic acid) and 12 (where Dab = L-2,4-diaminobutanoic acid) display a 4.3- and 4.7-fold enhancement in fluorescence, respectively, upon phosphorylation. Pronounced NOEs between the pyrene and tyrosine protons are displayed in 11 but not in the phosphorylated analogue 13.
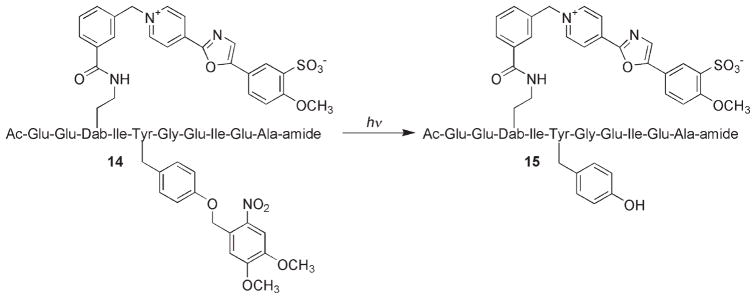
One of the primary advantages associated with peptide-derived PTK sensors is that multiple unnatural substituents can be easily introduced during solid-phase peptide synthesis. For example, the cascade yellow derivative 14 possesses a side-chain-modified tyrosine residue that cannot be phosphorylated (Scheme 6). However, since the substituent on the tyrosine is photolabile, the sensor can be loaded into cells (either by microinjection or by the use of cell-permeabilizing agents) and subsequently activated (to furnish 15) when convenient for the investigator. This is especially helpful when dealing with an intracellular enzyme that is constitutively active, such as in the human lung cancer A549 cell line in which the Src kinase is overexpressed. A light-activatable species provides a clear start point for observing kinase activity as a function of some treatment (e.g., a potential inhibitor) or a cell-based behavior (e.g., the various stages of mitosis). In vitro studies demonstrated that 14 is only susceptible to phosphorylation following photolysis and that the amount of available active sensor present can be controlled by varying the light-exposure time.[21] Furthermore, small quantities of active sensor can be released at multiple time points, thus allowing multiple readouts to be performed in a single experiment. Indeed, microinjection of 14 into A549 cells, followed by photolysis, furnished the expected sensor behavior in a light-controlled fashion (Figure 1).
Scheme 6.
The light-activatable cascade yellow sensor 14 and its conversion to the active (phosphorylatable) sensor 15.
Figure 1.
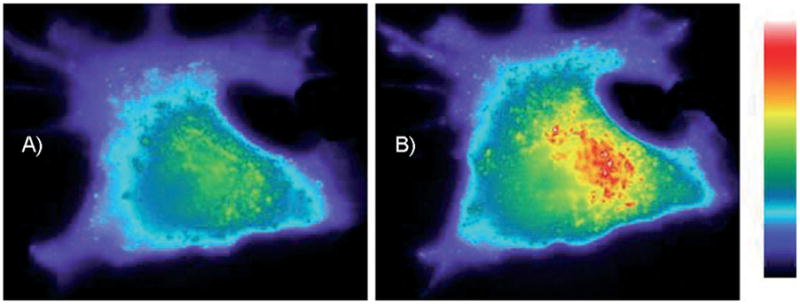
Light-induced time-dependent change in fluorescence intensity in A549 cells containing caged PTK sensor 14. Cells were exposed to a 100 W Hg-Arc lamp through the objective for 8 s to convert 14 to 15. Immediately after photolysis (A), images were collected at 1 min intervals. Final image (B) was obtained at 25 min following activation of the sensor (Reprinted with permission from ref. [21], Copyright the American Chemical Society, 2006).
One of the clear disadvantages of peptides is their general lack of cell permeability. However, peptides are now easily rendered cell permeable by the attachment of Arg-rich sequences.[23] Unfortunately, although these sequences can deliver peptides into cells, the peptide itself is often trapped within endosomes and therefore unavailable to interact with the intracellular milieu. Divita and his group have developed a series of peptide-carrier constructs that efficiently and noncovalently deliver peptides into mammalian cells.[24] Indeed, his research team has shown that 14 can be uniformly delivered into A549 cells.[25]
Although peptide-based sensors offer an attractive strategy for interrogating protein tyrosine kinase activity in living cells, several issues remain. Cells are irregularly shaped and often change their morphology as a function of time and in response to environmental stimuli. This leads to an uneven distribution of sensor, which can render fluorescence changes difficult to interpret, particularly if the changes are modest. One means to compensate for this potential difficulty is to compare the fluorescence of the reporter fluorophore to that of a second fluorophore whose photophysical properties are not altered by phosphorylation (ratiometric imaging). Although ratiometric peptide-based sensors of protein kinases have not (to the best of our knowledge) been described, it should be fairly straightforward to prepare a peptide containing both a reporter fluorophore and a second reference fluorophore. In addition, a second, spectacularly more difficult challenge remains to be addressed. Most, if not all, protein tyrosine kinases utilize peptides as substrates. Unfortunately, closely related tyrosine kinases tend to catalyze the phosphorylation of the same peptides. Consequently, the issue of selectivity, particularly with respect to peptide substrates, looms ever large. By contrast, substrate selectivity in the cell is controlled by a number of factors, including spatial localization of the PTK and its protein substrate as well as by multiple contacts between the PTK and the protein to be phosphorylated. Unfortunately, both of these mechanisms appear to be inaccessible to comparatively small peptide substrates. One solution to the issue of specificity is the construction of peptide/non-peptide hybrids. For example, we have found that highly selective peptide-derived inhibitors for protein kinases can be generated by adorning a conventional peptide framework with unnatural substituents.[26] It might be possible to apply this strategy to protein tyrosine kinase peptide substrates as well in order to create exquisitely selective sensors.
Conclusions
PTK sensors offer a number of opportunities for rapidly identifying inhibitors for specific tyrosine kinases, exploring the behavior of tyrosine kinases in living cells, and ultimately serving as novel diagnostics for a variety of disorders. Recently, several new design concepts have emerged that translate the appearance of a phosphorylated tyrosine into a fluorescent readout in peptide-based systems. All of these approaches take advantage of the inherent synthetic malleability of solid-phase peptide synthesis, which allows an array of unnatural moieties to be easily incorporated with pinpoint site-specific accuracy.
Acknowledgments
We thank the National Institutes of Health (CA79954), Panomics Inc., and Sigma–Aldrich for generous financial support.
References
- 1.Adams JA. Biochemistry. 2003;42:601–607. doi: 10.1021/bi020617o. [DOI] [PubMed] [Google Scholar]
- 2.Fukami Y, Nagao T, Iwasaki T, Sato KI. Pharmacol Ther. 2002;93:263–270. doi: 10.1016/s0163-7258(02)00195-x. [DOI] [PubMed] [Google Scholar]
- 3.For example, see Mendelsohn J, Baselga J. Semin Oncol. 2006;33:369–385. doi: 10.1053/j.seminoncol.2006.04.003.
- 4.Martin GS. Oncogene. 2004;23:7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- 5.Randolph TR. Clin Lab Sci. 2005;18:38–48. [PubMed] [Google Scholar]
- 6.Kurzrock R, Kantarjian HM, Druker BJ, Talpaz M. Ann Intern Med. 2003;138:819–830. doi: 10.7326/0003-4819-138-10-200305200-00010. [DOI] [PubMed] [Google Scholar]
- 7.Boggon TJ, Eck MJ. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 8.a) Kurokawa K, Mochizuki N, Ohba Y, Mizuno H, Miyawaki A, Matsuda M. J Biol Chem. 2001;276:31 305–31 310. doi: 10.1074/jbc.M104341200. [DOI] [PubMed] [Google Scholar]; b) Ting AY, Kain KH, Klemke RL, Tsien RY. Proc Natl Acad Sci USA. 2001;98:15 003–15 008. doi: 10.1073/pnas.211564598. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Sato M, Ozawa T, Inukai K, Asano T, Umezawa Y. Nat Biotechnol. 2002;20:287–294. doi: 10.1038/nbt0302-287. [DOI] [PubMed] [Google Scholar]; d) Miyawaki A. Dev Cell. 2003;4:295–305. doi: 10.1016/s1534-5807(03)00060-1. [DOI] [PubMed] [Google Scholar]; e) Sato M, Umezawa Y. Methods. 2004 ;32:451–455. doi: 10.1016/j.ymeth.2003.10.013. [DOI] [PubMed] [Google Scholar]; f) Wang Y, Botvinick EL, Zhao Y, Berns MW, Usami S, Tsien RY, Chien S. Nature. 2005;434:1040–1045. doi: 10.1038/nature03469. [DOI] [PubMed] [Google Scholar]
- 9.There is no theoretical reason why PTK protein sensors must be restricted to the FRET reporter mechanism. The fact that nearly all protein-based PTK sensors utilize FRET is likely due to a combination of synthetic expediency and the structural nature of GFPs. The primary advantage of protein sensors is that they are genetically encoded and thus need not be handled. In order to retain this advantage, one is limited to using genetically encoded fluorophores, such as the GFPs. However, the fluorophore buried within the GFP is physically sequestered and thus not able to directly interact with the tyrosine suffering phosphorylation. Consequently, although GFPs excel in reporting conformational changes relative to a second GFP partner, they are less effective in transmitting highly localized structural alterations in a non-FRET fashion. For an interesting non-FRET approach see Sao YM, Umezawa Y. Anal Chem. 2004;76:6144–6149. doi: 10.1021/ac040037s.
- 10.Zaccolo M. Circ Res. 2004;94:866–873. doi: 10.1161/01.RES.0000123825.83803.CD. [DOI] [PubMed] [Google Scholar]
- 11.Shults MD, Imperiali B. J Am Chem Soc. 2003;125:14 248–14 249. doi: 10.1021/ja0380502. [DOI] [PubMed] [Google Scholar]
- 12.Chen CA, Yeh RH, Lawrence DS. J Am Chem Soc. 2002;124:3840–3841. doi: 10.1021/ja017530v. [DOI] [PubMed] [Google Scholar]
- 13.Sun H, Low KE, Woo S, Noble RL, Graham RJ, Connaughton SS, Gee MA, Lee LG. Anal Chem. 2005;77:2043–2049. doi: 10.1021/ac048280e. [DOI] [PubMed] [Google Scholar]
- 14.Pertz O, Hahn KM. J Cell Sci. 2004;117:1313–1318. doi: 10.1242/jcs.01117. [DOI] [PubMed] [Google Scholar]
- 15.Wang Q, Lawrence DS. J Am Chem Soc. 2005;127:7684–7685. doi: 10.1021/ja050789j. [DOI] [PubMed] [Google Scholar]
- 16.Toutchkine A, Kraynov V, Hahn K. J Am Chem Soc. 2003;125:4132–4145. doi: 10.1021/ja0290882. [DOI] [PubMed] [Google Scholar]
- 17.Marme N, Knemeyer JP, Sauer M, Wolfrum J. Bioconjugate Chem. 2003;14:1133–1139. doi: 10.1021/bc0341324. [DOI] [PubMed] [Google Scholar]
- 18.Vaiana AC, Neuweiler H, Schulz A, Wolfrum J, Sauer M, Smith JC. J Am Chem Soc. 2003;125:14 564–14 572. doi: 10.1021/ja036082j. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, Cahill SM, Blumenstein M, Lawrence DS. J Am Chem Soc. 2006;128:1808–1809. doi: 10.1021/ja0577692. [DOI] [PubMed] [Google Scholar]
- 20.Doose S, Neuweiler H, Sauer M. Chem Phys Chem. 2005;6:2277–2285. doi: 10.1002/cphc.200500191. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Dai Z, Cahill SM, Blumenstein M, Lawrence DS. J Am Chem Soc. 2006;128:14 016–14 017. doi: 10.1021/ja065852z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Q, Lawrence DS. 2006 unpublished results. [Google Scholar]
- 23.Futaki S. Biopolymers. 2006;84:241–249. doi: 10.1002/bip.20421. [DOI] [PubMed] [Google Scholar]
- 24.Gros E, Deshayes S, Morris MC, Aldrian-Herrada G, Depollier J, Heitz F, Divita G. Biochim Biophys Acta. 2006;1758:384–393. doi: 10.1016/j.bbamem.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 25.Divita G. personal communication. 2006.
- 26.Lawrence DS. Biochim Biophys Acta. 2005;1754:50–57. doi: 10.1016/j.bbapap.2005.07.038. [DOI] [PubMed] [Google Scholar]