Abstract
The need for a new anti-Staphylococcus aureus therapy that can effectively cripple bacterial infection, neutralize secretory virulence factors, and lower the risk of creating bacterial resistance is undisputed. Here, we propose what is, to our knowledge, a previously unreported infectious mechanism by which S. aureus may commandeer Propionibacterium acnes, a key member of the human skin microbiome, to spread its invasion and highlight two secretory virulence factors (S. aureus β-hemolysin and P. acnes CAMP (Christie, Atkins, Munch-Peterson) factor) as potential molecular targets for immunotherapy against S. aureus infection. Our data demonstrate that the hemolysis and cytolysis by S. aureus were noticeably augmented when S. aureus was grown with P. acnes. The augmentation was significantly abrogated when the P. acnes CAMP factor was neutralized or β-hemolysin of S. aureus was mutated. In addition, the hemolysis and cytolysis of recombinant β-hemolysin were markedly enhanced by recombinant CAMP factor. Furthermore, P. acnes exacerbated S. aureus-induced skin lesions in vivo. The combination of CAMP factor neutralization and β-hemolysin immunization cooperatively suppressed the skin lesions caused by coinfection of P. acnes and S. aureus. These observations suggest a previously unreported immunotherapy targeting the interaction of S. aureus with a skin commensal.
INTRODUCTION
Staphylococcus aureus, a Gram-positive bacterium, is a major causes of fatal nosocomial infections (Homa and Palfreyman, 2000). It is estimated that S. aureus accounts for 12 million outpatient visits and 292,000 hospitalizations annually in the United States alone, of which 126,000 are due to methicillin-resistant S. aureus (MRSA) (Goetghebeur et al., 2007). Recent studies estimated that more people die from MRSA bacterium than from HIV in the United States (Klevens et al., 2008; Payne, 2008). In addition, MRSA infections have recently been found in increasing numbers among individuals in the community without health-care exposure (Wilson and Rinker, 2009). Although S. aureus, including MRSA and community-associated MRSA, can cause life-threatening and systemic infection, skin and soft tissue are the most common sites of S. aureus infection, comprising > 75% of MRSA disease (Cohen et al., 2007).
Nearly everyone hosts Propionibacterium acnes (Brook and Frazier, 1991; Ahn et al., 1996), which accounts for approximately half of the total skin microbiome (Tancrede, 1992), with an estimated density of 102–105–6cm2 (McGinley et al., 1978; Leyden et al., 1998). P. acnes predominates (> 60% of total bacteria) in facial skin (Grice et al., 2009); however, it can be found almost everywhere on the body (Yamada et al., 2002; Nishiwaki et al., 2004). There is growing evidence that P. acnes and S. aureus coexist in many human diseases, including acne lesions (Williams et al., 1992), implant infections (Ramage et al., 2003; Bashir et al., 2007), and sepsis (Duncan and Sperling, 2008). This indicates that the pathogenic S. aureus may interact with residential P. acnes during infection in humans, and it might therefore be important to understand the role of P. acnes in S. aureus infection.
Developing effective therapeutic approaches for S. aureus or MRSA treatment remains an unmet challenge because of its formidable resistance against multiple traditional antibiotics such as methicillin, cloxacillin, and flucloxacillin (Takizawa et al., 2005; Takano et al., 2007). Current antibiotic therapy nonspecifically eliminates the majority of bacteria, which impacts human microbiome homeostasis (Jeong et al., 2009) and increases the risk of developing resistant bacteria. Past efforts to generate vaccines against S. aureus have been unsuccessful. Anti–S. aureus vaccines targeting surface proteins (e.g., clumping factor) have failed in clinical trials (Shinefield and Black, 2005; DeJonge et al., 2007). Other anti–S. aureus vaccines targeting surface molecules (e.g., poly-N-succinyl β-1 –6-glucosamine (McKenney et al., 1999) and iron-regulated surface determinants A, B, D, and E (Stranger-Jones et al., 2006)) have subsequently been developed. A recent vaccine using a secretory toxin (α-hemolysin) as an antigen (Bubeck Wardenburg and Schneewind, 2008) emphasizes the notion that targeting secretory virulence factors rather than bacterial surface proteins creates a more effective therapy with a lower tendency to select for resistance. Although these vaccines had been demonstrated to be effective in mice, none of them was constructed taking into account the interaction of S. aureus with human commensal bacteria. Without this consideration, the efficacy of these vaccines becomes doubtful when they are translated from mice to humans.
Here, we have demonstrated that the secretory CAMP (Christie, Atkins, Munch-Peterson) factor of P. acnes enhances hemolysis and cytolysis by S. aureus β-hemolysin, suggesting that S. aureus may shrewdly utilize the secreted P. acnes CAMP factor to intensify its virulence. The results revealed an intriguing interaction of S. aureus with human commensals at the initial stage of infection and two potential therapeutic targets (P. acnes CAMP factor and S. aureus β-hemolysin) for treatment of S. aureus infection.
RESULTS
P. acnes amplified the hemolytic activity of S aureus
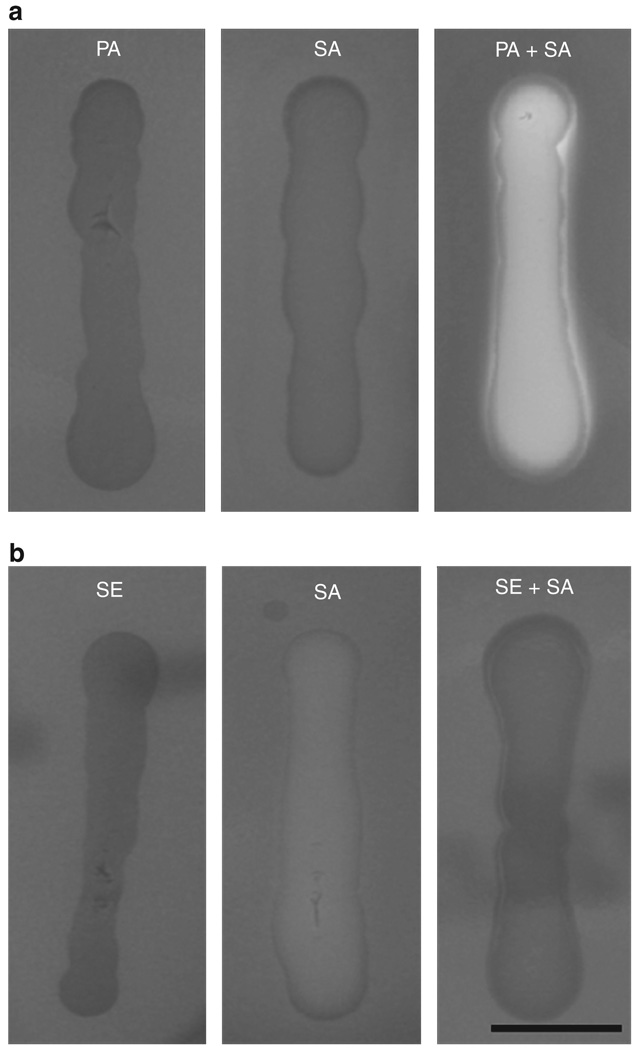
When S. aureus (S. aureus 113) was grown with P. acnes (ATCC 6919) on a sheep blood agar plate, we found that the hemolytic activity of S. aureus was dramatically augmented in comparison with that of S. aureus or P. acnes alone (Figure 1a). Accordingly, the colony-forming units (CFUs) of S. aureus in culture alone or in a coculture of S. aureus and P. acnes were not distinct (Supplementary Figure S1 online), suggesting that P. acnes did not influence the growth of S. aureus.However, this augmentation of hemolysis did not appear when S. aureus 113 was cocultured with Staphylococcus epidermidis (ATCC 12228), one of the human skin commensal bacteria (Figure 1b; Otto, 2009), which indicated that hemolytic augmentation of S. aureus was specifically mediated by P. acnes and was not a result of an increase in the growth of S. aureus.
Figure 1. Propionibacterium acnes, not Staphylococcus epidermidis, enhances the hemolytic activity of Staphylococcus aureus.
(a) P. acnes alone (PA, 2 × 107 colony-forming units (CFUs)), S. aureus 113 alone (SA, 2 × 107 CFUs), P. acnes plus S. aureus (PA + SA, 1:1 ratio with a total of 2 × 107 CFUs), (b) S. epidermidis alone (SE, 2 × 107 CFUs), or S. epidermidis plus S. aureus (SE + SA, 1:1 ratio with a total of 2 × 107 CFUs) were suspended in 10 µl PBS and streaked on sheep blood agar plates at 37°C for 2 days under anaerobic conditions. Hemolytic activity was determined as described in Materials and Methods. Bar = 1 cm. Data are representative of three separate experiments with similar results.
P. acnes CAMP factor is involved in the hemolysis and cytolysis of S. aureus
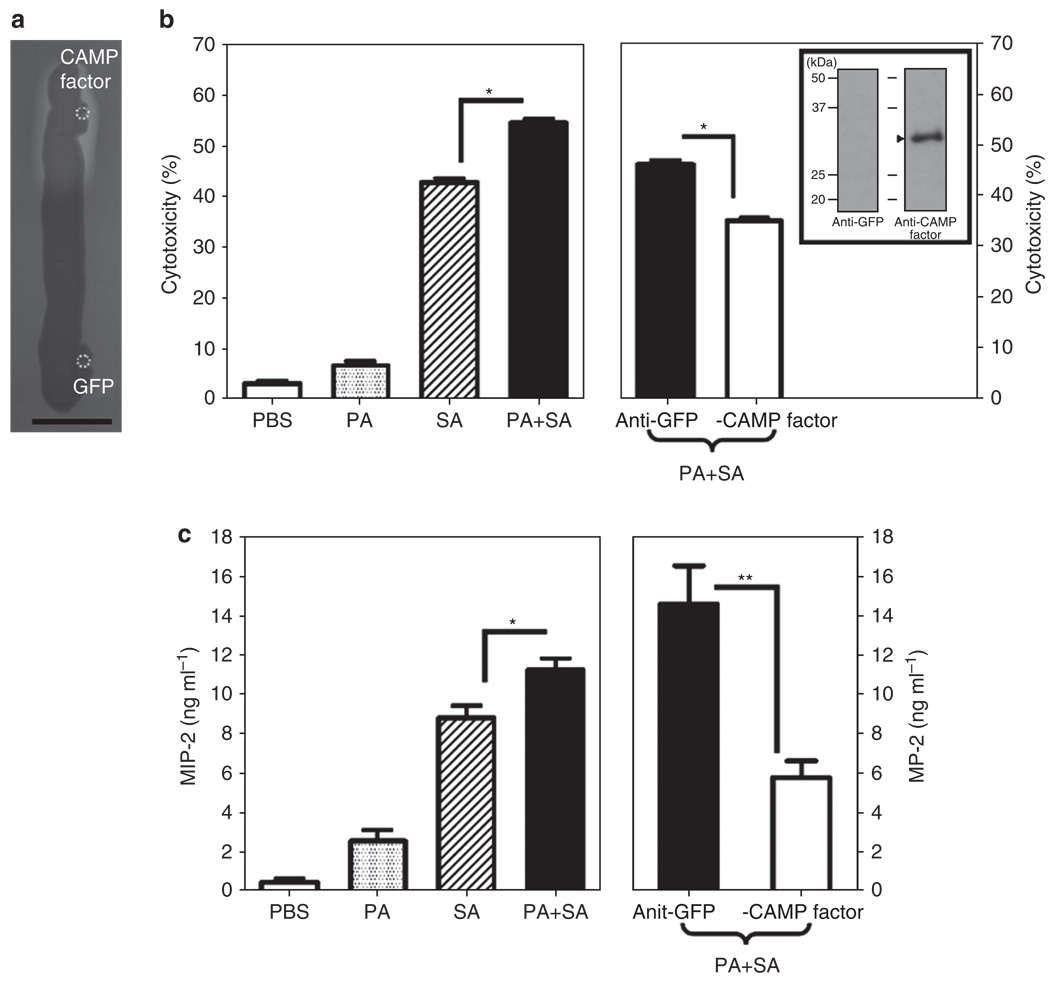
It has been reported that the CAMP factors of Streptococcus agalactiae (Schneewind et al., 1988), Streptococcus uberis (Jiang et al., 1996), and Streptococcus pyogenes (Gase et al., 1999) exhibit the CAMP reactions that can enhance the hemolytic activity of S. aureus. Spotting P. acnes CAMP factor (accession number AY726656), but not a control green fluorescence protein (GFP), considerably increased the intensity of the diffusion zone of the hemolysis of S. aureus (Figure 2a), supporting the notion that the P. acnes CAMP factor with a CAMP reaction exerts cohemolytic activity. It has been known that macrophages are essential for the initiation and execution of inflammatory response and subsequent resolution of bacterial infection (Kobayashi et al., 2006). We exposed murine RAW264.7 macrophage cells to culture supernatants of bacteria and found that secretory components from P. acnes and S. aureus cooperatively enhance the cytolysis of S. aureus (Figure 2b). In addition, neutralization of P. acnes CAMP factor markedly suppressed the cytotoxicity of macrophages induced by the supernatants of bacterial coculture (Figure 2b), indicating that CAMP factor was involved in the enhancement of cytolysis of S. aureus. The antisera were obtained from mice as described in Materials and Methods. The specificity of antiserum against CAMP factor was verified by western blotting (shown in the inset panel in Figure 2b).
Figure 2. Propionibacterium acnes Christie, Atkins, Munch-Peterson (CAMP) factor mediates the enhancement of hemolysis and cytolysis caused by a coculture of P. acnes with Staphylococcus aureus.
(a) A total of 10 µg of recombinant CAMP factor and green fluorescence protein (GFP) in 5 µl phosphate-buffered saline (PBS) was spotted (circles) adjacent to a S. aureus 113 (1 × 105 colony-forming units (CFUs) in 10 µl PBS) streak on a sheep blood agar plate grown overnight at 37°C. Bar = 1 cm. (b) Macrophage RAW264.7 cells were treated with PBS or bacterial culture supernatants (10 µg ml−1) of P. acnes (PA), S. aureus (SA), or P. acnes plus S. aureus (PA + SA). Bacterial culture supernatants of coculture of P. acnes and S. aureus were incubated with 5% (v/v) anti-CAMP factor or anti-GFP (as a negative control) antiserum. The in vitro cytotoxicity assay was performed after cells were treated with the mixture of bacterial culture supernatant and antiserum for 24 hours. Western blot analysis was conducted to validate the specificity of anti-CAMP factor antiserum (arrowhead in the inset panel). One microgram of recombinant CAMP factor was separated via 12% SDS-PAGE, transferred to an Immobilon-P polyvinylidene difluoride membrane, and reacted with anti-CAMP factor (right lane) or anti-GFP antiserum (left lane), in 1:2,000 dilution. (c) Neutralization of CAMP factor significantly diminishes the enhancement of P. acnes on the S. aureus-induced macrophage inflammatory protein-2 (MIP-2) production. PBS (25 µl), P. acnes (PA, 25 µl; 2 × 107 CFUs), S. aureus 113 (SA, 25 µl; 2 × 107 CFUs), or a mixture of P. acnes plus S. aureus 113 (PA + SA, 25 µl; 1:1 ratio with a total of 2 × 107 CFUs) was intradermally injected into the ears of imprinting control region (ICR) mice. The mixture of P. acnes and S. aureus (PA + SA) was preincubated with 5% (v/v) anti-CAMP factor or anti-GFP antiserum at 25°C for 1 hour to neutralize the P. acnes CAMP factor. After incubation, the bacteria alone or bacteria preincubated with antiserum were intradermally injected into ears of ICR mice. After injection for 24 hours, ears were excised, homogenized, and centrifuged. ELISA was performed to measure the proinflammatory MIP-2 cytokine in supernatant. The data are represented as mean ± SE (n = 3, *P<0.05 and **P<0.005 by Student’s t-test).
Induction of proinflammatory cytokines also has a crucial role in the progression of bacterial infection. We next examined whether P. acnes alters the levels of S. aureus–induced proinflammatory cytokines in vivo. Ears of imprinting control region (ICR) mice were intradermally injected with bacteria for 24 hours and subsequently excised to homogenize for detection of macrophage inflammatory protein-2 (MIP-2) levels in the supernatants. As shown in Figure 2c, coinjection of P. acnes and S. aureus significantly elevated the level of MIP-2 (11.87 ± 1.72 ng ml−1) compared with the injection of P. acnes (2.58 ± 0.94 ng ml−1) or S. aureus (8.78 ± 1.23 ng ml−1) alone. Subsequently, the neutralization of P. acnes with anti-CAMP factor (5.76 ± 1.48 ng ml−1), but not anti-GFP (14.61 ± 3.36 ng ml−1), antiserum notably reduced the level of MIP-2 induced by bacterial coinjection. The data in Figure 2 indicate that P. acnes CAMP factor exhibited cohemolytic activity and was an indispensable secretory protein in P. acnes that enhanced the virulence of S. aureus.
S. aureus β-hemolysin is associated with CAMP factor in the enhancement of hemolysis and cytolysis
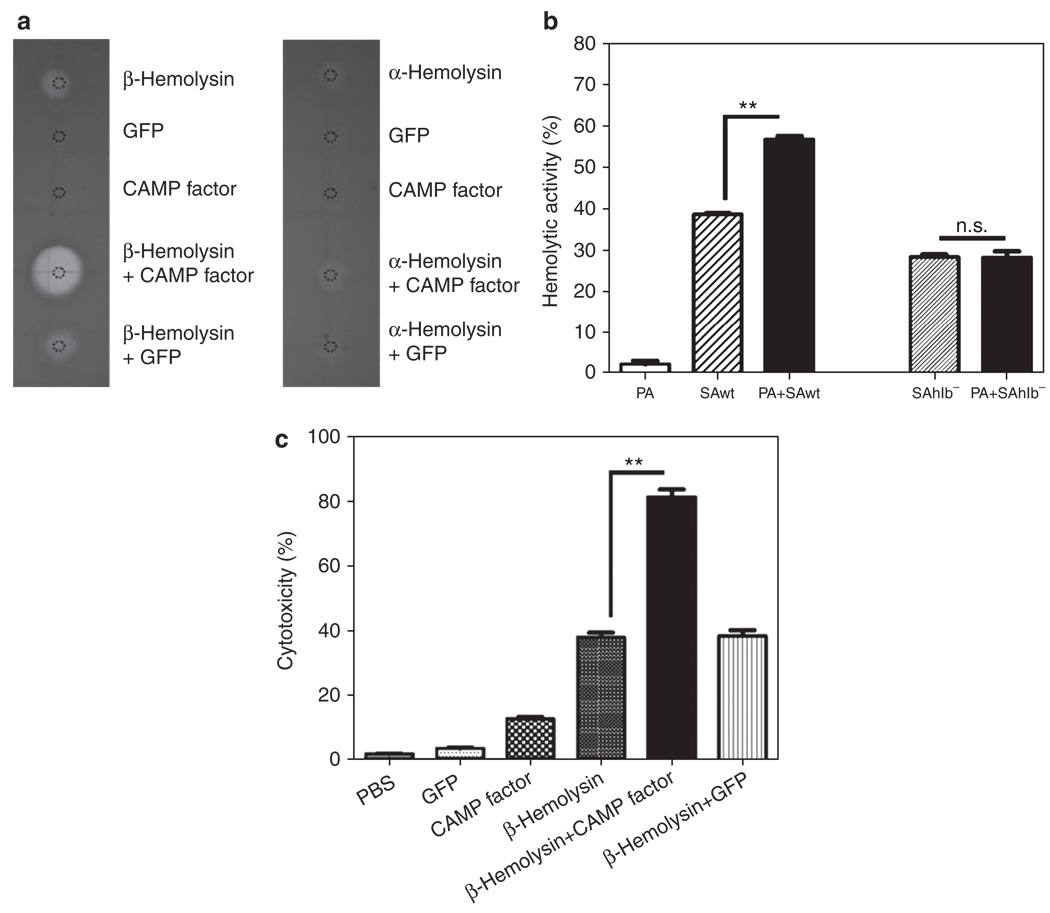
It has been documented that P. acnes CAMP factor can potentiate the hemolytic activity of S. aureus β-hemolysin, a secretory virulence factor (Huseby et al., 2007). Spotting recombinant CAMP factor with recombinant β-hemolysin, but not with recombinant α-hemolysin, on a sheep blood agar plate for 1 day showed synergistic enhancement of hemolysis (Figure 3a). The hemolysis assay showed that incubation of erythrocytes with wild-type S. aureus and P. acnes displayed greater hemolytic activity than incubation with individual S. aureus or P. acnes (Figure 3b). The enhancement of hemolytic activity was completely abrogated when P. acnes was coincubated with β-hemolysin-deficient (SAhlb−) strains (Figure 3b), but not with α-hemolysin (SAhla−) strains (Supplementary Figure S2 online). To further investigate whether β-hemolysin strengthened the cytolysis of CAMP factor, RAW264.7 cells were treated with 200 µgml−1 of recombinant GFP, CAMP factor, β-hemolysin, or a mixture of β-hemolysin plus CAMP factor or GFP for 18 hours (Figure 3c). As expected, the cytolysis of β-hemolysin (37.92 ± 2.4%) was strikingly reinforced when cells were treated with the mixture of β-hemolysin and CAMP factor (81.09 ± 5.45%).
Figure 3. Staphylococcus aureus β-hemolysin contributes to the augmentation of hemolysis and cytolysis caused by coculture of Propionibacterium acnes and S. aureus.
(a) Recombinant β-hemolysin (5 µg; Toxin Technology, Sarasota, FL), Christie, Atkins, Munch-Peterson (CAMP) factor (5 µg), and α-hemolysin (1 µg) in 5 µl phosphate-buffered saline (PBS) were spotted on sheep blood agar plates to examine the hemolytic activity. Recombinant green fluorescence protein (GFP; 5 µg) in 5 µl PBS was used as a control. (b) P. acnes (PA, 2 × 107 colony-forming units (CFUs)) alone, wild-type S. aureus alone (SAwt, 2 × 107 CFUs), or P. acnes plus wild-type S. aureus (1:1 ratio with a total of 2 × 107 CFUs) was incubated with sheep blood cells at 37°C with end-over-end rotation for 2 days. Hemolytic activity was detected by measuring the absorbance of hemoglobin release at 540 nm. To examine the essentiality of S. aureus β-hemolysin in the enhancement of hemolysis by coculture of P. acnes and S. aureus, a β-hemolysin-deficient S. aureus (SAhlb−, 2 × 107 CFUs) was incubated with sheep blood cells (PA + SAhlb− in 1:1 ratio with a total of 2 × 107 CFUs) in the absence and presence of P. acnes. . (c) To determine the synergistic effect of CAMP factor and β-hemolysin on cytotoxicity, macrophage RAW264.7 cells (1 × 105 per well) were incubated with 10 µl of 200 µg ml−1 recombinant proteins of GFP, CAMP factor, β-hemolysin, or the mixture of β-hemolysin plus CAMP factor or GFP at 37°C for 18 hours. An equal volume of PBS was used as a negative control. After incubation, the cytotoxicity of recombinant proteins to macrophages was determined by an in vitro cytotoxicity assay as described in Materials and Methods. Data are represented as mean ± SE (n = 3, **P<0.005 by Student’s t-test). NS, not significant.
P. acnes exacerbates S. aureus–induced skin lesions
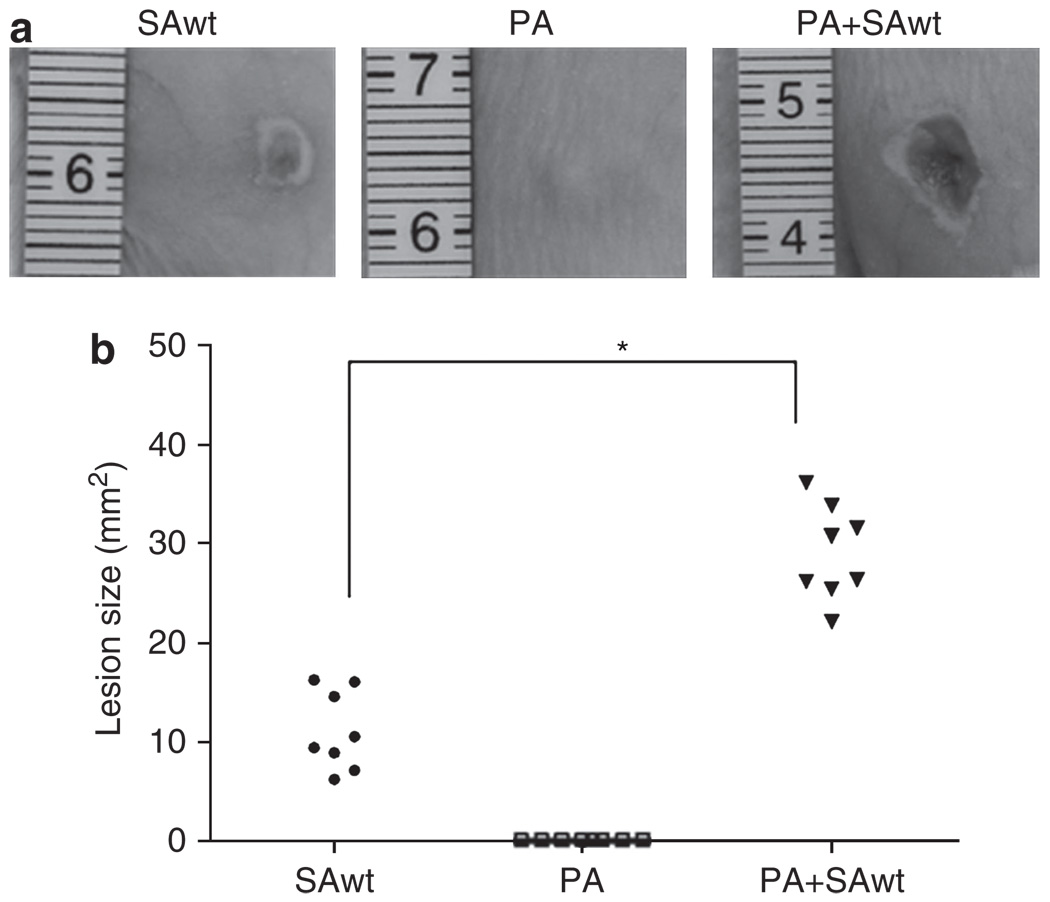
To gain insight into the interaction of S. aureus and P. acnes in vivo, skin lesions were made by injection of bacteria into the dorsal skin of ICR mice, a mouse strain with diversity in gene function that more closely resembles the human population. Injection of wild-type S. aureus (National Collection of Type Cultures (8325-4; 11.11 ± 3.96mm2), but not P. acnes, for 2 days induced the development of skin lesions (Figure 4) that recapitulate an ulcerative response of S. aureus infection in humans. The lesions (29.01 ± 4.75mm2) were significantly exacerbated when mice were coinjected with S. aureus and P. acnes.
Figure 4. Propionibacterium acnes intensifies Staphylococcus aureus-induced skin lesions.
(a) Wild-type S. aureus (SAwt, 1 × 107 colony-forming units (CFUs)), P. acnes (PA, 1 × 107 CFUs), or P. acnes plus wild-type S. aureus (PA + SAwt, 1:1 ratio with a total of 2 × 107 CFUs) in 50 µl phosphate-buffered saline was subcutaneously injected into the dorsal skins of imprinting control region mice to induce skin lesions. (b) Lesion sizes were examined and quantified 2 days after injection. Representative photographs of dorsal skin lesions are shown. Data are means of two independent experiments (n = 8, *P<0.05 by Student’s t-test).
S. aureus β-hemolysin is immunogenic
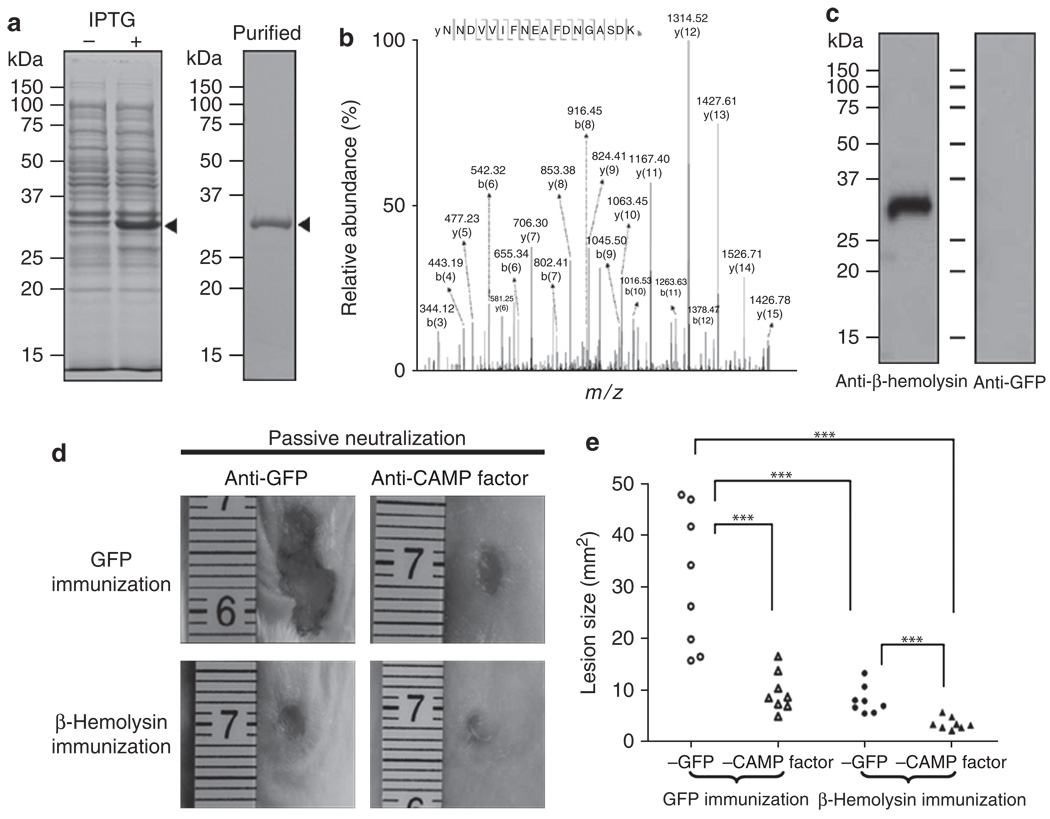
To explore the value of a therapy targeting β-hemolysin, we first examined the antigenicity of β-hemolysin. An expressed protein matching β-hemolysin with a molecular weight of ~33 kDa was detected by SDS-PAGE after isopropyl-β-d-thiogalactopyranoside induction (Figure 5a left panel) and protein purification (Figure 5a right panel). It was further validated via sequencing by Nano liquid chromatography linear trap quadrupole tandem mass spectrometry (West Palm Beach, FL) after in-gel trypsin digestion (Figure 5b). Twenty-seven peptides were fully sequenced and matched well with internal amino acids of S. aureus β-hemolysin (Supplementary Table S1 online). A sequenced peptide (NNDVVIFNEAFDNGASDK; 78–95 amino acid residues) of β-hemolysin is presented in Figure 5b. To examine the immunogenicity of β-hemolysin, we immunized ICR mice intranasally with UV-inactivated E. coli overexpressing β-hemolysin or GFP (a control protein). Immunization of UV-inactivated E. coli overexpressing antigens has been demonstrated in our previous efforts (Liu et al., 2008). A single band was detected in western blot analysis when recombinant β-hemolysin was immunoreacted with serum obtained from immunized mice, indicating that β-hemolysin was immunogenic (Figure 5c).
Figure 5. The immunogenicity of β-hemolysin and the combination of β-hemolysin vaccination and Christie, Atkins, Munch-Peterson (CAMP) factor neutralization confer immune protection against bacteria-induced skin lesions.
(a) Recombinant β-hemolysin (arrowheads) was expressed in E. coli BL21 (DE3). Competent cells transformed with the pEcoli-Nterm 6 × HN vector-inserted cDNA encoding β-hemolysin were incubated without (−) or with (+) isopropyl-β-d-thiogalactopyranoside (IPTG) for 4 hours and then subjected to 12% SDS-PAGE. Purified β-hemolysin is shown in the right panel. (b) The identity of recombinant β-hemolysin was analyzed by Nano liquid chromatography linear trap quadrupole tandem mass spectrometry (NanoLC-LTQ MS/MS). Tryptic digests of purified recombinant β-hemolysin were subjected to NanoLC-LTQ MS/MS. A sequenced internal peptide (NNDVVIFNEAFDNGASDK) of β-hemolysin is presented. The m/z value of each “y” and “b” ion in collision-induced dissociation spectra is indicated. All sequenced peptides are shown in Supplementary Table S1 online. (c) Immunogenicity of β-hemolysin was evaluated by western blotting. Imprinting control region mice were intranasally vaccinated with UV-inactivated E. coli BL21 (DE3) overexpressing β-hemolysin or green fluorescence protein (GFP). Anti-antisera were collected 1 week after second vaccination. One microgram of recombinant β-hemolysin was separated via 12% SDS-PAGE, transferred to an Immobilon-P polyvinylidene difluoride membrane, and reacted with anti-β-hemolysin (left lane) or anti-GFP antiserum (right lane), in 1:2,000 dilution. (d) The GFP-(upper row) and β-hemolysin-immunized mice (lower row) were used for evaluation of immune protection. Mice were injected subcutaneously with the mixture of wild-type Staphylococcus aureus and Propionibacterium acnes (1:1 ratio with a total of 2 × 107 colony-forming units in 50 µl phosphate-buffered saline) that were preincubated with 5% (v/v) anti-CAMP factor antiserum (right panel) or anti-GFP antiserum (left panel) at 25°C for 1 hour. Lesion sizes were examined 2 days after injection. (e) Skin lesions were measured and statistics compiled. Representative photographs of dorsal skin lesions are shown. Data are from two independent replicated experiments (***P<0.001; Tukey’s honest significant difference comparing the four treatments, in a two-way analysis of variance on log-transformed data comparing the four treatments, with day included as a blocking factor).
The combination of β-hemolysin immunization with passive neutralization of CAMP factor confers protection against skin lesions
To assess the effect of immunization with UV-inactivated E. coli overexpressing β-hemolysin and/or neutralization of CAMP factor on the bacteria-induced skin lesions, immunized mice were injected subcutaneously with both S. aureus and P. acnes in the presence of antiserum against CAMP factor or GFP. In GFP-immunized mice, a massive lesion size (30.01 ± 12.96mm2) developed after injection with bacteria in the presence of antiserum against GFP. However, the lesion (7.91 ± 2.67mm2) was significantly decreased in β-hemolysin-immunized mice (Figure 5d). Similarly, the lesion (9.53 ± 3.83mm2) was markedly attenuated in GFP-immunized mice accompanied by passive neutralization of antiserum against CAMP factor (Figure 5d and e). The results indicated that immunization targeting either S. aureus β-hemolysin or P. acnes CAMP factor can effectively protect mice from skin lesions caused by coinfection of S. aureus and P. acnes. To exclude the possibility of anti-CAMP factor antibody crossreacting with S. aureus directly, the S. aureus bacteria treated with and without anti-CAMP factor antiserum were subcutaneously injected into the dorsal skin of ICR mice to induce skin lesions. As shown in Supplementary Figure S3 online, there is no difference in the size of lesions caused by S. aureus treated with or without anti-CAMP factor antiserum, suggesting that anti-CAMP factor antiserum did not affect the S. aureus-induced skin lesions.
To investigate whether abrogation of both β-hemolysin and CAMP factor provided greater protection, mice immunized to β-hemolysin accompanied by passive neutralization of antiserum against CAMP factor were coinjected with S. aureus and P. acnes.The bacteria-induced lesion size (3.46 ± 1.17mm2) was considerably reduced only in the case of both S. aureus β-hemolysin immunization and P. acnes CAMP factor neutralization (Figure 5d and e). The result clearly illustrated that the combination of β-hemolysin immunization and passive neutralization of CAMP factor cooperatively suppressed skin lesions caused by co-infection of S. aureus and P. acnes.
DISCUSSION
In the human microbiome, the full spectrum of microbial species residing in humans, P. acnes is ubiquitous in the general population, whereas S. aureus is present in ~25% (Miller et al., 2007). It has been reported that pathogenic strains of MRSA bacteria have acquired genes from human commensal organisms that are present on the skin (Diep et al., 2006; Grice et al., 2009). Our data demonstrate that P. acnes enhances the hemolysis of S. aureus (Figure 1), suggesting that a specific interaction of the bacteria occurs in the human microbiome (Cogen et al., 2008; Young and Schmidt, 2008). We identified two secretory toxins—P. acnes CAMP factor and S. aureus β-hemolysin—that were critically engaged in the interaction of P. acnes and S. aureus. The hemolytic capacity is thought to be a virulence factor for numerous microbial pathogens to degrade tissue, invade host cells, disseminate themselves, and resist the host immune system. Thus, evidence of the enhancement of hemolysis of S. aureus by P. acnes raises the possibility that S. aureus benefits from P. acnes present on the skin to invade its human host.
Staphylococcal hemolysins are recognized as chief virulence factors that contribute to bacterial invasion and escape from the host immune response (Dinges et al., 2000). The β-hemolysin, also called sphingomyelinase, is highly active against sheep and bovine erythrocytes (Larsen et al., 2002). We observed an augmentation of hemolysis and cytolysis when cells were treated with both S. aureus β-hemolysin and P. acnes CAMP factor (Figure 3a and c), suggesting that S. aureus β-hemolysin may commandeer P. acnes CAMP factor to enhance the production of host ceramide for spreading infection in a cell-to-cell manner.
It has been documented that skin and soft tissue infections make up most of the cases of MRSA infections (Miller et al., 2005), although community-associated MRSA infections may cause more severe necrotizing pneumonia (Hageman et al., 2006) and bacteremia (Seybold et al., 2006). Skin can be the first place through which the S. aureus/MRSA bacterium enters a human host. Our data illustrated that P. acnes enhanced the S. aureus–induced skin lesions (Figure 4). Why passive neutralization of P. acnes CAMP factor significantly attenuated the skin lesions caused by the coinfection of S. aureus and P. acnes (Figure 5d and e) has two possible explanations. First, antiserum to P. acnes CAMP factor may exhibit cross-reactivity to S. aureus, leading to a decrease in skin lesions caused by S. aureus. Second, P. acnes CAMP factor may be massively secreted while S. aureus is present and thus exacerbate existing lesions. Our data (Supplementary Figure S3 online) suggest that anti-CAMP factor antiserum did not affect the skin lesions induced by S. aureus alone. However, we cannot rule out the second possibility that S. aureus stimulates secretion of P. acnes CAMP factor. Given that recombinant CAMP factor can potentiate the hemolytic activity of S. aureus (Figure 2a), it is possible that P. acnes does not need to be alive to exert this cohemolytic effect. Mixing killed P. acnes with S. aureus in future studies will determine whether CAMP factor secretion is necessary to enhance the virulence of S. aureus. Direct measurement of β-hemolysin and CAMP factor produced by these bacteria would be useful to determine whether an increase in the production and/or activities of virulence factors leads to the augmentation of cytolysis. In fact, it is worthwhile to clarify a signaling relationship among S. aureus β-hemolysin, P. acnes CAMP factor, and host ceramide. We have shown that humoral immunity against P. acnes CAMP factor can mitigate the in vitro hemolytic activity of S. aureus cocultured with P. acnes. Although we have shown protection against S. aureus infected mice through vaccination against β-hemolysin, we do not have enough evidence to conclude whether this is a T or B cell mediated immune response. Adoptive transfer experiments with sera and/or T cells are crucial to understanding the underlying mechanisms.
Although the overgrowth of P. acnes can cause many human diseases such as acne vulgaris and toxic shock syndrome (Yang et al., 2009), the bacterium is a major commensal in humans (Grice et al., 2009). Unlike active immunization, passive immunotherapy with local attenuation of CAMP factor will be less disruptive to P. acnes in distant locales. The genome of P. acnes has five homologs of the cohemolytic CAMP factor of S. agalactiae (Jurgens et al., 1987; Lang and Palmer, 2003; Valanne et al., 2005). Here, we have shown that neutralization of one (accession number AY726656) of the CAMP factors in P. acnes dramatically suppressed the coinfection-induced skin lesions (Figure 5d and e). Currently, we are determining whether other CAMP factors can also intensify the β-hemolysin toxicity and if they can be neutralized by their respective antisera. In conclusion, we have shown the potential benefit of targeting P. acnes CAMP factor for S. aureus infections using an animal model. Although disarming bacteria via neutralization of their secretory toxins may enhance the clearance of bacteria by immune cells, the immune-mediated blockade of CAMP factor and/or β-hemolysin in combination with small-molecule inhibitors directly targeting bacterial particles may be of value for complete eradication of bacterial infection.
Overall, the study provides the clinical relevance that passive administration of anti-CAMP factor antibody into S. aureus β-hemolysin-immunized patients may (i) prevent systemic bacterial invasion after local colonization at the initial site of infection; (ii) faciliate the host immune system’s eradication of disarmed/avirulent bacteria before antibody reaches the infected site; and (iii) instantly rescue the inefficiency of vaccines that provoke low titer of antibody in immunocompromised people or vaccines that have no activity against new strains of S. aureus/MRSA.
MATERIALS AND METHODS
Bacterial culture
P. acnes (ATCC 6919; Manassas, VA) was cultured on Brucella broth agar (BD, Sparks, MD) as described previously (Nakatsuji et al., 2009). Single colonies were inoculated in reinforced Clostridium medium(Oxford, Hampshire, UK) and cultured at 37 °C under anaerobic conditions using Gas-Pak (BD Biosciences, San Jose, CA). S. aureus 113 (ATCC 35556) and S. epidermidis (ATCC 12228) bacteria were from ATCC. A β-hemolysin-deficient S. aureus strain and its wild type (National Collection of Type Cultures 8325-4) were obtained from Pyong Park (Division of Respiratory Diseases, Children’s Hospital, Harvard Medical School, Boston, MA). An α-hemolysin-deficient S. aureus strain was obtained from Bill Schwan (Department of Microbiology, University of Wisconsin–La Crosse, La Cross, WI). S. aureus and S. epidermidis were cultured on 3% tryptic soy broth (Sigma-Aldrich, St Louis, MO) agar overnight at 37 °C. Bacteria were suspended in an appropriate amount of phosphate-buffered saline (PBS) for the further experiments.
Hemolytic activity of S. aureus enhanced by P. acnes or CAMP factor
Sheep blood agar plates were used to determine the cohemolytic activity of P. acnes or CAMP factor. P. acnes (2 × 107 CFUs), S. aureus 113 (2 × 107 CFUs), the mixture of P. acnes and S. aureus 113 (1:1 ratio with a total of 2 × 107 CFUs), S. epidermidis (2 × 107 CFUs), and the mixture of S. epidermidis and S. aureus 113 (1:1 ratio with a total of 2 × 107 CFUs) were all suspended in 10 µl PBS and then streaked on sheep blood agar plates and incubated for 2 days under anaerobic conditions at 37 °C. For determination of cohemolytic activity of CAMP factor, S. aureus 113 (10 µl, 2×107 CFUs in PBS) was streaked on the sheep blood agar plate. A total of 10 µg of recombinant CAMP factor or GFP within 5 µl PBS was spotted beside the S. aureus streak grown at 37°C for 18 hours.
Molecular cloning, expression, and purification of β-hemolysin and CAMP factor
The bacterial genomic DNA was prepared as described previously (Sohail, 1998). For purposes of constructing a plasmid carrying the hlb gene encoding the β-hemolysin (accession number: X61716), the gene was amplified by PCR from wild-type S. aureus genomic DNA as a template, using oligonucleotide primers of forward primer, 5′-AAGTCGACATGGTGAAAAAAACAAAATCC-3′ (SalI restriction site is underlined), and reverse primer, 5′-ATAAGCTTCTATTTACT ATAGGCTTTGATTGGG-3′ (HindIII restriction site is underlined). The oligonucleotide primers specific for the hlb gene were also used for PCR analysis of genomic DNA of various strains of S. aureus. The CAMP factor (accession number: AY726656) was cloned from P. acnes genomic DNA, using oligonucleotide primers of forward primer, 5′-ATGTCGACGTCGAGCCGACGACGACCATC TCG-3′ (SalI restriction site is underlined), and reverse primer, 5′-AT AAGCTTGGCAGCCTTCTTGACATCGGGGGAG-3′ (HindIII restriction site is underlined). The amplified PCR products and the In-Fusion Ready pEcoli-Nterm 6 × HN expression plasmid (Clontech, Mountain View, CA) were digested with SalI/HindIII and purified by agarose gel electrophoresis for ligation. The constructed plasmids were transformed and expressed in E. coli BL21 (DE3) (Invitrogen, Carlsbad, CA). A pEcoli-Nterm-GFP plasmid (Clontech) was used to express the GFP. A 2ml overnight culture of E. coli BL21 (DE3) containing expression plasmids of β-hemolysin, CAMP factor, or GFP, was inoculated into 200 ml Luria-Bertani and incubated with isopropyl-β-d-thiogalactopyranoside (1mm; Sigma-Aldrich) for 4 hours. A column with 2 ml Ni-NTA agarose (Qiagen, Valencia, CA) was used for protein expression and refolding. The purified and refolded protein was dialyzed overnight at 4 °C against 5 liters of 1 × PBS by using Spectra/Por molecular-porous membrane tubing (molecular weight cutoff: 3,500; Spectrum Laboratories, Rancho Dominguez, CA).
Protein identification via mass spectrometry
In-gel digestion with trypsin and protein identification via a Nano liquid chromatography linear trap quadrupole tandem mass spectrometry analysis were performed as described previously (Martin and Clynes, 1993; Shi et al., 2007). SEQUEST was searched with a fragment ion mass tolerance of 0.5Da and a parent ion tolerance of 1.0Da.
Vaccination and detection of antibodies to β-hemolysin and CAMP factor
Female ICR mice (Harlan, Indianapolis, IN) ~2 months old were used for vaccination. The mice were intranasally vaccinated with E. coli BL21 (DE3) overexpressing β-hemolysin, CAMP factor, or GFP (as the control), which were inactivated by UV (3,500 Jm−2) irradiation. The inactivation of irradiated E. coli BL21 (DE3) was indicated by its inability to grow on a Luria-Bertani agar plate (data not shown). For intranasal vaccination, 25 µl of irradiated (1 × 109 CFUs) E. coli BL21 (DE3) overexpressing β-hemolysin, CAMP factor, or GFP was pipetted into the nasal cavities of mice. ICR mice were vaccinated at 3-week intervals for 9 weeks. One week after the third boost, antisera against β-hemolysin or CAMP factor were collected individually for western blot analysis using a goat anti-mouse IgG (H + L) conjugated to horseradish peroxidase (Promega, Madison, WI) and the ECL Western Blotting Substrate (Pierce, Rockford, IL) for the detection of chemiluminescence.
Proinflammatory MIP-2 detection
The proinflammatory MIP-2 cytokine was determined by sandwich ELISA using a Quantikine M mouse MIP-2 set (R&D Systems, Minneapolis, MN).
Skin infection of S. aureus
The wild-type S. aureus (1 × 107 CFUs), P. acnes (1 × 107 CFUs), and P. acnes plus wild-type S. aureus (1:1 ratio with a total of 2 × 107 CFUs) were suspended in 50 µl PBS and subsequently injected subcutaneously into dorsal skin of the flank of 8- to 12-week-old female ICR mice, respectively. After injection for 2 days, the lesion sizes were examined and quantified by using ImageJ software (National Institutes of Health, Bethesda, MD).
Protective effect of a β-hemolysin-based vaccine along with CAMP factor neutralization
P. acnes (1 × 107 CFUs in 25µl PBS) was preincubated with 5% (v/v) anti-CAMP factor or anti-GFP antiserum (as the control) at 25°C for 1 hour and then mixed with wild-type S. aureus (1 × 107 CFUs in 25µl PBS). Subsequently, the mixtures were subcutaneously injected into the dorsal skin of ICR mice that were vaccinated with irradiated E. coli BL21 (DE3) overexpressing β-hemolysin or GFP, respectively. After injection for 2 days, the lesions were measured using ImageJ software.
Hemolysis assay
The defibrinated sheep blood cells (Lampire Biological Laboratories, Pipersville, PA) were incubated with individual bacteria (P. acnes or S. aureus; 2 × 107 CFUs) or a bacterial coculture (P. acnes with various strains of S. aureus; 2 × 107 CFUs) suspended in 10µl PBS for 2 days. Incubation of blood cells with Triton X-100 (2%; Sigma-Aldrich) served as a positive control. The samples were then centrifuged at 800g for 10 minutes. The absorbance of hemoglobin release was measured at 540 nm and is expressed as percentage of Triton X-100–induced hemolysis. Results given represent mean values from triplicate measurements.
Cell culture and in vitro cytotoxicity assay
A murine macrophage cell line, RAW264.7, was cultured in RPMI 1640 medium, supplemented with 10% heat-inactivated fetal bovine serum at 37°C. Cells (1 × 105) were infected with 1 × 106 CFUs (multiplicity of infection = 1:10) bacteria. For in vitro neutralization, complements in sera were deactivated by heating at 56°C for 30 minutes before adding into bacterial culture. The mixed bacterial culture media of P. acnes and S. aureus were preincubated in the presence of 5% (v/v) anti-GFP (as a negative control) or anti-CAMP factor antiserum at 25°C for 1 hour before treatment. Each well of cells was cocultured with bacterial culture media in 5 µl at 37 °C for 24 hours. Cell viability was determined by an acid phosphatase assay as described previously (Nakatsuji et al., 2009).
Statistical analysis
Data are presented as mean ± SE. Data are represented as means for measurement of lesion sizes. The Student t-test with criterion P<0.05 was used to determine statistical significance for all experiments. A two-way analysis of variance model was built to compare the treatment groups on the basis of log-transformed data with adjustment for the day of experiments (two independent replicates) shown in Figure 5e. A Tukey’s honest significant difference test (Miller, 1981) was performed for pairwise comparisons of the treatment groups at a family-wise error rate of 5%; the corresponding adjusted P-values were provided.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01-AI067395-01, R21-R022754-01, R21-I58002-01, 1R41AR056169-01, and 5R21AI088147-02. We thank Karen Messer for advice on statistical analyses, Pei-Feng Liu and Teruaki Nakatsuji for their technical assistance, and Jamie Bernard and Jason Steiner for their critical reading of the manuscript.
Abbreviations
- CAMP factor
Christie, Atkins, Munch-Peterson factor
- CFU
colony-forming unit
- GFP
green fluorescence protein
- HA-MRSA
hospital-acquired MRSA
- ICR
imprinting control region
- MIP-2
macrophage inflammatory protein-2
- MRSA
methicillin-resistant S. aureus
- PBS
phosphate-buffered saline
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Ahn CY, Ko CY, Wagar EA, et al. Microbial evaluation: 139 implants removed from symptomatic patients. Plast Reconstr Surg. 1996;98:1225–1229. doi: 10.1097/00006534-199612000-00016. [DOI] [PubMed] [Google Scholar]
- Bashir A, Mujahid TY, Jehan N. Antibiotic resistance profile: isolation and characterization of clinical isolates of staphylococci from patients with community-acquired skin infections. Pak J Pharm Sci. 2007;20:299–304. [PubMed] [Google Scholar]
- Brook I, Frazier EH. Infections caused by Propionibacterium species. Rev Infect Dis. 1991;13:819–822. doi: 10.1093/clinids/13.5.819. [DOI] [PubMed] [Google Scholar]
- Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J Exp Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogen AL, Nizet V, Gallo RL. Skin microbiota: a source of disease or defence? Br J Dermatol. 2008;158:442–455. doi: 10.1111/j.1365-2133.2008.08437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AL, Shuler C, McAllister S, et al. Methamphetamine use and methicillin-resistant Staphylococcus aureus skin infections. Emerg Infect Dis. 2007;13:1707–1713. doi: 10.3201/eid1311.070148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJonge M, Burchfield D, Bloom B, et al. Clinical trial of safety and efficacy of INH-A21 for the prevention of nosocomial staphylococcal bloodstream infection in premature infants. J Pediatr. 2007;151:260–265. doi: 10.1016/j.jpeds.2007.04.060. 265.e1. [DOI] [PubMed] [Google Scholar]
- Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet. 2006;367:731–739. doi: 10.1016/S0140-6736(06)68231-7. [DOI] [PubMed] [Google Scholar]
- Dinges MM, Orwin PM, Schlievert PM. Exotoxins of Staphylococcus aureus. Clin Microbiol Rev. 2000;13:16–34. doi: 10.1128/cmr.13.1.16-34.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan SF, Sperling JW. Treatment of primary isolated shoulder sepsis in the adult patient. Clin Orthop Relat Res. 2008;466:1392–1396. doi: 10.1007/s11999-008-0213-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gase K, Ferretti JJ, Primeaux C, et al. Identification, cloning, and expression of the CAMP factor gene (cfa) of group A streptococci. Infect Immun. 1999;67:4725–4731. doi: 10.1128/iai.67.9.4725-4731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetghebeur M, Landry PA, Han D, et al. Methicillin-resistant Staphylococcus aureus: a public health issue with economic consequences. Can J Infect Dis Med Microbiol. 2007;18:27–34. doi: 10.1155/2007/253947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grice EA, Kong HH, Conlan S, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–1192. doi: 10.1126/science.1171700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman JC, Uyeki TM, Francis JS, et al. Severe community-acquired pneumonia due to Staphylococcus aureus, 2003–04 influenza season. Emerg Infect Dis. 2006;12:894–899. doi: 10.3201/eid1206.051141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa DG, Palfreyman MA. Infectious diseases in the operating room. CRNA. 2000;11:8–14. [PubMed] [Google Scholar]
- Huseby M, Shi K, Brown CK, et al. Structure and biological activities of beta toxin from Staphylococcus aureus. J Bacteriol. 2007;189:8719–8726. doi: 10.1128/JB.00741-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SH, Song YK, Cho JH. Risk assessment of ciprofloxacin, flavomycin, olaquindox and colistin sulfate based on microbiological impact on human gut biota. Regul Toxicol Pharmacol. 2009;53:209–216. doi: 10.1016/j.yrtph.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Jiang M, Babiuk LA, Potter AA. Cloning, sequencing and expression of the CAMP factor gene of Streptococcus uberis. Microb Pathog. 1996;20:297–307. doi: 10.1006/mpat.1996.0028. [DOI] [PubMed] [Google Scholar]
- Jurgens D, Sterzik B, Fehrenbach FJ. Unspecific binding of group B streptococcal cocytolysin (CAMP factor) to immunoglobulins and its possible role in pathogenicity. J Exp Med. 1987;165:720–732. doi: 10.1084/jem.165.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens RM, Gorwitz RJ, Collins AS. Methicillin-resistant Staphylococcus aureus: a primer for dentists. J Am Dent Assoc. 2008;139:1328–1337. doi: 10.14219/jada.archive.2008.0044. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Tsuda Y, Yoshida T, et al. Bacterial sepsis and chemokines. Curr Drug Targets. 2006;7:119–134. doi: 10.2174/138945006775270169. [DOI] [PubMed] [Google Scholar]
- Lang S, Palmer M. Characterization of Streptococcus agalactiae CAMP factor as a pore-forming toxin. J Biol Chem. 2003;278:38167–38173. doi: 10.1074/jbc.M303544200. [DOI] [PubMed] [Google Scholar]
- Larsen HD, Aarestrup FM, Jensen NE. Geographical variation in the presence of genes encoding superantigenic exotoxins and beta-hemolysin among Staphylococcus aureus isolated from bovine mastitis in Europe and USA. Vet Microbiol. 2002;85:61–67. doi: 10.1016/s0378-1135(01)00478-3. [DOI] [PubMed] [Google Scholar]
- Leyden JJ, McGinley KJ, Vowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology. 1998;196:55–58. doi: 10.1159/000017868. [DOI] [PubMed] [Google Scholar]
- Liu YT, Lin SB, Huang CP, et al. A novel immunogenic spore coat-associated protein in Bacillus anthracis: characterization via proteomics approaches and a vector-based vaccine system. Protein Expr Purif. 2008;57:72–80. doi: 10.1016/j.pep.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Clynes M. Comparison of 5 microplate colorimetric assays for in vitro cytotoxicity testing and cell proliferation assays. Cytotechnology. 1993;11:49–58. doi: 10.1007/BF00749057. [DOI] [PubMed] [Google Scholar]
- McGinley KJ, Webster GF, Leyden JJ. Regional variations of cutaneous propionibacteria. Appl Environ Microbiol. 1978;35:62–66. doi: 10.1128/aem.35.1.62-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney D, Pouliot KL, Wang Y, et al. Broadly protective vaccine for Staphylococcus aureus based on an in vivo-expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–1453. doi: 10.1056/NEJMoa042683. [DOI] [PubMed] [Google Scholar]
- Miller RG. Simultaneous Statistical Inference. New York: Springer; 1981. p. 300. [Google Scholar]
- Miller SI, Hoffman LR, Sanowar S. Did bacterial sensing of host environments evolve from sensing within microbial communities? Cell Host Microbe. 2007;1:85–87. doi: 10.1016/j.chom.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Kao MC, Fang JY, et al. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129:2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiwaki T, Yoneyama H, Eishi Y, et al. Indigenous pulmonary Propionibacterium acnes primes the host in the development of sarcoid-like pulmonary granulomatosis in mice. Am J Pathol. 2004;165:631–639. doi: 10.1016/S0002-9440(10)63327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcus epidermidis – the “accidental” pathogen. Nat Rev Microbiol. 2009;7:555–567. doi: 10.1038/nrmicro2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne DJ. Microbiology. Desperately seeking new antibiotics. Science. 2008;321:1644–1645. doi: 10.1126/science.1164586. [DOI] [PubMed] [Google Scholar]
- Ramage G, Tunney MM, Patrick S, et al. Formation of Propionibacterium acnes biofilms on orthopaedic biomaterials and their susceptibility to antimicrobials. Biomaterials. 2003;24:3221–3227. doi: 10.1016/s0142-9612(03)00173-x. [DOI] [PubMed] [Google Scholar]
- Schneewind O, Friedrich K, Lutticken R. Cloning and expression of the CAMP factor of group B streptococci in Escherichia coli. Infect Immun. 1988;56:2174–2179. doi: 10.1128/iai.56.8.2174-2179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold U, Kourbatova EV, Johnson JG, et al. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care-associated blood stream infections. Clin Infect Dis. 2006;42:647–656. doi: 10.1086/499815. [DOI] [PubMed] [Google Scholar]
- Shi Y, Elmets CA, Smith JW, et al. Quantitative proteomes and in vivo secretomes of progressive and regressive UV-induced fibrosarcoma tumor cells: mimicking tumor microenvironment using a dermis-based cell-trapped system linked to tissue chamber. Proteomics. 2007;7:4589–4600. doi: 10.1002/pmic.200700425. [DOI] [PubMed] [Google Scholar]
- Shinefield HR, Black S. Prevention of Staphylococcus aureus infections: advances in vaccine development. Expert Rev Vaccines. 2005;4:669–676. doi: 10.1586/14760584.4.5.669. [DOI] [PubMed] [Google Scholar]
- Sohail M. A simple and rapid method for preparing genomic DNA from gram-positive bacteria. Mol Biotechnol. 1998;10:191–193. doi: 10.1007/BF02760866. [DOI] [PubMed] [Google Scholar]
- Stranger-Jones YK, Bae T, Schneewind O. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci USA. 2006;103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Saito K, Teng LJ, et al. Spread of community-acquired methicillin-resistant Staphylococcus aureus (MRSA) in hospitals in Taipei, Taiwan in 2005, and comparison of its drug resistance with previous hospital-acquired MRSA. Microbiol Immunol. 2007;51:627–632. doi: 10.1111/j.1348-0421.2007.tb03949.x. [DOI] [PubMed] [Google Scholar]
- Takizawa Y, Taneike I, Nakagawa S, et al. A Panton-Valentine leucocidin (PVL)-positive community-acquired methicillin-resistant Staphylococcus aureus (MRSA) strain, another such strain carrying a multiple-drug resistance plasmid, and other more-typical PVL-negative MRSA strains found in Japan. J Clin Microbiol. 2005;43:3356–3363. doi: 10.1128/JCM.43.7.3356-3363.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tancrede C. Role of human microflora in health and disease. Eur J Clin Microbiol Infect Dis. 1992;11:1012–1015. doi: 10.1007/BF01967791. [DOI] [PubMed] [Google Scholar]
- Valanne S, McDowell A, Ramage G, et al. CAMP factor homologues in Propionibacterium acnes: a new protein family differentially expressed by types I and II. Microbiology. 2005;151:1369–1379. doi: 10.1099/mic.0.27788-0. [DOI] [PubMed] [Google Scholar]
- Williams RE, Doherty VR, Perkins W, et al. Staphylococcus aureus and intra-nasal mupirocin in patients receiving isotretinoin for acne. Br J Dermatol. 1992;126:362–366. doi: 10.1111/j.1365-2133.1992.tb00679.x. [DOI] [PubMed] [Google Scholar]
- Wilson PC, Rinker B. The incidence of methicillin-resistant staphylococcus aureus in community-acquired hand infections. Ann Plast Surg. 2009;62:513–516. doi: 10.1097/SAP.0b013e31818a6665. [DOI] [PubMed] [Google Scholar]
- Yamada T, Eishi Y, Ikeda S, et al. In situ localization of Propionibacterium acnes DNA in lymph nodes from sarcoidosis patients by signal amplification with catalysed reporter deposition. J Pathol. 2002;198:541–547. doi: 10.1002/path.1243. [DOI] [PubMed] [Google Scholar]
- Yang D, Pornpattananangkul D, Nakatsuji T, et al. The antimicrobial activity of liposomal lauric acids against Propionibacterium acnes. Biomaterials. 2009;30:6035–6040. doi: 10.1016/j.biomaterials.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young VB, Schmidt TM. Overview of the gastrointestinal microbiota. Adv Exp Med Biol. 2008;635:29–40. doi: 10.1007/978-0-387-09550-9_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.