Abstract
We report the first application of a photoinduced 1,3-dipolar cycloaddition reaction to ‘staple’ a peptide dual inhibitor of the p53-Mdm2/Mdmx interactions. A series of stapled peptide inhibitors were efficiently synthesized and showed excellent dual inhibitory activity in ELISA assay. Furthermore, the positively charged, stapled peptides showed enhanced cellular uptake along with modest in vivo activity.
Keywords: inhibitors, protein-protein interaction, dipolar cycloaddition, cellular uptake, stapled peptides
The α-helix is one of the most common secondary structural elements in proteins; approximately 40% of amino acids in proteins are found as part of α-helices.1 Furthermore, α-helices frequently mediate the protein-protein interactions that regulate diverse cellular processes.2 Whereas short peptides may exhibit potent in vitro activity and are generally devoid of toxicity, they are rarely good drug candidates for intracellular targets because of their unfavorable pharmacological properties, e.g., low proteolytic stability and inefficient cellular uptake. While the disulfide bond 3 and lactam formations4 were known long ago to increase the helicity and proteolytic stability of peptides, the hydrocarbon stapling—direct cross-linking of peptide side-chains via ring-closing metathesis—has emerged as one of the most promising strategies reported to date.5 Despite its tremendous success, alternative stapling methods other than hydrocarbon stapling are still desirable because they may offer stapled peptides with distinct physicochemical properties. We recently reported a photoinduced 1,3-dipolar cycloaddition based stapling reaction for a model 310 helical peptide carrying the tetrazole and alkene moieties, affording enhanced peptide cell permeability.6 Here we report the application of this stapling chemistry to dual-active peptide inhibitors of p53-Mdm2/Mdmx interactions and the characterization of the inhibitory activities of the stapled peptides both in vitro and in cancer cells.
The p53 tumor suppressor is a potent inducer of cell cycle arrest, apoptosis, cellular senescence, and innate immunity. It is activated in response to oncogenic transformation, extrinsic stress, and viral infection to protect higher organisms from cancer. Mdm2 and Mdmx function as two key regulators of p53 by binding to its N-terminus, inhibiting its transcriptional activity, and promoting its degradation. Despite sharing high sequence and structural homology, Mdm2 and Mdmx regulate p53 in distinct manners. Mdm2 serves as an E3 ligase and downregulates p53 activity by promoting p53 ubiquitination and subsequent degradation.7 In contrast, Mdmx abrogates p53 function by sequestering it into the inactive p53-Mdmx complex.8 Both Mdm2 and Mdmx are overexpressed in human cancers, making them attractive anticancer drug targets.9 However, despite their prominent roles in cancer progression, potent dual-active small molecules that disrupt both p53-Mdm2 and p53-Mdmx interactions have not been reported. By screening a phage library, we have recently discovered a potent peptide dual inhibitor (PDI) of the p53-Mdm2/Mdmx interactions with the sequence of LTFEHYWAQLTS.10 However, PDI was found to be cell-impermeable, preventing its further development into therapeutics. To overcome this limitation, we hypothesized that by applying our photoinduced stapling chemistry,6 we could obtain cell-permeable analogs that retain dual inhibitory activity.
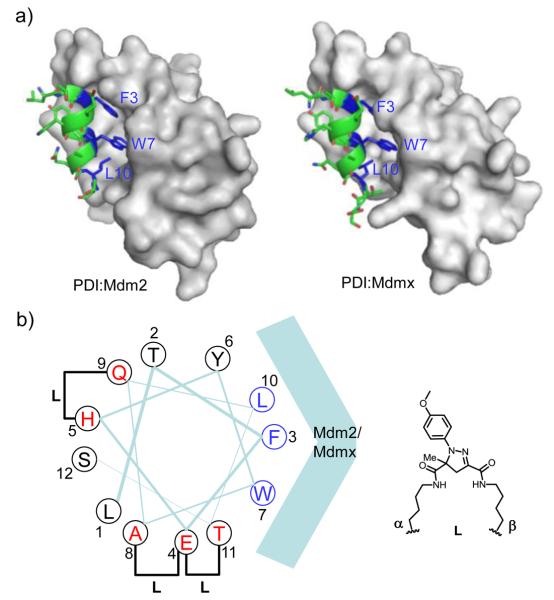
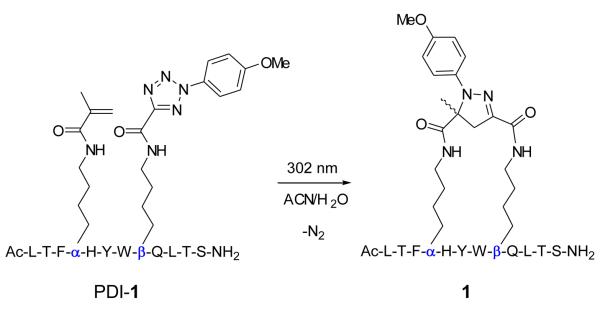
To identify suitable sites in the PDI sequence to append the alkene and tetrazole moieties, we first performed an acetyllysine scan and found that Glu-4, His-5, Ala-8, Gln-9, and Thr-11 can be substituted without significant loss in activity (Table S2 in Supplemental Material). This finding is consistent with the crystal structures of PDI in complexes with Mdm2 and Mdmx, in which these five residues are solvent exposed and do not make critical contacts with either Mdm2 or Mdmx (Figure 1a).11 After mapping these residues to the PDI helix wheel model (Figure 1b), three possible stapling schemes emerged: i,i+4 stapling between Glu-4 and Asp-8 and between His-5 and Gln-9, and i,i+7 stapling between Glu-4 and Thr-11.12 To expedite the synthesis of stapled peptides, alkene-(α) and tetrazole-modified lysine (β) were prepared from Fmoc-Lys-OH (Scheme S1 in Supplemental Material) and used directly in solid-phase peptide synthesis. After cleavage from the resin and purification by reverse-phase HPLC, linear peptides were exposed to a handheld UV lamp (UVM-57, 302 nm, 115 V, 0.16 Amps) in a mixed acetonitrile/water (1:1) solvent for 2 hr (Scheme 1). Three stapled peptides, 1-3, embodying three stapling patterns were obtained (Table 1). The conversions from linear precursors to stapled products ranged from 82% for 1, 75% for 2, to 89% for 3, based on HPLC analysis (Figure S1-S3 in Supplemental Material).
Figure 1.
(a) Crystal structure of PDI bound to Mdm2 (PDB code: 3G03) and Mdmx (PDB code: 3FDO). The proteins were rendered in surface model and PDI was shown in ribbon and tube model. The side chains of three canonical residues, F3, W7 and L10, were shown in blue. (b) Helical wheel diagram of PDI viewed from the N- to C-terminus. The five possible stapling sites were colored in red. The pyrazoline cross-linker structure formed after the cycloaddition reaction is shown on the right.
Scheme 1.
Representative synthesis of stapled peptide 1 using a photoinduced 1,3-dipolar cycloaddition reaction. α and β denote the alkene and tetrazole-modified lysine, respectively.
Table 1.
Sequences and Inhibitory Activity of PDI Analogs a
| name | sequence | charge | IC50, Mdm2 (nM) |
IC50, Mdmx (nM) |
|---|---|---|---|---|
| Nutlin-3b | 600 | No activity | ||
| PDI | LTFEHYWAQLTSc | −1 | 44 | 550 |
| 1 |
|
0 | 61 ± 5.2 | 540 ± 55 |
| 1a |
|
+1 | 71 ± 3.9 | 220 ± 19 |
| 1b |
|
+2 | 160 ± 8.6 | 390 ± 25 |
| 2 |
|
−1 | 170 ± 15 | 1,600 ± 290 |
| 3 |
|
0 | 6.2 ± 1.5 | 340 ± 27 |
| 3a |
|
+1 | 15 ± 0.6 | 240 ± 18 |
| 3b |
|
+1 | 18 ± 0.9 | 210 ± 23 |
| 3c |
|
+2 | 39 ± 1.4 | 550 ± 58 |
| 3d |
|
+2 | 28 ± 1.0 | 410 ± 38 |
| 3e |
|
+2 | 14 ± 1.2 | 810 ± 95 |
| 4 | LTFKAcRYWRQLKPyrSe | +2 | 2,300 ± 220 | 34,000 |
ELISA assays were repeated three times in deriving average IC50 values along with standard deviations.
IC50 values were taken from ref. 10 where the same ELISA assays were performed.
Residues tolerant of substitution were underlined.
WCl = 6-chlorotryptophane.
KAc = Nε-acetyllysine; Kpyr = Nε-pyrazolinelysine, see Table S1 in Supplemental Material for structure.
Previously, PDI was determined to inhibit the full-length p53 binding to Mdm2 and Mdmx with IC50 values of 44 nM and 550 nM, respectively, in an ELISA assay.11b To gauge the effect of stapling on inhibitory activity, stapled PDI analogs 1-3 were evaluated and their inhibitory activity data were collected in Table 1. Compared to PDI, 1 with the cross-linker connecting residues 4-8 showed similar activities against both Mdm2 and Mdmx. The other i,i+4 stapled PDI analog 2 with the cross-linker connecting residues 5-9 showed roughly fourfold drop in Mdm2 activity and threefold drop in Mdmx activity. Meanwhile, the i,i+7 stapled PDI analog 3 showed sevenfold increase in Mdm2 activity together with a slight improvement in Mdmx activity. To discern the effect of stapling from that of residue substitution, we determined the activity of its linear precursor, PDI-4α11β, with IC50 values to be 1200 nM against Mdm2 and 6300 nM against Mdmx. By comparing the activity of 3 with that of PDI-4α11β, stapling led to roughly 200-fold increase in the Mdm2 activity and 20-fold increase in the Mdmx activity.
Since increased positive charges generally lead to improved cellular uptake,5d, 13 we prepared a series of positively charged PDI analogs by substituting the remaining non-essential residues (His-5, Ala-8, Gln-9 and Thr-11) in 1 and 3 with either one (1a, 3a and 3b) or two arginines (1b, 3c and 3d) and determined their inhibitory activities (Table 1). Compared to their parent peptides, these positively charged analogs showed 2-6-fold drop in activity against Mdm2 and small changes against Mdmx (Table 1), indicating that arginine substitutions were largely tolerated. Furthermore, we probed the effect of increased hydrophobicity at the binding surface of PDI by replacing the canonical Trp with 6-chlorotryptophan to derive 3e.14 Interestingly, 3e showed roughly 2-fold increase in Mdm2 activity but 2-fold decrease in Mdmx activity relative to 3d (Table 1), which can be attributed to the interaction between 6-chlorine and Phe-86 of Mdm2, but not Mdmx, as detected previously.14b
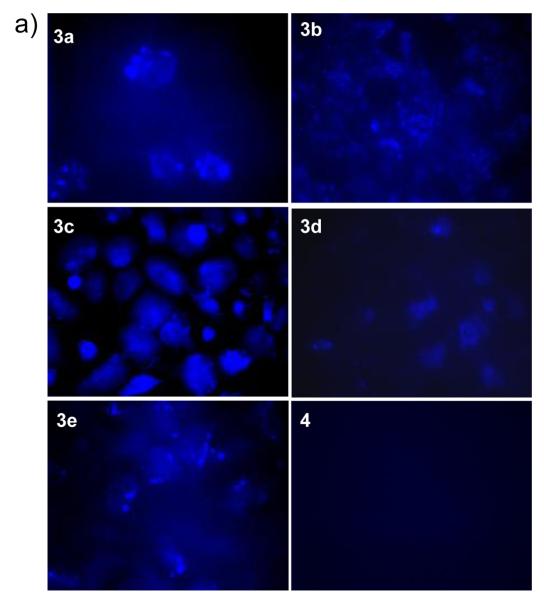
To examine whether stapled PDI analogs penetrate into cancer cells, we took advantage of the intrinsic fluorescence of the pyrazoline cross-linker4 and monitored their cellular uptake by fluorescence microscopy. Our initial study revealed that the charge neutral stapled peptide 3 was not cell-permeable (Figure S6 in Supplemental Material). To our delight, treatment of U2OS cells with +1 charged, stapled peptides 3a and 3b gave rise to a punctuated fluorescence pattern (Figure 2). This distribution pattern suggests that stapled peptides are taken up by cancer cells via a pinocytotic pathway where the majority of peptides are likely trapped in the endosomes.15 On the other hand, treatment of U2OS cells with +2 charged stapled peptides 3c, 3d and 3e produced more diffusive fluorescence patterns, suggesting that increased positive charge may facilitate the peptides to escape the acidic endosomes. Notably, stapled peptide 3c in which two arginines form a continuous positive patch in an i,i+4 fashion showed a strong and uniform fluorescence distribution in the cytosol, indicating that the charge locations are also important in addition to total charge. This charge location dependency also suggests that the observed intracellular fluorescence was not an artifact derived from the cell fixation. It is noteworthy that U2OS cells treated with a linear fluorescent peptide carrying +2 charge, 4 (see Table 1 for sequence), under identical conditions did not produce any intracellular fluorescence, confirming that stapling is essential for cellular uptake (Figure 2). In our preliminary circular dichroism study, stapled peptide 3e showed 21% helicity compared to 13% for peptide 4 of the same sequence (Figure S7), indicating that stapling indeed increases helicity, which is partly responsible for the enhanced cellular uptake.
Figure 2.
Fluorescence images of fixed U2OS cells after treated with 20 μM of stapled PDI-3a-e or linear PDI-4 for 4 hr at 37 °C. A 20 × oil immersion lens was used.
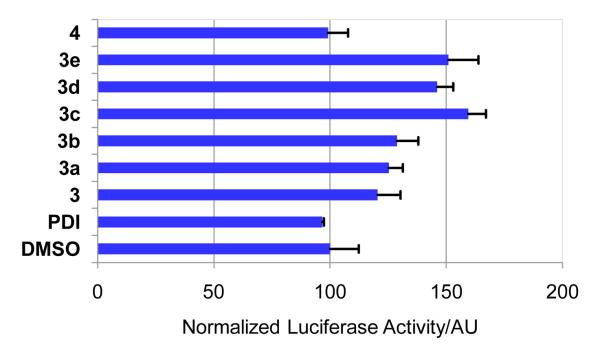
To assess whether enhanced cellular uptake imparts in vivo activity, U2OS cells stably expressing a p53-dependent luciferase reporter was treated with 20 μM of stapled peptides along with the linear control peptides PDI and 4. We found that compared to the linear control peptides which were inactive in this assay, all positively charged stapled peptides showed modest, yet reproducible activity in luciferase activation (Figure 3). Stapled peptide 3c showed the highest activity, giving rise to 1.6-fold increase in luciferase activation, which is in good agreement with its excellent cellular uptake (Figure 2). By comparison, treatment of 5 μM of nutlin-3, a cell-permeable small-molecule Mdm2 inhibitor, gave rise to 4-fold increase in luciferase activation, higher than all stapled peptides. Taken together, despite higher in vitro activity (Table 1), a large fraction of stapled peptides appear to get trapped in the endosomes and likely degraded before they can reach their targets.
Figure 3.
The p53-dependent luciferase transcriptional activation in U2OS cells: The luciferase activities were measured at least three times and the averaged activities along with standard deviations were plotted.
In summary, we have demonstrated a facile synthesis of stapled peptide dual inhibitors of the Mdm2/Mdmx interactions using a photoinduced cycloaddition reaction. Compared to PDI, stapled PDIs showed significantly higher inhibitory activities: up to sevenfold against Mdm2 and 2.5-fold against Mdmx. The inhibitory activity was found to depend on stapling site, charge number and distribution, and overall hydrophobicity. Moreover, the positively charged, stapled peptides showed enhanced cellular uptake along with modest in vivo activity.
Supplementary Material
Acknowledgments
We gratefully acknowledge the Pardee Foundation and the National Institutes of Health (GM 085092 to Q.L.; CA 109636 and 118210 to J.C.) for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
Supplemental table, scheme and figures, experimental procedures, and characterization of all peptides.
References and notes
- 1.Kabsch W, Sander C. Biopolymers. 1983;22:2577. doi: 10.1002/bip.360221211. [DOI] [PubMed] [Google Scholar]
- 2.(a) Meador WE, Means AR, Quiocho FA. Science. 1992;257:1251. doi: 10.1126/science.1519061. [DOI] [PubMed] [Google Scholar]; (b) Kussie PH, Gorina S, Marechal V, Elenbaas B, Moreau J, Levine AJ, Pavletich NP. Science. 1996;274:948. doi: 10.1126/science.274.5289.948. [DOI] [PubMed] [Google Scholar]; (c) Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Science. 1997;275:983. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 3.Jackson DY, King DS, Chmielewski J, Singh S, Schultz PG. J. Am. Chem. Soc. 1991;113:9391. [Google Scholar]
- 4.(a) Bracken C, Gulyas J, Taylor JW, Baum J. J. Am. Chem. Soc. 1994;116:6431. [Google Scholar]; (b) Phelan CJ, Skelton NJ, Braisted AC, McDowell RS. J. Am. Chem. Soc. 1997;119:455. [Google Scholar]; (c) Sia SK, Carr PA, Cochran AG, Malashkevich VN, Kim PS. Proc. Natl. Acad. Sci. USA. 2002;99:14664. doi: 10.1073/pnas.232566599. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Harrison RS, Shepherd NE, Hoang HN, Ruiz-Gomez G, Hill TA, Driver RW, Desai VS, Young PR, Abbenante G, Fairlie DP. Proc. Natl. Acad. Sci. USA. 2010;107:11686. doi: 10.1073/pnas.1002498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Blackwell HE, Grubbs RH. Angew. Chem. Int. Ed. 1998;37:3281. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3281::AID-ANIE3281>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]; (b) Schafmeister CE, Po J, Verdine GL. J. Am. Chem. Soc. 2000;122:5891. [Google Scholar]; (c) Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Bernal F, Tyler AF, Korsmeyer SJ, Walensky LD, Verdine GL. J. Am. Chem. Soc. 2007;129:2456. doi: 10.1021/ja0693587. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, Walensky LD. Nature. 2008;455:1076. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Moellering RE, Cornejo M, Davis TN, Del Bianco C, Aster JC, Blacklow SC, Kung AL, Gilliland DG, Verdine GL, Bradner JE. Nature. 2009;462:182. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Bautista AD, Appelbaum JS, Craig CJ, Michel J, Schepartz A. J. Am. Chem. Soc. 2010;132:2904. doi: 10.1021/ja910715u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madden MM, Rivera Vera CI, Song W, Lin Q. Chem. Commun. 2009:5588. doi: 10.1039/b912094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Wu X, Bayle JH, Olson D, Levine AJ. Genes Dev. 1993;7:1126. doi: 10.1101/gad.7.7a.1126. [DOI] [PubMed] [Google Scholar]; (b) Levine AJ. Cell. 1997;88:323. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 8.(a) Tanimura S, Ohtsuka S, Mitsui K, Shirouzu K, Yoshimura A, Ohtsubo M. FEBS Lett. 1999;447:5. doi: 10.1016/s0014-5793(99)00254-9. [DOI] [PubMed] [Google Scholar]; (b) Sharp DA, Kratowicz SA, Sank MJ, George DL. J. Biol. Chem. 1999;274:38189. doi: 10.1074/jbc.274.53.38189. [DOI] [PubMed] [Google Scholar]; (c) Linares LK, Hengstermann A, Ciechanover A, Muller S, Scheffner M. Proc. Natl. Acad. Sci. USA. 2003;100:12009. doi: 10.1073/pnas.2030930100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven v. O. W., Hateboer G, van der Eb AJ, Jochemsen AG. EMBO J. 1996;15:5349. [PMC free article] [PubMed] [Google Scholar]
- 9.Toledo F, Wahl GM. Nat. Rev. Cancer. 2006;6:909. doi: 10.1038/nrc2012. [DOI] [PubMed] [Google Scholar]
- 10.Hu B, Gilkes DM, Chen J. Cancer Res. 2007;67:8810. doi: 10.1158/0008-5472.CAN-07-1140. [DOI] [PubMed] [Google Scholar]
- 11.(a) Czarna A, Popowicz GM, Pecak A, Wolf S, Dubin G, Holak TA. Cell Cycle. 2009;8:1176. doi: 10.4161/cc.8.8.8185. [DOI] [PubMed] [Google Scholar]; (b) Phan J, Li Z, Kasprzak A, Li B, Sebti S, Guida W, Schönbrunn E, Chen J. J. Biol. Chem. 2010;285:2174. doi: 10.1074/jbc.M109.073056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Our molecular dynamic simulation revealed that the pyrazoline cross-linker shows a bimodal distribution, with the terminal C-C distance spanning from 4.0 Å to 11.6 Å, making it suitable for both i, i+4 and i, i+7 stapling: See Figure S5 in Supplemental Material for details.
- 13.Smith BA, Daniels DS, Coplin AE, Jordan GE, McGregor LM, Schepartz A. J. Am. Chem. Soc. 2008;130:2948. doi: 10.1021/ja800074v. [DOI] [PubMed] [Google Scholar]
- 14.(a) García-Echeverría C, Chène P, Blommers MJ, Furet P. J. Med. Chem. 2000;43:3205. doi: 10.1021/jm990966p. [DOI] [PubMed] [Google Scholar]; (b) Kallen J, Goepfert A, Blechschmidt A, Izaac A, Geiser M, Tavares G, Ramage P, Furet P, Masura K, Lisztwan J. J. Biol. Chem. 2009;284:8812. doi: 10.1074/jbc.M809096200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bird GH, Bernal F, Pitter K, Walensky LD. Methods Enzymol. 2008;446:369. doi: 10.1016/S0076-6879(08)01622-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.