Abstract
Kinesin-related Cin8p is the most important spindle-pole-separating motor in Saccharomyces cerevisiae but is not essential for cell viability. We identified 20 genes whose products are specifically required by cell deficient for Cin8p. All are associated with mitotic roles and represent at least four different functional pathways. These include genes whose products act in two spindle motor pathways that overlap in function with Cin8p, the kinesin-related Kip1p pathway and the cytoplasmic dynein pathway. In addition, genes required for mitotic spindle checkpoint function and for normal microtubule stability were recovered. Mutant alleles of eight genes caused phenotypes similar to dyn1 (encodes the dynein heavy chain), including a spindle-positioning defect. We provide evidence that the products of these genes function in concept with dynein. Among the dynein pathway gene products, we found homologues of the cytoplasmic dynein intermediate chain, the p150Glued subunit of the dynactin complex, and human LIS-1, required for normal brain development. These findings illustrate the complex cellular interactions exhibited by Cin8p, a member of a conserved spindle motor family.
Full text
PDF



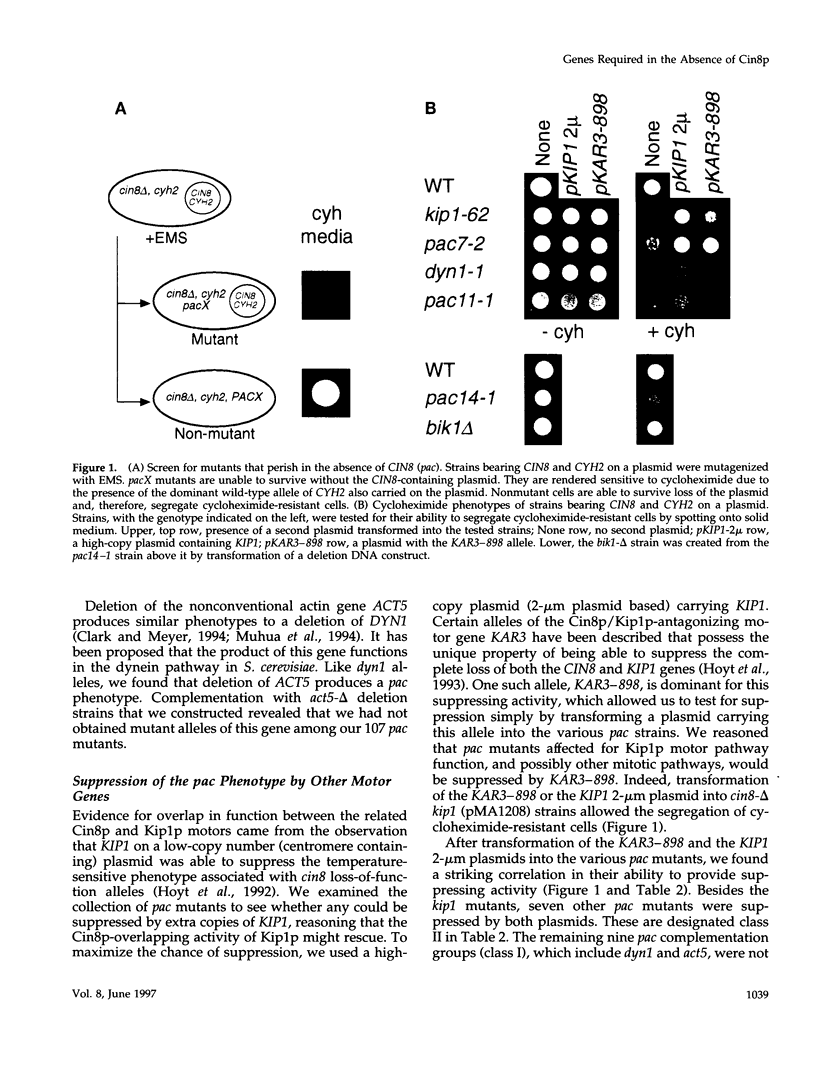
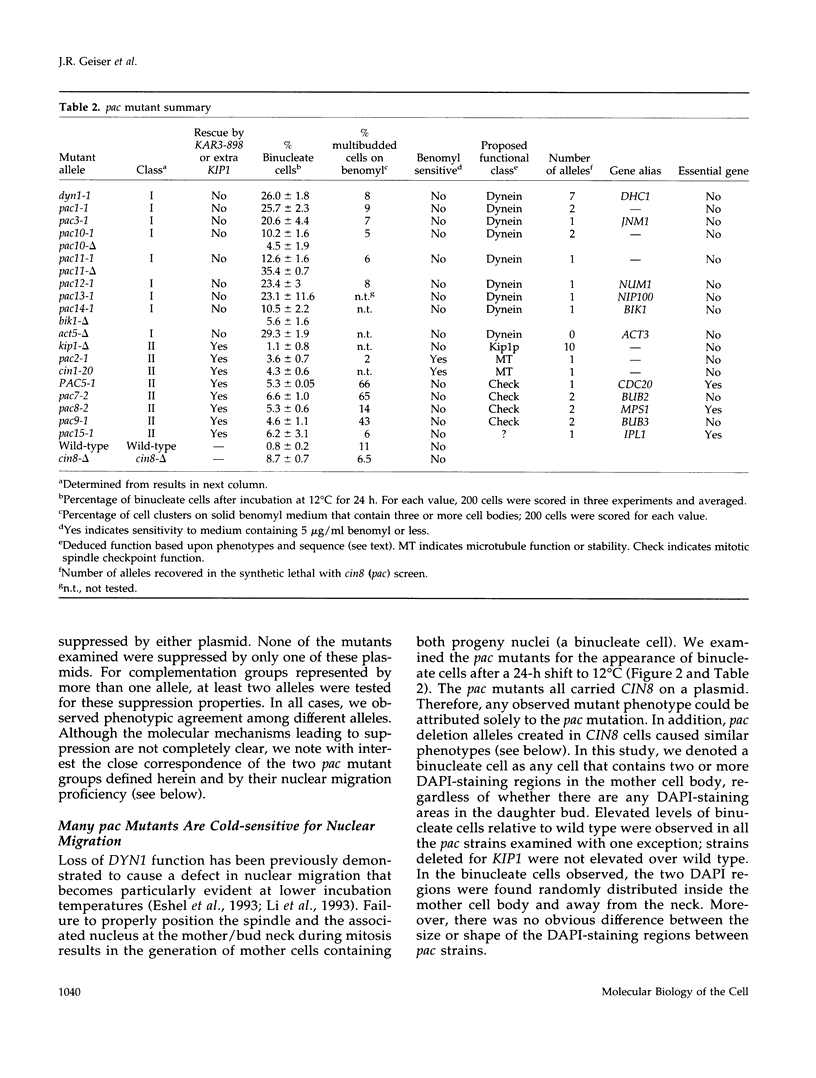
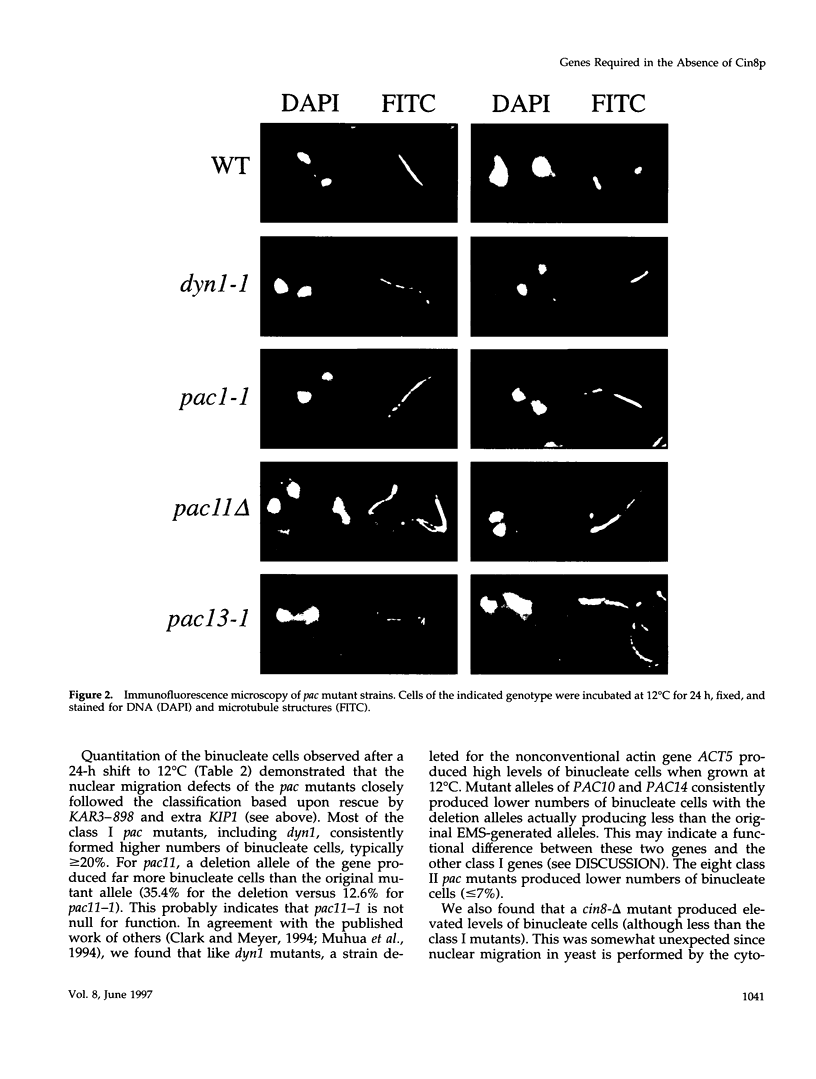








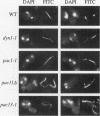
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton N. R., Goldstein L. S. Going mobile: microtubule motors and chromosome segregation. Proc Natl Acad Sci U S A. 1996 Mar 5;93(5):1735–1742. doi: 10.1073/pnas.93.5.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993 Jul 11;21(14):3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin V., Styles C. A., Fink G. R. BIK1, a protein required for microtubule function during mating and mitosis in Saccharomyces cerevisiae, colocalizes with tubulin. J Cell Biol. 1990 Dec;111(6 Pt 1):2573–2586. doi: 10.1083/jcb.111.6.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blangy A., Lane H. A., d'Hérin P., Harper M., Kress M., Nigg E. A. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell. 1995 Dec 29;83(7):1159–1169. doi: 10.1016/0092-8674(95)90142-6. [DOI] [PubMed] [Google Scholar]
- Book K. J., Howard R., Morest D. K. Direct observation in vitro of how neuroblasts migrate: medulla and cochleovestibular ganglion of the chick embryo. Exp Neurol. 1991 Feb;111(2):228–243. doi: 10.1016/0014-4886(91)90011-z. [DOI] [PubMed] [Google Scholar]
- Book K. J., Morest D. K. Migration of neuroblasts by perikaryal translocation: role of cellular elongation and axonal outgrowth in the acoustic nuclei of the chick embryo medulla. J Comp Neurol. 1990 Jul 1;297(1):55–76. doi: 10.1002/cne.902970105. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Botstein D. Isolation and characterization of chromosome-gain and increase-in-ploidy mutants in yeast. Genetics. 1993 Nov;135(3):677–691. doi: 10.1093/genetics/135.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark S. W., Meyer D. I. ACT3: a putative centractin homologue in S. cerevisiae is required for proper orientation of the mitotic spindle. J Cell Biol. 1994 Oct;127(1):129–138. doi: 10.1083/jcb.127.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole D. G., Saxton W. M., Sheehan K. B., Scholey J. M. A "slow" homotetrameric kinesin-related motor protein purified from Drosophila embryos. J Biol Chem. 1994 Sep 16;269(37):22913–22916. [PMC free article] [PubMed] [Google Scholar]
- Dobyns W. B., Reiner O., Carrozzo R., Ledbetter D. H. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993 Dec 15;270(23):2838–2842. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- Endow S. A., Kang S. J., Satterwhite L. L., Rose M. D., Skeen V. P., Salmon E. D. Yeast Kar3 is a minus-end microtubule motor protein that destabilizes microtubules preferentially at the minus ends. EMBO J. 1994 Jun 1;13(11):2708–2713. doi: 10.1002/j.1460-2075.1994.tb06561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enos A. P., Morris N. R. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell. 1990 Mar 23;60(6):1019–1027. doi: 10.1016/0092-8674(90)90350-n. [DOI] [PubMed] [Google Scholar]
- Eshel D., Urrestarazu L. A., Vissers S., Jauniaux J. C., van Vliet-Reedijk J. C., Planta R. J., Gibbons I. R. Cytoplasmic dynein is required for normal nuclear segregation in yeast. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11172–11176. doi: 10.1073/pnas.90.23.11172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkasovsky M., Küntzel H. Yeast Num1p associates with the mother cell cortex during S/G2 phase and affects microtubular functions. J Cell Biol. 1995 Nov;131(4):1003–1014. doi: 10.1083/jcb.131.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Francisco L., Wang W., Chan C. S. Type 1 protein phosphatase acts in opposition to IpL1 protein kinase in regulating yeast chromosome segregation. Mol Cell Biol. 1994 Jul;14(7):4731–4740. doi: 10.1128/mcb.14.7.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Synthetic enhancement in gene interaction: a genetic tool come of age. Trends Genet. 1993 Oct;9(10):362–366. doi: 10.1016/0168-9525(93)90042-g. [DOI] [PubMed] [Google Scholar]
- Hagan I., Yanagida M. Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature. 1992 Mar 5;356(6364):74–76. doi: 10.1038/356074a0. [DOI] [PubMed] [Google Scholar]
- Hagan I., Yanagida M. Novel potential mitotic motor protein encoded by the fission yeast cut7+ gene. Nature. 1990 Oct 11;347(6293):563–566. doi: 10.1038/347563a0. [DOI] [PubMed] [Google Scholar]
- Hager G., Dodt H. U., Zieglgänsberger W., Liesi P. Novel forms of neuronal migration in the rat cerebellum. J Neurosci Res. 1995 Feb 1;40(2):207–219. doi: 10.1002/jnr.490400209. [DOI] [PubMed] [Google Scholar]
- Hardwick K. G., Murray A. W. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J Cell Biol. 1995 Nov;131(3):709–720. doi: 10.1083/jcb.131.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck M. M., Pereira A., Pesavento P., Yannoni Y., Spradling A. C., Goldstein L. S. The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J Cell Biol. 1993 Nov;123(3):665–679. doi: 10.1083/jcb.123.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A. Cellular roles of kinesin and related proteins. Curr Opin Cell Biol. 1994 Feb;6(1):63–68. doi: 10.1016/0955-0674(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., Geiser J. R. Genetic analysis of the mitotic spindle. Annu Rev Genet. 1996;30:7–33. doi: 10.1146/annurev.genet.30.1.7. [DOI] [PubMed] [Google Scholar]
- Hoyt M. A., He L., Loo K. K., Saunders W. S. Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J Cell Biol. 1992 Jul;118(1):109–120. doi: 10.1083/jcb.118.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., He L., Totis L., Saunders W. S. Loss of function of Saccharomyces cerevisiae kinesin-related CIN8 and KIP1 is suppressed by KAR3 motor domain mutations. Genetics. 1993 Sep;135(1):35–44. doi: 10.1093/genetics/135.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Stearns T., Botstein D. Chromosome instability mutants of Saccharomyces cerevisiae that are defective in microtubule-mediated processes. Mol Cell Biol. 1990 Jan;10(1):223–234. doi: 10.1128/mcb.10.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyt M. A., Totis L., Roberts B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell. 1991 Aug 9;66(3):507–517. doi: 10.1016/0092-8674(81)90014-3. [DOI] [PubMed] [Google Scholar]
- Huffaker T. C., Hoyt M. A., Botstein D. Genetic analysis of the yeast cytoskeleton. Annu Rev Genet. 1987;21:259–284. doi: 10.1146/annurev.ge.21.120187.001355. [DOI] [PubMed] [Google Scholar]
- Kashina A. S., Baskin R. J., Cole D. G., Wedaman K. P., Saxton W. M., Scholey J. M. A bipolar kinesin. Nature. 1996 Jan 18;379(6562):270–272. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashina A. S., Scholey J. M., Leszyk J. D., Saxton W. M. An essential bipolar mitotic motor. Nature. 1996 Nov 21;384(6606):225–225. doi: 10.1038/384225a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kormanec J., Schaaff-Gerstenschläger I., Zimmermann F. K., Perecko D., Küntzel H. Nuclear migration in Saccharomyces cerevisiae is controlled by the highly repetitive 313 kDa NUM1 protein. Mol Gen Genet. 1991 Nov;230(1-2):277–287. doi: 10.1007/BF00290678. [DOI] [PubMed] [Google Scholar]
- Lauzé E., Stoelcker B., Luca F. C., Weiss E., Schutz A. R., Winey M. Yeast spindle pole body duplication gene MPS1 encodes an essential dual specificity protein kinase. EMBO J. 1995 Apr 18;14(8):1655–1663. doi: 10.1002/j.1460-2075.1995.tb07154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Murray A. W. Feedback control of mitosis in budding yeast. Cell. 1991 Aug 9;66(3):519–531. doi: 10.1016/0092-8674(81)90015-5. [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Yeh E., Hays T., Bloom K. Disruption of mitotic spindle orientation in a yeast dynein mutant. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10096–10100. doi: 10.1073/pnas.90.21.10096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupas A., Van Dyke M., Stock J. Predicting coiled coils from protein sequences. Science. 1991 May 24;252(5009):1162–1164. doi: 10.1126/science.252.5009.1162. [DOI] [PubMed] [Google Scholar]
- McMillan J. N., Tatchell K. The JNM1 gene in the yeast Saccharomyces cerevisiae is required for nuclear migration and spindle orientation during the mitotic cell cycle. J Cell Biol. 1994 Apr;125(1):143–158. doi: 10.1083/jcb.125.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R., Xiang X., Beckwith S. M. Nuclear migration advances in fungi. Trends Cell Biol. 1995 Jul;5(7):278–282. doi: 10.1016/s0962-8924(00)89039-x. [DOI] [PubMed] [Google Scholar]
- Muhua L., Karpova T. S., Cooper J. A. A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration. Cell. 1994 Aug 26;78(4):669–679. doi: 10.1016/0092-8674(94)90531-2. [DOI] [PubMed] [Google Scholar]
- Neer E. J., Schmidt C. J., Nambudripad R., Smith T. F. The ancient regulatory-protein family of WD-repeat proteins. Nature. 1994 Sep 22;371(6495):297–300. doi: 10.1038/371297a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. E., Sullivan D. S., Huffaker T., Koshland D. Role of astral microtubules and actin in spindle orientation and migration in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 1992 Nov;119(3):583–593. doi: 10.1083/jcb.119.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Mikami A., Pfister K. K., Vallee R. B. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J Cell Biol. 1992 Sep;118(5):1133–1143. doi: 10.1083/jcb.118.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plamann M., Minke P. F., Tinsley J. H., Bruno K. S. Cytoplasmic dynein and actin-related protein Arp1 are required for normal nuclear distribution in filamentous fungi. J Cell Biol. 1994 Oct;127(1):139–149. doi: 10.1083/jcb.127.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poch O., Schwob E., de Fraipont F., Camasses A., Bordonné R., Martin R. P. RPK1, an essential yeast protein kinase involved in the regulation of the onset of mitosis, shows homology to mammalian dual-specificity kinases. Mol Gen Genet. 1994 Jun 15;243(6):641–653. doi: 10.1007/BF00279573. [DOI] [PubMed] [Google Scholar]
- Reiner O., Carrozzo R., Shen Y., Wehnert M., Faustinella F., Dobyns W. B., Caskey C. T., Ledbetter D. H. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993 Aug 19;364(6439):717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Riehemann K., Sorg C. Sequence homologies between four cytoskeleton-associated proteins. Trends Biochem Sci. 1993 Mar;18(3):82–83. doi: 10.1016/0968-0004(93)90159-k. [DOI] [PubMed] [Google Scholar]
- Roberts B. T., Farr K. A., Hoyt M. A. The Saccharomyces cerevisiae checkpoint gene BUB1 encodes a novel protein kinase. Mol Cell Biol. 1994 Dec;14(12):8282–8291. doi: 10.1128/mcb.14.12.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof D. M., Meluh P. B., Rose M. D. Kinesin-related proteins required for assembly of the mitotic spindle. J Cell Biol. 1992 Jul;118(1):95–108. doi: 10.1083/jcb.118.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M. D., Novick P., Thomas J. H., Botstein D., Fink G. R. A Saccharomyces cerevisiae genomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60(2-3):237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Saunders W. S., Hoyt M. A. Kinesin-related proteins required for structural integrity of the mitotic spindle. Cell. 1992 Aug 7;70(3):451–458. doi: 10.1016/0092-8674(92)90169-d. [DOI] [PubMed] [Google Scholar]
- Saunders W. S., Koshland D., Eshel D., Gibbons I. R., Hoyt M. A. Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. J Cell Biol. 1995 Feb;128(4):617–624. doi: 10.1083/jcb.128.4.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin K. E., LeGuellec K., Philippe M., Mitchison T. J. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature. 1992 Oct 8;359(6395):540–543. doi: 10.1038/359540a0. [DOI] [PubMed] [Google Scholar]
- Sawin K. E., Mitchison T. J. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4289–4293. doi: 10.1073/pnas.92.10.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stearns T., Hoyt M. A., Botstein D. Yeast mutants sensitive to antimicrotubule drugs define three genes that affect microtubule function. Genetics. 1990 Feb;124(2):251–262. doi: 10.1093/genetics/124.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. S., Huffaker T. C. Astral microtubules are not required for anaphase B in Saccharomyces cerevisiae. J Cell Biol. 1992 Oct;119(2):379–388. doi: 10.1083/jcb.119.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinsley J. H., Minke P. F., Bruno K. S., Plamann M. p150Glued, the largest subunit of the dynactin complex, is nonessential in Neurospora but required for nuclear distribution. Mol Biol Cell. 1996 May;7(5):731–742. doi: 10.1091/mbc.7.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya H., Iseda T., Hino O. Identification of a novel protein (VBP-1) binding to the von Hippel-Lindau (VHL) tumor suppressor gene product. Cancer Res. 1996 Jul 1;56(13):2881–2885. [PubMed] [Google Scholar]
- Vaughan K. T., Vallee R. B. Cytoplasmic dynein binds dynactin through a direct interaction between the intermediate chains and p150Glued. J Cell Biol. 1995 Dec;131(6 Pt 1):1507–1516. doi: 10.1083/jcb.131.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak C. E., Mitchison T. J., Desai A. XKCM1: a Xenopus kinesin-related protein that regulates microtubule dynamics during mitotic spindle assembly. Cell. 1996 Jan 12;84(1):37–47. doi: 10.1016/s0092-8674(00)80991-5. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C. M., Karki S., Holzbaur E. L. The p150Glued component of the dynactin complex binds to both microtubules and the actin-related protein centractin (Arp-1). Proc Natl Acad Sci U S A. 1995 Feb 28;92(5):1634–1638. doi: 10.1073/pnas.92.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Winey M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J Cell Biol. 1996 Jan;132(1-2):111–123. doi: 10.1083/jcb.132.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson C. G., King S. M., Koutoulis A., Pazour G. J., Witman G. B. The 78,000 M(r) intermediate chain of Chlamydomonas outer arm dynein isa WD-repeat protein required for arm assembly. J Cell Biol. 1995 Apr;129(1):169–178. doi: 10.1083/jcb.129.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Beckwith S. M., Morris N. R. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2100–2104. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang X., Osmani A. H., Osmani S. A., Xin M., Morris N. R. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol Biol Cell. 1995 Mar;6(3):297–310. doi: 10.1091/mbc.6.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh E., Skibbens R. V., Cheng J. W., Salmon E. D., Bloom K. Spindle dynamics and cell cycle regulation of dynein in the budding yeast, Saccharomyces cerevisiae. J Cell Biol. 1995 Aug;130(3):687–700. doi: 10.1083/jcb.130.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]