Abstract
Various sebum free fatty acids (FFAs) have shown antibacterial activity against a broad range of Gram-positive bacteria, resulting in the suggestion that they are accountable, at least partially, for the direct antimicrobial activity of the skin surface. In this study, we examined the effects of sebum FFAs on the antimicrobial peptide (AMP)-mediated innate immune defense of human sebocytes. Incubation of lauric acid, palmitic acid, or oleic acid (OA) with human sebocytes dramatically enhanced their expression of human β-defensin (hBD)-2, one of the predominant AMPs found in the skin, whereas remarkable increases in hBD-1, hBD-3, and human cathelicidin LL-37 were not observed. Secreted hBD-2 was detectable by western blotting in the supernatant of sebocyte culture incubated with each FFA, but not with a vehicle control. The supernatant of FFA-incubated sebocyte culture showed antimicrobial activity against Propionibacterium acnes, whereas the enhanced antimicrobial activity of human sebocytes was neutralized by anti-hBD-2 IgG. In addition, the FFA-induced hBD-2 expression was suppressed by blocking the cluster of differentiation (CD)36 fatty acid translocase on the surface of sebocytes with anti-human CD36 IgG or blocking the NF-κB signaling pathway with BMS-345541, a highly selective inhibitor of inhibitory κB kinase. These data suggest that sebum FFAs upregulate the expression of hBD-2 in human sebocytes, which may enhance the disinfecting activity of the human sebaceous gland. The FFA-induced upregulation of hBD-2 is facilitated by CD36-mediated FFA uptake and NF-κB-mediated transactivation. The upregulation of mouse β-defensin 4, a mouse ortholog for hBD-2, was also observed in the hair follicle sebaceous glands of mouse ear skin after an epicutaneous application of OA, the most hBD-2-inducible FFA tested. This report highlights the potential of using FFAs as a multifunctional antimicrobial therapy agent for acne vulgaris treatment; FFAs may provide direct antibacterial activities against P. acnes and enhance the skin’s innate antibacterial defense by inducing the expression of hBD-2 in sebocytes as well.
INTRODUCTION
Propionibacterium acnes is a Gram-positive anaerobic bacterium residing in pilosebaceous follicles as a member of the resident bacterial flora in the human skin. Once it overgrows and colonizes in sebaceous hair follicles, P. acnes is pertinent to the development of inflammatory acne vulgaris, which is the most common skin disease, afflicting up to 80% of individuals throughout their lives (Nordstrom et al., 1986; Chronnell et al., 2001; Bojar and Holland, 2004). The inflammation of severe acne is initiated by disruption of the follicular epithelium due to the overgrowth of P. acnes, followed by the spread of the bacteria from the microcomedone to the dermis, in which they interact with various skin and immune cells such as keratinocytes and macrophages, triggering granulomatous reactions of inflammatory acne (Marples et al., 1971; Toyoda and Morohashi, 2001; Degitz et al., 2007). The bacteria stimulate the production of proinflammatory cytokines, including inter-leukins-1β, -8, and -12, and tumor necrosis factor-α. It is known that P. acnes-induced cytokine production is mediated by Toll-like receptor 2 (Kim et al., 2002; Kim, 2005; Nagy et al, 2006).
The skin epithelium participates in self-disinfection of the human skin surface through various modes of action. It has been widely accepted that antimicrobial peptides (AMPs) are major contributors to cutaneous innate nonspecific immunity, which is the first line of defense against invading pathogens (Nizet et al., 2001; Zasloff, 2002). Cathelicidins and β-defensins are the best-characterized AMPs found in the skin. Human β-defensin (hBD)-1 is constitutively expressed in keratinocytes, whereas hBD-2, hBD-3, and human cathelicidin LL-37 are typically upregulated in keratinocytes during inflammation and then accumulate in the skin (Gallo et al., 2002; Froy, 2005). Because of their direct antimicrobial action, the secretion of these peptides provides a defense against infectious pathogens. Recent evidence has indicated that human sebaceous glands may contribute to the skin immune defense by releasing AMPs. For example, hBDs are expressed in human pilosebaceous units and their expression is upregulated in acne lesions (Chronnell et al., 2001). Cathelicidin and hBD-2 are detected in cultured human sebocytes, the predominant cells residing in the sebaceous gland, and their expression levels are upregulated in the presence of P. acnes (Nagy et al., 2006; Lee et al., 2008). Free fatty acids (FFAs) are ubiquitously found on the surface of the human skin and are the predominant components in human sebum. FFAs are produced via hydrolysis of their precursors, sebum triacyl-glycerides secreted from sebaceous glands, by lipases that are secreted from commensal bacterial flora such as P. acnes and Staphylococcus epidermidis (Marples et al., 1971; Holland et al., 1981; Gotz et al., 1998); they can also be produced by sebocytes in the absence of bacteria (Fujie et al., 1996; Zouboulis et al., 1999). Because various FFAs have shown antibacterial activity against a broad range of Gram-positive bacteria in vitro, FFAs, together with AMPs, have been considered to be responsible for at least a part of the innate immune defense of the skin surface against microbial colonization and infection (Drake et al., 2008), Therefore, sebaceous glands may have a very significant role in skin self-disinfection by both providing antimicrobial agents to the external skin surface and contributing to the cutaneous innate immune defense.
Recently, we demonstrated that lauric acid (LA) (C12:0), one of the sebum FFAs (Bodoprost and Rosemeyer, 2007), has strong antimicrobial activity in vitro against skin bacteria, including P. acnes. Topical application or intradermal injection of LA in vivo shows remarkable therapeutic effectiveness against P. acnes-induced inflammation and significant reduction in the number of bacteria (Nakatsuji et al., 2009). In this study, we examined the effects of sebum FFAs on the AMP-mediated cutaneous innate immune defense of human sebocytes and mouse skin. We found that LA, palmitic acid (PA; 16:0), and oleic acid (OA; C18:1, cis-9), which are the typical FFAs found in human sebum, enhanced the hBD-2 expression and antimicrobial activity of human sebocytes against P. acnes. Our data suggest that sebum FFAs are involved in the disinfecting activity of the human skin both through their direct antimicrobial characteristics and by inducing AMPs in human sebocytes to enhance their innate immune defense ability.
RESULTS
FFA-induced increase of AMP expression in human sebocytes
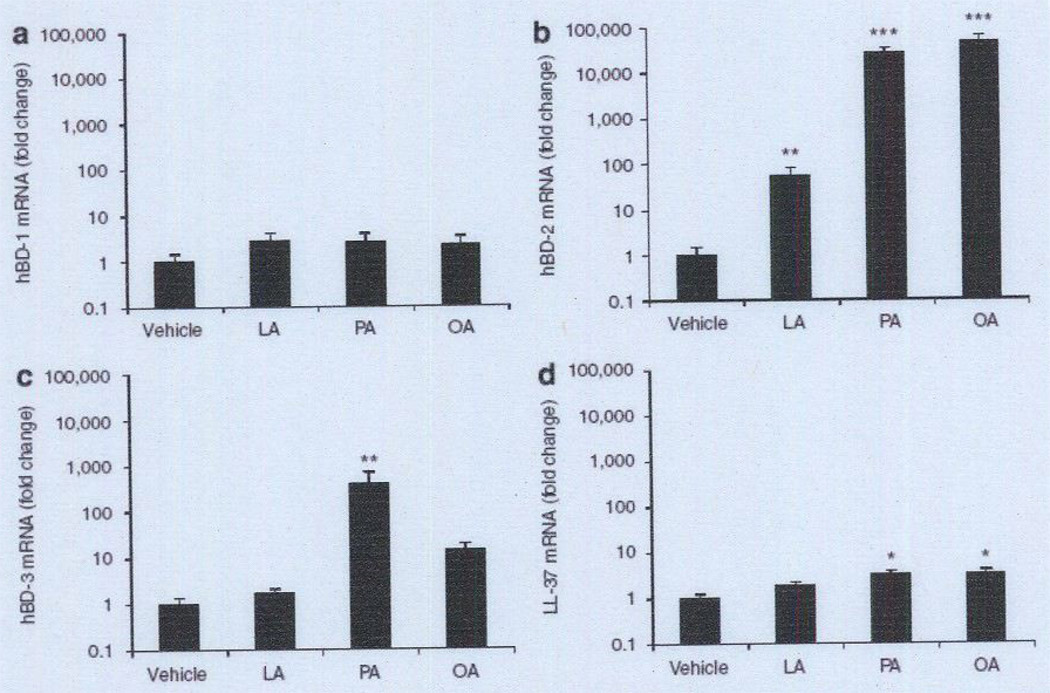
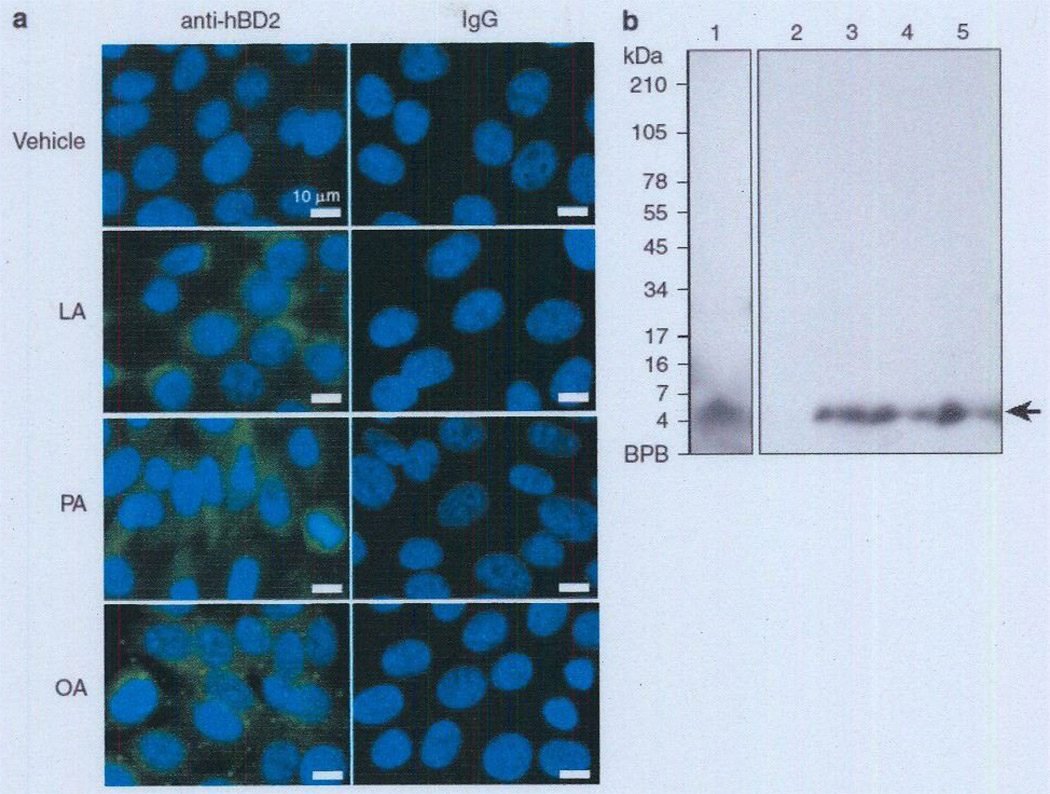
To examine the effects of sebum-containing FFAs on the expression of the predominant AMPs in the human skin, such as hBD-1, -2, and -3 and human cathelicidin LL-37, human SZ95 sebocytes were incubated with LA, PA, or OA for 24 hours. After incubation, we first demonstrated that FFAs had a negligible effect on the cell viability of human SZ95 sebocytes (see Supplementary Figure S1 online). We further found that FFAs did not enhance hBD-1 expression in comparison with vehicle contral (Figure 1a). In contrast, hBD-2 expression was remarkably enhanced by incubation with LA (54-fold), PA (25,170-fold), or OA (45,260-fold) (Figure 1b). hBD-3 expression was significantly enhanced by PA (382-fold), but not by LA or OA (Figure 1c). Cathelicidin expression was slightly enhanced by PA (3.3-fold) and OA (3.3-fold), but not by LA. Overall, the hBD-2 expression level was upregulated most when human sebocytes were incubated with the FFAs. An increase in the amount of hBD-2 peptide in SZ95 sebocytes after incubation with FFAs was further confirmed by immunofluorescent staining with anti-hBD-2 IgG (Figure 2a). In addition, an increase in hBD-2 secreted into the culture medium was confirmed by western blotting. A single band was detected at the expected size (5 kDa) after incubation with each FFA, whereas no bands were detected in the vehicle control, indicating that the basal secretion level of hBD-2 from the cell line is very low (Figure 2b), These data suggest the AMP inducibility of LA, PA, and OA in human sebocytes.
Figure 1. Effect of FFAs on the antimicrobial peptide expression in human sebocytes.
Immortalized human sebocytes, SZ95 (1 × l06 per well), were incubated with LA, PA, or OA (25 µg ml−1) in 1% FBS-Sebomed containing 0.5% (w/v) DMSO for 24 hours at 37 °C. The control received an equal amount of DMSO. (a–d) mRNA expression of hBD-1 (a), hBD-2 (b). hBD-3 (c), and human cathelicidin LL-37 (d) was evaluated by real-time qPCR, normalized to that of GAPDH. and then plotted as relative expression compared with that of vehicle-treated cells. Data represent means ± SE of four individual experiments (*P<0.05, **P <0.01, ***P<0.005 by Student’s t-test vs. vehicle control). FBS, fetal bovine serum; FFA, free fatty acid; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; hBD, human β-defensin; LA, lauric acid; OA, oleic acid; PA, palmitic acid; qPCR. quantitative PCR.
Figure 2. FFA-enhanced hBD-2 peptide level in human sebocytes.
(a) Immortalized human sebocytes, SZ95 (2 × 106 per well), were incubated with LA, PA, or OA (25 µg ml−1) in 1% FBS-Sebomed containing 0.5% (w/v) DMSO for 24 hours at 37 °C. The control received an equal amount of DMSO. After incubation, the cells were stained with rabbit anti-hBD-2, followed by FITC-labeled anti-rabbit IgCs. The nuclei were counterstained with DAPI. Bar = 10 µm. (b) To detect secreted hBD-2 from SZ95 sebocytes by western blotting, the supernatants of the cell culture media were concentrated using a Sep-Pak cartridge as described in the Materials and Methods section. The reconstituted samples (400 µl equivalent of medium) from cell culture media, incubated with vehicle, LA, PA, and OA (lanes 2–5. respectively), were separated by SDS-PACE (16% acrylamide). Synthetic hBD-2 (60 nmol) was used as a positive control (lane 1) for detection of hBD-2. hBD-2 was detected by rabbit anti-hBD-2 IgCs. Data are representative of three separate experiments with similar results. Lane 1 and lanes 2–5 are from the same original gel. Because of different film exposure times, two images were obtained and later merged again. DAPI, 40′-6-diamidino-2-phenylindole; FBS, fetal bovine serum; FFA, free fatty acid; hBD-2, human (β-defensin-2; LA, lauric acid; OA, oleic acid; PA, palmitic acid.
Neutralization of FFA-induced antimicrobial activity of human sebocytes with anti-hBD-2 IgG
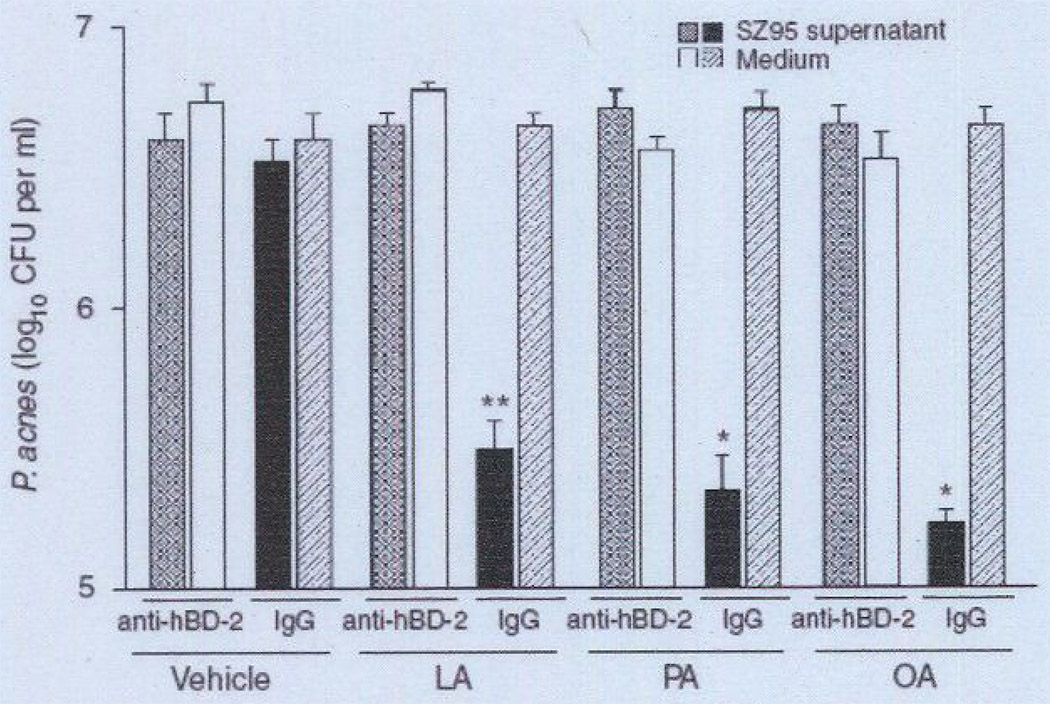
We first incubated human SZ95 sebocytes with LA, PA, or OA at 25 µg ml−1 for 24 hours to enhance their AMP expression. Separate experiments were conducted by incubating FFAs at various concentrations (0–100 µg ml−1) with P. acnes to confirm that FFAs alone at 25 µg ml−1 do not exert antimicrobial activity against P. acnes (see Supplementary Figure S2). To examine the antimicrobial activity of FFA itself contained in the medium, the FFA-conditioned medium was incubated without sebocytes. Next, we tried to neutralize the hBD-2-mediated antimicrobial activity of the FFA-treated human sebocytes. To this end, the supernatant of the culture medium of the FFA-treated sebocytes or the FFA-conditioned medium was preincubated with anti-hBD-2 IgG for 2 hours to produce a mixture solution; normal IgG was used as control. The antimicrobial activity of the mixture solution was then evaluated against P. acnes using a solution killing assay. The bacteria were incubated in the mixture solution for 5 hours at 37 °C and then diluted with PBS and spotted on an agar plate for the counting of colony-forming units (CFUs). As shown in Figure 3, the supernatant that was preincubated with normal IgG significantly reduced the number of P. acnes bacteria as compared with the corresponding control medium containing FFAs (Figure 3). However, the supernatant preincubated with anti-hBD-2 IgG neutralized the antimicrobial activity of the supernatant (Figure 3). These data suggest that hBD-2 is involved in the FFA-enhanced antimicrobial activity of human sebocytes.
Figure 3. Neutralization of FFA-induced antimicrobial activity of human sebocytes with anti-hBD-2 IgG.
Immortalized human sebocytes, SZ95 (2 × 106 per well), were incubated with LA, PA, or OA (25 µg ml−1) in 1% FBS-Sebomed containing 0.5% (w/v) DMSO for 24 hours at 37 °C. An equal amount of DMSO was used as vehicle control. To examine the antimicrobial activity of each FFA contained in the medium, the FFA-conditioned medium was incubated without sebocytes. After incubation, the supermatant of the cell culture was filtrated to remove cell debris and then preincubated with rabbit anti-hBD-2 IgG or normal rabbit IgG for 2 hours at 37 °C. The mixture was incubated with Propionibacterium acnes (1 × 106 CFU per ml) for 5 hours at 37 °C under anaerobic conditions. After incubation, the P. acnes suspension was diluted 1:1–1:104 with PBS. A volume of 5 µl of the diluted solution was spotted on a Brucella broth agar plate supplemented with 5% defibrinated sheep blood and hemin and with vitamin K. After the liquid in the P. acnes suspension was absorbed into the agar, the plate was incubated under anaerobic conditions to quantify the CFUs of P. acnes Data represent means ± SE of three individual experiments (*P<0.05, **P <0.01 by Student0’s t-test vs. medium control). CFU, colony-forming unit: FBS, fetal bovine serum: FFA, free fatty acid; hBD-2, human β-defensin-2; LA, lauric acid; OA, oleic acid; PA, palmitic acid; PBS, phosphate-buffered saline.
Antimicrobial activity of hBD-2 on P. acnes
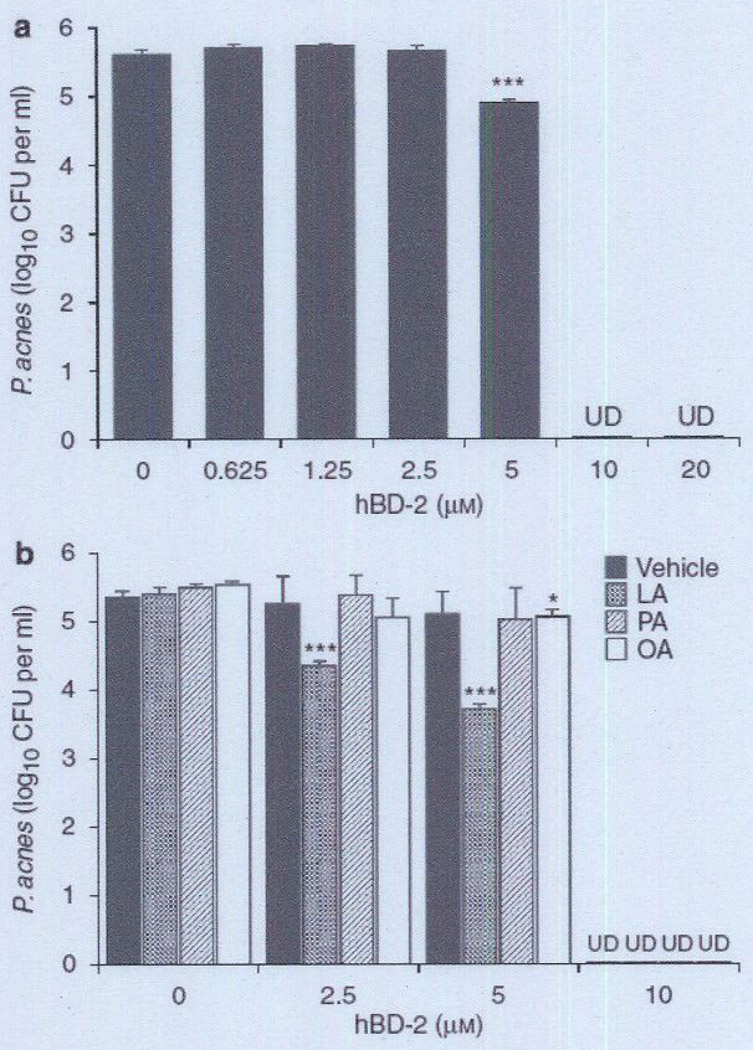
To examine the antimicrobial activity of the hBD-2 peptide on P. acnes, bacteria were incubated with several concentrations of synthetic hBD-2 for 5 hours at 37 °C. After incubation, the bacteria were diluted with PBS and spotted on an agar plate so that CFUs could be counted (Figure 4a). Strikingly, at concentrations higher than 10 µm, hBD-2 killed P. acnes. In contrast, no killing was observed when the hBD-2 concentration was 2.5 µm. Given that hBD-2 may be released from sebocytes along with FFAs in the human skin, the next experiment was conducted to examine synergistic antimicrobial activity of hBD-2 and FFAs. P. acnes was incubated with synthetic hBD-2 in combination with 25 µg ml−1 of LA, PA, or OA, a concentration at which each FFA alone does not exert antimicrobial activity (see Supplementary Figure S2). hBD-2 (2.5 and 5 µm) synergistically killed P. acnes in combination with LA, but not with PA or OA (Figure 4b), suggesting synergistic antimicrobial activity of hBD-2 and LA.
Figure 4. Bactericidal effect of synthetic hBD-2 on Propionibacterium acnes.
(a) P. acnes (1 × 106 CFU per ml) was incubated with 0–20 µm synthetic hBD-2 peptide in 20 mm phosphate buffer, pH 6.5, containing 100 mm NaCI for 5 hours under anaerobic conditions, (b) To enable examination of the synergistic antimicrobial activity of hBD-2 and FFAs, P. acnes (1 × 106 CFU per ml) was incubated with hBD-2 (0, 2.5, 5, 10 µm) in phosphate buffer in the presence of LA, PA, or OA (25 µg ml−1) on a 96-well microplate (100 µl per well) at 37 °C for 5 hours. The control received only DMSO (0.5%(v/v)) instead of FFA. After incubation, the P. acnes suspension was diluted 1:10–1:104 with PBS, and 5 µl of the diluted solution was spotted on a Brucella broth agar plate supplemented with 5% defibrinated sheep blood and hemin and with vitamin K. After the liquid in the P. acnes suspension was absorbed into the agar, the plate was incubated under anaerobic conditions to quantify the CFUs of P. acnes. Data represent means ± SE of three individual experiments (*P<0.05, ***P<0.005 by Student’s t-test). CFU, colony-forming unit; FFA, free fatty acid; hBD-2, human β-defensin-2; LA, lauric acid; OA, oleic acid: PA, palmitic acid; PBS, phosphate-buffered saline: UD, undetectable.
Detection of CD36 in human sebocytes by immunocytochemical analysis
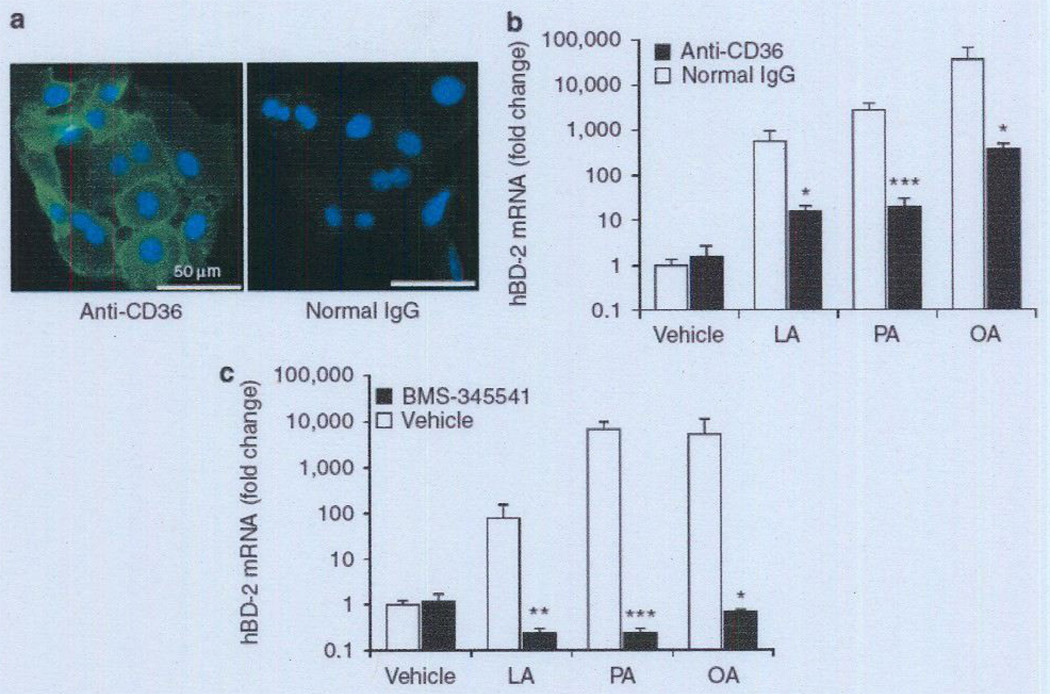
After discovering that FFAs enhance the antimicrobial activity of human sebocytes, we examined the mechanism of FFA translocation into sebocytes. Because the cluster of differentiation (CD)36 is known to be a key protein involved in regulating the uptake of FFAs across the plasma membrane in various tissues, we assessed the presence of CD36 in human SZ95 sebocytes using an immunocytochemical method. Using anti-CD36 IgG as the primary antibody, we found that immunoreactivity (green fluorescence) was widely revealed in the sebocytes, but when we used normal mouse IgG, no immunoreactivity was detected in the cells (Figure 5a). Moreover, CD36 immunoreactivity was located predominantly at the peripheral margins of cells, indicating that CD36 is a membrane-associated protein in human sebocytes.
Figure 5. FFA-induccd increase of hBD-2 expression was mediated by CD36 and the NF-κB signaling pathway.
(a) To reveal the cellular distribution of CD36 in human SZ95 sebocytes, cells were stained with mouse anti-CD36 IgG, followed by anti-mouse IgG-FITC conjugate (green) (left panel). Normal mouse IgG was used as negative control (right panel). The nuclei were counterstained with DAP1 (blue). Data are representative of four separate experiments with similar results. Bar = 50 µm. (b–c) To block the function of CD36 on the surface of SZ95 sebocytes, cells (2 × 106 per well) cultured on a 24-well plate were preincubated with mouse anti-CD36 IgG (5 µg m1−1) or normal mouse IgG in 1% FBS-Sebomed (250 µl) for 2 hours at 37 °C (b). To block NF-κB-mediated signaling in SZ95 sebocytes, the cells (2 × 106 per well) cultured on a 24-well plate were preincubated with BMS-345541 (20 µm) or with an equal amount of DMSO (0.1%) in 1% FBS-Sebomed (250 µl) for 1 hour at 37 °C (c). After preincubation, LA, PA, or OA (2.5 µg ml−1) was added to the cells, which were subsequently incubated for 24 hours at 37 °C. Control received an equal amount of DMSO (0.5% (v/v)). After incubation, the mRNA expression levels of hBD-2 were determined as described in the Materials and Methods section. Data represent means ± SE of four individual experiments (*P<0.05, **P<0.01, ***P<0.005 by Student’s t-test vs. normal IgG or vehicle control). CD36, cluster of differentiation 36; DAPI, 4′-6-diamidino-2-phenylindote; FBS, fetal bovine serum; FFA, free fatty acid; hBD-2, human β-defensin-2; LA, lauric acid; OA, oleic acid; PA, palmitic acid.
Involvement of CD36 and NF-κB signaling in FFA-enhanced hBD-2 expression
Subsequent experiments were designed to explore the connection between CD36-mediated uptake of FFAs and FFA-induced increase of hBD-2 expression. Human sebocytes were preincubated with anti-CD36 IgG or normal IgG for 2 hours. After the preincubation, LA, PA, or OA was added to the cell culture medium and subsequently incubated for 24 hours. Neither preincubation with anti-CD36 IgG nor subsequent incubation with each FFA affected the viability of sebocytes (see Supplementary Figure S3a). As shown in Figure 5, for cells preincubated with normal control IgG, the addition of LA, PA, or OA enhanced the hBD-2 expression by 561-, 2,763-, or 35,627-fold, respectively, in comparison with the vehicle control (Figure 5b). However, for cells preincubated with anti-CD36 IgG, the addition of LA, PA, or OA showed a marginal enhancement of hBD-2 expression, by 16-, 19-, or 361 -fold, respectively. These data suggest that the FFA-induced increase of hBD-2 expression in human sebocytes is mediated by CD36 on the cell surface.
Given that hBD-2 expression is facilitated by the NF-κB-mediated transactivation pathway in many cell types, we next examined the connection between the FFA-induced increase of hBD-2 expression and the NF-κB signal pathway in human SZ95 sebocytes. To block NF-κB-mediated signaling in sebocytes, cells were preincubated with BMS-345541, a highly selective inhibitor of inhibitory κB kinase(IKK), or with an equal amount of DMSO for 1 hour at 37 °C. The cells were then incubated with LA, PA, OA, or an equal amount of DMSO for 24 hours at 37 °C (Figure 5c). It was found that preincubation with BMS-345541 completely suppressed the FFA-induced increase of hBD-2 expression in human sebocytes. Neither treatment affected the viability of sebo-cytes (see Supplementary Figure S3b).
Effect of epicutaneous application of OA on mBD-4 expression in mouse skin
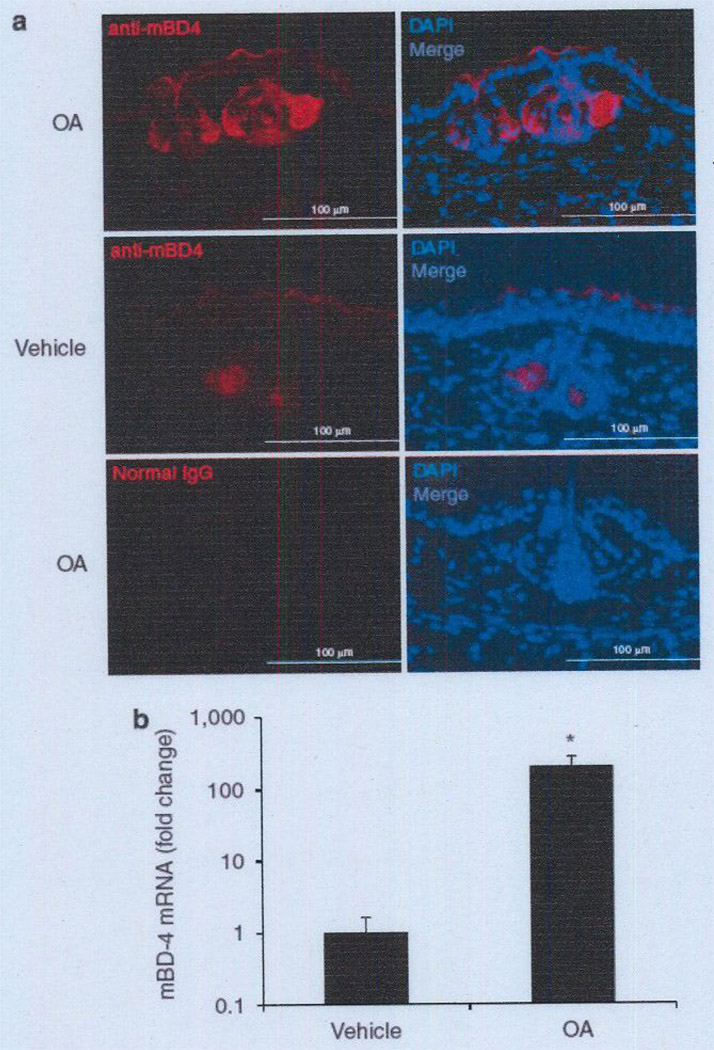
Because OA is the most hBD-2-inducible FFA tested (Figure 1), we examined its effect on the expression of mouse β-defensin-4 (mBD-4), a mouse ortholog for hBD-2 (Jia et al., 2000), in the skin of mouse ears. Immunoreactivity of mBD-4 in the hair follicle sebaceous glands of mouse ear skin was enhanced after epicutaneous application of OA, whereas little reaction was observed after vehicle treatment (Figure 6a). No immunoreactivity was found with a normal rabbit IgG used as a negative control. In addition, treatment with OA dramatically enhanced mBD-4 gene expression in mouse ear skin by 198-fold in comparison with the vehicle treatment (Figure 6b). As shown in Supplementary Figure S4, hBD-2 expression in keratinocytes was slightly enhanced by incubation with LA, PA, or OA, suggesting that sebocytes, and not keratinocytes, primarily contribute to the FFA-induced enhancement of defensin expression in the skin. All data were comparable to in vitro data using the human sebocyte cell line.
Figure 6. Effect of epicutaneous application of OA on mBD-4 expression in mouse sebaceous gland.
OA (150 µg in 5% acetone mixed with 15 mg of Vaseline) and 5% acetone mixed with 15 mg of Vaseline (vehicle) were epicutaneously applied to the left and right ears, respectively, at 0 and 12 hours. The ears were excised 12 hours after epicutaneous application. (a) Frozen sections of mouse ears were stained with rabbit anti-mBD-4 IgG, followed by goat anti-rabbit IgG-Alexa 568 conjugate (red). Normal rabbit IgG was used as negative control staining. Nuclei were stained with DAPI (blue). Bar = 100 µm. Data are representative of four separate experiments with similar results, (b) mRNA expression of mBD-4 in the ear skin was evaluated by real-time qPCR. normalized to that of GAPDH. and then plotted as relative expression compared with that of the vehicle-treated sample. Data represent means ± SE of four individual experiments (*P<0.05 by Student’s t-test vs. vehicle-treated control). DAPI, 4′-6-diamidino-2-phenylindole; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; mBD-4, mouse β-defensin-4; OA, oleic acid: qPCR, quantitative PCR.
DISCUSSION
Most microorganisms residing in the human skin, such as P. acnes and S. epidermidis, possess lipolytic activity, which is believed to be responsible for the hydrolysis of sebaceous lipids, liberating FFAs to the skin surface (Marples et al., 1971; Holland et al., 1981; Gotz et al., 1998), although genuine FFA production by human sebocytes is also possible (Fujie et al., 1996; Zouboulis et al., 1999). Because some middle- to long-chain FFAs (C8-C18) have shown antibacterial activity against a broad range of Gram-positive bacteria (Wille and Kydonieus, 2003; Georgel et al., 2005; Skrivanova et al., 2005; Drake et al., 2008), they are considered to be responsible for at least a part of the direct antimicrobial activity of the skin surface against pathogen colonization and infection. LA is a minor sebum component, but it is the most active antimicrobial FFA (Kabara et al., 1972; Kitahara et al., 2004; Rouse et al., 2005; Skrivanova et al., 2005). Our previous study has confirmed the potent antimicrobial activity of LA against P. acnes in vitro and the therapeutic potential against P. acnes-induced inflammation in vivo (Nakatsuji et al., 2009). Conversely, PA and OA, the most predominant FFAs found in human sebum (Bodoprost and Rosemeyer, 2007), did not exert potent bactericidal activity against P. acnes in vitro (data not shown). In the present study, we demonstrated that LA, PA, and OA dramatically enhanced the hBD-2 expression level in human sebocytes (Figures 1b and 2a) and the secretion of hBD-2 into the medium (Figure 2b). The supernatant of the sebocyte culture medium containing LA, PA, or OA showed significant antimicrobial activity against P. acnes when the supernatant was incubated with normal control IgG. However, the observed antimicrobial activity was neutralized when the supernatant was incubated with anti-hBD-2 IgG before its antimicrobial activity against P. acnes was evaluated (Figure 3). These results suggest that the FFA-induced antimicrobial activity of sebocytes is at least mediated by hBD-2. Consistent with our observation, Chronnell et al. (2001) found that hBD-2 expression was upregulated in acne lesions. Because increased quantities of FFAs have been observed in the skin of acne patients (Ito et al., 1996), the increase in hBD-2 expression in acne lesions might be associated with sebum FFAs. However, Nagy et al. (2005) reported that recombinant hBD-2, in concentrations of up to 2.5 µg ml−1 (0.5 µm), exerted no antimicrobial effect on the viability of any strains of P. acnes tested. We observed that synthetic hBD-2 peptide (5–20 µm) dose-dependently killed P. acnes in vitro at these higher concentrations (Figure 4a). The combination of hBD-2 and human cathelicidin LL-37, both of which are also produced in human sebocytes (Nagy et al., 2006; Lee et al., 2008), has shown synergistic antimicrobial activity against group B Streptococcus in vitro (Dorschner et al., 2003). Our group has recently demonstrated that histone H4 is an important functional AMP released by human sebocytes and has a synergistic antimicrobial effect with LA or OA at sublethal doses against Staphylococcus aureus (Lee et al., 2009). in this study, the combination of hBD-2 and LA at sublethal doses exerted synergistic antimicrobial activity against P. acnes (Figure 4b). These findings suggest that in the human pilosebaceous microenvironment, antimicrobial components secreted from sebocytes can synergistically enhance their antimicrobial activity, thereby providing potent skin antimicrobial defense against pathogen colonization and infection.
Several pathways allow the uptake of FFAs into cells. As a result of their hydrophobic nature and ability to transfer passively across the phospholipid bilayer, translocation of FFAs can occur in part simply by a diffusion mechanism (Hamilton et al., 2001). In addition, several lines of evidence suggest that FFA translocation involves a number of plasma membrane-associated proteins such as the scavenger receptor CD36, plasma membrane-bound fatty acid-binding protein, and fatty acid transport protein (Abumrad et al., 1999; Goldberg et al., 2008). CD36 has been well characterized as a fatty acid translocase and is known to be a key protein involved in regulating the uptake of FFAs across the plasma membrane in various tissues such as the heart, skeletal muscle, and adipose tissue (Goldberg et al., 2008).Our data showed that CD36 exists on the surface of SZ95 sebocytes (Figure 5a). The CD36 protein has two transmembrane segments, very short intracellular segments, and a large glycosylated extracellular domain that functions as a fatty acid-binding domain (Baillie et al., 1996). Blocking CD36 with specific monoclonal IgG against its extracellular fatty acid-binding domain partially suppressed the FFA-induced increase in hBD-2 expression in human sebocytes (Figure 5b), suggesting that the CD36-mediated translocation mechanism of FFAs is involved in the FFA-induced increase of hBD-2 expression in human sebocytes. CD36 has higher selectivity to longer-chain FFAs (Gaillard et al., 2008). This might explain why PA and OA have a higher hBD-2 inducibility than LA (Figure 1). In future experiments, it will be interesting to investigate whether the production of FFAs or triacylgly-cerides by sebocytes is influenced by treatment with FFAs.
The increase in the intracellular concentrations of FFAs and their metabolites can activate signal transduction pathways in various tissues. For example, they can induce inflammation and impair insulin signaling in the skeletal muscle and liver (Boden et al., 2005; de Luca and Olefsky, 2008). Intracellular FFAs are converted into acyl-coenzyme A’s by acyl-coenzyme A synthetases in the cytoplasm. Acyl-coenzyme A’s in turn activate several several/threonine kinases, such as protein kinase C and IKKβ (Delarue and Magnan, 2007). IKKβ is a member of the IKK complex, which is a key enzyme involved in the phosphorylation of IκB, a protein that suppresses the activation of NF-κB complexes, resulting in its degradation by the proteasome (Ghosh and Hayden, 2008). The released NF-κB complexes then translocate into the nucleus and regulate the expression of target genes involved in inflammatory reactions and initiation of innate immune responses. Because hBD-2 expression is facilitated by NF-κB-mediated transactivation in many cell types (Froy, 2005), we subsequently examined the potential connection between the FFA-induced increase of hBD-2 expression and NF-κB signal transduction in human sebocytes. When sebocytes were preincubated with BMS-345541, a highly selective inhibitor of IKK, the FFA-induced increase of hBD-2 expression in human sebocytes was completely suppressed (Figure 5c). This indicates that the FFA-induced hBD-2 expression is mediated by the NF-κB signaling pathway.
hBD-2 is expressed in a variety of tissues and is inducible by bacterial and viral products (Froy, 2005). In addition, hBD-2 is inducible by cytokines, such as tumor necrosis factor-α and IL-1β (Harder et al., 1997; Wehkamp et al., 2006), which are induced in keratinocytes by P. acnes. Thus, hBD-2 is expected to have a critical role in the host defense against pathogen infection as an inducible component existing in the epithelial barrier. To our knowledge, this study is the first to demonstrate that sebum FFAs upregulate hBD-2 expression in human sebocytes, which may enhance the disinfecting activity of the human sebaceous gland. Given that extracellular lipases secreted by bacteria can hydrolyze sebum triacylglycerides to produce FFAs, sebocytes may become an important sensor of abnormal overgrowth of pathogens such as P. acnes in pilosebaceous units, which induces local inflammation such as inflammatory acne vulgaris. Although LA is a potent antimicrobial FFA found in human sebum, it shows less potency in inducing hBD-2 expression in human sebocytes than do other FFAs, such as PA and OA. Conversely, PA and OA, the most predominant FFAs found in human sebum, do not have a strong antimicrobial activity against P. acnes (see Supplementary Figure S2), although they show a higher hBD-2-inducible activity than LA. These results suggest that LA may provide a direct antimicrobial defense, whereas both PA and OA induce antimicrobial defenses indirectly via the upregulation of hBD-2 expression in human sebocytes. In addition to the FFAs tested (Figure 1), other fatty acids may have the capability of inducing AMPs. For example, ceramide, a major lipid accounting for ~50% of the lipid in the stratum corneum (Downing et al., 1986), can trigger NF-κB activation (Schutze et al., 1992; Higuchi et al., 1996), leading to the induction of hBD-2 expression. Conversely, linoleic acid (C18:2, cis,cis-9,12), a polyunsaturated FFA with anti-inflammatory activity, can suppress NF-κB in human mono-cytes (Zhao et al., 2005), suggesting that linoleic acid attenuates the NF-κB-mediated host immune defense. Thus, the roles of FFAs in the antimicrobial defense of sebocytes may depend on the length of their carbon chain and degree of saturation. Most importantly, the obtained data highlight the potential of using FFAs as a multifunctional antimicrobial therapy for acne vulgaris; FFA therapies may concurrently provide direct antibacterial activity and enhance the skin’s innate antibacterial defense by inducing AMPs in sebocytes.
We also observed the accumulation of mBD-4, a mouse ortholog for hBD-2, in the hair follicle sebaceous glands and upregulation of mBD-4 mRNA in the mouse ear skin after epicutaneous application of OA (Figure 6). The data strongly suggest that FFAs enhance the skin’s innate antibacterial defense by inducing the expression of defensin AMP in vivo. Unlike human skin, murine skin produces a limited amount of triglycerides and FFAs on the skin surface (Wilkinson and Karasek, 1966). We therefore used this mouse model to observe the effects of externally applied FFAs in a system lacking substantial amounts of endogenous FFAs. We have developed a tissue chamber model integrated with a dermis-based; cell-trapped system to mimic the in vivo microenvironment of acne lesions. A tissue chamber bearing human sebocytes is implanted into ICR mice, and then P. acnes can be injected into the tissue chamber to induce a host immune response (Nakatsuji et al., 2008). Thus, we anticipate that the tissue chamber model will be a useful animal model for additional studies of the physiological functions of FFAs in the innate immune defense of human sebocytes.
MATERIALS AND METHODS
Preparation of bacteria
P. acnes (ATCC 6919; American Type Culture Collection, Manassas, VA) was cultured on Brucella broth agar (BD, Sparks, MD), supplemented with 5% (v/v) defibrinated sheep blood (LAMPIRE Biological Laboratories, Pipersville, PA), vitamin K (5 µg ml−1, Remel, Lenexa, KS), and hemin (50 µg ml−1, Remel), under anaerobic conditions using Gas-Pak (BD) at 37 °C. Single colonies were inoculated in reinforced Clostridium medium (Oxford, Hampshire, England) and cultured at 37 °C until an OD600 value of ~ 1.0 (logarithmic growth phase) was reached under anaerobic conditions. The bacteria were harvested by centrifugation at 5,000g for 10 minutes, washed with PBS, and suspended with an appropriate amount of PBS for the experiments.
Quantification of gene expression of AMP in human sebocytes
The immortalized human sebaceous gland cell line SZ95 (Zouboulis et al., 1999 was cultured on 24-well plates in Sebomed basal medium (Biochrom, Berlin, Germany) supplemented with 5 ng ml−1 human recombinant epidermal growth factor (Sigma, St Louis, MO) and 10% (v/v) heat-inactivated fetal bovine serum (FBS) (Mediatech, Hemdon, VA) at 37 °C under an atmosphere of 5% (v/v) CO2 in air. LA, PA, and OA (Sigma) were prepared in DMSO and added to the culture medium at 25 µg ml−1. Although the concentration of FFA may be lower than the physiological concentration of total FFAs in human sebum (approximately 25–45% of total lipids) (James and Wheatley, 1956; Nordstrom et al., 1986), we chose a dose that does not influence the viability of SZ95 sebocytes and P. acnes in vitro (see Supplementary Figures S1 and S2). SZ95 sebocytes (2 × 106 cells per well) were incubated with each FFA in 1% FBS-Sebomed for 24 hours at 37 °C As a negative control, an equal amount of DMSO (0.5% (w/v)) was added to the culture medium. After incubation, total RNA was extracted from the cells using TRIzol (Invitrogen, Carlsbad, CA) and 1 µg RNA was reverse transcribed using iScript (Bio-Rad, Hercules, CA). Predeveloped TaqMan assay probes (Applied Biosystems, foster City, CA) were used for analyzing the expression of hBD-1 (DEFB1) (gi/28839288), hBD-2 (DEFB4) (gi/62740068), and hBD-3 (DEFB103B) (gi/151555097). The expression of human cathelicidin LL-37 (gi/32967658) was evaluated using an FAM-CAGAGGATTGTCACTTCA-MGB probe with 5′-CTTCACCAGCCCGTCCTTC-3′ and 5′-CCAGGACGACAGCAGTCA-3′ primers. For expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (gi/20809306), a VIC-CATCCATGACAACTTTGGTA-MGB probe with primers 5′-CTTAGCACCCCTGGCCAAG-3′ and 5′-TGG TCATGAGTCCTTCCACC-3′ was used. The mRNA expression of hBD-2 was evaluated by real-time quantitative PCR, normalized to that of GAPDH, and then expressed relative to that of vehicle-treated cells.
Fluorescence immunocytochemistry
To detect an FFA-induced increase in the hBD-2 peptide in SZ95 sebocytes, cells were incubated with LA, PA, or OA in 1% FBS-Sebomed for 24 hours at 37 °C, After incubation, the cells were fixed by cold acetone and then blocked with PBS containing 3% (w/v) BSA and 2% (v/v) normal goat serum for 30 minutes, After blocking, the cells were incubated with rabbit anti-hBD-2 IgG (5 µg ml−1) (Santa Cruz Biotechnology, Santa Cruz, CA), followed by FITC-labeled anti-rabbit IgGs (5 µg ml−1) (Santa Cruz Biotechnology). Normal rabbit IgG (Jackson Immunoresearch Laboratories, West Grove, PA) was used as a negative control for detection.
To detect CD36 in SZ95 sebocytes, cells were fixed by cold acetone and then blocked as described above. After blocking, the cells were incubated with mouse anti-human CD36 monoclonal IgG (FA6-152) (2 µg ml−1) (Santa Cruz Biotechnology), which is an antagonist antibody clone against the extracellular domain, followed by FITC-labeled anti-mouse IgGs (5 µg ml−l) (Santa Cruz Biotechnology), Normal mouse IgG (Jackson Immunoresearch Laboratories) was used as a negative control for the detection. Nuclei were counterstained with 4′-6-diamidino-2-phenylindole (Sigma). Images were obtained using an Olympus BX41 fluorescence microscope (Olympus, Center Valley, PA).
Western blotting for hBD-2
To detect an FFA-induced increase in hBD-2 secretion from SZ95 sebocytes, cells were incubated in a six-well plate with LA, PA, or OA in 1% FBS-Sebomed for 24 hours at 37 °C. After incubation, the supernatant of the cell culture medium (2 ml) was added to a Sep-Pak cartridge (Waters, Milford, MA), washed with 10% (v/v) acetonitrile in 0.1% (v/v) trifluoroacetic acid, and eluted with 60% (v/v) acetonitrile in 0.1% (v/v) trifluoroacetic acid. The elution was lyophilized, reconstituted in sample buffer (50 µl) for SDS-PAGE, and then boiled for 2 minutes. The sample (10 µl) was electrophoresed in a 16% (w/v) polyacrylamide gel (NuSep, Austell, GA) and electrophoretically transferred onto polyvinylidene difluoride membranes (Millipore, Billerica, MA). The membranes were preincu-bated for 30 minutes in PBS containing 5% (w/v) skim milk and then incubated with anti-hBD-2 IgG (1 µg ml−1) at 4 °C overnight. Bound antibodies were detected with goat anti-rabbit peroxidase-conjugated IgG (1:2,000 dilution, Dako, Glostrup, Denmark), and peroxidase activity was developed using the Western Lighting Chemiluminescence kit (PerkinElmer, Boston, MA).
In vitro antimicrobial assay for FFA-induced antimicrobial activity of human sebocytes
To stimulate hBD-2 production in human SZ95 sebocytes, cells (2 × 106 per well) were incubated with LA, PA, OA (25 µg ml−1) or an equal amount of DMSO (0.5% (v/v)) (vehicle control) in 1% FBS-Sebomed (250 µl) in a 24-well plate for 24 hours, To examine the antimicrobial activity of each FFA contained in the medium, the FFA-conditioned medium was incubated without sebocytes. After incubation, the supernatant was centrifuged and filtered with a 0.22-µm-pore-size filter to remove cell debris. To neutralize the antimicrobial activity of FFA-induced hBD-2, the supernatant was preincubated with rabbit anti-hBD-2 IgG (20 µg ml−1) or normal rabbit IgG (20 µg ml−1) (negative control) for 2 hours at 37 °C. P. acnes (1 × 106 CFU per ml) was added to the supernatant of the cell culture and incubated for 5 hours at 37 °C under anaerobic conditions. FBS-Sebomed (1%) containing each FFA, which was incubated without sebocytes, was used as a control for this antimicrobial assay. After incubation, the reaction mixture was diluted 1:1–1:104 with PBS, and 5 µl of the diluted solution was spotted on a Brucella broth agar plate for counting CFUs.
In vitro antimicrobial assay for synthetic hBD-2
Synthetic hBD-2 (Peptide Institute, Osaka, Japan), which was dissolved in 20 mm phosphate buffer, pH 6.5, containing 100 mm NaCI, was added to the bacterial suspension. P. acnes (1 × 106 CFU per ml was incubated with hBD-2 (0–20 µm) on a 96-well microplate (100 µl per well) in the phosphate buffer at 37 °C for 5 hours. To examine the synergistic antimicrobial activity of hBD-2 and FFAs, P. acnes (1 × 106 CFU per ml) was incubated with hBD-2 (0, 2.5, 5, 10 µm) in the phosphate buffer in the presence of LA, PA, or OA (25 µg ml−1) on a 96-well microplate (100 µl per well) at 37 °C for 5 hours. The control received only DMSO (0.5% (v/v)) instead of FFA. After incubation, the CFUs of P. acnes were counted as described above.
Mechanism of signal transduction for FFA-enhanced hBD-2 expression
To block the function of CD36 on the surface of SZ95 sebocytes, cells (2 × 106 per well) cultured on a 24-well plate were preincubated with mouse anti-CD36 IgG (FA6-152) (5 µg ml−1), which is an antagonist antibody clone against the extracellular domain, or normal mouse igG in 1% FBS-Sebomed (250 µl) for 2 hours at 37 °C. LA, PA, or OA (25 µg ml−1) was added to the preincubated cell culture and incubated for 24 hours at 37 °C. An equal amount of DMSO (0.5% (v/v)) was used as a control. To block the NF-κB-mediated signaling pathway in SZ95 sebocytes, the cells (2 × 106 per well) cultured on a 24-well plate were preincubated with BMS-345541 (20 µm), a highly selective inhibitor of IKK (Burke et al., 2003), or with an equal amount of DMSO (0.1%) in 1% FBS-Sebomed (250 µl) for 1 hour at 37 °C. After preincubation, LA, PA, or OA (25 µg ml−1) was added to the cells, which were subsequently incubated for 24 hours at 37 °C. An equal amount of DMSO (0.5% (v/v)) was used as a control. After incubation, the mRNA expression of hBD-2 was determined as described above.
Epicutaneous application of FFA to mouse skin
Eight-week-old female ICR mice (Harlan, Indianapolis, IN) were used in all animal experiments. The mice were housed according to institutional guidelines. OA (150 µg in 5% acetone mixed with 15 mg of Vaseline (Sigma)) was applied to the surface of the left ear at 0 and 12 hours. Epicutaneous application of 5% acetone mixed with 15 mg of Vaseline was applied to the right ear as a vehicle control. To enable observation of mBD-4 immunoreactivity in sebaceous glands, the ears were embedded in an optimal cutting temperature compound (Sakura Finetek, Torrance, CA) 12 hours after epicutaneous application. Frozen sections (6 µm) were fixed in a 3.7% (w/v) paraformaldehyde solution in PBS, followed by permeabilization with 0.1% (w/v) Triton X-100 in PBS, and then blocked with PBS containing 3%., BSA and 2% (v/v) normal goat serum. The sections were then incubated with affinity-purified rabbit anti-mBD-4 (2 µg ml−1) (Alpha Diagnostic International, San Antonio, TX), followed by goat anti-rabbit IgG-Alexa 568 conjugate (5 µg ml−1) (Invitrogen), for 30 minutes. Normal rabbit IgG was used as a negative control for detection. Nuclei were counterstained with 4′-6-diamidino-2-phenylindole.
To determine the gene expression of mBD-4, total RNA was extracted and reverse transcribed as described above. Predeveloped TaqMan assay probes (Applied Biosysiems) were used for analyzing the expression of mBD-4 (DEFB4) (gi/142379735). For expression of mouse GAPDH (gi/126012538), a VIC-CATCCATGACAACTTTGG TA-MGB probe with primers 5′-CTTAGCACCCCTGGCCAAG-3′ and 5′-TGGTCATGAGTC:CTTCCACG-3′ was used. The mRNA expression of mBD-4 was evaluated by real-time quantitative PCR, normalized to that of GAPDH, and then expressed relative to that of vehicle-treated cells.
Statistical analysis
Data are presented as means ± SE. Student’s t-test was used to assess the significance of independent experiments. The criterion P<0.05 was used to determine statistical significance.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants R01-AI067395-01, R21-R022754-01,. 1R41AR056169, and R21-I58002-01. We thank Y-T Liu for valuable comments and Corbiri Clawson, Daniel MacLeod, and Beda Müehleisen for reviewing the manuscript.
Abbreviations
- AMP
antimicrobial peptide
- CD
cluster of differentiation
- CFU
colony-forming unit
- FBS
fetal bovine serum
- FFA
free fatty acid
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- hBD
human β-defensin
- IKK
inhibitory κB kinase
- LA
lauric acid
- mBD-4
mouse β-defensin-4
- OA
oleic acid
- PA
palmitic acid
- PBS
phosphate-buffered saline
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
REFERENCES
- Abumrad N, Coburn C, Ibrahimi A. Membrane proteins implicated in long-chain fatty acid uptake by mammalian cells: CD36, FATP and FABPm. Biochim Biophys Acta. 1999;1441:4–13. doi: 10.1016/s1388-1981(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Baillie AG, Coburn CT, Abumrad NA. Reversible binding of long-chain fatty acids to purified FAT, the adipose CD36 homolog. J Membr Biol. 1996;153:75–81. doi: 10.1007/s002329900111. [DOI] [PubMed] [Google Scholar]
- Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-kappaB pathway in rat liver. Diabetes. 2005;54:3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- Bodoprost J, Rosemeyer H. Analysis of phenacylester derivatives of fatty acids from human skin surface sebum by reversed-phase HPLC: chromatographic mobility as a function of physico-chemical properties. Int J Mol Sci. 2007;8:1111–1124. [Google Scholar]
- Bojar RA, Holland KT. Acne and Propionibacterium acnes. Clin Dermatol. 2004;22:375–379. doi: 10.1016/j.clindermatol.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Burke JR, Pattoli MA, Gregor KR, et al. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278:1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- Chronnell CM, Ghali LR, Ali RS, et al. Human beta defensin-1 and -2 expression in human pilosebaceous units: upregulation in acne vulgaris lesions. J Invest Dermatol. 2001;117:1120–1125. doi: 10.1046/j.0022-202x.2001.01569.x. [DOI] [PubMed] [Google Scholar]
- de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97–105. doi: 10.1016/j.febslet.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degitz K, Placzek M, Borelli C, et al. Pathophysiology of acne. J Dtsch Dermatol Ges. 2007;5:316–323. doi: 10.1111/j.1610-0387.2007.06274.x. [DOI] [PubMed] [Google Scholar]
- Delarue J, Magnan C. Free Fatty acids and insulin resistance. Curr Opin Clin Nutr Metab Care. 2007;10:142–148. doi: 10.1097/MCO.0b013e328042ba90. [DOI] [PubMed] [Google Scholar]
- Dorschner RA, Lin KH, Murakami M, et al. Neonatal skin in mice and humans expresses increased levels of antimicrobial peptides: innate immunity during development of the adaptive response. Pediatr Res. 2003;53:566–572. doi: 10.1203/01.PDR.0000057205.64451.B7. [DOI] [PubMed] [Google Scholar]
- Downing DT, Wertz PW, Stewart ME. The role of sebum and epidermal lipids in the cosmetic properties of skin. Int J Cosmet Sci. 1986;8:115–123. doi: 10.1111/j.1467-2494.1986.tb00439.x. [DOI] [PubMed] [Google Scholar]
- Drake DR, Brogden KA, Dawson DV, et al. Thematic review series: skin lipids. Antimicrobial lipids at the skin surface. J Lipid Res. 2008;49:4–11. doi: 10.1194/jlr.R700016-JLR200. [DOI] [PubMed] [Google Scholar]
- Froy O. Regulation of mammalian defensin expression by Toll-like receptor-dependent and independent signalling pathways. Cell Micro-biol. 2005;7:1387–1397. doi: 10.1111/j.1462-5822.2005.00590.x. [DOI] [PubMed] [Google Scholar]
- Fujie T, Shikiji T, Uchida N, et al. Culture of cells derived from the human sebaceous gland under serum-free conditions without a biological feeder layer or specific matrices. Arch Dermatol Res. 1996;288:703–708. doi: 10.1007/BF02505281. [DOI] [PubMed] [Google Scholar]
- Gaillard D, Laugerette F, Darcel N, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- Gallo RL, Murakami M, Ohtake T, et al. Biology and clinical relevance of naturally occurring antimicrobial peptides. J Allergy Clin Immunol. 2002;110:823–831. doi: 10.1067/mai.2002.129801. [DOI] [PubMed] [Google Scholar]
- Georgel P, Crozat K, Lauth X, et al. A toll-like receptor 2-responsive lipid effector pathway protects mammals against skin infections with gram-positive bacteria. Infect Immun. 2005;73:4512–4521. doi: 10.1128/IAI.73.8.4512-4521.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nat Rev Immunol. 2008;8:837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- Goldberg IG, Eckel RH, Abumrad NA. Regulation of fatty acid uptake into tissues: lipoprotein lipase- and CD36-mediated pathways. J Lipid Res. 2008;50 Suppl:S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz F, Verheij HM, Rosenstein R. Staphylococcal lipases: molecular characterisation, secretion, and processing. Chem Phys Lipids. 1998;93:15–25. doi: 10.1016/s0009-3084(98)00025-5. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Johnson RA, Corkey B, et al. Fatty acid transport: the diffusion mechanism in model and biological membranes. J Mol Neurosci. 2001;16:99–108. doi: 10.1385/JMN:16:2-3:99. [DOI] [PubMed] [Google Scholar]
- Harder J, Bartels J, Christophers E, et al. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Singh S, Jaffrezou JP, et al. Acidic sphingomyelinase-generated ceramide is needed but not sufficient for TNF-induced apoptosis and nuclear factor-kappa B activation. J Immunol. 1996;157:297–304. [PubMed] [Google Scholar]
- Holland KT, Ingham E, Cunliffe WJ. A review, the microbiology of acne. J Appl Bacteriol. 1981;51:195–215. doi: 10.1111/j.1365-2672.1981.tb01234.x. [DOI] [PubMed] [Google Scholar]
- Ito A, Kitamura K, Sato K, et al. A novel enzymatic assay for the quantification of skin surface lipids. J Int Med Res. 1996;24:69–83. doi: 10.1177/030006059602400109. [DOI] [PubMed] [Google Scholar]
- James AT, Wheatley VR. Studies of sebum. 6. The determination of the component fatty acids of human forearm sebum by gas-liquid chromatography. Biochem J. 1956;63:269–273. doi: 10.1042/bj0630269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia HP, Wowk SA, Schutte BC, et al. A novel murine beta-defensin expressed in tongue, esophagus, and trachea. J Biol Chem. 2000;275:33314–33320. doi: 10.1074/jbc.M006603200. [DOI] [PubMed] [Google Scholar]
- Kabara JJ, Swieczkowski DM, Conley AJ, et al. Fatty acids and derivatives as antimicrobial agents. Antimicrob Agents Chemother. 1972;2:23–28. doi: 10.1128/aac.2.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Review of the innate immune response in acne vulgaris: activation of Toll-like receptor 2 in acne triggers inflammatory cytokine responses. Dermatology. 2005;211:193–198. doi: 10.1159/000087011. [DOI] [PubMed] [Google Scholar]
- Kim J, Ochoa MT, Krutzik SR, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitahara T, Koyama N, Matsuda J, et al. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol Pharm Bull. 2004;27:1321–1326. doi: 10.1248/bpb.27.1321. [DOI] [PubMed] [Google Scholar]
- Lee DY, Huang CM, Nakatsuji T, et al. Histone H4 is a major component of the antimicrobial action of human sebocytes. J Invest Dermatol. 2009;129:2489–2496. doi: 10.1038/jid.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Yamasaki K, Rudsil J, et al. Sebocytes express functional cathelicidin antimicrobial peptides and can act to kill Propionibacterium acnes. J Invest Dermatol. 2008;128:1863–1866. doi: 10.1038/sj.jid.5701235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marples RR, Downing DT, Kligman AM. Control of free fatty acids in human surface lipids by Corynebacterium acnes. J Invest Dermatol. 1971;56:127–131. doi: 10.1111/1523-1747.ep12260695. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Kis K, et al. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006;8:2195–2205. doi: 10.1016/j.micinf.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Koreck A, et al. Distinct strains ot Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- Nakatsuji T, Kao MC, Fang JY, et al. Antimicrobial property of lauric acid against Propionibacterium acnes: its therapeutic potential for inflammatory acne vulgaris. J Invest Dermatol. 2009;129:2480–2488. doi: 10.1038/jid.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatsuji T, Shi Y, Zhu W, et al. Bioengineering a humanized acne microenvironment model: proteomics analysis of host responses to P. acnes infection in vivo. Proteomics. 2008;8:3406–3415. doi: 10.1002/pmic.200800044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414:454–457. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- Nordstrom KM, Labows JN, McGinley KJ, et al. Characterization of wax esters, triglycerides, and free fatty acids of follicular casts. J Invest Dermatol. 1986;86:700–705. doi: 10.1111/1523-1747.ep12276314. [DOI] [PubMed] [Google Scholar]
- Rouse MS, Rotger M, Piper KE, et al. In vitro and in vivo evaluations of the activities of lauric acid monoester formulations against Staphylo-coccus aureus. Antimicrob Agents Chemother. 2005;49:3187–3191. doi: 10.1128/AAC.49.8.3187-3191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutze S, Potthoff K, Machleidt T, et al. TNF activates NF-kappa B by phosphatidylcholine-spedfic phospholipase C-induced “acidic” sphin-gomyelin breakdown. Cell. 1992;71:765–766. doi: 10.1016/0092-8674(92)90553-o. [DOI] [PubMed] [Google Scholar]
- Skrivanova E, Marounek M, Dlouha G, et al. Susceptibility of Clostridium perfringens to C-C fatty acids. Lett Appl Microbiol. 2005;41:77–81. doi: 10.1111/j.1472-765X.2005.01709.x. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Morohashi M. Pathogenesis of acne. Med Electron Microsc. 2001;34:29–40. doi: 10.1007/s007950100002. [DOI] [PubMed] [Google Scholar]
- Wehkamp K, Schwichtenberg L, Schroder JM, et al. Pseudomonas aeruginosa- and IL-1beta-mediated induction of human beta-defensin-2 in keratinocytes is controlled by NF-kappaB and AP-1. J Invest Dermatol. 2006;126:121–127. doi: 10.1038/sj.jid.5700020. [DOI] [PubMed] [Google Scholar]
- Wilkinson DI, Karasek MA. Skin lipids of a normal and mutant (asebic) mouse strain. J Invest Dermatol. 1966;47:449–455. doi: 10.1038/jid.1966.168. [DOI] [PubMed] [Google Scholar]
- Wille JJ, Kydonieus A. Palmitoleic acid isomer (C16:1 delta6) in human skin sebum is effective against gram-positive bacteria. Skin Pharmacol Appl Skin Physiol. 2003;16:176–187. doi: 10.1159/000069757. [DOI] [PubMed] [Google Scholar]
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- Zhao G, Etherton TD, Martin KR, et al. Anti-inflammatory effects of polyunsaturated fatty acids in THP-1 cells. Biochem Biophys Res Commun. 2005;336:909–917. doi: 10.1016/j.bbrc.2005.08.204. [DOI] [PubMed] [Google Scholar]
- Zouboulis CC, Seltmann H, Neitzel H, et al. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95) J Invest Dermatol. 1999;113:1011–1020. doi: 10.1046/j.1523-1747.1999.00771.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.