Abstract
Objective
To determine if eclampsia has a different circulating profile of angiogenic [placental growth factor (PlGF)] and anti-angiogenic factors [soluble vascular endothelial growth factor receptor-1 (sVEGFR-1) and soluble endoglin (sEng)] than severe preeclampsia.
Study Design
This cross-sectional study included women in the following groups: 1) normal pregnancy (n=40); 2) severe preeclampsia (n=40); and 3) eclampsia (n=20). Maternal serum PlGF, sVEGFR-1, and sEng concentrations were determined using ELISA.
Results
1) The median concentration of sVEGFR-1 and sEng was higher and of PlGF lower in severe preeclampsia or eclampsia than in normal pregnancy (p<0.001 for all); 2) the median concentrations of these 3 analytes did not differ significantly between the severe preeclampsia and eclampsia groups.
Conclusions
Eclampsia is associated with higher maternal circulating concentrations of sVEGFR-1 and sEng and lower concentrations of PlGF than normal pregnancy, but with similar concentrations to severe preeclampsia. These findings suggest that eclampsia shares a common pathogenic pathway as severe preeclampsia.
Keywords: placental growth factor, PlGF, preeclampsia, pregnancy, sEng, sFlt-1, soluble endoglin, soluble vascular endothelial growth factor receptor-1, sVEGFR-1
INTRODUCTION
Preeclampsia, one of the “great obstetrical syndromes,”1;2 complicates about 2 to 7% of pregnancies,3 and is a major contributor to maternal and neonatal morbidity and mortality worldwide.4-8 In recent years, an imbalance between circulating angiogenic and anti-angiogenic factors has emerged as a potential key pathway in the pathophysiology of preeclampsia.9-69 Specifically, patients with preeclampsia have a higher circulating concentration of anti-angiogenic factors [i.e., soluble vascular endothelial growth factor receptor-1 (sVEGFR-1, also called soluble fms-like tyrosine kinase 1 (sFlt1))15-17;20-22;25;31-33;37;39;41;42;45;48-50;57;58;60;69;70 and soluble endoglin (sEng)]34;35;49;51;52;57;60;63;69;70 and a lower maternal circulating concentration of free angiogenic factors [i.e., vascular endothelial growth factor (VEGF)15;71 and placental growth factor (PlGF)]11;12;14;15;19;20;23;39;41;48;50;53;54;56-58;60;61;68;71;72 than patients with a normal pregnancy. These findings have been demonstrated both at the time of the clinical diagnosis of preeclampsia and also prior to the clinical manifestation of the disease.12;14;19;20;23;25;44;47;48;50;51;59;60;63;68;72-78
In addition, the degree of the angiogenic/anti-angiogenic imbalance in preeclampsia has been associated with disease severity. Indeed, Venkatesha et al.35 reported that serum sEng concentrations were three-, five- and ten-fold higher in individuals with mild preeclampsia, severe preeclampsia and HELLP (hemolysis, elevated liver enzymes, and low platelets) syndrome, respectively, compared to gestational age-matched controls. Therefore, sEng has been suggested to be a marker for severity of disease.35
Eclampsia, defined as the occurrence of new-onset grand mal seizures or unexplained coma in a woman with preeclampsia,6;79 is a serious and life-threatening complication of pregnancy with an incidence of about 1 in 2000 pregnancies.80;81 In approximately two-thirds of cases the onset of seizures is during pregnancy (antepartum or intrapartum); however, eclampsia can occur in the post-partum period in about one-third of cases (range 11% to 44%).79;81-84 One of the signs characterizing women as having severe preeclampsia is the eclamptic seizures. However, the classical diagnostic signs of preeclampsia (i.e., hypertension and proteinuria) may not precede the eclamptic seizures in more than one-third of the cases,81;82 and hypertension may be absent in about 20% of cases.85;86 Katz et al.82 analyzed a series of 53 cases of eclampsia and found that seizures were the first signs of preeclampsia in 60% of cases. The authors concluded that eclampsia seems not to be a progression from severe preeclampsia.82
The underlying pathophysiologic process predisposing pregnant women to eclamptic seizures is not clear. Several mechanism of disease have been implicated in the pathogenesis of eclampsia including hypertensive encephalopathy, cerebral edema, infarction, or hemorrhage, endothelial dysfunction, and others.79;87-94 To our knowledge, there is no data regarding the concentrations of angiogenic/anti-angiogenic factors in serum from patients with eclampsia, and whether the concentrations of angiogenic and anti-angiogenic factors in these patients differ from those of patients with severe preeclampsia. Thus, the aim of this study was to compare the profile of circulating angiogenic (i.e., PlGF) and anti-angiogenic (i.e. sVEGFR-1 and sEng) factors in patients with eclampsia and those with severe preeclampsia.
PATIENTS AND METHODS
Study design
A cross-sectional study was conducted by searching our clinical database and bank of biologic samples, and included 100 pregnant women in the following groups: 1) normal pregnancy (n=40); 2) severe preeclampsia (n=40); and 3) eclampsia (n=20). Women with multiple pregnancies, fetuses with chromosomal and / or congenital anomalies, as well as women with HELLP syndrome or in which eclampsia occurred after the delivery of the placenta were excluded.
All participants provided written informed consent prior to the collection of blood samples. The collection of blood and its utilization for research purposes was approved by the Institutional Review Boards of Wayne State University. Many of these samples have been previously used to study the biology of inflammation, hemostasis, angiogenesis regulation and growth factor concentrations in normal pregnant women and those with pregnancy complications.
Clinical definitions
Women with a normal pregnancy were defined as those without medical, obstetrical, or surgical complications at the time of the study and who subsequently delivered an appropriate-for-gestational age infant at term (≥37 weeks of gestation)95 without neonatal complications. Preeclampsia was defined as the onset of hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg on at least two occasions, 4 hours to 1 week apart) after 20 weeks of gestation with proteinuria (≥300 mg in a 24-hour urine collection or at least one dipstick measurement ≥2+).6;96 Severe preeclampsia was diagnosed according to the criteria proposed by the American College of Obstetricians and Gynecologists (ACOG) committee6 as systolic blood pressure ≥160mmHg and/or diastolic blood pressure ≥110mmHg and/or proteinuria greater than 5g in a 24h collection or ≥3+ protein on dipstick, or in the presence of multi-organ involvement.3;6 HELLP syndrome was defined as hemolysis (serum LDH >600IU/l; bilirubin >1.2mg/dl; presence of schistocytes in peripheral blood), elevated liver enzymes (serum ALT and/or AST >70IU/l) and thrombocytopenia (platelet count <100,000/mm3).97 Eclampsia was defined as the occurrence of convulsions in pregnant women who had preexisting gestational hypertension or preeclampsia or as new-onset convulsions in women without a previously known hypertensive or seizure disorder, however in whom hypertension (systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg) was part of the clinical presentation.
Blood sample collection and determination of PlGF, sVEGFR-1 and sEng in maternal serum
Maternal blood samples were obtained from normal pregnant women either during an antenatal clinic visit or before a scheduled cesarean section, and from women with preeclampsia or eclampsia at the time of diagnosis. Samples of peripheral blood were obtained by venipuncture, centrifuged at 1300 × g for 10 min at 4°C, and stored at −70°C until assayed.
Concentrations of PlGF, sVEGFR-1 and sEng in maternal serum were determined using specific and sensitive immunoassays (R&D Systems, Minneapolis, MN, USA). All three immunoassays utilized the quantitative sandwich enzyme immunoassay technique. Immunoassays were carried out according to the manufacturer’s recommendations. The calculated inter-assay coefficients of variation (CV) for PlGF, sVEGFR-1 and sEng immunoassays in our laboratory were 5.7%, 6.2% and 3.8%. Calculated intra-assay CVs for PlGF, sVEGFR-1 and sEng were 4.4%, 2.2% and 2.8%. The calculated detection limit (sensitivity) for PlGF, sVEGFR-1 and sEng assays were 9.2 pg/ml, 16.8 pg/ml, and 0.11 ng/ml, respectively.
Statistical analysis
Kolmogorov–Smirnov tests were used to test for normal distribution of the data. Since maternal serum concentrations of PlGF, sVEGFR-1, and sEng were not normally distributed, Kruskal-Wallis test with post-hoc Mann-Whitney U test were used for comparisons of continuous variables among and between groups. Comparison of proportions was performed using Fisher’s exact test. Correlations between continuous variables were examined using Spearman’s rank correlation test. A multivariable logistic regression analysis was applied to determine the association between serum concentrations of PlGF, sVEGFR-1, and sEng (and their ratios) and eclampsia while adjusting for maternal age and gestational age at blood drawn among patients with severe preeclampsia and those with eclampsia (pooled together). A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 12 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics
The demographic and clinical characteristics of the study population are shown in Table 1. There were no significant differences among the study groups in the medians of any of the demographic characteristics, as well as in the median gestational age at blood drawn. As expected, patients with severe preeclampsia or eclampsia had significantly higher medians of maximal systolic and diastolic blood pressure and lower medians of gestational age at delivery and neonatal birthweight than women with a normal pregnancy. None of these variables differ significantly between patients with severe preeclampsia and those with eclampsia.
Table 1.
Demographic and clinical characteristics of the study population
| Variable | Normal pregnancy (n=40) |
pa | Severe preeclampsia (n=40) |
pb | Eclampsia (n=20) |
pc |
|---|---|---|---|---|---|---|
|
Maternal age
(years) * |
20 (19-23) | 0.1 | 23.5 (18-27) | 0.2 | 20 (17-25) | 0.8 |
|
African American
Ethnic origin |
80 (32) | 0.3 | 90 (36) | 0.4 | 80 (16) | 0.99 |
| Nulliparity | 87 (35) | 0.005 | 57.5 (23) | 0.3 | 75 (15) | 0.3 |
|
Pre-pregnancy BMI
(kg/m2) * |
25.8 (22.7- 28.3) |
0.3 | 27.6 (21.7-36.6) | 0.8 | 25.5 (24.0- 29.3) |
0.6 |
|
BMI at blood draw (kg/m2)* |
30.7 (27.6- 35.2) |
0.2 | 35.0 (27.6-44) | 0.9 | 34.8 (30.2- 37.3) |
0.3 |
| Delta BMI (kg/m2)* | 4.8 (3.2-8.2) | 0.99 | 5.1 (3.0-9.0) | 0.2 | 7.9 (5.1- 12.2) |
0.1 |
|
Maximum Systolic
BP (mm Hg) † |
120 (110-130) | <0.001 | 165 (151-180) | >0.99 | 159 (150- 174) |
<0.001 |
|
Maximum Diastolic
BP (mm Hg) † |
68 (60-72) | <0.001 | 99 (88-104) | >0.99 | 96 (85-107) | <0.001 |
|
Gestational age at
blood sampling (weeks) ** |
38.6 (34.5-39.9) |
0.1 | 36.7 (34.7-38.9) |
0.6 | 36.5 (32.2-39) |
0.2 |
|
Gestational age at
delivery (weeks) † |
39.9 (39.0-40.4) |
<0.001 | 36.8 (34.9-38.9) |
>0.99 | 37 (33.2- 39.2) |
<0.001 |
|
Birthweight
(grams) † |
3400 (3122-3674) |
<0.001 | 2624 (2030-3062) |
0.6 | 2569 (1546-3227) |
<0.001 |
|
Sample starage
time (years) *** |
12.7 (11.5- 13.4) |
0.4 | 12.2 (11.5-13.1) | 0.09 | 11.5 (9.8- 12.8) |
0.0 |
Values are expressed as median (interquartile range) or percent (number)
BMI, body mass index; BP, blood pressure
Kruskal-Wallis, p = NS
Kruskal-Wallis, p < 0.001
Kruskal-Wallis, p = 0.04
ANOVA, p < 0.001
pa: Normal pregnancy vs. Severe preeclampsia
pb: Severe preeclampsia vs. Eclampsia
pc: Normal pregnancy vs. Eclampsia
Maternal serum concentration of PlGF in women with a normal pregnancy, severe preeclampsia, or eclampsia
The median maternal serum PlGF concentration was lower in patients with severe preeclampsia (190 pg/mL, interquartile range (IQR) 63.1-190.0) or eclampsia (84.3 pg/mL, IQR 44.9-145.9) than that of women with a normal pregnancy (335.6 pg/mL, IQR 220.6-646.0; p<0.001 for both comparisons) (Figure 1). The median maternal serum concentration of PlGF was not significantly different between patients with severe preeclampsia and those with eclampsia (p=0.3). The association between maternal serum concentrations of PlGF and eclampsia remained non-significant after adjusting for maternal age and gestational age at blood drawn (OR 1.002, 95% CI 0.998-1.005; p=0.37).
Figure 1. Comparison of serum concentration of placental growth factor (PlGF) between women with a normal pregnancy, severe preeclampsia, or eclampsia.
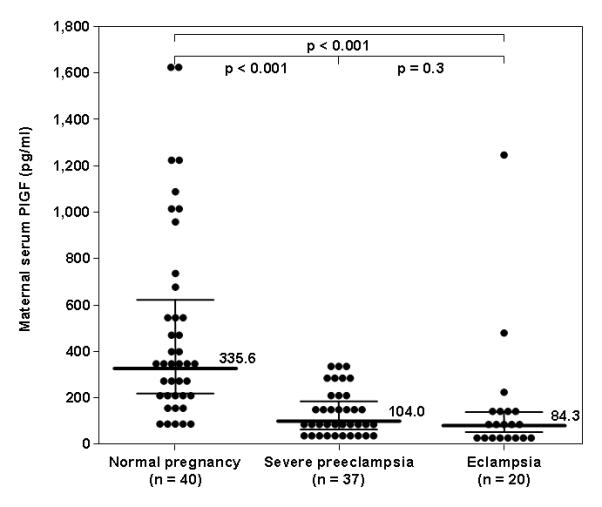
The median maternal serum PlGF concentration was lower in patients with severe preeclampsia (190 pg/mL, interquartile range (IQR) 63.1-190.0) or eclampsia (84.3 pg/mL, IQR 44.9-145.9) than that of women with a normal pregnancy (335.6 pg/mL, IQR 220.6-646.0; p<0.001 for both comparisons). The median maternal serum concentration of PlGF was not significantly different between patients with severe preeclampsia and those with eclampsia (p=0.3).
Maternal serum concentration of sVEGFR-1 in women with a normal pregnancy, severe preeclampsia, or eclampsia
The median maternal serum sVEGFR-1 concentration was higher in patients with severe preeclampsia (19,070.6 pg/mL, interquartile range (IQR) 12,425.9-35,456.5) or eclampsia (28,527.9 pg/mL, IQR 14,441.1-53,197.3) than that of women with a normal pregnancy (6,483.3 pg/mL, IQR 3,464-8,585.5; p<0.001 for both comparisons) (Figure 2). The median maternal serum concentration of sVEGFR-1 was not significantly different between patients with severe preeclampsia and those with eclampsia (p=0.2). The association between maternal serum concentrations of sVEGFR-1 and eclampsia remained non-significant after adjusting for maternal age and gestational age at blood drawn (OR 1.000, 95% CI 1.000-1.000; p=0.5).
Figure 2. Comparison of serum concentration of soluble vascular endothelial growth factor receptor (sVEGFR)-1 between women with a normal pregnancy, severe preeclampsia, or eclampsia.
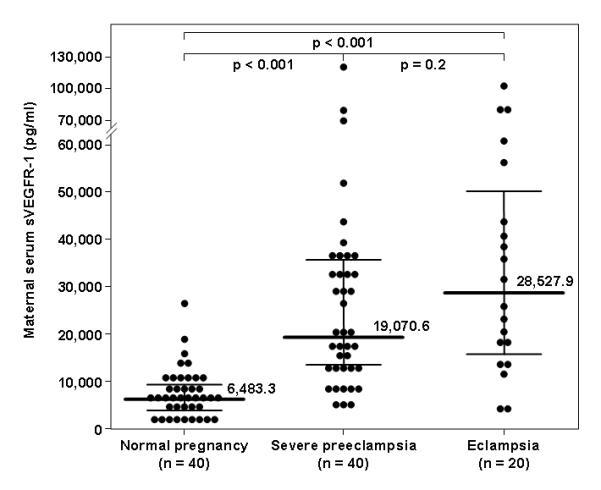
The median maternal serum sVEGFR-1 concentration was higher in patients with severe preeclampsia (19,070.6 pg/mL, interquartile range (IQR) 12,425.9-35,456.5) or eclampsia (28,527.9 pg/mL, IQR 14,441.1-53,197.3) than that of women with a normal pregnancy (6,483.3 pg/mL, IQR 3,464-8,585.5; p<0.001 for both comparisons). The median maternal serum concentration of sVEGFR-1 was not significantly different between patients with severe preeclampsia and those with eclampsia (p=0.2).
Maternal serum concentration of sEng in women with a normal pregnancy, severe preeclampsia, or eclampsia
The median maternal serum sEng concentration was higher in patients with severe preeclampsia (37.2 pg/mL, interquartile range (IQR) 21.9-47.8) or eclampsia (46.7 pg/mL, IQR 25.6-107.6) than that of women with a normal pregnancy (12.7 pg/mL, IQR 8.8-19.6; p<0.001 for both comparisons) (Figure 3). The median maternal serum concentration of sEng was not significantly different between patients with severe preeclampsia and those with eclampsia (p=0.2). The association between maternal serum concentrations of sEng (pg/mL) and eclampsia remained non-significant after adjusting for maternal age and gestational age at blood drawn (OR 1.013, 95% CI 0.998-1.029; p=0.08).
Figure 3. Comparison of serum concentration of soluble endoglin (sEng) between women with a normal pregnancy, severe preeclampsia, or eclampsia.
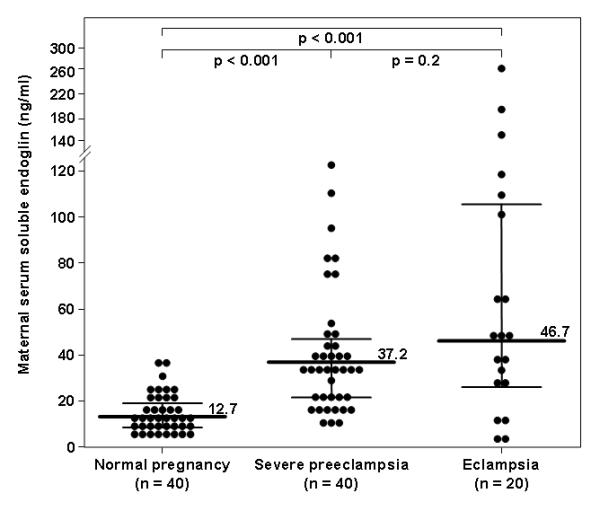
The median maternal serum sEng concentration was higher in patients with severe preeclampsia (37.2 pg/mL, interquartile range (IQR) 21.9-47.8) or eclampsia (46.7 pg/mL, IQR 25.6-107.6) than that of women with a normal pregnancy (12.7 pg/mL, IQR 8.8-19.6; p<0.001 for both comparisons). The median maternal serum concentration of sEng was not significantly different between patients with severe preeclampsia and those with eclampsia (p=0.2).
The ratios between PlGF, VEGFR-1, and sEng in women with a normal pregnancy, severe preeclampsia, or eclampsia
The ratios of PlGF/sVEGFR-1, PlGF/sEng, PlGF/(sEng X VEGFR-1), and PlGF/(sEng+VEGFR-1)68 were all significantly lower in patients with severe preeclampsia or eclampsia than patients with a normal pregnancy (p<0.001 for all comparisons; Table 2). However, these ratios did not differ significantly between patients with severe preeclampsia and those with eclampsia (Table 2). In addition, the different ratios were not significantly associated with eclampsia after adjusting for maternal age and gestational age at blood drawn (data not shown).
Table 2.
Comparison of the ratios of PlGF, VEGFR-1, and sEng in normal pregnancy, severe preeclampsia and eclampsia.
| Ratio | Normal pregnancy (n=40) |
pa | Severe preeclampsia (n=40) |
pb | Eclampsia (n=20) |
pc |
|---|---|---|---|---|---|---|
| PlGF/sVEGFR-1* | 0.0580 (0.0240-0.167) |
<0.001 | 0.0047 (0.0025-0.0161) |
0.27 | 0.0043 (0.0011-0.008) |
<0.001 |
| PlGF/sEng* | 29.59 (9.86-64.98) |
<0.001 | 2.69 (1.30-9.45) |
0.22 | 1.56 (0.46-5.73) |
<0.001 |
| PlGF/(sEng × sVEGFR-1)* |
0.0048 (0.0011-0.021) |
<0.001 | 0.0002 (0.0001-0.0007) |
0.19 | 0.0001 (0.0000-0.0004 |
<0.001 |
| PlGF/(sEng + sVEGFR-1)* |
0.0578 (0.0239- 0.1669) |
<0.001 | 0.0047 (0.0025-0.016) |
0.27 | 0.0043 (0.0011-0.008) |
<0.001 |
Values are expressed as median (interquartile range)
Kruskal-Wallis, p < 0.001
pa: Normal pregnancy vs. Severe preeclampsia
pb: Severe preeclampsia vs. Eclampsia
pc: Normal pregnancy vs. Eclampsia
COMMENT
Principal findings of the study
1) At the time of diagnosis, the median maternal serum concentrations of sVEGFR-1 and sEng were significantly higher and those of PlGF significantly lower in patients with severe preeclampsia or eclampsia than in women with a normal pregnancy; 2) Similarly, the ratios PlGF/sVEGFR-1, PlGF/sEng, PlGF/(sEng × sVEGFR-1), and PlGF/(sEng + sVEGFR-1) were significantly lower in patients with severe preeclampsia or eclampsia than those with a normal pregnancy; 3) In contrast, all 3 analytes and their ratios were comparable at the time of diagnosis in maternal circulation of patients with severe preeclampsia and those with eclampsia.
A large body of evidence supports the notion that an imbalance between angiogenic and anti-angiogenic factors in maternal circulation plays a critical role in the pathogenesis of preeclampsia. Increased concentrations of placental-derived anti-angiogenic factors (i.e., sVEGFR-1 and sEng) and decreased concentrations of free angiogenic factors (i.e., VEGF and PlGF) have been reported in maternal blood at the time of the clinical diagnosis of preeclampsia.9-11;13;15-17;21;22;24;26-46;49;52-56;58;65-67;70;71 Levine et al.20 reported that the concentrations of sVEGFR-1 begin to increase about five weeks prior to the manifestation of preeclampsia with a parallel decrease in free PlGF and free VEGF concentrations. Moreover, the authors reported that the degree of angiogenic/anti-angiogenic imbalance was associated with disease severity. Indeed, women with preterm preeclampsia or preeclampsia with a small-for-gestational (SGA) neonate had a more severe imbalance between these angiogenic/anti-angiogenic factors than women with term preeclampsia of preeclampsia without SGA.20 A correlation between severity of preeclampsia and elevated concentrations of anti-angiogenic factors has been also demonstrated by others.22;35;39;67 Venkatesha et al.35 reported that the concentrations of sEng were three-, five-, and tenfold higher in women with mild preeclampsia, severe preeclampsia and HELLP syndrome, respectively, than gestational-age matched normal pregnant women. Similarly, Chaiworapongsa et al.22 (sVEGFR-1), Masuyama at al.39 (sVEGFR-1 and sEng), and Kim et al.67 (sEng) reported an association between elevated circulating concentrations of anti-angiogenic factors and severity of preeclampsia. However, to our knowledge, this is the first study that was specifically design to determine the concentration of angiogenic and anti-angiogenic factors in patients with eclampsia.
Eclampsia, the ultimate life-threatening complication of preeclampsia, is characterized by the occurrence of seizures in association with signs and symptoms of preeclampsia; yet, seizures as the first clinical manifestation of this complication of pregnancy is not an infrequent event.79;81;82;86 The eclamptic seizures are commonly attributed to hypertensive encephalopathy,79 and indeed, frequent findings in autopsy of women who died from eclampsia are cerebral edema and intracranial hemorrhage.87;88 However, Schwartz et al.91 reported that the baseline and maximal mean systolic and diastolic blood pressures were not significantly different between women with eclampsia with and without cerebral edema on MRI, and it has been proposed that the edema may be secondary to endothelial dysfunction.64;91 The increased concentrations of anti-angiogenic factors in maternal circulation have been hypothesized to be related to the extensive endothelial dysfunction implicated in the pathogenesis of preeclampsia.15 Indeed overexpression of sFlt1 and sEng in animals models have been associated not only with features of severe preeclampsia, but also cerebral edema that resembles what is noted in human eclampsia.98
In the present study, and consistent with previous reports, women with severe preeclampsia had a higher median serum concentration of sVEGFR-1 and sEng and a lower median PlGF concentration than normal pregnant women. Our study further demonstrates that women with eclampsia have a similar imbalance in angiogenic/anti-angiogenic factors as women with preeclampsia. However, there was no significant difference in the degree of angiogenic/anti-angiogenic imbalance (i.e., the median concentration of sVEGFR-1, sEng, PlGF, and their ratios) between women with severe preeclampsia and those with eclampsia. These findings suggest that the degree of angiogenic/anti-angiogenic imbalance per se is not associated with eclampsia. Thus, it seems that other factors rather than the maternal circulating concentration of angiogenic and anti-angiogenic factors may be involved in predisposing women to eclampsia.
Strength and limitation of the study
To our knowledge, this is the first study that specifically examines the changes in maternal serum concentrations of PlGF, sVEGFR-1, and sEng in patients with antepartum eclampsia. This is not the first report regarding angiogenic/anti angiogenic factors in preeclampsia to include patients with eclampsia; however, previous studies included such patients as part of the severe preeclampsia group.20;25 Furthermore, we excluded patients with HELLP syndrome or post-partum eclampsia from this study, because both HELLP syndrome35 and the delivery of the placenta69;99 have been previously shown to be associated with changes in angiogenic/ anti-angiogenic factors. Thus, one of the limitations of this study relies on the relative small number of subjects with antepartum eclampsia without concomitant HELLP syndrome. Indeed, there was a trend toward higher median concentrations of sVEGFR-1 and sEng and a lower median concentration of PlGF in patients with eclampsia than in those with severe preeclampsia. It is possible that a higher sample size would reveal significant difference between these two entities. Another limitation is the fact that some patients with eclampsia were in active labor at the time of seizures and blood drawn. To date, the only study to examine the effect of the process of labor on anti-angiogenic factors concentration demonstrated a higher concentration of sVEGFR-1 (but not of sEng) in women with pre-eclampsia at full-dilatation compared to the pre-labor concentration.69 The small sample size and different gestational age at blood drawn of the current study preclude us from determining whether labor itself could have influence our results.
In conclusion, our findings suggest that, similar to patients with severe preeclampsia, patients with eclampsia have increased maternal circulating concentration of the anti-angiogenic factors, sVEGFR-1 and sEng, and decreased concentrations of the angiogenic factor PlGF compared to women with a normal pregnancy. However, the concentrations of these 3 analytes and their ratios could not differentiate between patients with severe preeclampsia and those who experienced eclampsia during pregnancy. Collectively, these findings suggest that the severity of the angiogenic imbalance is not associated (at least not independently) with the presence of eclampsia. Further studies may be warranted, with a larger sample size as well as with a longitudinal design in order to determine whether one, or more, of the angiogenic/anti-angiogenic factors have a predictive value and a role in the pathophysiology of eclampsia.
CONDENSATION.
Eclampsia, similar to severe preeclampsia, is associated with maternal circulating concentration of sVEGFR-1 and sEng and lower concentrations of PlGF than normal pregnant women.
Acknowledgment
This research was supported in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern.Fetal Neonatal Med. 2009;22:636–39. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 2.Di Renzo GC. The great obstetrical syndromes. J Matern.Fetal Neonatal Med. 2009;22:633–35. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 3.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SA, Schiff E, Kao L, Sibai BM. Neonatal outcome after preterm delivery for preeclampsia. Am J Obstet Gynecol. 1995;172:1785–88. doi: 10.1016/0002-9378(95)91412-9. [DOI] [PubMed] [Google Scholar]
- 5.MacKay AP, Berg CJ, Atrash HK. Pregnancy-related mortality from preeclampsia and eclampsia. Obstet.Gynecol. 2001;97:533–38. doi: 10.1016/s0029-7844(00)01223-0. [DOI] [PubMed] [Google Scholar]
- 6.ACOG practice bulletin Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 7.Kullima AA, Kawuwa MB, Audu BM, Geidam AD, Mairiga AG. Trends in maternal mortality in a tertiary institution in Northern Nigeria. Ann.Afr.Med. 2009;8:221–24. doi: 10.4103/1596-3519.59575. [DOI] [PubMed] [Google Scholar]
- 8.Schutte JM, Steegers EA, Schuitemaker NW, Santema JG, de BK, Pel M, et al. Rise in maternal mortality in the Netherlands. BJOG. 2009 doi: 10.1111/j.1471-0528.2009.02382.x. [DOI] [PubMed] [Google Scholar]
- 9.Kupferminc MJ, Daniel Y, Englender T, Baram A, Many A, Jaffa AJ, et al. Vascular endothelial growth factor is increased in patients with preeclampsia. Am.J.Reprod.Immunol. 1997;38:302–06. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 10.Lyall F, Greer IA, Boswell F, Fleming R. Suppression of serum vascular endothelial growth factor immunoreactivity in normal pregnancy and in pre-eclampsia. Br.J.Obstet.Gynaecol. 1997;104:223–28. doi: 10.1111/j.1471-0528.1997.tb11050.x. [DOI] [PubMed] [Google Scholar]
- 11.Torry DS, Wang HS, Wang TH, Caudle MR, Torry RJ. Preeclampsia is associated with reduced serum levels of placenta growth factor. Am.J.Obstet.Gynecol. 1998;179:1539–44. doi: 10.1016/s0002-9378(98)70021-3. [DOI] [PubMed] [Google Scholar]
- 12.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am.J.Obstet.Gynecol. 2001;184:1267–72. doi: 10.1067/mob.2001.113129. [DOI] [PubMed] [Google Scholar]
- 13.Bosio PM, Wheeler T, Anthony F, Conroy R, O’herlihy C, McKenna P. Maternal plasma vascular endothelial growth factor concentrations in normal and hypertensive pregnancies and their relationship to peripheral vascular resistance. Am J Obstet.Gynecol. 2001;184:146–52. doi: 10.1067/mob.2001.108342. [DOI] [PubMed] [Google Scholar]
- 14.Tjoa ML, van Vugt JM, Mulders MA, Schutgens RB, Oudejans CB, van Wijk IJ. Plasma placenta growth factor levels in midtrimester pregnancies. Obstet Gynecol. 2001;98:600–07. doi: 10.1016/s0029-7844(01)01497-1. [DOI] [PubMed] [Google Scholar]
- 15.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin.Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsatsaris V, Goffin F, Munaut C, Brichant JF, Pignon MR, Noel A, et al. Overexpression of the soluble vascular endothelial growth factor receptor in preeclamptic patients: pathophysiological consequences 84. J.Clin.Endocrinol.Metab. 2003;88:5555–63. doi: 10.1210/jc.2003-030528. [DOI] [PubMed] [Google Scholar]
- 17.Koga K, Osuga Y, Yoshino O, Hirota Y, Ruimeng X, Hirata T, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin.Endocrinol.Metab. 2003;88:2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 18.Ahmad S, Ahmed A. Elevated placental soluble vascular endothelial growth factor receptor-1 inhibits angiogenesis in preeclampsia. Circ.Res. 2004;95:884–91. doi: 10.1161/01.RES.0000147365.86159.f5. [DOI] [PubMed] [Google Scholar]
- 19.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens.Pregnancy. 2004;23:101–11. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 20.Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, et al. Circulating angiogenic factors and the risk of preeclampsia. N.Engl.J.Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 21.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–46. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Chaiworapongsa T, Romero R, Espinoza J, Bujold E, Mee KY, Goncalves LF, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am.J Obstet Gynecol. 2004;190:1541–47. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 23.Thadhani R, Mutter WP, Wolf M, Levine RJ, Taylor RN, Sukhatme VP, et al. First trimester placental growth factor and soluble fms-like tyrosine kinase 1 and risk for preeclampsia. J Clin.Endocrinol.Metab. 2004;89:770–75. doi: 10.1210/jc.2003-031244. [DOI] [PubMed] [Google Scholar]
- 24.Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46:1077–85. doi: 10.1161/01.HYP.0000187899.34379.b0. [DOI] [PubMed] [Google Scholar]
- 25.Chaiworapongsa T, Romero R, Kim YM, Kim GJ, Kim MR, Espinoza J, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J.Matern.Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 26.Bdolah Y, Karumanchi SA, Sachs BP. Recent advances in understanding of preeclampsia. Croat.Med.J. 2005;46:728–36. [PubMed] [Google Scholar]
- 27.Levine RJ, Karumanchi SA. Circulating Angiogenic Factors in Preeclampsia. Clin.Obstet Gynecol. 2005;48:372–86. doi: 10.1097/01.grf.0000160313.82606.d7. [DOI] [PubMed] [Google Scholar]
- 28.Maynard SE, Venkatesha S, Thadhani R, Karumanchi SA. Soluble Fms-like tyrosine kinase 1 and endothelial dysfunction in the pathogenesis of preeclampsia. Pediatr.Res. 2005;57:1R–7R. doi: 10.1203/01.PDR.0000159567.85157.B7. [DOI] [PubMed] [Google Scholar]
- 29.Rajakumar A, Michael HM, Rajakumar PA, Shibata E, Hubel CA, Karumanchi SA, et al. Extra-placental expression of vascular endothelial growth factor receptor-1, (Flt-1) and soluble Flt-1 (sFlt-1), by peripheral blood mononuclear cells (PBMCs) in normotensive and preeclamptic pregnant women. Placenta. 2005;26:563–73. doi: 10.1016/j.placenta.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–94. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 31.Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J.Clin.Endocrinol.Metab. 2005;90:4895–903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- 32.Staff AC, Braekke K, Harsem NK, Lyberg T, Holthe MR. Circulating concentrations of sFlt1 (soluble fms-like tyrosine kinase 1) in fetal and maternal serum during pre-eclampsia. Eur.J.Obstet.Gynecol.Reprod.Biol. 2005;122:33–39. doi: 10.1016/j.ejogrb.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Shen H, Liu H, Chen H, Guo Y, Zhang M, Xu X, et al. Analysis of placental growth factor in placentas of normal pregnant women and women with hypertensive disorders of pregnancy. J Huazhong.Univ Sci.Technolog.Med Sci. 2006;26:116–19. doi: 10.1007/BF02828055. [DOI] [PubMed] [Google Scholar]
- 34.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N.Engl.J Med. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat.Med. 2006;12:642–49. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 36.Aggarwal PK, Jain V, Sakhuja V, Karumanchi SA, Jha V. Low urinary placental growth factor is a marker of pre-eclampsia. Kidney Int. 2006;69:621–24. doi: 10.1038/sj.ki.5000075. [DOI] [PubMed] [Google Scholar]
- 37.Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am.J.Obstet.Gynecol. 2006;195:201–07. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Levine RJ, Qian C, Maynard SE, Yu KF, Epstein FH, Karumanchi SA. Serum sFlt1 concentration during preeclampsia and mid trimester blood pressure in healthy nulliparous women. Am.J.Obstet.Gynecol. 2006;194:1034–41. doi: 10.1016/j.ajog.2005.10.192. [DOI] [PubMed] [Google Scholar]
- 39.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am.J.Obstet.Gynecol. 2006;194:551–56. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Muller PR, James AH, Murtha AP, Yonish B, Jamison MG, Dekker G. Circulating angiogenic factors and abnormal uterine artery Doppler velocimetry in the second trimester. Hypertens.Pregnancy. 2006;25:183–92. doi: 10.1080/10641950600912968. [DOI] [PubMed] [Google Scholar]
- 41.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am.J.Obstet.Gynecol. 2006;195:255–59. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 42.Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, et al. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation 158. J.Clin.Endocrinol.Metab. 2006;91:180–84. doi: 10.1210/jc.2005-1076. [DOI] [PubMed] [Google Scholar]
- 43.Tjoa ML, Levine RJ, Karumanchi SA. Angiogenic factors and preeclampsia. Front Biosci. 2007;12:2395–402. doi: 10.2741/2241. 2395-402. [DOI] [PubMed] [Google Scholar]
- 44.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, et al. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertens.Res. 2007;30:151–59. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 45.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–74. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 46.Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stepan H, Unversucht A, Wessel N, Faber R. Predictive value of maternal angiogenic factors in second trimester pregnancies with abnormal uterine perfusion. Hypertension. 2007;49:818–24. doi: 10.1161/01.HYP.0000258404.21552.a3. [DOI] [PubMed] [Google Scholar]
- 48.Simas TA Moore, Crawford SL, Solitro MJ, Frost SC, Meyer BA, Maynard SE. Angiogenic factors for the prediction of preeclampsia in high-risk women. Am J Obstet.Gynecol. 2007;197:244–48. doi: 10.1016/j.ajog.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 49.Rana S, Karumanchi SA, Levine RJ, Venkatesha S, Rauh-Hain JA, Tamez H, et al. Sequential changes in antiangiogenic factors in early pregnancy and risk of developing preeclampsia. Hypertension. 2007;50:137–42. doi: 10.1161/HYPERTENSIONAHA.107.087700. [DOI] [PubMed] [Google Scholar]
- 50.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia 3168. Am J Obstet.Gynecol. 2007;196:239–6. doi: 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- 51.Robinson CJ, Johnson DD. Soluble endoglin as a second-trimester marker for preeclampsia. Am.J.Obstet.Gynecol. 2007;197:174–75. doi: 10.1016/j.ajog.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 52.Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am.J.Obstet.Gynecol. 2007;197:176. doi: 10.1016/j.ajog.2007.03.036. [DOI] [PubMed] [Google Scholar]
- 53.Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia 3183. Am J Obstet.Gynecol. 2007;197:211–14. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern.Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc.Res. 2008;75:1–8. doi: 10.1016/j.mvr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Teixeira PG, Cabral AC, Andrade SP, Reis ZS, da Cruz LP, Pereira JB, et al. Placental growth factor (PlGF) is a surrogate marker in preeclamptic hypertension. Hypertens.Pregnancy. 2008;27:65–73. doi: 10.1080/10641950701825937. [DOI] [PubMed] [Google Scholar]
- 57.De Vivo A, Baviera G, Giordano D, Todarello G, Corrado F, D’anna R. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet.Gynecol.Scand. 2008;87:837–42. doi: 10.1080/00016340802253759. [DOI] [PubMed] [Google Scholar]
- 58.Diab AE, El-Behery MM, Ebrahiem MA, Shehata AE. Angiogenic factors for the prediction of pre-eclampsia in women with abnormal midtrimester uterine artery Doppler velocimetry. Int.J Gynaecol.Obstet. 2008;102:146–51. doi: 10.1016/j.ijgo.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 59.Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet.Gynecol. 2008;31:303–09. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 60.Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstet.Gynecol. 2008;111:1403–09. doi: 10.1097/AOG.0b013e3181719b7a. [DOI] [PubMed] [Google Scholar]
- 61.Erez O, Romero R, Espinoza J, Fu W, Todem D, Kusanovic JP, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern.Fetal Neonatal Med. 2008;21:279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sibai BM, Koch MA, Freire S, Pinto e Silva JL, Rudge MV, Martins-Costa S, et al. Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet.Gynecol. 2008;199:268–69. doi: 10.1016/j.ajog.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 63.Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet.Gynecol. 2008;198:175–76. doi: 10.1016/j.ajog.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 64.Maynard S, Epstein FH, Karumanchi SA. Preeclampsia and angiogenic imbalance. Annu.Rev.Med. 2008;59:61–78. doi: 10.1146/annurev.med.59.110106.214058. [DOI] [PubMed] [Google Scholar]
- 65.Foidart JM, Schaaps JP, Chantraine F, Munaut C, Lorquet S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia--a step forward but not the definitive answer. J Reprod.Immunol. 2009;82:106–11. doi: 10.1016/j.jri.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 66.Smith GC, Wear H. The perinatal implications of angiogenic factors. Curr.Opin.Obstet Gynecol. 2009;21:111–16. doi: 10.1097/GCO.0b013e328328cf7d. [DOI] [PubMed] [Google Scholar]
- 67.Kim YN, Lee DS, Jeong DH, Sung MS, Kim KT. The relationship of the level of circulating antiangiogenic factors to the clinical manifestations of preeclampsia. Prenat.Diagn. 2009;29:464–70. doi: 10.1002/pd.2203. [DOI] [PubMed] [Google Scholar]
- 68.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, et al. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern.Fetal Neonatal Med. 2009;22:1021–38. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reddy A, Suri S, Sargent IL, Redman CW, Muttukrishna S. Maternal circulating levels of activin A, inhibin A, sFlt-1 and endoglin at parturition in normal pregnancy and pre-eclampsia. PLoS.One. 2009;4:e4453. doi: 10.1371/journal.pone.0004453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salahuddin S, Lee Y, Vadnais M, Sachs BP, Karumanchi SA, Lim KH. Diagnostic utility of soluble fms-like tyrosine kinase 1 and soluble endoglin in hypertensive diseases of pregnancy. Am.J.Obstet.Gynecol. 2007;197:28–6. doi: 10.1016/j.ajog.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 71.Reuvekamp A, Velsing-Aarts FV, Poulina IE, Capello JJ, Duits AJ. Selective deficit of angiogenic growth factors characterises pregnancies complicated by pre-eclampsia. Br.J.Obstet.Gynaecol. 1999;106:1019–22. doi: 10.1111/j.1471-0528.1999.tb08107.x. [DOI] [PubMed] [Google Scholar]
- 72.Taylor RN, Grimwood J, Taylor RS, McMaster MT, Fisher SJ, North RA. Longitudinal serum concentrations of placental growth factor: evidence for abnormal placental angiogenesis in pathologic pregnancies. Am.J.Obstet.Gynecol. 2003;188:177–82. doi: 10.1067/mob.2003.111. [DOI] [PubMed] [Google Scholar]
- 73.Livingston JC, Haddad B, Gorski LA, Neblett P, Ahokas RA, Ramsey R, et al. Placenta growth factor is not an early marker for the development of severe preeclampsia. Am J Obstet.Gynecol. 2001;184:1218–20. doi: 10.1067/mob.2001.113877. [DOI] [PubMed] [Google Scholar]
- 74.Su YN, Lee CN, Cheng WF, Shau WY, Chow SN, Hsieh FJ. Decreased maternal serum placenta growth factor in early second trimester and preeclampsia. Obstet.Gynecol. 2001;97:898–904. doi: 10.1016/s0029-7844(01)01341-2. [DOI] [PubMed] [Google Scholar]
- 75.Chappell LC, Seed PT, Briley A, Kelly FJ, Hunt BJ, Charnock-Jones DS, et al. A longitudinal study of biochemical variables in women at risk of preeclampsia. Am J Obstet Gynecol. 2002;187:127–36. doi: 10.1067/mob.2002.122969. [DOI] [PubMed] [Google Scholar]
- 76.Parra M, Rodrigo R, Barja P, Bosco C, Fernandez V, Munoz H, et al. Screening test for preeclampsia through assessment of uteroplacental blood flow and biochemical markers of oxidative stress and endothelial dysfunction. Am J Obstet Gynecol. 2005;193:1486–91. doi: 10.1016/j.ajog.2005.02.109. [DOI] [PubMed] [Google Scholar]
- 77.Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet.Gynecol. 2008;32:732–39. doi: 10.1002/uog.6244. [DOI] [PubMed] [Google Scholar]
- 78.Baumann MU, Bersinger NA, Mohaupt MG, Raio L, Gerber S, Surbek DV. First-trimester serum levels of soluble endoglin and soluble fms-like tyrosine kinase-1 as first-trimester markers for late-onset preeclampsia. Am J Obstet.Gynecol. 2008;199:266. doi: 10.1016/j.ajog.2008.06.069. [DOI] [PubMed] [Google Scholar]
- 79.Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105:402–10. doi: 10.1097/01.AOG.0000152351.13671.99. [DOI] [PubMed] [Google Scholar]
- 80.Saftlas AF, Olson DR, Franks AL, Atrash HK, Pokras R. Epidemiology of preeclampsia and eclampsia in the United States, 1979-1986. Am J Obstet Gynecol. 1990;163:460–65. doi: 10.1016/0002-9378(90)91176-d. [DOI] [PubMed] [Google Scholar]
- 81.Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309:1395–400. doi: 10.1136/bmj.309.6966.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Katz VL, Farmer R, Kuller JA. Preeclampsia into eclampsia: toward a new paradigm. Am J Obstet Gynecol. 2000;182:1389–96. doi: 10.1067/mob.2000.106178. [DOI] [PubMed] [Google Scholar]
- 83.Mattar F, Sibai BM. Eclampsia. VIII. Risk factors for maternal morbidity. Am J Obstet Gynecol. 2000;182:307–12. doi: 10.1016/s0002-9378(00)70216-x. [DOI] [PubMed] [Google Scholar]
- 84.Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM. Late postpartum eclampsia: a preventable disease? Am J Obstet Gynecol. 2002;186:1174–77. doi: 10.1067/mob.2002.123824. [DOI] [PubMed] [Google Scholar]
- 85.Pritchard JA, Cunningham FG, Pritchard SA. The Parkland Memorial Hospital protocol for treatment of eclampsia: evaluation of 245 cases. Am J Obstet Gynecol. 1984;148:951–63. doi: 10.1016/0002-9378(84)90538-6. [DOI] [PubMed] [Google Scholar]
- 86.Noraihan MN, Sharda P, Jammal AB. Report of 50 cases of eclampsia. J Obstet Gynaecol.Res. 2005;31:302–09. doi: 10.1111/j.1447-0756.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 87.Richards AM, Moodley J, Graham DI, Bullock MR. Active management of the unconscious eclamptic patient. Br.J Obstet Gynaecol. 1986;93:554–62. doi: 10.1111/j.1471-0528.1986.tb07953.x. [DOI] [PubMed] [Google Scholar]
- 88.Lopez-Llera M. Main clinical types and subtypes of eclampsia. Am J Obstet Gynecol. 1992;166:4–9. doi: 10.1016/0002-9378(92)91816-s. [DOI] [PubMed] [Google Scholar]
- 89.Dahmus MA, Barton JR, Sibai BM. Cerebral imaging in eclampsia: magnetic resonance imaging versus computed tomography. Am J Obstet Gynecol. 1992;167:935–41. doi: 10.1016/s0002-9378(12)80015-9. [DOI] [PubMed] [Google Scholar]
- 90.Belfort MA, Grunewald C, Saade GR, Varner M, Nisell H. Preeclampsia may cause both overperfusion and underperfusion of the brain: a cerebral perfusion based model. Acta Obstet Gynecol Scand. 1999;78:586–91. [PubMed] [Google Scholar]
- 91.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, et al. Preeclampsia-eclampsia: clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217:371–76. doi: 10.1148/radiology.217.2.r00nv44371. [DOI] [PubMed] [Google Scholar]
- 92.Cunningham FG, Twickler D. Cerebral edema complicating eclampsia. Am J Obstet Gynecol. 2000;182:94–100. doi: 10.1016/s0002-9378(00)70496-0. [DOI] [PubMed] [Google Scholar]
- 93.Sibai BM. Hypertension. In: Gabbe SG, Niebyl JR, Simpson JL, editors. Obstetrics: normal and problem pregnancies. Churchill Livingstone; New York (NY): 2002. pp. 945–1004. [Google Scholar]
- 94.Zeeman GG, Fleckenstein JL, Twickler DM, Cunningham FG. Cerebral infarction in eclampsia. Am J Obstet Gynecol. 2004;190:714–20. doi: 10.1016/j.ajog.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 95.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–68. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 96.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 97.Barton JR, Sibai BM. Diagnosis and management of hemolysis, elevated liver enzymes, and low platelets syndrome. Clin.Perinatol. 2004;31:807–33. vii. doi: 10.1016/j.clp.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 98.Maharaj AS, Walshe TE, Saint-Geniez M, Venkatesha S, Maldonado AE, Himes NC, et al. VEGF and TGF-beta are required for the maintenance of the choroid plexus and ependyma. J Exp.Med. 2008;205:491–501. doi: 10.1084/jem.20072041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Powers RW, Roberts JM, Cooper KM, Gallaher MJ, Frank MP, Harger GF, et al. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. Am J Obstet Gynecol. 2005;193:185–91. doi: 10.1016/j.ajog.2004.11.038. [DOI] [PubMed] [Google Scholar]


