Abstract
Rationale
The phospholipid Platelet-activating Factor (PAF) stimulates all cells of the innate immune system, and numerous cardiovascular cells. A single enzyme [plasma PAF acetylhydrolase (PAF-AH) or Lipoprotein-associated Phospholipase A2 (Lp-PLA2)] in plasma hydrolyzes PAF, but significant controversy exists whether its action is pro- or anti-inflammatory and accordingly whether its inhibition will slow cardiovascular disease.
Objective
We sought to define how PAF and related short chain oxidized phospholipids turnover in vivo and the role of PAF acetylhydrolase/Lp-PLA2 in this process.
Methods and Results
[3H-acetyl]PAF was hydrolyzed by murine or human plasma (t1/2 3 and 7 min, respectively), but injected [3H-acetyl]PAF disappeared from murine circulation more quickly (t1/2 < 30 sec). [3H]PAF clearance was unchanged in PAF receptor−/− animals, or over the 1st two half-lives in PAF-AH−/− animals. [3H]PAF turnover was reduced by co-injecting excess unlabeled PAF or an oxidatively truncated phospholipid, and [3H]PAF clearance was slowed in hyperlipidemic apoE−/− mice with excess circulating oxidatively truncated phospholipids. [3H]PAF, fluorescent NBD-PAF, or fluorescent oxidatively truncated phospholipid were primarily accumulated by liver and lung, and were transported into endothelium as intact phospholipids through a common mechanism involving TMEM30a.
Conclusions
Circulating PAF and oxidized phospholipids are continually and rapidly cleared, and hence continually and rapidly produced. Saturable PAF receptor-independent transport, rather than just intravascular hydrolysis, controls circulating inflammatory and pro-apoptotic oxidized phospholipid mediators. Intravascular PAF has access to intracellular compartments. Inflammatory and pro-apoptotic phospholipids may accumulate in the circulation as transport is overwhelmed by substrates in hyperlipidemia.
Keywords: PAF, oxidized phospholipids, phospholipid transport, lipoprotein-associated phospholipase A2
The phospholipid Platelet-activating Factor (PAF) stimulates a single receptor (PAFR) expressed by platelets, but also by nearly every other cell of the innate immune system, and by numerous cells of the cardiovascular system. PAF and its receptor regulate inflammation, atherogenesis, cardiac rhythm, liver contractility, body temperature, and vascular tone.1–4 PAF is remarkably potent—activating cells at concentrations of 10−12 M5—suggesting its presence is tightly controlled. However its blood concentration can increase, for instance after ischemic stroke6 and inflammatory stress7.
A single enzyme in blood metabolizes PAF8, 9 and bioactive10 oxidatively-modified phospholipids27 that accumulate in atherosclerosis11 and in response to other oxidative insults12. Common names for this lipoprotein-associated enzyme are plasma PAF acetylhydrolase (PAF-AH) or lipoprotein-associated phospholipase (Lp-PLA2), but its gene name is group VII phospholipase A2 (PLA2G7). PAF as a relatively soluble phospholipid is bound to lipoproteins, mainly albumin13, with rapid exchange from lipoprotein particles14 allowing access both to PLA2G7 and the PAF receptor.
There is significant controversy whether PLA2G7 is protective or promotes chronic inflammation, cardiovascular disease and atherogenesis,15 and direct manipulation of this activity in humans has not clarified the issue. A phase III trial administering recombinant human plasma PAF acetylhydrolase to septic patients did not decrease mortality.16 Conversely, chronic suppression of circulating enzymatic activity with the experimental drug Darapladib® did not reduce circulating oxidized phospholipids nor alter human plaque volume.17 Still, plasma PAF acetylhydrolase is a strong and independent risk factor for cardiovascular disease,18 and a large (~15,000 individuals) phase III trial is underway to determine if Lp-PLA2 inhibition reduces incidence of first occurrence of major adverse cardiovascular events.
The focus on PAF-AH/Lp-PLA2 in controlling the amounts of circulating PAF or oxidized phospholipids has yet to incorporate the results of a single early report indicating that PAF is rapidly cleared from rat circulation by transport into tissues.19 Whether this uptake process is slower or faster than PLA2G7 action on PAF or oxidized phospholipids in plasma has not been examined, nor is it known whether transport of PAF and short chain lipids occurs in species other than rat.
Here, we show that PAF is cleared from murine circulation in seconds as an intact molecule through saturable uptake by endothelium. This disappearance was faster than PAF hydrolysis in human or murine blood, and ablation of PLA2G7 had no immediate effect on PAF clearance. The implications are that transport rather than intravascular hydrolysis primarily controls circulating PAF levels; that PAF and oxidized phospholipids are continually—and rapidly—released to the circulation to achieve steady state levels; that an excess of oxidized phospholipids promotes inflammation by competitively slowing PAF clearance; and, that PAF catabolism primarily occurs in the intracellular compartment of tissues, reducing the role for circulating—although perhaps not intracellular—PLA2G7 in in vivo PAF catabolism.
Methods
An expanded Methods section is available in the Online Data Supplement at http://circ.ahajournals.org.
In vivo Phospholipid Metabolism
[3H-acetyl]PAF in 0.5% albumin in PBS, with or without a 1,000-molar excess of inactive enantiomeric PAF or a 2000-fold excess of synthetic AzPC, was injected into the retro-orbital plexus.
Phospholipid Mass Spectrometry
Mass spectrometric analyses were performed on-line using electrospray ionization tandem mass spectrometry in the positive ion mode with multiple reaction monitoring using the molecular cation [MH]+ and the m/z 184 daughter phosphocholine ion.
Immunohistochemistry
Fluorescent NBD-PAF or NBD-AzLPAF were introduced by retro-orbital injection. Organs were recovered 5 min later after extensively flushing the vasculature with PBS. Organs were excised, immediately frozen in liquid nitrogen, and embedded in OTC media for sectioning.
Statistics
The data represent the means ± S.D. of the stated number of samples. The statistical analyses used a paired Student’s t test. For all of these hypotheses, the significance level was 0.05.
Results
PAF Disappeared from Circulation Faster than Hydrolysis in Plasma
The half life of [3H-acetyl]PAF in human plasma was approximately 7 min (Fig. 1A), as we previously observed.22 We also observed that the turnover of [3H-acetyl]PAF was somewhat quicker in plasma than in whole blood where some 40% of plasma volume is displaced by blood cell volume. A similar pattern of efficient hydrolysis of [3H]PAF in plasma relative to blood held when murine material was examined (Fig. 1A) where [3H]PAF was hydrolyzed in plasma with a t1/2 of ~ 3 min.
Figure 1. [3H]PAF Clearance in vitro and in vivo.
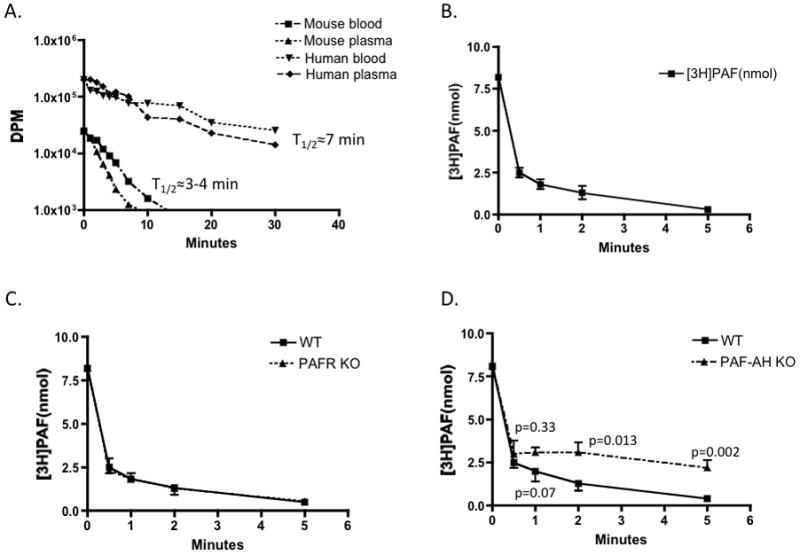
A. In vitro PAF hydrolysis by human or mouse (10 weeks old; n=5) plasma or blood. [3H-acetyl]PAF (3.4 μCi) was added to 500 μl of plasma or whole blood to retain the ratio used in the following in vivo clearance experiments. Hydrolysis in aliquots taken at the stated times was stopped by mixing into an organic phase (methanol/chloroform; 2.5;1.25 vol/vol), splitting the resulting monophase with additional 0.1M acetic acid and chloroform (1 vol each) and recovering the hydrolyzed [3H]acetate in the aqueous phase for quantitation by liquid scintillation counting. Data represent two independent experiments. B. Half-life of [3H-acetyl]PAF in vivo. [3H-acetyl]PAF (10 μCi) in 100 μl of PBS containing 0.5% human serum albumin was injected into 10 week old wild-type or PAF receptor null male mice (n=5) through the retro-orbital plexus. At the stated post-injection times, 100 μl of blood was collected by cardiac puncture, and intact [3H-acetyl]PAF was recovered by organic extraction for quantitation by liquid scintillation counting. C. [3H]PAF turnover in PAFR−/− mice. [3H-acetyl]PAF turnover in PAFR−/− mice and wild-type BL6 mice (n=5) performed on the same day with the same [3H]PAF preparation. Representative of two independent experiments. D. [3H]PAF turnover in PLA2G7−/− mice. [3H-acetyl]PAF turnover in PLA2G7−/− mice (n=5) and wild-type BL6 mice (n=3) was assessed on the same day as above.
We examined the rate of [3H-acetyl]PAF turnover in vivo with the expectation from the above observations that it would require approximately 3 min to hydrolyze half the [3H-acetyl]PAF we introduced into murine circulation by retro-orbital injection. Instead we found that the rate of [3H-acetyl]PAF disappearance was significantly faster than this, with a t1/2 of less than 30 seconds (Fig. 1B). We were technically unable to perform more blood collections over these short times to better define the precise t1/2, but it is apparent that the turnover in vivo is far faster than in ex vivo blood samples.
In Vivo PAF Clearance was Independent of the PAF Receptor
PAF internalization by murine macrophages is a function of the PAF receptor,23 the only known entity to selectively recognize the structural features of PAF. The PAF receptor of circulating cells can account for little PAF clearance, as shown above, but microvascular endothelial cells express PAF receptors that are positioned to recognize and potentially clear circulating PAF. The rate of [3H]PAF clearance, however, was identical in parental BL6 mice and those with genetically ablated PAF receptors (Fig. 1C).
In Vivo PAF Clearance initially was Independent of PAF-AH/ Lp-PLA2
Plasma PLA2G7 (PAF-AH/ Lp-PLA2) is the sole enzyme in blood to effectively degrade PAF25, and a global PLA2G7 knockout has now been found to sensitize animals to necrotizing enterocolitis26. [3H]PAF clearance in PLA2G7−/− mice was not different over the 1st minute when ~75% of the label was cleared (Fig. 1D). After this time, however, turnover was significantly delayed by loss of this enzyme.
Tissue Uptake and Intracellular PAF Catabolism
PAF that rapidly disappeared from the circulation might be internalized by tissues as the intact phospholipid and then rapidly metabolized as it enters, might be fully recovered from one or more of these organs as the intact phospholipid, or may be distributed between these two outcomes. We collected the major organs 5 min after [3H-acetyl]PAF injection, a time when vascular PAF had been fully cleared. We first extensively perfused the animals with buffer to remove residual blood-borne label before harvesting the organs. Most soft tissues accumulated intact [3H]PAF, with liver and lung accounting for the bulk of this accumulation (Fig. 2A). In part, preferential accumulation of [3H]PAF by liver reflected organ size, and when accumulation was normalized by wet weight it is apparent that [3H]PAF was preferentially accumulated by lung and spleen (Fig. 2B). These data show PAF was accumulated by least some organs as the intact phospholipid because the solvent extraction separates this lipid from its aqueous [3H]acetate hydrolytic product. When tissue [3H]acetate was quantified, it was apparent that the [3H-acetyl]PAF had been extensively hydrolyzed in liver (Fig. 2C) where only about 3% of the label remained as the intact phospholipid 5 min post-injection (compare 2A with 2C). Similarly, only about 7% of the label remained as PAF in kidney, while lung retained nearly half its label as intact PAF. This pattern is congruent with the abundance of type II PAF acetylhydrolase (an intracellular enzyme with 41% identity to PLA2G7 with a similar substrate preference) in liver = kidney ≫ lung.27
Figure 2. Tissue Accumulation of [3H-acetyl]PAF and its [3H]Acetate Hydrolytic Product.
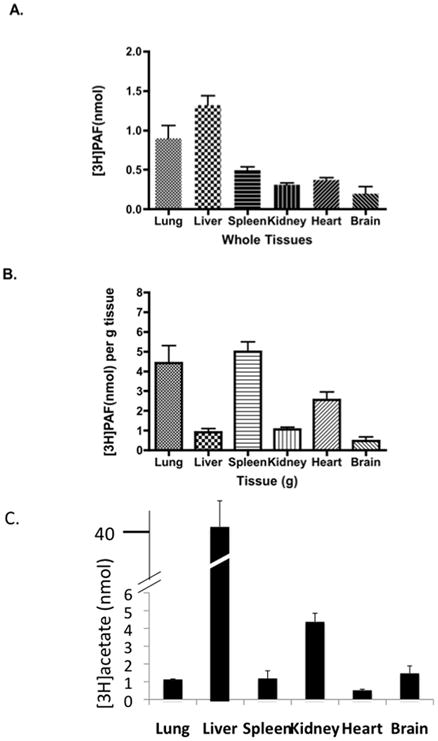
[3H-acetyl]PAF (10 μCi) in 0.5% human serum albumin in PBS was injected (100 μl) into 10 week old anesthetized wild-type mice (n=5) through the retro-orbital plexus. After 5 minutes, the mice were perfused with 30 ml PBS, the organs were rapidly excised in the sequence presented from left to right in the figure, and immediately frozen in liquid nitrogen. The entire organ was weighed, PAF was extracted from weighed tissue specimens after mincing and separating [3H]PAF from its [3H]acetate hydrolytic product by methanol/chloroform extraction20. A. Intact [3H-acetyl]PAF in whole organs. B. Intact [3H-acetyl]PAF per gram of organ weight. C. Total [3H]acetate present in each tissue. Data are from one of two independent experiments.
Vascular Endothelium Accumulated Vascular PAF
We sought to identify where intravascular PAF accumulated in the soft organs by introducing NBD-labeled PAF by the retro-orbital route and collecting the organs 5 min later after exsanguination and buffer perfusion as before. We found sections of lung, liver and kidney fluoresced brightly in this experiment, heart less so, and brain not at all (Fig. 3A). We also found clearly delineated patches of bright fluorescence in spleen. The accumulated fluorescence marks intact PAF, and its NBD-lysoPAF and NBD-phosphatidylcholine metabolites, since the NBD label in the lyso-PAF backbone is in the non-hydrolyzable sn-1 alkyl residue. Thin layer chromatography confirmed (not shown) fluorescence was confined to these complex phospholipids and had not been converted to a neutral lipid.
Figure 3. Tissue Distribution of Fluorescent NBD-PAF and Co-localization with Endothelium.
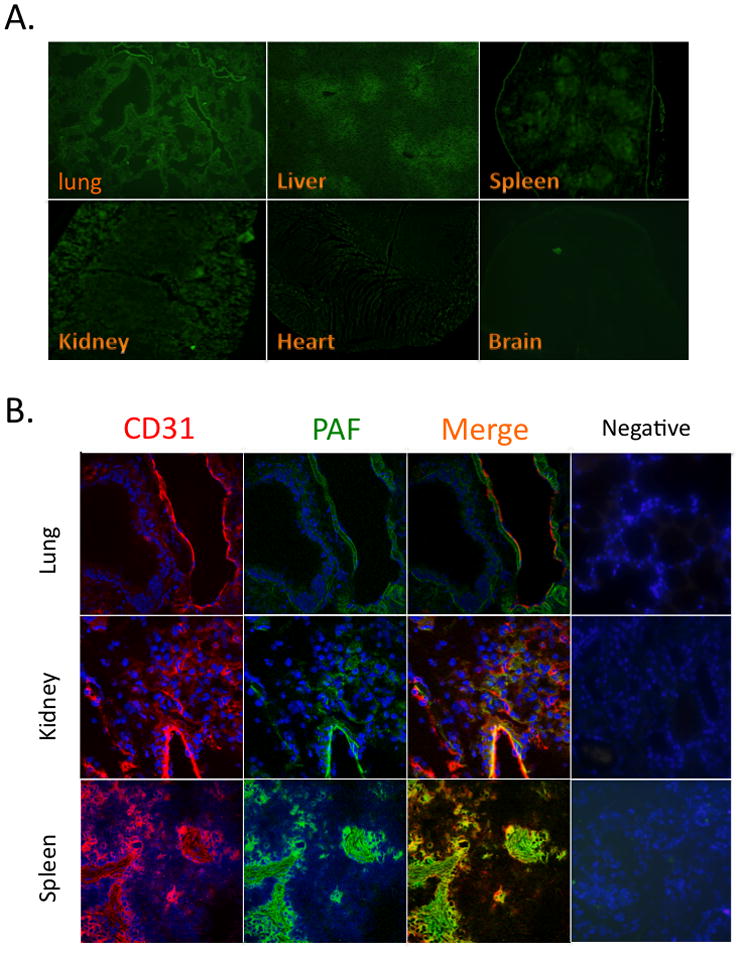
A. Tissue distribution. Fluorescent NBD labeled-PAF (10 μg in 100 μl PBS containing 0.5% human serum albumin) was injected, or nothing, into 10 week old wild-type male mice through the retro-orbital plexus as before. The mice were extensively perfused with PBS 5 min post-injection, organs were extracted in sequence, and frozen immediately. The tissue was OTC embedded, sectioned, stained for endothelial cell CD31, and fluorescent micrographs were captured at 4X magnification. B. Endothelium and PAF co-staining. NBD-PAF labeled sections (top: lung; middle, kidney; lower, spleen) were stained, or not (right), with anti-CD31 and Alexa647-conjugated secondary antibody before fluorescent micrographs (63X magnification) were generated by confocal laser scanning microscopy.
Immunohistochemical detection of endothelial CD31 indicated that fluorescent PAF and its phospholipid metabolites primarily accumulated in endothelium (Fig. 3B). The large vessel adjacent to an unstained bronchiole shows strong co-localization of fluorescent PAF and CD31 (an endothelial cell and platelet specific marker), but also that the phospholipid had been released into the subluminal compartment. Similarly, endothelium of a large renal vessel was strongly positive for the NBD label, as were numerous smaller vessels. The strongly punctate staining of spleen was revealed to reflect the distribution of white pulp vessels with little staining away from the vessels (not shown).
A Short-chain Phospholipid Competed for in vivo PAF Clearance
We wished to determine whether clearance of trace quantities of [3H]PAF was saturable, and so would be slowed by high PAF concentrations. We cannot test this in vivo with PAF because of its strong vasoactivity, but the stereoisomer of PAF, while chemically identical, is not recognized by the PAF receptor28 and is not vasoactive. We observed that a 1000-fold molar excess of the entiomeric stereoisomer of PAF reduced the rate of clearance of [3H]PAF as the t1/2 increased from less than 30 seconds to about 3 min (Fig. 4Aleft).
Figure 4. PAF and an Oxidatively Truncated Phospholipid Compete for Uptake in vivo.
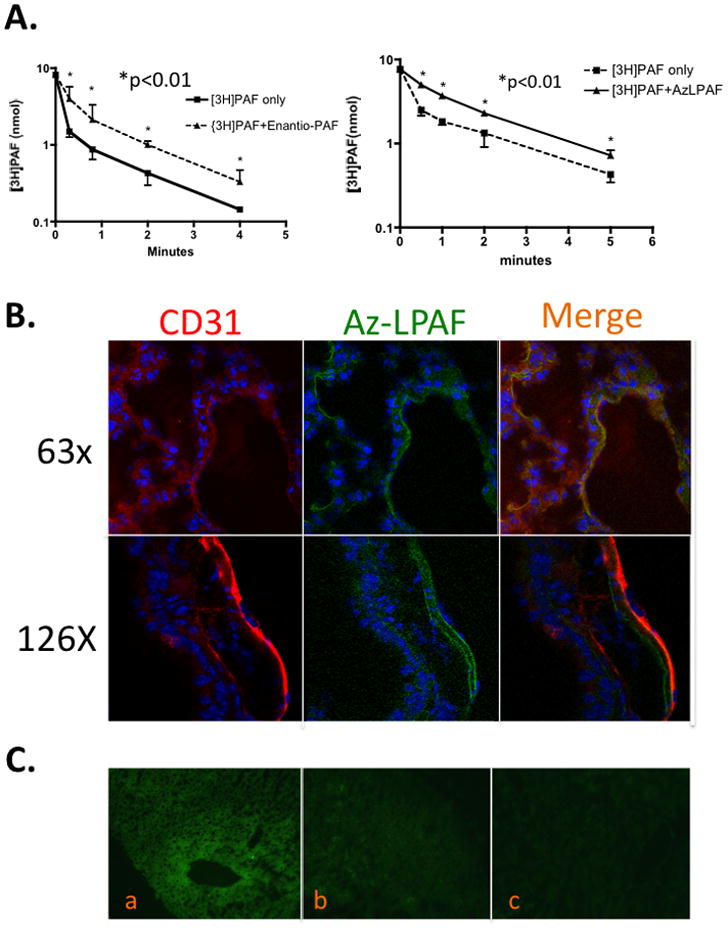
A. PAF or Az-lysoPAF competition for PAF [3H]PAF clearance. Left A trace concentration of [3H]PAF (10 μ Ci) was mixed with excess (10−6 M) enantiomeric PAF (3-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine) in 0.5% human serum albumin in PBS. Enantiomeric PAF is chemically identical to PAF, but the PAF receptor is not activated by this stereoisomer and so blood pressure and the inflammatory response are not altered as they would be in response to micromolar PAF. Blood samples were collected at the stated times and processed to quantitate [3H-acetyl]PAF as in Fig. 1. Right. A trace quantity of [3H]PAF (10 μ Ci) was mixed with excess (2×10−6 M) synthetic Az-lysoPAF and [3H]PAF clearance measured as before. B. Lung endothelium accumulates circulating Az-lysoPAF. NBD-labeled Az-lysoPAF was injected, animals were perfused with PBS 5 min later, and organs were harvested and processed and co-stained with anti-CD31 as in Fig. 3B. C. Short-chain phospholipids compete for labeled PAF uptake in precision-cut liver slices. Freshly isolated mouse liver was sectioned into 1,000 micron thick sections with a Krumdieck Tissue Slicer. The liver slices were treated for 1 minute with 100 ng/ml of fluorescent NBD-PAF in the absence (a) or presence (10 μg/ml) of unlabeled PAF (b), or Az-lysoPAF (c). The treated slices were placed in 2 ml PBS containing 5% human serum albumin, washed 3 times, immediately frozen in liquid nitrogen, and then embedded in OTC. Sections were examined by fluorescence microscopy and micrographs captured at 10X magnification.
An Oxidatively Truncated Phospholipid and PAF Share a Clearance Mechanism
PAF is a short-chain phospholipid—the sn-2 residue is a two carbon acetyl residue—and oxidatively truncated phospholipids with short sn-2 residues accumulate in the circulation in response to hyperlipidemia11 or oxidative stress12, which might slow PAF clearance through competition. We repeated the in vivo [3H]PAF clearance experiments in the presence of a large molar excess of chemically synthesized Az-LysoPAF, an abundant pro-apoptotic oxidatively truncated phospholipid29. Excess Az-LysoPAF also significantly reduced the rate of clearance of intravascular [3H-acetyl]PAF (Fig. 4A right). We next injected fluorescent Az-lysoPAF to determine whether this oxidatively truncated phospholipid was internalized, and whether this was by the same type of cells that acquired circulating PAF, to find that it also accumulated in endothelium and sub-endothelial structures (Fig. 4B).
We determined whether isolated liver tissue was able to accumulate extracellular PAF using precision-cut liver slices. In this approach, liver was sectioned into 1,000 micron thick slices with a Krumdieck Tissue Slicer that maintain organ ultrastructure while allowing cellular access to extracellular materials. Incubation of precision cut liver slices with NBD-PAF for one minute showed this fluorescent phospholipid was rapidly accumulated by liver cells (Fig. 4C), particularly in areas around the central vein. Inclusion of a 100-fold molar excess of PAF (here using biologically active PAF) greatly reduced fluorescent PAF uptake. The oxidatively truncated phospholipid Az-lysoPAF was similarly effective in reducing fluorescent PAF uptake. Both PAF and an oxidatively truncated phospholipid thus appear to compete for PAF uptake ex vivo in a structurally intact tissue.
Short Chain Choline Phospholipid Import Shares TMEM30a
Phospholipid import is undefined in mammals, but genetic approaches in S. cerevisiae show choline phospholipid uptake requires Lem3/DRS1 or DRS2 heterodimers.30 Humans express TMEM30 mRNA whose sequence is similar to Lem3, but unknown protein function. We find phospholipid uptake is reconstituted by human TMEM30a or human TMEM30a/yeast Lem3 chimeras in Lem3 deletion mutants, and TMEM30a knockdown reduces PAF uptake by CHO and Jurkat cells (Chen et al, submitted). We found that human endothelial cells also express mRNA encoding TMEM30a, and that siRNA to this sequence reduced its mRNA compared to cognate scrambled RNA (Fig. 5A). siRNA knockdown of TMEM30a also reduced uptake of fluorescent NBD-labeled phosphatidylcholine compared to cells transfected with scrambled RNA (Fig. 5B) in a quantitatively significant way (Fig. 5C). Endothelial cells internalize fluorescent BODIPY-labeled PAF (Fig. 5Dtop), which was suppressed in the presence of excess unlabeled PAF (Fig. 5D middle) or the short chain phospholipid Az-PC (Fig. 5D bottom). Short chain choline phospholipids enter endothelial cells, in part, through a common carrier that includes TMEM30a.
Figure 5. Endothelial Cell TMEM30a Aids Choline Phospholipid Uptake.
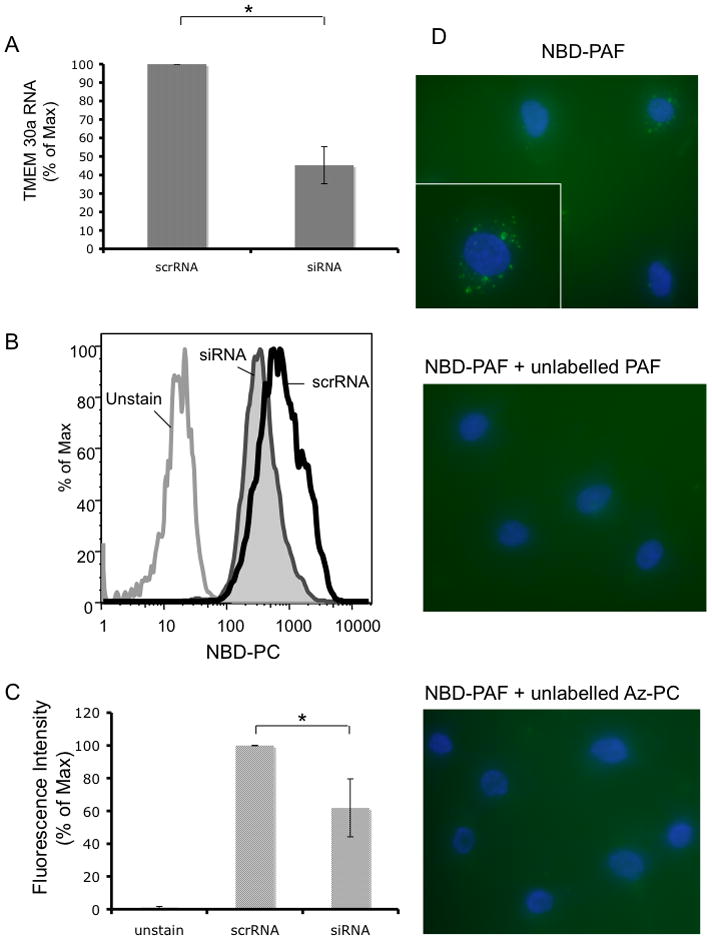
(A) HUVEC were transfected by nuclear poration with human TMEM30a or scrambled siRNA, and 48 h later the amount of TMEM30a mRNA was determined by qPCR (n=3; p< 0.01). (B) Cells were suspended, washed with HBSS twice and resuspended (2 × 106 cells/ml) in 1 μM NBD-PC for 10 min. The labeled cells were washed twice with HBSS containing 1% (w/v) albumin before flow cytometry. (C) Summation of NBD-PC uptake in three experiments (p<0.05). (D) HUVEC on glass cover slips were treated with BODIPY-PAF (100 ng/ml) alone (top), or in the presence (10 μg/ml) of unlabeled PAF (middle) or Az-PC (bottom) for 1 min before the media was removed, the cells washed with 5% albumin in PBS and the cells imaged at 60× or 100× (inset) Shown is representative data from one of two independent experiments.
[3H]PAF Clearance is Decreased in apoE−/− Mice with Enhanced Intravascular Levels of Short-chain Phospholipids
A bolus of short chain phospholipids slowed [3H]PAF clearance in vivo, and a series of such short chain phospholipids circulate in hyperlipidemic apoE−/− animals.11 We found PAF and Az-PC concentrations also were higher in the circulation of apoE−/− animals fed a high fat diet for 6 weeks compared to wild type animals on this diet (Fig 6A). We injected trace amounts of [3H]PAF into animals maintained on the high fat diet for 6 weeks to determine whether endogenous short chained phospholipids slowed PAF clearance. Indeed, [3H-acetyl]PAF disappeared significantly more slowly from the circulation of apoE−/− mice than BL6 control animals (Fig. 6B). We examined the tissue distribution of [3H]PAF in apoE−/− and wild-type animals on a high fat diet to determine whether uptake into all organs was uniformly altered. The data show all tissues of apoE−/− animals, except brain where significance was not attained, accumulated [3H]PAF more slowly than their wild-type counterparts (Fig. 6C).
Figure 6. Hyperlipidemia Slows in vivo PAF Clearance.
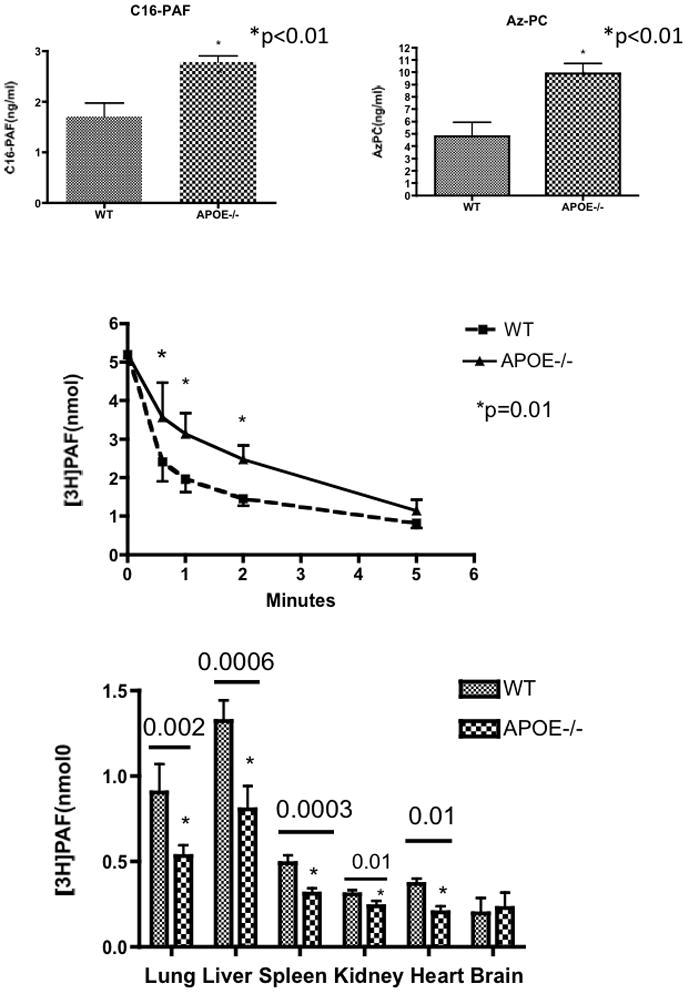
A. Circulating PAF and Az-PC are increased in apoE−/− mice on a high fat diet. Plasma was isolated from wild-type and apoE−/− mice (n=5) after six weeks on a high fat diet, deuterated d4-PAF was added as an internal standard, and phospholipids were extracted20 and purified over an HyperSep NH2 cartridge prior to reverse phase HPLC separation and analysis by electrospray ionization tandem mass spectrometry. C16:0-PAF was separated from isobaric lysophosphatidylcholine by normal phase chromatography prior to reverse phase chromatography. B. [3H-acetyl]PAF turnover. Clearance of [3H-acetyl]PAF was determined as in Fig. 1 in wild-type or ApoE−/− male mice after six weeks of a high fat diet (n=5). C. Tissue distribution of intact [3H-acetyl]PAF. [3H]PAF turnover was determined 5 min post-injection as in Fig. 2. All three panels present one of two independent experiments.
Discussion
PLA2G7 is the sole enzyme in plasma to appreciably catabolize PAF and short-chain phospholipid oxidation products. This is established by mutations in Japanese and other Asian populations where plasma from homozygous individuals who lack this enzyme cannot hydrolyze PAF, while plasma from heterozygous individuals hydrolyze PAF at half the rate of individuals with two wild-type alleles.31, 32 Despite this, individuals with reduced levels of PLA2G7 activity do not display rampant inflammatory responses anticipated from uncontrolled PAF accumulation,33, 34 nor does acute bronchoconstriction to inhaled PAF vary in these individuals.35 Additionally, a recent meta-analysis of ~26,000 individuals revealed PLA2G7 variants, such as 379V, were associated with modest changes in enzymatic activity, but were not associated with cardiovascular risk markers, coronary atheroma, or coronary heart disease.36
Here, we find that circulating PLA2G7 is not the only way PAF is cleared from blood. [3H]PAF clearance initially occurred through tissue uptake by a system employing TMEM30a that accepts choline phospholipids as transport substrates. Accordingly, at early times [3H]PAF clearance was unaffected by ablation of PLA2G7. However, the knockout also shows this enzyme does significantly participate to [3H]PAF turnover at later times or lower concentrations. This observation elucidates a basis for incongruence between circulating PLA2G7 enzymatic activity and pathophysiologic measures.
We propose that circulating PAF and phospholipid oxidation products accumulate in the inflammatory apoE−/− hyperlipidemic model because saturable transport limits their clearance. In hyperlipidemia, a plethora of short chain phospholipid oxidation products are available to compete and slow transport of PAF and the other biologically active phospholipids. In this way, hyperlipidemia can promote inflammation. Our studies were conducted in mice, where the PLA2G7 activity is 8.6 times that of humans44, suggesting hydrolysis in human circulation would be even less effective, although human PAF turnover is yet to be investigated.
PAF was primarily transported as the intact molecule since a significant portion of PAF and Az-LPAF were recovered from tissue as intact molecules, particularly in lung. Additionally, intact PAF was recovered well after it would have been hydrolyzed had it remained in the circulation. By example, 3 min post-injection when ~98% of intravascular PAF had been cleared from the circulation, only about half of this could have been hydrolyzed in plasma by this time.
In contrast, the majority of PAF was hydrolyzed in liver and kidney after internalization, and both hepatocytes and renal cells abundantly express type II intracellular PAF acetylhydrolase that also specifically hydrolyzes PAF.2, 4 Liver Kupffer cells, as differentiated tissue macrophages, additionally retain a portion of the PLA2G7 they make45, so intracellular PLA2G7 may contribute to PAF metabolism.
Uptake of intact PAF can have a biologic consequence because the PAF receptor is present in intracellular compartments,46 and the PAF receptor of isolated nuclei stimulates a Ca++ flux and initiates inflammatory gene transcription.47 These observations indicate intracellular PAF receptors in cells lacking robust hydrolytic activity have the potential to respond to extracellular PAF.
PAF is cleared from the circulation with great rapidity, so the presence of PAF in blood48 requires equally rapid secretion to counterbalance turnover. The concentration of circulating PAF increases with inflammatory or pathologic insults7, 12, 49, indicating either or both increased production and release in response to these insults. Pathways contributing to circulating PAF remain opaque, but likely include the combination of reduced PLA2G7 hydrolytic activity50, increased intracellular PAF synthesis51, and, at least in yeast, export facilitated by P-glycoprotein ABC transporters52. Circulating PAF may be the product of mononuclear cells since of all the cells known to synthesize PAF, only monocytes release PAF53, 54.
Reduced phospholipid uptake might also enhance circulating PAF concentrations, but molecular details of this internalization process are just now being defined. Uptake of PAF and a related structure Edelfosine (PAF with an alkyl sn-2 residue) by genetically tractable yeast requires a heterodimeric complex of the P4-type ATPase DRS1 or DRS243, and lem3 (also discovered as ros3)55. Mutational analysis of the corresponding human ATPase homolog ATP8B1 shows it has no role in phospholipid import56, but we find TMEM30a—a human lem3p homolog—reconstitutes phospholipid import in S. cerevisiae and aids PAF uptake by cell lines (Chen, submitted). The finding here that TMEM30a is expressed by endothelial cells and facilitates phospholipid import suggest that the rapid clearance of circulating PAF reflects transport into endothelial cell rich organs including lung, liver and kidney.
Supplementary Material
Acknowledgments
We gratefully acknowledge the generous gift of PLA2G7−/− mice from Diana M. Stafforini (Huntsman Cancer Center, University of Utah). We greatly appreciate the gift of PAFR−/− mice from Takao Shimizu (Tokyo University) and Jeffery Travers (University of Indiana) whose colony supplied the animals. We also appreciate the gift of HUVEC from Dr. Stephan Nicholls (Cleveland Clinic), and the qPCR performed by Erin Brady. We are also very thankful for critical early experiments and insights from Dr. Ravi Misra at an early stage in this project before his tragic, early death.
Sources of funding
This work was supported by NIH 1P01 HL087018, 1 R01 HL092747, and 1 R01 AA017748.
Non-standard Abbreviations and Acronyms
- Az
azelaoyl (nonadioyl)
- Lp-PLA2
lipoprotein-associated phospholipase A2
- LPAF
lyso-platelet-activating Factor, 1-O-hexadecyl-sn-glycero-3-phosphocholine
- NBD
nitrobenzoxadiazole
- PAF
Platelet-activating Factor, 1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine
- PAF-AH
PAF acetylhydrolase
- PAFR
Platelet-activating Factor Receptor
Footnotes
Disclosures
None
References
- 1.Ishii S, Shimizu T. Platelet-activating factor (PAF) receptor and genetically engineered PAF receptor mutant mice. Prog Lipid Res. 2000;39:41–82. doi: 10.1016/s0163-7827(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 2.Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet-activating factor and related lipid mediators. Annu Rev Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 3.Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev. 2000;80:1669–1699. doi: 10.1152/physrev.2000.80.4.1669. [DOI] [PubMed] [Google Scholar]
- 4.Honda Z, Ishii S, Shimizu T. Platelet-activating factor receptor. J Biochem. 2002;131:773–779. doi: 10.1093/oxfordjournals.jbchem.a003164. [DOI] [PubMed] [Google Scholar]
- 5.Marathe GK, Davies SS, Harrison KA, Silva AR, Murphy RC, Castro-Faria Neto H, Prescott SM, Zimmerman GA, McIntyre TM. Inflammatory PAF-like lipids in oxidized low density lipoproteins are fragmented alkyl phosphatidylcholines. J Biol Chem. 1999;274:28395–28405. doi: 10.1074/jbc.274.40.28395. [DOI] [PubMed] [Google Scholar]
- 6.Satoh K, Imaizumi T-a, Yoshida H, Hiramoto M, Takamatsu S. Increased levels of blood platelet-activating factor (PAF) and PAF-like lipids in patients with ischemic stroke. Acta Neurol Scand. 1992;85:122–127. doi: 10.1111/j.1600-0404.1992.tb04010.x. [DOI] [PubMed] [Google Scholar]
- 7.Callea L, Arese M, Orlandini A, Bargnani C, Priori A, Bussolino F. Platelet activating factor is elevated in cerebral spinal fluid and plasma of patients with relapsing-remitting multiple sclerosis. J Neuroimmunol. 1999;94:212–221. doi: 10.1016/s0165-5728(98)00246-x. [DOI] [PubMed] [Google Scholar]
- 8.Stafforini DM. Biology of platelet-activating factor acetylhydrolase (PAF-AH, lipoprotein associated phospholipase A2) Cardiovasc Drugs Ther. 2009;23:73–83. doi: 10.1007/s10557-008-6133-8. [DOI] [PubMed] [Google Scholar]
- 9.Tselepis AD, Chapman MJ. Inflammation, bioactive lipids and atherosclerosis: potential roles of a lipoprotein-associated phospholipase A2, platelet activating factor-acetylhydrolase. Atheroscler Suppl. 2002;3:57–68. doi: 10.1016/s1567-5688(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 10.Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, Prescott SM. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest. 1995;96:2322–2330. doi: 10.1172/JCI118288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Podrez EA, Byzova TV, Febbraio M, Salomon RG, Ma Y, Valiyaveettil M, Poliakov E, Sun M, Finton PJ, Curtis BR, Chen J, Zhang R, Silverstein RL, Hazen SL. Platelet CD36 links hyperlipidemia, oxidant stress and a prothrombotic phenotype. Nat Med. 2007;13:1086–1095. doi: 10.1038/nm1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang L, Latchoumycandane C, McMullen MR, Pratt BT, Zhang R, Papouchado BG, Nagy LE, Feldstein AE, McIntyre TM. Chronic alcohol exposure increases circulating bioactive oxidized phospholipids. J Biol Chem. 2010;285:22211–22220. doi: 10.1074/jbc.M110.119982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clay KL, Johnson C, Henson P. Binding of platelet activating factor to albumin. Biochim Biophys Acta. 1990;1046:309–314. doi: 10.1016/0005-2760(90)90246-t. [DOI] [PubMed] [Google Scholar]
- 14.Kulikov VI, Bergel'son LD. Binding of 1-0-alkyl-2-0-acetyl-sn-glycero-3-phosphocholine (thrombocyte activating factor) by plasma components. Exchange of the thrombocyte activating factor between lipoproteins and thrombocytes. Biokhimiia. 1984;49:1310–1315. [PubMed] [Google Scholar]
- 15.Karabina SA, Ninio E. Plasma PAF-acetylhydrolase: an unfulfilled promise? Biochim Biophys Acta. 2006;1761:1351–1358. doi: 10.1016/j.bbalip.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Opal S, Laterre PF, Abraham E, Francois B, Wittebole X, Lowry S, Dhainaut JF, Warren B, Dugernier T, Lopez A, Sanchez M, Demeyer I, Jauregui L, Lorente JA, McGee W, Reinhart K, Kljucar S, Souza S, Pribble J. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit Care Med. 2004;32:332–341. doi: 10.1097/01.CCM.0000108867.87890.6D. [DOI] [PubMed] [Google Scholar]
- 17.Serruys PW, Garcia-Garcia HM, Buszman P, Erne P, Verheye S, Aschermann M, Duckers H, Bleie O, Dudek D, Botker HE, von Birgelen C, D'Amico D, Hutchinson T, Zambanini A, Mastik F, van Es GA, van der Steen AF, Vince DG, Ganz P, Hamm CW, Wijns W, Zalewski A. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 18.Corson MA, Jones PH, Davidson MH. Review of the evidence for the clinical utility of lipoprotein-associated phospholipase A2 as a cardiovascular risk marker. Am J Cardiol. 2008;101:41F–50F. doi: 10.1016/j.amjcard.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Blank ML, Cress EA, Whittle T, Snyder F. In vivo metabolism of a new class of biologically active phospholipids: 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a platelet activating-hypotensive phospholipid. Life Sci. 1981;29:769–775. doi: 10.1016/0024-3205(81)90031-x. [DOI] [PubMed] [Google Scholar]
- 20.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 21.Stafforini DM, McIntyre TM, Prescott SM. Platelet-activating factor acetylhydrolase from human plasma. Methods Enzymol. 1990;187:344–357. doi: 10.1016/0076-6879(90)87041-z. [DOI] [PubMed] [Google Scholar]
- 22.Stafforini DM, Carter ME, Zimmerman GA, McIntyre TM, Prescott SM. Lipoproteins alter the catalytic behavior of the platelet-activating factor acetylhydrolase in human plasma. Proc Natl Acad Sci U S A. 1989;86:2393–2397. doi: 10.1073/pnas.86.7.2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohshima N, Ishii S, Izumi T, Shimizu T. Receptor-dependent metabolism of platelet-activating factor in murine macrophages. J Biol Chem. 2002;277:9722–9727. doi: 10.1074/jbc.M112406200. [DOI] [PubMed] [Google Scholar]
- 24.Stafforini DM, Rollins EN, Prescott SM, McIntyre TM. The platelet-activating factor acetylhydrolase from human erythrocytes. Purification and properties. J Biol Chem. 1993;268:3857–3865. [PubMed] [Google Scholar]
- 25.Yoshida H, Satoh K, Koyama M, Hiramoto M, Takamatsu S. Deficiency of plasma platelet-activating factor acetylhydrolase: roles of blood cells. Am J Hematol. 1996;53:158–164. doi: 10.1002/(SICI)1096-8652(199611)53:3<158::AID-AJH2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Pierce M, Franklin A, Jilling T, Stafforini DM, Caplan M. Dual roles of endogenous platelet-activating factor acetylhydrolase in a murine model of necrotizing enterocolitis. Pediatric Res. 2010;68:225–230. doi: 10.1203/PDR.0b013e3181eb2efe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hattori K, Adachi H, Matsuzawa A, Yamamoto K, Tsujimoto M, Aoki J, Hattori M, Arai H, Inoue K. cDNA cloning and expression of intracellular platelet-activating factor (PAF) acetylhydrolase II. Its homology with plasma PAF acetylhydrolase. J Biol Chem. 1996;271:33032–33038. doi: 10.1074/jbc.271.51.33032. [DOI] [PubMed] [Google Scholar]
- 28.O'Flaherty JT, Salzer WL, Cousart S, McCall CE, Piantadosi C, Surles JR, Hammett MJ, Wykle RL. Platelet-activating factor and analogues: comparative studies with human neutrophils and rabbit platelets. Res Commun Chem Pathol Pharmacol. 1983;39:291–309. [PubMed] [Google Scholar]
- 29.Chen R, Yang L, McIntyre TM. Cytotoxic phospholipid oxidation products. Cell death from mitochondrial damage and the intrinsic caspase cascade. J Biol Chem. 2007;282:24842–24850. doi: 10.1074/jbc.M702865200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elvington SM, Bu F, Nichols JW. Fluorescent, acyl chain-labeled phosphatidylcholine analogs reveal novel transport pathways across the plasma membrane of yeast. J Biol Chem. 2005;280:40957–40964. doi: 10.1074/jbc.M507926200. [DOI] [PubMed] [Google Scholar]
- 31.Miwa M, Miyake T, Yamanaka T, Sugatani J, Suzuki Y, Sakata S, Araki Y, Matsumoto M. Characterization of serum platelet-activating factor (PAF) acetylhydrolase: Correlation between deficiency of serum PAF acetylhydrolase and respiratory symptoms in asthmatic children. J Clin Invest. 1988;82:1983–1991. doi: 10.1172/JCI113818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stafforini DM, Satoh K, Atkinson DL, Tjoelker LW, Eberhardt C, Yoshida H, Imaizumi T, Takamatsu S, Zimmerman GA, McIntyre TM, Gray PW, Prescott SM. Platelet-activating factor acetylhydrolase deficiency. A missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest. 1996;97:2784–2791. doi: 10.1172/JCI118733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karasawa K, Harada A, Satoh N, Inoue K, Setaka M. Plasma platelet activating factor-acetylhydrolase (PAF-AH) Prog Lipid Res. 2003;42:93–114. doi: 10.1016/s0163-7827(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 34.Karasawa K. Clinical aspects of plasma platelet-activating factor-acetylhydrolase. Biochim Biophys Acta. 2006;1761:1359–1372. doi: 10.1016/j.bbalip.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 35.Naoki K, Asano K, Satoh N, Fukunaga K, Oguma T, Shiomi T, Suzuki Y, Nakajima T, Niimi K, Shiraishi Y, Ishizaka A, Yamaguchi K. PAF responsiveness in Japanese subjects with plasma PAF acetylhydrolase deficiency. Biochem Biophys Res Commun. 2004;317:205–210. doi: 10.1016/j.bbrc.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Casas JP, Ninio E, Panayiotou A, Palmen J, Cooper JA, Ricketts SL, Sofat R, Nicolaides AN, Corsetti JP, Fowkes FG, Tzoulaki I, Kumari M, Brunner EJ, Kivimaki M, Marmot MG, Hoffmann MM, Winkler K, Marz W, Ye S, Stirnadel HA, Khaw KT, Humphries SE, Sandhu MS, Hingorani AD, Talmud PJ. PLA2G7 Genotype, Lipoprotein-Associated Phospholipase A2 Activity, and Coronary Heart Disease Risk in 10 494 Cases and 15 624 Controls of European Ancestry. Circulation. 2010;121:2284–2293. doi: 10.1161/CIRCULATIONAHA.109.923383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marathe GK, Zimmerman GA, Prescott SM, McIntyre TM. Activation of vascular cells by PAF-like lipids in oxidized LDL. Vascul Pharmacol. 2002;38:193–200. doi: 10.1016/s1537-1891(02)00169-6. [DOI] [PubMed] [Google Scholar]
- 38.Minneci PC, Deans KJ, Banks SM, Eichacker PQ, Natanson C. Should we continue to target the platelet-activating factor pathway in septic patients? Crit Care Med. 2004;32:585–588. doi: 10.1097/01.CCM.0000110730.38696.9C. [DOI] [PubMed] [Google Scholar]
- 39.Schmitz G, Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2009 doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 40.Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, Wesselborg S, Lauber K. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–5305. doi: 10.1074/jbc.M706586200. [DOI] [PubMed] [Google Scholar]
- 41.Yang LV, Radu CG, Wang L, Riedinger M, Witte ON. Gi-independent macrophage chemotaxis to lysophosphatidylcholine via the immunoregulatory GPCR G2A. Blood. 2005;105:1127–1134. doi: 10.1182/blood-2004-05-1916. [DOI] [PubMed] [Google Scholar]
- 42.McConnell JP, Jaffe AS. The spin stops here: inhibition of lipoprotein-associated phospholipase A2-- a promising target but a negative initial trial? Clin Chem. 2009;55:21–23. doi: 10.1373/clinchem.2008.118364. [DOI] [PubMed] [Google Scholar]
- 43.Muthusamy BP, Natarajan P, Zhou X, Graham TR. Linking phospholipid flippases to vesicle-mediated protein transport. Biochim Biophys Acta. 2009;1791:612–619. doi: 10.1016/j.bbalip.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner AA, Reichert EC, Topham MK, Stafforini DM. Identification of a domain that mediates association of platelet-activating factor acetylhydrolase with high density lipoprotein. J Biol Chem. 2008;283:17099–17106. doi: 10.1074/jbc.M802394200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elstad MR, Stafforini DM, Prescott SM, McIntyre TM, Zimmerman GA. Human macrophages secrete platelet-activating factor acetylhydrolase. A mechanism for resolution of pulmonary inflammation. Chest. 1991;99:9S–10S. doi: 10.1378/chest.99.3_supplement.9s. [DOI] [PubMed] [Google Scholar]
- 46.Ihida K, Predescu D, Czekay RP, Palade GE. Platelet activating factor receptor (PAF-R) is found in a large endosomal compartment in human umbilical vein endothelial cells. J Cell Sci. 1999;112 ( Pt 3):285–295. doi: 10.1242/jcs.112.3.285. [DOI] [PubMed] [Google Scholar]
- 47.Marrache AM, Gobeil F, Zhu T, Chemtob S. Intracellular signaling of lipid mediators via cognate nuclear G protein-coupled receptors. Endothelium. 2005;12:63–72. doi: 10.1080/10623320590933815. [DOI] [PubMed] [Google Scholar]
- 48.Demopoulos CA, Andrikopoulos NK, Antonopoulou S. A simple and precise method for the routine determination of platelet-activating factor in blood and urine. Lipids. 1994;29:305–309. doi: 10.1007/BF02536336. [DOI] [PubMed] [Google Scholar]
- 49.Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T, Simons FE, Simons KJ, Cass D, Yeung J. Platelet-activating factor, PAF acetylhydrolase, and severe anaphylaxis. New England J Med. 2008;358:28–35. doi: 10.1056/NEJMoa070030. [DOI] [PubMed] [Google Scholar]
- 50.Chen J, Yang L, Foulks JM, Weyrich AS, Marathe GK, McIntyre TM. Intracellular PAF catabolism by PAF acetylhydrolase counteracts continual PAF synthesis. J Lipid Res. 2007;48:2365–2376. doi: 10.1194/jlr.M700325-JLR200. [DOI] [PubMed] [Google Scholar]
- 51.Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem. 2007;282:6532–6539. doi: 10.1074/jbc.M609641200. [DOI] [PubMed] [Google Scholar]
- 52.Ruetz S, Brault M, Dalton W, Gros P. Functional interactions between synthetic alkyl phospholipids and the ABC transporters P-glycoprotein, Ste-6, MRP, and Pgh 1. Biochemistry. 1999;38:2860. [PubMed] [Google Scholar]
- 53.Elstad MR, Prescott SM, McIntyre TM, Zimmerman GA. Synthesis and release of platelet-activating factor by stimulated human mononuclear phagocytes. J Immunol. 1988;140:1618–1624. [PubMed] [Google Scholar]
- 54.Elstad MR, Stafforini DM, McIntyre TM, Prescott SM, Zimmerman GA. Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J Biol Chem. 1989;264:8467–8470. [PubMed] [Google Scholar]
- 55.Hanson PK, Malone L, Birchmore JL, Nichols JW. Lem3p is essential for the uptake and potency of alkylphosphocholine drugs, edelfosine and miltefosine. J Biol Chem. 2003;278:36041–36050. doi: 10.1074/jbc.M305263200. [DOI] [PubMed] [Google Scholar]
- 56.Verhulst PM, van der Velden LM, Oorschot V, van Faassen EE, Klumperman J, Houwen RH, Pomorski TG, Holthuis JC, Klomp LW. A flippase-independent function of ATP8B1, the protein affected in familial intrahepatic cholestasis type 1, is required for apical protein expression and microvillus formation in polarized epithelial cells. Hepatology. 2010;51:2049–2060. doi: 10.1002/hep.23586. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


