Abstract
Invasive carcinoma cells use specialized actin polymerization-driven protrusions called invadopodia to degrade and possibly invade through the extracellular matrix during metastasis. Phosphorylation of the invadopodium protein cortactin is a master switch that activates invadopodium maturation and function. Cortactin was originally identified as a hyperphosphorylated protein in v-Src-transformed cells, but the kinase or kinases which are directly responsible for cortactin phosphorylation in invadopodia remain unknown. In this study, we provide evidence that the Abl-related non-receptor tyrosine kinase Arg mediates EGF-induced cortactin phosphorylation, triggering actin polymerization in invadopodia, extracellular matrix degradation, and matrix proteolysis-dependent tumor cell invasion. Both Src and Arg localize to invadopodia and are required for EGF-induced actin polymerization. Notably, Arg overexpression in Src knockdown cells can partially rescue actin polymerization in invadopodia, while Src overexpression can not compensate for loss of Arg, arguing that Src indirectly regulates invadopodium maturation through Arg activation. Our findings suggest a novel mechanism by which an EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion. Further, they identify Arg as a novel mediator of invadopodium function and a candidate therapeutic target to inhibit tumor invasion in vivo.
Keywords: invadopodia, Arg, Src, phosphorylation, actin polymerization
INTRODUCTION
Complications from metastasis are the major cause of death in breast cancer patients. Metastatic carcinoma cells must initially invade through the basement membrane, a network of extracellular matrix proteins that support the overlaying epithelium (1). Once they have escaped the primary tumor, cancer cells must also degrade the vascular sub-endothelial basement membrane to gain entry into the bloodstream. Directed invasion of cancer cells through the ECM and their intravasation into the bloodstream is mediated by chemoattractants such as EGF, which are produced by other cell types including tumor-associated macrophages (2, 3). It is believed that invasive cancer cells penetrate these barriers by forming specialized F-actin rich protrusions called invadopodia that localize matrix degrading activity to cell-substrate contact points (4–8).
Invadopodia proceed through several different stages during their maturation into functional structures. First, small punctate clusters, or invadopodium precursors, uniquely containing actin and the actin polymerization regulators cortactin, N-WASp, and cofilin are formed (9–12). These precursors can have two fates: they can disappear or become stabilized and mature into functional invadopodia. Stabilization and maturation requires cortactin tyrosine phosphorylation, which leads to generation of free actin barbed ends in invadopodia and increased invadopodial lifetime (9, 10). The final maturation stage involves acquisition of matrix degrading ability through delivery of matrix metalloproteinases such as MT1-MMP into invadopodia (9, 10, 13, 14). Understanding the mechanisms that govern invadopodium formation and function is an essential step in the prevention of invasion and metastasis.
The cortactin gene (CTTN), located in chromosome 11q13, is amplified in various human carcinomas and is usually correlated with poor patient prognosis (15, 16). Cortactin localizes to invadopodia in invasive breast cancer cells, where it regulates both their formation and function (10, 17). Cortactin tyrosine phosphorylation is essential for generation of free actin barbed ends required for actin polymerization in invadopodia (9) and for efficient ECM degradation (17–19) and metastasis in vivo (20). Cortactin phosphorylation promotes release of cofilin from cortactin, leading to free actin barbed end generation (9, 21), and also enables binding of proteins such as Nck1, leading to enhancement of N-WASp-mediated Arp2/3-dependent actin polymerization (9, 21–24). Combination of both pathways is important for actin polymerization in invadopodia, which is believed to mediate invadopodium-dependent protrusion into the ECM. Cortactin phosphorylation is therefore a critical regulator of invadopodial function and tumor metastasis, but how this phosphorylation is achieved specifically in invadopodia has not been established.
We show here that Arg and Src, but not Abl, localize to invadopodia of mammary carcinoma cells, where they specifically mediate cortactin tyrosine phosphorylation. Arg is not required for initial invadopodium precursor formation, but is critical for regulating invadopodial activation in response to EGF by promoting the generation of free actin barbed ends. Both Src and Arg localize to invadopodia and are essential for their activation and function. Interestingly, Arg overexpression in Src knockdown cells can partially rescue invadopodial activation and function, while Src overexpression cannot compensate for loss of Arg function. These experiments demonstrate an EGFR-Src-Arg-cortactin pathway mediates functional maturation of invadopodia and breast cancer cell invasion, and identify Arg as a novel mediator of invadopodia function in breast cancer cells.
MATERIALS AND METHODS
Reagents and antibodies
The following antibodies were used: anti-Tks5, anti-Abl (Santa Cruz, Santa Cruz, CA), anti-cortactin, anti-actin (Millipore, Billerica, MA), anti-Src (Calbiochem, Gibbstown, NJ), anti-pY418-Src (Cell Signaling, Beverly, MA), FITC-anti-biotin (Jackson Laboratories, Bar Harbor, ME), anti-GFP (Rockland Immunochemicals, Gilbertsville, PA), pY421-cortactin (Sigma, St. Louis, MO), Anti-Arg antibody (Clone AR11) was a generous gift from Dr. Peter Davies (Albert Einstein School of Medicine, NY) (25). Human recombinant EGF was from Invitrogen (Carlsbad, CA). Alexa-405-gelatin and Alexa-568-fibronectin/gelatin coated dishes were prepared as previously described (9, 10, 26).
RNAi
Control non-silencing siRNA pool (D-001810-10), Arg siRNA pool (L-003101-00), Abl siRNA pool (L-003100-00) and Src siRNA pool (L-003175-00) were from Dharmacon (Thermo Fischer, Lafayette, CO). MDA-MB-231 cells were nucleofected with 2 μM siRNA according to the manufacturer’s instructions (Lonza, Basel, Switzerland). siRNA-treated cells were used 72 hours post-transfection, a period of maximal knockdown as confirmed by immunoblot.
Constructs and cell lines
MDA-MB-231 human breast carcinoma cells were from the American Type Culture Collection. AmphoPack 293 packaging cell line was from Clontech. The rat mammary adenocarcinoma cell line MTLn3 was from Dr. Garth Nicolson (Institute for Molecular Medicine, Huntington Beach, CA)(9). Previously described murine YFP rescue constructs of Arg (27) were subcloned into pLXSN retroviral vector. Murine cortactin-TagRFP was previously described (28). Src-YFP and Src-TagRFP were subcloned from mouse Src cDNA into pEYFP-N1, pTagRFP-N1, and pLXSN vectors. Packaging cells were transfected using Lipofectamine 2000 and retroviral sups were used to infect MDA-MB-231 cells followed by selection with 600μg/mL G418 to generate stable cell-lines. MDA-MB-231 cells were cultured in DMEM supplemented with10% FBS and antibiotics.
Co-localization analysis
MDA-MB-231 cells were plated on Alexa-405-labeled gelatin matrix for 4 hours, fixed using 3.7% PFA, permeabilized with 0.1% TX-100, and fluorescently labeled for either Arg, Abl, or Src-TagRFP and Tks5. Images were acquired on an Olympus IX81 microscope (Olympus, Center Valley, PA) with a Sensicam QE cooled CCD (Cooke, Romulus, MI) camera using IP Lab 4.0 and processed using the ImageJ Jacop colocalization plug-in.
Invadopodium precursor formation analysis
Arg, Abl, Src, or non-silencing control knockdown cells were stained for invadopodium precursors as punctate structures in which F-actin and cortactin co-localize, as previously described (9).
Barbed end assay
The barbed end assay was performed as previously described (9).
Immunoprecipitation and western blot analysis
Cells were immunoprecipitated and/or blotted as described previously (28). Quantification was performed using Quantity One software (BioRad, Hercules, CA).
Immunofluorescence analysis of cortactin tyrosine phosphorylation in invadopodia
Cells stably expressing cortactin-TagRFP were knocked down using control, Arg, Abl, or Src specific siRNA, plated on fibronectin/gelatin matrix, starved for 12-16 hours and stimulated with EGF for 0 or 3 minutes. Cells were fixed and labeled with anti-pY421 cortactin antibodies. The intensity of cortactin-TagRFP and pY421-cortactin in invadopodia was quantified, the ratio of pY421-cortactin/cortactin-TagRFP was expressed as the relative fold change.
Matrix Degradation Assay
Cells were plated on Alexa-568-fibronectin/gelatin-coated matrix for 22 hours. The cells were fixed and imaged using a Nikon TE2000 microscope with a Retiga cooled CCD camera (Q Imaging, Surrey, BC, Canada) and Nikon Elements software (Nikon, Melville, NY). Degradation area per field was measured using ImageJ.
Transwell Invasion Assay
The assay was performed as previously described (26).
Statistical Analysis
Unless otherwise noted, for all experiments containing single comparisons, One-Way ANOVA with Newman-Kuels post-tests were performed. For grouped samples, Two-Way ANOVA with Bonferroni post-tests were performed using GraphPad Prism. All graphs are mean ± SEM and *=P<0.05, **=P<0.01, ***=P<0.001.
RESULTS
Arg localizes to invadopodia in metastatic breast cancer cells
Invadopodia initially form as punctate structures, or precursors, enriched in F-actin, cortactin, Tks5, and the Arp2/3 complex, which mature to acquire matrix degrading capabilities (9, 10, 14, 18, 24, 29, 30). Based on our previous demonstration that Arg interacts with cortactin via a series of binding and phosphorylation events to promote F-actin-based cell edge protrusion in fibroblasts (22), we hypothesized that Arg may have a similar role in invadopodia.
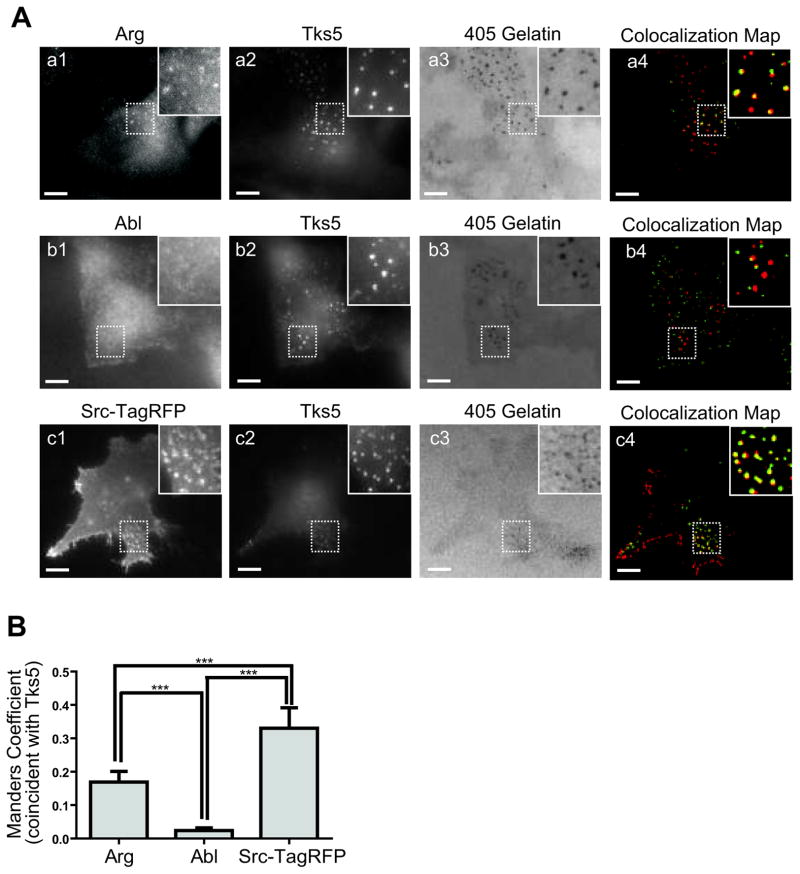
Immunofluorescence labeling of endogenous Arg in MDA-MB-231 cells plated on a fluorescent substrate showed co-localization with the invadopodium marker Tks5, and with degradation puncta, suggesting that Arg localizes to active, degrading invadopodia in these cells (Figure 1A, a1-4). Consistent with these findings, a functional Arg-YFP fusion protein co-localized with F-actin and cortactin in invadopodia of MTLn3 cells, both while growing in constant 10% serum (steady state) and in serum starved cells acutely stimulated with EGF (Figure S1). Surprisingly, despite its abundance in MDA-MB-231 cells, the closely related Abl kinase did not localize to Tks5-containing, matrix-degrading invadopodia (Figure 1A, b1-4). In agreement with previous studies (9, 10, 24, 29, 31), Src-TagRFP also localized to invadopodia both at steady state (Figure 1Ac1-4) and following EGF stimulation (Figure S2). These data suggest that Arg and Src may regulate invadopodial formation or function in invasive human breast cancer cells.
Figure 1. Arg and Src, but not Abl, localize to invadopodia.
(A) MDA-MB-231 cells were plated on Alexa-405 labeled gelatin matrix, fixed, and labeled for Arg (a1) or Abl (a2), and the invadopodia marker, Tks5 (a2 and b2). Cells expressing Src-TagRFP and knocked down for endogenous Src (c1) were plated on Alexa-405 gelatin, fixed, and labeled for Tks5 as above (c2). Co-localization masks are shown for the kinases with Tks5 at invadopodia actively degrading Alexa-405 gelatin matrix (a3,4, b3,4, c3,4). (B) Quantification of the co-localization (Manders coefficient) between Arg, Abl, Src, and Tks5 at matrix-degrading invadopodia. n=24 (Arg), n=17 (Abl), n=13 (Src). Scale bars=10 μm.
Invadopodium precursor formation does not depend on Abl family kinases or Src
We next sought to determine if Abl family kinases or Src regulate the initial formation of invadopodium precursors. Using siRNA, we knocked down Arg, Abl, or Src in these cells (Figure S3A), along with Tks5, which is required for invadopodium precursor formation, as a control (Figure S3B). Arg, Abl, and Src knockdown cells plated on fibronectin/gelatin substrates revealed no significant difference in the number of F-actin- and cortactin-positive invadopodium precursors compared to control cells expressing non-silencing siRNA whereas knockdown of Tks5 reduced precursor formation by 80% (Figure S3C,D). These experiments suggest that despite their localization to invadopodia, Arg and Src are not required for the formation of invadopodium precursors in breast cancer cells.
Arg is required for cortactin phosphorylation at invadopodium precursors
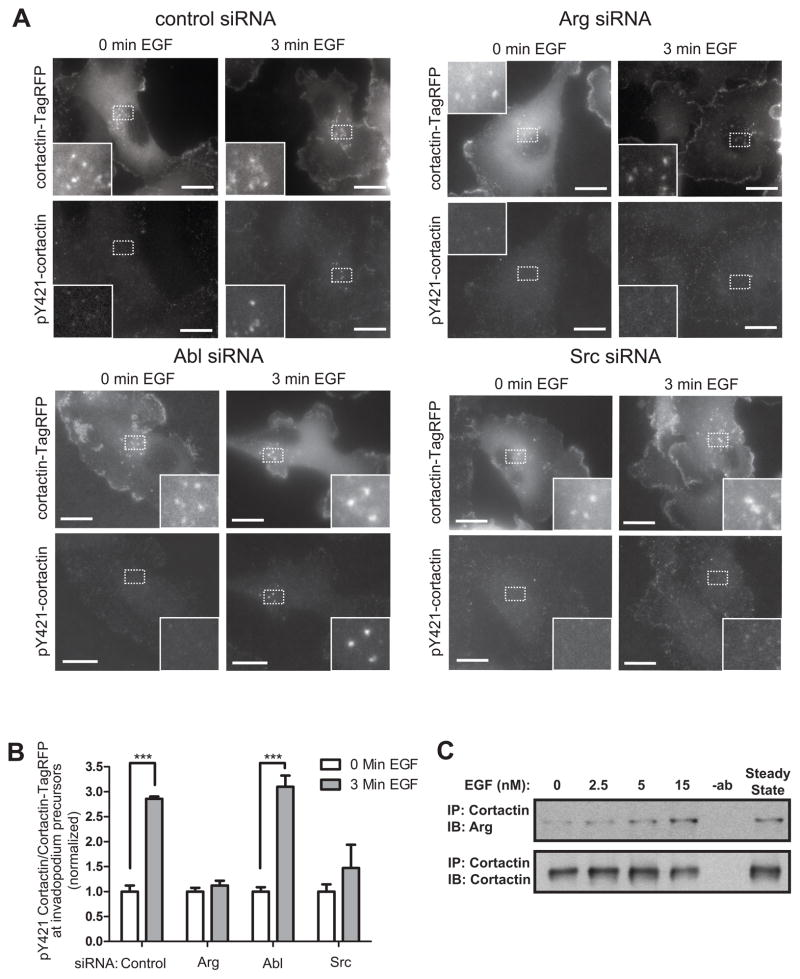
Stimulation with the growth factor EGF, a major chemoattractant for invading cancer cells, induces phosphorylation of the invadopodial core protein cortactin, which is essential for maturation of invadopodia (9). Activated EGF receptor has been shown to bind to and activate the Abl family kinases (32, 33). Based on these observations, we hypothesized that Arg may phosphorylate cortactin in invadopodia in response to EGF. Control and knockdown cells were treated with EGF and labeled with a phosphorylation-specific antibody for cortactin. Cortactin phosphorylation was increased in invadopodium precursors of EGF-stimulated control or Abl siRNA treated cells, while almost no cortactin phosphorylation was detected in either Arg or Src knockdown cells (Figure 2A,B). Supporting these data, and confirming our previous in vitro data (22, 28), Arg also co-immunoprecipitated with cortactin in cells treated with EGF in a concentration-dependent manner (Figure 2C). These data suggest that Arg and Src, but not Abl, regulate cortactin phosphorylation within invadopodium precursors in response to EGF.
Figure 2. Arg is required for cortactin phosphorylation at invadopodium precursors.
(A) MDA-MB-231 cells stably expressing cortactin-TagRFP were treated with siRNA specific for control, Arg, Abl, or Src, plated on fibronectin/gelatin matrix and starved before EGF stimulation. Cells were fixed and labeled for phosphorylated cortactin using a phosphospecific cortactin antibody (anti-pY421) before (0min.) or after (3min.) EGF stimulation. (B) Cortactin phosphorylation was measured as the ratio of pY421 signal/cortactin-TagRFP signal at invadopodium precursors. Control siRNA: n=105(0min.)/n=127(3min.), Abl siRNA: n=110(0min.)/n=97(3min.), Arg siRNA: n=138(0min.)/n=136(3min.), Src siRNA: n=122(0min.)/n=160(3min.). Scale bars=10 μm. (C) MDA-MB-231 cells were treated with increasing concentrations of EGF for 3 minutes, lysed, immunoprecipitated with anti-cortactin antibodies followed by immunoblotting with anti-Arg antibodies. The samples were blotted with anti-cortactin as control.
Arg and Src, but not Abl, are required for stimulation of actin barbed end generation in invadopodia
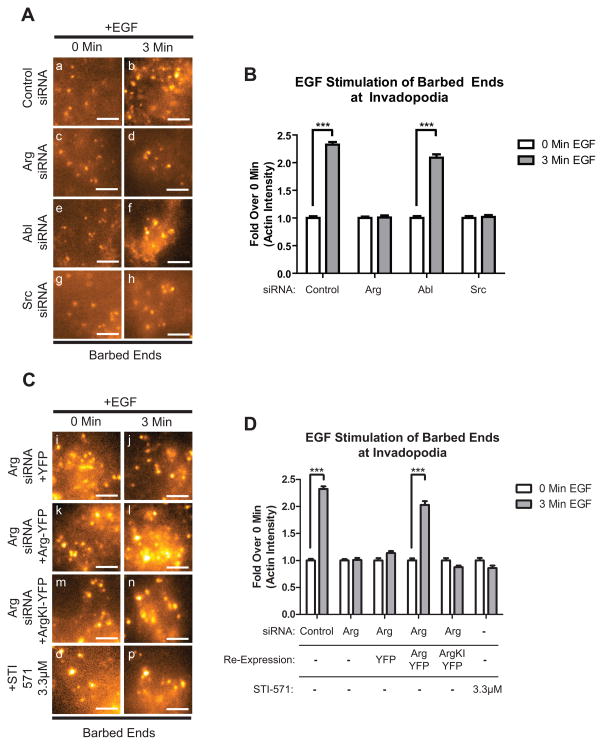
EGF stimulation of breast cancer cells triggers downstream signaling cascades leading to actin polymerization in invadopodia, a process that requires cortactin tyrosine phosphorylation (9, 24, 26). Because they are required for cortactin tyrosine phosphorylation in response to EGF in invadopodia (Figure 2), we hypothesized that Arg and Src might regulate actin polymerization in these structures. For this assay, we stimulated serum-starved cells with EGF in order to synchronize the generation of free actin barbed ends within invadopodium precursors in control and knockdown cells. We then quantified free actin barbed ends specifically within these structures following EGF stimulation, as described previously (9). Cells treated with control siRNA or Abl siRNA yielded a 2.3-fold increase in barbed end intensity in invadopodium precursors in response to EGF (Figure 3A, a-d, 3B) suggesting that Abl is not essential for this process. Interestingly, knockdown of either Arg or Src disrupted the EGF-dependent generation of barbed ends in invadopodium precursors (Figure 3Ae-h, 3B). These data suggest that both Arg and Src are essential for the EGF-dependent burst in actin polymerization in stimulated invadopodia.
Figure 3. Arg kinase activity is required for actin barbed end generation at invadopodia.
(A) MDA-MB-231 cells were knocked down using control, Arg, Abl, or Src siRNA and either left untreated (a,c,e,g) or stimulated with EGF for 3 minutes (b,d,f,h). Cells were fixed and labeled for biotin-actin and Arp2 as an invadopodium marker. (B) Quantification of free actin barbed ends as measured by average biotin-actin intensity in stimulated invadopodia containing Arp2. Control siRNA: n=404(0min.)/n=365(3min.), Abl siRNA: n=312(0min.)/n=271(3min.), Arg siRNA: n=283(0min.)/n=252(3min.), Src siRNA: n=296(0min.)/n=256(3min.). (C) Cells stably expressing RNAi-resistant rescue mutants of Arg (Arg-YFP or Arg KI-YFP=kinase inactive) or YFP alone were knocked down using Arg siRNA, stimulated and labeled as in (A) (i-n). Alternatively, MDA-MB-231 cells were treated with 3.3 μM STI-571 prior to EGF stimulation (o, p). (D) Quantification of free actin barbed ends from experiments in (C). Arg-YFP: n=308(0min.)/n=209(3min.), YFP: n=127(0min.)/n=275(3min.), ArgKI-YFP: n=196(0min.)/n=181(3min.), STI-571: n=289(0min.)/n=415(3min.). Scale bars=5μm.
Arg kinase activity is required for new F-actin barbed end generation at invadopodia
Given that Arg is required for cortactin phosphorylation in invadopodia (Figure 2A, B), which is important for actin polymerization in these structures, we also hypothesized that Arg kinase activity would be required for new barbed end formation. To test this hypothesis, we expressed Arg-YFP or a kinase-inactive Arg-YFP (Arg KI-YFP) in cells knocked down for endogenous Arg (Figure S4A). Arg knockdown cells rescued with Arg-YFP exhibited a 2-fold increase in actin barbed end formation within invadopodium precursors in response to EGF. In contrast, EGF treated Arg KI-YFP cells or wild-type cells treated with STI-571, an inhibitor of Abl family kinases, did not induce increased actin barbed end formation in these structures (Figure 3Ci-p, 3D). These data strongly support the idea that Arg kinase activity is required for new actin barbed end generation within developing invadopodia.
Arg is required for Src-mediated stimulation of cortactin phosphorylation and actin barbed end generation in invadopodia
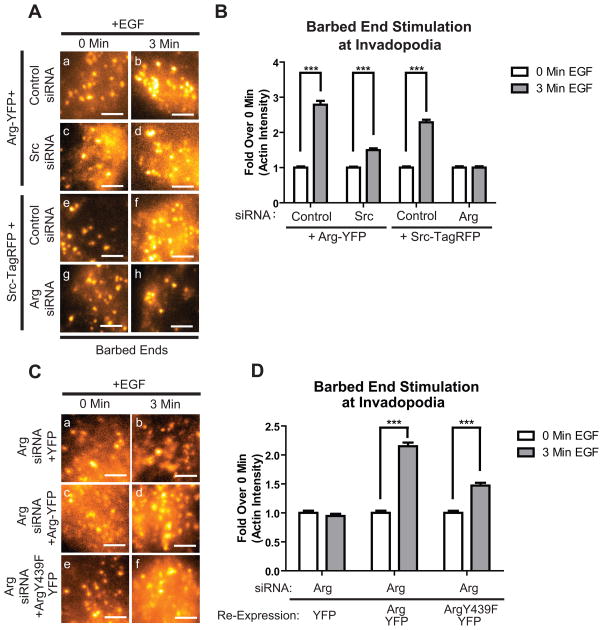
Both Src and Arg were required for EGF-dependent stimulation of barbed end formation, suggesting they may act in a sequential pathway leading to actin polymerization in invadopodia (Figure 3). Previous findings from both our lab and the Van Etten lab have established that full Arg activation requires both autophosphorylation of a tyrosine in the Arg linker region (Y272) as well as Src-dependent phosphorylation of a tyrosine in the Arg activation loop (34, 35). To determine whether Src plays this same regulatory role in Arg activation in invadopodia, we used cells stably overexpressing Arg-YFP treated with Src siRNA or a non-silencing control siRNA (Figure S4B). Cells overexpressing Arg-YFP and control siRNA showed almost 3-fold induction of free barbed ends in invadopodium precursors following EGF stimulation. When Src was knocked down in cells overexpressing Arg-YFP, only a partial (1.4-fold) EGF-induced increase in actin incorporation was observed (Figure 4Aa-d, 4B), suggesting that Arg requires Src expression for full stimulation of EGF-induced actin polymerization at invadopodia. To further validate this hypothesis, we expressed an Arg-YFP mutant with the activation loop tyrosine Y439, which is phosphorylated by Src (34), mutated to phenylalanine (Y439F) (Figure S4C). This mutant only partially rescued the barbed end formation in cells following EGF stimulation, even when expressed in the presence of endogenous Src (Figure 4C, D). We suggest that this partial rescue of barbed end generation results from partial activation of Arg via Arg-mediated autophosphorylation. However, Arg cannot reach its full activation state in the absence of Src (34).
Figure 4. Arg is required for Src-mediated stimulation of actin barbed end formation in invadopodia.
(A) Representative images of MDA-MB-231 cells stably expressing Src-TagRFP (a-d) or cells stably expressing Arg-YFP (e-h), treated with control siRNA (a,b,e,f), Arg siRNA (c,d), or Src siRNA (g,h). Cells were either left untreated (a,c,e,g), or stimulated with EGF for 3 min. (b,d,f,h), fixed and stained for biotin-actin and Arp2 as an invadopodium marker. (B) Quantification of barbed ends as measured by the average actin intensity at stimulated invadopodia in response to EGF (all conditions n=200). The difference between all 3 minute stimulation values are statistically significant as measured by individual Post-Hoc student’s t-test (p<0.01). Scale bars=5μm. (C) Representative images of cells knocked down for endogenous Arg and re-expressing YFP, Arg-YFP, or mutant Arg Y439F-YFP. Scale bars=5μm. (D) Cells were stimulated and treated as above, and actin barbed ends were quantified as in B. YFP: n=127(0min.)/n=275(3min.), Arg-YFP: n=308(0min.)/n=209(3min.), ArgKI-YFP: n=196(0min.)/n=181(3min.), STI-571: n=289(0min.)/n=415(3min.).
If Src lies upstream of Arg in the EGF-receptor signaling pathway, then knockdown of Arg, even in Src overexpressing cells, should eliminate any barbed end response to EGF. To examine this possibility, we used cells stably expressing Src-TagRFP treated with either Arg siRNA or a non-silencing control siRNA. As predicted, knockdown of Arg in cells overexpressing Src-TagRFP blocked the generation of actin barbed ends at invadopodium precursors in response to EGF (Figure 4Ae-h, 4B).
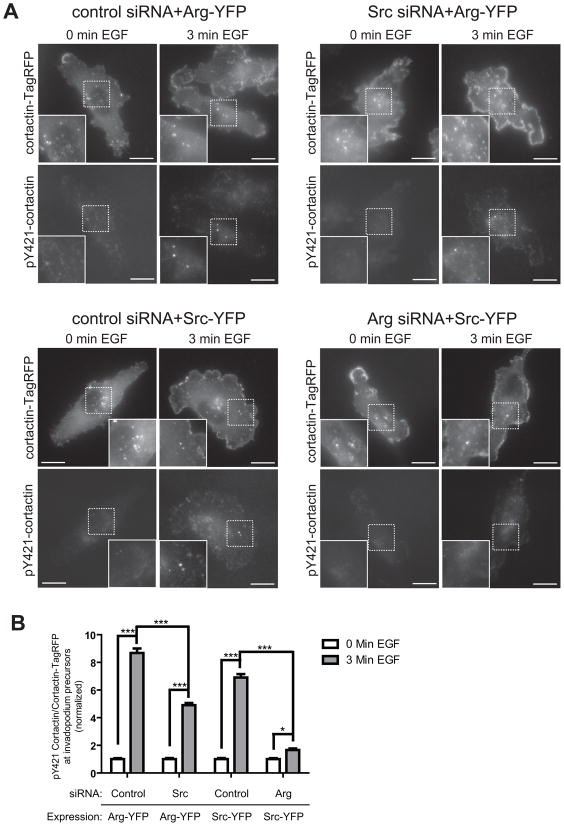
Consistent with the data described above, cortactin phosphorylation within invadopodia was only partially rescued when Arg-YFP was expressed in Src knockdown cells (Figure 5) or when Arg Y439F-YFP was expressed in presence of endogenous Src (Figure S5). No cortactin phosphorylation was observed when Src was overexpressed in Arg knockdown cells (Figure 5). Together with the results described above, these data suggest that Src lies upstream of Arg in regulating cortactin phosphorylation and barbed end formation in response to EGF at invadopodia.
Figure 5. Arg is required for Src-mediated cortactin phosphorylation in invadopodia.
(A) MDA-MB-231 cells stably expressing cortactin-TagRFP were treated with control siRNA, Arg siRNA, or Src siRNA and transfected with either Arg-YFP (two upper panels) or Src-YFP (two lower panels), plated on fibronectin/gelatin matrix, and starved before EGF stimulation. The cells were fixed and labeled for phosphorylated cortactin using a phosphospecific cortactin antibody (anti-pY421) before (0min.) or after (3min.) EGF stimulation. Scale bars=20μm. (B) Cortactin phosphorylation was measured as the ratio of pY421 signal/cortactin-TagRFP signal at invadopodium precursors. Control siRNA+ArgYFP: n=50(0min.)/n=49(3min.), Src siRNA+ArgYFP: n=48(0min.)/n=53(3min.), Control siRNA+SrcYFP: n=55(0min.)/n=48(3min.), Arg siRNA+SrcYFP: n=48(0min.)/n=48(3min.).
Knockdown of Arg and Src reduces extracellular matrix degradation and tumor cell invasion
In the final stage of maturation, invadopodia acquire the ability to focally degrade the extracellular matrix via matrix metalloproteinases (9, 10, 18). This process allows the cells to escape through the basement membrane surrounding the primary tumor (36, 37). We therefore tested whether Arg, Abl, or Src knockdown cell lines form mature invadopodia that are capable of degrading a fibronectin/gelatin matrix (24). Interestingly, Arg knockdown cells, and to a lesser extent Src knockdown cells, exhibited decreased ability to degrade fluorescently-labeled fibronectin/gelatin matrix compared to control cells, while Abl knockdown cells were able to degrade matrix at control levels, both when cultured in constant 10% serum (Figure 6A,B) and when serum starved followed by acute EGF stimulation (Figure S6).
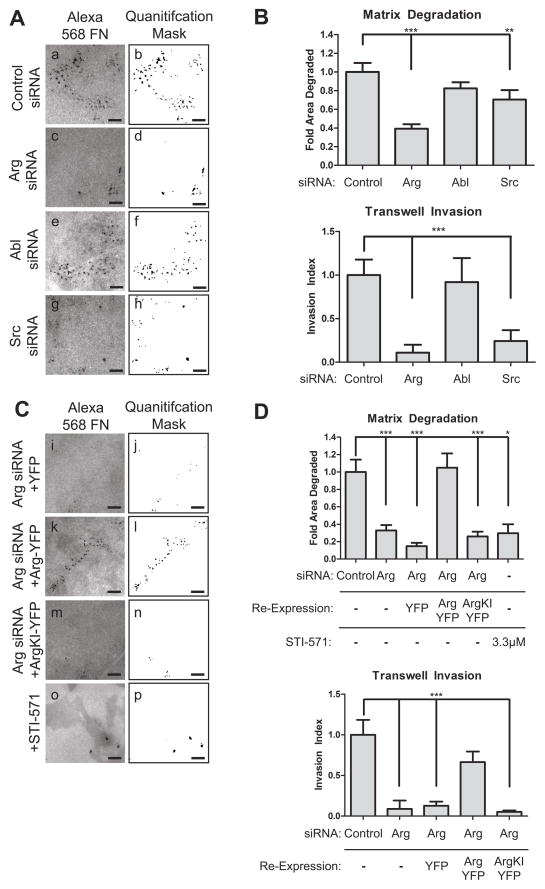
Figure 6. Arg kinase activity is required for extracellular matrix degradation and invasion.
(A) MDA-MB-231 cells treated with control, Arg, Abl, or Src siRNA were plated on Alexa-568 fibronectin/gelatin matrix and allowed to degrade for 22 hours. Shown are representative images (left panels) and quantification masks (right panels) of degradation areas. (B) Quantification of matrix degradation from conditions as in A; n=60 fields (upper graph) and MDA-MB-231 cells knocked down as in A and B were plated on Matrigel-coated membranes and allowed to invade for 20 hours; n=12 (lower graph) (C) MDA-MB-231 cells stably expressing Arg-YFP, Arg KI-YFP, or YFP control were treated with Arg siRNA, control siRNA, or with STI-571, and plated on fibronectin/gelatin matrix and allowed to degrade as in A. Shown are representative images (left panels) and quantification masks (right panels). (D) Quantification of matrix degradation area from conditions in C. YFP n=31, Arg-YFP n=30, ArgKI-YFP n=30, STI-571 n=10. MDA-MB-231 cells were plated on Matrigel-coated membranes and allowed to invade, as described in C. YFP n=8, Arg-YFP n=6, ArgKI-YFP n=8. Invasion was normalized to migration rate, proteolysis-independent invasion, and protein re-expression levels where appropriate. Scale bars=10μm.
As Arg and Src regulate matrix degradation in breast cancer cells, we hypothesized that they might also regulate the ability of breast cancer cells to invade through an extracellular matrix barrier. To test this hypothesis, we assayed the ability of serum-starved MDA-MB-231 cells treated with Arg, Abl, or Src siRNA to invade towards serum-containing media through Matrigel-coated membranes. To control for matrix proteolysis-independent amoeboid-like migration (37–41), we also measured invasion of identically treated cells with the general matrix metalloproteinase inhibitor GM6001. Cells treated with either Arg or Src siRNA, but not Abl siRNA, exhibited a dramatic reduction in proteolysis-dependent invasion through Matrigel-coated chambers (Figure 6B). Importantly, Arg, Abl, or Src knockdown cell lines displayed no significant differences in migration rates as measured with uncoated migration wells (data not shown). These data suggest that Arg and Src both play critical roles in matrix degradation and subsequent tumor cell invasion.
Arg kinase activity is required for extracellular matrix degradation and tumor cell invasion
Because Arg kinase activity was required for actin barbed end generation in developing invadopodia (Fig. 3), we also tested whether Arg kinase activity was required for subsequent matrix degradation and tumor cell invasion. Re-expression of full length Arg-YFP, but not a kinase inactive mutant (Arg KI-YFP), in Arg knockdown cells rescued both matrix degradation and invasion through Matrigel to control levels (Figure 6C,D). Similar to the kinase inactive mutant, chemical inhibition of Arg kinase activity with 3.3 μM of the Abl family kinase inhibitor STI-571 significantly reduced both matrix degradation and tumor cell invasion (Figure 6Co,p, 6D). Together, these observations indicate that Arg kinase activity is required for matrix degradation and proteolysis-dependent tumor cell invasion.
DISCUSSION
Free actin barbed end generation followed by actin polymerization is required for the stabilization and maturation of invadopodia into functional structures. While it has been shown that cortactin phosphorylation is critical for barbed end formation and actin polymerization in invadopodia (9, 18, 26, 29), it is not clear which kinase is responsible for this phosphorylation and subsequent regulation of actin polymerization. Here, we present evidence that the Arg non-receptor tyrosine kinase is responsible for regulating actin polymerization in invadopodia. Arg localizes to and is required for cortactin phosphorylation in invadopodia. The kinase activity of Arg is required for efficient barbed end generation and for the matrix degrading ability of mature invadopodia. Our data suggest that while Src alone is not capable of stimulating actin polymerization at invadopodia, EGF signaling through Src is required for full Arg-mediated cortactin phosphorylation and actin polymerization at invadopodia. Taken together, these data show that Arg is a novel, central regulator of the actin polymerization machinery in invadopodia and subsequent matrix degrading and invasive behavior of human breast cancer cells.
Arg, but not Abl, localizes to and is required for invadopodium function
It was previously shown that Abl family kinases are highly expressed in breast cancer cell lines, and their expression levels and activity directly correlate with cell invasiveness (42). Arg and Abl share extensive similarity in their N-terminal and kinase domains and both have been shown to phosphorylate cortactin on tyrosine residues in vitro and following growth factor or integrin signaling in fibroblasts (22, 28). It is therefore somewhat surprising that only Arg, but not Abl, regulates cortactin phosphorylation and polymerization of actin in invadopodia, processes that are critical for the invasiveness of breast cancer cells. The differential effect of Arg versus Abl on invadopodial function may lie in their divergent patterns of localization in the cell - while Arg localizes to invadopodia, Abl is excluded from these structures. Arg and Abl differ most extensively in their C-terminal extensions, suggesting that structural differences may account for their non-redundant roles in breast cancer cells.
Arg is a novel regulator of tyrosine phosphorylation in invadopodia
Tyrosine kinase signaling has an important role in regulating invadopodial function, such as matrix degradation and actin polymerization (5, 29), however little is known about the role of specific kinases in these processes. Overexpressed Src stimulates invadopodial formation and function in breast carcinoma cells, while the Src kinase family inhibitor PP2 reduces these functions (9–11, 21, 29, 31, 43). Cortactin is central to invadopodial function and requires phosphorylation to promote actin polymerization and matrix degradation of these structures. Because cortactin was originally identified as a Src substrate (44, 45), has been shown to promote cortactin phosphorylation when overexpressed in cells (9, 10, 45, 46), and can promote actin polymerization in vitro through interactions with Nck1 and N-WASp (23), it has been inferred that Src is solely mediating cortactin phosphorylation at invadopodia, thereby mediating invadopodial actin polymerization and function.
Previously, we have demonstrated that Arg has a lower KM for cortactin than Src in vitro, suggesting that Arg may be more adept than Src at cortactin phosphorylation (28). We also have demonstrated that Src phosphorylation of Arg is required for full Arg activation (34, 35). Here, we show that both Src and Arg are required for downstream cortactin phosphorylation. However, since Arg kinase activity is critical for actin polymerization and Src overexpression cannot compensate for Arg knockdown, we favor a model in which Src acts upstream to activate Arg. Consistent with this model, several observations suggest that Src family kinases can activate Arg in vitro (34, 35) and following growth factor stimulation in cells (28, 32, 42, 47, 48). Taken together, these observations strongly suggest that Arg is a central kinase responsible for direct cortactin phosphorylation at invadopodia, while Src may regulate invadopodia function through promotion of Arg activity as well as additional independent pathways.
Model for regulation of actin polymerization in invadopodia by a Src-Arg relay
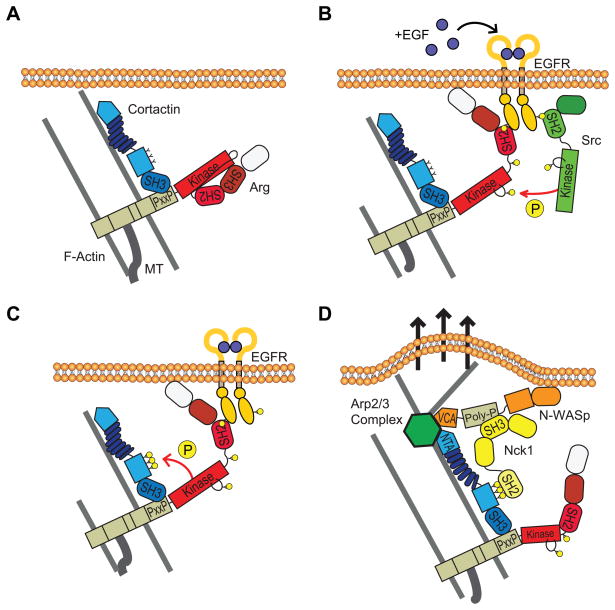
Abl family kinases are highly expressed in breast cancer cell lines, where they signal downstream of EGFR or Src kinases (32). Abl and Arg bind directly to phosphorylated EGFR through their SH2 domains, an interaction which activates the kinases and is important for their ability to regulate migration and invasion towards growth factors in vitro (33, 42). Based on these studies and on the data presented here, we propose a model by which EGF signaling through Src activates Arg to phosphorylate cortactin and stimulate actin polymerization in invadopodia (Figure 7). According to our model, Arg is recruited to invadopodium precursors, where it uses its PXXP1 motif to bind to the cortactin SH3 domain (22) (Figure 7A). EGF stimulation leads to EGF receptor autophosphorylation, providing potential binding sites for both Arg and Src SH2 domains (33, 42, 46). Binding of the Src and Arg SH2 domains relieves their autoinhibited conformation, enabling Src and Arg to autophosphorylate. In this particular pathway, activated Src can further stimulate Arg via phosphorylation of an additional tyrosine in its activation loop, enabling full Arg kinase activity (34, 35) (Figure 7B). Fully activated Arg phosphorylates cortactin on its three major tyrosine residues (Y421, Y466, Y482) (Figure 7C). Phosphorylated cortactin promotes free barbed end generation in invadopodia through activation of N-WASp-Arp2/3-mediated actin polymerization (9, 26) (Figure 7D). These coordinated activities lead to formation of an invadopodial protrusive force that enables cancer cells to penetrate and degrade through the matrix and metastasize to distant tissues and organs.
Figure 7. Model for EGF-stimulated activation of actin polymerization at invadopodia.
(A) Cortactin and actin form invadopodium precursors, to which Arg is recruited via a phosphorylation-independent binding interaction. (B) EGF stimulation promotes dimerization and tyrosine phosphorylation of the receptor. The phosphorylated receptor binds to the Arg SH2 domain, allowing for autophosphorylation of its linker tyrosine (Y272) and weak activation. The phosphorylated receptor also binds and activates Src kinase, which can phosphorylate Arg on its activation loop tyrosine (Y439), resulting in full Arg kinase activation. (C) Fully activated Arg phosphorylates cortactin at invadopodium precursors. (D) Phosphorylated cortactin induces Arp2/3-dependent actin polymerization. NTA, N-terminal acidic region; VCA, verprolin-cofilin-acidic domain; PolyP, poly-proline domain.
Supplementary Material
Acknowledgments
We wish to thank the Koleske Lab for helpful discussions and comments on the manuscript and the Einstein AIF for help with imaging. We are grateful to Xianyun Ye for technical assistance.
AJK: NIH grants NS39475, CA133346, and an American Heart Established Investigator Award
JC: NIH grants CA150344, CA113395
CM: NSF Graduate Research Fellowship
References
- 1.Barsky SH, Siegal GP, Jannotta F, Liotta LA. Loss of basement membrane components by invasive tumors but not by their benign counterparts. Lab Invest. 1983;49:140–7. [PubMed] [Google Scholar]
- 2.Yamaguchi H, Wyckoff J, Condeelis J. Cell migration in tumors. Curr Opin Cell Biol. 2005;17:559–64. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Wyckoff J, Wang W, Lin EY, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. Cancer Res. 2004;64:7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 4.Gimona M, Buccione R, Courtneidge SA, Linder S. Assembly and biological role of podosomes and invadopodia. Curr Opin Cell Biol. 2008;20:235–41. doi: 10.1016/j.ceb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Linder S. Invadosomes at a glance. J Cell Sci. 2009;122:3009–13. doi: 10.1242/jcs.032631. [DOI] [PubMed] [Google Scholar]
- 6.Stylli SS, Kaye AH, Lock P. Invadopodia: at the cutting edge of tumour invasion. J Clin Neurosci. 2008;15:725–37. doi: 10.1016/j.jocn.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 7.Weaver AM. Invadopodia: specialized cell structures for cancer invasion. Clin Exp Metastasis. 2006;23:97–105. doi: 10.1007/s10585-006-9014-1. [DOI] [PubMed] [Google Scholar]
- 8.Buccione R, Caldieri G, Ayala I. Invadopodia: specialized tumor cell structures for the focal degradation of the extracellular matrix. Cancer Metastasis Rev. 2009;28:137–49. doi: 10.1007/s10555-008-9176-1. [DOI] [PubMed] [Google Scholar]
- 9.Oser M, Yamaguchi H, Mader CC, et al. Cortactin regulates cofilin and N-WASp activities to control the stages of invadopodium assembly and maturation. J Cell Biol. 2009;186:571–87. doi: 10.1083/jcb.200812176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 11.Chan KT, Cortesio CL, Huttenlocher A. FAK alters invadopodia and focal adhesion composition and dynamics to regulate breast cancer invasion. J Cell Biol. 2009;185:357–70. doi: 10.1083/jcb.200809110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desmarais V, Yamaguchi H, Oser M, et al. N-WASP and cortactin are involved in invadopodium-dependent chemotaxis to EGF in breast tumor cells. Cell Motil Cytoskeleton. 2009;66:303–16. doi: 10.1002/cm.20361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabeh F, Ota I, Holmbeck K, et al. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. J Cell Biol. 2004;167:769–81. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldassarre M, Ayala I, Beznoussenko G, et al. Actin dynamics at sites of extracellular matrix degradation. Eur J Cell Biol. 2006;85:1217–31. doi: 10.1016/j.ejcb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Hui R, Campbell DH, Lee CS, et al. EMS1 amplification can occur independently of CCND1 or INT-2 amplification at 11q13 and may identify different phenotypes in primary breast cancer. Oncogene. 1997;15:1617–23. doi: 10.1038/sj.onc.1201311. [DOI] [PubMed] [Google Scholar]
- 16.Ormandy CJ, Musgrove EA, Hui R, Daly RJ, Sutherland RL. Cyclin D1, EMS1 and 11q13 amplification in breast cancer. Breast Cancer Res Treat. 2003;78:323–35. doi: 10.1023/a:1023033708204. [DOI] [PubMed] [Google Scholar]
- 17.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 18.Ayala I, Baldassarre M, Giacchetti G, et al. Multiple regulatory inputs converge on cortactin to control invadopodia biogenesis and extracellular matrix degradation. J Cell Sci. 2008;121:369–78. doi: 10.1242/jcs.008037. [DOI] [PubMed] [Google Scholar]
- 19.Clark ES, Weaver AM. A new role for cortactin in invadopodia: regulation of protease secretion. Eur J Cell Biol. 2008;87:581–90. doi: 10.1016/j.ejcb.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y, Tondravi M, Liu J, et al. Cortactin potentiates bone metastasis of breast cancer cells. Cancer Res. 2001;61:6906–11. [PubMed] [Google Scholar]
- 21.Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009 doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapetina S, Mader CC, Machida K, Mayer BJ, Koleske AJ. Arg interacts with cortactin to promote adhesion-dependent cell edge protrusion. J Cell Biol. 2009;185:503–19. doi: 10.1083/jcb.200809085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tehrani S, Tomasevic N, Weed S, Sakowicz R, Cooper JA. Src phosphorylation of cortactin enhances actin assembly. Proc Natl Acad Sci U S A. 2007;104:11933–8. doi: 10.1073/pnas.0701077104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamaguchi H, Lorenz M, Kempiak S, et al. Molecular mechanisms of invadopodium formation: the role of the N-WASP-Arp2/3 complex pathway and cofilin. J Cell Biol. 2005;168:441–52. doi: 10.1083/jcb.200407076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tremblay MA, Acker CM, Davies P. Tau Phosphorylated at Tyrosine 394 is Found in Alzheimer's Disease Tangles and Can Be a Product of the Abl-Related Kinase, Arg. J Alzheimers Dis. 2009 doi: 10.3233/JAD-2010-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oser M, Mader CC, Gil-Henn H, et al. Specific tyrosine phosphorylation sites on cortactin regulate Nck1-dependent actin polymerization in invadopodia. 2010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller AL, Wang Y, Mooseker MS, Koleske AJ. The Abl-related gene (Arg) requires its F-actin-microtubule cross-linking activity to regulate lamellipodial dynamics during fibroblast adhesion. J Cell Biol. 2004;165:407–19. doi: 10.1083/jcb.200308055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyle SN, Michaud GA, Schweitzer B, Predki PF, Koleske AJ. A critical role for cortactin phosphorylation by Abl-family kinases in PDGF-induced dorsal-wave formation. Curr Biol. 2007;17:445–51. doi: 10.1016/j.cub.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 29.Bowden ET, Onikoyi E, Slack R, et al. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res. 2006;312:1240–53. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 30.Seals DF, Azucena EF, Jr, Pass I, et al. The adaptor protein Tks5/Fish is required for podosome formation and function, and for the protease-driven invasion of cancer cells. Cancer Cell. 2005;7:155–65. doi: 10.1016/j.ccr.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 31.Cortesio CL, Chan KT, Perrin BJ, et al. Calpain 2 and PTP1B function in a novel pathway with Src to regulate invadopodia dynamics and breast cancer cell invasion. J Cell Biol. 2008;180:957–71. doi: 10.1083/jcb.200708048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM. c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev. 1999;13:2400–11. doi: 10.1101/gad.13.18.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu G, Decker SJ, Mayer BJ, Saltiel AR. Direct analysis of the binding of the abl Src homology 2 domain to the activated epidermal growth factor receptor. J Biol Chem. 1993;268:1775–9. [PubMed] [Google Scholar]
- 34.Tanis KQ, Veach D, Duewel HS, Bornmann WG, Koleske AJ. Two distinct phosphorylation pathways have additive effects on Abl family kinase activation. Mol Cell Biol. 2003;23:3884–96. doi: 10.1128/MCB.23.11.3884-3896.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brasher BB, Van Etten RA. c-Abl has high intrinsic tyrosine kinase activity that is stimulated by mutation of the Src homology 3 domain and by autophosphorylation at two distinct regulatory tyrosines. J Biol Chem. 2000;275:35631–7. doi: 10.1074/jbc.M005401200. [DOI] [PubMed] [Google Scholar]
- 36.Poincloux R, Lizarraga F, Chavrier P. Matrix invasion by tumour cells: a focus on MT1-MMP trafficking to invadopodia. J Cell Sci. 2009;122:3015–24. doi: 10.1242/jcs.034561. [DOI] [PubMed] [Google Scholar]
- 37.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–9. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–23. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 39.Friedl P, Wolf K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. Biochem Soc Symp. 2003:277–85. doi: 10.1042/bss0700277. [DOI] [PubMed] [Google Scholar]
- 40.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–74. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 41.Carragher NO, Walker SM, Scott Carragher LA, et al. Calpain 2 and Src dependence distinguishes mesenchymal and amoeboid modes of tumour cell invasion: a link to integrin function. Oncogene. 2006;25:5726–40. doi: 10.1038/sj.onc.1209582. [DOI] [PubMed] [Google Scholar]
- 42.Srinivasan D, Plattner R. Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res. 2006;66:5648–55. doi: 10.1158/0008-5472.CAN-06-0734. [DOI] [PubMed] [Google Scholar]
- 43.Liu S, Yamashita H, Weidow B, Weaver AM, Quaranta V. Laminin-332-beta1 integrin interactions negatively regulate invadopodia. J Cell Physiol. 2009;223:134–42. doi: 10.1002/jcp.22018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu H, Reynolds AB, Kanner SB, Vines RR, Parsons JT. Identification and characterization of a novel cytoskeleton-associated pp60src substrate. Mol Cell Biol. 1991;11:5113–24. doi: 10.1128/mcb.11.10.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H, Parsons JT. Cortactin, an 80/85-kilodalton pp60src substrate, is a filamentous actin-binding protein enriched in the cell cortex. J Cell Biol. 1993;120:1417–26. doi: 10.1083/jcb.120.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luttrell DK, Lee A, Lansing TJ, et al. Involvement of pp60c-src with two major signaling pathways in human breast cancer. Proc Natl Acad Sci U S A. 1994;91:83–7. doi: 10.1073/pnas.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Srinivasan D, Kaetzel DM, Plattner R. Reciprocal regulation of Abl and receptor tyrosine kinases. Cell Signal. 2009;21:1143–50. doi: 10.1016/j.cellsig.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasan D, Sims JT, Plattner R. Aggressive breast cancer cells are dependent on activated Abl kinases for proliferation, anchorage-independent growth and survival. Oncogene. 2008;27:1095–105. doi: 10.1038/sj.onc.1210714. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.